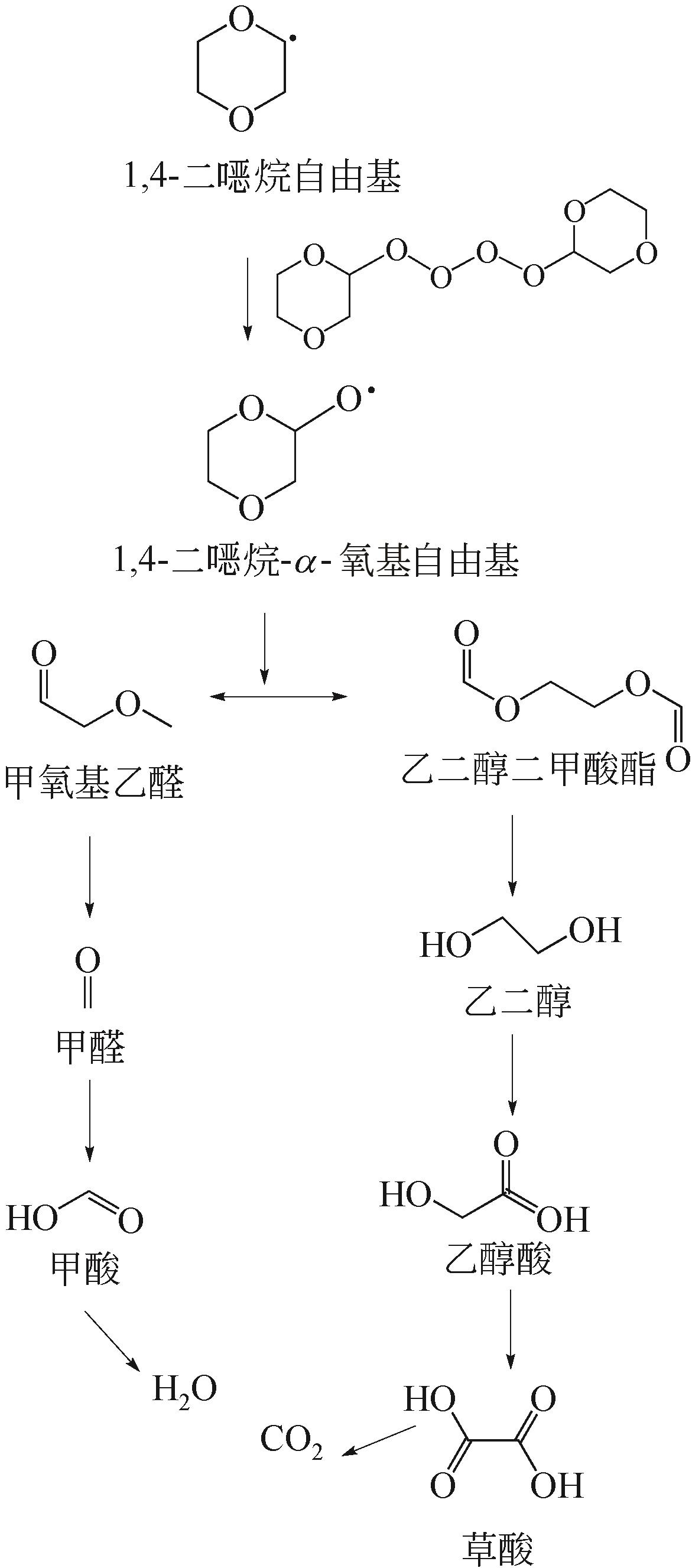
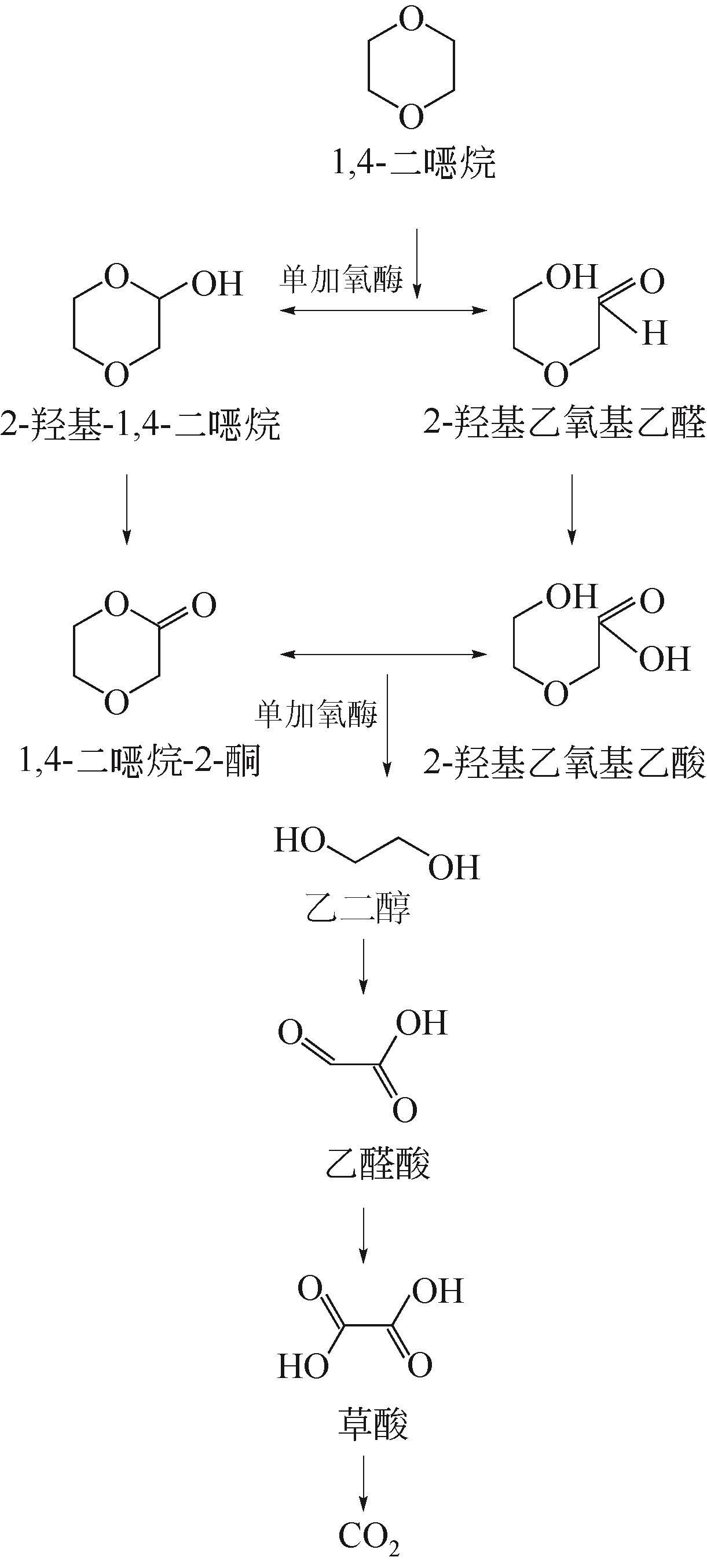
| 1 |
ANDERSON R H, ANDERSON J K, BOWER P A. Co-occurrence of 1,4-dioxane with trichloroethylene in chlorinated solvent groundwater plumes at US air force installations: fact or fiction[J]. Integr. Environ. Assess. Manag., 2012, 8(4): 731-737.
|
| 2 |
MOHR T K, STICKNEY J A, DIGUISEPPI W H. Environmental investigation and remediation: 1,4-dioxane and other solvent stabilizers[M]. Los Angeles: CRC Press, 2010.
|
| 3 |
USEPA. Integrated risk information system (IRIS) on 1,4-dioxane[R]. National Center for Environmental Assessment, Office of Research and Development, Washington D C, 2013.
|
| 4 |
ZENKER M J, BORDEN R C, BARLAZ M A. Occurrence and treatment of 1,4-dioxane in aqueous environments[J]. Environmental Engineering Science, 2003, 20(5): 423-432.
|
| 5 |
International Agency for Research on Cancer. Re-evaluation of some organic chemicals, hydrazine and hydrogen peroxide[C]//IARC. Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Humans. Lyon, 1999: 247.
|
| 6 |
STEPIEN D K, DIEHL P, HELM J, et al. Fate of 1,4-dioxane in the aquatic environment: from sewage to drinking water[J]. Water Research, 2014, 48: 406-419.
|
| 7 |
HOWARD P. Handbook of environmental fate and exposure data: for organic chemicals, volume Ⅲ pesticides[M]. Florida: Routledge & CRC Press, 1991: 216-221.
|
| 8 |
ADAMSON D T, PIÑA E A, CARTWRIGHT A E, et al. 1,4-Dioxane drinking water occurrence data from the third unregulated contaminant monitoring rule[J]. Science of the Total Environment, 2017, 596/597: 236-245.
|
| 9 |
ADAMSON D T, MAHENDRA S, WALKER K L, et al. A multisite survey to identify the scale of the 1,4-dioxane problem at contaminated groundwater sites[J]. Environmental Science & Technology Letters, 2014, 1(5): 254-258.
|
| 10 |
KARGES U, BECKER J, PÜTTMANN W. 1,4-Dioxane pollution at contaminated groundwater sites in western Germany and its distribution within a TCE plume[J]. Science of the Total Environment, 2018, 619/620: 712-720.
|
| 11 |
KARGES U, OTT D, DE BOER S, et al. 1,4-Dioxane contamination of German drinking water obtained by managed aquifer recharge systems: distribution and main influencing factors[J]. The Science of the Total Environment, 2020, 711: 134783.
|
| 12 |
KWAK Jin Il, Sun-Hwa NAM, AN Youn-Joo. Water quality standards for the protection of human health and aquatic ecosystems in Korea: current state and future perspective[J]. Environmental Science and Pollution Research, 2018, 25(4): 3108-3119.
|
| 13 |
AN Y-J, KWAK J, S-H NAM, et al. Development and implementation of surface water quality standards for protection of human health in Korea[J]. Environmental Science and Pollution Research, 2014, 21(1): 77-85.
|
| 14 |
TANABE A, TSUCHIDA Y, IBARAKI T, et al. Impact of 1,4-dioxane from domestic effluent on the Agano and Shinano Rivers, Japan[J]. Bulletin of Environmental Contamination and Toxicology, 2006, 76(1): 44-51.
|
| 15 |
POLLITT K J G, KIM Jae-Hong, PECCIA J, et al. 1,4-Dioxane as an emerging water contaminant: state of the science and evaluation of research needs[J]. Science of the Total Environment, 2019, 690: 853-866.
|
| 16 |
LIN Wan-Ting, CHEN Wun-Ling, CHENG Wei-Chih, et al. Determining the residual characteristics of alkylphenols, arsenic, and lead as well as assessing the exposures of 1,4-dioxane from household food detergents[J]. Journal of AOAC International, 2017, 100(4): 1086-1093.
|
| 17 |
KINGSTON J L T, DAHLEN P R, JOHNSON P C. State-of-the-practice review of in situ thermal technologies[J]. Groundwater Monitoring & Remediation, 2010, 30(4): 64-72.
|
| 18 |
CHEN Ruihuan, LIU Cun, JOHNSON N W, et al. Removal of 1,4-dioxane by titanium silicalite-1: separation mechanisms and bioregeneration of sorption sites[J]. Chemical Engineering Journal, 2019, 371: 193-202.
|
| 19 |
KISHIMOTO N, NAKAGAWA T, ASANO M, et al. Ozonation combined with electrolysis of 1,4-dioxane using a two-compartment electrolytic flow cell with solid electrolyte[J]. Water Research, 2008, 42(1): 379-385.
|
| 20 |
YAMAZAKI S, YAMABE N, NAGANO S, et al. Adsorption and photocatalytic degradation of 1,4-dioxane on TiO2[J]. Journal of Photochemistry and Photobiology A: Chemistry, 2007, 185(2/3): 150-155.
|
| 21 |
EBERLE D, BALL R, BOVING T B. Peroxone activated persulfate treatment of 1,4-dioxane in the presence of chlorinated solvent co-contaminants[J]. Chemosphere, 2016, 144: 728-735.
|
| 22 |
ISACOFF E G, NICKELSEN M G. Removal of 1,4-dioxane from water using carbonaceous adsorbents: US20130220935A1[S]. 2013-08-29.
|
| 23 |
WOODARD S, MOHR T, NICKELSEN M G. Synthetic media: a promising new treatment technology for 1,4‐dioxane[J]. Remediation Journal, 2014, 24(4): 27-40.
|
| 24 |
MYERS M A, JOHNSON N W, MARIN E Z, et al. Abiotic and bioaugmented granular activated carbon for the treatment of 1,4-dioxane-contaminated water[J]. Environmental Pollution, 2018, 240: 916-924.
|
| 25 |
LIU Yun, JOHNSON N W, LIU Cun, et al. Mechanisms of 1,4-dioxane biodegradation and adsorption by bio-zeolite in the presence of chlorinated solvents: experimental and molecular dynamics simulation studies[J]. Environmental Science & Technology, 2019, 53(24): 14538-14547.
|
| 26 |
CORTÉS-ARRIAGADA D. Expanding the environmental applications of metal (Al, Ti, Mn, Fe) doped graphene: adsorption and removal of 1,4-dioxane[J]. Physical Chemistry Chemical Physics, 2016, 18(47): 32281-32292.
|
| 27 |
CORTÉS-ARRIAGADA D, MIRANDA-ROJAS S, ORTEGA D E, et al. Oxidized and Si-doped graphene: emerging adsorbents for removal of dioxane[J]. Physical Chemistry Chemical Physics, 2017, 19(27): 17587-17597.
|
| 28 |
IZAKMEHRI Z, ARDJMAND M, GANJI M D, et al. Removal of dioxane pollutants from water by using Al-doped single walled carbon nanotubes[J]. RSC Advances, 2015, 5(59): 48124-48132.
|
| 29 |
RÖDER A, SKOV A, BOGUSLAVSKIY A E, et al. VUV Excited-state dynamics of cyclic ethers as a function of ring size[J]. Physical Chemistry Chemical Physics, 2020, 22(45): 26241-26254.
|
| 30 |
COLEMAN H M, VIMONSES V, LESLIE G, et al. Degradation of 1,4-dioxane in water using TiO2 based photocatalytic and H2O2/UV processes[J]. Journal of Hazardous Materials, 2007, 146(3): 496-501.
|
| 31 |
MIN Byoung Koun, Jung Eun HEO, YOUN Na Kyoung, et al. Tuning of the photocatalytic 1,4-dioxane degradation with surface plasmon resonance of gold nanoparticles on titania[J]. Catalysis Communications, 2009, 10(5): 712-715.
|
| 32 |
VESCOVI T, COLEMAN H M, AMAL R. The effect of pH on UV-based advanced oxidation technologies – 1,4-dioxane degradation[J]. Journal of Hazardous Materials, 2010, 182(1): 75-79.
|
| 33 |
KRUITHOF J C, KAMP P C, MARTIJN B J. UV/H2O2 Treatment: a practical solution for organic contaminant control and primary disinfection[J]. Ozone: Science & Engineering, 2007, 29(4): 273-280.
|
| 34 |
COOPER W J, CRAMER C J, MARTIN N H, et al. Free radical mechanisms for the treatment of methyl tert-butyl ether (MTBE) via advanced oxidation/reductive processes in aqueous solutions[J]. Chemical Reviews, 2009, 109(3): 1302-1345.
|
| 35 |
ZHANG Shu, GEDALANGA P B, MAHENDRA S. Advances in bioremediation of 1,4-dioxane-contaminated waters[J]. Journal of Environmental Management, 2017, 204: 765-774.
|
| 36 |
BARNDÕK H, HERMOSILLA D, HAN Changseok, et al. Degradation of 1,4-dioxane from industrial wastewater by solar photocatalysis using immobilized NF-TiO2 composite with monodisperse TiO2 nanoparticles[J]. Applied Catalysis B: Environmental, 2016, 180: 44-52.
|
| 37 |
BECKETT M A, HUA I. Elucidation of the 1,4-dioxane decomposition pathway at discrete ultrasonic frequencies[J]. Environmental Science & Technology, 2000, 34(18): 3944-3953.
|
| 38 |
BECKETT M A, HUA I. Enhanced sonochemical decomposition of 1,4-dioxane by ferrous iron[J]. Water Research, 2003, 37(10): 2372-2376.
|
| 39 |
Hyun-Seok SON, CHOI Seok-Bong, KHAN E, et al. Removal of 1,4-dioxane from water using sonication: effect of adding oxidants on the degradation kinetics[J]. Water Research, 2006, 40(4): 692-698.
|
| 40 |
CHOI Jong Young, LEE You-Jin, SHIN J, et al. Anodic oxidation of 1,4-dioxane on boron-doped diamond electrodes for wastewater treatment[J]. Journal of Hazardous Materials, 2010, 179(1): 762-768.
|
| 41 |
NAKAGAWA H, TAKAGI S, MAEKAWA J. Fered-Fenton process for the degradation of 1,4-dioxane with an activated carbon electrode: a kinetic model including active radicals[J]. Chemical Engineering Journal, 2016, 296: 398-405.
|
| 42 |
WANG Huijiao, BAKHEET B, YUAN Shi, et al. Kinetics and energy efficiency for the degradation of 1,4-dioxane by electro-peroxone process[J]. Journal of Hazardous Materials, 2015, 294: 90-98.
|
| 43 |
KISHIMOTO N, KATAYAMA Y, KATO M, et al. Technical feasibility of UV/electro-chlorine advanced oxidation process and pH response[J]. Chemical Engineering Journal, 2018, 334: 2363-2372.
|
| 44 |
ZHANG B T, ZHANG Y, TENG Y G, et al. Sulfate radical and its application in decontamination technologies[J]. Critical Reviews in Environmental Science and Technology, 2015, 45(16): 1756-1800.
|
| 45 |
黄智辉, 纪志永, 陈希, 等. 过硫酸盐高级氧化降解水体中有机污染物研究进展[J]. 化工进展, 2019, 38(5): 2461-2470.
|
|
HUANG Zhihui, JI Zhiyong, CHEN Xi, et al. Degradation of organic pollutants in water by persulfate advanced oxidation[J]. Chemical Industry and Engineering Progress, 2019, 38(5): 2461-2470.
|
| 46 |
ZHU Jiang, LI Bingzhi. Degradation kinetic and remediation effectiveness of 1,4-dioxane-contaminated groundwater by a sono-activated persulfate process[J]. Journal of Environmental Engineering, 2018, 144(10): 04018098.
|
| 47 |
Hyun-Seok SON, Jong-Kwon IM, Kyung-Duk ZOH. A Fenton-like degradation mechanism for 1,4-dioxane using zero-valent iron (Fe0) and UV light[J]. Water Research, 2009, 43(5): 1457-1463.
|
| 48 |
TAKAHASHI N, HIBINO T, TORII H, et al. Evaluation of O3/UV and O3/H2O2 as practical advanced oxidation processes for degradation of 1,4-dioxane[J]. Ozone: Science and Engineering, 2013, 35(5): 331-337.
|
| 49 |
ZHONG Hua, BRUSSEAU M L, WANG Yake, et al. In-situ activation of persulfate by iron filings and degradation of 1,4-dioxane[J]. Water Research, 2015, 83: 104-111.
|
| 50 |
BARNDÕK H, BLANCO L, HERMOSILLA D, et al. Heterogeneous photo-Fenton processes using zero valent iron microspheres for the treatment of wastewaters contaminated with 1,4-dioxane[J]. Chemical Engineering Journal, 2016, 284: 112-121.
|
| 51 |
LI Bingzhi, ZHU Jiang. Simultaneous degradation of 1,1,1-trichloroethane and solvent stabilizer 1,4-dioxane by a sono-activated persulfate process[J]. Chemical Engineering Journal, 2016, 284: 750-763.
|
| 52 |
OUYANG Da, YAN Jingchun, QIAN Linbo, et al. Degradation of 1,4-dioxane by biochar supported nano magnetite particles activating persulfate[J]. Chemosphere, 2017, 184: 609-617.
|
| 53 |
KAMBHU A, GREN M, TANG Wei, et al. Remediating 1,4-dioxane-contaminated water with slow-release persulfate and zerovalent iron[J]. Chemosphere, 2017, 175: 170-177.
|
| 54 |
FENG Yong, LEE Po-Heng, WU Deli, et al. Surface-bound sulfate radical-dominated degradation of 1,4-dioxane by alumina-supported palladium (Pd/Al2O3) catalyzed peroxymonosulfate[J]. Water Research, 2017, 120: 12-21.
|
| 55 |
KANG Yu-Gyeong, YOON Hakwon, LEE Woojin, et al. Comparative study of peroxide oxidants activated by nZVI: removal of 1,4-dioxane and arsenic(Ⅲ) in contaminated waters[J]. Chemical Engineering Journal, 2018, 334: 2511-2519.
|
| 56 |
OUYANG Da, CHEN Yun, YAN Jingchun, et al. Activation mechanism of peroxymonosulfate by biochar for catalytic degradation of 1,4-dioxane: important role of biochar defect structures[J]. Chemical Engineering Journal, 2019, 370: 614-624.
|
| 57 |
PARK Young-kwon, CHUNG Kyong-Hwan, PARK In-Soo, et al. Photocatalytic degradation of 1,4-dioxane using liquid phase plasma on visible light photocatalysts[J]. Journal of Hazardous Materials, 2020, 399: 123087.
|
| 58 |
ZENKER M J, BORDEN R C, BARLAZ M A. Mineralization of 1,4-dioxane in the presence of a structural analog[J]. Biodegradation, 2000, 11(4): 239-246.
|
| 59 |
SEI K, KAKINOKI T, INOUE D, et al. Evaluation of the biodegradation potential of 1,4-dioxane in river, soil and activated sludge samples[J]. Biodegradation, 2010, 21(4): 585-591.
|
| 60 |
CHIANG S Y D, MORA R, DIGUISEPPI W H, et al. Characterizing the intrinsic bioremediation potential of 1,4-dioxane and trichloroethene using innovative environmental diagnostic tools[J]. Journal of Environmental Monitoring, 2012, 14(9): 2317-2326.
|
| 61 |
LI M Y, ORDEN E T VAN, DEVRIES D J, et al. Bench-scale biodegradation tests to assess natural attenuation potential of 1,4-dioxane at three sites in California[J]. Biodegradation, 2015, 26(1): 39-50.
|
| 62 |
FUTUGHE A E, PURCHASE D, JONES H. Phytoremediation: phytoremediation using native plants[M]. Switzerland: Springer, Cham, 2020: 285-327.
|
| 63 |
CRISTALDI A, CONTI G O, Eun Hea JHO, et al. Phytoremediation of contaminated soils by heavy metals and PAHs. A brief review[J]. Environmental Technology & Innovation, 2017, 8: 309-326.
|
| 64 |
AITCHISON E W, KELLEY S L, ALVAREZ P J J, et al. Phytoremediation of 1,4-dioxane by hybrid poplar trees[J]. Water Environment Research, 2000, 72(3): 313-321.
|
| 65 |
KELLEY S L, AITCHISON E W, DESHPANDE M, et al. Biodegradation of 1,4-dioxane in planted and unplanted soil: effect of bioaugmentation with Amycolata sp. CB1190[J]. Water Research, 2001, 35(16): 3791-3800.
|
| 66 |
FERRO A M, KENNEDY J, LARUE J C. Phytoremediation of 1,4-dioxane-containing recovered groundwater[J]. International Journal of Phytoremediation, 2013, 15(10): 911-923.
|
| 67 |
SIMMER R, MATHIEU J, SILVA M L B DA, et al. Bioaugmenting the poplar rhizosphere to enhance treatment of 1,4-dioxane[J]. Science of the Total Environment, 2020, 744: 140823.
|
| 68 |
OSAMA R, AWAD H M, ZHA Shanshan, et al. Greenhouse gases emissions from duckweed pond system treating polyester resin wastewater containing 1,4-dioxane and heavy metals[J]. Ecotoxicology and Environmental Safety, 2020, 207: 111253.
|
| 69 |
OUYANG Ying. Phytoremediation: modeling plant uptake and contaminant transport in the soil-plant-atmosphere continuum[J]. Journal of Hydrology, 2002, 266(1): 66-82.
|
| 70 |
SORENSEN H. 1,4-Dioxane and the application of phytoremediation at North Carolina hazardous waste groundwater contaminated sites[D]. North Carolina State University, 2013.
|
| 71 |
沈源源, 滕应, 骆永明, 等. 几种豆科、禾本科植物对多环芳烃复合污染土壤的修复[J]. 土壤, 2011, 43(2): 253-257.
|
|
SHEN Yuanyuan, TENG Ying, LUO Yongming, et al. Remediation efficiency of several legumes and grasses in PAH-contaminated soils[J]. Soil, 2011, 43(2): 253-257.
|
| 72 |
陈静. 小麦草对芘-镍污染土壤的修复潜力及耐性机理研究[D]. 上海: 上海大学, 2019.
|
|
CHEN Jing. Study on the remediation potential and tolerance mechanism of wheatgrass to pyrene-Ni contaminated soil[D]. Shanghai: Shanghai University, 2019.
|
| 73 |
朱灿, 刘慧刚, 顾彦, 等. 外源硒对萘胁迫下车前草生长及土壤修复能力的影响[J]. 农业环境科学学报, 2019, 38(11): 2511-2519.
|
|
ZHU Can, LIU Huigang, GU Yan, et al. Effects of selenium on the growth and phytoremediation efficiency of Plantago asiatica L. in soil exposed to naphthalene[J]. Journal of Agro-Environment Science, 2019, 38(11): 2511-2519.
|
| 74 |
GLICK B R. Using soil bacteria to facilitate phytoremediation[J]. Biotechnology Advances, 2010, 28(3): 367-374.
|
| 75 |
PARALES R E, ADAMUS J E, WHITE N, et al. Degradation of 1,4-dioxane by an actinomycete in pure culture[J]. Applied and Environmental Microbiology, 1994, 60(12): 4527.
|
| 76 |
MAHENDRA S, ALVAREZ-COHEN L. Pseudonocardia dioxanivorans sp. nov., a novel actinomycete that grows on 1,4-dioxane[J]. International Journal of Systematic and Evolutionary Microbiology, 2005, 55(2): 593-598.
|
| 77 |
NAKAMIYA K, HASHIMOTO S, ITO H, et al. Degradation of 1,4-dioxane and cyclic ethers by an isolated fungus[J]. Applied and Environmental Microbiology, 2005, 71(3): 1254.
|
| 78 |
MAHENDRA S, ALVAREZ-COHEN L. Kinetics of 1,4-dioxane biodegradation by monooxygenase-expressing bacteria[J]. Environmental Science & Technology, 2006, 40(17): 5435-5442.
|
| 79 |
KIM Young Mo, JEON Jong Rok, MURUGESAN K, et al. Biodegradation of 1,4-dioxane and transformation of related cyclic compounds by a newly isolated Mycobacterium sp. PH-06[J]. Biodegradation, 2008, 20(4): 511.
|
| 80 |
SKINNER K, CUIFFETTI L, HYMAN M. Metabolism and cometabolism of cyclic ethers by a filamentous fungus, a Graphium sp[J]. Applied and Environmental Microbiology, 2009, 75(17): 5514.
|
| 81 |
MASUDA H, MCCLAY K, STEFFAN R J, et al. Biodegradation of tetrahydrofuran and 1,4-dioxane by soluble diiron monooxygenase in Pseudonocardia sp. strain ENV478[J]. J. Mol. Microbiol. Biotechnol., 2012, 22(5): 312-316.
|
| 82 |
SEI K, MIYAGAKI K, KAKINOKI T, et al. Isolation and characterization of bacterial strains that have high ability to degrade 1,4-dioxane as a sole carbon and energy source[J]. Biodegradation, 2013, 24(5): 665-674.
|
| 83 |
HUANG Huanlin, SHEN Dongsheng, LI Na, et al. Biodegradation of 1,4-dioxane by a novel strain and its biodegradation pathway[J]. Water, Air, & Soil Pollution, 2014, 225(9): 2135.
|
| 84 |
LIPPINCOTT D, STREGER S H, SCHAEFER C E, et al. Bioaugmentation and propane biosparging for in situ biodegradation of 1,4-dioxane[J]. Groundwater Monitoring and Remediation, 2015, 35(2): 81-92.
|
| 85 |
PUGAZHENDI A, RAJESH BANU J, DHAVAMANI J, et al. Biodegradation of 1,4-dioxane by Rhodanobacter AYS5 and the role of additional substrates[J]. Annals of Microbiology, 2015, 65(4): 2201-2208.
|
| 86 |
CHEN Dongzhi, JIN Xiaojun, CHEN Jing, et al. Intermediates and substrate interaction of 1,4-dioxane degradation by the effective metabolizer Xanthobacter flavus DT8[J]. International Biodeterioration & Biodegradation, 2016, 106: 133-140.
|
| 87 |
MATSUI R, TAKAGI K, SAKAKIBARA F, et al. Identification and characterization of 1,4-dioxane-degrading microbe separated from surface seawater by the seawater-charcoal perfusion apparatus[J]. Biodegradation, 2016, 27(2): 155-163.
|
| 88 |
INOUE D, TSUNODA T, YAMAMOTO N, et al. 1,4-Dioxane degradation characteristics of Rhodococcus aetherivorans JCM 14343[J]. Biodegradation, 2018, 29(3): 301-310.
|
| 89 |
TUSHER T R, SHIMIZU T, INOUE C, et al. Enrichment and analysis of stable 1,4-dioxane-degrading microbial consortia consisting of novel dioxane-degraders[J]. Microorganisms, 2020, 8(1): 50.
|
| 90 |
SHEN Weirong, CHEN Hong, PAN Shanshan. Anaerobic biodegradation of 1,4-dioxane by sludge enriched with iron-reducing microorganisms[J]. Bioresource Technology, 2008, 99(7): 2483-2487.
|
| 91 |
RODRIGUEZ F J B. Evaluation of 1,4-dioxane biodegradation under aerobic and anaerobic conditions[D]. Clemson: Clemson University, 2016.
|
| 92 |
MAHENDRA S, PETZOLD C J, BAIDOO E E, et al. Identification of the intermediates of in vivo oxidation of 1,4-dioxane by monooxygenase-containing bacteria[J]. Environmental Science & Technology, 2007, 41(21): 7330-7336.
|
| 93 |
金小君, 陈东之, 朱润晔, 等. Xanthobacter flavus DT8降解二 烷的特性研究[J]. 环境科学, 2012, 33(5): 1657-1662. 烷的特性研究[J]. 环境科学, 2012, 33(5): 1657-1662.
|
|
JIN Xiaojun, CHEN Dongzhi, ZHU Runye, et al. Characteristics of 1,4-dioxane degradation by Xanthobacter flavus DT8.[J]. Environmental Science, 2012, 33(5): 1657-1662.
|
| 94 |
ZENKER M J, BORDEN R C, BARLAZ M A. Biodegradation of 1,4-dioxane using trickling filter[J]. Journal of Environmental Engineering, 2004, 130(9): 926-931.
|
| 95 |
SAUL M T. Bioaugmentation to remediate dioxane in groundwater: US8241500B2[P]. 2012-08-14.
|
| 96 |
Ji-Hyun NAM, VENTURA J-R S, YEOM Ick Tae, et al. Structural and kinetic characteristics of 1,4-dioxane-degrading bacterial consortia containing the Phylum TM7[J]. Journal of Microbiology and Biotechnology, 2016, 26(11): 1951-1964.
|
| 97 |
SEKAR R, DICHRISTINA T J. Microbially driven Fenton reaction for degradation of the widespread environmental contaminant 1,4-dioxane[J]. Environmental Science & Technology, 2014, 48(21): 12858-12867.
|
| 98 |
MAHENDRA S, GROSTERN A, ALVAREZ-COHEN L. The impact of chlorinated solvent co-contaminants on the biodegradation kinetics of 1,4-dioxane[J]. Chemosphere, 2013, 91(1): 88-92.
|
| 99 |
ZHANG Shu, GEDALANGA P B, MAHENDRA S. Biodegradation kinetics of 1,4-dioxane in chlorinated solvent mixtures[J]. Environmental Science & Technology, 2016, 50(17): 9599-9607.
|
| 100 |
MATTES T E, ALEXANDER A K, COLEMAN N V. Aerobic biodegradation of the chloroethenes: pathways, enzymes, ecology, and evolution[J]. FEMS Microbiology Reviews, 2010, 34(4): 445-475.
|
| 101 |
PORNWONGTHONG P, MULCHANDANI A, GEDALANGA P B, et al. Transition metals and organic ligands influence biodegradation of 1,4-dioxane[J]. Applied Biochemistry and Biotechnology, 2014, 173(1): 291-306.
|
| 102 |
YAN Ni, LIU Fei, LIU Boyang, et al. Treatment of 1,4-dioxane and trichloroethene co-contamination by an activated binary persulfate-peroxide oxidation process[J]. Environmental Science and Pollution Research, 2018, 25(32): 32088-32095.
|
| 103 |
POLASKO A L, ZULLI A, GEDALANGA P B, et al. A mixed microbial community for the biodegradation of chlorinated ethenes and 1,4-dioxane[J]. Environmental Science & Technology Letters, 2019, 6(1): 49-54.
|
| 104 |
SEKAR R, TAILLEFERT M, DICHRISTINA T J. Simultaneous transformation of commingled trichloroethylene, tetrachloroethylene, and 1,4-dioxane by a microbially driven Fenton reaction in batch liquid cultures[J]. Applied and Environmental Microbiology, 2016, 82(21): 6335.
|
| 105 |
DENG Daiyong, PHAM Dung Ngoc, LI Fei, et al. Discovery of an inducible toluene monooxygenase that cooxidizes 1,4-dioxane and 1,1-dichloroethylene in propanotrophic Azoarcus sp. strain DD4[J]. Applied and Environmental Microbiology, 2020, 86(17).
|
| 106 |
JASMANN J R, GEDALANGA P B, BORCH T, et al. Synergistic treatment of mixed 1,4-dioxane and chlorinated solvent contaminations by coupling electrochemical oxidation with aerobic biodegradation[J]. Environmental Science & Technology, 2017, 51(21): 12619-12629.
|
| 107 |
MIAO Yu, JOHNSON N W, GEDALANGA P B, et al. Response and recovery of microbial communities subjected to oxidative and biological treatments of 1,4-dioxane and co-contaminants[J]. Water Research, 2019, 149: 74-85.
|
| 108 |
MIAO Yu, JOHNSON N W, PHAN Thien, et al. Monitoring, assessment, and prediction of microbial shifts in coupled catalysis and biodegradation of 1,4-dioxane and co-contaminants[J]. Water Research, 2020, 173: 115540.
|
 ), YAO Dandan2,3, ZHAO Yuantian2,3, GUO Lili1, DONG Yuanhua2,3, LIU Yun1,2,3(
), YAO Dandan2,3, ZHAO Yuantian2,3, GUO Lili1, DONG Yuanhua2,3, LIU Yun1,2,3( )
)
 烷污染处理技术研究进展
烷污染处理技术研究进展
 ), 姚丹丹2,3, 赵元添2,3, 郭丽莉1, 董元华2,3, 刘云1,2,3(
), 姚丹丹2,3, 赵元添2,3, 郭丽莉1, 董元华2,3, 刘云1,2,3( )
)
 烷污染处理技术研究进展[J]. 化工进展, 2021, 40(10): 5708-5719.
烷污染处理技术研究进展[J]. 化工进展, 2021, 40(10): 5708-5719. 

 烷初始浓度
烷初始浓度 烷初始浓度
烷初始浓度 烷初始浓度
烷初始浓度 烷初始浓度
烷初始浓度 烷的特性研究[J]. 环境科学, 2012, 33(5): 1657-1662.
烷的特性研究[J]. 环境科学, 2012, 33(5): 1657-1662.