Chemical Industry and Engineering Progress ›› 2023, Vol. 42 ›› Issue (3): 1341-1352.DOI: 10.16085/j.issn.1000-6613.2022-0952
• Industrial catalysis • Previous Articles Next Articles
Cu-Zn catalyzed hydrogenation of furfural with methanol as hydrogen donor
XIAO Yaoxin1,2( ), ZHANG Jun2,3,4(
), ZHANG Jun2,3,4( ), HU Sheng5, SHAN Rui2,3,4, YUAN Haoran2,3,4(
), HU Sheng5, SHAN Rui2,3,4, YUAN Haoran2,3,4( ), CHEN Yong1,2,3,4
), CHEN Yong1,2,3,4
- 1.Institute of Biomass Engineering, South China Agricultural University, Guangzhou 510642, Guangdong, China
2.Guangzhou Institute of Energy Conversion, Chinese Academy of Sciences (CAS), Guangzhou 510640, Guangdong, China
3.CAS Key Laboratory of Renewable Energy, Guangzhou 510640, Guangdong, China
4.Guangdong Provincial Key Laboratory of New and Renewable Energy Research and Development, Guangzhou 510640, Guangdong, China
5.School of Engineering Science, University of Science and Technology of China, Hefei 230026, Anhui, China
-
Received:2022-05-23Revised:2022-11-22Online:2023-04-10Published:2023-03-15 -
Contact:YUAN Haoran
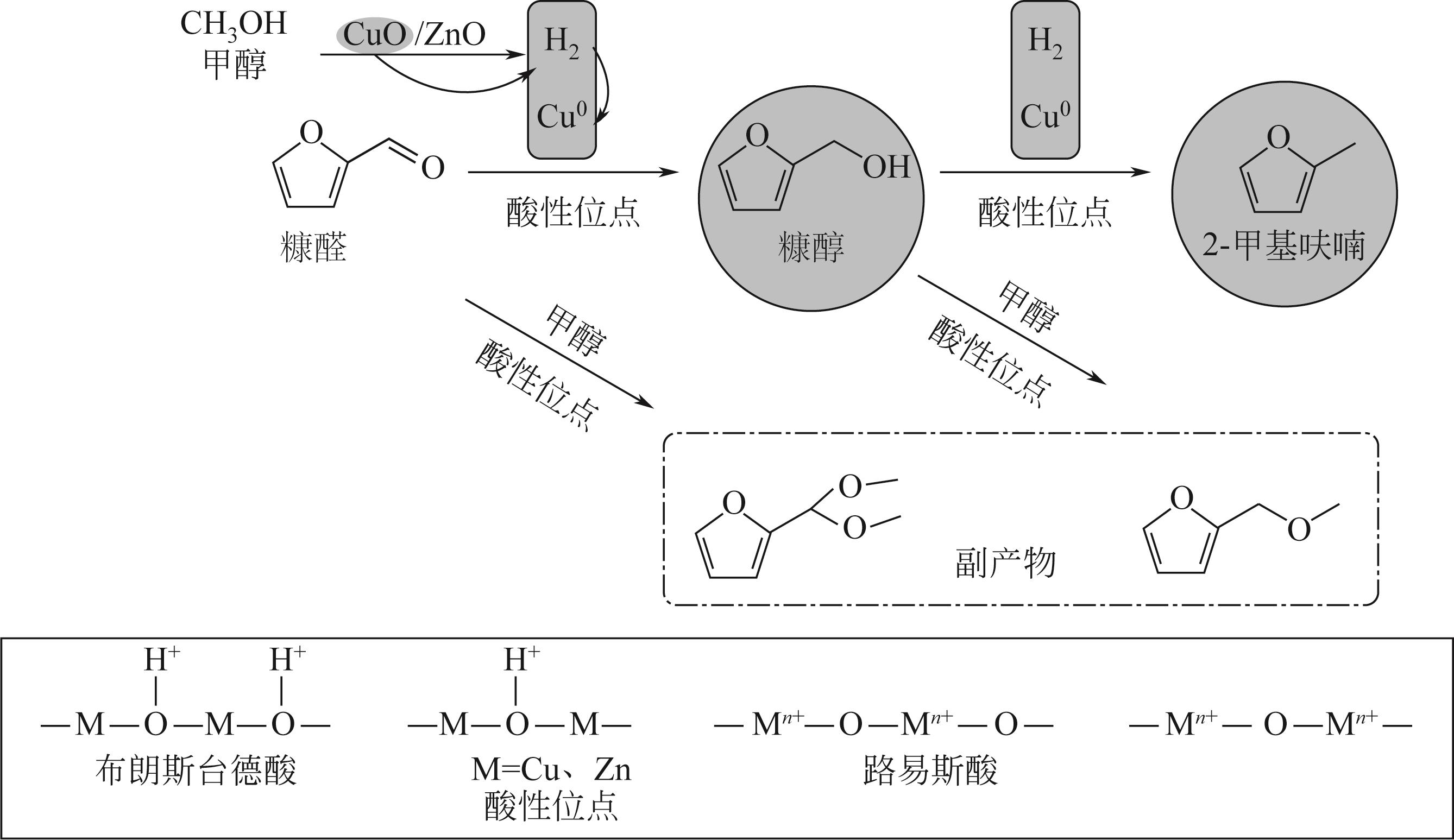
甲醇供氢体系铜锌双金属催化糠醛加氢转化
萧垚鑫1,2( ), 张军2,3,4(
), 张军2,3,4( ), 胡升5, 单锐2,3,4, 袁浩然2,3,4(
), 胡升5, 单锐2,3,4, 袁浩然2,3,4( ), 陈勇1,2,3,4
), 陈勇1,2,3,4
- 1.华南农业大学生物质工程研究院,广东 广州 510642
2.中国科学院广州能源研究所,广东 广州 510640
3.中国科学院可再生能源重点实验室,广东 广州 510640
4.广东省新能源和可再生能源研究开发与应用重点 实验室,广东 广州 510640
5.中国科学技术大学工程科学学院,安徽 合肥 230026
-
通讯作者:袁浩然 -
作者简介:萧垚鑫(1998—),男,硕士研究生,研究方向为生物质高值资源化利用。E-mail:2252164032@qq.com
张军(1987—),男,博士,副研究员,研究方向为有机固废/农林废弃生物质高值资源化利用。E-mail:zhagnjun@ms.giec.ac.cn。 -
基金资助:国家自然科学基金面上项目(51976222);能源清洁利用国家重点实验室开放基金课题(ZJU-CEU2020023)
CLC Number:
Cite this article
XIAO Yaoxin, ZHANG Jun, HU Sheng, SHAN Rui, YUAN Haoran, CHEN Yong. Cu-Zn catalyzed hydrogenation of furfural with methanol as hydrogen donor[J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1341-1352.
萧垚鑫, 张军, 胡升, 单锐, 袁浩然, 陈勇. 甲醇供氢体系铜锌双金属催化糠醛加氢转化[J]. 化工进展, 2023, 42(3): 1341-1352.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2022-0952
| 组别 | 前体溶液的Cu/Zn比 | Cu浓度 /mol·L-1 | Zn浓度 /mol·L-1 | Cu/Zn | 简记 |
|---|---|---|---|---|---|
| 1 | 0.25 | 0.90 | 4.04 | 0.22 | CZ-0.22 |
| 2 | 0.43 | 1.28 | 3.39 | 0.38 | CZ-0.38 |
| 3 | 0.67 | 1.78 | 2.96 | 0.60 | CZ-0.60 |
| 4 | 1.00 | 2.19 | 2.44 | 0.90 | CZ-0.90 |
| 5 | 1.50 | 2.79 | 2.04 | 1.30 | CZ-1.30 |
| 组别 | 前体溶液的Cu/Zn比 | Cu浓度 /mol·L-1 | Zn浓度 /mol·L-1 | Cu/Zn | 简记 |
|---|---|---|---|---|---|
| 1 | 0.25 | 0.90 | 4.04 | 0.22 | CZ-0.22 |
| 2 | 0.43 | 1.28 | 3.39 | 0.38 | CZ-0.38 |
| 3 | 0.67 | 1.78 | 2.96 | 0.60 | CZ-0.60 |
| 4 | 1.00 | 2.19 | 2.44 | 0.90 | CZ-0.90 |
| 5 | 1.50 | 2.79 | 2.04 | 1.30 | CZ-1.30 |
| 样品 | 比表面积/m2·g-1 | 孔容/m3·g-1 | 孔径/nm |
|---|---|---|---|
| CZ-0.22 | 113.59 | 6.97 | 3.42 |
| CZ-0.38 | 46.67 | 2.66 | 17.07 |
| CZ-0.60 | 51.96 | 2.62 | 15.35 |
| CZ-0.90 | 38.07 | 1.83 | 15.31 |
| CZ-1.30 | 48.25 | 2.36 | 16.57 |
| 样品 | 比表面积/m2·g-1 | 孔容/m3·g-1 | 孔径/nm |
|---|---|---|---|
| CZ-0.22 | 113.59 | 6.97 | 3.42 |
| CZ-0.38 | 46.67 | 2.66 | 17.07 |
| CZ-0.60 | 51.96 | 2.62 | 15.35 |
| CZ-0.90 | 38.07 | 1.83 | 15.31 |
| CZ-1.30 | 48.25 | 2.36 | 16.57 |
| 序号 | 样品 | 糠醛转化率 /% | 产物收率/% | 副产物收率 /% | |
|---|---|---|---|---|---|
| 糠醇 | 2-甲基呋喃 | ||||
| 1 | CZ-0.22 | 100.0 | 70.3 | 6.5 | 23.2 |
| 2 | CZ-0.38 | 100.0 | 71.4 | 6.6 | 22.0 |
| 3 | CZ-0.60 | 100.0 | 69.0 | 12.3 | 18.7 |
| 4 | CZ-0.90 | 100.0 | 72.8 | 4.4 | 22.8 |
| 5 | CZ-1.30 | 100.0 | 70.3 | 8.2 | 21.5 |
| 序号 | 样品 | 糠醛转化率 /% | 产物收率/% | 副产物收率 /% | |
|---|---|---|---|---|---|
| 糠醇 | 2-甲基呋喃 | ||||
| 1 | CZ-0.22 | 100.0 | 70.3 | 6.5 | 23.2 |
| 2 | CZ-0.38 | 100.0 | 71.4 | 6.6 | 22.0 |
| 3 | CZ-0.60 | 100.0 | 69.0 | 12.3 | 18.7 |
| 4 | CZ-0.90 | 100.0 | 72.8 | 4.4 | 22.8 |
| 5 | CZ-1.30 | 100.0 | 70.3 | 8.2 | 21.5 |
| 1 | 李全生, 张凯. 我国能源绿色开发利用路径研究[J]. 中国工程科学, 2021, 23(1): 101-111. |
| LI Quansheng, ZHANG Kai. The path for green development and utilization of energy in China[J]. Strategic Study of CAE, 2021, 23(1): 101-111. | |
| 2 | Thomas Bejoy, Midhun C RAJ, Athira K B, et al. Nanocellulose, a versatile green platform: From biosources to materials and their applications[J]. Chemical Reviews, 2018, 118(24): 11575-11625. |
| 3 | MA Jiping, SHI Song, JIA Xiuquan, et al. Advances in catalytic conversion of lignocellulose to chemicals and liquid fuels[J]. Journal of Energy Chemistry, 2019, 36: 74-86. |
| 4 | GALKIN Maxim V, SAMEC Joseph S M. Lignin valorization through catalytic lignocellulose fractionation: A fundamental platform for the future biorefinery[J]. ChemSusChem, 2016, 9(13): 1544-1558. |
| 5 | TESTA Maria Luisa, TUMMINO Maria Laura. Lignocellulose biomass as a multifunctional tool for sustainable catalysis and chemicals: An overview[J]. Catalysts, 2021, 11(1): 125. |
| 6 | YUAN Haoran, LI Chengyu, SHAN Rui, et al. Municipal sludge derived solid acids for levoglucosenone production via cellulose fast pyrolysis[J]. Journal of Analytical and Applied Pyrolysis, 2022, 167: 105663. |
| 7 | ZHANG Jun, LI Chengyu, YUAN Haoran, et al. Enhancement of aromatics production via cellulose fast pyrolysis over Ru modified hierarchical zeolites[J]. Renewable Energy, 2022, 184: 280-290. |
| 8 | 石宁, 唐文勇, 唐石云, 等. 木质纤维素衍生平台化学品制备液态烷烃的研究进展[J]. 化工进展, 2019, 38(7): 3097-3110. |
| SHI Ning, TANG Wenyong, TANG Shiyun, et al. Advances in the catalytic conversion of lignocellulosic derived platform chemicals into liquid alkanes[J]. Chemical Industry and Engineering Progress, 2019, 38(7): 3097-3110. | |
| 9 | 刘志斌, 张学勤. 木质纤维素生物质催化转化为高附加值产品的研究进展[J]. 纤维素科学与技术, 2019, 27(3): 77-82. |
| LIU Zhibin, ZHANG Xueqin. Progress of lignocellulose biomass catalytic conversion to high added-value products[J]. Journal of Cellulose Science and Technology, 2019, 27(3): 77-82. | |
| 10 | 李诗琪, 刘蝈蝈, 张雅静, 等. 糠醛转移加氢制糠醇催化剂的研究进展[J]. 当代化工, 2021, 50(7): 1724-1727. |
| LI Shiqi, LIU Guoguo, ZHANG Yajing, et al. Research progress of catalysts for furfural transfer hydrogenation to furfuryl alcohol[J]. Contemporary Chemical Industry, 2021, 50(7): 1724-1727. | |
| 11 | 陈佳宁, 王慧, 刘慰, 等. 木质纤维素的酸催化精炼研究进展[J]. 中国造纸, 2021, 40(8): 75-82. |
| CHEN Jianing, WANG Hui, LIU Wei, et al. Research progress on acid catalytic refining of lignocellulose[J]. China Pulp & Paper, 2021, 40(8): 75-82. | |
| 12 | 朱晨杰, 张会岩, 肖睿, 等. 木质纤维素高值化利用的研究进展[J]. 中国科学: 化学, 2015, 45(5): 454-478. |
| ZHU Chenjie, ZHANG Huiyan, XIAO Rui, et al. Research progress in catalytic valorization of lignocellulose[J]. Scientia Sinica Chimica, 2015, 45(5): 454-478. | |
| 13 | 熊健, 吕学斌, 任国权, 等. 生物质材料制备糠醛和5-羟甲基糠醛的研究进展[J]. 现代化工, 2022, 42(5): 30-34. |
| XIONG Jian, LV Xuebin, REN Guoquan, et al. Research progress in preparation of furfural and 5-hydroxymethylfurfural from biomass materials[J]. Modern Chemical Industry, 2022, 42(5): 30-34. | |
| 14 | 邓理, 廖兵, 郭庆祥. 纤维素选择性催化转化为重要平台化合物的研究进展[J]. 化工进展, 2013, 32(2): 245-254. |
| DENG Li, LIAO Bing, GUO Qingxiang. Recent progress in selective catalytic conversion of cellulose into key platform molecules[J]. Chemical Industry and Engineering Progress, 2013, 32(2): 245-254. | |
| 15 | 张军, 李丹妮, 袁浩然, 等. 生物质基糠醛和5-羟甲基糠醛加氢转化研究进展[J]. 燃料化学学报, 2021, 49(12): 1752-1767. |
| ZHANG Jun, LI Danni, YUAN Haoran, et al. Advances on the catalytic hydrogenation of biomass-derived furfural and 5-hydroxymethylfurfural[J]. Journal of Fuel Chemistry and Technology, 2021, 49(12): 1752-1767. | |
| 16 | LONG Jingxuan, XU Yufei, ZHAO Wenfeng, et al. Heterogeneous catalytic upgrading of biofuranic aldehydes to alcohols[J]. Frontiers in Chemistry, 2019, 7: 529. |
| 17 | HOANG Anh Tuan, VAN VIET PHAM. 2-Methylfuran (MF) as a potential biofuel: A thorough review on the production pathway from biomass, combustion progress, and application in engines[J]. Renewable and Sustainable Energy Reviews, 2021, 148: 111265. |
| 18 | 聂一凡, 候其东, 李维尊, 等. 糠醛的水解制备和应用研究进展[J]. 化工进展, 2019, 38(5): 2164-2178. |
| NIE Yifan, HOU Qidong, LI Weizun, et al. Advances in production furfural via hydrolysis and application of furfural[J]. Chemical Industry and Engineering Progress, 2019, 38(5): 2164-2178. | |
| 19 | 陈伦刚, 张兴华, 张琦, 等. 木质纤维素解聚平台分子催化合成航油技术的进展[J]. 化工进展, 2019, 38(3): 1269-1282. |
| CHEN Lungang, ZHANG Xinghua, ZHANG Qi, et al. Progress in aviation biofuel technology by catalysis synthesis of platform molecules from lignocelluloses depolymerization[J]. Chemical Industry and Engineering Progress, 2019, 38(3): 1269-1282. | |
| 20 | 闫瑞, 郭勇, 李杨, 等. 生物质衍生物催化转化制喷气燃料组分的研究进展[J]. 化工进展, 2016, 35(9): 2735-2745. |
| YAN Rui, GUO Yong, LI Yang, et al. Progress in catalytic production of jet fuel range alkanes from biomass-derivatives[J]. Chemical Industry and Engineering Progress, 2016, 35(9): 2735-2745. | |
| 21 | SHIVHARE Atal, KUMAR Abhinav, SRIVASTAVA Rajendra. An account of the catalytic transfer hydrogenation and hydrogenolysis of carbohydrate-derived renewable platform chemicals over non-precious heterogeneous metal catalysts[J]. ChemCatChem, 2021, 13(1): 59-80. |
| 22 | MARISCAL R, MAIRELES-TORRES P, OJEDA M, et al. Furfural: A renewable and versatile platform molecule for the synthesis of chemicals and fuels[J]. Energy & Environmental Science, 2016, 9(4): 1144-1189. |
| 23 | FANG Wenting, RIISAGER Anders. Recent advances in heterogeneous catalytic transfer hydrogenation/hydrogenolysis for valorization of biomass-derived furanic compounds[J]. Green Chemistry, 2021, 23(2): 670-688. |
| 24 | AN Zhidong, LI Jiang. Recent advances in the catalytic transfer hydrogenation of furfural to furfuryl alcohol over heterogeneous catalysts[J]. Green Chemistry, 2022, 24(5): 1780-1808. |
| 25 | 王庄清, 张弨, 赵凤玉. 糠醛及其衍生物催化加氢制备1, 5-戊二醇研究进展[J]. 科学通报, 2019, 64(31): 3165-3172. |
| WANG Zhuangqing, ZHANG Chao, ZHAO Fengyu. Recent advances in the hydrogenolysis of furfural and its derivatives to 1, 5-pentanediol[J]. Chinese Science Bulletin, 2019, 64(31): 3165-3172. | |
| 26 | ZHANG Jun, CHEN Jinzhu. Selective transfer hydrogenation of biomass-based furfural and 5-hydroxymethylfurfural over hydrotalcite-derived copper catalysts using methanol as a hydrogen donor[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(7): 5982-5993. |
| 27 | LI Bolong, LI Lulu, SUN Hao, et al. Selective deoxygenation of aqueous furfural to 2-methylfuran over Cu0/Cu2O·SiO2 sites via a copper phyllosilicate precursor without extraneous gas[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(9): 12096-12103. |
| 28 | HANSEN Thomas S, BARTA Katalin, ANASTAS Paul T, et al. One-pot reduction of 5-hydroxymethylfurfural via hydrogen transfer from supercritical methanol[J]. Green Chemistry, 2012, 14(9): 2457-2461. |
| 29 | NAGARAJA Bhari Mallanna, PADMASRI Aytam Hari, RAJU Burri David, et al. Production of hydrogen through the coupling of dehydrogenation and hydrogenation for the synthesis of cyclohexanone and furfuryl alcohol over different promoters supported on Cu-MgO catalysts[J]. International Journal of Hydrogen Energy, 2011, 36(5): 3417-3425. |
| 30 | 李聪明, 陈阔, 王晓月, 等. 探究Cu/ZnO相互作用对CO2加氢制甲醇反应性能的影响[J]. 物理化学学报, 2021, 37(5): 201-212. |
| LI Congming, CHEN Kuo, WANG Xiaoyue, et al. Understanding the role of Cu/ZnO interaction in CO2 hydrogenation to methanol[J]. Acta Physico-Chimica Sinica, 2021, 37(5): 201-212. | |
| 31 | SEBASTIAN P J, QUINTANA J, AVILA F. Retention of the high optical absorptance in thermally aged black chrome on variably sensitized Cu[J]. Solar Energy Materials and Solar Cells, 1997, 45(1): 65-74. |
| 32 | HU Yubing, ZHANG Yajing, DU Jie, et al. The influence of composition on the functionality of hybrid CuO-ZnO-Al2O3/HZSM-5 for the synthesis of DME from CO2 hydrogenation[J]. RSC Advances, 2018, 8(53): 30387-30395. |
| 33 | EL-SHOBAKY G A, AHMED A S, FAGAL G A, et al. Solid–solid interaction in CuO-ZnO/Al2O3 system under varying conditions[J]. Thermochimica Acta, 1998, 319(1/2): 67-74. |
| 34 | 姚志龙, 闵恩泽. ZnO对CuO-ZnO/Al2O3催化剂催化甘油氢解性能的影响[J]. 精细化工, 2011, 28(9): 866-869, 874. |
| YAO Zhilong, MIN Enze. Effect of ZnO on the hydrogenolysis performance of CuO-ZnO/Al2O3 in catalyzing glycerol[J]. Fine Chemicals, 2011, 28(9): 866-869, 874. | |
| 35 | 郭馨馨, 张希清. BiOCl/Zn-Al LDHs复合材料的制备及光催化性能研究[J]. 石家庄铁道大学学报(自然科学版), 2018, 31(4): 88-95. |
| GUO Xinxin, ZHANG Xiqing. Preparation and photocatalytic activity of BiOCl/Zn-Al layered double hydroxides composites[J]. Journal of Shijiazhuang Tiedao University (Natural Science Edition), 2018, 31(4): 88-95. | |
| 36 | POURMORTAZAVI Seied Mahdi, MARASHIANPOUR Zahra, KARIMI Meisam Sadeghpour, et al. Electrochemical synthesis and characterization of zinc carbonate and zinc oxide nanoparticles[J]. Journal of Molecular Structure, 2015, 1099: 232-238. |
| 37 | EL-SHOBAKY G A, AHMAD A S, AL-NOAIMI A N, et al. Thermal decomposition of basic cobalt and copper carbonates[J]. Journal of Thermal Analysis, 1996, 46(6): 1801-1808. |
| 38 | SONG Hyun-tae, FAZELI Ali, KIM Hyun Dong, et al. Effect of lanthanum group promoters on Cu/(mixture of ZnO and Zn-Al-spinnel-oxides) catalyst for methanol synthesis by hydrogenation of CO and CO2 mixtures[J]. Fuel, 2021, 283: 118987. |
| 39 | DING Wen, LIU Yingwei, WANG Fang, et al. Promoting effect of a Cu-Zn binary precursor on a ternary Cu-Zn-Al catalyst for methanol synthesis from synthesis gas[J]. RSC Advances, 2014, 4(58): 30677-30682. |
| 40 | WANG Jianqiang, WANG Youzhen, XIE Songhai, et al. Partial hydrogenation of benzene to cyclohexene on a Ru-Zn/m-ZrO2 nanocomposite catalyst[J]. Applied Catalysis A: General, 2004, 272(1/2): 29-36. |
| 41 | LIU Hailong, HUANG Zhiwei, KANG Haixiao, et al. Selective hydrogenolysis of biomass-derived furfuryl alcohol into 1, 2- and 1, 5-pentanediol over highly dispersed Cu-Al2O3 catalysts[J]. Chinese Journal of Catalysis, 2016, 37(5): 700-710. |
| 42 | MENG Hao, LIU Jiangning, DU Yali, et al. Novel Cu-based oxides catalyst from one-step carbothermal reduction decomposition method for selective catalytic reduction of NO with NH3 [J]. Catalysis Communications, 2019, 119: 101-105. |
| 43 | LU Ping, ZHOU Wei, LI Ying, et al. CuO nanosheets/ZnO nanorods synthesized by a template-free hydrothermal approach and their optical and magnetic characteristics[J]. Ceramics International, 2017, 43(13): 9798-9805. |
| 44 | WANG Yue, LIAO Junyu, ZHANG Jian, et al. Hydrogenation of methyl acetate to ethanol by Cu/ZnO catalyst encapsulated in SBA-15[J]. AIChE Journal, 2017, 63(7): 2839-2849. |
| 45 | WANG Wen, XU Linhua, ZHANG Ruofan, et al. Coexistence of ferromagnetism and paramagnetism in ZnO/CuO nanocomposites[J]. Chemical Physics Letters, 2019, 721: 57-61. |
| 46 | LI Yandong, LIANG Guangfen, WANG Chengrui, et al. Effect of precipitated precursor on the catalytic performance of mesoporous carbon supported CuO-ZnO catalysts[J]. Crystals, 2021, 11(6): 582. |
| 47 | LI Li, MAO Dongsen, YU Jun, et al. Highly selective hydrogenation of CO2 to methanol over CuO-ZnO-ZrO2 catalysts prepared by a surfactant-assisted co-precipitation method[J]. Journal of Power Sources, 2015, 279: 394-404. |
| 48 | MATSON Theodore D, Barta Katalin, IRETSKII Alexei V, et al. One-pot catalytic conversion of cellulose and of woody biomass solids to liquid fuels[J]. Journal of the American Chemical Society, 2011, 133(35): 14090-14097. |
| 49 | GILKEY Matthew J, PANAGIOTOPOULOU Paraskevi, MIRONENKO Alexander V, et al. Mechanistic insights into metal lewis acid-mediated catalytic transfer hydrogenation of furfural to 2-methylfuran[J]. ACS Catalysis, 2015, 5(7): 3988-3994. |
| [1] | SHI Yongxing, LIN Gang, SUN Xiaohang, JIANG Weigeng, QIAO Dawei, YAN Binhang. Research progress on active sites in Cu-based catalysts for CO2 hydrogenation to methanol [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 287-298. |
| [2] | XU Jiaheng, LI Yongsheng, LUO Chunhuan, SU Qingquan. Optimization of methanol steam reforming process [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 41-46. |
| [3] | SHU Bin, CHEN Jianhong, XIONG Jian, WU Qirong, YU Jiangtao, YANG Ping. Necessity analysis of promoting the development of green methanol under the goal of carbon neutrality [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4471-4478. |
| [4] | MAO Shanjun, WANG Zhe, WANG Yong. Group recognition hydrogenation: From concept to application [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 3917-3922. |
| [5] | WANG Zijian, KE Ming, SONG Zhaozheng, LI Jiahan, TONG Yanbing, SUN Jinru. Progress in alkylation of gasoline with molecular sieve catalyst for benzene reduction [J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2371-2389. |
| [6] | HUANG Qizhong, LIU Bing, MA Hongpeng, LYU Wenjie. Methanol to olefin wastewater treatment based on a novel microchannel separation technology [J]. Chemical Industry and Engineering Progress, 2023, 42(2): 669-676. |
| [7] | GUO Feng, ZHANG Shangjie, JIANG Yujia, JIANG Wankui, XIN Fengxue, ZHANG Wenming, JIANG Min. Biotransformation of one-carbon resources by yeast [J]. Chemical Industry and Engineering Progress, 2023, 42(1): 30-39. |
| [8] | LI Bin, PAN Qinggang, JIANG Shuang, ZHANG Tianyong. Green synthesis of fluopyram intermediates in high yield [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 469-476. |
| [9] | LIU Penglong, XU Xiongfei, ZHANG Wei, XU Xin, ZHANG Kan, WANG Junwen. Local modeling and optimization of K-means-PSO-SVR for methanol to aromatics [J]. Chemical Industry and Engineering Progress, 2022, 41(9): 4691-4700. |
| [10] | XIANG Sheng, WANG Chao, ZHUANG Yu, GU Siwen, ZHANG Lei, DU Jian. Design and control of pressure-swing distillation for separating methyl acetate-methanol-ethyl acetate azeotropic system [J]. Chemical Industry and Engineering Progress, 2022, 41(8): 4065-4076. |
| [11] | ZHANG Jiaqi, LIN Lina, GAO Wengui, ZHU Xing. Effect of CeO2 morphology on the performance of CuO/CeO2 catalyst for CO2 hydrogenation to methanol [J]. Chemical Industry and Engineering Progress, 2022, 41(8): 4213-4223. |
| [12] | ZHAO Jianbing, YANG Dan, SHU Yuancao, ZHU Junbo, PU Shiping, SONG Xiaodan, LIU Shouqing, CHAI Xijuan, LI Xuemei. Preparation of Na2CO3 /CF solid base and its catalytic transesterification of rapeseed oil [J]. Chemical Industry and Engineering Progress, 2022, 41(7): 3608-3614. |
| [13] | ZHUANG Yuting, WANG Jianhua, XIANG Zhiyan, ZHAO Juan, XU Qiong, LIU Xianxiang, YIN Dulin. Research progress in preparation and kinetics of γ-valerolactone synthesis from hemicellulose and its derivatives [J]. Chemical Industry and Engineering Progress, 2022, 41(7): 3519-3533. |
| [14] | LI Guixian, ZHANG Junqiang, YANG Yong, FAN Xueying, WANG Dongliang. A novel PX production shortcut through PX selectivity intensification in toluene and methanol methylation [J]. Chemical Industry and Engineering Progress, 2022, 41(6): 2939-2947. |
| [15] | WANG Jijie, HAN Zhe, CHEN Siyu, TANG Chizhou, SHA Feng, TANG Shan, YAO Tingting, LI Can. Liquid sunshine methanol [J]. Chemical Industry and Engineering Progress, 2022, 41(3): 1309-1317. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||