Chemical Industry and Engineering Progress ›› 2022, Vol. 41 ›› Issue (12): 6586-6605.DOI: 10.16085/j.issn.1000-6613.2022-0369
• Resources and environmental engineering • Previous Articles Next Articles
Preparation of magnetic biochar and its application in polluted water
SONG Shaohua1( ), XU Jinlan2, SONG Xiaoqiao1, YU Yuan1
), XU Jinlan2, SONG Xiaoqiao1, YU Yuan1
- 1.Huaqing College, Xi’an University of Architecture and Technology, Xi’an 710043, Shaanxi, China
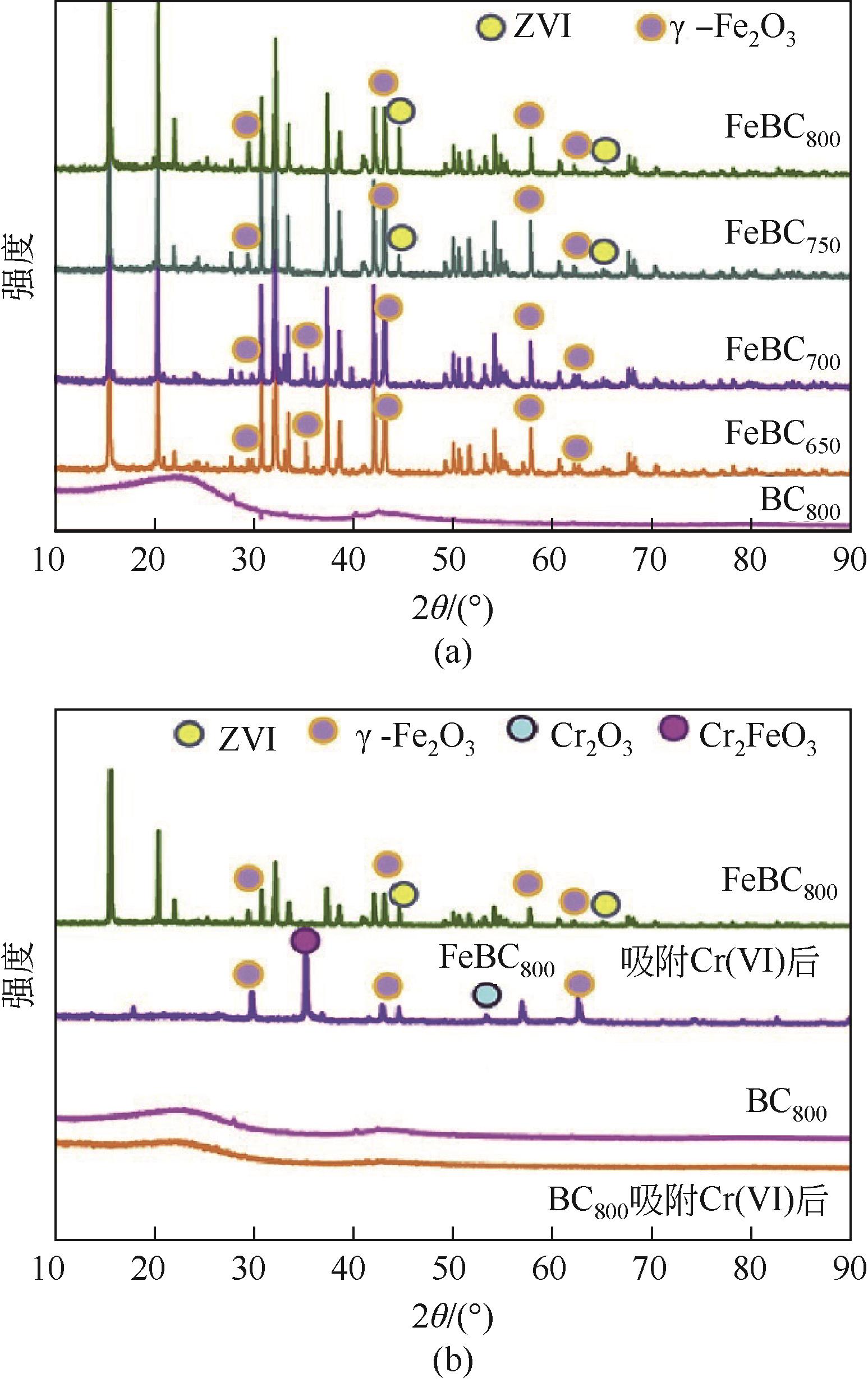
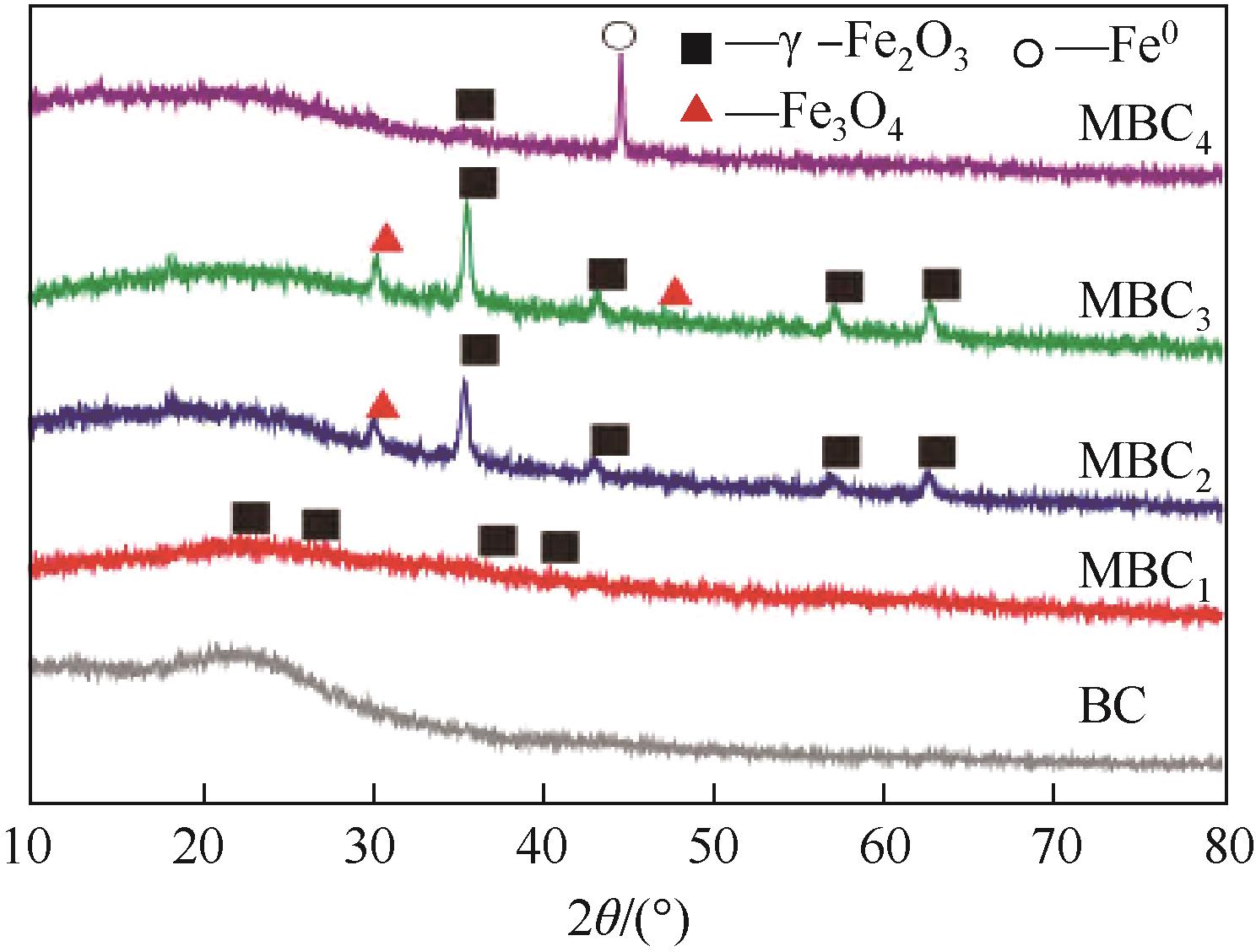
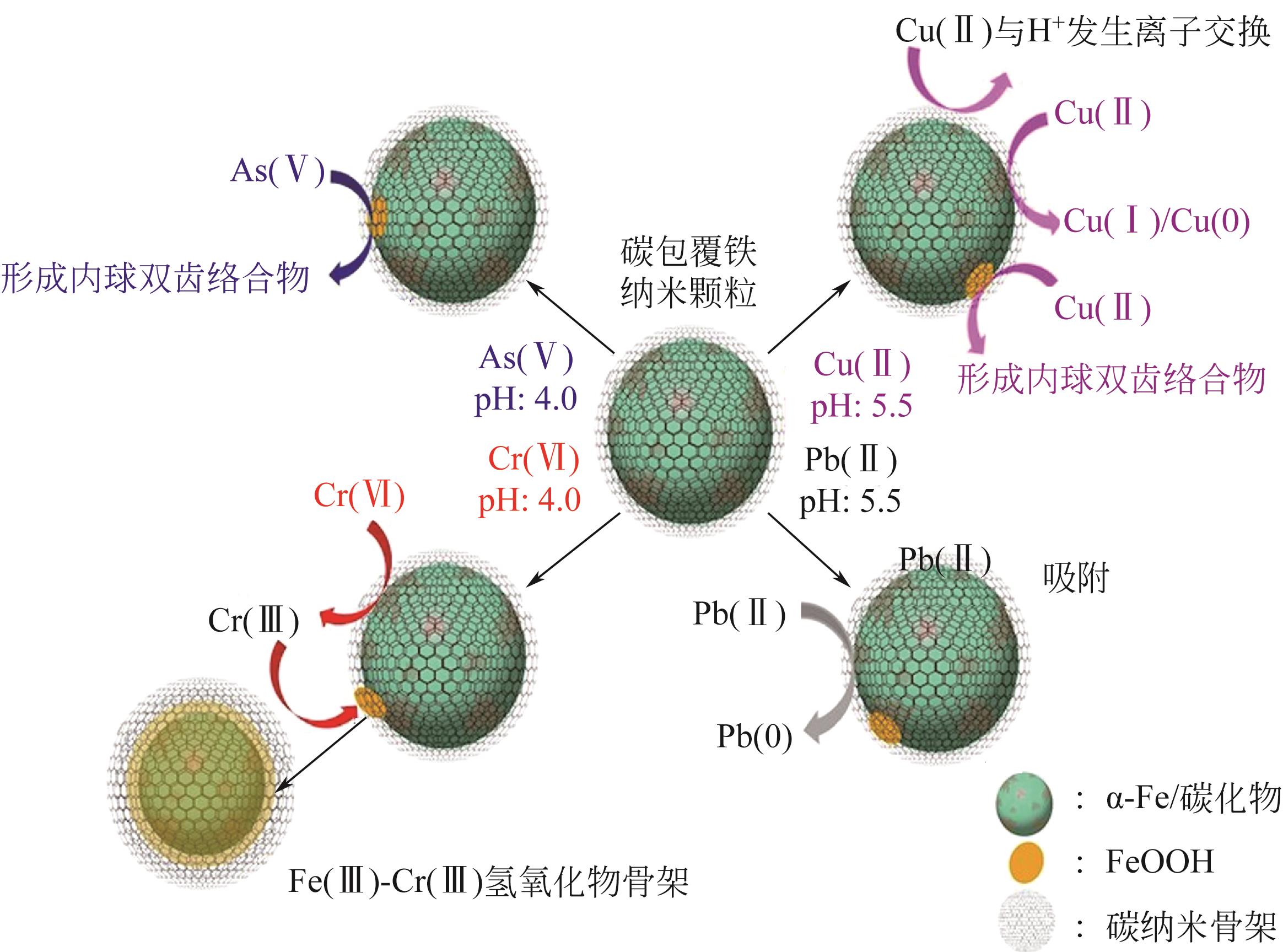
2.School of Environmental and Municipal Engineering, Xi’an University of Architecture & Technology, Xi’an 710055, Shaanxi, China
-
Received:2022-03-11Revised:2022-07-02Online:2022-12-29Published:2022-12-20 -
Contact:XU Jinlan
磁性生物质炭的制备及在污染水体中的应用
- 1.西安建筑科技大学华清学院,陕西 西安 710043
2.西安建筑科技大学环境与市政工程学院,陕西 西安 710055
-
通讯作者:徐金兰 -
作者简介:宋少花(1988—),女,讲师,硕士,研究方向为石油污染土壤修复技术及水污染控制。E-mail:915998273@qq.com。 -
基金资助:国家自然科学基金(51778524);陕西省教育厅专项科研计划(21JK0738);2021年西安建筑科技大学华清学院大学生创新训练项目(S202113679014)
CLC Number:
Cite this article
SONG Shaohua, XU Jinlan, SONG Xiaoqiao, YU Yuan. Preparation of magnetic biochar and its application in polluted water[J]. Chemical Industry and Engineering Progress, 2022, 41(12): 6586-6605.
宋少花, 徐金兰, 宋晓乔, 于媛. 磁性生物质炭的制备及在污染水体中的应用[J]. 化工进展, 2022, 41(12): 6586-6605.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2022-0369
| 名称 | 原材料 | 污染物 | 吸附量/mg·g-1 | 参考文献 |
|---|---|---|---|---|
| 磁性玉米秸秆生物质炭 | 玉米秸秆 | Cd(Ⅱ) | 33.45 | [ |
| 磁性小麦秸秆生物质炭 | 小麦秸秆 | Cd(Ⅱ) | 23. 44 | [ |
| 磁性谷壳生物质炭 | 谷壳 | Pb(Ⅱ) | 45. 7 | [ |
| NH2磁性米糠生物质炭复合材料 | 米糠 | Ni(Ⅱ) | 201.62 | [ |
| 乙二胺四乙酸(EDTA)磁性落叶生物质炭纳米复合材料 | 落叶 | Pb(Ⅱ) | 146.84 | [ |
| 磁性生物质炭负载Mg-Fe水滑石复合材料 | 油茶树果壳 | Cd(Ⅱ) Ni(Ⅱ) | 263.156 43.291 | [ |
| 磁性铁酸盐/生物质炭复合材料 | 松木屑 | Pb(Ⅱ) | 99.5 | [ |
| 磁性黑藻生物质炭复合材料 | 黑藻 | Cu(Ⅱ) | 24.28 | [ |
| 磁性生物质炭矿物复合材料 | 养猪场污泥 | Pb(Ⅱ) | 450.58 | [ |
| 磁性生物质炭 | 活性污泥 | Cd(Ⅱ) Pb(Ⅱ) | 8.5 48.05 | [ |
磁性废骨粉生物质炭 | 废骨粉 | Cd(Ⅱ) Cu(Ⅱ) Pb(Ⅱ) | 151.3 219.8 271.9 | [ |
| 磁性蘑菇渣生物质炭 | 蘑菇渣 | Cr(Ⅵ) | 47.62 | [ |
| 名称 | 原材料 | 污染物 | 吸附量/mg·g-1 | 参考文献 |
|---|---|---|---|---|
| 磁性玉米秸秆生物质炭 | 玉米秸秆 | Cd(Ⅱ) | 33.45 | [ |
| 磁性小麦秸秆生物质炭 | 小麦秸秆 | Cd(Ⅱ) | 23. 44 | [ |
| 磁性谷壳生物质炭 | 谷壳 | Pb(Ⅱ) | 45. 7 | [ |
| NH2磁性米糠生物质炭复合材料 | 米糠 | Ni(Ⅱ) | 201.62 | [ |
| 乙二胺四乙酸(EDTA)磁性落叶生物质炭纳米复合材料 | 落叶 | Pb(Ⅱ) | 146.84 | [ |
| 磁性生物质炭负载Mg-Fe水滑石复合材料 | 油茶树果壳 | Cd(Ⅱ) Ni(Ⅱ) | 263.156 43.291 | [ |
| 磁性铁酸盐/生物质炭复合材料 | 松木屑 | Pb(Ⅱ) | 99.5 | [ |
| 磁性黑藻生物质炭复合材料 | 黑藻 | Cu(Ⅱ) | 24.28 | [ |
| 磁性生物质炭矿物复合材料 | 养猪场污泥 | Pb(Ⅱ) | 450.58 | [ |
| 磁性生物质炭 | 活性污泥 | Cd(Ⅱ) Pb(Ⅱ) | 8.5 48.05 | [ |
磁性废骨粉生物质炭 | 废骨粉 | Cd(Ⅱ) Cu(Ⅱ) Pb(Ⅱ) | 151.3 219.8 271.9 | [ |
| 磁性蘑菇渣生物质炭 | 蘑菇渣 | Cr(Ⅵ) | 47.62 | [ |
| 名称 | 磁性前体 | 污染物 | 吸附量/mg·g-1 | 参考文献 |
|---|---|---|---|---|
| 磁性海藻生物质炭复合材料 | FeCl3·6H2O、FeSO4·7H2O | 甲基橙 | 240.3 | [ |
| 磁性污泥生物质炭 | FeCl3·6H2O | Cd(Ⅱ) | 167.42 | [ |
| 磁性明胶改性生物质炭 | FeCl2、FeCl3 | 双氯芬酸钠 | 266 | [ |
| 磁性玉米生物质炭 | FeCl3 | As(Ⅲ) | 51.81 | [ |
| 磁性稻壳生物质炭 | FeSO4·7H2O | 菲 | 89. 64 | [ |
| 磁性花生壳生物质炭复合材料 | FeSO4·7H2O、Fe2(SO4)3 | 磷 | 22. 32 | [ |
| 磁性松木生物炭 | 赤铁矿 | As(Ⅲ) | 429 | [ |
| 天然铁矿石-生物质炭复合材料 | 赤铁矿 黄铁矿 | 诺氟沙星 | 1.676 1.978 | [ |
| 磁性生物炭复合材料 | 菱铁矿 | U(Ⅵ) | 52.63 | [ |
| 磁性生物质炭 | Fe2O3 | 磷酸盐 | 330.86 | [ |
| 磁性花生壳生物质炭 | Fe3O4 | 亚甲基蓝 | 666.67 | [ |
| 名称 | 磁性前体 | 污染物 | 吸附量/mg·g-1 | 参考文献 |
|---|---|---|---|---|
| 磁性海藻生物质炭复合材料 | FeCl3·6H2O、FeSO4·7H2O | 甲基橙 | 240.3 | [ |
| 磁性污泥生物质炭 | FeCl3·6H2O | Cd(Ⅱ) | 167.42 | [ |
| 磁性明胶改性生物质炭 | FeCl2、FeCl3 | 双氯芬酸钠 | 266 | [ |
| 磁性玉米生物质炭 | FeCl3 | As(Ⅲ) | 51.81 | [ |
| 磁性稻壳生物质炭 | FeSO4·7H2O | 菲 | 89. 64 | [ |
| 磁性花生壳生物质炭复合材料 | FeSO4·7H2O、Fe2(SO4)3 | 磷 | 22. 32 | [ |
| 磁性松木生物炭 | 赤铁矿 | As(Ⅲ) | 429 | [ |
| 天然铁矿石-生物质炭复合材料 | 赤铁矿 黄铁矿 | 诺氟沙星 | 1.676 1.978 | [ |
| 磁性生物炭复合材料 | 菱铁矿 | U(Ⅵ) | 52.63 | [ |
| 磁性生物质炭 | Fe2O3 | 磷酸盐 | 330.86 | [ |
| 磁性花生壳生物质炭 | Fe3O4 | 亚甲基蓝 | 666.67 | [ |
| 制备方法 | 优点 | 缺点 | 参考文献 |
|---|---|---|---|
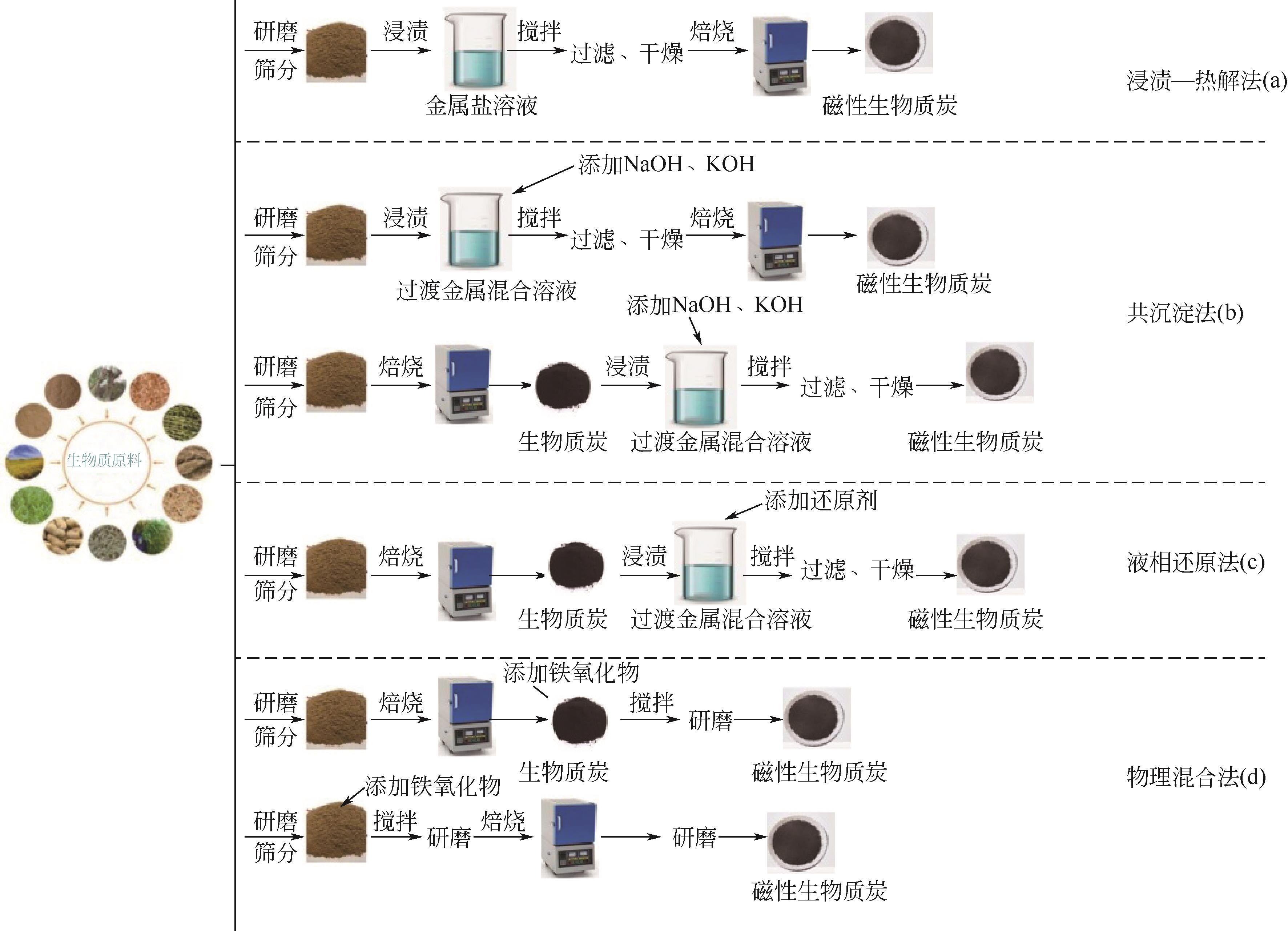
| 浸渍-热解法 | 生物质炭的磁化和热解同时完成;操作简单;所得磁性生物质炭稳定性好;金属浸出少;制备过程中产生的废液较少 | 热解过程中会产生气体污染物和焦油,处理不当会造成二次污染;需要较高的热解温度(300~1000℃),导致能耗较高 | [ |
| 共沉淀法 | 所得磁性生物质炭稳定性好,金属浸出少;无需过高温度;高生产率;操作简单;可控性强 | 需要引入大量碱性试剂,增加了成本;碱性废水需要处理 | [ |
| 液相还原法 | 可以得到负载有零价金属的磁性生物质炭;所得磁性生物质炭还原性强,稳定性好;可控性强;生产效率高 | 添加的还原剂有毒,需要妥善储存和使用,并考虑后续处理;氢气通常在制备过程中产生,存在一定的安全隐患 | [ |
| 物理混合法 | 制备方法绿色且经济,而且可制备有效的低成本磁性吸附剂 | 混合不均匀;制备的磁性生物质炭有团聚的可能 | [ |
| 制备方法 | 优点 | 缺点 | 参考文献 |
|---|---|---|---|
| 浸渍-热解法 | 生物质炭的磁化和热解同时完成;操作简单;所得磁性生物质炭稳定性好;金属浸出少;制备过程中产生的废液较少 | 热解过程中会产生气体污染物和焦油,处理不当会造成二次污染;需要较高的热解温度(300~1000℃),导致能耗较高 | [ |
| 共沉淀法 | 所得磁性生物质炭稳定性好,金属浸出少;无需过高温度;高生产率;操作简单;可控性强 | 需要引入大量碱性试剂,增加了成本;碱性废水需要处理 | [ |
| 液相还原法 | 可以得到负载有零价金属的磁性生物质炭;所得磁性生物质炭还原性强,稳定性好;可控性强;生产效率高 | 添加的还原剂有毒,需要妥善储存和使用,并考虑后续处理;氢气通常在制备过程中产生,存在一定的安全隐患 | [ |
| 物理混合法 | 制备方法绿色且经济,而且可制备有效的低成本磁性吸附剂 | 混合不均匀;制备的磁性生物质炭有团聚的可能 | [ |
| 原料 | 焙烧温度/℃ | 焙烧时间/h | 比表面积/m2·g-1 | 总孔容/cm3·g-1 | 重金属 | 最大吸附容量/mg·g-1 | 参考文献 |
|---|---|---|---|---|---|---|---|
| 玉米秸秆 | 500 | 3 | 23.58 | — | Cd(Ⅱ) | 33.45 | [ |
| 小麦秸秆 | 450 | 2 | 152.24 | 0.13 | Cd(Ⅱ) | 23.44 | [ |
| 城市污泥 | 500 | 3 | 82.39 | 0.18 | Cd(Ⅱ) | 167.42 | [ |
| 棕榈纤维 | 400 | 2 | 117.62 | — | Cd(Ⅱ) | 197.96 | [ |
| 谷壳 | 400 | 4 | 72.94 | — | Pb(Ⅱ) | 43.9 | [ |
| 落叶 | 450 | 1 | — | — | Pb(Ⅱ) | 146.84 | [ |
| 松木屑 | 200 | 8 | 138.5 | 0.288 | Pb(Ⅱ) | 99.5 | [ |
| 贝壳 | 200 | 8 | 57.5 | — | Pb(Ⅱ) | 129.31 | [ |
| 杉木屑 | 700 | 2 | 168 | 0.181 | Pb(Ⅱ) | 817.64 | [ |
| 玉米秸秆 | 600 | 3 | — | — | Pb(Ⅱ) | 210.85 | [ |
| 小麦秸秆 | 800 | 2 | 44.31 | 0.0652 | Pb(Ⅱ) | 196.91 | [ |
| 松木屑 | 200 | 8 | 138.5 | — | Pb(Ⅱ) | 99.5 | [ |
| 养猪场污泥 | 200 | 4 | 23.32 | — | Pb(Ⅱ) | 459.24 | [ |
| 300 | 39.99 | — | 355.89 | ||||
| 400 | 27.63 | — | 369.02 | ||||
| 500 | 18.59 | — | 450.58 | ||||
| 600 | 22.61 | — | 402.78 | ||||
| 赤泥 | 350 | 1 | 9.31 | 0.05 | Cr(Ⅵ) | 125.37 | [ |
| 650 | 75.01 | 0.10 | 325.35 | ||||
| 850 | 106.03 | 0.15 | 363.35 | ||||
| 浒苔 | 400 | 2 | 708.27 | — | Cr(Ⅵ) | 53.08 | [ |
| 800 | 780.14 | — | 95.23 | ||||
| 花生壳 | 650 | 1 | 2.40 | 0.02 | Cr(Ⅵ) | 182.32 | [ |
| 700 | 1.80 | 0.01 | 195.22 | ||||
| 750 | 7.74 | 0.03 | 208.33 | ||||
| 800 | 8.81 | 0.05 | 223.21 | ||||
| 小麦秸秆 | 500 | 1 | 33.73 | 0.128 | Cr(Ⅵ) | 23.94 | [ |
| 香菇废基质 | 600 | 4 | 92.78 | 0.112 | Cr(Ⅵ) | 47.62 | [ |
| 纳氏叶栅藻 | 200 | 8 | 142.49 | 0.38 | Cr(Ⅵ) | 56.79 | [ |
| 污水厂污泥 | 500 | 1 | — | — | Cr(Ⅵ) | 11.56 | [ |
| 竹子 | 700 | 2 | — | — | Cr(Ⅵ) | 127 | [ |
| 花生壳 | 220 | 12 | 62.4 | 0.34 | Cr(Ⅵ) | 142.86 | [ |
| 柚皮 | 300 | 1 | 35.88 | 0.024 | Cr(Ⅵ) | 209.64 | [ |
| 稻壳 | 500 | 2 | 17.6 | — | Cr(Ⅵ) | 23.25 | [ |
| 凤凰树叶 | 500 | 2 | 83.6 | — | Cr(Ⅵ) | 55.0 | [ |
| 马缨丹 | 800 | 2 | 607.53 | — | Cr(Ⅵ) | 161.23 | [ |
| 米糠 | 500 | 3 | — | — | Ni(Ⅱ) | 201.62 | [ |
| 黑藻 | 450 | 2 | — | — | Cu(Ⅱ) | 24.28 | [ |
| 丝瓜络 | 700 | 5 | — | — | Cu(Ⅱ) | 54.68 | [ |
| 马尾松锯末 | 240 | 8 | 63.75 | 0.35 | Cu(Ⅱ) | 9.58 | [ |
| 木屑 | 500 | 3 | 25.654 | 0.13 | Cu(Ⅱ) | 3.23 | [ |
| 苹果渣 | 600 | 1 | 102.18 | 0.09 | Ag(Ⅰ) | 818.4 | [ |
| 西瓜皮 | 500 | 1 | — | — | Tl(Ⅰ) | 1123 | [ |
| 蘑菇废料 | 750 | — | 71 | 0.19 | Sb(Ⅲ) | 56.49 | [ |
| 茶树果壳 | 600 | 1 | 257.795 | 1.141 | Cd(Ⅱ) | 263.156 | [ |
| Ni(Ⅱ) | 43.291 | ||||||
| 活性污泥 | 220 | 4 | 114.57 | 0.9 | Cd(Ⅱ) | 48.05 | [ |
| Pb(Ⅱ) | 8.5 | ||||||
| 废骨粉 | 450 | 2 | 288.0 | 0.117 | Cd(Ⅱ) | 151.31 | [ |
| Cu(Ⅱ) | 219.84 | ||||||
| Pb(Ⅱ) | 219.84 | ||||||
| 棕榈纤维粉 | 700 | 3 | 574 | — | Cd(Ⅱ) | 363 | [ |
| As(Ⅲ) | 92.8 | ||||||
| 棕榈仁蛋糕 | 350 | 2 | 89.39 | — | Cd(Ⅱ) | 18.60 | [ |
| Cr(Ⅲ) | 19.92 | ||||||
| Pb(Ⅱ) | 49.64 | ||||||
| Hg(Ⅱ) | 13.69 | ||||||
| 玉米秸秆 | 500 | 2 | — | — | Cd(Ⅱ) | 33.81 | [ |
| As(Ⅲ) | 148.5 | ||||||
| 稻草 | 400 | 2 | — | — | Cd(Ⅱ) | 6.34 | [ |
| As(Ⅲ) | 10.07 | ||||||
| 松木 | 300 | 8 | 1.676 | — | Cd(Ⅱ) | 173 | [ |
| Cu(Ⅱ) | 359 | ||||||
| 450 | 4 | 14.3 | — | Cd(Ⅱ) | 138 | ||
| Cu(Ⅱ) | 172 | ||||||
| 600 | 1 | 75.48 | — | Cd(Ⅱ) | 130 | ||
| Cu(Ⅱ) | 197 |
| 原料 | 焙烧温度/℃ | 焙烧时间/h | 比表面积/m2·g-1 | 总孔容/cm3·g-1 | 重金属 | 最大吸附容量/mg·g-1 | 参考文献 |
|---|---|---|---|---|---|---|---|
| 玉米秸秆 | 500 | 3 | 23.58 | — | Cd(Ⅱ) | 33.45 | [ |
| 小麦秸秆 | 450 | 2 | 152.24 | 0.13 | Cd(Ⅱ) | 23.44 | [ |
| 城市污泥 | 500 | 3 | 82.39 | 0.18 | Cd(Ⅱ) | 167.42 | [ |
| 棕榈纤维 | 400 | 2 | 117.62 | — | Cd(Ⅱ) | 197.96 | [ |
| 谷壳 | 400 | 4 | 72.94 | — | Pb(Ⅱ) | 43.9 | [ |
| 落叶 | 450 | 1 | — | — | Pb(Ⅱ) | 146.84 | [ |
| 松木屑 | 200 | 8 | 138.5 | 0.288 | Pb(Ⅱ) | 99.5 | [ |
| 贝壳 | 200 | 8 | 57.5 | — | Pb(Ⅱ) | 129.31 | [ |
| 杉木屑 | 700 | 2 | 168 | 0.181 | Pb(Ⅱ) | 817.64 | [ |
| 玉米秸秆 | 600 | 3 | — | — | Pb(Ⅱ) | 210.85 | [ |
| 小麦秸秆 | 800 | 2 | 44.31 | 0.0652 | Pb(Ⅱ) | 196.91 | [ |
| 松木屑 | 200 | 8 | 138.5 | — | Pb(Ⅱ) | 99.5 | [ |
| 养猪场污泥 | 200 | 4 | 23.32 | — | Pb(Ⅱ) | 459.24 | [ |
| 300 | 39.99 | — | 355.89 | ||||
| 400 | 27.63 | — | 369.02 | ||||
| 500 | 18.59 | — | 450.58 | ||||
| 600 | 22.61 | — | 402.78 | ||||
| 赤泥 | 350 | 1 | 9.31 | 0.05 | Cr(Ⅵ) | 125.37 | [ |
| 650 | 75.01 | 0.10 | 325.35 | ||||
| 850 | 106.03 | 0.15 | 363.35 | ||||
| 浒苔 | 400 | 2 | 708.27 | — | Cr(Ⅵ) | 53.08 | [ |
| 800 | 780.14 | — | 95.23 | ||||
| 花生壳 | 650 | 1 | 2.40 | 0.02 | Cr(Ⅵ) | 182.32 | [ |
| 700 | 1.80 | 0.01 | 195.22 | ||||
| 750 | 7.74 | 0.03 | 208.33 | ||||
| 800 | 8.81 | 0.05 | 223.21 | ||||
| 小麦秸秆 | 500 | 1 | 33.73 | 0.128 | Cr(Ⅵ) | 23.94 | [ |
| 香菇废基质 | 600 | 4 | 92.78 | 0.112 | Cr(Ⅵ) | 47.62 | [ |
| 纳氏叶栅藻 | 200 | 8 | 142.49 | 0.38 | Cr(Ⅵ) | 56.79 | [ |
| 污水厂污泥 | 500 | 1 | — | — | Cr(Ⅵ) | 11.56 | [ |
| 竹子 | 700 | 2 | — | — | Cr(Ⅵ) | 127 | [ |
| 花生壳 | 220 | 12 | 62.4 | 0.34 | Cr(Ⅵ) | 142.86 | [ |
| 柚皮 | 300 | 1 | 35.88 | 0.024 | Cr(Ⅵ) | 209.64 | [ |
| 稻壳 | 500 | 2 | 17.6 | — | Cr(Ⅵ) | 23.25 | [ |
| 凤凰树叶 | 500 | 2 | 83.6 | — | Cr(Ⅵ) | 55.0 | [ |
| 马缨丹 | 800 | 2 | 607.53 | — | Cr(Ⅵ) | 161.23 | [ |
| 米糠 | 500 | 3 | — | — | Ni(Ⅱ) | 201.62 | [ |
| 黑藻 | 450 | 2 | — | — | Cu(Ⅱ) | 24.28 | [ |
| 丝瓜络 | 700 | 5 | — | — | Cu(Ⅱ) | 54.68 | [ |
| 马尾松锯末 | 240 | 8 | 63.75 | 0.35 | Cu(Ⅱ) | 9.58 | [ |
| 木屑 | 500 | 3 | 25.654 | 0.13 | Cu(Ⅱ) | 3.23 | [ |
| 苹果渣 | 600 | 1 | 102.18 | 0.09 | Ag(Ⅰ) | 818.4 | [ |
| 西瓜皮 | 500 | 1 | — | — | Tl(Ⅰ) | 1123 | [ |
| 蘑菇废料 | 750 | — | 71 | 0.19 | Sb(Ⅲ) | 56.49 | [ |
| 茶树果壳 | 600 | 1 | 257.795 | 1.141 | Cd(Ⅱ) | 263.156 | [ |
| Ni(Ⅱ) | 43.291 | ||||||
| 活性污泥 | 220 | 4 | 114.57 | 0.9 | Cd(Ⅱ) | 48.05 | [ |
| Pb(Ⅱ) | 8.5 | ||||||
| 废骨粉 | 450 | 2 | 288.0 | 0.117 | Cd(Ⅱ) | 151.31 | [ |
| Cu(Ⅱ) | 219.84 | ||||||
| Pb(Ⅱ) | 219.84 | ||||||
| 棕榈纤维粉 | 700 | 3 | 574 | — | Cd(Ⅱ) | 363 | [ |
| As(Ⅲ) | 92.8 | ||||||
| 棕榈仁蛋糕 | 350 | 2 | 89.39 | — | Cd(Ⅱ) | 18.60 | [ |
| Cr(Ⅲ) | 19.92 | ||||||
| Pb(Ⅱ) | 49.64 | ||||||
| Hg(Ⅱ) | 13.69 | ||||||
| 玉米秸秆 | 500 | 2 | — | — | Cd(Ⅱ) | 33.81 | [ |
| As(Ⅲ) | 148.5 | ||||||
| 稻草 | 400 | 2 | — | — | Cd(Ⅱ) | 6.34 | [ |
| As(Ⅲ) | 10.07 | ||||||
| 松木 | 300 | 8 | 1.676 | — | Cd(Ⅱ) | 173 | [ |
| Cu(Ⅱ) | 359 | ||||||
| 450 | 4 | 14.3 | — | Cd(Ⅱ) | 138 | ||
| Cu(Ⅱ) | 172 | ||||||
| 600 | 1 | 75.48 | — | Cd(Ⅱ) | 130 | ||
| Cu(Ⅱ) | 197 |
| 原料 | 焙烧温度/℃ | 焙烧时间/h | 比表面积/m2·g-1 | 总孔容/cm3·g-1 | 有机污染物 | 最大吸附容量/mg·g-1 | 参考文献 |
|---|---|---|---|---|---|---|---|
| 城市污泥 | 800 | 2 | — | — | 四环素 | 159.26 | [ |
| 甘蔗渣 | 400 | 2 | 25.45 | — | 四环素 | 48.35 | [ |
| 锯屑 | 800 | 1 | 1710.3 | 0.969 | 四环素 | 423.7 | [ |
| 椰子、松子和核桃壳 | 500 | 1.5 | 365 | 0.54 | 卡马西平 | 62.7 | [ |
| 四环素 | 94.2 | ||||||
| 鸡骨 | 500 | 2 | 328 | 0.285 | 四环素 | 98.89 | [ |
| 罗丹明B | 13.31 | ||||||
| 樟脑叶 | 650 | 2 | 915 | 0.552 | 环丙沙星 | 449.40 | [ |
| 柳木锯末 | 600 | 3 | 662.68 | 0.153 | 达托霉素 | 217.39 | [ |
| 松木锯末 | 634.22 | 0.126 | 212.77 | ||||
| 城市污泥 | 500 | 2 | 39.1 | 0.129 | 环丙沙星 | 83.7 | [ |
| 诺氟沙星 | 39.3 | ||||||
| 氧氟沙星 | 25.4 | ||||||
| 黄芪草药渣 | 700 | 3 | 203.70 | — | 环丙沙星 | 68.9 | [ |
| 玉米秸秆 | 500 | 1.5 | 760.7 | 0.3698 | 诺氟沙星 | 7.63 | [ |
| 松木屑 | — | — | 125.8 | — | 磺胺甲𫫇唑 | 111.2 | [ |
| 甘蔗渣 | 800 | 2 | 613.87 | 0.29 | 磺胺甲𫫇唑 | 187.31 | [ |
| 竹子 | 550 | 1 | 61.48 | 0.157 | 双酚A | 101.5 | [ |
| 磺胺甲𫫇唑 | 99.99 | ||||||
| 橙皮 | 500 | 4 | 857.42 | 0.12 | 布洛芬 | 58.12 | [ |
| 磺胺甲𫫇唑 | 60.90 | ||||||
| 海藻粉 | 700 | 1.5 | 622.88 | 0.733 | 甲基橙 | 622.88 | [ |
| 香蕉皮 | 600 | 1 | — | — | 甲基蓝 | 862 | [ |
| 花生壳 | 750 | 1.5 | 1219 | 0.5767 | 亚甲基蓝 | 666.67 | [ |
| 裙带菜 | 800 | 2 | 744.15 | 0.451 | 亚甲基蓝 | 479.49 | [ |
| 苹果树废枝 | 200 | 12 | 15.17 | 0.071 | 亚甲基蓝 | 46.32 | [ |
| 果树残枝 | 200 | 12 | 135.3 | 0.285 | 亚甲基蓝 | 81.9 | [ |
| 核桃壳 | 700 | 4 | — | — | 亚甲基蓝 | 710 | [ |
| 松仁壳 | 700 | 3 | — | — | 酸性铬蓝K | 79.81 | [ |
| 花生壳 | 800 | 1.5 | 722.34 | 0.336 | 孔雀石绿 | 1106.40 | [ |
| 玉米秸秆 | 500 | 3 | 80.1 | 0.127 | 孔雀石绿 | 515.77 | [ |
| 玉米秸秆 | 400 | 2 | — | — | 结晶紫 | 349.40 | [ |
| 稻草 | 600 | 1 | 145.96 | 0.06549 | 结晶紫 | 199 | [ |
| 褐藻 | 600 | 1 | 336.97 | 0.2323 | 酸性橙7 | 382.01 | [ |
| 油菜秸秆 | 600 | 2.5 | — | — | 双氯芬酸钠 | 266 | [ |
| 稻壳 | 500 | 2 | 108.53 | 0.05 | 菲 | 89.64 | [ |
| 棉花秸秆 | 500 | 6 | 41.91 | 0.076 | 对硝基苯酚 | 48.94 | [ |
| 芦苇 | 600 | 2 | 254.6 | 0.257 | 氟苯尼考 | 9.29 | [ |
| 柳枝稷 | 450 | 1 | 1.1 | — | 灭蚁灵 | 155 | [ |
| 琼脂 | 900 | 2 | 563.0 | 0.30 | 4-硝基苯酚 | 227.27 | [ |
| 稻壳 | 350 | 6 | 184.91 | — | 三氯乙烯 | 98.9 | [ |
| 道格拉斯冷杉 | 600 | 1 | 494 | — | 苯胺 | 338 | [ |
| 硝基苯 | 178 | ||||||
| 菌丝球团 | 400 | 2 | 1986 | 1.04 | 双氯芬酸 | 361.25 | [ |
| 甘蔗渣 | 400 | 1 | 166.87 | 0.14 | 17β-雌二醇 | 50.24 | [ |
| 600 | 1 | 339.12 | 0.14 | 41.71 | |||
| 800 | 1 | 321.68 | 0.13 | 34.06 | |||
| 稻草 | 500 | 2 | 190.28 | — | 17β-雌二醇 | 46.22 | [ |
| 道格拉斯冷杉 | 600~700 | 1.5 | 745.3 | 0.258 | 4-硝基苯胺水杨酸 | 114 | [ |
| 苯甲酸 | 109 | ||||||
| 90 | |||||||
| 邻苯二甲酸 | 86 | ||||||
| 玉米秸秆 | 600 | 1 | 14.26 | — | 阿特拉津 | 143.15 | [ |
| 原料 | 焙烧温度/℃ | 焙烧时间/h | 比表面积/m2·g-1 | 总孔容/cm3·g-1 | 有机污染物 | 最大吸附容量/mg·g-1 | 参考文献 |
|---|---|---|---|---|---|---|---|
| 城市污泥 | 800 | 2 | — | — | 四环素 | 159.26 | [ |
| 甘蔗渣 | 400 | 2 | 25.45 | — | 四环素 | 48.35 | [ |
| 锯屑 | 800 | 1 | 1710.3 | 0.969 | 四环素 | 423.7 | [ |
| 椰子、松子和核桃壳 | 500 | 1.5 | 365 | 0.54 | 卡马西平 | 62.7 | [ |
| 四环素 | 94.2 | ||||||
| 鸡骨 | 500 | 2 | 328 | 0.285 | 四环素 | 98.89 | [ |
| 罗丹明B | 13.31 | ||||||
| 樟脑叶 | 650 | 2 | 915 | 0.552 | 环丙沙星 | 449.40 | [ |
| 柳木锯末 | 600 | 3 | 662.68 | 0.153 | 达托霉素 | 217.39 | [ |
| 松木锯末 | 634.22 | 0.126 | 212.77 | ||||
| 城市污泥 | 500 | 2 | 39.1 | 0.129 | 环丙沙星 | 83.7 | [ |
| 诺氟沙星 | 39.3 | ||||||
| 氧氟沙星 | 25.4 | ||||||
| 黄芪草药渣 | 700 | 3 | 203.70 | — | 环丙沙星 | 68.9 | [ |
| 玉米秸秆 | 500 | 1.5 | 760.7 | 0.3698 | 诺氟沙星 | 7.63 | [ |
| 松木屑 | — | — | 125.8 | — | 磺胺甲𫫇唑 | 111.2 | [ |
| 甘蔗渣 | 800 | 2 | 613.87 | 0.29 | 磺胺甲𫫇唑 | 187.31 | [ |
| 竹子 | 550 | 1 | 61.48 | 0.157 | 双酚A | 101.5 | [ |
| 磺胺甲𫫇唑 | 99.99 | ||||||
| 橙皮 | 500 | 4 | 857.42 | 0.12 | 布洛芬 | 58.12 | [ |
| 磺胺甲𫫇唑 | 60.90 | ||||||
| 海藻粉 | 700 | 1.5 | 622.88 | 0.733 | 甲基橙 | 622.88 | [ |
| 香蕉皮 | 600 | 1 | — | — | 甲基蓝 | 862 | [ |
| 花生壳 | 750 | 1.5 | 1219 | 0.5767 | 亚甲基蓝 | 666.67 | [ |
| 裙带菜 | 800 | 2 | 744.15 | 0.451 | 亚甲基蓝 | 479.49 | [ |
| 苹果树废枝 | 200 | 12 | 15.17 | 0.071 | 亚甲基蓝 | 46.32 | [ |
| 果树残枝 | 200 | 12 | 135.3 | 0.285 | 亚甲基蓝 | 81.9 | [ |
| 核桃壳 | 700 | 4 | — | — | 亚甲基蓝 | 710 | [ |
| 松仁壳 | 700 | 3 | — | — | 酸性铬蓝K | 79.81 | [ |
| 花生壳 | 800 | 1.5 | 722.34 | 0.336 | 孔雀石绿 | 1106.40 | [ |
| 玉米秸秆 | 500 | 3 | 80.1 | 0.127 | 孔雀石绿 | 515.77 | [ |
| 玉米秸秆 | 400 | 2 | — | — | 结晶紫 | 349.40 | [ |
| 稻草 | 600 | 1 | 145.96 | 0.06549 | 结晶紫 | 199 | [ |
| 褐藻 | 600 | 1 | 336.97 | 0.2323 | 酸性橙7 | 382.01 | [ |
| 油菜秸秆 | 600 | 2.5 | — | — | 双氯芬酸钠 | 266 | [ |
| 稻壳 | 500 | 2 | 108.53 | 0.05 | 菲 | 89.64 | [ |
| 棉花秸秆 | 500 | 6 | 41.91 | 0.076 | 对硝基苯酚 | 48.94 | [ |
| 芦苇 | 600 | 2 | 254.6 | 0.257 | 氟苯尼考 | 9.29 | [ |
| 柳枝稷 | 450 | 1 | 1.1 | — | 灭蚁灵 | 155 | [ |
| 琼脂 | 900 | 2 | 563.0 | 0.30 | 4-硝基苯酚 | 227.27 | [ |
| 稻壳 | 350 | 6 | 184.91 | — | 三氯乙烯 | 98.9 | [ |
| 道格拉斯冷杉 | 600 | 1 | 494 | — | 苯胺 | 338 | [ |
| 硝基苯 | 178 | ||||||
| 菌丝球团 | 400 | 2 | 1986 | 1.04 | 双氯芬酸 | 361.25 | [ |
| 甘蔗渣 | 400 | 1 | 166.87 | 0.14 | 17β-雌二醇 | 50.24 | [ |
| 600 | 1 | 339.12 | 0.14 | 41.71 | |||
| 800 | 1 | 321.68 | 0.13 | 34.06 | |||
| 稻草 | 500 | 2 | 190.28 | — | 17β-雌二醇 | 46.22 | [ |
| 道格拉斯冷杉 | 600~700 | 1.5 | 745.3 | 0.258 | 4-硝基苯胺水杨酸 | 114 | [ |
| 苯甲酸 | 109 | ||||||
| 90 | |||||||
| 邻苯二甲酸 | 86 | ||||||
| 玉米秸秆 | 600 | 1 | 14.26 | — | 阿特拉津 | 143.15 | [ |
| 原料 | 焙烧温度/℃ | 焙烧时间/h | 比表面积/m2·g-1 | 总孔容/cm3·g-1 | 无机污染物 | 最大吸附容量/mg·g-1 | 参考文献 |
|---|---|---|---|---|---|---|---|
| 花生壳 | 600 | 1 | 178.15 | 0.15 | 磷酸盐 | 2.906 | [ |
| 裙带菜根 | 800 | 1 | 172.81 | 0.247 | 磷酸盐 | 428.87 | [ |
| 废催化剂 | 350 | 4 | — | — | 磷酸盐 | 6.32 | [ |
| 玉米秸秆 | 800 | 2 | 20.61 | — | 磷酸盐 | 330.86 | [ |
| 水葫芦 | 450 | — | — | — | 磷酸盐 | 5.07 | [ |
| 梭菌 | 600 | 1 | 233.29 | 0.13 | 磷酸盐 | 205.7 | [ |
| 稻壳 | 600 | — | 50.66 | 0.069 | 磷酸盐 | 11.52 | [ |
| 厌氧消化残渣 | 800 | 1 | 41.79 | 0.0690 | 磷酸盐 | 149.25 | [ |
| 烟秆 | 600 | 1 | 229.71 | 0.16 | 磷酸盐 | 106.52 | [ |
| 玉米芯 | 500 | 2 | 6.19 | — | 磷 | 1.99 | [ |
| 园林木屑 | 500 | 2 | 9.42 | — | 磷 | 2.75 | |
| 木屑 | 500 | 2 | 11.08 | — | 磷 | 3.20 | |
| 菠萝皮 | 300 | 1 | 84.89 | — | 磷 | 101.16 | [ |
| 芦苇秸秆 | 500 | 3 | 19.76 | 0.061 | 磷 | 9.05 | [ |
| 污泥 | 500 | 1 | 126.74 | 0.97 | 氨氮 | 3.18 | [ |
| 道格拉斯冷杉 | 600 | 1 | 494 | — | 硝酸盐 | 15.5 | [ |
| 氟化物 | 9.04 | ||||||
| 小麦秸秆 | 600 | 1 | 3.9 | — | 硝酸盐 | 24.8 | [ |
| 原料 | 焙烧温度/℃ | 焙烧时间/h | 比表面积/m2·g-1 | 总孔容/cm3·g-1 | 无机污染物 | 最大吸附容量/mg·g-1 | 参考文献 |
|---|---|---|---|---|---|---|---|
| 花生壳 | 600 | 1 | 178.15 | 0.15 | 磷酸盐 | 2.906 | [ |
| 裙带菜根 | 800 | 1 | 172.81 | 0.247 | 磷酸盐 | 428.87 | [ |
| 废催化剂 | 350 | 4 | — | — | 磷酸盐 | 6.32 | [ |
| 玉米秸秆 | 800 | 2 | 20.61 | — | 磷酸盐 | 330.86 | [ |
| 水葫芦 | 450 | — | — | — | 磷酸盐 | 5.07 | [ |
| 梭菌 | 600 | 1 | 233.29 | 0.13 | 磷酸盐 | 205.7 | [ |
| 稻壳 | 600 | — | 50.66 | 0.069 | 磷酸盐 | 11.52 | [ |
| 厌氧消化残渣 | 800 | 1 | 41.79 | 0.0690 | 磷酸盐 | 149.25 | [ |
| 烟秆 | 600 | 1 | 229.71 | 0.16 | 磷酸盐 | 106.52 | [ |
| 玉米芯 | 500 | 2 | 6.19 | — | 磷 | 1.99 | [ |
| 园林木屑 | 500 | 2 | 9.42 | — | 磷 | 2.75 | |
| 木屑 | 500 | 2 | 11.08 | — | 磷 | 3.20 | |
| 菠萝皮 | 300 | 1 | 84.89 | — | 磷 | 101.16 | [ |
| 芦苇秸秆 | 500 | 3 | 19.76 | 0.061 | 磷 | 9.05 | [ |
| 污泥 | 500 | 1 | 126.74 | 0.97 | 氨氮 | 3.18 | [ |
| 道格拉斯冷杉 | 600 | 1 | 494 | — | 硝酸盐 | 15.5 | [ |
| 氟化物 | 9.04 | ||||||
| 小麦秸秆 | 600 | 1 | 3.9 | — | 硝酸盐 | 24.8 | [ |
| 1 | LI Xiang, QIN Yang, JIA Yan, et al. Preparation and application of Fe/biochar (Fe-BC) catalysts in wastewater treatment: a review[J]. Chemosphere, 2021, 274: 129766. |
| 2 | WANG Qing, YANG Zhiming. Industrial water pollution, water environment treatment, and health risks in China[J]. Environmental Pollution, 2016, 218: 358-365. |
| 3 | LI Xiang, ZHOU Minghua, PAN Yuwei. Enhanced degradation of 2, 4-dichlorophenoxyacetic acid by pre-magnetization Fe-C activated persulfate: influential factors, mechanism and degradation pathway[J]. Journal of Hazardous Materials, 2018, 353: 454-465. |
| 4 | LI Xiang, ZHOU Minghua, PAN Yuwei. Degradation of diclofenac by H2O2 activated with pre-magnetization Fe0: influencing factors and degradation pathways[J]. Chemosphere, 2018, 212: 853-862. |
| 5 | CAI Ruquan, ZHANG Baogang, SHI Jiaxin, et al. Rapid photocatalytic decolorization of methyl orange under visible light using VS4/carbon powder nanocomposites[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(9): 7690-7699. |
| 6 | WANG Yue, YANG Qixia, CHEN Jiacheng, et al. Adsorption behavior of Cr(VI) by magnetically modified Enteromorpha prolifera based biochar and the toxicity analysis[J]. Journal of Hazardous Materials, 2020, 395: 122658. |
| 7 | HAN Yanhe, LI Han, LIU Meili, et al. Purification treatment of dyes wastewater with a novel micro-electrolysis reactor[J]. Separation and Purification Technology, 2016, 170: 241-247. |
| 8 | CHEN Weiming, ZHANG Aiping, GU Zhepei, et al. Enhanced degradation of refractory organics in concentrated landfill leachate by Fe0/H2O2 coupled with microwave irradiation[J]. Chemical Engineering Journal, 2018, 354: 680-691. |
| 9 | YAO Bin, LUO Zirui, ZHI Dan, et al. Current progress in degradation and removal methods of polybrominated diphenyl ethers from water and soil: a review[J]. Journal of Hazardous Materials, 2021, 403: 123674. |
| 10 | NADEEM R, ZAFAR M N, AFZAL A, et al. Potential of NaOH pretreated Mangifera indica waste biomass for the mitigation of Ni(II) and Co(II) from aqueous solutions[J]. Journal of the Taiwan Institute of Chemical Engineers, 2014, 45(3): 967-972. |
| 11 | HADDAD M, OIE C, VO DUY S, et al. Adsorption of micropollutants present in surface waters onto polymeric resins: impact of resin type and water matrix on performance[J]. Science of the Total Environment, 2019, 660: 1449-1458. |
| 12 | KHATAEE A R, KASIRI M B. Photocatalytic degradation of organic dyes in the presence of nanostructured titanium dioxide: influence of the chemical structure of dyes[J]. Journal of Molecular Catalysis A: Chemical, 2010, 328(1/2): 8-26. |
| 13 | XU Huacheng, JI Li, KONG Ming, et al. Molecular weight-dependent adsorption fractionation of natural organic matter on ferrihydrite colloids in aquatic environment[J]. Chemical Engineering Journal, 2019, 363: 356-364. |
| 14 | XIONG X N, YU I K M, TSANG D C W, et al. Value-added chemicals from food supply chain wastes: state-of-the-art review and future prospects[J]. Chemical Engineering Journal, 2019, 375: 121983. |
| 15 | YI Yunqiang, TU Guoquan, ZHAO Dongye, et al. Biomass waste components significantly influence the removal of Cr(VI) using magnetic biochar derived from four types of feedstocks and steel pickling waste liquor[J]. Chemical Engineering Journal, 2019, 360: 212-220. |
| 16 | ZHANG Lianke, GUO Jinyue, HUANG Xuemin, et al. Functionalized biochar-supported magnetic MnFe2O4 nanocomposite for the removal of Pb(ii) and Cd(ii)[J]. RSC Advances, 2019, 9(1): 365-376. |
| 17 | YI Yunqiang, HUANG Zhexi, LU Baizhou, et al. Magnetic biochar for environmental remediation: a review[J]. Bioresource Technology, 2020, 298: 122468. |
| 18 | WANG Jianlong, WANG Shizong. Preparation, modification and environmental application of biochar: a review[J]. Journal of Cleaner Production, 2019, 227: 1002-1022. |
| 19 | JING Xiangrong, WANG Yuanying, LIU Wujun, et al. Enhanced adsorption performance of tetracycline in aqueous solutions by methanol-modified biochar[J]. Chemical Engineering Journal, 2014, 248: 168-174. |
| 20 | INYANG M, GAO B, ZIMMERMAN A, et al. Synthesis, characterization, and dye sorption ability of carbon nanotube-biochar nanocomposites[J]. Chemical Engineering Journal, 2014, 236: 39-46. |
| 21 | SHI Jingxin, HAN Hongjun, XU Chunyan. A novel enhanced anaerobic biodegradation method using biochar and Fe(OH)3@biochar for the removal of nitrogen heterocyclic compounds from coal gasification wastewater[J]. Science of the Total Environment, 2019, 697: 134052. |
| 22 | LIU Banghai, GUO Wanqian, WANG Huazhe, et al. Activation of peroxymonosulfate by cobalt-impregnated biochar for atrazine degradation: the pivotal roles of persistent free radicals and ecotoxicity assessment[J]. Journal of Hazardous Materials, 2020, 398: 122768. |
| 23 | FENG Yu, LIU Peng, WANG Yanxin, et al. Distribution and speciation of iron in Fe-modified biochars and its application in removal of As(V), As(III), Cr(VI), and Hg(II): an X-ray absorption study[J]. Journal of Hazardous Materials, 2020, 384: 121342. |
| 24 | LEE T, NAM I H, JUNG S, et al. Synthesis of nickel/biochar composite from pyrolysis of Microcystis aeruginosa and its practical use for syngas production[J]. Bioresource Technology, 2020, 300: 122712. |
| 25 | LUO Junmei, BO Shufeng, QIN Yanan, et al. Transforming goat manure into surface-loaded cobalt/biochar as PMS activator for highly efficient ciprofloxacin degradation[J]. Chemical Engineering Journal, 2020, 395: 125063. |
| 26 | DAI Shijin, ZHAO Youcai, NIU Dongjie, et al. Preparation and reactivation of magnetic biochar by molten salt method: relevant performance for chlorine-containing pesticides abatement[J]. Journal of the Air & Waste Management Association, 2019, 69(1): 58-70. |
| 27 | FENG Zhuqing, YUAN Rongfang, WANG Fei, et al. Preparation of magnetic biochar and its application in catalytic degradation of organic pollutants: a review[J]. Science of the Total Environment, 2021, 765: 142673. |
| 28 | 胡学玉, 陈窈君, 张沙沙, 等. 磁性玉米秸秆生物炭对水体中Cd的去除作用及回收利用[J]. 农业工程学报, 2018, 34(19): 208-218. |
| HU Xueyu, CHEN Yaojun, ZHANG Shasha, et al. Cd removal from aqueous solution using magnetic biochar derived from maize straw and its recycle[J]. Transactions of the Chinese Society of Agricultural Engineering, 2018, 34(19): 208-218. | |
| 29 | 崔志文, 任艳芳, 王伟, 等. 碱和磁复合改性小麦秸秆生物炭对水体中镉的吸附特性及机制[J]. 环境科学, 2020, 41(7): 3315-3325. |
| CUI Zhiwen, REN Yanfang, WANG Wei, et al. Adsorption characteristics and mechanism of cadmium in water by alkali and magnetic composite modified wheat straw biochar[J]. Environmental Science, 2020, 41(7): 3315-3325. | |
| 30 | 曹玮, 周航, 邓贵友, 等. 改性谷壳生物炭负载磁性Fe去除废水中Pb2+的效果及机制[J]. 环境工程学报, 2017, 11(3): 1437-1444. |
| CAO Wei, ZHOU Hang, DENG Guiyou, et al. Effects and mechanisms of magnetic iron supported on rice husk biochar removing Pb2+ in wastewater[J]. Chinese Journal of Environmental Engineering, 2017, 11(3): 1437-1444. | |
| 31 | GUO Zhiqiang, CHEN Rui, YANG Rongrong, et al. Synthesis of amino-functionalized biochar/spinel ferrite magnetic composites for low-cost and efficient elimination of Ni(II) from wastewater[J]. Science of the Total Environment, 2020, 722: 137822. |
| 32 | WANG Chongqing, WANG Hui. Pb(II) sorption from aqueous solution by novel biochar loaded with nano-particles[J]. Chemosphere, 2018, 192: 1-4. |
| 33 | 符剑刚, 贾阳, 李政, 等. 磁性生物炭负载Mg-Fe水滑石的制备及其吸附水中Cd(Ⅱ)和Ni(Ⅱ)的性能[J]. 化工环保, 2019, 39(5): 574-580. |
| FU Jiangang, JIA Yang, LI Zheng, et al. Preparation of Mg-Fe hydrotalcite supported on magnetic biochar and its adsorption capacity to Cd(Ⅱ) and Ni(Ⅱ) from water[J]. Environmental Protection of Chemical Industry, 2019, 39(5): 574-580. | |
| 34 | NIU Zhirui, FENG Wenli, HUANG Hua, et al. Green synthesis of a novel Mn-Zn ferrite/biochar composite from waste batteries and pine sawdust for Pb2+ removal[J]. Chemosphere, 2020, 252: 126529. |
| 35 | 高海荣, 姜明月, 黄振旭, 等. 磁性黑藻生物炭复合材料的制备及其对水体Cu2+的吸附[J]. 化工新型材料, 2021, 49(10): 186-190. |
| GAO Hairong, JIANG Mingyue, HUANG Zhenxu, et al. Preparation of magnetic black algae biochar composite material and its adsorption of Cu2+ in water[J]. New Chemical Materials, 2021, 49(10): 186-190. | |
| 36 | LUO Xuewen, SHEN Minxian, HUANG Zhujian, et al. Efficient removal of Pb(II) through recycled biochar-mineral composite from the coagulation sludge of swine wastewater[J]. Environmental Research, 2020, 190: 110014. |
| 37 | 袁健, 钱雅洁, 薛罡, 等. 活性污泥水热碳化法制备磁性炭及对水体Cd2+及Pb2+的去除[J]. 环境工程, 2020, 38(2): 55-62. |
| YUAN Jian, QIAN Yajie, XUE Gang, et al. Removal of cadmium and lead in water by magnetic carbon prepared from activated sludge with hydrothermal carbonization[J]. Environmental Engineering, 2020, 38(2): 55-62. | |
| 38 | AJMAL Z, MUHMOOD A, DONG Renjie, et al. Probing the efficiency of magnetically modified biomass-derived biochar for effective phosphate removal[J]. Journal of Environmental Management, 2020, 253: 109730. |
| 39 | XIAO Jiang, HU Rui, CHEN Guangcai, et al. Facile synthesis of multifunctional bone biochar composites decorated with Fe/Mn oxide micro-nanoparticles: physicochemical properties, heavy metals sorption behavior and mechanism[J]. Journal of Hazardous Materials, 2020, 399: 123067. |
| 40 | WANG Can, TAN Hang, LIU Huakang, et al. A nanoscale ferroferric oxide coated biochar derived from mushroom waste to rapidly remove Cr(VI) and mechanism study[J]. Bioresource Technology Reports, 2019, 7: 100253. |
| 41 | 王向辉, 石建军, 游诚航, 等. Fe3O4@ABc复合材料的制备及其对水中甲基橙的吸附[J]. 精细化工, 2020, 37(7): 1422-1428. |
| WANG Xianghui, SHI Jianjun, YOU Chenghang, et al. Preparation of Fe3O4@ABc composite and its adsorption for methyl orange in water[J]. Fine Chemicals, 2020, 37(7): 1422-1428. | |
| 42 | 周雅兰, 周冰. Fe浸渍污泥生物炭对含Cd(Ⅱ)废水的吸附性能研究[J]. 工业水处理, 2021, 41(5): 80-85. |
| ZHOU Yalan, ZHOU Bing. Adsorption performance of Fe-impregnated sludge biochar for removing Cd(Ⅱ)-containing wastewater[J]. Industrial Water Treatment, 2021, 41(5): 80-85. | |
| 43 | 杨期鑫, 俞璐军, 董余兵, 等. 磁功能化多孔生物质炭复合材料的制备及吸波性能[J]. 新型炭材料, 2019, 34(5): 455-463. |
| YANG Qixin, YU Lujun, DONG Yubing, et al. Preparation and microwave absorption properties of magnetic functional porous biomass carbon composites[J]. New Carbon Materials, 2019, 34(5): 455-463. | |
| 44 | 陈昌诚, 罗米娜, 黄超, 等. 磁性明胶改性秸秆生物炭对双氯芬酸钠的吸附研究[J]. 应用化工, 2021, 50(7): 1850-1854. |
| CHEN Changcheng, LUO Mina, HUANG Chao, et al. Study on sorption of diclofenac sodium using magnetic-gelatin supported on Brassica-straw biochar[J]. Applied Chemical Industry, 2021, 50(7): 1850-1854. | |
| 45 | 郑景华, 王宇, 王聪, 等. 磁性生物炭对As(Ⅲ)的吸附行为研究[J]. 离子交换与吸附, 2018, 34(2): 116-126. |
| ZHENG Jinghua, WANG Yu, WANG Cong, et al. Adsoption of As(ⅲ) on magnetic biochar[J]. Ion Exchange and Adsorption, 2018, 34(2): 116-126. | |
| 46 | 赵旭, 王淑娟, 郭伟, 等. 磁性稻壳生物炭对水体中菲的去除特性[J]. 水资源保护, 2019, 35(5): 70-77. |
| ZHAO Xu, WANG Shujuan, GUO Wei, et al. Characteristics of removing phenanthrene (PHE) from water by magnetic rice husk biochar[J]. Water Resources Protection, 2019, 35(5): 70-77. | |
| 47 | 万霞, 梅昌艮, 何俐臻, 等. 磁性生物炭的制备、表征及对磷的吸附特性[J]. 安全与环境学报, 2017, 17(3): 1069-1075. |
| WAN Xia, MEI Changgen, HE Lizhen, et al. On the synthesis, characterization and phosphate removal of the biocharbased magnetic composites[J]. Journal of Safety and Environment, 2017, 17(3): 1069-1075. | |
| 48 | WANG Shengsen, GAO Bin, ZIMMERMAN A R, et al. Removal of arsenic by magnetic biochar prepared from pinewood and natural hematite[J]. Bioresource Technology, 2015, 175: 391-395. |
| 49 | YANG Xing, ZHANG Xiaoli, WANG Zhaowei, et al. Mechanistic insights into removal of norfloxacin from water using different natural iron ore - biochar composites: more rich free radicals derived from natural pyrite-biochar composites than hematite-biochar composites[J]. Applied Catalysis B: Environmental, 2019, 255: 117752. |
| 50 | LU Jing, YANG Yaqian, LIU Pengxiao, et al. Iron-montmorillonite treated corn straw biochar: interfacial chemical behavior and stability[J]. Science of the Total Environment, 2020, 708: 134773. |
| 51 | LI Mengxue, LIU Haibo, CHEN Tianhu, et al. Synthesis of magnetic biochar composites for enhanced uranium(VI) adsorption[J]. Science of the Total Environment, 2019, 651: 1020-1028. |
| 52 | QU Jianhua, AKINDOLIE M S, FENG Yan, et al. One-pot hydrothermal synthesis of NaLa(CO3)2 decorated magnetic biochar for efficient phosphate removal from water: kinetics, isotherms, thermodynamics, mechanisms and reusability exploration[J]. Chemical Engineering Journal, 2020, 394: 124915. |
| 53 | 陶利春, 陈豪宇, 刘杰, 等. 磁性花生壳基活性炭对亚甲基蓝的吸附特性[J]. 环境污染与防治, 2018, 40(6): 639-644, 692. |
| TAO Lichun, CHEN Haoyu, LIU Jie, et al. Adsorption characteristics of methylene blue onto magnetic peanut shell based activated carbon[J]. Environmental Pollution & Control, 2018, 40(6): 639-644, 692. | |
| 54 | ZHANG Sijing, JI Yongliang, DANG Jing, et al. Magnetic apple pomace biochar: Simple preparation, characterization, and application for enriching Ag(I) in effluents[J]. Science of the Total Environment, 2019, 668: 115-123. |
| 55 | WANG He, WANG Han, ZHAO Hui, et al. Adsorption and Fenton-like removal of chelated nickel from Zn-Ni alloy electroplating wastewater using activated biochar composite derived from Taihu blue algae[J]. Chemical Engineering Journal, 2020, 379: 122372. |
| 56 | LIU Shaobo, LI Meifang, LIU Yunguo, Liu, et al. Removal of 17β-estradiol from aqueous solution by graphene oxide supported activated magnetic biochar: adsorption behavior and mechanism[J]. Journal of the Taiwan Institute of Chemical Engineers, 2019, 102:330-339. |
| 57 | LIU Kai, LI Fangbai, CUI Jianghu, et al. Simultaneous removal of Cd(Ⅱ) and As(Ⅲ) by graphene-like biochar-supported zero-valent iron from irrigation waters under aerobic conditions: synergistic effects and mechanisms[J]. Journal of Hazardous Materials, 2020, 395: 122623. |
| 58 | 肖芳芳, 张莹莹, 程建华, 等. 壳聚糖/磁性生物碳对重金属Cu(Ⅱ)的吸附性能[J]. 环境工程学报, 2019, 13(5): 1048-1055. |
| XIAO Fangfang, ZHANG Yingying, CHENG Jianhua, et al. Adsorption properties of chitosan/magnetic biochar for Cu(Ⅱ) removal from solution[J]. Chinese Journal of Environmental Engineering, 2019, 13(5): 1048-1055. | |
| 59 | ZHOU Xiaohui, ZHOU Jianjun, LIU Yaochi, et al. Preparation of iminodiacetic acid-modified magnetic biochar by carbonization, magnetization and functional modification for Cd(Ⅱ) removal in water[J]. Fuel, 2018, 233: 469-479. |
| 60 | LI Mingrun, WEI Dong, LIU Ting, et al. EDTA functionalized magnetic biochar for Pb( Ⅱ ) removal: adsorption performance, mechanism and SVM model prediction[J]. Separation and Purification Technology, 2019, 227: 115696. |
| 61 | MANEECHAKR P, MONGKOLLERTLOP S. Investigation on adsorption behaviors of heavy metal ions (Cd2+, Cr3+, Hg2+ and Pb2+) through low-cost/active manganese dioxide-modified magnetic biochar derived from palm kernel cake residue[J]. Journal of Environmental Chemical Engineering, 2020, 8(6): 104467. |
| 62 | WANG Huabin, CAI Jiayi, LIAO Zhuwei, et al. Black liquor as biomass feedstock to prepare zero-valent iron embedded biochar with red mud for Cr( Ⅵ ) removal: mechanisms insights and engineering practicality[J]. Bioresource Technology, 2020, 311: 123553. |
| 63 | YU Jiangfang, TANG Lin, PANG Ya, et al. Magnetic nitrogen-doped sludge-derived biochar catalysts for persulfate activation: internal electron transfer mechanism[J]. Chemical Engineering Journal, 2019, 364: 146-159. |
| 64 | GAN Quan, HOU Huijie, LIANG Sha, et al. Sludge-derived biochar with multivalent iron as an efficient Fenton catalyst for degradation of 4-chlorophenol[J]. Science of the Total Environment, 2020, 725: 138299. |
| 65 | WANG Jia, SHEN Min, GONG Qing, et al. One-step preparation of ZVI-sludge derived biochar without external source of iron and its application on persulfate activation[J]. Science of the Total Environment, 2020, 714: 136728. |
| 66 | LIU Y Y, SOHI S P, LIU S Y, et al. Adsorption and reductive degradation of Cr(Ⅵ) and TCE by a simply synthesized zero valent iron magnetic biochar[J]. Journal of Environmental Management, 2019, 235: 276-281. |
| 67 | ZHANG Xiaoying, SUN Peizhe, WEI Kajia, et al. Enhanced H2O2 activation and sulfamethoxazole degradation by Fe-impregnated biochar[J]. Chemical Engineering Journal, 2020, 385: 123921. |
| 68 | 吴卫蔚, 毛磊, 胡慧兰, 等. 不同铁改性剂对磁性麦秆生物炭吸附Cr(Ⅵ)的影响[J]. 有色金属(冶炼部分), 2022(2): 90-98. |
| WU Weiwei, MAO Lei, HU Huilan, et al. Effect of Fe-bearing modifying agents on adsorption performance of magnetic straw-derived biochars for Cr(Ⅵ)[J]. Nonferrous Metals (Extractive Metallurgy), 2022(2): 90-98. | |
| 69 | 沈玲芳, 董隽, 单胜道, 等. 磁性生物质炭制备方法及其对水体Pb2+吸附特性的影响[J]. 环境工程, 2021, 39(9): 48-55. |
| SHEN Lingfang, DONG Jun, SHAN Shengdao, et al. Influence of magnetic biochar preparation methods on adsorption characteristics of pb2+ in wastewater[J]. Environmental Engineering, 2021, 39(9): 48-55. | |
| 70 | ZHOU Jingyao, LIU Yuyan, HAN Yitong, et al. Bone-derived biochar and magnetic biochar for effective removal of fluoride in groundwater: effects of synthesis method and coexisting chromium[J]. Water Environment Research, 2019, 91(7): 588-597. |
| 71 | YANG Dong, WANG Lu, LI Zhangtao, et al. Simultaneous adsorption of Cd(Ⅱ) and As(Ⅲ) by a novel biochar-supported nanoscale zero-valent iron in aqueous systems[J]. Science of the Total Environment, 2020, 708: 134823. |
| 72 | QIAN Linbo, ZHANG Wenying, YAN Jingchun, et al. Nanoscale zero-valent iron supported by biochars produced at different temperatures: synthesis mechanism and effect on Cr(Ⅵ) removal[J]. Environmental Pollution, 2017, 223: 153-160. |
| 73 | YI Yan, WANG Xiangyu, MA Jun, et al. An efficient Egeria najas-derived biochar supported nZVI composite for Cr( Ⅵ ) removal: characterization and mechanism investigation based on visual MINTEQ model[J]. Environmental Research, 2020, 189: 109912. |
| 74 | WANG Kun, SUN Yuebing, TANG Jingchun, et al. Aqueous Cr(Ⅵ) removal by a novel ball milled Fe0-biochar composite: role of biochar electron transfer capacity under high pyrolysis temperature[J]. Chemosphere, 2020, 241: 125044. |
| 75 | HEO J, YOON Y, LEE G, et al. Enhanced adsorption of bisphenol A and sulfamethoxazole by a novel magnetic CuZnFe2O4-biochar composite[J]. Bioresource Technology, 2019, 281: 179-187. |
| 76 | LI Yanfei, ZIMMERMAN A R, HE Feng, et al. Solvent-free synthesis of magnetic biochar and activated carbon through ball-mill extrusion with Fe3O4 nanoparticles for enhancing adsorption of methylene blue[J]. Science of the Total Environment, 2020, 722: 137972. |
| 77 | ZHANG Han, XIAO Ran, LI Ronghua, et al. Enhanced aqueous Cr(Ⅵ) removal using chitosan-modified magnetic biochars derived from bamboo residues[J]. Chemosphere, 2020, 261: 127694. |
| 78 | FENG Zhuqing, CHEN Huilun, LI Haiqing, et al. Preparation, characterization, and application of magnetic activated carbon for treatment of biologically treated papermaking wastewater[J]. Science of the Total Environment, 2020, 713: 136423. |
| 79 | GAO Jie, HAN Dongqiang, XU Yun, et al. Persulfate activation by sulfide-modified nanoscale iron supported by biochar (S-nZVI/BC) for degradation of ciprofloxacin[J]. Separation and Purification Technology, 2020, 235: 116202. |
| 80 | IFTHIKAR J, WANG Jia, WANG Qiliang, et al. Highly efficient lead distribution by magnetic sewage sludge biochar: sorption mechanisms and bench applications[J]. Bioresource Technology, 2017, 238: 399-406. |
| 81 | MEI Jinfeng, ZHANG Hui, LI Zhongyu, et al. A novel tetraethylenepentamine crosslinked chitosan oligosaccharide hydrogel for total adsorption of Cr( Ⅵ )[J]. Carbohydrate Polymers, 2019, 224: 115154. |
| 82 | WANG Bo, JIANG Yansong, LI Fayun, et al. Preparation of biochar by simultaneous carbonization, magnetization and activation for norfloxacin removal in water[J]. Bioresource Technology, 2017, 233: 159-165. |
| 83 | ZHANG Yuting, LIU Na, YANG Yadong, et al. Novel carbothermal synthesis of Fe, N co-doped oak wood biochar (Fe/N-OB) for fast and effective Cr( Ⅵ ) removal[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2020, 600: 124926. |
| 84 | LI Xiangping, WANG Chuanbin, ZHANG Jianguang, et al. Preparation and application of magnetic biochar in water treatment: a critical review[J]. Science of the Total Environment, 2020, 711: 134847. |
| 85 | JUNG K W, LEE S, LEE Y J. Synthesis of novel magnesium ferrite (MgFe2O4)/biochar magnetic composites and its adsorption behavior for phosphate in aqueous solutions[J]. Bioresource Technology, 2017, 245: 751-759. |
| 86 | 肖作义, 肖宇, 肖明慧, 等. 磁性水滑石/生物炭复合材料的制备及其对水溶液中磷的吸附性能[J]. 环境污染与防治, 2020, 42(9): 1090-1095, 1101. |
| XIAO Zuoyi, XIAO Yu, XIAO Minghui, et al. Preparation of magnetic hydrotalcite/biochar composite and its adsorption performance for phosphorus in aqueous solution[J]. Environmental Pollution & Control, 2020, 42(9): 1090-1095, 1101. | |
| 87 | LIU Liheng, LIU Xiu, WANG Dunqiu, et al. Removal and reduction of Cr(Ⅵ) in simulated wastewater using magnetic biochar prepared by co-pyrolysis of nano-zero-valent iron and sewage sludge[J]. Journal of Cleaner Production, 2020, 257: 120562. |
| 88 | 王芳君, 桑倩倩, 邓颖, 等. 磁性铁基改性生物炭去除水中氨氮[J]. 环境科学, 2021, 42(4): 1913-1922. |
| WANG Fangjun, SANG Qianqian, DENG Ying, et al. Synthesis of magnetic iron modifying biochar for ammonia nitrogen removal from water[J]. Environmental Science, 2021, 42(4): 1913-1922. | |
| 89 | WU Jizi, HUANG Dan, LIU Xingmei, et al. Remediation of As(Ⅲ) and Cd(Ⅱ) co-contamination and its mechanism in aqueous systems by a novel calcium-based magnetic biochar[J]. Journal of Hazardous Materials, 2018, 348: 10-19. |
| 90 | CAI Weiquan, WEI Jiahao, LI Zhonglei, et al. Preparation of amino-functionalized magnetic biochar with excellent adsorption performance for Cr(Ⅵ) by a mild one-step hydrothermal method from peanut hull[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2019, 563: 102-111. |
| 91 | NEELI S T, RAMSURN H, NG C Y, et al. Removal of Cr(Ⅵ), As (Ⅴ), Cu( Ⅱ ), and Pb( Ⅱ ) using cellulose biochar supported iron nanoparticles: a kinetic and mechanistic study[J]. Journal of Environmental Chemical Engineering, 2020, 8(5): 103886. |
| 92 | QIU Bingbing, TAO Xuedong, WANG Hao, et al. Biochar as a low-cost adsorbent for aqueous heavy metal removal: a review[J]. Journal of Analytical and Applied Pyrolysis, 2021, 155: 105081. |
| 93 | 马锋锋, 赵保卫, 刁静茹, 等. 磁性生物炭对水体中对硝基苯酚的吸附特性[J]. 中国环境科学, 2019, 39(1): 170-178. |
| MA Fengfeng, ZHAO Baowei, DIAO Jingru, et al. Adsorption characteristics of p-nitrophenol removal by magnetic biochar[J]. China Environmental Science, 2019, 39(1): 170-178. | |
| 94 | ZHANG P, O’CONNOR D, WANG Y N, et al. A green biochar/iron oxide composite for methylene blue removal[J]. Journal of Hazardous Materials, 2020, 384: 121286. |
| 95 | ZHAO Yueling, ZHANG Ruyu, LIU Haibo, et al. Green preparation of magnetic biochar for the effective accumulation of Pb(Ⅱ): performance and mechanism[J]. Chemical Engineering Journal, 2019, 375: 122011. |
| 96 | 张连科, 王洋, 王维大, 等. 磁性羟基磷灰石/生物炭复合材料的制备及对Pb2+的吸附性能[J]. 环境科学学报, 2018, 38(11): 4360-4370. |
| ZHANG Lianke, WANG Yang, WANG Weida, et al. Preparation of magnetic hydroxyapatite/biochar composite and its adsorption behavior of Pb2+ and recycling performance[J]. Acta Scientiae Circumstantiae, 2018, 38(11): 4360-4370. | |
| 97 | CAI Ru, WANG Xin, JI Xionghui, et al. Phosphate reclaim from simulated and real eutrophic water by magnetic biochar derived from water hyacinth[J]. Journal of Environmental Management, 2017, 187: 212-219. |
| 98 | MA Yongfei, LI Ming, LI Ping, et al. Hydrothermal synthesis of magnetic sludge biochar for tetracycline and ciprofloxacin adsorptive removal[J]. Bioresource Technology, 2021, 319: 124199. |
| 99 | AGRAFIOTI E, KALDERIS D, DIAMADOPOULOS E. Ca and Fe modified biochars as adsorbents of arsenic and chromium in aqueous solutions[J]. Journal of Environmental Management, 2014, 146: 444-450. |
| 100 | SU Chunli, WANG Sheng, ZHOU Ziyi, et al. Chemical processes of Cr( Ⅵ ) removal by Fe-modified biochar under aerobic and anaerobic conditions and mechanism characterization under aerobic conditions using synchrotron-related techniques[J]. Science of the Total Environment, 2021, 768: 144604. |
| 101 | LIAO Taiwan, LI Ting, SU Xiangde, et al. La(OH)3-modified magnetic pineapple biochar as novel adsorbents for efficient phosphate removal[J]. Bioresource Technology, 2018, 263: 207-213. |
| 102 | PENG Yaru, AZEEM M, LI Ronghua, et al. Zirconium hydroxide nanoparticle encapsulated magnetic biochar composite derived from rice residue: application for As( Ⅲ ) and As( Ⅴ ) polluted water purification[J]. Journal of Hazardous Materials, 2022, 423: 127081. |
| 103 | YU Yang, AN Qiang, JIN Lin, et al. Unraveling sorption of Cr(Ⅵ) from aqueous solution by FeCl3 and ZnCl2-modified corn stalks biochar: implicit mechanism and application[J]. Bioresource Technology, 2020, 297: 122466. |
| 104 | YIN Zhibing, XU Shuang, LIU Sen, et al. A novel magnetic biochar prepared by K2FeO4-promoted oxidative pyrolysis of pomelo peel for adsorption of hexavalent chromium[J]. Bioresource Technology, 2020, 300: 122680. |
| 105 | WANG Xuedong, XU Jin, LIU Jia, et al. Mechanism of Cr(Ⅵ) removal by magnetic greigite/biochar composites[J]. Science of the Total Environment, 2020, 700: 134414. |
| 106 | LIANG Sha, SHI Shunquan, ZHANG Haohao, et al. One-pot solvothermal synthesis of magnetic biochar from waste biomass: formation mechanism and efficient adsorption of Cr(Ⅵ) in an aqueous solution[J]. Science of the Total Environment, 2019, 695: 133886. |
| 107 | NARZARI R, PODDAR M K, BORDOLOI N, et al. A comprehensive study to understand removal efficiency for Cr6+ using magnetic and activated biochar through response surface methodology[J]. Biomass Conversion and Biorefinery, 2021, DOI: 10.10071s13399-021-01448-3 . |
| 108 | 盖希坤, 马晓锋, 骆美宇, 等. 马尾松基磁性水热炭的制备及其吸附性能[J]. 化工进展, 2022, 41(4): 1994-1999. |
| GAI Xikun, MA Xiaofeng, LUO Meiyu, et al. Preparation and adsorption properties of magnetic hydrothermal carbon based on Pinus Massoniana[J]. Chemical Industry and Engineering Progress, 2022, 41(4): 1994-1999. | |
| 109 | 郭丰艳, 刘迎, 金昌磊. 果胶@生物炭-Fe3O4的制备及其对Cu2+的吸附性能[J]. 唐山学院学报, 2020, 33(6): 24-30. |
| GUO Fengyan, LIU Ying, JIN Changlei. Making of pectin @ biochar Fe3O4 and its adsorption property for Cu2+ [J]. Journal of Tangshan University, 2020, 33(6): 24-30. | |
| 110 | LI Huosheng, XIONG Jingfang, ZHANG Gaosheng, et al. Enhanced thallium(I) removal from wastewater using hypochlorite oxidation coupled with magnetite-based biochar adsorption[J]. Science of the Total Environment, 2020, 698: 134166. |
| 111 | ZHU Guocheng, LIN Jialin, YUAN Qian, et al. A biochar supported magnetic metal organic framework for the removal of trivalent antimony[J]. Chemosphere, 2021, 282: 131068. |
| 112 | WANG Shengsen, ZHAO Mingyue, ZHOU Min, et al. Biomass facilitated phase transformation of natural hematite at high temperatures and sorption of Cd2+ and Cu2+ [J]. Environment International, 2019, 124: 473-481. |
| 113 | GUO Feiqiang, LI Xiaolei, JIANG Xiaochen, et al. Characteristics and toxic dye adsorption of magnetic activated carbon prepared from biomass waste by modified one-step synthesis[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2018, 555: 43-54. |
| 114 | ZHOU Yaoyu, HE Yangzhou, HE Yangzhuo, et al. Analyses of tetracycline adsorption on alkali-acid modified magnetic biochar: Site energy distribution consideration[J]. Science of the Total Environment, 2019, 650: 2260-2266. |
| 115 | RATTANACHUESKUL N, SANING A, KAOWPHONG S, et al. Magnetic carbon composites with a hierarchical structure for adsorption of tetracycline, prepared from sugarcane bagasse via hydrothermal carbonization coupled with simple heat treatment process[J]. Bioresource Technology, 2017, 226: 164-172. |
| 116 | CHEN Siqin, CHEN Yali, JIANG Hong. Slow pyrolysis magnetization of hydrochar for effective and highly stable removal of tetracycline from aqueous solution[J]. Industrial & Engineering Chemistry Research, 2017, 56(11): 3059-3066. |
| 117 | SHAN Danna, DENG Shubo, ZHAO Tianning, et al. Preparation of ultrafine magnetic biochar and activated carbon for pharmaceutical adsorption and subsequent degradation by ball milling[J]. Journal of Hazardous Materials, 2016, 305: 156-163. |
| 118 | OLADIPO A A, IFEBAJO A O. Highly efficient magnetic chicken bone biochar for removal of tetracycline and fluorescent dye from wastewater: two-stage adsorber analysis[J]. Journal of Environmental Management, 2018, 209: 9-16. |
| 119 | HU Yi, ZHU Yuan, ZHANG Yi, et al. An efficient adsorbent: Simultaneous activated and magnetic ZnO doped biochar derived from camphor leaves for ciprofloxacin adsorption[J]. Bioresource Technology, 2019, 288: 121511. |
| 120 | AI Tian, JIANG Xiaojun, LIU Qingyu, et al. Daptomycin adsorption on magnetic ultra-fine wood-based biochars from water: kinetics, isotherms, and mechanism studies[J]. Bioresource Technology, 2019, 273: 8-15. |
| 121 | MA Yongfei, LI Ping, YANG Lie, et al. Iron/zinc and phosphoric acid modified sludge biochar as an efficient adsorbent for fluoroquinolones antibiotics removal[J]. Ecotoxicology and Environmental Safety, 2020, 196: 110550. |
| 122 | KONG Xiangrui, LIU Yaoxuan, PI Jiachang, et al. Low-cost magnetic herbal biochar: characterization and application for antibiotic removal[J]. Environmental Science and Pollution Research International, 2017, 24(7): 6679-6687. |
| 123 | REGUYAL F, SARMAH A K. Adsorption of sulfamethoxazole by magnetic biochar: Effects of pH, ionic strength, natural organic matter and 17α-ethinylestradiol[J]. Science of the Total Environment, 2018, 628/629: 722-730. |
| 124 | ZHANG Runyuan, ZHENG Xiaoxian, CHEN Bohan, et al. Enhanced adsorption of sulfamethoxazole from aqueous solution by Fe-impregnated graphited biochar[J]. Journal of Cleaner Production, 2020, 256: 120662. |
| 125 | AI Tian, JIANG Xiaojun, ZHONG Zhenxia, et al. Methanol-modified ultra-fine magnetic orange peel powder biochar as an effective adsorbent for removal of ibuprofen and sulfamethoxazole from water[J]. Adsorption Science & Technology, 2020, 38: 304-321. |
| 126 | YAO Xinxin, JI Lili, GUO Jian, et al. Magnetic activated biochar nanocomposites derived from wakame and its application in methylene blue adsorption[J]. Bioresource Technology, 2020, 302: 122842. |
| 127 | 李茜, 封温俐, 牛志睿, 等. 绿色合成磁性水热炭及对亚甲基蓝的吸附研究[J]. 安全与环境学报, 2021, 21(4): 1769-1776. |
| LI Qian, FENG Wenli, NIU Zhirui, et al. Green synthesis of magnetic hydrothermal biochar formethylene blue removal[J]. Journal of Safety and Environment, 2021, 21(4): 1769-1776. | |
| 128 | 王博, 牛志睿, 黄华, 等. 绿色合成磁性生物炭及其对亚甲基蓝的吸附[J]. 环境污染与防治, 2021, 43(4): 427-431, 435. |
| WANG Bo, NIU Zhirui, HUANG Hua, et al. Green synthesis of magnetic biochar and its adsorption capacity for methylene blue[J]. Environmental Pollution & Control, 2021, 43(4): 427-431, 435. | |
| 129 | 黄超, 罗米娜, 陈馥, 等. 核桃壳基磁性生物炭对亚甲基蓝的吸附特性研究[J]. 应用化工, 2020, 49(8): 1956-1961, 1965. |
| HUANG Chao, LUO Mina, CHEN Fu, et al. Study on sorption characteristics of methylene blue by magnetic biochar derived from walnut shell[J]. Applied Chemical Industry, 2020, 49(8): 1956-1961, 1965. | |
| 130 | WANG Huan, WANG Shan, GAO Yihong. Cetyl trimethyl ammonium bromide modified magnetic biochar from pine nut shells for efficient removal of acid chrome blue K[J]. Bioresource Technology, 2020, 312: 123564. |
| 131 | ELTAWEIL A S, MOHAMED H A, EL-MONAEM E M ABD, et al. Mesoporous magnetic biochar composite for enhanced adsorption of malachite green dye: characterization, adsorption kinetics, thermodynamics and isotherms[J]. Advanced Powder Technology, 2020, 31(3): 1253-1263. |
| 132 | SUN Pengfei, HUI Cai, AZIM KHAN R, et al. Efficient removal of crystal violet using Fe3O4-coated biochar: the role of the Fe3O4 nanoparticles and modeling study their adsorption behavior[J]. Scientific Reports, 2015, 5: 12638. |
| 133 | DU Cong, SONG Yonghui, SHI Shengnan, et al. Preparation and characterization of a novel Fe3O4-graphene-biochar composite for crystal violet adsorption[J]. Science of the Total Environment, 2020, 711: 134662. |
| 134 | JUNG K W, CHOI B H, JEONG T U, et al. Facile synthesis of magnetic biochar/Fe3O4 nanocomposites using electro-magnetization technique and its application on the removal of acid orange 7 from aqueous media[J]. Bioresource Technology, 2016, 220: 672-676. |
| 135 | ZHAO Huaxuan, LANG Yinhai. Adsorption behaviors and mechanisms of florfenicol by magnetic functionalized biochar and reed biochar[J]. Journal of the Taiwan Institute of Chemical Engineers, 2018, 88: 152-160. |
| 136 | ESSANDOH M, WOLGEMUTH D, PITTMAN C U, et al. Adsorption of metribuzin from aqueous solution using magnetic and nonmagnetic sustainable low-cost biochar adsorbents[J]. Environmental Science and Pollution Research International, 2017, 24(5): 4577-4590. |
| 137 | GAO Xiangyu, LIU Ruilin, MA Jin, et al. Combined dual-metal templates for fabrication of magnetic hierarchical porous carbon for highly efficient removal of 4-nitrophenol[J]. Journal of Porous Materials, 2016, 23(1): 157-164. |
| 138 | YAN Jingchun, QIAN Linbo, GAO Weiguo, et al. Enhanced Fenton-like degradation of trichloroethylene by hydrogen peroxide activated with nanoscale zero valent iron loaded on biochar[J]. Scientific Reports, 2017, 7: 43051. |
| 139 | BOMBUWALA DEWAGE N, LIYANAGE A S, SMITH Q, et al. Fast aniline and nitrobenzene remediation from water on magnetized and nonmagnetized Douglas fir biochar[J]. Chemosphere, 2019, 225: 943-953. |
| 140 | LUO Haiqiong, ZHANG Yongkui, XIE Yi, et al. Iron-rich microorganism-enabled synthesis of magnetic biocarbon for efficient adsorption of diclofenac from aqueous solution[J]. Bioresource Technology, 2019, 282: 310-317. |
| 141 | DONG Xinwei, HE Lingzhi, HU Hui, et al. Removal of 17β-estradiol by using highly adsorptive magnetic biochar nanoparticles from aqueous solution[J]. Chemical Engineering Journal, 2018, 352: 371-379. |
| 142 | KARUNANAYAKE A G, TODD O A, CROWLEY M L, et al. Rapid removal of salicylic acid, 4-nitroaniline, benzoic acid and phthalic acid from wastewater using magnetized fast pyrolysis biochar from waste Douglas fir[J]. Chemical Engineering Journal, 2017, 319: 75-88. |
| 143 | WANG Yifan, KANG Jiaming, JIANG Simeng, et al. A composite of Ni–Fe–Zn layered double hydroxides/biochar for atrazine removal from aqueous solution[J]. Biochar, 2020, 2(4): 455-464. |
| 144 | YUAN Ling, QIU Zhaofu, YUAN Lin, et al. Adsorption and mechanistic study for phosphate removal by magnetic Fe3O4-doped spent FCC catalysts adsorbent[J]. Chemosphere, 2019, 219: 183-190. |
| 145 | CUI Qingliang, XU Jinling, WANG Wei, et al. Phosphorus recovery by core-shell γ-Al2O3/Fe3O4 biochar composite from aqueous phosphate solutions[J]. Science of the Total Environment, 2020, 729: 138892. |
| 146 | LIU Jiwei, JIANG Jianguo, AIHEMAITI A, et al. Removal of phosphate from aqueous solution using MgO-modified magnetic biochar derived from anaerobic digestion residue[J]. Journal of Environmental Management, 2019, 250: 109438. |
| 147 | 余佳敏, 赵宇, 肖勇, 等. 烟秆基Fe/Mg磁性生物炭复合材料的制备、表征及吸附性能[J]. 江苏农业科学, 2019, 47(9): 257-262. |
| YU Jiamin, ZHAO Yu, XIAO Yong, et al. Study on preparation and characterization of tobacco stems magnetic biochar composites and its adsorbing capacity[J]. Jiangsu Agricultural Sciences, 2019, 47(9): 257-262. | |
| 148 | MICHÁLEKOVÁ-RICHVEISOVÁ B, FRIŠTÁK V, PIPÍŠKA M, et al. Iron-impregnated biochars as effective phosphate sorption materials[J]. Environmental Science and Pollution Research International, 2017, 24(1): 463-475. |
| 149 | BOMBUWALA DEWAGE N, LIYANAGE A S, PITTMAN C U, et al. Fast nitrate and fluoride adsorption and magnetic separation from water on α-Fe2O3 and Fe3O4 dispersed on Douglas fir biochar[J]. Bioresource Technology, 2018, 263: 258-265. |
| 150 | XUE Lihong, GAO Bin, WAN Yongshan, et al. High efficiency and selectivity of MgFe-LDH modified wheat-straw biochar in the removal of nitrate from aqueous solutions[J]. Journal of the Taiwan Institute of Chemical Engineers, 2016, 63: 312-317. |
| 151 | WANG Binliang, LI Yingying, ZHENG Junli, et al. Efficient removal of U(Ⅵ) from aqueous solutions using the magnetic biochar derived from the biomass of a bloom-forming cyanobacterium (Microcystis aeruginosa)[J]. Chemosphere, 2020, 254: 126898. |
| 152 | LI Mengxue, LIU Haibo, CHEN Tianhu, et al. Synthesis of magnetic biochar composites for enhanced uranium(Ⅵ) adsorption[J]. Science of the Total Environment, 2019, 651: 1020-1028. |
| 153 | ZHU Yuling, ZHENG Cong, WU Siying, et al. Interaction of Eu(III) on magnetic biochar investigated by batch, spectroscopic and modeling techniques[J]. Journal of Radioanalytical and Nuclear Chemistry, 2018, 316(3): 1337-1346. |
| 154 | HU Qingyuan, ZHU Yuling, HU Baowei, et al. Mechanistic insights into sequestration of U(Ⅵ) toward magnetic biochar: batch, XPS and EXAFS techniques[J]. Journal of Environmental Sciences, 2018, 70: 217-225. |
| [1] | CUI Shoucheng, XU Hongbo, PENG Nan. Simulation analysis of two MOFs materials for O2/He adsorption separation [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 382-390. |
| [2] | CHEN Chongming, CHEN Qiu, GONG Yunqian, CHE Kai, YU Jinxing, SUN Nannan. Research progresses on zeolite-based CO2 adsorbents [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 411-419. |
| [3] | XU Chunshu, YAO Qingda, LIANG Yongxian, ZHOU Hualong. Research progress on functionalization strategies of covalent organic frame materials and its adsorption properties for Hg(Ⅱ) and Cr(Ⅵ) [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 461-478. |
| [4] | GU Yongzheng, ZHANG Yongsheng. Dynamic behavior and kinetic model of Hg0 adsorption by HBr-modified fly ash [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 498-509. |
| [5] | GUO Qiang, ZHAO Wenkai, XIAO Yonghou. Numerical simulation of enhancing fluid perturbation to improve separation of dimethyl sulfide/nitrogen via pressure swing adsorption [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 64-72. |
| [6] | WANG Shengyan, DENG Shuai, ZHAO Ruikai. Research progress on carbon dioxide capture technology based on electric swing adsorption [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 233-245. |
| [7] | GE Quanqian, XU Mai, LIANG Xian, WANG Fengwu. Research progress on the application of MOFs in photoelectrocatalysis [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4692-4705. |
| [8] | GE Yafen, SUN Yu, XIAO Peng, LIU Qi, LIU Bo, SUN Chengying, GONG Yanjun. Research progress of zeolite for VOCs removal [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4716-4730. |
| [9] | YANG Ying, HOU Haojie, HUANG Rui, CUI Yu, WANG Bing, LIU Jian, BAO Weiren, CHANG Liping, WANG Jiancheng, HAN Lina. Coal tar phenol-based carbon nanosphere prepared by Stöber method for adsorption of CO2 [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 5011-5018. |
| [10] | JIANG Jing, CHEN Xiaoyu, ZHANG Ruiyan, SHENG Guangyao. Research progress of manganese-loaded biochar preparation and its application in environmental remediation [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4385-4397. |
| [11] | ZHANG Zhen, LI Dan, CHEN Chen, WU Jinglan, YING Hanjie, QIAO Hao. Separation and purification of salivary acids with adsorption resin [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4153-4158. |
| [12] | WANG Zhicai, LIU Weiwei, ZHOU Cong, PAN Chunxiu, YAN Honglei, LI Zhanku, YAN Jingchong, REN Shibiao, LEI Zhiping, SHUI Hengfu. Synthesis and performance of a superplasticizer based on coal-based humic acid [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3634-3642. |
| [13] | YU Jingwen, SONG Luna, LIU Yanchao, LYU Ruidong, WU Mengmeng, FENG Yu, LI Zhong, MI Jie. An indole-bearing hypercrosslinked polymer In-HCP for iodine adsorption from water [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3674-3683. |
| [14] | LI Yanling, ZHUO Zhen, CHI Liang, CHEN Xi, SUN Tanglei, LIU Peng, LEI Tingzhou. Research progress on preparation and application of nitrogen-doped biochar [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3720-3735. |
| [15] | BAI Yadi, DENG Shuai, ZHAO Ruikai, ZHAO Li, YANG Yingxia. Exploration on standardized test scheme and experimental performance of temperature swing adsorption carbon capture unit [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3834-3846. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||