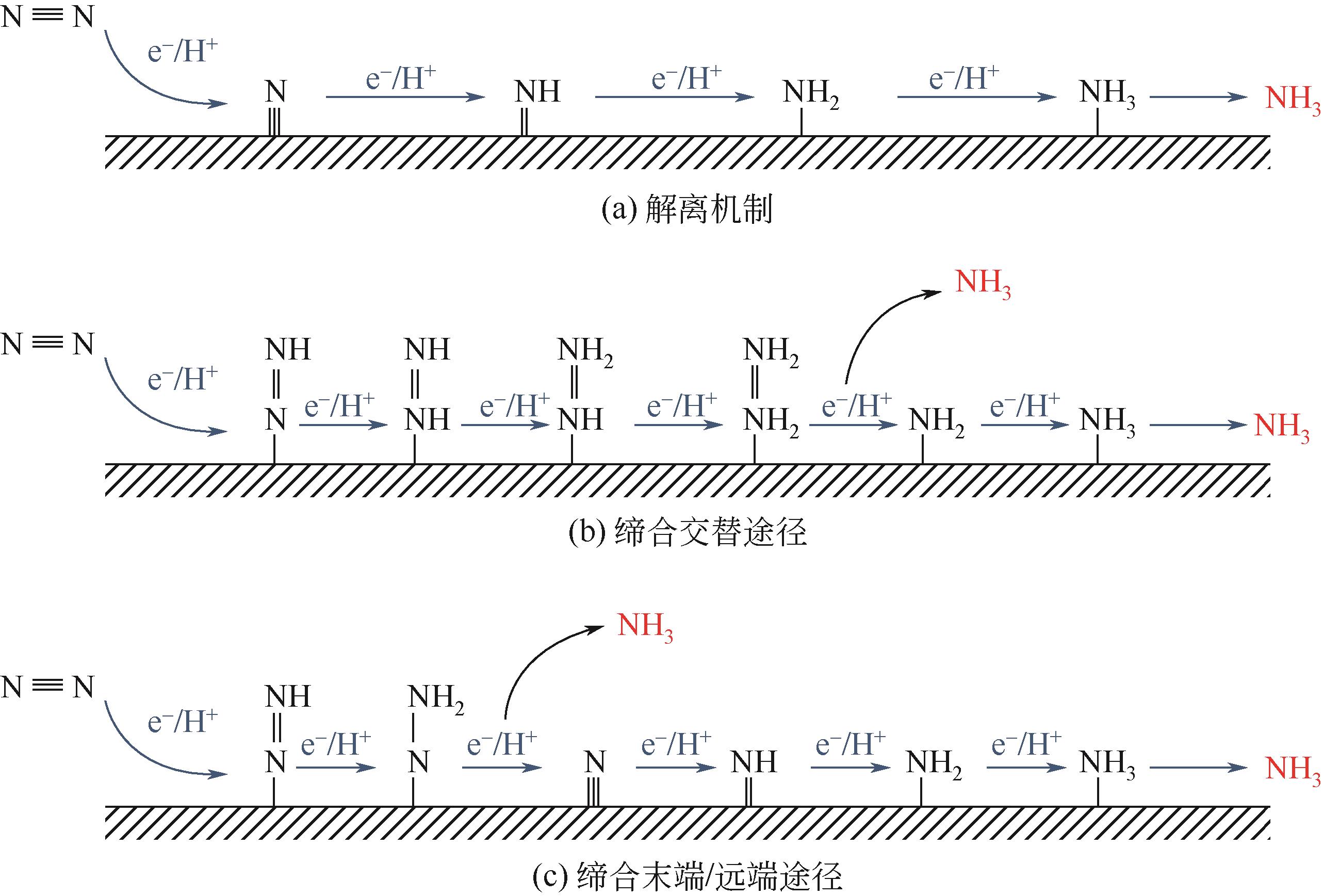
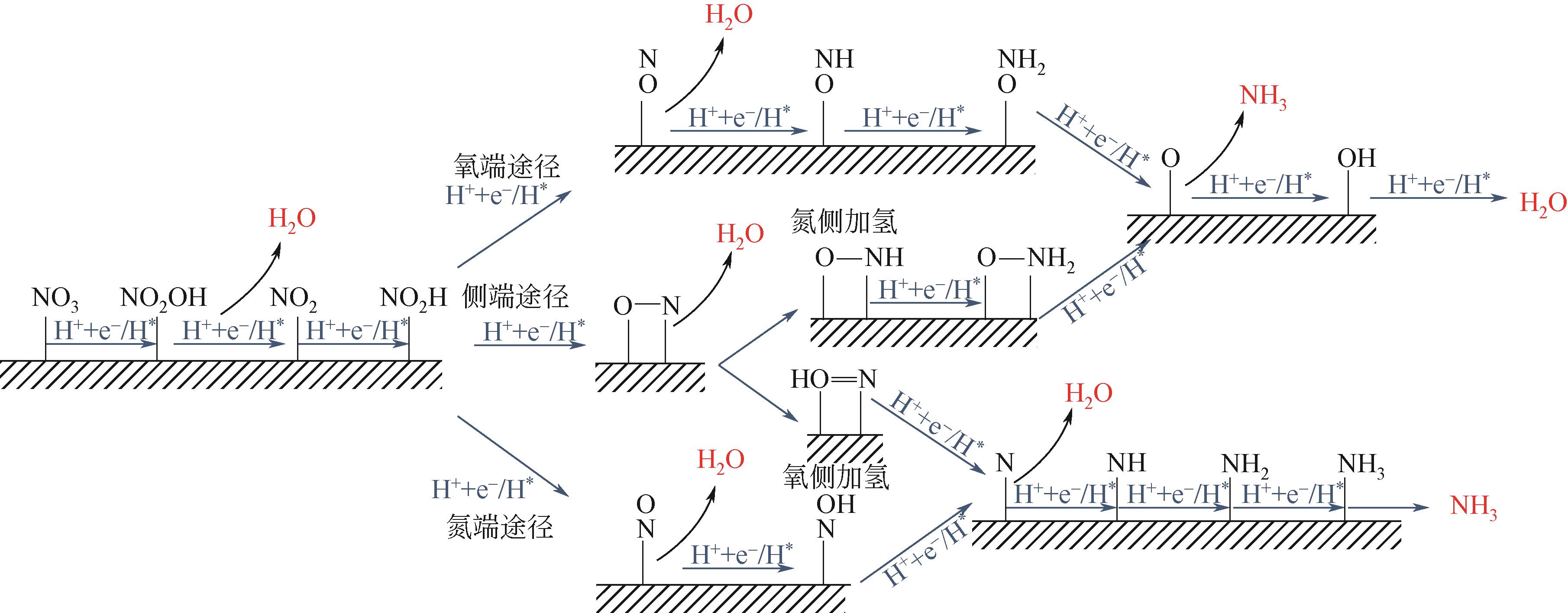
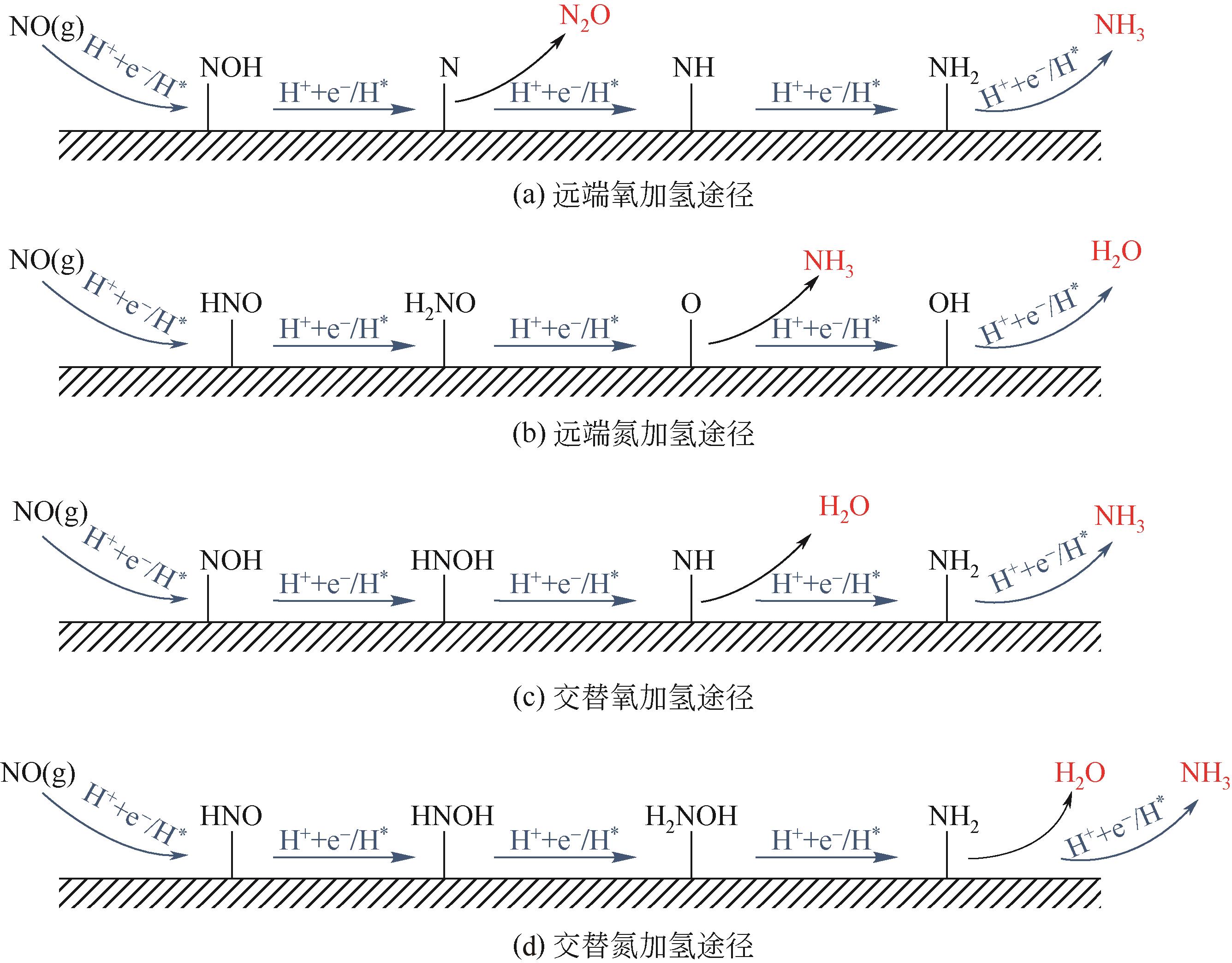
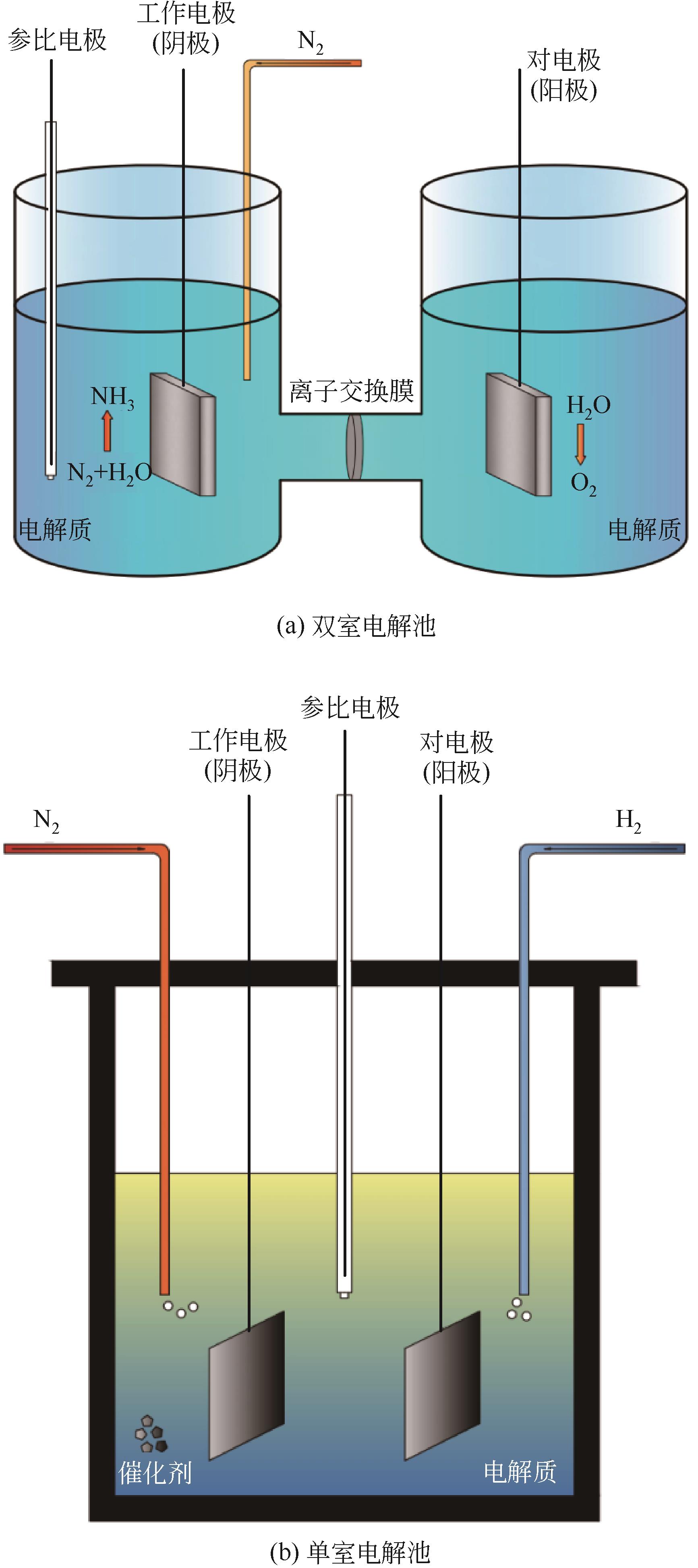
Chemical Industry and Engineering Progress ›› 2022, Vol. 41 ›› Issue (8): 4098-4110.DOI: 10.16085/j.issn.1000-6613.2021-2178
• Energy processes and technology • Previous Articles Next Articles
Progress and challenges of electrochemical synthesis of ammonia from different hydrogen and nitrogen sources
GUAN Haoran1( ), ZHU Lina2, ZHU Lingyue1, YUAN Dandan1, ZHANG Yuqing1, WANG Baohui1(
), ZHU Lina2, ZHU Lingyue1, YUAN Dandan1, ZHANG Yuqing1, WANG Baohui1( )
)
- 1.College of Chemistry and Chemical Engineering, Northeast Petroleum University, Daqing 163000, Heilongjiang, China
2.Daqing Chemical Research Institute of Petrochemical Research Institute of PetroChina Co. , Ltd. , Daqing 163714, Heilongjiang, China
-
Received:2021-10-25Revised:2022-01-12Online:2022-08-22Published:2022-08-25 -
Contact:WANG Baohui
利用不同氢源及氮源电化学合成氨研究进展与挑战
关浩然1( ), 朱丽娜2, 朱凌岳1, 苑丹丹1, 张雨晴1, 王宝辉1(
), 朱丽娜2, 朱凌岳1, 苑丹丹1, 张雨晴1, 王宝辉1( )
)
- 1.东北石油大学化学化工学院,黑龙江 大庆 163000
2.中国石油天然气股份有限公司石油化工研究院大庆化工研究中心,黑龙江 大庆 163714
-
通讯作者:王宝辉 -
作者简介:关浩然(1998—),男,硕士研究生,研究方向为电化学合成氨。E-mail:guanhr1998@163.com 。
CLC Number:
Cite this article
GUAN Haoran, ZHU Lina, ZHU Lingyue, YUAN Dandan, ZHANG Yuqing, WANG Baohui. Progress and challenges of electrochemical synthesis of ammonia from different hydrogen and nitrogen sources[J]. Chemical Industry and Engineering Progress, 2022, 41(8): 4098-4110.
关浩然, 朱丽娜, 朱凌岳, 苑丹丹, 张雨晴, 王宝辉. 利用不同氢源及氮源电化学合成氨研究进展与挑战[J]. 化工进展, 2022, 41(8): 4098-4110.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2021-2178
| 温度/℃ | 阴极/催化剂 | 阳极 | 还原电位(vs.RHE)/V | 电解质 |  /mol·s-1·cm-2 /mol·s-1·cm-2 | FE/% | 参考文献 |
|---|---|---|---|---|---|---|---|
| 贵金属 | |||||||
| RT | THH Au NRs | 石墨棒 | -0.2 | 0.1mol/L KOH | 2.7×10-11 | 3.9 | [ |
| AT | pAu/NF | Ag/AgCl(饱和KCl溶液) | -0.2 | 0.1mol/L Na2SO4 | 1.54×10-10 | 13.36 | [ |
| AT | TA-Au NW | — | -0.3 | 0.1mol/L Na2SO4 | 4.36×10-9g·s-1· | 14.83 | [ |
| AT | NCM-Au NPs | Pt | -0.3 | 0.1mol/L HCl | 5.88×10-10 | 22 | [ |
| AT | Rh NNs | 碳棒 | -0.2 | 0.1mol/L KOH | 6.63×10-9g·s-1· | 0.7 | [ |
| AT | Pd/C | Pt | 0.1 | 0.05mol/L H2SO4 | 1.25×10-9g·s-1· | 8.2 | [ |
| 过渡金属(铁基) | |||||||
| 65 | γ-Fe2O3/CP | 石墨棒 | -0.4 | 0.1mol/L KOH | 1.64×10-11 | 1.96 | [ |
| AT | 磁性Fe3O4 | 石墨片 | -0.15 | 0.1mol/L Na2SO4 | 3.36×10-9g·s-1· | 16.9 | [ |
| 250 | Fe2O3/AC | Ni | 1.55 | 熔融NaOH-KOH | 8.27×10-9 | 13.7 | [ |
| RT | Fe2O3/CNT | Pt | -2.0(vs.Ag/AgCl) | KHCO3 | 3.59×10-12 | 95.1 | [ |
| 25 | o-Fe2O3-CNT/CP | 石墨棒 | -0.9(vs.Ag/AgCl) | 0.1mol/L KOH | 2.37×10-11 | 8.28 | [ |
| 90 | MOF(Fe) | Pt | 1.2 | 2mol/L KOH | 2.12×10-9 | 1.43 | [ |
| AT | α-Fe/Fe3O4 | Pt | 0.15 | [C4mpyr][eFAP] | 2.35×10-11 | 32 | [ |
| AT | Fe-SS | Pt | -0.8(vs.NHE) | [C4mpyr][eFAP] | 2.2×10-11 | 35 | [ |
| AT | Fe-FTO | Pt | -0.8(vs.NHE) | [P6,6,6,14][eFAP] | 6.5×10-12 | 60 | [ |
| 过渡金属(钼基) | |||||||
| RT | Mo-D-R-5h | Pt | -0.49 | 0.01mol/L H2SO4 | 3.09×10-11 | 0.72 | [ |
| RT | MoS2/CC | 石墨棒 | -0.5 | 0.1mol/L HCl | 8.08×10-11 | 1.17 | [ |
| AT | Mo2C/C | Pt | -0.3 | 0.5mol/L Li2SO4 | 3.14×10-9g·s-1· | 7.8 | [ |
| 过渡金属(钒基) | |||||||
| RT | VN/CC | 石墨棒 | -0.3 | 0.1mol/L HCl | 2.48×10-10 | 3.58 | [ |
| 25 | VN/钛网 | 石墨棒 | -0.5 | 0.1mol/L HCl | 1.21×10-8g·s-1· | 2.25 | [ |
| AT | VO2(空心微球) | Ag/AgCl(饱和KCl溶液) | -0.7 | 0.1mol/L Na2SO4 | 4.13×10-9g·s-1· | 3.97 | [ |
| AT | V2CTx MXene | — | -0.7 | 0.1mol/L Na2SO4 | 3.5×10-9g·s-1· | 4 | [ |
| 过渡金属(钴基) | |||||||
| RT | CoP HNC | Pt | -0.4 | 1.0mol/L KOH | 8.8×10-11 | 7.36 | [ |
| AT | CMO-NR | — | -0.1 | 0.1mol/L Na2SO4 或0.1mol/L KOH | 1.31×10-9g·s-1· | 22.76 | [ |
| AT | Co4N/Co2C@rGO | 石墨棒 | -0.1 | 0.1mol/L HCl | 6.7×10-9g·s-1· | 24.97 | [ |
| 90 | MOF(Co) | Pt | 1.2 | 2mol/L KOH | 1.64×10-9 | 1.06 | [ |
| 其他过渡金属 | |||||||
| RT | Sn(Ⅱ)肽菁碳箔电极 | Pt | -0.3 | 1mol/L KOH | 1.4×10-11 | 2 | [ |
| AT | Nb2O5 | 石墨棒 | -0.55 | 0.1mol/L HCl | 6.8×10-11 | 9.26 | [ |
| AT | Nb2O5 NA/CC | 石墨棒 | -0.6 | 0.1mol/L Na2SO4 | 1.58×10-10 | 2.26 | [ |
| AT | Ni-MOF-74 | Pt | -0.7(vs.Ag/AgCl) | 0.1mol/L Na2SO4 | 6.68×10-11 | 23.69 | [ |
| 90 | MOF(Cu) | Pt | 1.2 | 2mol/L KOH | 1.24×10-9 | 0.96 | [ |
| 非金属 | |||||||
| RT | B4C/CP | 石墨棒 | -0.75 | 0.1mol/L HCl | 4.34×10-11 | 15.95 | [ |
| AT | NPC-750 | Pt | 0.9 | 0.05mol/L H2SO4 | 2.33×10-10 | 1.42 | [ |
| AT | CNS | Pt | -1.19 | 0.25mol/L LiClO4 | (1.59±0.12)×10-9 | 11.56 | [ |
| 复合材料 | |||||||
| 25 | Li+/PEBCD | Pt | -0.6 | 0.5mol/L Li2SO4 | 3.28×10-11 | 0.42 | [ |
| RT | Au/C3N4 | Pt | -0.1 | 0.05mol/L H2SO4 | 3.63×10-7g·s-1· | 11.1 | [ |
| AT | Au/TiO2 | Pt | -0.2 | 0.05mol/L H2SO4 | 3.5×10-10 | 8.11 | [ |
| 60 | Au/TiO2 | Pt | -0.2 | 0.05mol/L H2SO4 | 5×10-10 | 13.5 | [ |
| RT | Ag-Au/ZIF | Pt | -2.9 | THF | 1×10-11 | 18±4 | [ |
| AT | NiS@MoS2 | 碳棒 | -0.1 | 0.1mol/L Na2SO4 | N/A | 14.8 | [ |
| AT | NiS@MoS2 | 碳棒 | -0.3 | 0.1mol/L Na2SO4 | 2.68×10-9g·s-1· | N/A | [ |
| AT | 非晶型Au/CeO x -rGO | Pt | -0.2 | 0.1mol/L HCl | 2.7×10-8 | 10.1 | [ |
| 200~250 | Ru/Cs+/MgO|Pd-Ag | Pt | — | CsH2PO4/SiP2O7 复合材料 | 9×10-9 | 2.6 | [ |
| 温度/℃ | 阴极/催化剂 | 阳极 | 还原电位(vs.RHE)/V | 电解质 |  /mol·s-1·cm-2 /mol·s-1·cm-2 | FE/% | 参考文献 |
|---|---|---|---|---|---|---|---|
| 贵金属 | |||||||
| RT | THH Au NRs | 石墨棒 | -0.2 | 0.1mol/L KOH | 2.7×10-11 | 3.9 | [ |
| AT | pAu/NF | Ag/AgCl(饱和KCl溶液) | -0.2 | 0.1mol/L Na2SO4 | 1.54×10-10 | 13.36 | [ |
| AT | TA-Au NW | — | -0.3 | 0.1mol/L Na2SO4 | 4.36×10-9g·s-1· | 14.83 | [ |
| AT | NCM-Au NPs | Pt | -0.3 | 0.1mol/L HCl | 5.88×10-10 | 22 | [ |
| AT | Rh NNs | 碳棒 | -0.2 | 0.1mol/L KOH | 6.63×10-9g·s-1· | 0.7 | [ |
| AT | Pd/C | Pt | 0.1 | 0.05mol/L H2SO4 | 1.25×10-9g·s-1· | 8.2 | [ |
| 过渡金属(铁基) | |||||||
| 65 | γ-Fe2O3/CP | 石墨棒 | -0.4 | 0.1mol/L KOH | 1.64×10-11 | 1.96 | [ |
| AT | 磁性Fe3O4 | 石墨片 | -0.15 | 0.1mol/L Na2SO4 | 3.36×10-9g·s-1· | 16.9 | [ |
| 250 | Fe2O3/AC | Ni | 1.55 | 熔融NaOH-KOH | 8.27×10-9 | 13.7 | [ |
| RT | Fe2O3/CNT | Pt | -2.0(vs.Ag/AgCl) | KHCO3 | 3.59×10-12 | 95.1 | [ |
| 25 | o-Fe2O3-CNT/CP | 石墨棒 | -0.9(vs.Ag/AgCl) | 0.1mol/L KOH | 2.37×10-11 | 8.28 | [ |
| 90 | MOF(Fe) | Pt | 1.2 | 2mol/L KOH | 2.12×10-9 | 1.43 | [ |
| AT | α-Fe/Fe3O4 | Pt | 0.15 | [C4mpyr][eFAP] | 2.35×10-11 | 32 | [ |
| AT | Fe-SS | Pt | -0.8(vs.NHE) | [C4mpyr][eFAP] | 2.2×10-11 | 35 | [ |
| AT | Fe-FTO | Pt | -0.8(vs.NHE) | [P6,6,6,14][eFAP] | 6.5×10-12 | 60 | [ |
| 过渡金属(钼基) | |||||||
| RT | Mo-D-R-5h | Pt | -0.49 | 0.01mol/L H2SO4 | 3.09×10-11 | 0.72 | [ |
| RT | MoS2/CC | 石墨棒 | -0.5 | 0.1mol/L HCl | 8.08×10-11 | 1.17 | [ |
| AT | Mo2C/C | Pt | -0.3 | 0.5mol/L Li2SO4 | 3.14×10-9g·s-1· | 7.8 | [ |
| 过渡金属(钒基) | |||||||
| RT | VN/CC | 石墨棒 | -0.3 | 0.1mol/L HCl | 2.48×10-10 | 3.58 | [ |
| 25 | VN/钛网 | 石墨棒 | -0.5 | 0.1mol/L HCl | 1.21×10-8g·s-1· | 2.25 | [ |
| AT | VO2(空心微球) | Ag/AgCl(饱和KCl溶液) | -0.7 | 0.1mol/L Na2SO4 | 4.13×10-9g·s-1· | 3.97 | [ |
| AT | V2CTx MXene | — | -0.7 | 0.1mol/L Na2SO4 | 3.5×10-9g·s-1· | 4 | [ |
| 过渡金属(钴基) | |||||||
| RT | CoP HNC | Pt | -0.4 | 1.0mol/L KOH | 8.8×10-11 | 7.36 | [ |
| AT | CMO-NR | — | -0.1 | 0.1mol/L Na2SO4 或0.1mol/L KOH | 1.31×10-9g·s-1· | 22.76 | [ |
| AT | Co4N/Co2C@rGO | 石墨棒 | -0.1 | 0.1mol/L HCl | 6.7×10-9g·s-1· | 24.97 | [ |
| 90 | MOF(Co) | Pt | 1.2 | 2mol/L KOH | 1.64×10-9 | 1.06 | [ |
| 其他过渡金属 | |||||||
| RT | Sn(Ⅱ)肽菁碳箔电极 | Pt | -0.3 | 1mol/L KOH | 1.4×10-11 | 2 | [ |
| AT | Nb2O5 | 石墨棒 | -0.55 | 0.1mol/L HCl | 6.8×10-11 | 9.26 | [ |
| AT | Nb2O5 NA/CC | 石墨棒 | -0.6 | 0.1mol/L Na2SO4 | 1.58×10-10 | 2.26 | [ |
| AT | Ni-MOF-74 | Pt | -0.7(vs.Ag/AgCl) | 0.1mol/L Na2SO4 | 6.68×10-11 | 23.69 | [ |
| 90 | MOF(Cu) | Pt | 1.2 | 2mol/L KOH | 1.24×10-9 | 0.96 | [ |
| 非金属 | |||||||
| RT | B4C/CP | 石墨棒 | -0.75 | 0.1mol/L HCl | 4.34×10-11 | 15.95 | [ |
| AT | NPC-750 | Pt | 0.9 | 0.05mol/L H2SO4 | 2.33×10-10 | 1.42 | [ |
| AT | CNS | Pt | -1.19 | 0.25mol/L LiClO4 | (1.59±0.12)×10-9 | 11.56 | [ |
| 复合材料 | |||||||
| 25 | Li+/PEBCD | Pt | -0.6 | 0.5mol/L Li2SO4 | 3.28×10-11 | 0.42 | [ |
| RT | Au/C3N4 | Pt | -0.1 | 0.05mol/L H2SO4 | 3.63×10-7g·s-1· | 11.1 | [ |
| AT | Au/TiO2 | Pt | -0.2 | 0.05mol/L H2SO4 | 3.5×10-10 | 8.11 | [ |
| 60 | Au/TiO2 | Pt | -0.2 | 0.05mol/L H2SO4 | 5×10-10 | 13.5 | [ |
| RT | Ag-Au/ZIF | Pt | -2.9 | THF | 1×10-11 | 18±4 | [ |
| AT | NiS@MoS2 | 碳棒 | -0.1 | 0.1mol/L Na2SO4 | N/A | 14.8 | [ |
| AT | NiS@MoS2 | 碳棒 | -0.3 | 0.1mol/L Na2SO4 | 2.68×10-9g·s-1· | N/A | [ |
| AT | 非晶型Au/CeO x -rGO | Pt | -0.2 | 0.1mol/L HCl | 2.7×10-8 | 10.1 | [ |
| 200~250 | Ru/Cs+/MgO|Pd-Ag | Pt | — | CsH2PO4/SiP2O7 复合材料 | 9×10-9 | 2.6 | [ |
| 1 | 刘化章. 合成氨工业: 过去、现在和未来——合成氨工业创立100周年回顾、启迪和挑战[J]. 化工进展, 2013, 32(9): 1995-2005. |
| LIU Huazhang. Ammonia synthesis industry: past, present and future—Retrospect, enlightenment and challenge from 100 years of ammonia synthesis industry[J]. Chemical Industry and Engineering Progress, 2013, 32(9): 1995-2005. | |
| 2 | GIDDEY S, BADWAL S P S, KULKARNI A. Review of electrochemical ammonia production technologies and materials[J]. International Journal of Hydrogen Energy, 2013, 38(34): 14576-14594. |
| 3 | Philibert CÉDRIC. Renewable energy for industry: from green energy to green materials and 1078 fuels[R]. IEA Report, 2017. |
| 4 | SURYANTO Bryan H R, DU Hoanglong, WANG Dabin, et al. Challenges and prospects in the catalysis of electroreduction of nitrogen to ammonia[J]. Nature Catalysis, 2019, 2(4): 290-296. |
| 5 | HOWALT J G, BLIGAARD T, ROSSMEISL J, et al. DFT based study of transition metal nano-clusters for electrochemical NH3 production[J]. Physical Chemistry Chemical Physics, 2013, 15(20): 7785-7795. |
| 6 | ABGHOUI Younes, GARDEN Anna Louise, HOWALT Jakob G, et al. Electroreduction of N2 to ammonia at ambient conditions on mononitrides of Zr, Nb, Cr, and V: a DFT guide for experiments[J]. ACS Catalysis, 2016, 6(2): 635-646. |
| 7 | DENG Jiao, IÑIGUEZ Jesus A, LIU Chong. Electrocatalytic nitrogen reduction at low temperature[J]. Joule, 2018, 2(5): 846-856. |
| 8 | Anna MENCIÓ, Josep MAS-PLA, OTERO Neus, et al. Nitrate pollution of groundwater; all right…, but nothing else?[J]. Science of the Total Environment, 2016, 539: 241-251. |
| 9 | Sergi GARCIA-SEGURA, Mariana LANZARINI-LOPES, HRISTOVSKI Kiril, et al. Electrocatalytic reduction of nitrate: fundamentals to full-scale water treatment applications[J]. Applied Catalysis B: Environmental, 2018, 236: 546-568. |
| 10 | STIRLING András, PÁPAI I, János MINK, et al. Density functional study of nitrogen oxides[J]. The Journal of Chemical Physics, 1994, 100(4):2910-2923. |
| 11 | HIRAKAWA Hiroaki, HASHIMOTO Masaki, SHIRAISHI Yasuhiro, et al. Selective nitrate-to-ammonia transformation on surface defects of titanium dioxide photocatalysts[J]. ACS Catalysis, 2017, 7(5): 3713-3720. |
| 12 | REN Haitao, JIA Shaoyi, ZOU Jijun, et al. A facile preparation of Ag2O/P25 photocatalyst for selective reduction of nitrate[J]. Applied Catalysis B: Environmental, 2015,176: 53-61. |
| 13 | NIU Huan, ZHANG Zhaofu, WANG Xiting, et al. Theoretical insights into the mechanism of selective nitrate-to-ammonia electroreduction on single-atom catalysts[J]. Advanced Functional Materials, 2021, 31(11): 2008533. |
| 14 | YU Yu, WANG Changhong, YU Yifu, et al. Promoting selective electroreduction of nitrates to ammonia over electron-deficient Co modulated by rectifying Schottky contacts[J]. Science China-Chemistry, 2020, 63(10): 1469-1476. |
| 15 | WANG Yuting, YU YIfu, JIA Rannran, et al. Electrochemical synthesis of nitric acid from air and ammonia through waste utilization[J]. National Science Review, 2019, 6 (4): 730-738. |
| 16 | JIA Ranran, WANG Yuting, WANG Changhong, et al. Boosting selective nitrate electroreduction to ammonium by constructing oxygen vacancies in TiO2 [J]. ACS Catalysis, 2020, 10(6): 3533-3540. |
| 17 | FU Xianbiao, ZHAO Xingang, HU Xiaobing, et al. Alternative route for electrochemical ammonia synthesis by reduction of nitrate on copper nanosheets[J]. Applied Materials Today, 2020, 19: 100620. |
| 18 | CHEN Gaofeng, YUAN Yifei, JIANG Haifeng, et al. Electrochemical reduction of nitrate to ammonia via direct eight-electron transfer using a copper-molecular solid catalyst[J]. Nature Energy, 2020, 5(8): 605-613. |
| 19 | LI Jie, ZHAN Guangming, YANG Jianhua, et al. Efficient ammonia electrosynthesis from nitrate on strained ruthenium nanoclusters[J]. Journal of the American Chemical Society, 2020, 142(15): 7036-7046. |
| 20 | LONG Jun, CHEN Shiming, ZHANG Yunlong, et al. Direct electrochemical ammonia synthesis from nitric oxide[J]. Angewandte Chemie International Edition, 2020, 59(24): 9711-9718. |
| 21 | ZHANG Longcheng, LIANG Jie, WANG Yuanyuan, et al. High-performance electrochemical NO reduction into NH3 by MoS2 nanosheet[J]. Angewandte Chemie International Edition, 2021, 60(48): 25263-25268. |
| 22 | SHIPMAN M A, SYMES M D. Recent progress towards the electrosynthesis of ammonia from sustainable resources[J]. Catalysis Today, 2017, 286: 57-68. |
| 23 | CHEN Cheng, MA Guilin. Preparation, proton conduction, and application in ammonia synthesis at atmospheric pressure of La0.9Ba0.1Ga1– x Mg x O3– α [J]. Journal of Materials Science, 2008, 43(15): 5109-5114. |
| 24 | GUO Yingxin, LIU Baoxin, YANG Qing, et al. Preparation via microemulsion method and proton conduction at intermediate-temperature of BaCe1– x Y x O3– α [J]. Electrochemistry Communications, 2009, 11(1): 153-156. |
| 25 | KISHIRA Shota, QING Geletu, shuya SUZU, et al. Ammonia synthesis at intermediate temperatures in solid-state electrochemical cells using cesium hydrogen phosphate based electrolytes and noble metal catalysts[J]. International Journal of Hydrogen Energy, 2017, 42(43): 26843-26854. |
| 26 | KOSAKA Fumihiko, NAKAMURA Takehisa, OIKAWA Akio, et al. Electrochemical acceleration of ammonia synthesis on Fe-based alkali-promoted electrocatalyst with proton conducting solid electrolyte[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(11): 10439-10446. |
| 27 | DÍEZ-RAMÍREZ J, KYRIAKOU V, GARAGOUNIS I, et al. Enhancement of ammonia synthesis on a Co3Mo3N-Ag electrocatalyst in a K-β-Al2O3 solid electrolyte cell[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(10): 8844-8851. |
| 28 | MURAKAMI Tsuyoshi, NISHIKIORI Tokujiro, NOHIRA Toshiyuki, et al. Electrolytic synthesis of ammonia in molten salts under atmospheric pressure[J]. Journal of the American Chemical Society, 2003, 125(2): 334-335. |
| 29 | AMAR IBRAHIM A, LAN Rong, PETIT Christophe T G, et al. Electrochemical synthesis of ammonia based on a carbonate-oxide composite electrolyte[J]. Solid State Ionics, 2011, 182(1): 133-138. |
| 30 | BICER Yusuf, DINCER Ibrahim. Electrochemical synthesis of ammonia in molten salt electrolyte using hydrogen and nitrogen at ambient pressure[J]. Journal of the Electrochemical Society, 2017, 164(8): H5036-H5042. |
| 31 | HATTORI Masashi, IIJIMA Shinya, NAKAO Takuya, et al. Solid solution for catalytic ammonia synthesis from nitrogen and hydrogen gases at 50℃[J]. Nature Communications, 2020, 11(1): 2001. |
| 32 | BAO Di, ZHANG Qi, MENG Fanlu, et al. Electrochemical reduction of N2 under ambient conditions for artificial N2 fixation and renewable energy storage using N2/NH3 cycle[J]. Advanced Materials, 2017, 29(3). |
| 33 | WANG Hongjing, YU Hongjie, WANG Ziqiang, et al. Electrochemical fabrication of porous Au film on Ni foam for nitrogen reduction to ammonia[J]. Small, 2019, 15(6): 1804769. |
| 34 | LIU Songliang, YIN Shuli, JIAO Shiqian, et al. Au nanowire modified with tannic acid for enhanced electrochemical synthesis of ammonia[J]. Materials Today Energy, 2021, 21: 100828. |
| 35 | WANG Hong, WANG Lu, WANG Qiang, et al. Ambient electrosynthesis of ammonia: electrode porosity and composition engineering[J]. Angewandte Chemie International Edition, 2018, 57(38): 12360-12364. |
| 36 | LIU Huimin, HAN Shuhe, ZHAO Yue, et al. Surfactant-free atomically ultrathin rhodium nanosheet nanoassemblies for efficient nitrogen electroreduction[J]. Journal of Materials Chemistry A, 2018, 6(7): 3211-3217. |
| 37 | WANG Jun, YU Liang, HU Lin, et al. Ambient ammonia synthesis via palladium-catalyzed electrohydrogenation of dinitrogen at low overpotential[J]. Nature Communications, 2018, 9: 1795. |
| 38 | KONG Jimin, Ahyoun LIM, YOON Changwon, et al. Electrochemical synthesis of NH3 at low temperature and atmospheric pressure using a γ-Fe2O3 catalyst[J]. Acs Sustainable Chemistry & Engineering, 2017, 5(11): 10986-10995. |
| 39 | HE Xiaojia, GUO Haoran, ZHANG Xinglong, et al. Facile electrochemical fabrication of magnetic Fe3O4 for electrocatalytic synthesis of ammonia used for hydrogen storage application[J]. International Journal of Hydrogen Energy, 2021, 46(47): 24128-24134. |
| 40 | CUI Baochen, ZHANG Jianhua, LIU Shuzhi, et al. Electrochemical synthesis of ammonia directly from N2 and water over iron-based catalysts supported on activated carbon[J]. Green Chemistry, 2017, 19(1): 298-304. |
| 41 | CHEN Shiming, PERATHONER Siglinda, CLAUDIO Ampelli, et al. Electrocatalytic synthesis of ammonia at room temperature and atmospheric pressure from water and nitrogen on a carbon-nanotube-based electrocatalyst[J]. Angewandte Chemie International Edition, 2017, 56(10):2699-2703. |
| 42 | CUI Xiaoyang, TANG Cheng, LIU Xiaomeng, et al. Highly selective electrochemical reduction of dinitrogen to ammonia at ambient temperature and pressure over iron oxide catalysts[J]. Chemistry, 2018, 24(69): 18494-18501. |
| 43 | ZHAO Xinran, YIN Fengxiang, LIU Ning, et al. Highly efficient metal-organic-framework catalysts for electrochemical synthesis of ammonia from N2 (air) and water at low temperature and ambient pressure[J]. Journal of Materials Science, 2017, 52(17): 10175-10185. |
| 44 | SURYANTO Bryan H R, KANG Colin S M, WANG Dabin, et al. Rational electrode-electrolyte design for efficient ammonia electrosynthesis under ambient conditions[J]. ACS Energy Letters, 2018, 3(6): 1219-1224. |
| 45 | ZHOU Fengling, AZOFRA L M, Muataz ALI, et al. Electro-synthesis of ammonia from nitrogen at ambient temperature and pressure in ionic liquid[J]. Energy & Environmental Science. 2017, 10(12): 2516-2520. |
| 46 | YANG Dashuai, CHEN Ting, WANG Zhijiang. Electrochemical reduction of aqueous nitrogen (N2) at a low overpotential on (110)-oriented Mo nanofilm[J]. Journal of Materials Chemistry A, 2017, 5(36): 18967-18971. |
| 47 | ZHANG Ling, JI Xuqiang, REN Xiang, et al. Electrochemical ammonia synthesis via nitrogen reduction reaction on a MoS2 catalyst: theoretical and experimental studies[J]. Advanced Materials, 2018, 30(28): e1800191. |
| 48 | CHENG Hui, DING Liangxin, CHEN Gaofeng, et al. Molybdenum carbide nanodots enable efficient electrocatalytic nitrogen fixation under ambient conditions[J]. Advanced Materials, 2018, 30(46): e1803694. |
| 49 | ZHANG Xiaoping, KONG Rongmei, DU Huitong, et al. Highly efficient electrochemical ammonia synthesis via nitrogen reduction reactions on a VN nanowires array under ambient conditions[J]. Chemical Communications, 2018, 54(42): 5323-5325. |
| 50 | ZHANG Rong, ZHANG Ya, REN Xiang, et al. High-efficiency electrosynthesis of ammonia with high selectivity under ambient conditions enabled by VN nanosheet array[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(8): 9545-9549. |
| 51 | ZHANG Rong, GUO Haoran, YANG Li, et al. Electrocatalytic N2 fixation over hollow VO2 microspheres at ambient conditions[J]. ChemElectroChem, 2019, 6(4): 1014-1018. |
| 52 | XIA Jiaojiao, GUO Haoran, YU Guangsen, et al. 2D vanadium carbide (MXene) for electrochemical synthesis of ammonia under ambient conditions[J]. Catalysis Letters, 2021, 151(12): 3516-3522. |
| 53 | GUO Wenhan, LIANG Zibin, ZHAO Junliang, et al.Hierarchical cobalt phosphide hollow nanocages toward electrocatalytic ammonia synthesis under ambient pressure and room temperature[J]. Small Methods, 2018, 2(12): 1800204. |
| 54 | ZHANG Yizhen, HU Jue, ZHANG Chengxu, et al. Electrochemical synthesis of ammonia from nitrogen catalyzed by CoMoO4 nanorods under ambient conditions[J]. Journal of Materials Chemistry A, 2021, 9(8): 5060-5066. |
| 55 | QIAO Huici, YU Jie, LU Jinwei, et al. Co4N/Co2C@rGO with abundant Co—C and N—C bonds as highly efficient electrocatalyst for N2 reduction[J]. ACS Sustainable Chemistry & Engineering, 2021, 9(3): 1373-1382. |
| 56 | SHIPMAN M A, SYMES M D. A re-evaluation of Sn (Ⅱ) phthalocyanine as a catalyst for the electrosynthesis of ammonia[J]. Electrochimica Acta, 2017, 258: 618-622. |
| 57 | HAN Jingrui, LIU Zaichun, MA Yongjun, et al. Ambient N2 fixation to NH3 at ambient conditions: using Nb2O5 nanofiber as a high-performance electrocatalyst[J]. Nano Energy, 2018, 52: 264-270. |
| 58 | KONG Wenhan, LIU Zaichun, HAN Jingrui, et al. Ambient electrochemical N2-to-NH3 fixation enabled by Nb2O5 nanowire array[J]. Inorganic Chemistry Frontiers, 2019, 6(2): 423-427. |
| 59 | 杨通, 何小波, 银凤翔. M-MOF-74(M=Ni,Co,Zn)的制备及其电化学催化合成氨性能[J]. 化工学报, 2020, 71(6): 2857-2870. |
| YANG Tong, HE Xiaobo, YIN Fengxiang. Preparation of M-MOF-74 (M = Ni, Co, Zn) and its performance in electrocatalytic synthesis of ammonia[J]. CIESC Journal, 2020, 71(6): 2857-2870. | |
| 60 | QIU Weibin, XIE Xiaoying, QIU Jianding, et al. High-performance artificial nitrogen fixation at ambient conditions using a metal-free electrocatalyst[J]. Nature Communications, 2018, 9: 3485. |
| 61 | LIU Yanming, SU Yan, QUAN Xie, et al. Facile ammonia synthesis from electrocatalytic N2 reduction under ambient conditions on N-doped porous carbon[J]. ACS Catalysis, 2018, 8(2): 1186-1191. |
| 62 | SONG Yang, JOHNSON Daniel, PENG Rui, et al. A physical catalyst for the electrolysis of nitrogen to ammonia[J]. Science Advances, 2018, 4(4): e1700336. |
| 63 | CHEN Gaofeng, CAO Xinrui, WU Shunqing, et al. Ammonia electrosynthesis with high selectivity under ambient conditions via a Li+ incorporation strategy[J]. Journal of the American Chemical Society, 2017, 139(29): 9771-9774. |
| 64 | WANG Xiaoqian, WANG Wenyu, QIAO Man, et al. Atomically dispersed Au1 catalyst towards efficient electrochemical synthesis of ammonia[J]. Science Bulletin, 2018, 63(19): 1246-1253. |
| 65 | SHI Miaomiao, BAO Di, WULAN Bari, et al. Au sub-nanoclusters on TiO2 toward highly efficient and selective electrocatalyst for N2 conversion to NH3 at ambient conditions[J]. Advanced Materials, 2017, 29(17): e1606550. |
| 66 | LEE Hiang Kwee, Charlynn Sher Lin KOH, LEE Yinhong, et al. Favoring the unfavored: selective electrochemical nitrogen fixation using a reticular chemistry approach[J]. Science Advances, 2018, 4(3): eaar3208. |
| 67 | HUANG Shoushuang, GAO Chunyan, XIN Peijun, et al. An advanced electrocatalyst for efficient synthesis of ammonia based on chemically coupled NiS@MoS2 heterostructured nanospheres[J]. Sustainable Energy & Fuels, 2021, 5(10): 2640-2648. |
| 68 | LI Sijia, BAO Di, SHI Miaomiao, et al. Amorphizing of Au nanoparticles by CeO x -RGO hybrid support towards highly efficient electrocatalyst for N2 reduction under ambient conditions[J]. Advanced Materials, 2017, 29(33): 1700001. |
| 69 | IMAMURA Kanako, KUBOTA Jun. Electrochemical membrane cell for NH3 synthesis from N2 and H2O by electrolysis at 200 to 250℃ using a Ru catalyst, hydrogen-permeable Pd membrane and phosphate-based electrolyte[J]. Sustainable Energy & Fuels, 2018, 2(6): 1278-1286. |
| 70 | LIU Ruiquan, XIE Yahong, WANG Jide, et al. Synthesis of ammonia at atmospheric pressure with Ce0.8M0.2O2- δ (M=La, Y, Gd, Sm) and their proton conduction at intermediate temperature[J]. Solid State Ionics, 2006, 177(1/2): 73-76. |
| 71 | MURAKAMI Tsuyoshi, NOHIRA Toshiyuki, OGATA Yukio H, et al. Electrolytic ammonia synthesis in molten salts under atmospheric pressure using methane as a hydrogen source[J]. Electrochemical and Solid-State Letters, 2005, 8(4). |
| 72 | MURAKAMI Tsuyoshi, NOHIRA Toshiyuki, OGATA Yukio H, et al. Electrochemical synthesis of ammonia and coproduction of metal sulfides from hydrogen sulfide and nitrogen under atmospheric pressure[J]. Journal of the Electrochemical Society, 2005, 152(6): D109. |
| 73 | KIM Kwiyong, LEE Nara, YOO Chung-Yul, et al. Communication-electrochemical reduction of nitrogen to ammonia in 2-propanol under ambient temperature and pressure[J]. Journal of the Electrochemical Society, 2016, 163(7): F610-F612. |
| 74 | TSUNETO Akira, KUDO Akihiko, SAKATA Tadayoshi. Lithium-mediated electrochemical reduction of high pressure N2 to NH3 [J]. Journal of Electroanalytical Chemistry, 1994, 367(1/2): 183-188. |
| 75 | PAPPENFUS TED M, LARAMY Janice K, THOMA Laura M, et al. Wind to ammonia: electrochemical processes in room temperature ionic liquids[J]. ECS Transactions, 2009, 16(49): 89-93. |
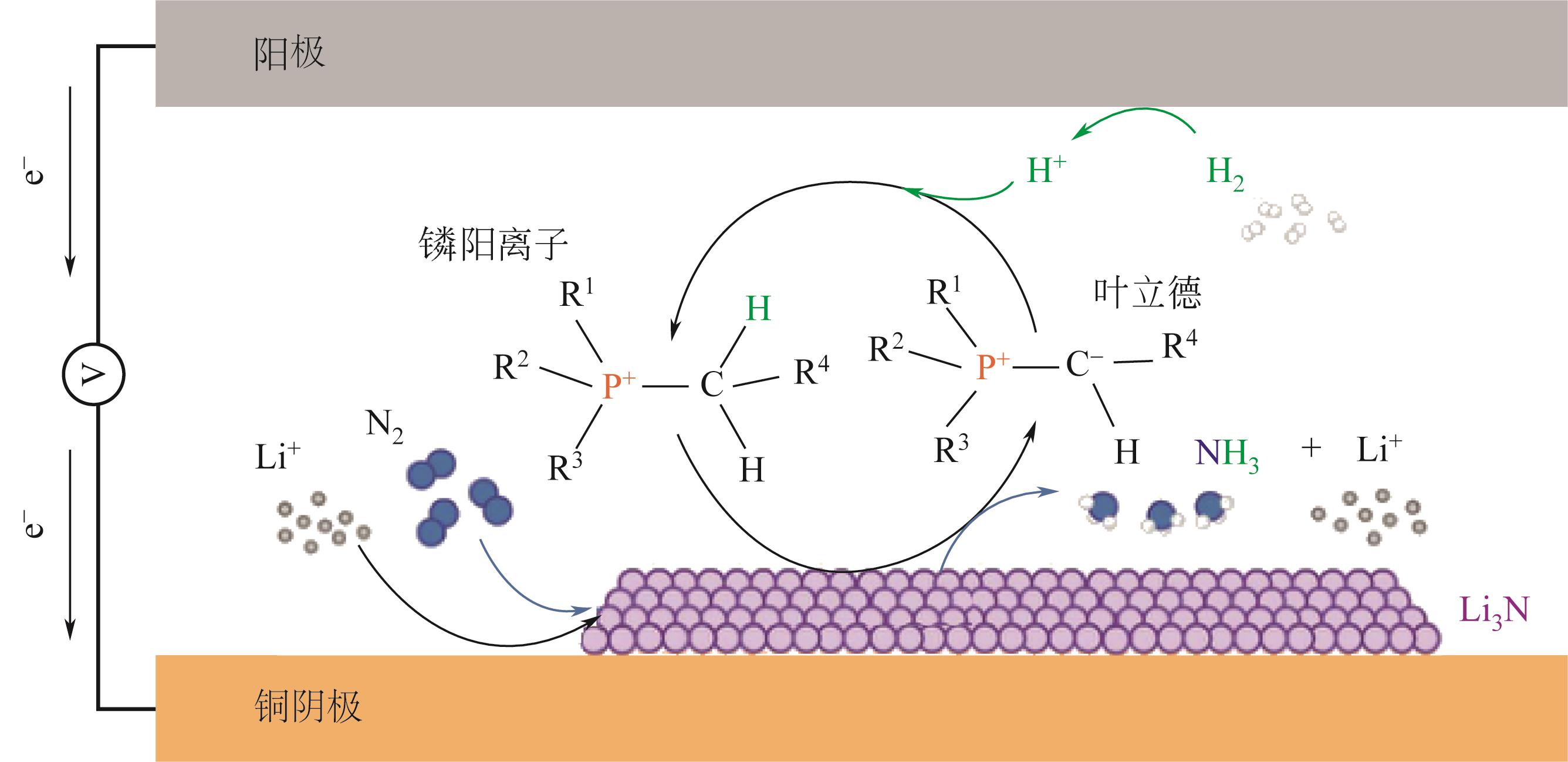
| 76 | SURYANTO B H R, MATUSZEK K, CHOI J, et al. Nitrogen reduction to ammonia at high efficiency and rates based on a phosphonium proton shuttle[J]. Science, 2021, 372(6547): 1187-1191. |
| [1] | ZHANG Mingyan, LIU Yan, ZHANG Xueting, LIU Yake, LI Congju, ZHANG Xiuling. Research progress of non-noble metal bifunctional catalysts in zinc-air batteries [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 276-286. |
| [2] | SHI Yongxing, LIN Gang, SUN Xiaohang, JIANG Weigeng, QIAO Dawei, YAN Binhang. Research progress on active sites in Cu-based catalysts for CO2 hydrogenation to methanol [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 287-298. |
| [3] | XIE Luyao, CHEN Songzhe, WANG Laijun, ZHANG Ping. Platinum-based catalysts for SO2 depolarized electrolysis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 299-309. |
| [4] | YANG Xiazhen, PENG Yifan, LIU Huazhang, HUO Chao. Regulation of active phase of fused iron catalyst and its catalytic performance of Fischer-Tropsch synthesis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 310-318. |
| [5] | HU Xi, WANG Mingshan, LI Enzhi, HUANG Siming, CHEN Junchen, GUO Bingshu, YU Bo, MA Zhiyuan, LI Xing. Research progress on preparation and sodium storage properties of tungsten disulfide composites [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 344-355. |
| [6] | ZHANG Jie, BAI Zhongbo, FENG Baoxin, PENG Xiaolin, REN Weiwei, ZHANG Jingli, LIU Eryong. Effect of PEG and its compound additives on post-treatment of electrolytic copper foils [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 374-381. |
| [7] | ZHAO Wei, ZHAO Deyin, LI Shihan, LIU Hongda, SUN Jin, GUO Yanqiu. Synthesis and application of triazine drag reducing agent for nature gas pipeline [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 391-399. |
| [8] | WANG Zhengkun, LI Sifang. Green synthesis of gemini surfactant decyne diol [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 400-410. |
| [9] | ZHANG Jie, WANG Fangfang, XIA Zhonglin, ZHAO Guangjin, MA Shuangchen. Current SF6 emission, emission reduction and future prospects under “carbon peaking and carbon neutrality” [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 447-460. |
| [10] | WANG Lele, YANG Wanrong, YAO Yan, LIU Tao, HE Chuan, LIU Xiao, SU Sheng, KONG Fanhai, ZHU Canghai, XIANG Jun. Influence of spent SCR catalyst blending on the characteristics and deNO x performance for new SCR catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 489-497. |
| [11] | ZHAO Jingchao, TAN Ming. Effect of surfactants on the reduction of industrial saline wastewater by electrodialysis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 529-535. |
| [12] | DENG Liping, SHI Haoyu, LIU Xiaolong, CHEN Yaoji, YAN Jingying. Non-noble metal modified vanadium titanium-based catalyst for NH3-SCR denitrification simultaneous control VOCs [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 542-548. |
| [13] | SHU Bin, CHEN Jianhong, XIONG Jian, WU Qirong, YU Jiangtao, YANG Ping. Necessity analysis of promoting the development of green methanol under the goal of carbon neutrality [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4471-4478. |
| [14] | LAI Shini, JIANG Lixia, LI Jun, HUANG Hongyu, KOBAYASHI Noriyuki. Research progress of ammonia blended fossil fuel [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4603-4615. |
| [15] | CHENG Tao, CUI Ruili, SONG Junnan, ZHANG Tianqi, ZHANG Yunhe, LIANG Shijie, PU Shi. Analysis of impurity deposition and pressure drop increase mechanisms in residue hydrotreating unit [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4616-4627. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||