Chemical Industry and Engineering Progress ›› 2022, Vol. 41 ›› Issue (1): 365-372.DOI: 10.16085/j.issn.1000-6613.2021-0136
• Biochemical and pharmaceutical engineering • Previous Articles Next Articles
Adsorption and controlled release behavior for thermosensitive imprinted silica microspheres toward diosgenin
MAO Lin( ), XU Guoqiang, LUO Xiaoyue, TIAN Haixi, LI Hui(
), XU Guoqiang, LUO Xiaoyue, TIAN Haixi, LI Hui( )
)
- College of Chemistry and Chemical Engineeering, Jishou University, Jishou 416000, Hunan, China
-
Received:2021-01-20Revised:2021-02-07Online:2022-01-24Published:2022-01-05 -
Contact:LI Hui
温敏印迹硅胶微球对薯蓣皂素的吸附及控制释放
- 吉首大学化学化工学院,湖南 吉首 416000
-
通讯作者:李辉 -
作者简介:毛琳(1999—),女,硕士研究生,研究方向为分析化学。E-mail:745018407@qq.com 。 -
基金资助:国家自然科学基金(21865011);湖南省湘西自治州科技创新重点研发计划(2019SF2003);吉首大学研究生校级科研项目(Jdy20005)
CLC Number:
Cite this article
MAO Lin, XU Guoqiang, LUO Xiaoyue, TIAN Haixi, LI Hui. Adsorption and controlled release behavior for thermosensitive imprinted silica microspheres toward diosgenin[J]. Chemical Industry and Engineering Progress, 2022, 41(1): 365-372.
毛琳, 许国强, 罗晓玥, 田海希, 李辉. 温敏印迹硅胶微球对薯蓣皂素的吸附及控制释放[J]. 化工进展, 2022, 41(1): 365-372.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2021-0136
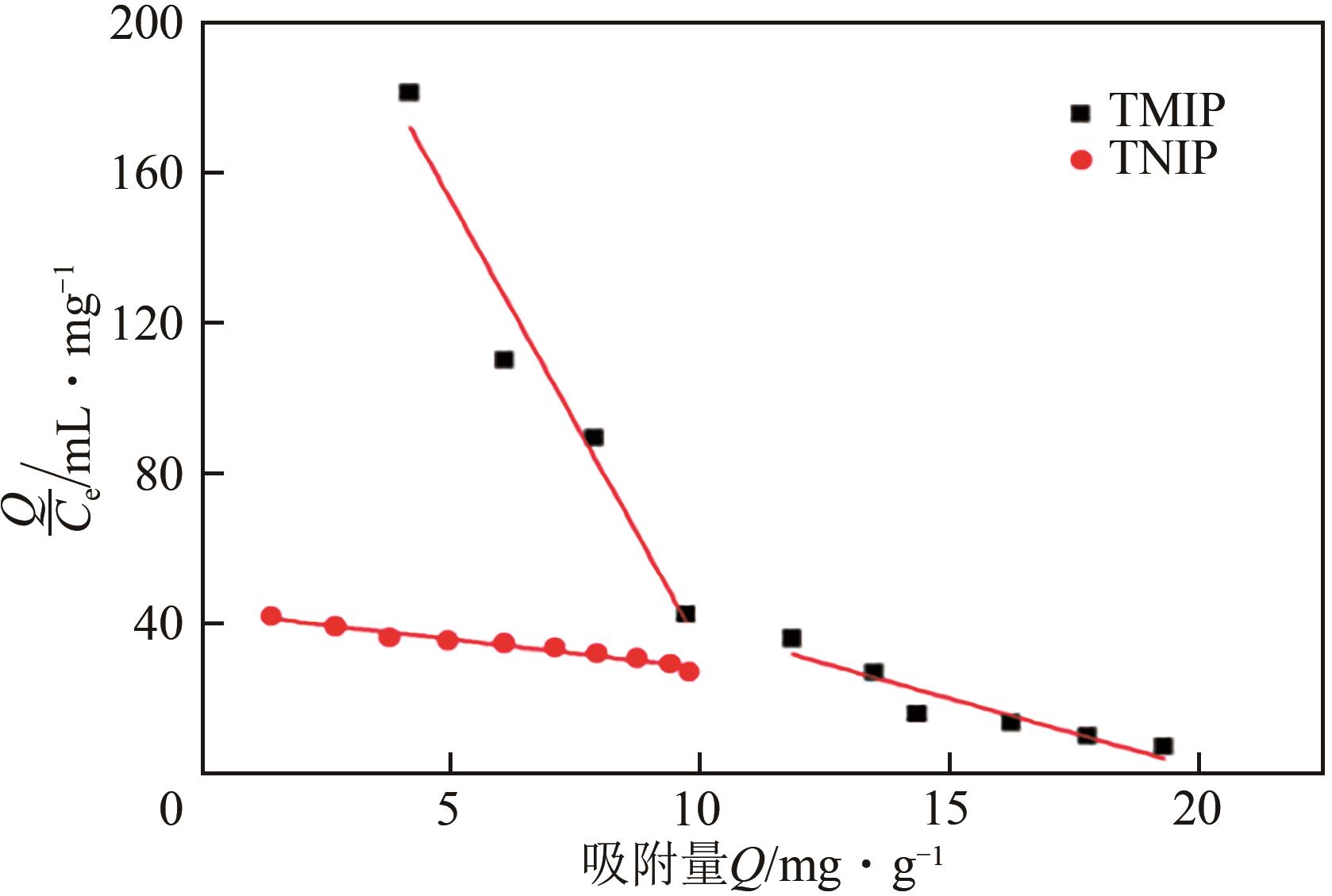
| 聚合物 | 位点类型 | 拟合直线 | Kd | Qmax |
|---|---|---|---|---|
| TMIP | 高亲和位点 | Y=-23.8167x+272.1389,R2=0.9398 | 0.04199 | 11.4271 |
| 低亲和位点 | Y=-3.7349x+145.0156,R2=0.9075 | 0.2677 | 38.8208 | |
| TNIP | Y=-1.6078x+43.7897,R2=0.9613 | 0.6220 | 27.2372 |
| 聚合物 | 位点类型 | 拟合直线 | Kd | Qmax |
|---|---|---|---|---|
| TMIP | 高亲和位点 | Y=-23.8167x+272.1389,R2=0.9398 | 0.04199 | 11.4271 |
| 低亲和位点 | Y=-3.7349x+145.0156,R2=0.9075 | 0.2677 | 38.8208 | |
| TNIP | Y=-1.6078x+43.7897,R2=0.9613 | 0.6220 | 27.2372 |
| 1 | SETHI G, SHANMUGAM M K, WARRIER S, et al. Pro-apoptotic and anti-cancer properties of diosgenin: a comprehensive and critical review[J]. Nutrients, 2018, 10(5): E645-E658. |
| 2 | FULLER S, STEPHENS J M. Diosgenin, 4-hydroxyisoleucine, and fiber from fenugreek: mechanisms of actions and potential effects on metabolic syndrome[J]. Advances in Nutrition, 2015, 6(2): 189-197. |
| 3 | GONG G H, QIN Y, HUANG W. Anti-thrombosis effect of diosgenin extract from Dioscorea zingiberensis C.H. Wright in vitro and in vivo[J]. Phytomedicine, 2011, 18(6): 458-463. |
| 4 | LI C, LU Y, DU S, et al. Dioscin exerts protective effects against crystalline silica-induced pulmonary fibrosis in mice[J]. Theranostics, 2017, 7(17): 4255-4275. |
| 5 | YAO H, TAO X F, XU L N, et al. Dioscin alleviates non-alcoholic fatty liver disease through adjusting lipid metabolism via SIRT1/AMPK signaling pathway[J]. Pharmacological Research, 2018, 131: 51-60. |
| 6 | CHEN L, LI Q N, LEI L, et al. Dioscin ameliorates cardiac hypertrophy through inhibition of the MAPK and Akt/GSK3β/mTOR pathways[J]. Life Sciences, 2018, 209: 420-429. |
| 7 | YANG B, XU B, ZHAO H, et al. Dioscin protects against coronary heart disease by reducing oxidative stress and inflammation via Sirt1/Nrf2 and p38 MAPK pathways[J]. Molecular Medicine Reports, 2018, 18(1): 973-980. |
| 8 | PARAMA D, BORUAH M, YACHNA K, et al. Diosgenin, a steroidal saponin, and its analogs: effective therapies against different chronic diseases[J]. Life Sciences, 2020, 260: 118182. |
| 9 | SHI J, KANTOFF P W, WOOSTER R, et al. Cancer nanomedicine: progress, challenges and opportunities[J]. Nature Reviews Cancer, 2017, 17(1): 20-37. |
| 10 | CUNLIFFE D, KIRBY A, ALEXANDER C. Molecularly imprinted drug delivery systems[J]. Advanced Drug Delivery Reviews, 2005, 57(12): 1836-1853. |
| 11 | SHI Y, MA C B, PENG L L, et al. Conductive “smart” hybrid hydrogels with PNIPAM and nanostructured conductive polymers[J]. Advanced Functional Materials, 2015, 25(8): 1219-1225. |
| 12 | SAKAGUCHI N, KOJIMA C, HARADA A, et al. The correlation between fusion capability and transfection activity in hybrid complexes of lipoplexes and pH-sensitive liposomes[J]. Biomaterials, 2008, 29(29): 4029-4036. |
| 13 | HUA Z, CHEN Z, LI Y, et al. Thermosensitive and salt-sensitive molecularly imprinted hydrogel for bovine serum albumin[J]. Langmuir, 2008, 24(11): 5773-5780. |
| 14 | ZHAO X Y, CHEN L G, LI B. Magnetic molecular imprinting polymers based on three-dimensional (3D) graphene-carbon nanotube hybrid composites for analysis of melamine in milk powder[J]. Food Chemistry, 2018, 255: 226-234. |
| 15 | ZIMMERMAN S C, LEMCOFF N G. Synthetic hosts via molecular imprinting—Are universal synthetic antibodies realistically possible?[J]. Chemical Communications, 2004(1): 5-14. |
| 16 | DMITRIENKO S G, IRKHA V V, KUZNETSOVA A Y, et al. Use of molecular imprinted polymers for the separation and preconcentration of organic compounds[J]. Journal of Analytical Chemistry, 2004, 59(9): 808-817. |
| 17 | FEIL H, BAE Y H, FEIJEN J, et al. Effect of comonomer hydrophilicity and ionization on the lower critical solution temperature of N-isopropylacrylamide copolymers[J]. Macromolecules, 1993, 26(10): 2496-2500. |
| 18 | MAKINO K, HIYOSHI J, OHSHIMA H. Kinetics of swelling and shrinking of poly(N-isopropylacrylamide) hydrogels at different temperatures[J]. Colloids and Surfaces B: Biointerfaces, 2000, 19(2): 197-204. |
| 19 | LOWE T L, VIRTANEN J, TENHU H. Interactions of drugs and spin probes with hydrophobically modified polyelectrolyte hydrogels based on N-isopropylacrylamide[J]. Polymer, 1999, 40(10): 2595-2603. |
| 20 | WULFF G. Enzyme-like catalysis by molecularly imprinted polymers[J]. Chemical Reviews, 2002, 102(1): 1-27. |
| 21 | CHEN T, SHAO M W, XU H Y, et al. Molecularly imprinted polymer-coated silicon nanowires for protein specific recognition and fast separation[J]. Journal of Materials Chemistry, 2012, 22(9): 3990-3996. |
| 22 | 李龙飞. 温敏型磁性表面分子印迹聚合物识别5-氟尿嘧啶的机理及其缓释行为[D]. 太原: 太原理工大学, 2016. |
| LI Longfei. Temperature and magnetic dual responsive surface molecularly imprinted polymers: the recognition mechanism and sustainedly release towards 5-fluorouracil[D]. Taiyuan: Taiyuan University of Technology, 2016. | |
| 23 | 何典雄. 基于pH/温度双响应的生物相容性水飞蓟宾印迹材料研究[D]. 衡阳: 南华大学, 2019. |
| HE Dianxiong. Research of biocmpatible silybin impeinted materials based on dual pH/temperature response[D]. Hengyang: University of South China, 2019. | |
| 24 | KOBAYASHI Y, NAKAMITSU Y, ZHENG Y T, et al. Preparation of cyclodextrin-based porous polymeric membrane by bulk polymerization of ethyl acrylate in the presence of cyclodextrin[J]. Polymer, 2019, 177: 208-213. |
| 25 | KAN X W, ZHAO Q, ZHANG Z, et al. Molecularly imprinted polymers microsphere prepared by precipitation polymerization for hydroquinone recognition[J]. Talanta, 2008, 75(1): 22-26. |
| 26 | LIN Z Z, ZHANG H Y, LI L, et al. Application of magnetic molecularly imprinted polymers in the detection of malachite green in fish samples[J]. Reactive and Functional Polymers, 2016, 98: 24-30. |
| 27 | GUO H Q, LIU Y, MA W T, et al. Surface molecular imprinting on carbon microspheres for fast and selective adsorption of perfluorooctane sulfonate[J]. Journal of Hazardous Materials, 2018, 348: 29-38. |
| [1] | CUI Shoucheng, XU Hongbo, PENG Nan. Simulation analysis of two MOFs materials for O2/He adsorption separation [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 382-390. |
| [2] | CHEN Chongming, CHEN Qiu, GONG Yunqian, CHE Kai, YU Jinxing, SUN Nannan. Research progresses on zeolite-based CO2 adsorbents [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 411-419. |
| [3] | XU Chunshu, YAO Qingda, LIANG Yongxian, ZHOU Hualong. Research progress on functionalization strategies of covalent organic frame materials and its adsorption properties for Hg(Ⅱ) and Cr(Ⅵ) [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 461-478. |
| [4] | GU Yongzheng, ZHANG Yongsheng. Dynamic behavior and kinetic model of Hg0 adsorption by HBr-modified fly ash [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 498-509. |
| [5] | GUO Qiang, ZHAO Wenkai, XIAO Yonghou. Numerical simulation of enhancing fluid perturbation to improve separation of dimethyl sulfide/nitrogen via pressure swing adsorption [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 64-72. |
| [6] | WANG Shengyan, DENG Shuai, ZHAO Ruikai. Research progress on carbon dioxide capture technology based on electric swing adsorption [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 233-245. |
| [7] | GE Yafen, SUN Yu, XIAO Peng, LIU Qi, LIU Bo, SUN Chengying, GONG Yanjun. Research progress of zeolite for VOCs removal [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4716-4730. |
| [8] | YANG Ying, HOU Haojie, HUANG Rui, CUI Yu, WANG Bing, LIU Jian, BAO Weiren, CHANG Liping, WANG Jiancheng, HAN Lina. Coal tar phenol-based carbon nanosphere prepared by Stöber method for adsorption of CO2 [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 5011-5018. |
| [9] | JIANG Jing, CHEN Xiaoyu, ZHANG Ruiyan, SHENG Guangyao. Research progress of manganese-loaded biochar preparation and its application in environmental remediation [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4385-4397. |
| [10] | ZHANG Zhen, LI Dan, CHEN Chen, WU Jinglan, YING Hanjie, QIAO Hao. Separation and purification of salivary acids with adsorption resin [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4153-4158. |
| [11] | WANG Zhicai, LIU Weiwei, ZHOU Cong, PAN Chunxiu, YAN Honglei, LI Zhanku, YAN Jingchong, REN Shibiao, LEI Zhiping, SHUI Hengfu. Synthesis and performance of a superplasticizer based on coal-based humic acid [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3634-3642. |
| [12] | YU Jingwen, SONG Luna, LIU Yanchao, LYU Ruidong, WU Mengmeng, FENG Yu, LI Zhong, MI Jie. An indole-bearing hypercrosslinked polymer In-HCP for iodine adsorption from water [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3674-3683. |
| [13] | GUAN Hongling, YANG Hui, JING Hongquan, LIU Yuqiong, GU Shouyu, WANG Haobin, HOU Cuihong. Lignin-based controlled release materials and application in drug delivery and fertilizer controlled-release [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3695-3707. |
| [14] | LI Yanling, ZHUO Zhen, CHI Liang, CHEN Xi, SUN Tanglei, LIU Peng, LEI Tingzhou. Research progress on preparation and application of nitrogen-doped biochar [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3720-3735. |
| [15] | BAI Yadi, DENG Shuai, ZHAO Ruikai, ZHAO Li, YANG Yingxia. Exploration on standardized test scheme and experimental performance of temperature swing adsorption carbon capture unit [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3834-3846. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||