Chemical Industry and Engineering Progress ›› 2021, Vol. 40 ›› Issue (8): 4196-4204.DOI: 10.16085/j.issn.1000-6613.2021-0097
• Chemical processes and equipment • Previous Articles Next Articles
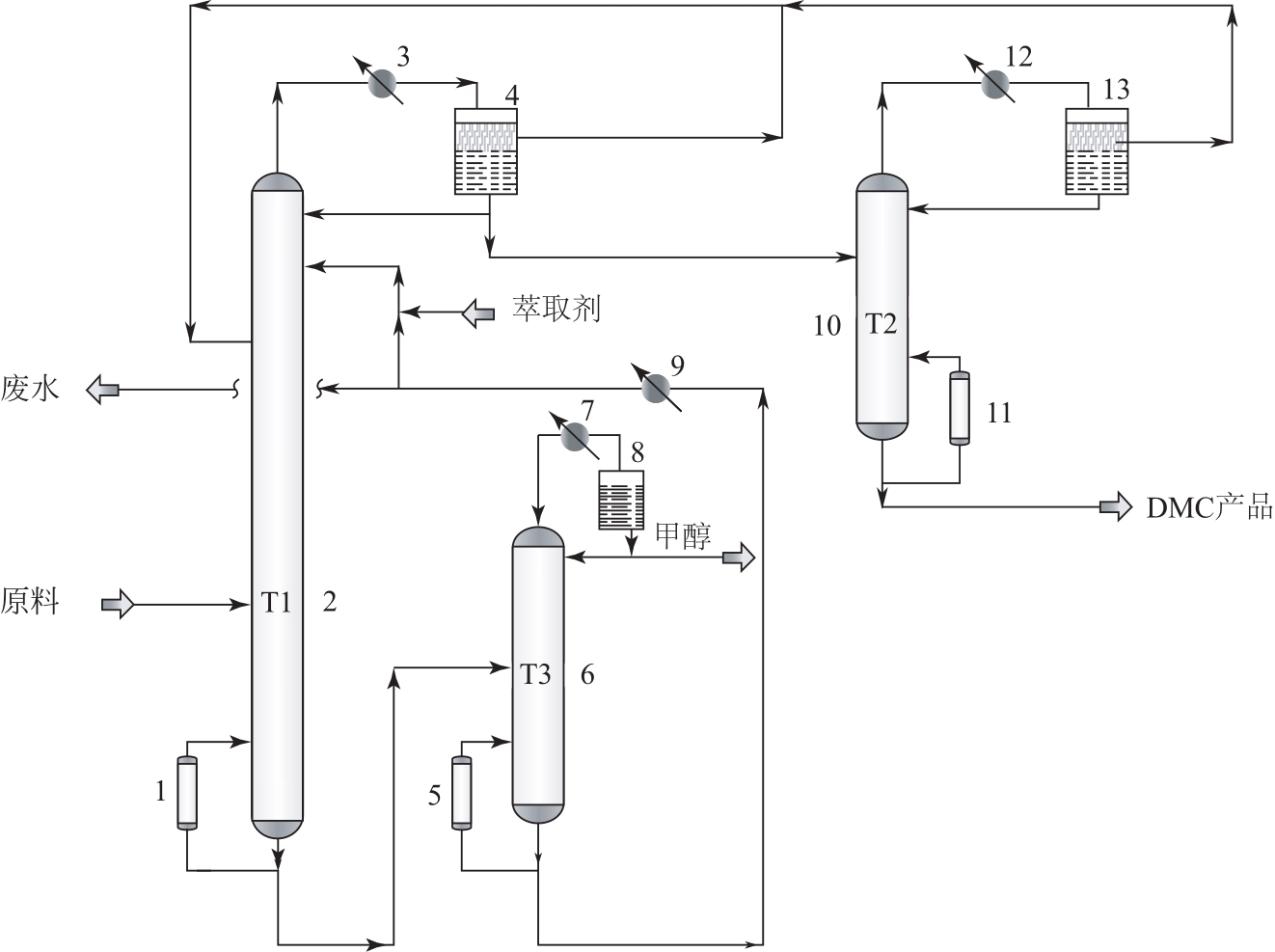
Extractive distillation separation process of DMC-methanol-water ternary mixture
WANG Yuchun1( ), ZHANG Zhihao1, GAO Yuan2, LI Zhong1, ZHENG Huayan1(
), ZHANG Zhihao1, GAO Yuan2, LI Zhong1, ZHENG Huayan1( )
)
- 1.State Key Laboratory of Clean and Efficient Coal Utilization, Taiyuan University of Technology, Taiyuan 030024, Shanxi, China
2.Chemical Division, Lu’an Chemical Group Co. , Ltd. , Changzhi 046299, Shanxi, China
-
Received:2021-01-15Online:2021-08-12Published:2021-08-05 -
Contact:ZHENG Huayan
DMC-甲醇-水三元混合物的萃取精馏分离工艺
王玉春1( ), 张志浩1, 高源2, 李忠1, 郑华艳1(
), 张志浩1, 高源2, 李忠1, 郑华艳1( )
)
- 1.太原理工大学省部共建煤基能源清洁高效利用国家重点实验室,山西 太原 030024
2.潞安化工集团有限公司化工事业部,山西 长治 046299
-
通讯作者:郑华艳 -
作者简介:王玉春(1990—),男,硕士研究生,研究方向为碳一化学。E-mail:Aruinen@sina.cn 。 -
基金资助:国家自然科学基金(U1510203);山西省重点研发计划(国际科技合作)(201803D421011)
CLC Number:
Cite this article
WANG Yuchun, ZHANG Zhihao, GAO Yuan, LI Zhong, ZHENG Huayan. Extractive distillation separation process of DMC-methanol-water ternary mixture[J]. Chemical Industry and Engineering Progress, 2021, 40(8): 4196-4204.
王玉春, 张志浩, 高源, 李忠, 郑华艳. DMC-甲醇-水三元混合物的萃取精馏分离工艺[J]. 化工进展, 2021, 40(8): 4196-4204.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2021-0097
| 序号 | 萃取剂 | 沸点/℃ | 密度/g·mL-1 | 熔点/℃ | 萃取精馏塔原料 | 塔顶馏分 | 参考文献 |
|---|---|---|---|---|---|---|---|
| 1 | 糠醛 | 161.8 | 1.16 | -36.5 | DMC-甲醇 | 甲醇 | [ |
| 2 | 邻二甲苯 | 144.4 | 0.88 | -25.2 | DMC-甲醇 | 甲醇 | [ |
| 3 | 乙酸己酯 | 171.5 | 0.87 | -80.9 | DMC-甲醇 | 甲醇 | [ |
| 4 | 碳酸丙烯酯 | 242.0 | 1.20 | -48.8 | DMC-甲醇 | 甲醇 | [ |
| 5 | 碳酸乙烯酯 | 248.0 | 1.32 | 38.0 | DMC-甲醇 | 甲醇 | [ |
| 6 | 草酸二甲酯 | 163.5 | 1.15 | 54.0 | DMC-甲醇 | 甲醇 | [ |
| 7 | 水 | 100.0 | 1.00 | 0.0 | DMC-甲醇-水(或DMC-甲醇) | DMC-水混合物 | [ |
| 8 | 苯酚 | 181.9 | 1.07 | 43.0 | DMC-甲醇 | 甲醇 | [ |
| 9 | 苯胺 | 184.4 | 1.02 | -6.2 | DMC-甲醇 | 甲醇 | [ |
| 10 | 乙二醇 | 197.3 | 1.11 | -12.9 | DMC-甲醇 | 甲醇 | [ |
| 序号 | 萃取剂 | 沸点/℃ | 密度/g·mL-1 | 熔点/℃ | 萃取精馏塔原料 | 塔顶馏分 | 参考文献 |
|---|---|---|---|---|---|---|---|
| 1 | 糠醛 | 161.8 | 1.16 | -36.5 | DMC-甲醇 | 甲醇 | [ |
| 2 | 邻二甲苯 | 144.4 | 0.88 | -25.2 | DMC-甲醇 | 甲醇 | [ |
| 3 | 乙酸己酯 | 171.5 | 0.87 | -80.9 | DMC-甲醇 | 甲醇 | [ |
| 4 | 碳酸丙烯酯 | 242.0 | 1.20 | -48.8 | DMC-甲醇 | 甲醇 | [ |
| 5 | 碳酸乙烯酯 | 248.0 | 1.32 | 38.0 | DMC-甲醇 | 甲醇 | [ |
| 6 | 草酸二甲酯 | 163.5 | 1.15 | 54.0 | DMC-甲醇 | 甲醇 | [ |
| 7 | 水 | 100.0 | 1.00 | 0.0 | DMC-甲醇-水(或DMC-甲醇) | DMC-水混合物 | [ |
| 8 | 苯酚 | 181.9 | 1.07 | 43.0 | DMC-甲醇 | 甲醇 | [ |
| 9 | 苯胺 | 184.4 | 1.02 | -6.2 | DMC-甲醇 | 甲醇 | [ |
| 10 | 乙二醇 | 197.3 | 1.11 | -12.9 | DMC-甲醇 | 甲醇 | [ |
温度 /℃ | 实验值[ | 内置二元交互作用 参数计算值 | 回归二元交互作用参数计算值 | |||||
|---|---|---|---|---|---|---|---|---|
有机相 /% | 水相 /% | 有机相 /% | 水相 /% | 有机相 /% | 水相 /% | |||
| 10 | 97.61 | 5.27 | 97.21 | 14.36 | 97.60 | 5.41 | ||
| 15 | 97.39 | 5.27 | 97.07 | 13.86 | 97.38 | 5.38 | ||
| 20 | 97.20 | 5.27 | 96.91 | 13.43 | 97.14 | 5.42 | ||
| 30 | 96.57 | 5.73 | 96.58 | 12.71 | 96.60 | 5.67 | ||
| 40 | 95.93 | 6.63 | 96.23 | 12.17 | 95.97 | 6.16 | ||
| 50 | 95.24 | 6.63 | 95.85 | 11.75 | 95.23 | 6.91 | ||
| 60 | 94.36 | 7.96 | 95.45 | 11.45 | 94.37 | 7.96 | ||
温度 /℃ | 实验值[ | 内置二元交互作用 参数计算值 | 回归二元交互作用参数计算值 | |||||
|---|---|---|---|---|---|---|---|---|
有机相 /% | 水相 /% | 有机相 /% | 水相 /% | 有机相 /% | 水相 /% | |||
| 10 | 97.61 | 5.27 | 97.21 | 14.36 | 97.60 | 5.41 | ||
| 15 | 97.39 | 5.27 | 97.07 | 13.86 | 97.38 | 5.38 | ||
| 20 | 97.20 | 5.27 | 96.91 | 13.43 | 97.14 | 5.42 | ||
| 30 | 96.57 | 5.73 | 96.58 | 12.71 | 96.60 | 5.67 | ||
| 40 | 95.93 | 6.63 | 96.23 | 12.17 | 95.97 | 6.16 | ||
| 50 | 95.24 | 6.63 | 95.85 | 11.75 | 95.23 | 6.91 | ||
| 60 | 94.36 | 7.96 | 95.45 | 11.45 | 94.37 | 7.96 | ||
| i | j | aij | aji | bij | bji | 来源 |
|---|---|---|---|---|---|---|
| DMC | 水 | 41.2183 | -74.0176 | -2741.0 | 3419.72 | 本文回归 |
| DMC | 甲醇 | 0 | 0 | -349.494 | 18.91 | [ |
| 甲醇 | 水 | 0 | 0 | 165.26 | -254.731 | [ |
| i | j | aij | aji | bij | bji | 来源 |
|---|---|---|---|---|---|---|
| DMC | 水 | 41.2183 | -74.0176 | -2741.0 | 3419.72 | 本文回归 |
| DMC | 甲醇 | 0 | 0 | -349.494 | 18.91 | [ |
| 甲醇 | 水 | 0 | 0 | 165.26 | -254.731 | [ |
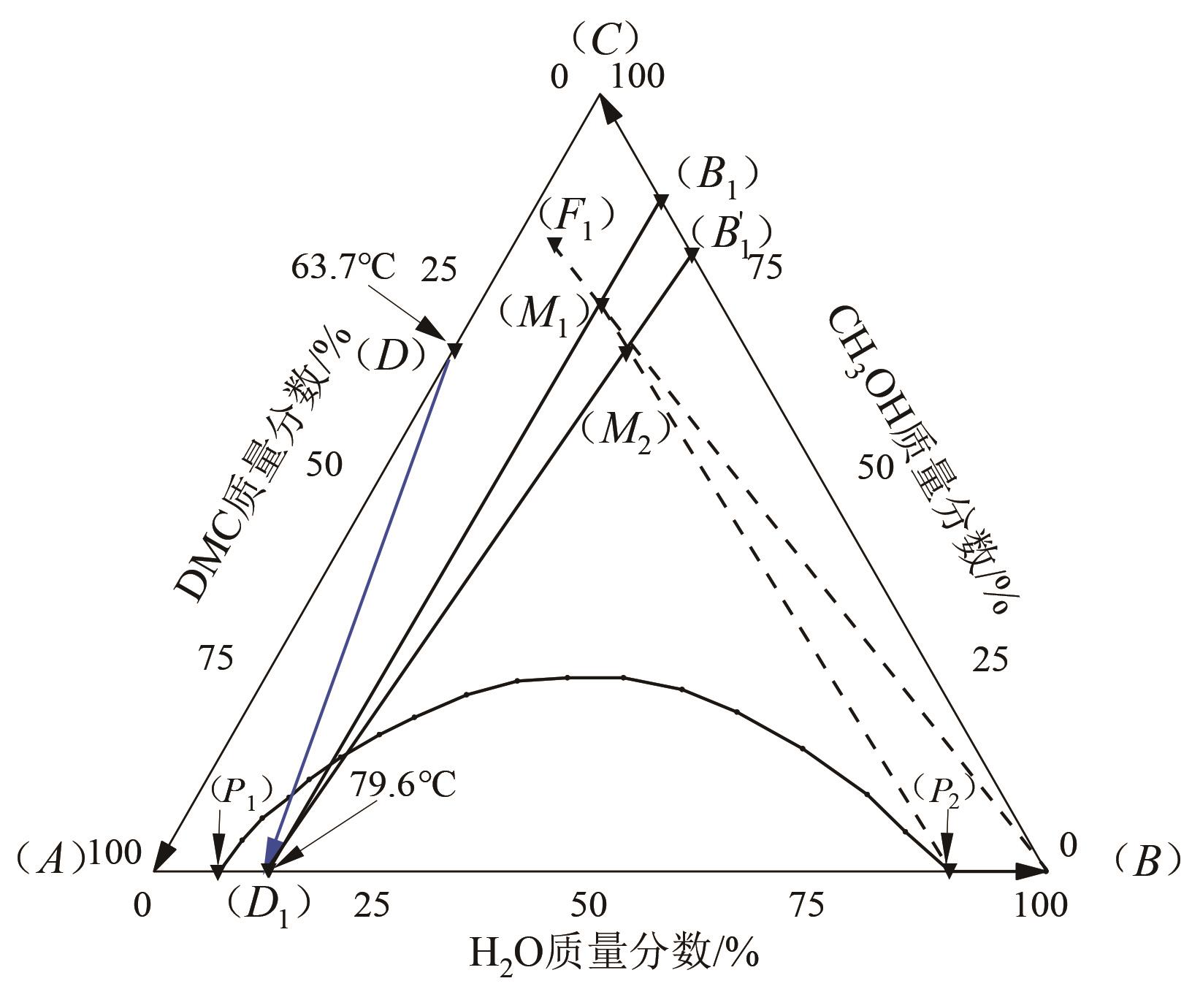
| 质量分数/% | 共沸温度 /℃ | 共沸类型 | 备注 | ||
|---|---|---|---|---|---|
| 甲醇 | DMC | 水 | |||
| 67.2 | 32.8 | 0.0 | 63.7 | 均相 | 计算值 |
| 70.0 | 30.0 | 0.0 | 63.5 | 文献[ | |
| 0.0 | 86.8 | 13.2 | 79.6 | 非均相 | 计算值 |
| 0.0 | 89.0 | 11.0 | 77.5 | 文献[ | |
| 质量分数/% | 共沸温度 /℃ | 共沸类型 | 备注 | ||
|---|---|---|---|---|---|
| 甲醇 | DMC | 水 | |||
| 67.2 | 32.8 | 0.0 | 63.7 | 均相 | 计算值 |
| 70.0 | 30.0 | 0.0 | 63.5 | 文献[ | |
| 0.0 | 86.8 | 13.2 | 79.6 | 非均相 | 计算值 |
| 0.0 | 89.0 | 11.0 | 77.5 | 文献[ | |
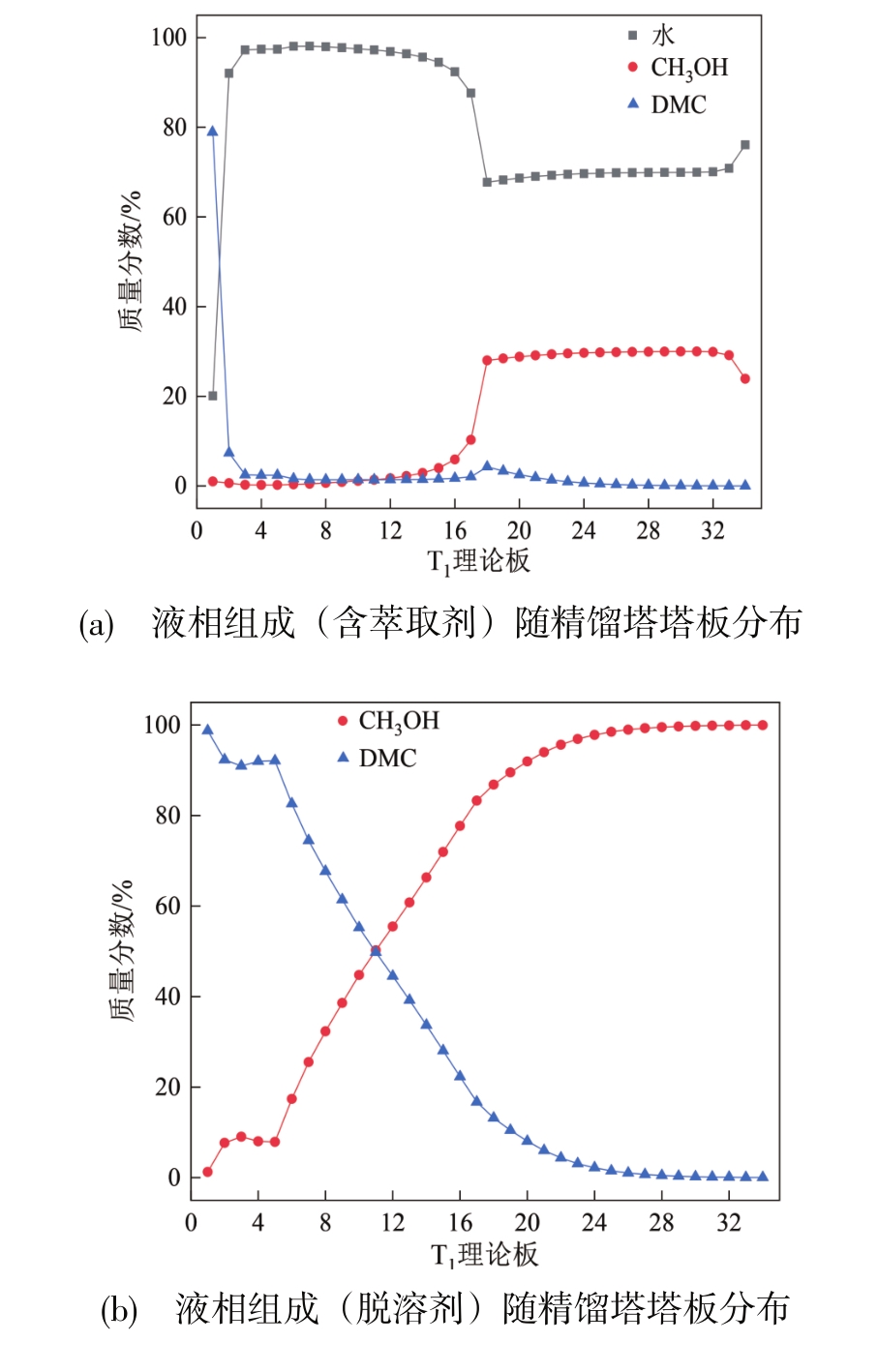
| 设备所属工艺 | 理论板数 | 原料进料位置 | 操作压力 /MPa | 塔顶温度 /℃ | 塔底温度 /℃ | 回流比 | 冷凝器负荷 /kW | 再沸器负荷 /kW | 总冷凝负荷 /kW | 总加热负荷 /kW |
|---|---|---|---|---|---|---|---|---|---|---|
| 萃取精馏 | ||||||||||
| T1 | 34 | 18 | 常压 | 78.7 | 85.9 | 1.5 | 110.5 | 212.3 | 888.7 | 898.2 |
| T2 | 8 | 2 | 常压 | 78.7 | 88.0 | 0.9 | 16.3 | 19.7 | ||
| T3 | 32 | 15 | 常压 | 64.7 | 101.4 | 1.6 | 761.9 | 666.2 | ||
| 变压精馏 | ||||||||||
| T4 | 42 | 35 | 常压 | 64.0 | 99.7 | 1.5 | 653.9 | 664.1 | 1683.4 | 1704.0 |
| T5 | 34 | 11 | 常压 | 63.4 | 65.4 | 3.0 | 1029.5 | 1007.7 | ||
| T6 | 32 | 22 | 1.2 | 144.0 | 189.0 | 1.8 | 541.5 | 573.7 |
| 设备所属工艺 | 理论板数 | 原料进料位置 | 操作压力 /MPa | 塔顶温度 /℃ | 塔底温度 /℃ | 回流比 | 冷凝器负荷 /kW | 再沸器负荷 /kW | 总冷凝负荷 /kW | 总加热负荷 /kW |
|---|---|---|---|---|---|---|---|---|---|---|
| 萃取精馏 | ||||||||||
| T1 | 34 | 18 | 常压 | 78.7 | 85.9 | 1.5 | 110.5 | 212.3 | 888.7 | 898.2 |
| T2 | 8 | 2 | 常压 | 78.7 | 88.0 | 0.9 | 16.3 | 19.7 | ||
| T3 | 32 | 15 | 常压 | 64.7 | 101.4 | 1.6 | 761.9 | 666.2 | ||
| 变压精馏 | ||||||||||
| T4 | 42 | 35 | 常压 | 64.0 | 99.7 | 1.5 | 653.9 | 664.1 | 1683.4 | 1704.0 |
| T5 | 34 | 11 | 常压 | 63.4 | 65.4 | 3.0 | 1029.5 | 1007.7 | ||
| T6 | 32 | 22 | 1.2 | 144.0 | 189.0 | 1.8 | 541.5 | 573.7 |
| 设备所属工艺 | 塔费用 /USD | 换热器费用 /USD | 设备总费用 /USD | 冷却水费用 /USD·a-1 | 蒸汽费用 /USD·a-1 | 总操作费用 /USD·a-1 | TAC /USD·a-1 |
|---|---|---|---|---|---|---|---|
| 萃取精馏 | |||||||
| T1 | 22897.1 | 56379.8 | 79276.8 | 12688.3 | 42481.7 | 55170.0 | 342347.6 |
| T2 | 7448.4 | 18744.7 | 26193.1 | 1871.7 | 3942.0 | 5813.7 | |
| T3 | 43847.7 | 153533.1 | 197380.8 | 87485.6 | 133308.2 | 220793.8 | |
| 变压精馏 | |||||||
| T4 | 34958.0 | 145117.1 | 180075.1 | 75084.5 | 132888.0 | 207972.5 | 668796.8 |
| T5 | 60456.0 | 190275.0 | 250731.0 | 118155.5 | 93447.8 | 211603.4 | |
| T6 | 30253.2 | 50068.6 | 80321.8 | 0.0 | 146995.4 | 146995.4 |
| 设备所属工艺 | 塔费用 /USD | 换热器费用 /USD | 设备总费用 /USD | 冷却水费用 /USD·a-1 | 蒸汽费用 /USD·a-1 | 总操作费用 /USD·a-1 | TAC /USD·a-1 |
|---|---|---|---|---|---|---|---|
| 萃取精馏 | |||||||
| T1 | 22897.1 | 56379.8 | 79276.8 | 12688.3 | 42481.7 | 55170.0 | 342347.6 |
| T2 | 7448.4 | 18744.7 | 26193.1 | 1871.7 | 3942.0 | 5813.7 | |
| T3 | 43847.7 | 153533.1 | 197380.8 | 87485.6 | 133308.2 | 220793.8 | |
| 变压精馏 | |||||||
| T4 | 34958.0 | 145117.1 | 180075.1 | 75084.5 | 132888.0 | 207972.5 | 668796.8 |
| T5 | 60456.0 | 190275.0 | 250731.0 | 118155.5 | 93447.8 | 211603.4 | |
| T6 | 30253.2 | 50068.6 | 80321.8 | 0.0 | 146995.4 | 146995.4 |
| 1 | ESAN A O, ADEYEMI A D, GANESAN S. A review on the recent application of dimethyl carbonate in sustainable biodiesel production[J]. Journal of Cleaner Production, 2020, 257: 120561. |
| 2 | SELVA M, PEROSA A, RODRÍGUEZ-PADRÓN D, et al. Applications of dimethyl carbonate for the chemical upgrading of biosourced platform chemicals[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(7): 6471-6479. |
| 3 | ARICÒ F, TUNDO P. Dimethyl carbonate as a modern green reagent and solvent[J]. Russian Chemical Reviews, 2010, 79(6): 479-489. |
| 4 | SCHÄFFNER B, SCHÄFFNER F, VEREVKIN S P, et al. Organic carbonates as solvents in synthesis and catalysis[J]. Chemical Reviews, 2010, 110(8): 4554-4581. |
| 5 | DING X S, DONG X M, KUANG D T, et al. Highly efficient catalyst PdCl2-CuCl2-KOAc/AC@Al2O3 for gas-phase oxidative carbonylation of methanol to dimethyl carbonate: preparation and reaction mechanism[J]. Chemical Engineering Journal, 2014, 240: 221-227. |
| 6 | REN J, GUO C J, YANG L L, et al. Synthesis of dimethyl carbonate over starch-based carbon-supported Cu nanoparticles catalysts[J]. Chinese Journal of Catalysis, 2013, 34(9): 1734-1744. |
| 7 | TAN H Z, CHEN Z N, XU Z N, et al. Synthesis of high-performance and high-stability Pd(II)/NaY catalyst for CO direct selective conversion to dimethyl carbonate by rational design[J]. ACS Catalysis, 2019, 9(4): 3595-3603. |
| 8 | SHI L, WANG S J, WONG D S H, et al. Novel process design of synthesizing propylene carbonate for dimethyl carbonate production by indirect alcoholysis of urea[J]. Industrial & Engineering Chemistry Research, 2017, 56(40): 11531-11544. |
| 9 | RODRı́GUEZ A, CANOSA J, DOMı́NGUEZ A, et al. Vapour-liquid equilibria of dimethyl carbonate with linear alcohols and estimation of interaction parameters for the UNIFAC and ASOG method[J]. Fluid Phase Equilibria, 2002, 201(1): 187-201. |
| 10 | BAIK J H, VENTE J F, MOTELICA A. Method and apparatus for purification of dimethyl carbonate using pervaporation: US10377696[P]. 2019-08-13. |
| 11 | PASSONI G A. Purification of dimethyl carbonate: DE2450856[P]. 1973-10-26. |
| 12 | 卫红梅, 王峰, 赵宁, 等. 加压-常压双塔分离碳酸二甲酯-甲醇共沸物的动态模拟研究[J]. 石油化工, 2014, 43(2): 169-175. |
| WEI H M, WANG F, ZHAO N, et al. Dynamic simulation for separation of dimethyl carbonate-methanol azeotrope by pressurized-atmospheric distillation[J]. Petrochemical Technology, 2014, 43(2): 169-175. | |
| 13 | 栾锋, 刘焕香, 刘满仓, 等. 碳酸二甲酯-甲醇共沸物萃取精馏过程模拟研究[J]. 兰州大学学报(自然科学版), 2003, 39(5): 53-56. |
| LUAN F, LIU H X, LIU M C, et al. Extractive distillation process simulation for DMC-MEOH azeotropic system[J]. Journal of Lanzhou University (Natural Sciences), 2003, 39(5): 53-56. | |
| 14 | 李群生, 朱炜, 付永泉, 等. 常压下甲醇-碳酸二甲酯汽液平衡测定及其萃取剂选择[J]. 化学工程, 2011, 39(8): 44-47. |
| LI Q S, ZHU W, FU Y Q, et al. Vapor-liquid equilibrium of methanol-dimethyl carbonate at normal pressure and selection of extractant[J]. Chemical Engineering, 2011, 39(8): 44-47. | |
| 15 | GINNASI A, PASSONI G. Extractive distillation of a dimethyl carbonate feed with water: US3963586[P]. 1976-06-15. |
| 16 | HSU K Y, HSIAO Y C, CHIEN I L. Design and control of dimethyl carbonate-methanol separation via extractive distillation in the dimethyl carbonate reactive-distillation process[J]. Industrial & Engineering Chemistry Research, 2010, 49(2): 735-749. |
| 17 | 张军亮, 王峰, 彭伟才, 等. 分离碳酸二甲酯和甲醇的常压-加压精馏工艺流程的模拟[J]. 石油化工, 2010, 39(6): 646-650. |
| ZHANG J L, WANG F, PENG W C, et al. Process simulation for separation of dimethyl carbonate and methanol through atmospheric-pressurized rectification[J]. Petrochemical Technology, 2010, 39(6): 646-650. | |
| 18 | YEH A I, BERG L, WARREN K J. The separation of acetone-methanol mixture by extractive distillation[J]. Chemical Engineering Communications, 1988, 68(1): 69-79. |
| 19 | 陈洪钫, 刘家祺. 化工分离过程[M]. 2版. 北京: 化学工业出版社, 2014: 78. |
| CHEN H F, LIU J Q. Chemical separation process[M]. Beijing: Chemical Industry Press, 2014: 78. | |
| 20 | DE LA TORRE J, CHÁFER A, BERNA A, et al. Liquid-liquid equlibria of the system dimethyl carbonate+methanol+water at different temperatures[J]. Fluid Phase Equilibria, 2006, 247(1/2): 40-46. |
| 21 | 王奎. 煤制乙二醇工艺技术及工艺流程简述[J]. 广东化工, 2017, 44(17): 240-241. |
| WANG K. A brief review of the technology and process of coal to ethylene glycol[J]. Guangdong Chemical Industry, 2017, 44(17): 240-241. | |
| 22 | WANG Y L, CUI P Z, ZHANG Z. Heat-integrated pressure-swing-distillation process for separation of tetrahydrofuran/methanol with different feed compositions[J]. Industrial & Engineering Chemistry Research, 2014, 53(17): 7186-7194. |
| 23 | DOUGLAS J M. Conceptual design of chemical processes[M]. New York: McGraw-Hill Book Company, 1988. |
| 24 | 李春山, 张香平, 张锁江, 等. 加压-常压精馏分离甲醇-碳酸二甲酯的相平衡和流程模拟[J]. 过程工程学报, 2003, 3(5): 453-458. |
| LI C S, ZHANG X P, ZHANG S J, et al. Vapor-liquid equilibria and process simulation for separation of dimethyl carbonate and methanol azeotropic system[J]. The Chinese Journal of Process Engineering, 2003, 3(5): 453-458. | |
| 25 | CHEN N N, XU D M, GAO J, et al. Measurement and correlation of phase equilibria for isobutyl acetate + {ethanol or methanol} + water at 303.15K and 323.15K[J]. Journal of Chemical & Engineering Data, 2017, 62(5): 1587-1593. |
| 26 | HORSLEY L H. Azeotropic data-II[M]. 2nd ed. Washington: American Chemical Society, 1962. |
| 27 | YOU X Q, GU J L, PENG C J, et al. Improved design and optimization for separating azeotropes with heavy component as distillate through energy-saving extractive distillation by varying pressure[J]. Industrial & Engineering Chemistry Research, 2017, 56(32): 9156-9166. |
| 28 | KOSSACK S, KRAEMER K, GANI R, et al. A systematic synthesis framework for extractive distillation processes[J]. Chemical Engineering Research and Design, 2008, 86(7): 781-792. |
| 29 | EWELL R H, HARRISON J M, BERG L. Azeotropic distillation[J]. Industrial & Engineering Chemistry, 1944, 36(10): 871-875. |
| 30 | 黄旭, 罗祎青, 袁希钢. 带共沸的乙醇/乙酸乙酯/2-丁酮三元物系变压精馏分离过程及其参数优化[J]. 化工学报, 2018, 69(5): 2089-2099. |
| HUANG X, LUO Y Q, YUAN X G. Separation of C2H5OH/C4H8O2-3/C4H8O-3 ternary mixture with azeotropes by pressure swing distillation and its parameter optimization[J]. CIESC Journal, 2018, 69(5): 2089-2099. |
| [1] | HE Meijin. Application and development trend of molecular management in separation technology in petrochemical field [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 260-266. |
| [2] | SHI Yongxing, LIN Gang, SUN Xiaohang, JIANG Weigeng, QIAO Dawei, YAN Binhang. Research progress on active sites in Cu-based catalysts for CO2 hydrogenation to methanol [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 287-298. |
| [3] | CUI Shoucheng, XU Hongbo, PENG Nan. Simulation analysis of two MOFs materials for O2/He adsorption separation [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 382-390. |
| [4] | XU Jiaheng, LI Yongsheng, LUO Chunhuan, SU Qingquan. Optimization of methanol steam reforming process [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 41-46. |
| [5] | LI Shilin, HU Jingze, WANG Yilin, WANG Qingji, SHAO Lei. Research progress in separation and extraction of high value components by electrodialysis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 420-429. |
| [6] | WANG Ying, HAN Yunping, LI Lin, LI Yanbo, LI Huili, YAN Changren, LI Caixia. Research status and future prospects of the emission characteristics of virus aerosols in urban wastewater treatment plants [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 439-446. |
| [7] | XU Chunshu, YAO Qingda, LIANG Yongxian, ZHOU Hualong. Research progress on functionalization strategies of covalent organic frame materials and its adsorption properties for Hg(Ⅱ) and Cr(Ⅵ) [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 461-478. |
| [8] | ZHAO Jingchao, TAN Ming. Effect of surfactants on the reduction of industrial saline wastewater by electrodialysis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 529-535. |
| [9] | GUO Qiang, ZHAO Wenkai, XIAO Yonghou. Numerical simulation of enhancing fluid perturbation to improve separation of dimethyl sulfide/nitrogen via pressure swing adsorption [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 64-72. |
| [10] | SHU Bin, CHEN Jianhong, XIONG Jian, WU Qirong, YU Jiangtao, YANG Ping. Necessity analysis of promoting the development of green methanol under the goal of carbon neutrality [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4471-4478. |
| [11] | LIAO Zhixin, LUO Tao, WANG Hong, KONG Jiajun, SHEN Haiping, GUAN Cuishi, WANG Cuihong, SHE Yucheng. Application and progress of solvent deasphalting technology [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4573-4586. |
| [12] | WANG Chen, BAI Haoliang, KANG Xue. Performance study of high power UV-LED heat dissipation and nano-TiO2 photocatalytic acid red 26 coupling system [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4905-4916. |
| [13] | WANG Qi, KOU Lihong, WANG Guanyu, WANG Jikun, LIU Min, LI Lanting, WANG Hao. Molecular recognition of dissolved organic matter in bio-treated effluent of coking wastewater [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4984-4993. |
| [14] | SHI Tianxi, SHI Yonghui, WU Xinying, ZHANG Yihao, QIN Zhe, ZHAO Chunxia, LU Da. Effects of Fe2+ on the performance of Anammox EGSB reactor [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 5003-5010. |
| [15] | WANG Haoran, YIN Quanyu, FANG Ming, HOU Jianlin, LI Jun, HE Bin, ZHANG Mingyue. Optimization of near critical-water treatment process of tobacco stems [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 5019-5027. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||