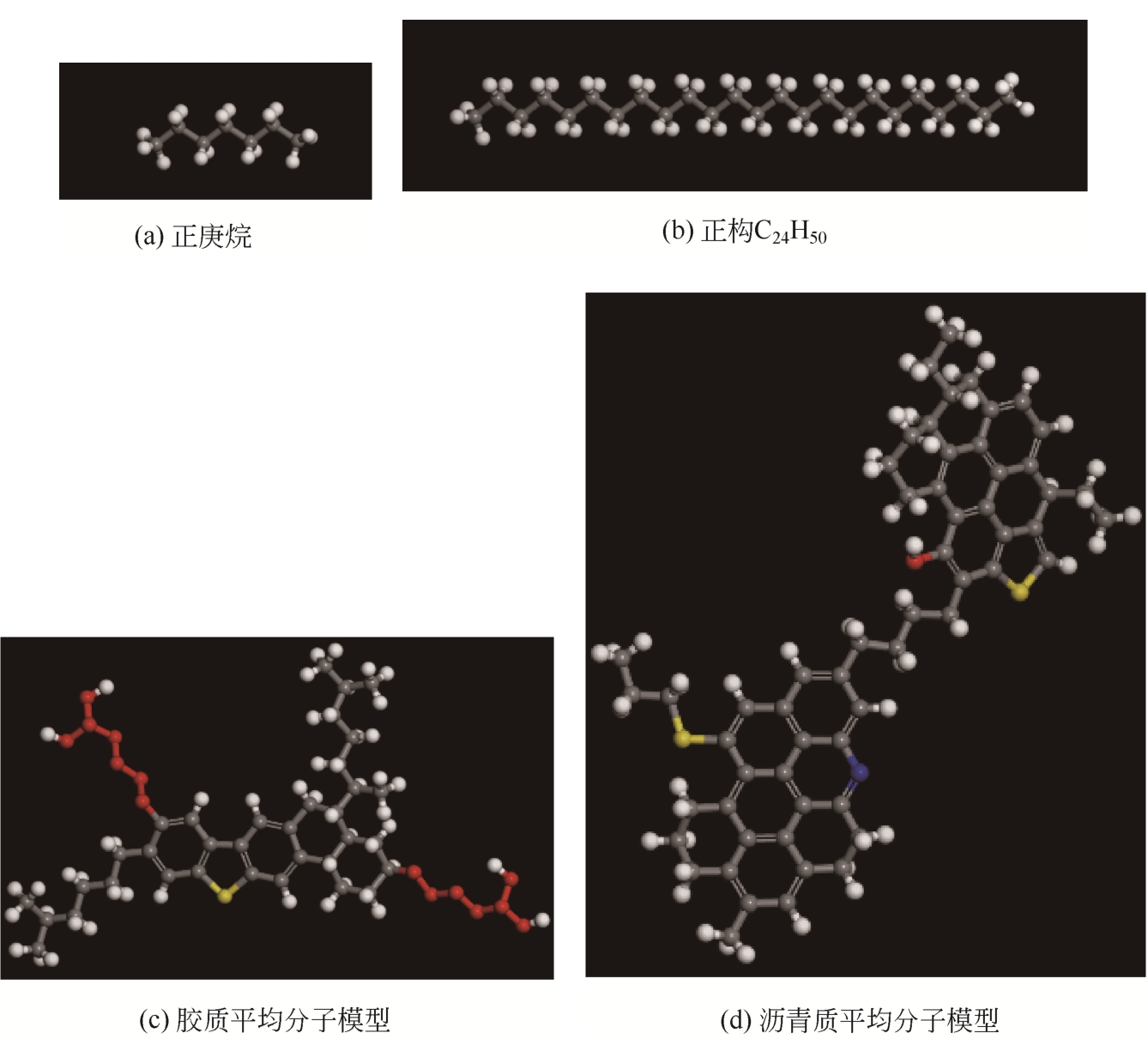
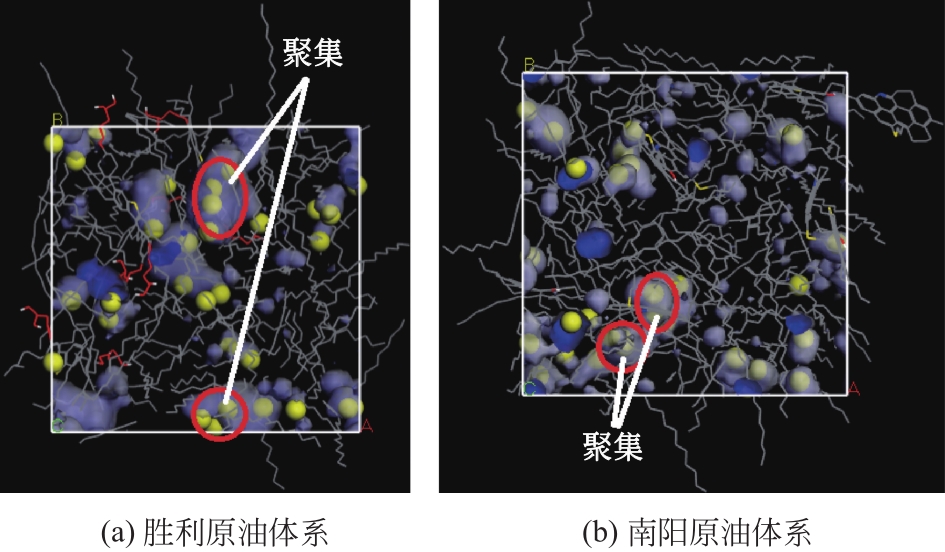
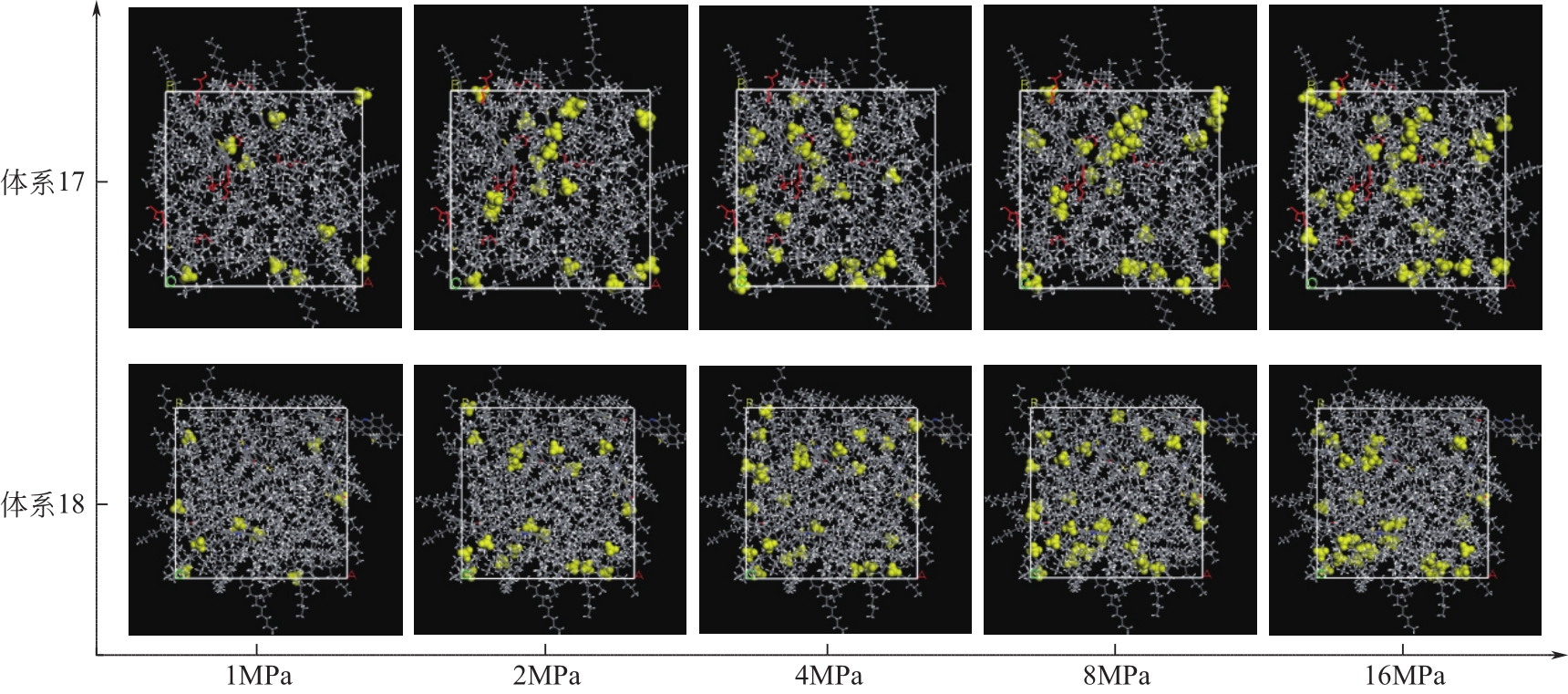
Chemical Industry and Engineering Progress ›› 2021, Vol. 40 ›› Issue (8): 4205-4222.DOI: 10.16085/j.issn.1000-6613.2020-1801
• Energy processes and technology • Previous Articles Next Articles
Dissolution of CH4 in the crude oil system: behaviors and mechanisms
LI Bingfan( ), LIU Gang(
), LIU Gang( ), CHEN Lei
), CHEN Lei
- University of Petroleum(East China), Shandong Provincial Key Laboratory of Oil & Gas Storage and Transportation Safety, Qingdao 266580, Shandong, China
-
Received:2020-09-08Online:2021-08-12Published:2021-08-05 -
Contact:LIU Gang
CH4在原油体系中溶解规律及影响机理
- 中国石油大学(华东),山东省油气储运安全省级重点实验室,山东 青岛 266580
-
通讯作者:刘刚 -
作者简介:李秉繁(1991—),男,博士研究生,研究方向为油气长距离管输技术。E-mail:670264522@qq.com 。 -
基金资助:国家自然科学基金(51774315)
CLC Number:
Cite this article
LI Bingfan, LIU Gang, CHEN Lei. Dissolution of CH4 in the crude oil system: behaviors and mechanisms[J]. Chemical Industry and Engineering Progress, 2021, 40(8): 4205-4222.
李秉繁, 刘刚, 陈雷. CH4在原油体系中溶解规律及影响机理[J]. 化工进展, 2021, 40(8): 4205-4222.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2020-1801
| 油样 | 20℃密度 /kg·m-3 | 凝点 /℃ | 析蜡点 /℃ | 含蜡量 /% | 胶质 质量分数/% | 沥青质 质量分数/% |
|---|---|---|---|---|---|---|
| 胜利原油 | 878.2 | 34 | 47 | 24.5 | 18.03 | 0.12 |
| 南阳原油 | 875.0 | 47 | 62 | 38.2 | — | 15 |
| 油样 | 20℃密度 /kg·m-3 | 凝点 /℃ | 析蜡点 /℃ | 含蜡量 /% | 胶质 质量分数/% | 沥青质 质量分数/% |
|---|---|---|---|---|---|---|
| 胜利原油 | 878.2 | 34 | 47 | 24.5 | 18.03 | 0.12 |
| 南阳原油 | 875.0 | 47 | 62 | 38.2 | — | 15 |
试验 压力 | 胜利原油 | 南阳原油 | ||
|---|---|---|---|---|
表观黏度(10s-1) /Pa·s | 平均降黏率 /% | 表观黏度(10s-1) /Pa·s | 平均降黏率 /% | |
| 常压 | 1.65 | — | 32.18 | — |
| 1 | 1.52 | 7.88 | 30.84 | 4.16 |
| 2 | 1.40 | 15.15 | 29.55 | 8.17 |
试验 压力 | 胜利原油 | 南阳原油 | ||
|---|---|---|---|---|
表观黏度(10s-1) /Pa·s | 平均降黏率 /% | 表观黏度(10s-1) /Pa·s | 平均降黏率 /% | |
| 常压 | 1.65 | — | 32.18 | — |
| 1 | 1.52 | 7.88 | 30.84 | 4.16 |
| 2 | 1.40 | 15.15 | 29.55 | 8.17 |
| 体系 | 组合情况 | 体系内分子个数 | ||
|---|---|---|---|---|
| 蜡分子 | 胶质 | 沥青质 | ||
| 1 | 111 | 0 | 0 | 0 |
| 2 | 122 | 0 | 5 | 5 |
| 3 | 133 | 0 | 11 | 11 |
| 4 | 144 | 0 | 25 | 25 |
| 5 | 212 | 5 | 0 | 5 |
| 6 | 221 | 5 | 5 | 0 |
| 7 | 234 | 5 | 11 | 25 |
| 8 | 243 | 5 | 25 | 11 |
| 9 | 313 | 11 | 0 | 11 |
| 10 | 324 | 11 | 5 | 25 |
| 11 | 331 | 11 | 11 | 0 |
| 12 | 342 | 11 | 25 | 5 |
| 13 | 414 | 25 | 0 | 25 |
| 14 | 423 | 25 | 5 | 11 |
| 15 | 432 | 25 | 11 | 5 |
| 16 | 441 | 25 | 25 | 0 |
| 17 | 胜利原油 | 13 | 4 | 0 |
| 18 | 南阳原油 | 25 | 0 | 4 |
| 体系 | 组合情况 | 体系内分子个数 | ||
|---|---|---|---|---|
| 蜡分子 | 胶质 | 沥青质 | ||
| 1 | 111 | 0 | 0 | 0 |
| 2 | 122 | 0 | 5 | 5 |
| 3 | 133 | 0 | 11 | 11 |
| 4 | 144 | 0 | 25 | 25 |
| 5 | 212 | 5 | 0 | 5 |
| 6 | 221 | 5 | 5 | 0 |
| 7 | 234 | 5 | 11 | 25 |
| 8 | 243 | 5 | 25 | 11 |
| 9 | 313 | 11 | 0 | 11 |
| 10 | 324 | 11 | 5 | 25 |
| 11 | 331 | 11 | 11 | 0 |
| 12 | 342 | 11 | 25 | 5 |
| 13 | 414 | 25 | 0 | 25 |
| 14 | 423 | 25 | 5 | 11 |
| 15 | 432 | 25 | 11 | 5 |
| 16 | 441 | 25 | 25 | 0 |
| 17 | 胜利原油 | 13 | 4 | 0 |
| 18 | 南阳原油 | 25 | 0 | 4 |
| 体系 | 组分 | 密度/g·cm-3 | 相对误差/% | |
|---|---|---|---|---|
| 模拟值 | 试验值 | |||
| 1 | 111 | 0.662 | 0.684 | 3.22 |
| 2 | 122 | 0.85 | 0.87 | 2.30 |
| 3 | 133 | 0.885 | 0.933 | 5.14 |
| 4 | 144 | 1.035 | 1.012 | 2.2 |
| 5 | 212 | 0.843 | 0.857 | 1.63 |
| 6 | 221 | 0.777 | 0.798 | 2.63 |
| 7 | 234 | 0.994 | 0.996 | 0.20 |
| 8 | 243 | 0.951 | 0.973 | 2.26 |
| 9 | 313 | 0.862 | 0.899 | 4.12 |
| 10 | 324 | 0.97 | 0.986 | 1.62 |
| 11 | 331 | 0.854 | 0.874 | 2.29 |
| 12 | 342 | 0.938 | 0.969 | 3.20 |
| 13 | 414 | 0.966 | 0.98 | 1.43 |
| 14 | 423 | 0.925 | 0.946 | 2.22 |
| 15 | 432 | 0.875 | 0.915 | 4.37 |
| 16 | 441 | 0.905 | 0.942 | 3.93 |
| 17 | 胜利原油 | 0.862 | 0.878 | 1.82 |
| 18 | 南阳原油 | 0.842 | 0.875 | 3.77 |
| 体系 | 组分 | 密度/g·cm-3 | 相对误差/% | |
|---|---|---|---|---|
| 模拟值 | 试验值 | |||
| 1 | 111 | 0.662 | 0.684 | 3.22 |
| 2 | 122 | 0.85 | 0.87 | 2.30 |
| 3 | 133 | 0.885 | 0.933 | 5.14 |
| 4 | 144 | 1.035 | 1.012 | 2.2 |
| 5 | 212 | 0.843 | 0.857 | 1.63 |
| 6 | 221 | 0.777 | 0.798 | 2.63 |
| 7 | 234 | 0.994 | 0.996 | 0.20 |
| 8 | 243 | 0.951 | 0.973 | 2.26 |
| 9 | 313 | 0.862 | 0.899 | 4.12 |
| 10 | 324 | 0.97 | 0.986 | 1.62 |
| 11 | 331 | 0.854 | 0.874 | 2.29 |
| 12 | 342 | 0.938 | 0.969 | 3.20 |
| 13 | 414 | 0.966 | 0.98 | 1.43 |
| 14 | 423 | 0.925 | 0.946 | 2.22 |
| 15 | 432 | 0.875 | 0.915 | 4.37 |
| 16 | 441 | 0.905 | 0.942 | 3.93 |
| 17 | 胜利原油 | 0.862 | 0.878 | 1.82 |
| 18 | 南阳原油 | 0.842 | 0.875 | 3.77 |
| 正庚烷(25℃) | 胜利原油(40℃) | 南阳原油(40℃) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
压力 /MPa | 试验值 /mol·mol-1 | 计算值 /mol·mol-1 | 相对误差 /% | 压力 /MPa | 试验值 /mmol·g-1 | 计算值 /mmol·g-1 | 相对误差 /% | 压力 /MPa | 试验值 /mmol·g-1 | 计算值 /mmol·g-1 | 相对误差 /% | ||
| 3.67 | 0.163 | 0.161 | 4.294 | 0.5 | 0.262 | 0.263 | 0.382 | 0.5 | 0.236 | 0.179 | 24.175 | ||
| 6.96 | 0.276 | 2.657 | 7.246 | 1 | 0.433 | 0.498 | 15.067 | 1 | 0.399 | 0.422 | 5.793 | ||
| 10.3 | 0.376 | 0.355 | 7.181 | 1.5 | 0.629 | 0.695 | 10.576 | 1.5 | 0.530 | 0.602 | 13.518 | ||
| 13.7 | 0.470 | 0.455 | 5.319 | 2 | 0.758 | 0.837 | 10.490 | 2 | 0.633 | 0.712 | 12.405 | ||
| 17.01 | 0.539 | 0.525 | 4.453 | — | — | — | — | — | — | — | — | ||
| 正庚烷(25℃) | 胜利原油(40℃) | 南阳原油(40℃) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
压力 /MPa | 试验值 /mol·mol-1 | 计算值 /mol·mol-1 | 相对误差 /% | 压力 /MPa | 试验值 /mmol·g-1 | 计算值 /mmol·g-1 | 相对误差 /% | 压力 /MPa | 试验值 /mmol·g-1 | 计算值 /mmol·g-1 | 相对误差 /% | ||
| 3.67 | 0.163 | 0.161 | 4.294 | 0.5 | 0.262 | 0.263 | 0.382 | 0.5 | 0.236 | 0.179 | 24.175 | ||
| 6.96 | 0.276 | 2.657 | 7.246 | 1 | 0.433 | 0.498 | 15.067 | 1 | 0.399 | 0.422 | 5.793 | ||
| 10.3 | 0.376 | 0.355 | 7.181 | 1.5 | 0.629 | 0.695 | 10.576 | 1.5 | 0.530 | 0.602 | 13.518 | ||
| 13.7 | 0.470 | 0.455 | 5.319 | 2 | 0.758 | 0.837 | 10.490 | 2 | 0.633 | 0.712 | 12.405 | ||
| 17.01 | 0.539 | 0.525 | 4.453 | — | — | — | — | — | — | — | — | ||
| 体系 | 原油体系体积/?3 | 体系 | 原油体系体积/?3 |
|---|---|---|---|
| 体系1 | 22777.56 | 体系10 | 68951.63 |
| 体系2 | 38182.82 | 体系11 | 45008.31 |
| 体系3 | 50821.36 | 体系12 | 66071.80 |
| 体系4 | 80052.22 | 体系13 | 73341.38 |
| 体系5 | 33501.69 | 体系14 | 53874.80 |
| 体系6 | 32650.47 | 体系15 | 62628.34 |
| 体系7 | 77681.58 | 体系16 | 63042.01 |
| 体系8 | 74932.23 | 体系17 | 36543.51 |
| 体系9 | 43913.76 | 体系18 | 41314.62 |
| 体系 | 原油体系体积/?3 | 体系 | 原油体系体积/?3 |
|---|---|---|---|
| 体系1 | 22777.56 | 体系10 | 68951.63 |
| 体系2 | 38182.82 | 体系11 | 45008.31 |
| 体系3 | 50821.36 | 体系12 | 66071.80 |
| 体系4 | 80052.22 | 体系13 | 73341.38 |
| 体系5 | 33501.69 | 体系14 | 53874.80 |
| 体系6 | 32650.47 | 体系15 | 62628.34 |
| 体系7 | 77681.58 | 体系16 | 63042.01 |
| 体系8 | 74932.23 | 体系17 | 36543.51 |
| 体系9 | 43913.76 | 体系18 | 41314.62 |
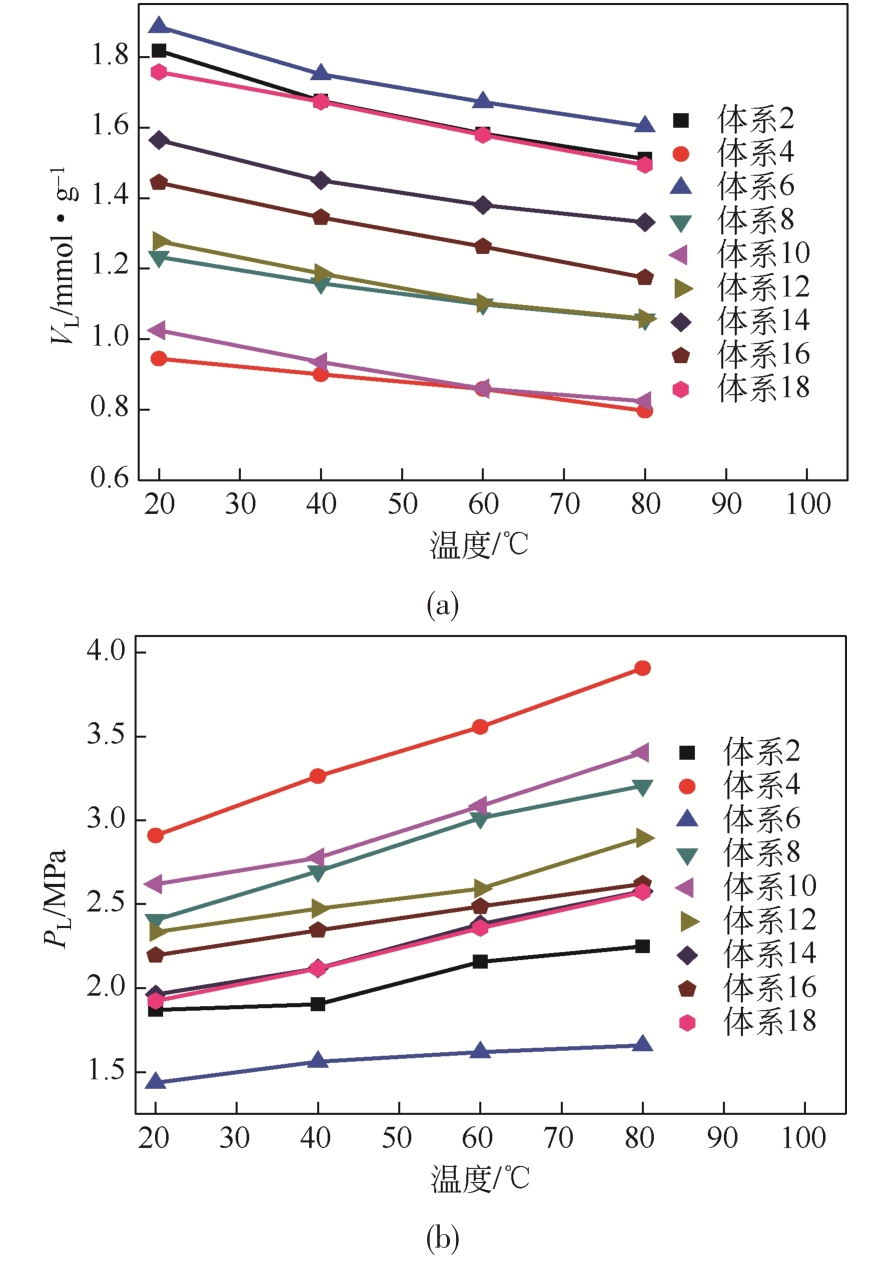
| 体系 | 参数 | 20℃ | 40℃ | 60℃ | 80℃ | 体系 | 参数 | 20℃ | 40℃ | 60℃ | 80℃ |
|---|---|---|---|---|---|---|---|---|---|---|---|
2 | VL | 1.817 | 1.675 | 1.582 | 1.511 | 11 | VL | 1.756 | 1.656 | 1.568 | 1.515 |
| PL | 1.870 | 1.904 | 2.157 | 2.250 | PL | 1.906 | 2.111 | 2.302 | 2.568 | ||
| R2 | 0.997 | 0.997 | 0.996 | 0.995 | R2 | 0.992 | 0.987 | 0.988 | 0.985 | ||
3 | VL | 1.479 | 1.403 | 1.330 | 1.258 | 12 | VL | 1.277 | 1.186 | 1.103 | 1.058 |
| PL | 2.020 | 2.258 | 2.438 | 2.595 | PL | 2.335 | 2.573 | 2.593 | 2.894 | ||
| R2 | 0.998 | 0.998 | 0.998 | 0.997 | R2 | 0.997 | 0.998 | 0.999 | 0.998 | ||
4 | VL | 0.945 | 0.900 | 0.859 | 0.797 | 13 | VL | 1.122 | 1.052 | 1.008 | 0.965 |
| PL | 2.910 | 3.263 | 3.556 | 3.908 | PL | 2.572 | 2.714 | 3.020 | 3.306 | ||
| R2 | 0.997 | 0.998 | 0.999 | 0.998 | R2 | 0.998 | 0.998 | 0.995 | 0.995 | ||
5 | VL | 1.855 | 1.725 | 1.608 | 1.527 | 14 | VL | 1.564 | 1.450 | 1.380 | 1.331 |
| PL | 1.863 | 1.899 | 1.926 | 2.064 | PL | 1.962 | 2.118 | 2.381 | 2.578 | ||
| R2 | 0.992 | 0.991 | 0.989 | 0.989 | R2 | 0.998 | 0.996 | 0.993 | 0.993 | ||
6 | VL | 1.885 | 1.751 | 1.672 | 1.603 | 15 | VL | 1.391 | 1.289 | 1.205 | 1.150 |
| PL | 1.436 | 1.561 | 1.619 | 1.659 | PL | 2.225 | 2.376 | 2.541 | 2.820 | ||
| R2 | 0.995 | 0.991 | 0.991 | 0.990 | R2 | 0.998 | 0.999 | 0.998 | 0.999 | ||
7 | VL | 0.992 | 0.926 | 0.858 | 0.822 | 16 | VL | 1.443 | 1.345 | 1.262 | 1.174 |
| PL | 2.694 | 3.007 | 3.194 | 3.431 | PL | 2.196 | 2.344 | 2.488 | 2.622 | ||
| R2 | 0.999 | 0.997 | 0.998 | 0.999 | R2 | 0.997 | 0.997 | 0.997 | 0.995 | ||
8 | VL | 1.233 | 1.158 | 1.099 | 1.056 | 17 | VL | 1.869 | 1.734 | 1.652 | 1.586 |
| PL | 2.406 | 2.696 | 3.014 | 3.207 | PL | 1.627 | 1.710 | 1.836 | 2.047 | ||
| R2 | 0.998 | 0.997 | 0.997 | 0.996 | R2 | 0.991 | 0.988 | 0.989 | 0.986 | ||
9 | VL | 1.702 | 1.612 | 1.510 | 1.421 | 18 | VL | 1.757 | 1.673 | 1.578 | 1.493 |
| PL | 2.016 | 2.164 | 2.391 | 2.582 | PL | 1.924 | 2.117 | 2.358 | 2.572 | ||
| R2 | 0.984 | 0.983 | 0.983 | 0.978 | R2 | 0.997 | 0.995 | 0.997 | 0.995 | ||
10 | VL | 1.025 | 0.935 | 0.860 | 0.824 | ||||||
| PL | 2.620 | 2.778 | 3.086 | 3.404 | |||||||
| R2 | 0.998 | 0.996 | 0.993 | 0.993 |
| 体系 | 参数 | 20℃ | 40℃ | 60℃ | 80℃ | 体系 | 参数 | 20℃ | 40℃ | 60℃ | 80℃ |
|---|---|---|---|---|---|---|---|---|---|---|---|
2 | VL | 1.817 | 1.675 | 1.582 | 1.511 | 11 | VL | 1.756 | 1.656 | 1.568 | 1.515 |
| PL | 1.870 | 1.904 | 2.157 | 2.250 | PL | 1.906 | 2.111 | 2.302 | 2.568 | ||
| R2 | 0.997 | 0.997 | 0.996 | 0.995 | R2 | 0.992 | 0.987 | 0.988 | 0.985 | ||
3 | VL | 1.479 | 1.403 | 1.330 | 1.258 | 12 | VL | 1.277 | 1.186 | 1.103 | 1.058 |
| PL | 2.020 | 2.258 | 2.438 | 2.595 | PL | 2.335 | 2.573 | 2.593 | 2.894 | ||
| R2 | 0.998 | 0.998 | 0.998 | 0.997 | R2 | 0.997 | 0.998 | 0.999 | 0.998 | ||
4 | VL | 0.945 | 0.900 | 0.859 | 0.797 | 13 | VL | 1.122 | 1.052 | 1.008 | 0.965 |
| PL | 2.910 | 3.263 | 3.556 | 3.908 | PL | 2.572 | 2.714 | 3.020 | 3.306 | ||
| R2 | 0.997 | 0.998 | 0.999 | 0.998 | R2 | 0.998 | 0.998 | 0.995 | 0.995 | ||
5 | VL | 1.855 | 1.725 | 1.608 | 1.527 | 14 | VL | 1.564 | 1.450 | 1.380 | 1.331 |
| PL | 1.863 | 1.899 | 1.926 | 2.064 | PL | 1.962 | 2.118 | 2.381 | 2.578 | ||
| R2 | 0.992 | 0.991 | 0.989 | 0.989 | R2 | 0.998 | 0.996 | 0.993 | 0.993 | ||
6 | VL | 1.885 | 1.751 | 1.672 | 1.603 | 15 | VL | 1.391 | 1.289 | 1.205 | 1.150 |
| PL | 1.436 | 1.561 | 1.619 | 1.659 | PL | 2.225 | 2.376 | 2.541 | 2.820 | ||
| R2 | 0.995 | 0.991 | 0.991 | 0.990 | R2 | 0.998 | 0.999 | 0.998 | 0.999 | ||
7 | VL | 0.992 | 0.926 | 0.858 | 0.822 | 16 | VL | 1.443 | 1.345 | 1.262 | 1.174 |
| PL | 2.694 | 3.007 | 3.194 | 3.431 | PL | 2.196 | 2.344 | 2.488 | 2.622 | ||
| R2 | 0.999 | 0.997 | 0.998 | 0.999 | R2 | 0.997 | 0.997 | 0.997 | 0.995 | ||
8 | VL | 1.233 | 1.158 | 1.099 | 1.056 | 17 | VL | 1.869 | 1.734 | 1.652 | 1.586 |
| PL | 2.406 | 2.696 | 3.014 | 3.207 | PL | 1.627 | 1.710 | 1.836 | 2.047 | ||
| R2 | 0.998 | 0.997 | 0.997 | 0.996 | R2 | 0.991 | 0.988 | 0.989 | 0.986 | ||
9 | VL | 1.702 | 1.612 | 1.510 | 1.421 | 18 | VL | 1.757 | 1.673 | 1.578 | 1.493 |
| PL | 2.016 | 2.164 | 2.391 | 2.582 | PL | 1.924 | 2.117 | 2.358 | 2.572 | ||
| R2 | 0.984 | 0.983 | 0.983 | 0.978 | R2 | 0.997 | 0.995 | 0.997 | 0.995 | ||
10 | VL | 1.025 | 0.935 | 0.860 | 0.824 | ||||||
| PL | 2.620 | 2.778 | 3.086 | 3.404 | |||||||
| R2 | 0.998 | 0.996 | 0.993 | 0.993 |
| 体系 | 组合 情况 | 分子个数 | 溶解量 /mmol·g-1 | ||
|---|---|---|---|---|---|
| 蜡分子 | 胶质 | 沥青质 | |||
| 1 | 111 | 0 | 0 | 0 | 13.959 |
| 2 | 122 | 0 | 5 | 5 | 1.817 |
| 3 | 133 | 0 | 11 | 11 | 1.479 |
| 4 | 144 | 0 | 25 | 25 | 0.945 |
| 5 | 212 | 5 | 0 | 5 | 1.855 |
| 6 | 221 | 5 | 5 | 0 | 1.885 |
| 7 | 234 | 5 | 11 | 25 | 0.992 |
| 8 | 243 | 5 | 25 | 11 | 1.233 |
| 9 | 313 | 11 | 0 | 11 | 1.702 |
| 10 | 324 | 11 | 5 | 25 | 1.025 |
| 11 | 331 | 11 | 11 | 0 | 1.756 |
| 12 | 342 | 11 | 25 | 5 | 1.277 |
| 13 | 414 | 25 | 0 | 25 | 1.122 |
| 14 | 423 | 25 | 5 | 11 | 1.564 |
| 15 | 432 | 25 | 11 | 5 | 1.391 |
| 16 | 441 | 25 | 25 | 0 | 1.443 |
| 水平K1 | 4.550 | 4.660 | 4.761 | — | |
| 水平K2 | 1.491 | 1.573 | 1.585 | — | |
| 水平K3 | 1.440 | 1.405 | 1.495 | — | |
| 水平K4 | 1.380 | 1.225 | 1.021 | — | |
| 极差R | 3.170 | 3.435 | 3.740 | — | |
| 因子主次 | 3 | 2 | 1 | — | |
| 体系 | 组合 情况 | 分子个数 | 溶解量 /mmol·g-1 | ||
|---|---|---|---|---|---|
| 蜡分子 | 胶质 | 沥青质 | |||
| 1 | 111 | 0 | 0 | 0 | 13.959 |
| 2 | 122 | 0 | 5 | 5 | 1.817 |
| 3 | 133 | 0 | 11 | 11 | 1.479 |
| 4 | 144 | 0 | 25 | 25 | 0.945 |
| 5 | 212 | 5 | 0 | 5 | 1.855 |
| 6 | 221 | 5 | 5 | 0 | 1.885 |
| 7 | 234 | 5 | 11 | 25 | 0.992 |
| 8 | 243 | 5 | 25 | 11 | 1.233 |
| 9 | 313 | 11 | 0 | 11 | 1.702 |
| 10 | 324 | 11 | 5 | 25 | 1.025 |
| 11 | 331 | 11 | 11 | 0 | 1.756 |
| 12 | 342 | 11 | 25 | 5 | 1.277 |
| 13 | 414 | 25 | 0 | 25 | 1.122 |
| 14 | 423 | 25 | 5 | 11 | 1.564 |
| 15 | 432 | 25 | 11 | 5 | 1.391 |
| 16 | 441 | 25 | 25 | 0 | 1.443 |
| 水平K1 | 4.550 | 4.660 | 4.761 | — | |
| 水平K2 | 1.491 | 1.573 | 1.585 | — | |
| 水平K3 | 1.440 | 1.405 | 1.495 | — | |
| 水平K4 | 1.380 | 1.225 | 1.021 | — | |
| 极差R | 3.170 | 3.435 | 3.740 | — | |
| 因子主次 | 3 | 2 | 1 | — | |
| 体系 | 碳占比 | 体系 | 碳占比 |
|---|---|---|---|
| 体系1 | 7.00 | 体系10 | 18.54 |
| 体系2 | 10.64 | 体系11 | 11.06 |
| 体系3 | 14.21 | 体系12 | 15.13 |
| 体系4 | 20.33 | 体系13 | 18.50 |
| 体系5 | 10.14 | 体系14 | 15.06 |
| 体系6 | 9.05 | 体系15 | 14.04 |
| 体系7 | 19.01 | 体系16 | 14.50 |
| 体系8 | 16.62 | 体系17 | 9.85 |
| 体系9 | 13.22 | 体系18 | 11.91 |
| 体系 | 碳占比 | 体系 | 碳占比 |
|---|---|---|---|
| 体系1 | 7.00 | 体系10 | 18.54 |
| 体系2 | 10.64 | 体系11 | 11.06 |
| 体系3 | 14.21 | 体系12 | 15.13 |
| 体系4 | 20.33 | 体系13 | 18.50 |
| 体系5 | 10.14 | 体系14 | 15.06 |
| 体系6 | 9.05 | 体系15 | 14.04 |
| 体系7 | 19.01 | 体系16 | 14.50 |
| 体系8 | 16.62 | 体系17 | 9.85 |
| 体系9 | 13.22 | 体系18 | 11.91 |
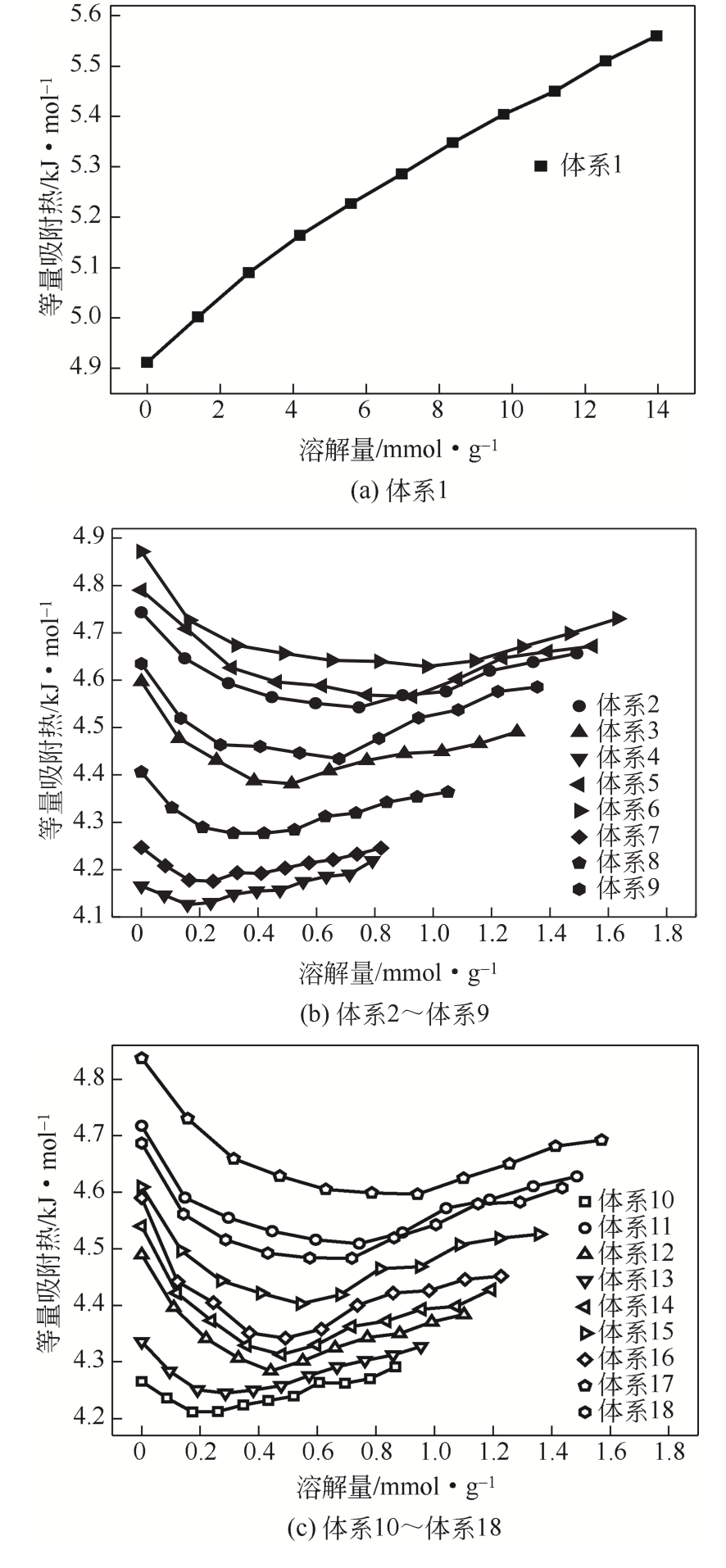
| 体系 | 等量吸附热/kJ·mol-1 | 平均值/kJ·mol-1 |
|---|---|---|
| 体系1 | 4.912~5.564 | 5.268 |
| 体系2 | 4.543~4.743 | 4.609 |
| 体系3 | 4.381~4.597 | 4.451 |
| 体系4 | 4.126~4.219 | 4.163 |
| 体系5 | 4.565~4.790 | 4.639 |
| 体系6 | 4.629~4.872 | 4.689 |
| 体系7 | 4.175~4.247 | 4.210 |
| 体系8 | 4.277~4.406 | 4.323 |
| 体系9 | 4.434~4.635 | 4.514 |
| 体系10 | 4.211~4.291 | 4.246 |
| 体系11 | 4.509~4.718 | 4.577 |
| 体系12 | 4.284~4.490 | 4.354 |
| 体系13 | 4.245~4.336 | 4.285 |
| 体系14 | 4.313~4.540 | 4.387 |
| 体系15 | 4.403~4.609 | 4.480 |
| 体系16 | 4.342~4.590 | 4.421 |
| 体系17 | 4.597~4.837 | 4.664 |
| 体系18 | 4.48~4.687 | 4.551 |
| 体系 | 等量吸附热/kJ·mol-1 | 平均值/kJ·mol-1 |
|---|---|---|
| 体系1 | 4.912~5.564 | 5.268 |
| 体系2 | 4.543~4.743 | 4.609 |
| 体系3 | 4.381~4.597 | 4.451 |
| 体系4 | 4.126~4.219 | 4.163 |
| 体系5 | 4.565~4.790 | 4.639 |
| 体系6 | 4.629~4.872 | 4.689 |
| 体系7 | 4.175~4.247 | 4.210 |
| 体系8 | 4.277~4.406 | 4.323 |
| 体系9 | 4.434~4.635 | 4.514 |
| 体系10 | 4.211~4.291 | 4.246 |
| 体系11 | 4.509~4.718 | 4.577 |
| 体系12 | 4.284~4.490 | 4.354 |
| 体系13 | 4.245~4.336 | 4.285 |
| 体系14 | 4.313~4.540 | 4.387 |
| 体系15 | 4.403~4.609 | 4.480 |
| 体系16 | 4.342~4.590 | 4.421 |
| 体系17 | 4.597~4.837 | 4.664 |
| 体系18 | 4.48~4.687 | 4.551 |
| 体系 | 液体分子所占有的体积/?3 | 自由的体积/?3 | 自由体积分数/% |
|---|---|---|---|
| 体系1 | 21210.66 | 1966.90 | 8.486 |
| 体系2 | 36588.23 | 1594.59 | 4.176 |
| 体系3 | 48823.01 | 1998.35 | 3.932 |
| 体系4 | 78514.80 | 1537.42 | 1.921 |
| 体系5 | 32011.95 | 1489.74 | 4.447 |
| 体系6 | 30797.41 | 1853.06 | 5.675 |
| 体系7 | 76103.78 | 1577.80 | 2.031 |
| 体系8 | 73082.61 | 1849.62 | 2.468 |
| 体系9 | 42138.28 | 1775.48 | 4.043 |
| 体系10 | 67452.92 | 1498.71 | 2.174 |
| 体系11 | 43092.17 | 1916.14 | 4.257 |
| 体系12 | 64227.34 | 1844.46 | 2.792 |
| 体系13 | 71665.13 | 1676.25 | 2.286 |
| 体系14 | 52325.56 | 1549.24 | 2.876 |
| 体系15 | 60674.83 | 1953.51 | 3.119 |
| 体系16 | 60820.33 | 2221.68 | 3.524 |
| 体系17 | 34698.63 | 1844.88 | 5.048 |
| 体系18 | 39594.58 | 1720.04 | 4.163 |
| 体系 | 液体分子所占有的体积/?3 | 自由的体积/?3 | 自由体积分数/% |
|---|---|---|---|
| 体系1 | 21210.66 | 1966.90 | 8.486 |
| 体系2 | 36588.23 | 1594.59 | 4.176 |
| 体系3 | 48823.01 | 1998.35 | 3.932 |
| 体系4 | 78514.80 | 1537.42 | 1.921 |
| 体系5 | 32011.95 | 1489.74 | 4.447 |
| 体系6 | 30797.41 | 1853.06 | 5.675 |
| 体系7 | 76103.78 | 1577.80 | 2.031 |
| 体系8 | 73082.61 | 1849.62 | 2.468 |
| 体系9 | 42138.28 | 1775.48 | 4.043 |
| 体系10 | 67452.92 | 1498.71 | 2.174 |
| 体系11 | 43092.17 | 1916.14 | 4.257 |
| 体系12 | 64227.34 | 1844.46 | 2.792 |
| 体系13 | 71665.13 | 1676.25 | 2.286 |
| 体系14 | 52325.56 | 1549.24 | 2.876 |
| 体系15 | 60674.83 | 1953.51 | 3.119 |
| 体系16 | 60820.33 | 2221.68 | 3.524 |
| 体系17 | 34698.63 | 1844.88 | 5.048 |
| 体系18 | 39594.58 | 1720.04 | 4.163 |
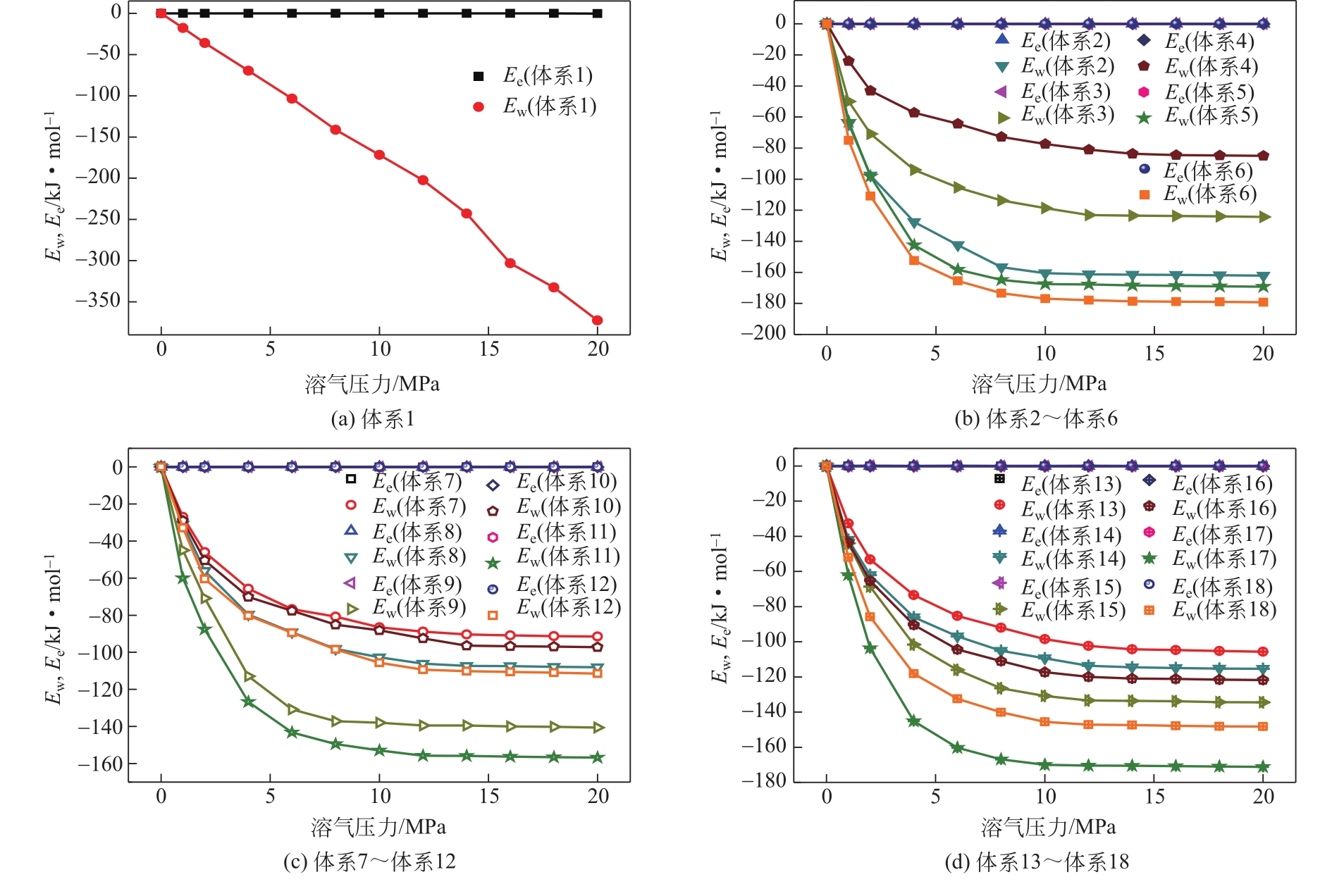
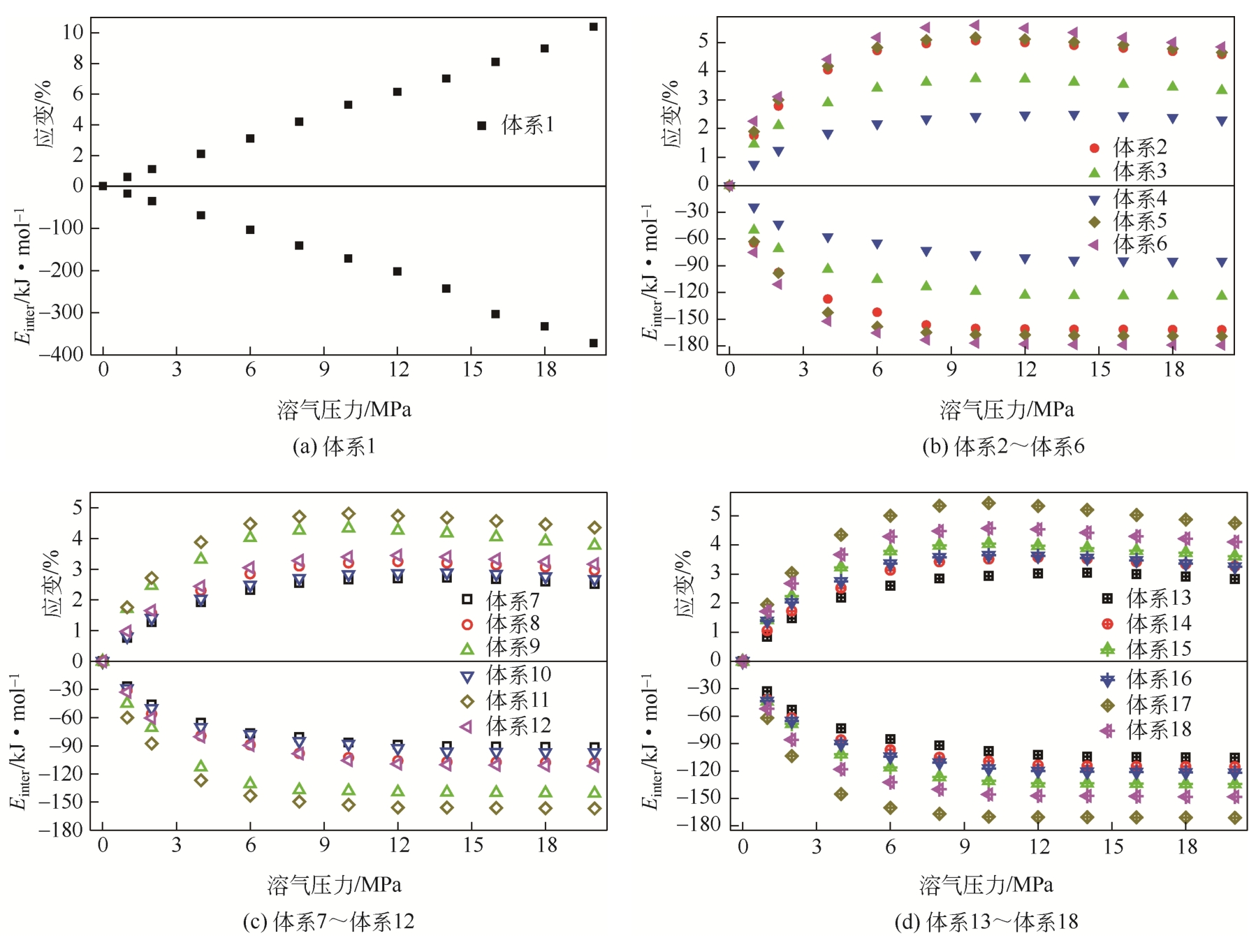
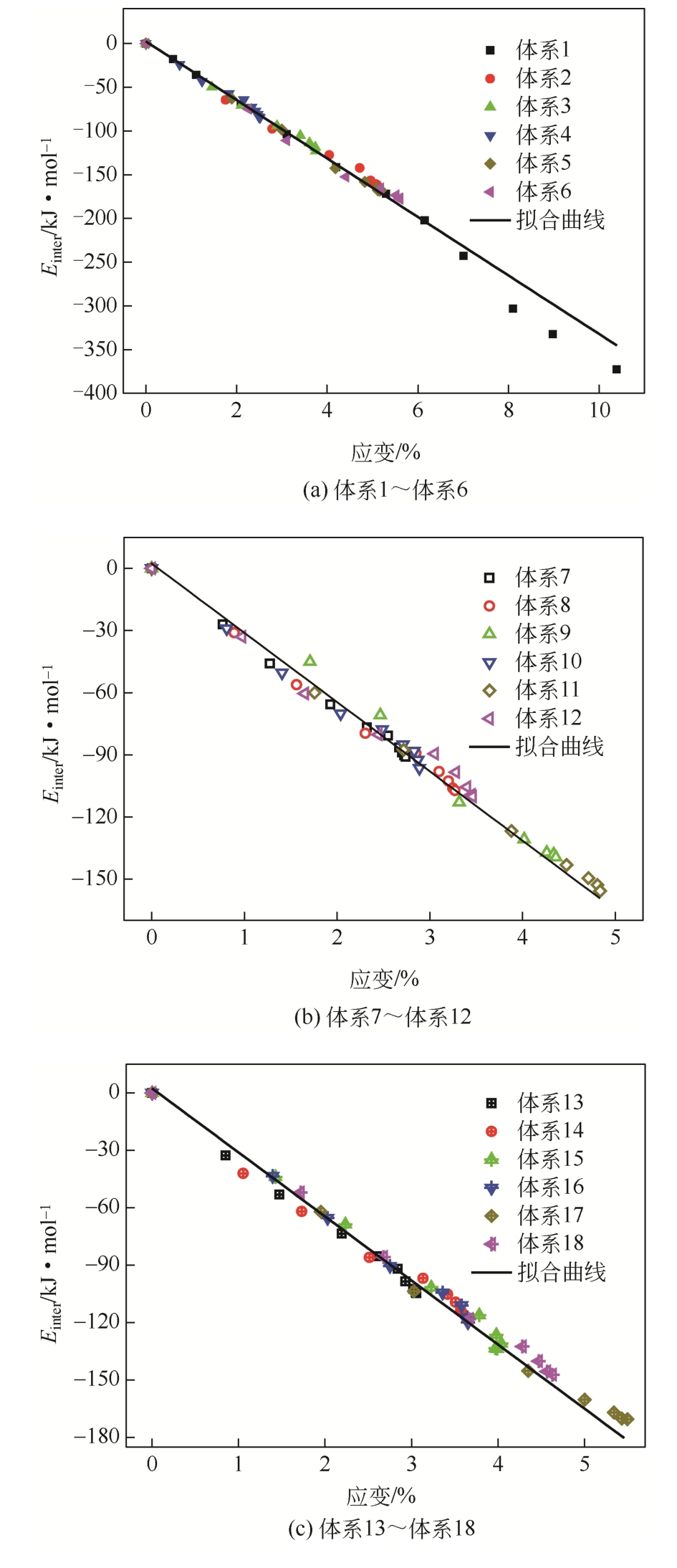
| 体系 | 原油体系体积模量/GPa | 体积膨胀系数 |
|---|---|---|
| 体系1 | 1.925 | 17.382 |
| 体系2 | 2.195 | 15.243 |
| 体系3 | 2.526 | 13.240 |
| 体系4 | 4.001 | 8.361 |
| 体系5 | 2.102 | 15.917 |
| 体系6 | 1.972 | 16.966 |
| 体系7 | 3.731 | 8.966 |
| 体系8 | 2.966 | 11.280 |
| 体系9 | 2.378 | 14.068 |
| 体系10 | 3.725 | 8.980 |
| 体系11 | 2.304 | 14.516 |
| 体系12 | 2.867 | 11.667 |
| 体系13 | 3.449 | 9.700 |
| 体系14 | 2.803 | 11.933 |
| 体系15 | 2.448 | 13.667 |
| 体系16 | 2.625 | 12.746 |
| 体系17 | 2.052 | 16.301 |
| 体系18 | 2.308 | 14.493 |
| 体系 | 原油体系体积模量/GPa | 体积膨胀系数 |
|---|---|---|
| 体系1 | 1.925 | 17.382 |
| 体系2 | 2.195 | 15.243 |
| 体系3 | 2.526 | 13.240 |
| 体系4 | 4.001 | 8.361 |
| 体系5 | 2.102 | 15.917 |
| 体系6 | 1.972 | 16.966 |
| 体系7 | 3.731 | 8.966 |
| 体系8 | 2.966 | 11.280 |
| 体系9 | 2.378 | 14.068 |
| 体系10 | 3.725 | 8.980 |
| 体系11 | 2.304 | 14.516 |
| 体系12 | 2.867 | 11.667 |
| 体系13 | 3.449 | 9.700 |
| 体系14 | 2.803 | 11.933 |
| 体系15 | 2.448 | 13.667 |
| 体系16 | 2.625 | 12.746 |
| 体系17 | 2.052 | 16.301 |
| 体系18 | 2.308 | 14.493 |
| 1 | SHIRYAEVA R, KUDASHEVA F, GIMAEV R, et al. Rheological properties of crude oils with a high resin and asphaltene content: effect of an electromagnetic field and modifiers[J]. Chemistry and Technology of Fuels and Oils, 2006, 42(3): 202-205. |
| 2 | LI Bingfan, LIU Gang, XING Xiao, et al. Molecular dynamics simulation of CO2 dissolution in heavy oil resin-asphaltene[J]. Journal of CO2 Utilization, 2019, 33: 303-310. |
| 3 | HINAI Nasser M, MYERS Matthew B, DEHGHANI Ali M, et al. Effects of oligomers dissolved in CO2 or associated gas on IFT and miscibility pressure with a gas-light crude oil system[J]. Journal of Petroleum Science and Engineering, 2019, 181: 106210. |
| 5 | YANG Hongqun, XU Zhenghe, FAN Maohong, et al. Progress in carbon dioxide separation and capture: a review[J]. Journal of Environmental Sciences, 2008, 20 (1): 14-27. |
| 6 | LEUNG Dennis Y, CARAMANNA Giorgio, MAROTO Mercedes M. An overview of current status of carbon dioxide capture and storage technologies[J]. Renewable and Sustainable Energy Reviews, 2014, 39: 426-443. |
| 7 | SABOORIAN-JOOYBARI H. A novel methodology for simultaneous estimation of gas diffusivity and solubility in bitumens and heavy oils[C]//SPE Heavy Oil Conference, Canada: Society of Petroleum Engineers, 2012. |
| 8 | YANG Fei, LI Chuanxian, XIA Binghuan, et al. Solubility and rheological properties of live crude oils[J]. Advanced Materials Research, 2012, 524-527: 1881-1888. |
| 9 | 鲁彦伯. 溶气原油流变性研究及模型的建立[D]. 东营: 中国石油大学(华东), 2011. |
| LU Yanbo. Research on peological property of live crude oil and foundation of model[D]. Dongying: China University of Petroleum (East China), 2011. | |
| 10 | 李保平. 溶气原油溶气规律及流变规律研究[D]. 青岛: 中国石油大学(华东), 2012. |
| LI Baoping. Research on resolve law and reological law of live crude oil[D]. Qingdao: China University of Petroleum (East China), 2012. | |
| 11 | 夏炳焕. 天然气在原油中的溶解性及溶气原油流变性研究[D]. 青岛: 中国石油大学(华东), 2010. |
| XIA Binghuan. Research on natural gas solubility in crude oil and rheological property of live crude oil[D]. Qingdao: China University of Petroleum (East China), 2010. | |
| 12 | 李传宪, 阎孔尧, 杨爽, 等. CO2溶胀和CH4协同作用下长庆原油流动性的改善[J]. 石油化工高等学校学报, 2017, 30(5): 88-94. |
| LI Chuanxian, YAN Kongyao, YANG Shuang, et al. CO2 swelling and synergistic effect of CH4 on rheological improvement of Changqing crude oil[J]. Journal of Petrochemical Universities, 2017, 30(5): 88-94. | |
| 13 | RABITZ Herschel. Strong-arming molecular dynamics[J]. Science, 2006, 314 (5797): 264-265. |
| 14 | DONG Zejiao, LIU Zhiyang, WANG Peng, et al. Nanostructure characterization of asphalt-aggregate interface through molecular dynamics simulation and atomic force microscopy[J]. Fuel, 2017, 189: 155-163. |
| 15 | XU Meng, YI Junyan, FENG Decheng, et al. Analysis of adhesive characteristics of asphalt based on atomic force microscopy and molecular dynamics simulation[J]. ACS Applied Materials & Interfaces, 2016, 8 (19): 12393-12403. |
| 16 | YANG Xiaoning, ZHANG Cuijuan. Structure and diffusion behavior of dense carbon dioxide fluid in clay-like slit pores by molecular dynamics simulation[J]. Chemical Physics Letters, 2005, 407(4/5/6): 427-432. |
| 17 | JIN Zhehui, FIROOZABADI Abbas. Effect of water on methane and carbon dioxide sorption in clay minerals by Monte Carlo simulations[J]. Fluid Phase Equilibria, 2014, 382: 10-20. |
| 18 | BROCHARD Laurent, VANDAMME Matthieu, PELLENQ Roland, et al. Adsorption-induced deformation of microporous materials: coal swelling induced by CO2-CH4 competitive adsorption[J]. Langmuir, 2012, 28(5): 2659-2670. |
| 19 | OTTIGER Stefan, PINI Ronny, STORTI Giuseppe, et al. Competitive adsorption equilibria of CO2 and CH4 on a dry coal[J]. Adsorption, 2008, 14(4/5): 539-556. |
| 20 | GEORGE J D ST, BARAKAT M A. The change in effective stress associated with shrinkage from gas desorption in coal[J]. International Journal of Coal Geology, 2001, 45(2/3): 105-113. |
| 21 | FAURE Francois, ROUSSEAU Bernard, VERONIQUE Lachet, et al. Molecular simulation of the solubility and diffusion of carbon dioxide and hydrogen sulfide in polyethylene melts[J]. Fluid Phase Equilibria, 2007, 261(1/2): 168-175. |
| 22 | ZHANG Junfang, PAN Zhejun, LIU Keyu, et al. Molecular simulation of CO2 solubility and its effect on octane swelling[J]. Energy & Fuels, 2013, 27 (5): 2741-2747. |
| 23 | 刘沺. 溶气原油流变性研究及流变模型的建立[D]. 东营: 中国石油大学(华东), 2015. |
| LIU Tian. Research on reological property of live oil and establishment of reological model[D]. Dongying: China University of Petroleum (East China), 2015. | |
| 24 | 白帆. 原油组成对结蜡规律影响的研究[D]. 东营: 中国石油大学(华东), 2014. |
| BAI Fan. Effect of crude oil composition on wax deposition[D]. Dongying: China University of Petroleum (East China), 2014. | |
| 25 | JENNINGS P W. Binder characterization and evaluation by nuclear magnetic resonance spectroscopy[R]. Washington D C: Strategic Highway Research Program of National Research Council, 1993: 1-21. |
| 26 | BOUHADDA Y, BORMANN D, SHEU E, et al. Characterization of Algerian Hassi-Messaoud asphaltene structure using Raman spectrometry and X-ray diffraction[J]. Fuel, 2007, 86 (12/13): 1855-1864. |
| 27 | KOWALEWSKI I, VANDENBROUCKE M, HUC A Y, et al. Preliminary results on molecular modeling of asphaltenes using structure elucidation programs in conjunction with molecular simulation programs[J]. Energy & Fuels, 1996, 10 (1): 97-107. |
| 28 | CLEVER H L, YOMG C L. Solubility data series v27/28[M]. Oxford: The International Union of Pure and Applied Chemistry, Pergamon Press, 1987. |
| 29 | EVERETT Douglas H, POWL John C. Adsorption in slit-like and cylindrical micropores in the henry's law region. A model for the microporosity of carbons[J]. Journal of the Chemical Society, Faraday Transactions 1, 1976, 72: 619-636. |
| 30 | 田力. CH4、CO2在烟煤结构模型中吸附的分子模拟研究[D]. 东营: 中国石油大学(华东), 2014. |
| TIAN Li. Molecular simulation study on adsorption of CH4 and CO2 in bituminous coal structure model[D]. Dongying: China University of Petroleum (East China), 2014. | |
| 31 | BILLEMONT Pierre, COASNE Benoit, DE WEIRELD Guy. An experimental and molecular simulation study of the adsorption of carbon dioxide and methane in nanoporous carbons in the presence of water[J]. Langmuir, 2011, 27(3): 1015-1024. |
| 32 | BILLEMONT Pierre, COASNE Benoit, DE WEIRELD Guy. Adsorption of carbon dioxide, methane, and their mixtures in porous carbons: effect of surface chemistry, water content, and pore disorder[J]. Langmuir, 2013, 29(10): 3328-3338. |
| 33 | NODZENSKI Adam. Sorption and desorption of gases (CH4, CO2) on hard coal and active carbon at elevated pressures[J]. Fuel, 1998, 77(11): 1243-1246. |
| 34 | 傅献彩, 沈文霞, 姚天扬. 物理化学[M]. 北京: 高等教育出版牡, 2006. |
| FU Xiancai, SHEN Wenxia, YAO Tianyang. Physical chemistry[M]. Beijing: Higher Education Press, 2006. | |
| 35 | Thomas G FOX, FLORY Paul J. Viscosity-molecular eight and viscosity-temperature relationships for polystyrene and polyisobutylene1, 2[J]. Journal of the American Chemical Society, 1948, 70(7): 2384-2395. |
| 36 | HE Yabin, ZHOU Wei, QIAN Guodong, et al. Methane storage in metal-organic frameworks[J]. Chemical Society Reviews, 2014, 43 (16): 5657-5678. |
| 37 | LI Ben, PAN Fusheng, FANG Zhiping, et al. Molecular dynamics simulation of diffusion behavior of benzene/water in PDMS-Calix[4]arene hybrid pervaporation membranes[J]. Industrial & Engineering Chemistry Research, 2008, 47 (13): 4440-4447. |
| 38 | COUSSY O. Poromechanics[M]. Chichester, UK:John Wiley & Sons, 2004. |
| 39 | BROCHARD L, VANDAMME M, PELLENQ J M. Poromechanics of microporous media[J]. Journal of the Mechanics and Physics of Solids, 2012, 60(4): 606-622. |
| 40 | SONG J, CURTIN W A. Atomic mechanism and prediction of hydrogen embrittlement in iron[J]. Nature Materials, 2013, 12(2): 145-151. |
| 41 | ZHOU Xiao, MARCHANG Daniel, MCDOWELL David L, et al. Chemomechanical origin of hydrogen trapping at grain boundaries in fcc metals[J]. Physical Review Letters, 2016, 116(7): 075502. |
| [1] | CUI Shoucheng, XU Hongbo, PENG Nan. Simulation analysis of two MOFs materials for O2/He adsorption separation [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 382-390. |
| [2] | LAI Shini, JIANG Lixia, LI Jun, HUANG Hongyu, KOBAYASHI Noriyuki. Research progress of ammonia blended fossil fuel [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4603-4615. |
| [3] | HUANG Yufei, LI Ziyi, HUANG Yangqiang, JIN Bo, LUO Xiao, LIANG Zhiwu. Research progress on catalysts for photocatalytic CO2 and CH4 reforming [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4247-4263. |
| [4] | ZHAO Jian, ZHUO Zewen, DONG Hang, GAO Wenjian. A new method for observation of microstructure of waxy crude oil and its emulsion system [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4372-4384. |
| [5] | WU Ya, ZHAO Dan, FANG Rongmiao, LI Jingyao, CHANG Nana, DU Chunbao, WANG Wenzhen, SHI Jun. Research progress on highly efficient demulsifiers for complex crude oil emulsions and their applications [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4398-4413. |
| [6] | XI Yonglan, WANG Chengcheng, YE Xiaomei, LIU Yang, JIA Zhaoyan, CAO Chunhui, HAN Ting, ZHANG Yingpeng, TIAN Yu. Research progress on the application of micro/nano bubbles in anaerobic digestion [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4414-4423. |
| [7] | ZHANG Kai, LYU Qiunan, LI Gang, LI Xiaosen, MO Jiamei. Morphology and occurrence characteristics of methane hydrates in the mud of the South China Sea [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3865-3874. |
| [8] | LIU Yang, YE Xiaomei, MIAO Xiao, WANG Chengcheng, JIA Zhaoyan, CAO Chunhui, XI Yonglan. Pilot-scale process research on dry digestion of rural organic household waste under ammonia stress [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3847-3854. |
| [9] | OUYANG Sufang, ZHOU Daowei, HUANG Wei, JIA Feng. Research progress on novel anti-migration rubber antioxidants [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3708-3719. |
| [10] | YANG Farong, GU Lili, LIU Yang, LI Weixue, CAI Jieyun, WANG Huiping. Preparation and application of molecularly imprinted polymers of terbutylazine assisted by computer simulation [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 3157-3166. |
| [11] | ZHAO Yi, YANG Zhen, ZHANG Xinwei, WANG Gang, YANG Xuan. Molecular simulation of self-healing behavior of asphalt under different crack damage and healing temperature [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 3147-3156. |
| [12] | SUN Zhengnan, LI Hongjing, JING Guolin, ZHANG Funing, YAN Biao, LIU Xiaoyan. Application of EVA and its modified polymer in crude oil pour point depressant field [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 2987-2998. |
| [13] | LI Ruidong, HUANG Hui, TONG Guohu, WANG Yueshe. Hygroscopic properties and corrosion behavior of ammonium salt in a crude oil distillation column [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 2809-2818. |
| [14] | RUAN Peng, YANG Runnong, LIN Zirong, SUN Yongming. Advances in catalysts for catalytic partial oxidation of methane to syngas [J]. Chemical Industry and Engineering Progress, 2023, 42(4): 1832-1846. |
| [15] | HE Yangdong, CHANG Honggang, WANG Dan, CHEN Changjie, LI Yaxin. Development of methane pyrolysis based on molten metal technology for coproduction of hydrogen and solid carbon products [J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1270-1280. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||