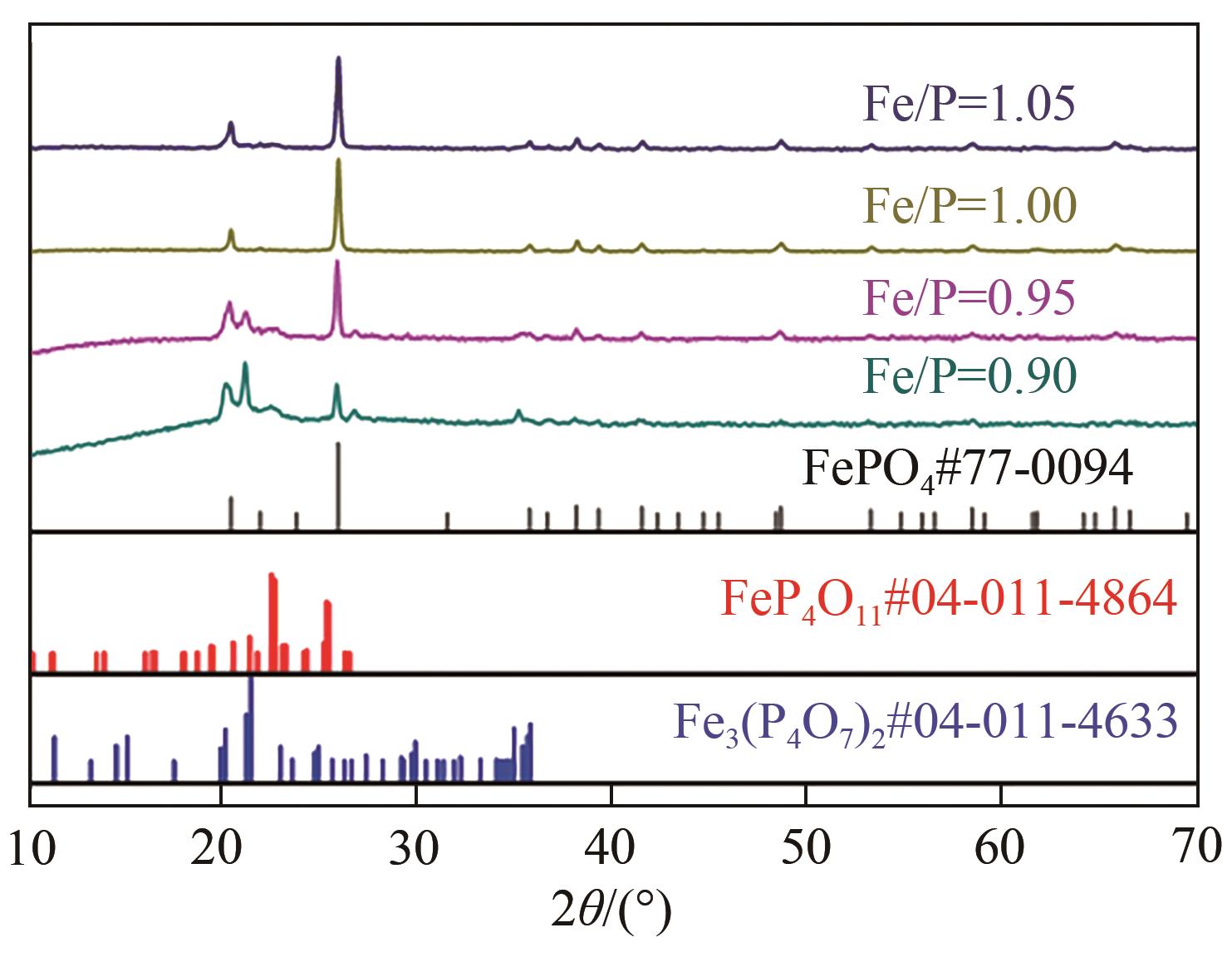
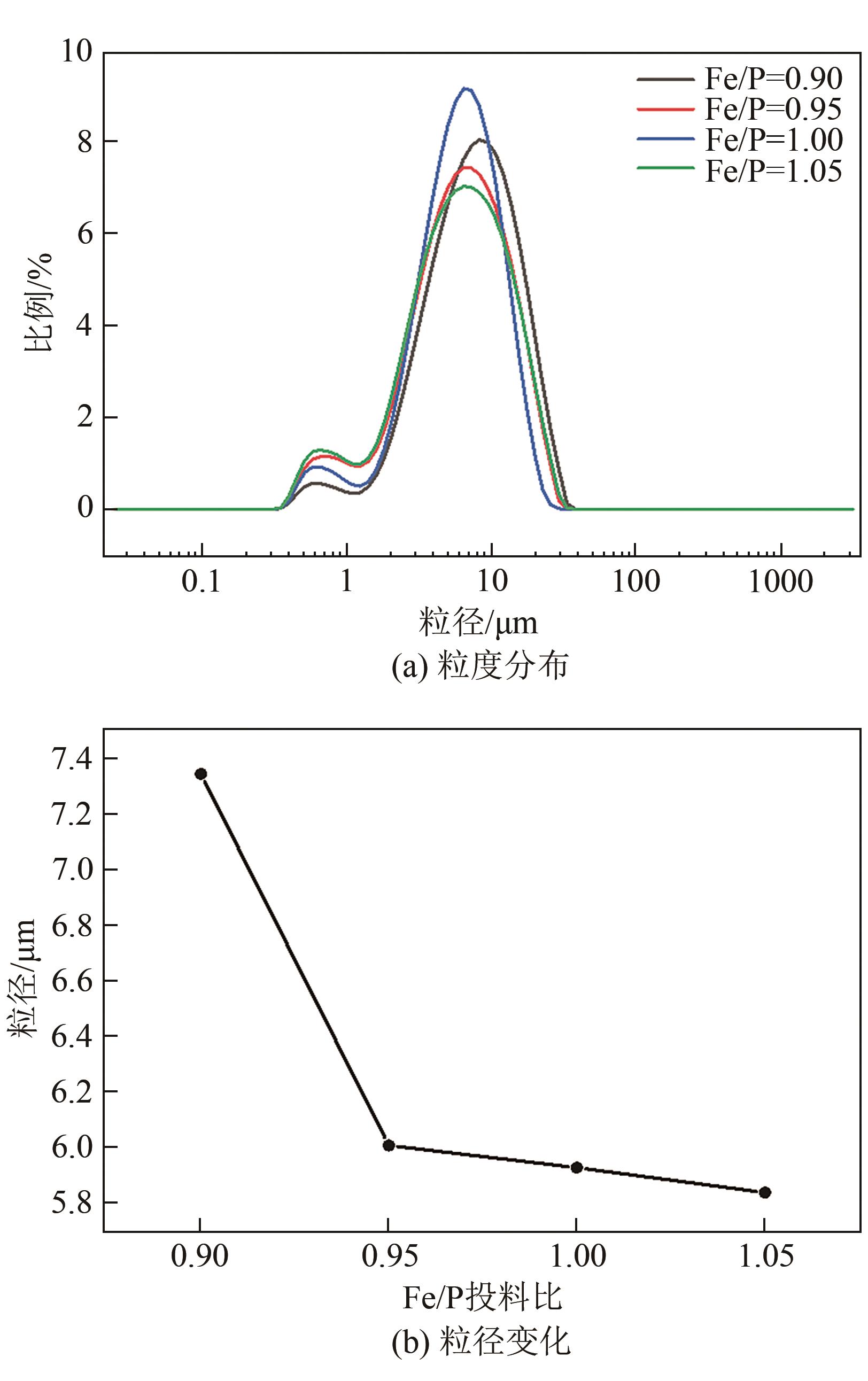
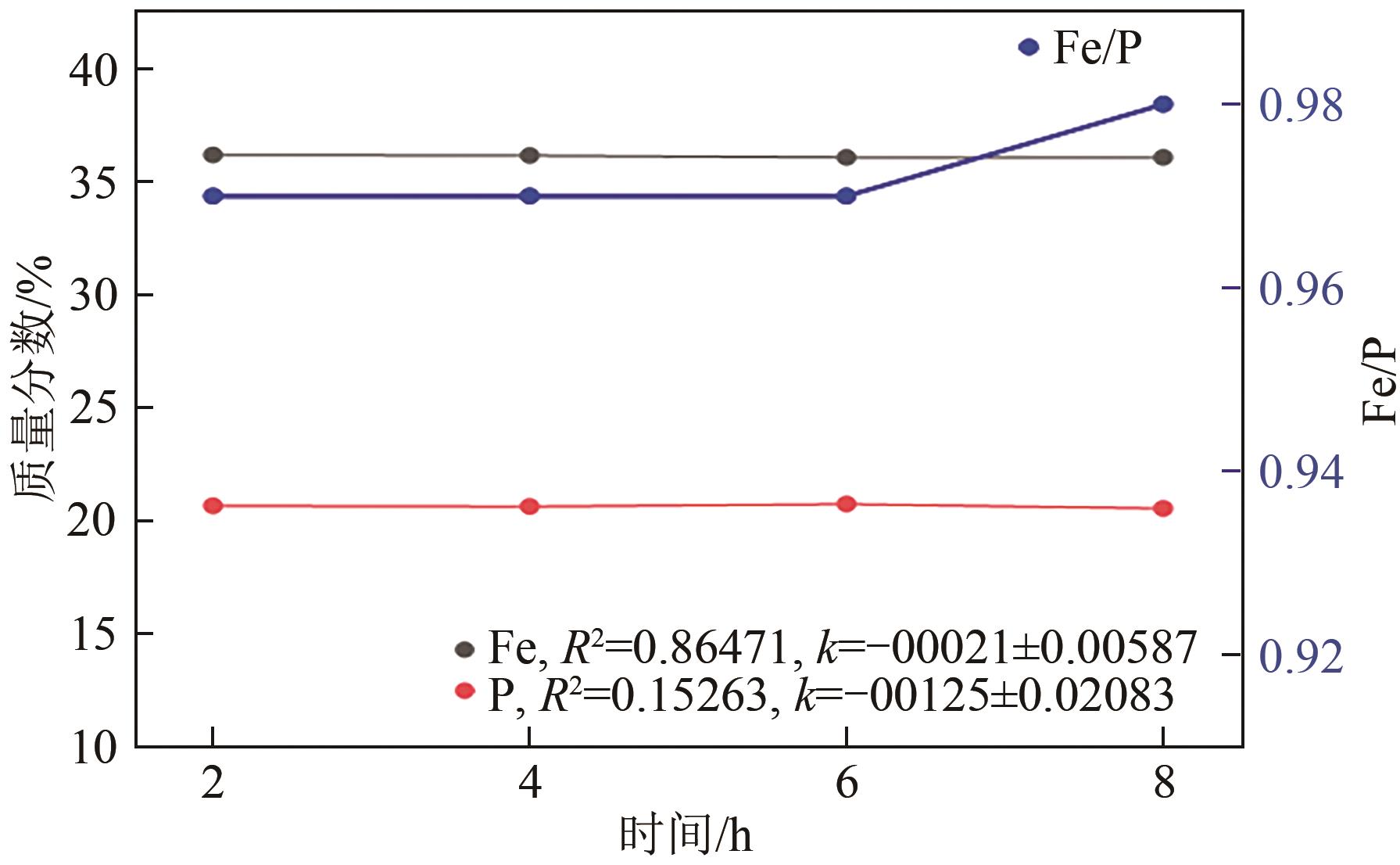
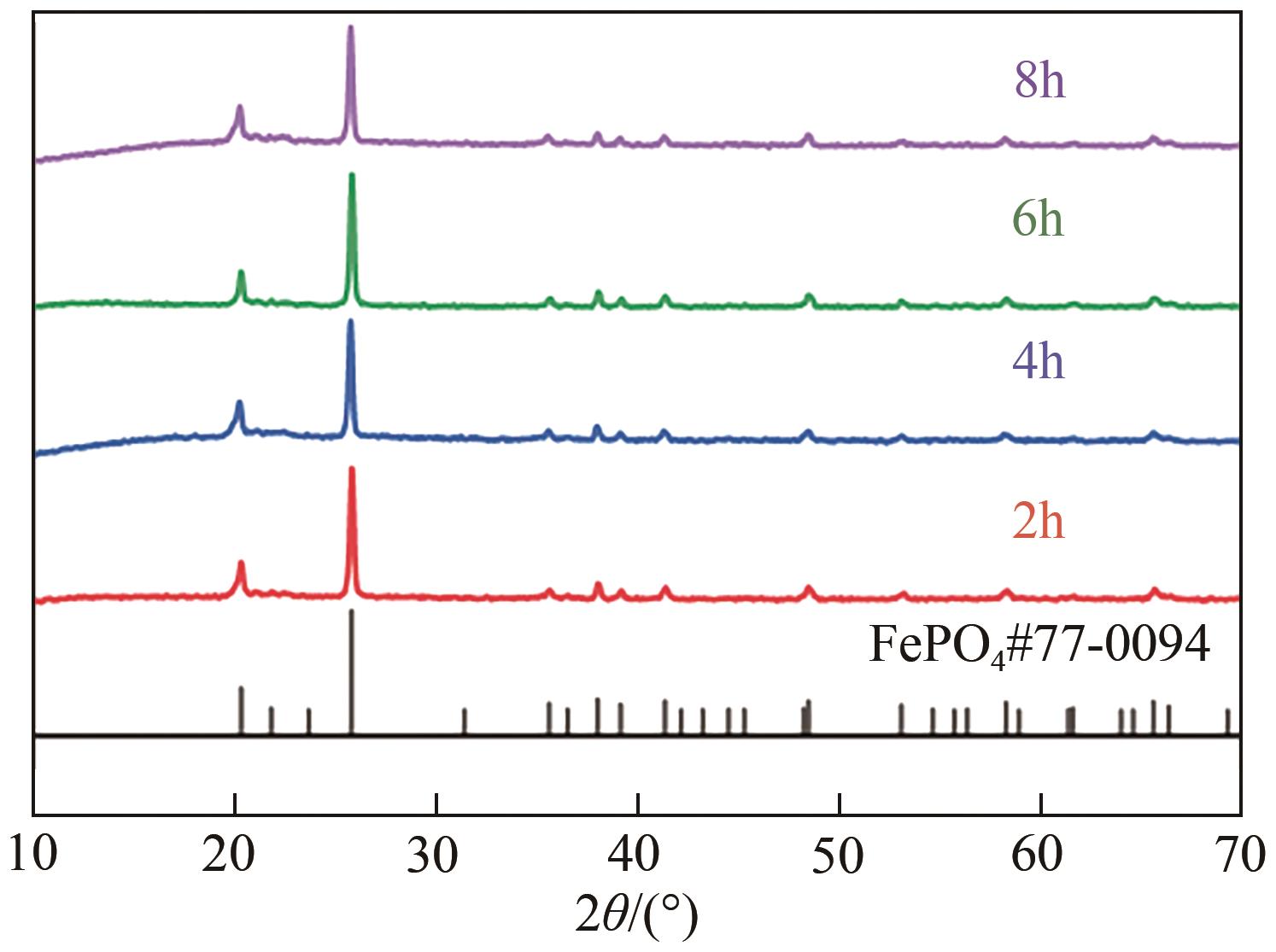
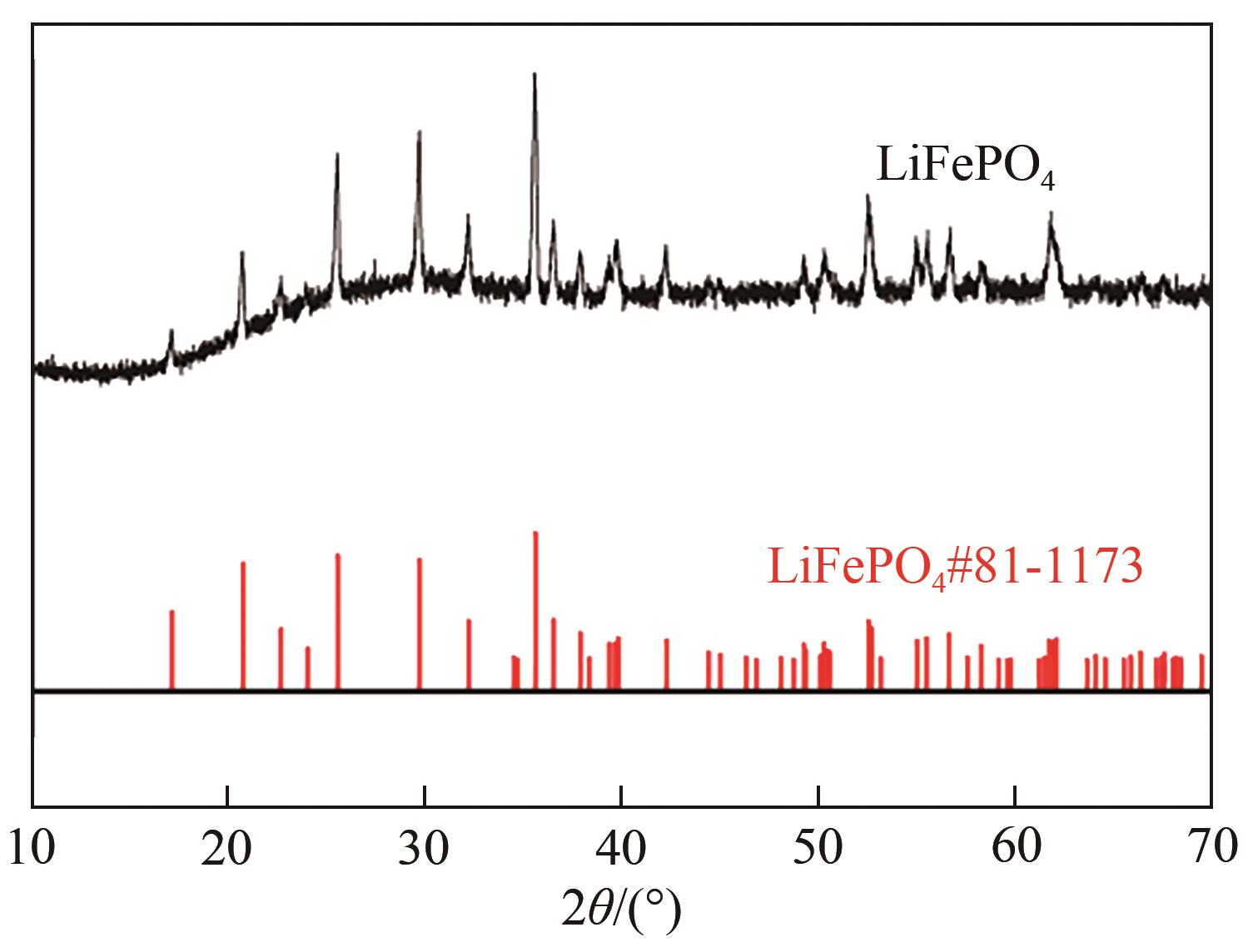
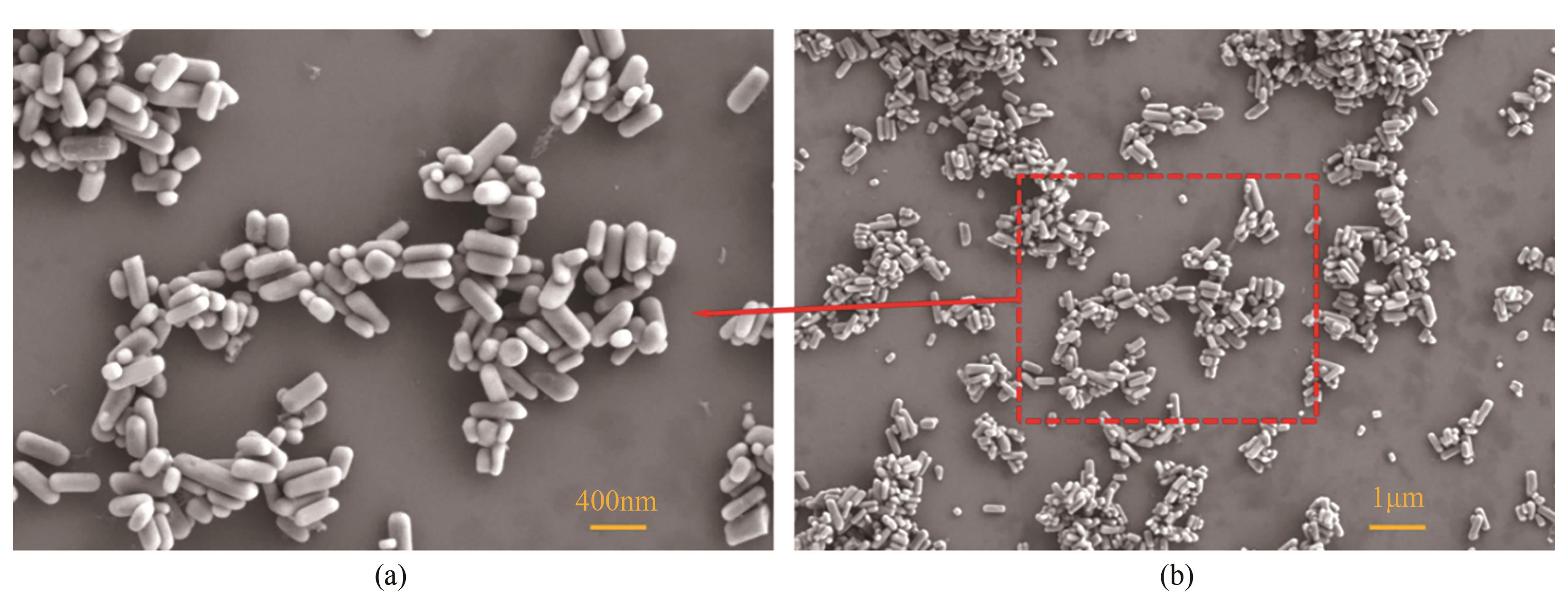
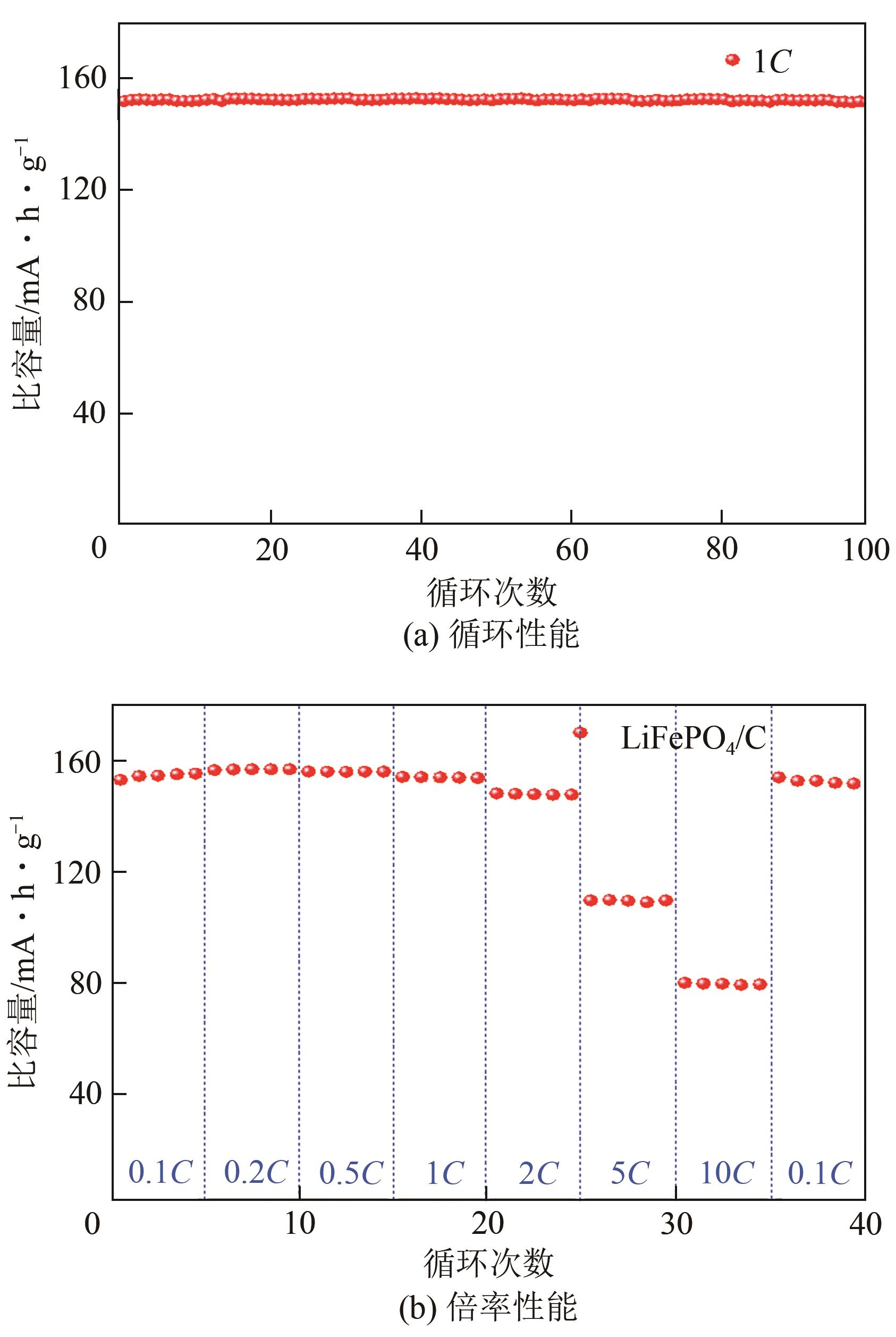
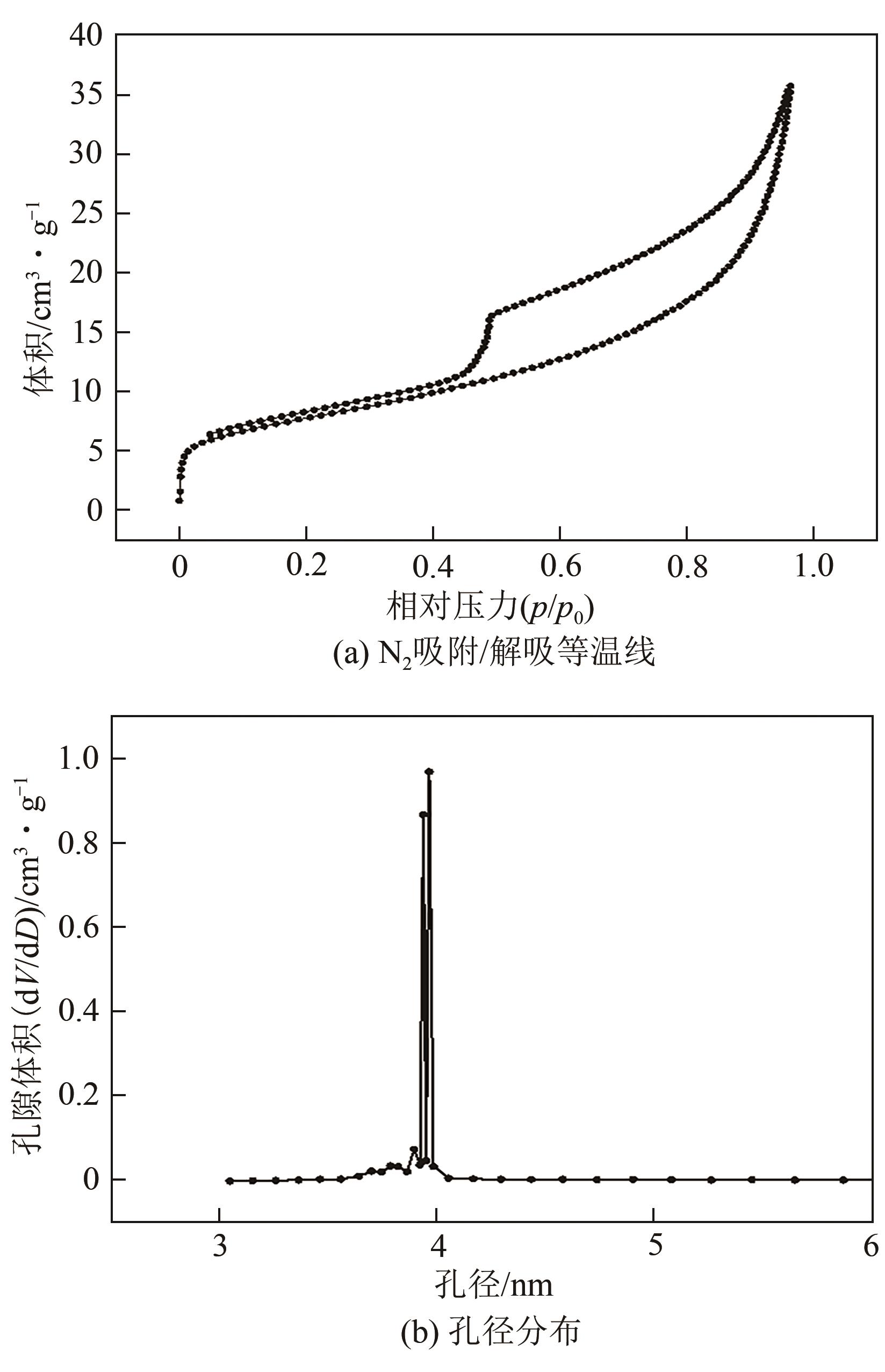
| 1 |
陈善继. 黄磷副产物磷铁的综合利用[J]. 磷肥与复肥, 2010, 25(6): 62-64.
|
|
CHEN Shanji. Comprehensive utilization of by-product ferrophosphorus in yellow phosphorus production[J]. Phosphate & Compound Fertilizer, 2010, 25(6): 62-64.
|
| 2 |
高旭伟, 吴勇生. 黄磷渣资源化利用的现状及发展趋势[J]. 中国资源综合利用, 2010, 28(1): 28-30.
|
|
GAO Xuwei, WU Yongsheng. The current comprehensive utilization and developing tendency of phosphorus slag[J]. China Resources Comprehensive Utilization, 2010, 28(1): 28-30.
|
| 3 |
马毅, 沈文喆, 袁梅梅, 等. 磷铁渣制备电池级纳米磷酸铁[J]. 化工进展, 2019, 38(11): 5015-5023.
|
|
MA Yi, SHEN Wenzhe, YUAN Meimei, et al. Preparation of battery grade nano iron phosphate by using ferro-phosphorus as raw material[J]. Chemical Industry and Engineering Progress, 2019, 38(11): 5015-5023.
|
| 4 |
赵曼, 肖仁贵, 廖霞, 等. 水热法以磷铁制备电池级磷酸铁的研究[J]. 材料导报, 2017, 31(10): 25-31.
|
|
ZHAO Man, XIAO Rengui, LIAO Xia, et al. Hydrothermal preparation of battery-grade iron phosphate from ferro-phosphorus[J]. Materials Review, 2017, 31(10): 25-31.
|
| 5 |
Dongyeon SON, KIM Eunjin, KIM Tae-Gon, et al. Nanoparticle iron-phosphate anode material for Li-ion battery[J]. Applied Physics Letters, 2004, 85(24): 5875-5877.
|
| 6 |
ZAGHIB K, JULIEN C M. Structure and electrochemistry of FePO4·2H2O hydrate[J]. Journal of Power Sources, 2005, 142(1/2): 279-284.
|
| 7 |
李成佳, 李根, 郭和一, 等. 磷化工副产磷铁渣电解制备FePO4 [J]. 磷肥与复肥, 2014, 29(4): 13-14.
|
|
LI Chengjia, LI Gen, GUO Heyi, et al. Synthesis of FePO4 by electrolyzing ferrophosphorus[J]. Phosphate & Compound Fertilizer, 2014, 29(4): 13-14.
|
| 8 |
张伟伟, 肖仁贵, 曹建新, 等. 微波消解磷铁制备电池级磷酸铁研究[J]. 无机盐工业, 2015, 47(3): 23-26.
|
|
ZHANG Weiwei, XIAO Rengui, CAO Jianxin, et al. Preparation of battery level ironic phosphate by microwave dissolving the ferro-phosphorus[J]. Inorganic Chemicals Industry, 2015, 47(3): 23-26.
|
| 9 |
任鹏文, 杜柯, 江剑兵, 等. 以磷铁粉为原料制备磷酸铁锂的工艺研究[J]. 轻金属, 2023(3): 53-59.
|
|
REN Pengwen, DU Ke, JIANG Jianbing, et al. Process study on the preparation of lithium iron phosphate from iron phosphate powder[J]. Light Metals, 2023(3): 53-59.
|
| 10 |
吴林君, 张家新, 管道安, 等. 水热法制备高密度超细磷酸铁锂多晶粉[J]. 武汉工程大学学报, 2012, 34(9): 26-29.
|
|
WU Linjun, ZHANG Jiaxin, GUAN Dao’an, et al. Hydrothermal synthesis of ultra-fine lithium iron phosphate powders with high tap densities[J]. Journal of Wuhan Institute of Technology, 2012, 34(9): 26-29.
|
| 11 |
苏晓飞, 张校刚. 水热法制备LiFePO4正极材料[J]. 化学进展, 2011, 23(6): 1090-1099.
|
|
SU Xiaofei, ZHANG Xiaogang. Hydrothermal synthesis of LiFePO4 cathode[J]. Progress in Chemistry, 2011, 23(6): 1090-1099.
|
| 12 |
马航, 查坐统, 王君婷, 等. 锂离子电池前体磷酸铁合成方法研究现状及展望[J]. 磷肥与复肥, 2023, 38(3): 19-22.
|
|
MA Hang, ZHA Zuotong, WANG Junting, et al. Research status and prospect of synthesis method of iron phosphate as lithium battery precursor[J]. Phosphate & Compound Fertilizer, 2023, 38(3): 19-22.
|
| 13 |
马志鸣, 肖仁贵, 廖霞, 等. 片层纳米结构磷酸铁制备及对磷酸铁锂电性能的影响[J]. 材料导报, 2018, 32(19): 3325-3331.
|
|
MA Zhiming, XIAO Rengui, LIAO Xia, et al. Preparation of the lameller nanostructure iron phosphate and its effect on the electrochemical performance of lithium iron phosphate[J]. Materials Review, 2018, 32(19): 3325-3331.
|
| 14 |
骆艳华, 佘世杰, 曹卫国, 等. 反应条件对磷酸铁粒度分布的影响[J]. 山东大学学报(工学版), 2015, 45(1): 82-87.
|
|
LUO Yanhua, SHE Shijie, CAO Weiguo, et al. Effects of reaction conditions on the size distribution of iron phosphate[J]. Journal of Shandong University (Engineering Science), 2015, 45(1): 82-87.
|
 ), QIN Anrui1,2, ZHOU Guimin1,2, CHEN Qiulin1,2, YUAN Yajie1,2, YAO Yaochun1,2(
), QIN Anrui1,2, ZHOU Guimin1,2, CHEN Qiulin1,2, YUAN Yajie1,2, YAO Yaochun1,2( ), LI Yin1,2(
), LI Yin1,2( )
)
 ), 秦安瑞1,2, 周桂民1,2, 陈秋霖1,2, 袁亚杰1,2, 姚耀春1,2(
), 秦安瑞1,2, 周桂民1,2, 陈秋霖1,2, 袁亚杰1,2, 姚耀春1,2( ), 李银1,2(
), 李银1,2( )
)