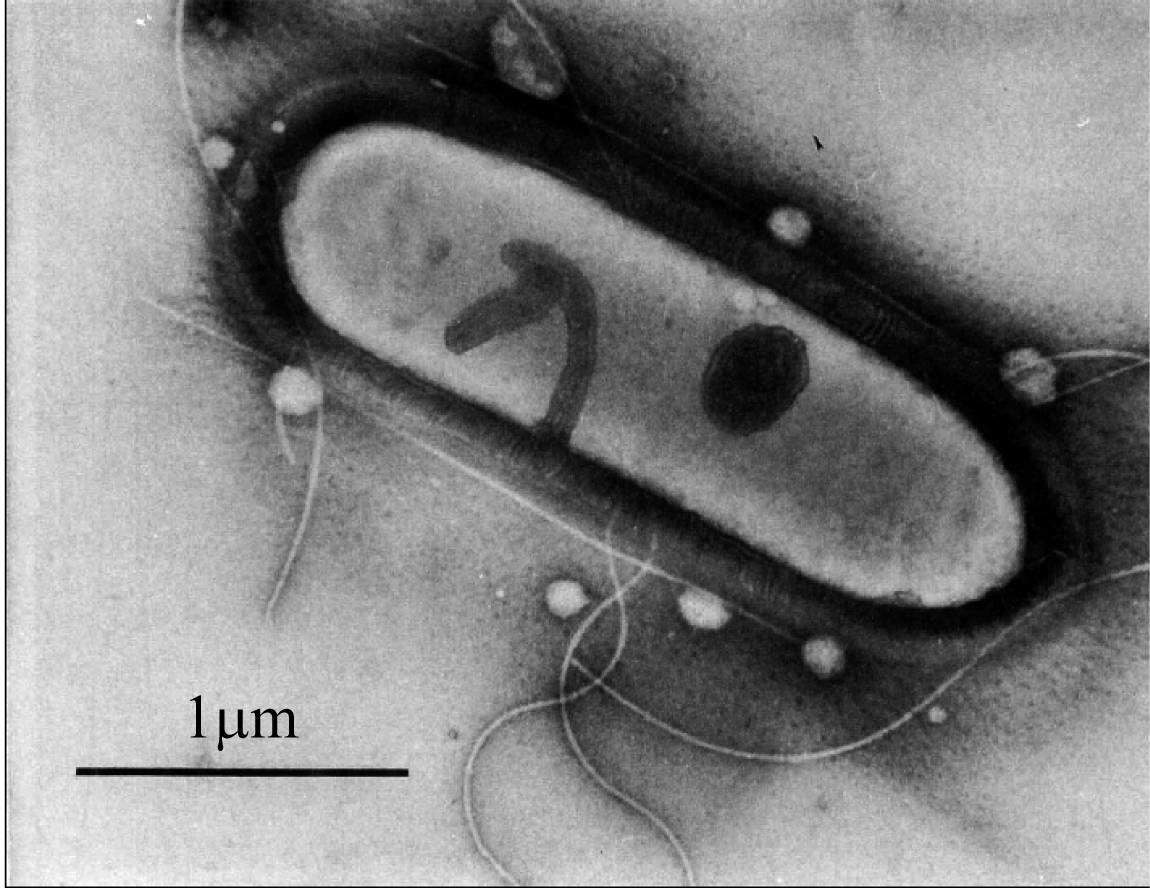
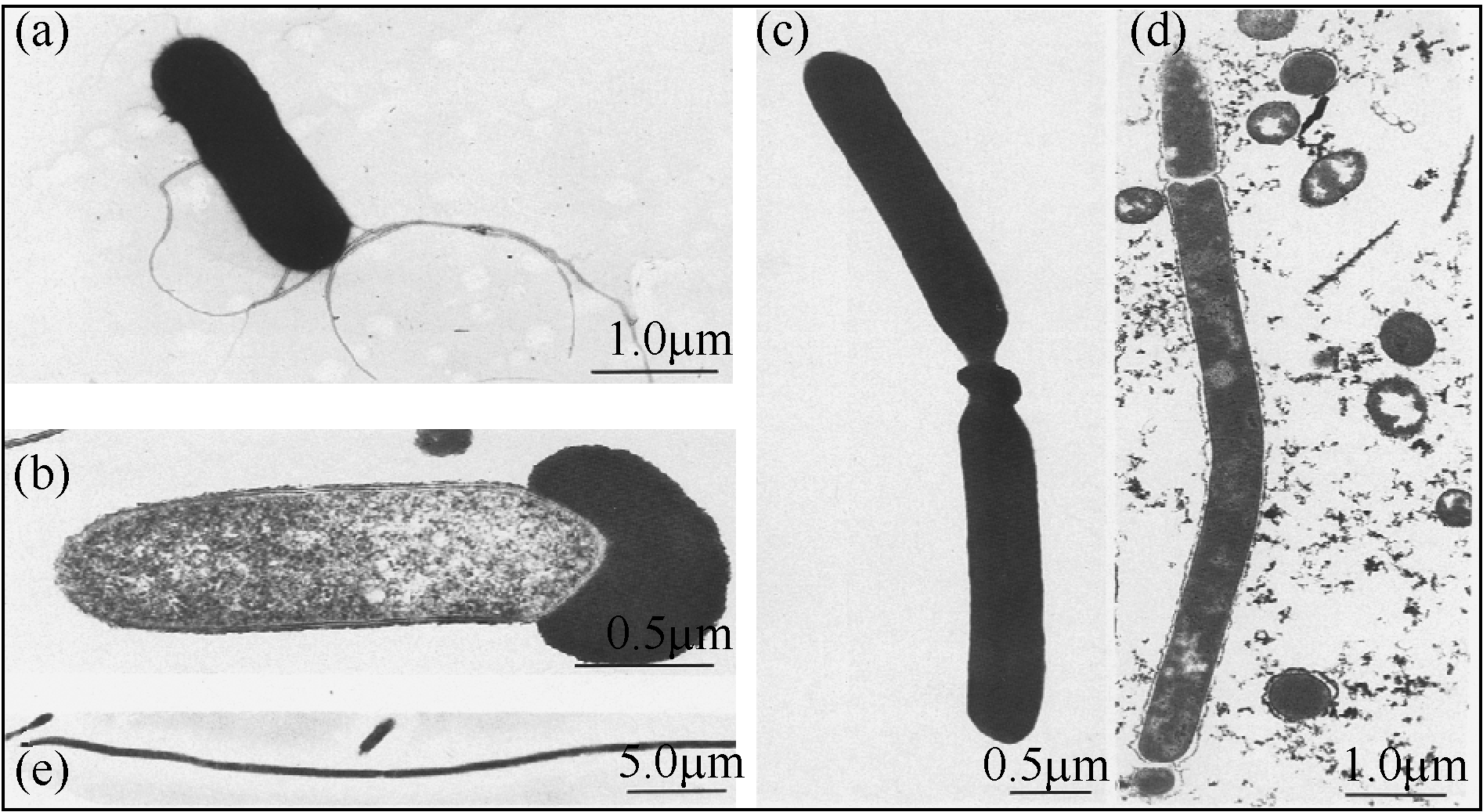
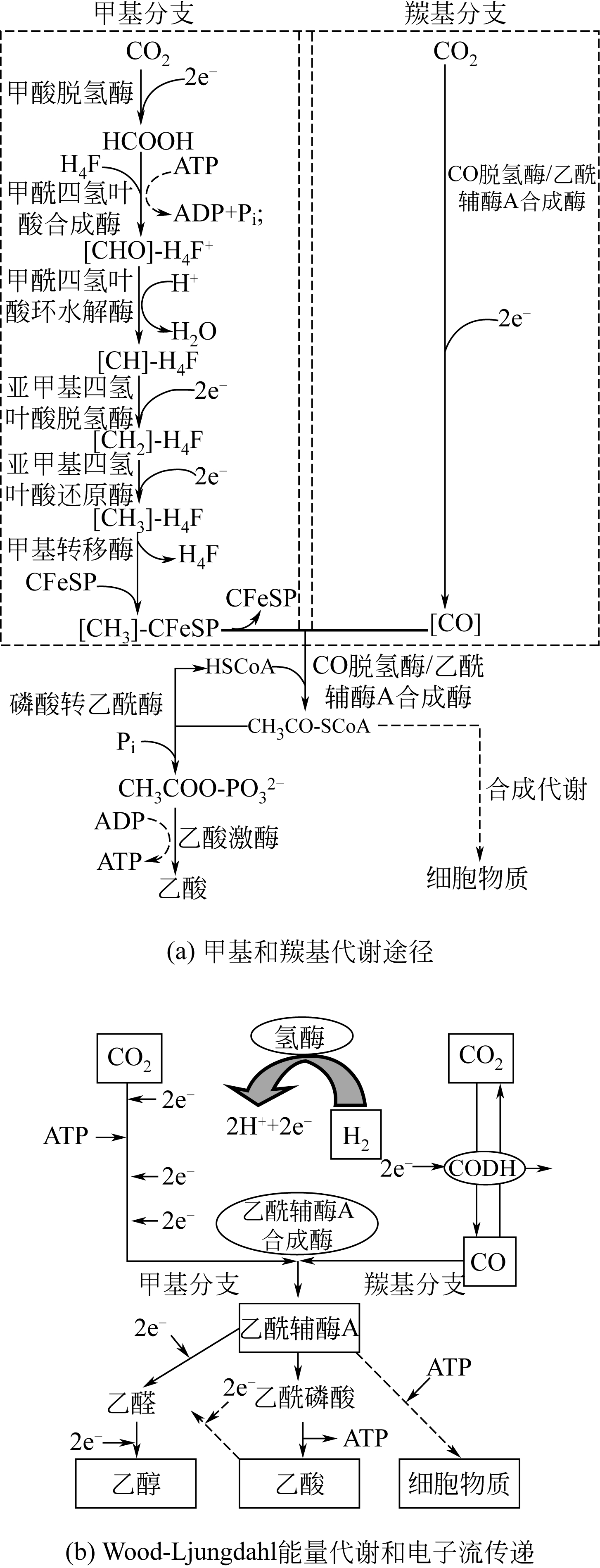
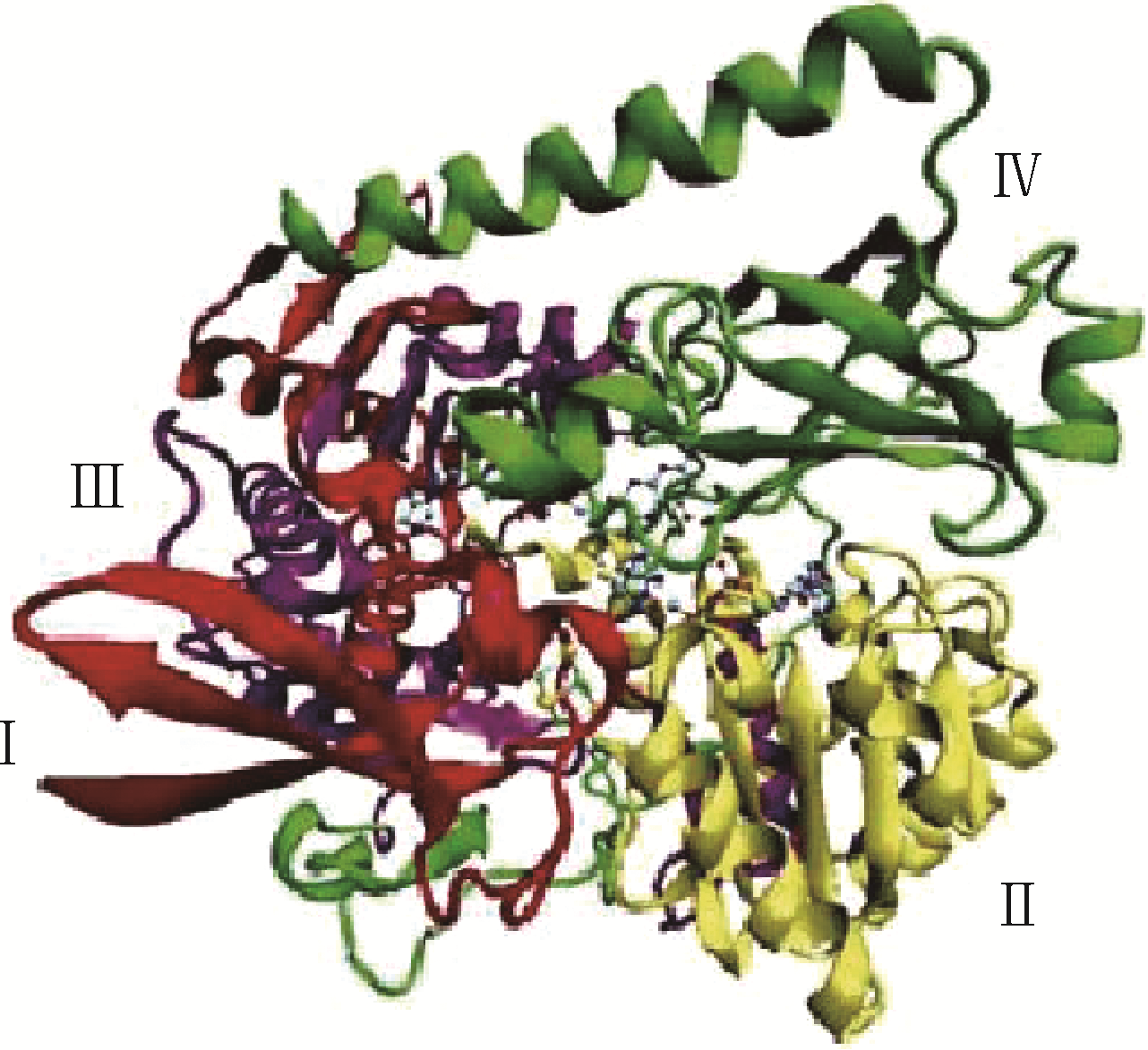
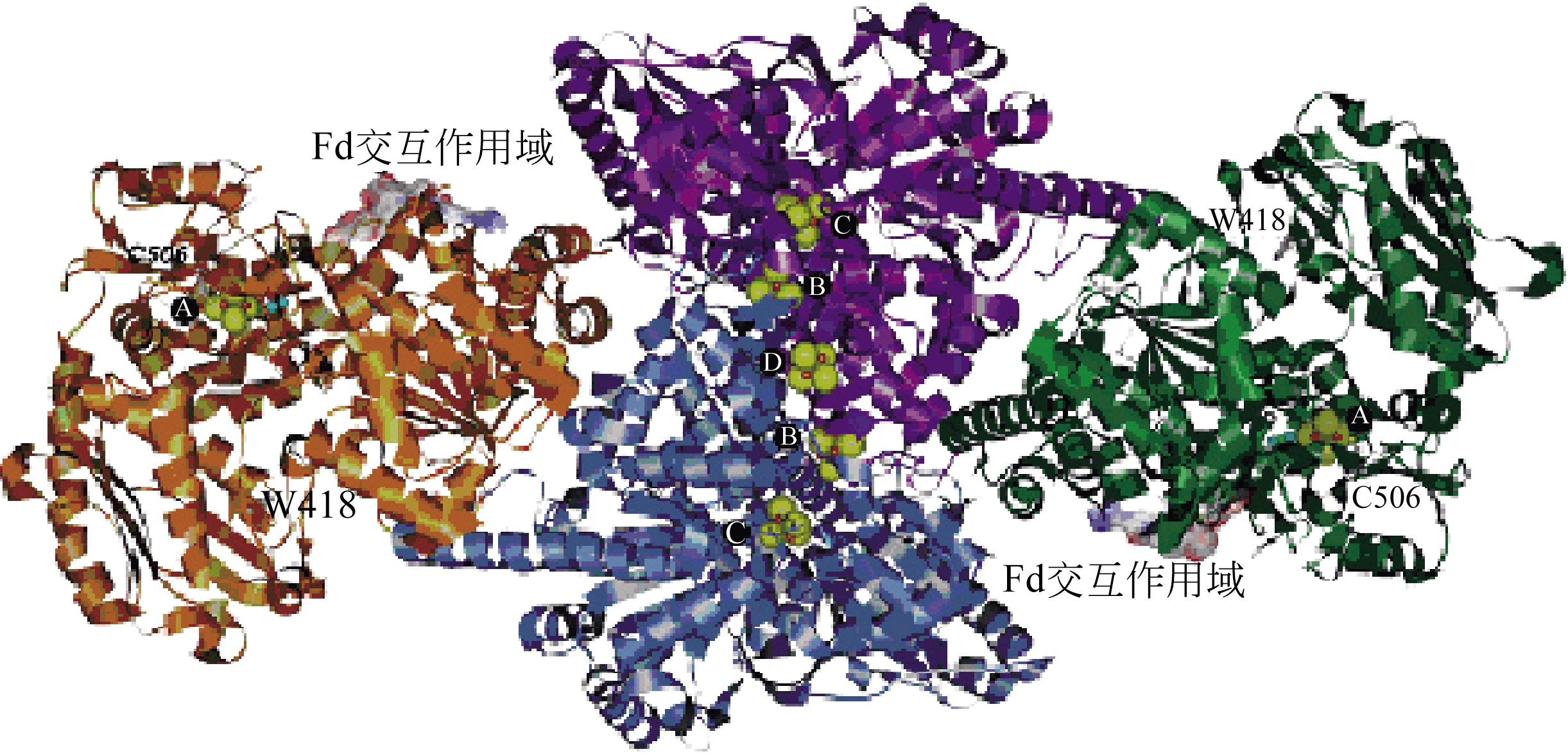
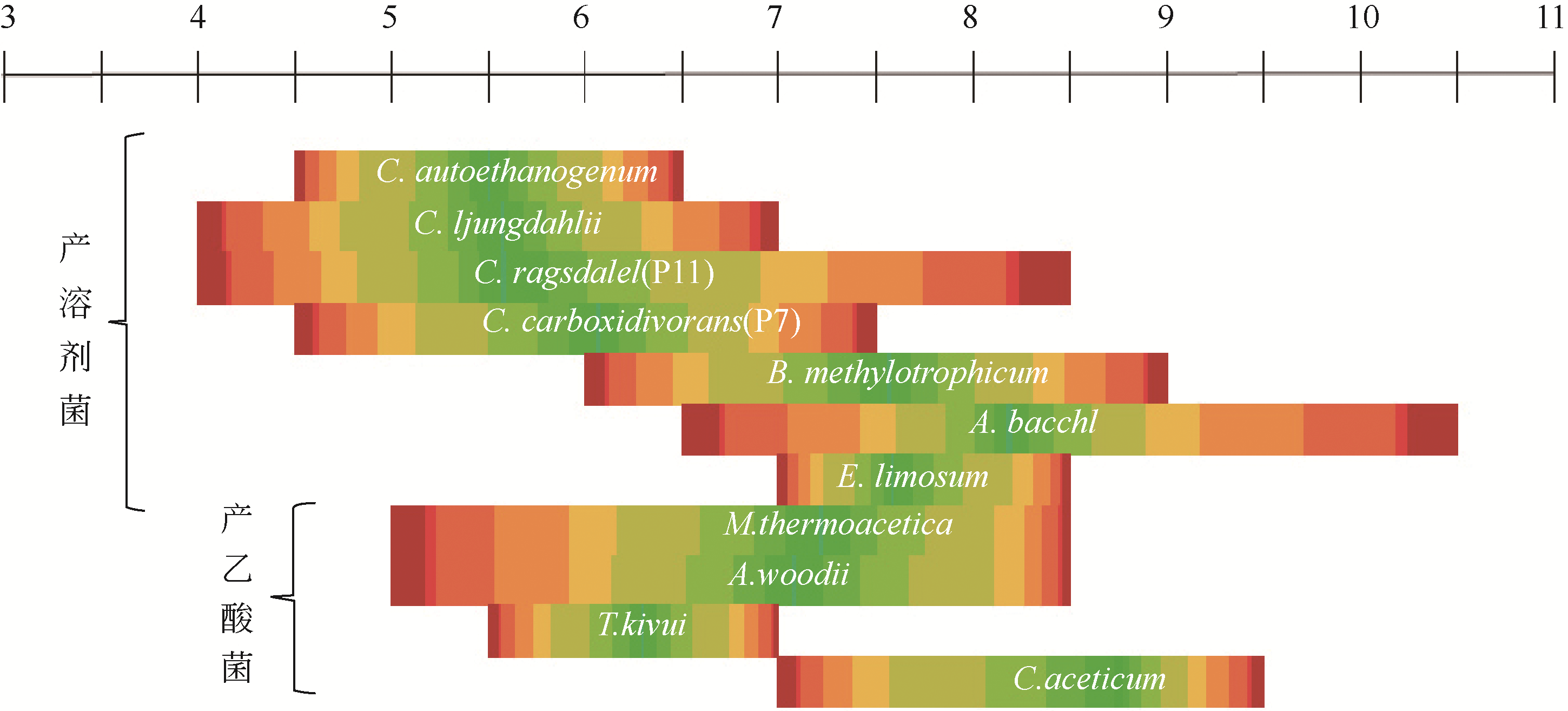
Chemical Industry and Engineering Progress ›› 2019, Vol. 38 ›› Issue (01): 586-597.DOI: 10.16085/j.issn.1000-6613.2018-1129
• Biochemical and pharmaceutical engineering • Previous Articles Next Articles
Research progress on bioethanol production technologies through syngas fermentation
Jingliang XU( ),Chun CHANG,Xiuli HAN,Yifan HAN(
),Chun CHANG,Xiuli HAN,Yifan HAN( )
)
- School of Chemical Engineering and Energy, Zhengzhou University, Zhengzhou 450001, Henan, China
-
Received:2018-05-31Revised:2018-08-13Online:2019-01-05Published:2019-01-05 -
Contact:Yifan HAN
合成气乙醇发酵技术研究进展
- 郑州大学化工与能源学院,河南 郑州 450001
-
通讯作者:韩一帆 -
作者简介:许敬亮(1977—),男,教授,博士生导师,研究方向为生物质生化转化与高值化利用。E-mail:<email>xujl@ zzu.edu.cn</email>。|韩一帆,教授,博士生导师,研究方向为C1资源化利用。E-mail:<email>yifanhan@zzu.edu.cn</email>。 -
基金资助:中国科学院对外合作重点项目(182344KYSB20160056);广东省工业高新技术领域科技计划(2017A010105018);中国科学院对外合作重点项目(182344KYSB20160056);广东省工业高新技术领域科技计划(2017A010105018)。
CLC Number:
Cite this article
Jingliang XU, Chun CHANG, Xiuli HAN, Yifan HAN. Research progress on bioethanol production technologies through syngas fermentation[J]. Chemical Industry and Engineering Progress, 2019, 38(01): 586-597.
许敬亮, 常春, 韩秀丽, 韩一帆. 合成气乙醇发酵技术研究进展[J]. 化工进展, 2019, 38(01): 586-597.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2018-1129
| 微生物 | 分离地点 | 最适温度 /℃ | 最适pH | 倍增时间 /h | 产物 |
|---|---|---|---|---|---|
| Acetobacterium woodii | 黑色沉积物 | 30 | 6.8 | 13 | 乙酸 |
| Alkalibaculum bacchi | 牧场土壤 | 37 | 8.0~8.5 | — | 乙酸、乙醇 |
| Archaeoglobus fulgidus | — | 83 | 6.4 | — | 乙酸、甲酸、H2S |
| Butyribacterium methylotrophicum | 下水道污泥 | 37 | 6 | 12~20 | 乙酸、乙醇、丁酸、丁醇 |
| Clostridium aceticum | — | 30 | 8.5 | — | 乙酸 |
| Clostridium autoethanogenum | 兔粪 | 37 | 5.8~6.0 | — | 乙酸、乙醇 |
| Clostridium carboxidivorans | 污水池沉积物 | 38 | 6.2 | 6.25 | 乙酸、乙醇、丁酸、丁醇 |
| Clostridium drakei | 沉积物 | 30~37 | 5.5~7.5 | — | 乙醇 |
| Clostridium ljungdahlii | 鸡粪 | 37 | 6.0 | 3.8 | 乙酸、乙醇 |
| Clostridium ragsdalei P11 | 鸭塘底泥 | 37 | 6.3 | — | 乙醇 |
| Desulfotomaculum kuznetsovii | — | 60 | 7 | — | 乙酸、H2S |
| Desulfotomaculum thermobenzoicum subsp. thermosyntrophicum | — | 55 | 7 | — | 乙酸、H2S |
| Eubacterium limosum | 绵羊食物 | 38~39 | 7.0~7.2 | 7 | 乙酸 |
| Eubacterium limosum KIST612 | 厌氧消化液 | 37 | 7.0 | — | 乙酸、丁酸 |
| Mesophilic bacterium P7 | 泻湖 | 37 | 5.7~5.8 | — | 乙醇 |
| Methanosarcina acetivorans C2A | — | 37 | 7.0 | 24 | 乙酸、甲酸、甲烷 |
| Moorella sp. HUC22-1 | 地下热泥浆 | 55 | 6.3 | — | 乙醇 |
| Moorella thermoacetica (原Clostridium thermoaceticum) | — | 55 | 6.5~6.8 | 10 | 乙酸 |
| Moorella thermoautotrophica (原Clostridium thermoautotrophicum) | — | 58 | 6.1 | 7 | 乙酸 |
| Oxobacter pfennigii | 食物 | 36~38 | 7.2 | 13.9 | 乙酸、正丁酸 |
| Peptostreptococcus productus | — | 37 | 7 | 1.5 | 乙酸 |
| 微生物 | 分离地点 | 最适温度 /℃ | 最适pH | 倍增时间 /h | 产物 |
|---|---|---|---|---|---|
| Acetobacterium woodii | 黑色沉积物 | 30 | 6.8 | 13 | 乙酸 |
| Alkalibaculum bacchi | 牧场土壤 | 37 | 8.0~8.5 | — | 乙酸、乙醇 |
| Archaeoglobus fulgidus | — | 83 | 6.4 | — | 乙酸、甲酸、H2S |
| Butyribacterium methylotrophicum | 下水道污泥 | 37 | 6 | 12~20 | 乙酸、乙醇、丁酸、丁醇 |
| Clostridium aceticum | — | 30 | 8.5 | — | 乙酸 |
| Clostridium autoethanogenum | 兔粪 | 37 | 5.8~6.0 | — | 乙酸、乙醇 |
| Clostridium carboxidivorans | 污水池沉积物 | 38 | 6.2 | 6.25 | 乙酸、乙醇、丁酸、丁醇 |
| Clostridium drakei | 沉积物 | 30~37 | 5.5~7.5 | — | 乙醇 |
| Clostridium ljungdahlii | 鸡粪 | 37 | 6.0 | 3.8 | 乙酸、乙醇 |
| Clostridium ragsdalei P11 | 鸭塘底泥 | 37 | 6.3 | — | 乙醇 |
| Desulfotomaculum kuznetsovii | — | 60 | 7 | — | 乙酸、H2S |
| Desulfotomaculum thermobenzoicum subsp. thermosyntrophicum | — | 55 | 7 | — | 乙酸、H2S |
| Eubacterium limosum | 绵羊食物 | 38~39 | 7.0~7.2 | 7 | 乙酸 |
| Eubacterium limosum KIST612 | 厌氧消化液 | 37 | 7.0 | — | 乙酸、丁酸 |
| Mesophilic bacterium P7 | 泻湖 | 37 | 5.7~5.8 | — | 乙醇 |
| Methanosarcina acetivorans C2A | — | 37 | 7.0 | 24 | 乙酸、甲酸、甲烷 |
| Moorella sp. HUC22-1 | 地下热泥浆 | 55 | 6.3 | — | 乙醇 |
| Moorella thermoacetica (原Clostridium thermoaceticum) | — | 55 | 6.5~6.8 | 10 | 乙酸 |
| Moorella thermoautotrophica (原Clostridium thermoautotrophicum) | — | 58 | 6.1 | 7 | 乙酸 |
| Oxobacter pfennigii | 食物 | 36~38 | 7.2 | 13.9 | 乙酸、正丁酸 |
| Peptostreptococcus productus | — | 37 | 7 | 1.5 | 乙酸 |
| 底物 | 结果 | 底物 | 结果 |
|---|---|---|---|
| H2/CO2 | + | 核糖 | + |
| CO | + | 木糖 | + |
| 甲酸钠 | +/- | 葡萄糖 | + |
| 甲醇 | - | 果糖 | + |
| 乙醇 | + | 半乳糖 | - |
| 丙酮酸钠 | + | 甘露糖 | - |
| 乳酸钠 | - | 山梨醇 | - |
| 甘油 | - | 蔗糖 | - |
| 柠檬酸钠 | - | 乳糖 | - |
| 琥珀酸钠 | - | 麦芽糖 | - |
| 富马酸钠 | + | 淀粉 | - |
| 苹果酸 | - | 阿魏酸 | - |
| 赤藓糖 | + | 三甲氧基苯甲酸 | - |
| 苏糖 | + | 酪蛋白氨基酸 | +/- |
| 阿拉伯糖 | + | 丙氨酸 | - |
| 底物 | 结果 | 底物 | 结果 |
|---|---|---|---|
| H2/CO2 | + | 核糖 | + |
| CO | + | 木糖 | + |
| 甲酸钠 | +/- | 葡萄糖 | + |
| 甲醇 | - | 果糖 | + |
| 乙醇 | + | 半乳糖 | - |
| 丙酮酸钠 | + | 甘露糖 | - |
| 乳酸钠 | - | 山梨醇 | - |
| 甘油 | - | 蔗糖 | - |
| 柠檬酸钠 | - | 乳糖 | - |
| 琥珀酸钠 | - | 麦芽糖 | - |
| 富马酸钠 | + | 淀粉 | - |
| 苹果酸 | - | 阿魏酸 | - |
| 赤藓糖 | + | 三甲氧基苯甲酸 | - |
| 苏糖 | + | 酪蛋白氨基酸 | +/- |
| 阿拉伯糖 | + | 丙氨酸 | - |
| 反应模式 | 转速/r·min-1 | 微生物 | 底物气体 | 体积传质系数K L a/h~1 |
|---|---|---|---|---|
| 滴流床 | n/a | n/a | 合成气 | 22 |
| 连续搅拌罐 | n/a | n/a | 合成气 | 38 |
| 连续搅拌罐 | 200 | B.methylotrophicum | CO | 14.2 |
| 连续搅拌罐 | 300 | SRB混合培养 | 合成气 | CO 31,H2 75 |
| 连续搅拌罐 | 300 | C.ljungdahlii | 合成气 | CO 35 |
| 连续搅拌罐 | 300 | R.rubrum | 合成气 | CO 28.1 |
| 连续搅拌罐 | 450 | R.rubrum | 合成气 | CO 101 |
| 搅拌罐-微泡喷雾器 | 200 | B.methylotrophicum | CO | 90.6 |
| 搅拌罐-微泡喷雾器 | 300 | SRB混合培养 | 合成气 | CO 104, H2 190 |
| 填充柱气泡反应器 | n/a | R.rubrum | 合成气 | 2.1 |
| 滴流床 | n/a | R.rubrum | 合成气 | 55.5 |
| 滴流床 | n/a | SRB混合培养 | 合成气 | CO 121, H2 335 |
| 滴流床 | n/a | C.ljungdahlii | 合成气 | CO 137 |
| 分批搅拌罐 | n/a | P.productus | CO | 7.15 |
| 搅拌罐 | 300 | C.ljungdahlii | CO | 14.9 |
| 搅拌罐 | 400 | C.ljungdahlii | CO | 21.5 |
| 搅拌罐 | 500 | C.ljungdahlii | CO | 22.8 |
| 搅拌罐 | 600 | C.ljungdahlii | CO | 23.8 |
| 搅拌罐 | 700 | C.ljungdahlii | CO | 35.5 |
| 气泡柱反应器 | n/a | n/a | CO | 72 |
| 搅拌罐 | n/a | n/a | CO | 10.8~155 |
| 搅拌罐 | 500 | R.rubrum | 合成气 | 71.8 |
| 柱状分散反应器 | n/a | n/a | CO | 2.5~40.0 |
| 20μm气泡分散器 | n/a | n/a | CO | 31.7~78.8 |
| 单喷式反应器 | n/a | n/a | CO | 29.5~50.4 |
| 机械混合喷雾反应器 | 150 | n/a | CO | 33.5~53.3 |
| 机械混合喷雾反应器 | 300 | n/a | CO | 34.9~55.8 |
| 浸没式复合中空纤维膜反应器 | n/a | n/a | CO | 0.4~1.1 |
| 气升式20μm气泡分散器 | n/a | n/a | CO | 49.0~91.1 |
| 单点输入气体气升式反应器 | n/a | n/a | CO | 16.6~45.0 |
| 反应模式 | 转速/r·min-1 | 微生物 | 底物气体 | 体积传质系数K L a/h~1 |
|---|---|---|---|---|
| 滴流床 | n/a | n/a | 合成气 | 22 |
| 连续搅拌罐 | n/a | n/a | 合成气 | 38 |
| 连续搅拌罐 | 200 | B.methylotrophicum | CO | 14.2 |
| 连续搅拌罐 | 300 | SRB混合培养 | 合成气 | CO 31,H2 75 |
| 连续搅拌罐 | 300 | C.ljungdahlii | 合成气 | CO 35 |
| 连续搅拌罐 | 300 | R.rubrum | 合成气 | CO 28.1 |
| 连续搅拌罐 | 450 | R.rubrum | 合成气 | CO 101 |
| 搅拌罐-微泡喷雾器 | 200 | B.methylotrophicum | CO | 90.6 |
| 搅拌罐-微泡喷雾器 | 300 | SRB混合培养 | 合成气 | CO 104, H2 190 |
| 填充柱气泡反应器 | n/a | R.rubrum | 合成气 | 2.1 |
| 滴流床 | n/a | R.rubrum | 合成气 | 55.5 |
| 滴流床 | n/a | SRB混合培养 | 合成气 | CO 121, H2 335 |
| 滴流床 | n/a | C.ljungdahlii | 合成气 | CO 137 |
| 分批搅拌罐 | n/a | P.productus | CO | 7.15 |
| 搅拌罐 | 300 | C.ljungdahlii | CO | 14.9 |
| 搅拌罐 | 400 | C.ljungdahlii | CO | 21.5 |
| 搅拌罐 | 500 | C.ljungdahlii | CO | 22.8 |
| 搅拌罐 | 600 | C.ljungdahlii | CO | 23.8 |
| 搅拌罐 | 700 | C.ljungdahlii | CO | 35.5 |
| 气泡柱反应器 | n/a | n/a | CO | 72 |
| 搅拌罐 | n/a | n/a | CO | 10.8~155 |
| 搅拌罐 | 500 | R.rubrum | 合成气 | 71.8 |
| 柱状分散反应器 | n/a | n/a | CO | 2.5~40.0 |
| 20μm气泡分散器 | n/a | n/a | CO | 31.7~78.8 |
| 单喷式反应器 | n/a | n/a | CO | 29.5~50.4 |
| 机械混合喷雾反应器 | 150 | n/a | CO | 33.5~53.3 |
| 机械混合喷雾反应器 | 300 | n/a | CO | 34.9~55.8 |
| 浸没式复合中空纤维膜反应器 | n/a | n/a | CO | 0.4~1.1 |
| 气升式20μm气泡分散器 | n/a | n/a | CO | 49.0~91.1 |
| 单点输入气体气升式反应器 | n/a | n/a | CO | 16.6~45.0 |
| 反应器 | 微生物 | 培养时间 | 反应体积 /L | 搅拌速度 /r·min-1 | 气体停留时间 /min | 稀释速率 /h–1 | 细胞浓度 /g·L–1 | 乙醇浓度 /g·L–1 |
|---|---|---|---|---|---|---|---|---|
| STB | C. ljungdahlii | 1d | 0.6① | 1000 | 1.4 | 0.208 | 7.1 | 12 |
| C. carboxidivorans P7T | 17d | 3 | 400 | 18.75 | 0.0069 | 0.215② | 0.75② | |
| Clostridium strain P11 | 59d | 70 | 150 | 77.78 | — | 0.87 | 25.26 | |
| B. methylotrophicum | 9d | 1.25 | 50 | 25 | 0.015 | 0.286 | 0.056 | |
| B. methylotrophicum | 56d | 1.5① | 200 | NR | NR | 9② | TA | |
| Moorella Sp. HUC22–1 | 220h | 0.5 | 500 | 8.34 | — | 0.28② | 0.221 | |
| C. carboxidivorans P7T | 100h | 0.123 | 120 | NR | — | 1.08 | 2 | |
| BCR | E. limosum KIST612 | 233h | 0.2 | — | 2.5 | 0.15 | 4.01 | 0.092 |
| C. carboxidivorans P7T | 10d | 4.5 | — | 22.5 | 0.027 | NR | 0.16%③ | |
| C. carboxidivorans P7T | 20d | 4 | — | 22.22 | 0.023 | 0.215② | 2.75② | |
| MBBR | C. ragsdalei | 30d | 18000 | — | 5.14 | 1.33 | NR | 30 |
| MBR | C. ragsdalei | 20d | 0.18① | — | NR | NR | NR | 15 |
| 反应器 | 微生物 | 培养时间 | 反应体积 /L | 搅拌速度 /r·min-1 | 气体停留时间 /min | 稀释速率 /h–1 | 细胞浓度 /g·L–1 | 乙醇浓度 /g·L–1 |
|---|---|---|---|---|---|---|---|---|
| STB | C. ljungdahlii | 1d | 0.6① | 1000 | 1.4 | 0.208 | 7.1 | 12 |
| C. carboxidivorans P7T | 17d | 3 | 400 | 18.75 | 0.0069 | 0.215② | 0.75② | |
| Clostridium strain P11 | 59d | 70 | 150 | 77.78 | — | 0.87 | 25.26 | |
| B. methylotrophicum | 9d | 1.25 | 50 | 25 | 0.015 | 0.286 | 0.056 | |
| B. methylotrophicum | 56d | 1.5① | 200 | NR | NR | 9② | TA | |
| Moorella Sp. HUC22–1 | 220h | 0.5 | 500 | 8.34 | — | 0.28② | 0.221 | |
| C. carboxidivorans P7T | 100h | 0.123 | 120 | NR | — | 1.08 | 2 | |
| BCR | E. limosum KIST612 | 233h | 0.2 | — | 2.5 | 0.15 | 4.01 | 0.092 |
| C. carboxidivorans P7T | 10d | 4.5 | — | 22.5 | 0.027 | NR | 0.16%③ | |
| C. carboxidivorans P7T | 20d | 4 | — | 22.22 | 0.023 | 0.215② | 2.75② | |
| MBBR | C. ragsdalei | 30d | 18000 | — | 5.14 | 1.33 | NR | 30 |
| MBR | C. ragsdalei | 20d | 0.18① | — | NR | NR | NR | 15 |
| 公司名称 | 过程 | 容量 /百万加仑·年-1 | 系统规模 | 气体 | 产物 | 地点/年份 | 状态 |
|---|---|---|---|---|---|---|---|
| BRI | 生物质气化/气体发酵 | — | 试用 | 气化合成气 | 乙醇 | 费耶特维尔,美国/2003年 | 运行 |
| INEOS Bio | 生物质气化/气体发酵 | 8 | 商业化 | 气化合成气 | 乙醇 | 佛罗里达,美国/2013年 | 运行 |
| INEOS Bio | 生物质气化/气体发酵 | 7.9 | 商业化 | 气化合成气 | 乙醇 | 蒂赛德,英国 | 运行 |
| Coskata Inc. | 生物质气化/气体发酵 | 0.04 | 示范 | 气化合成气 | 乙醇 | 麦迪逊,美国/2009年 | 运行 |
| LanzaTech | 气体发酵 | 0.015 | 试用 | 钢厂烟气 | 乙醇 | 格兰布,新西兰/2008年 | 运行 |
| LanzaTech | 气体发酵 | 0.1 | 示范 | 钢厂烟气 | 乙醇 | 上海,中国,宝山钢铁厂/2012年 | 运行 |
| LanzaTech | 气体发酵 | 50 | 商业化 | 钢厂烟气 | 乙醇 | 上海,中国,宝山钢铁厂/2013年 | 计划 |
| LanzaTech | 气体发酵 | 0.1 | 示范 | 钢厂烟气 | 乙醇 | 北京,中国,首都钢铁厂/2013年 | 运行 |
| LanzaTech | 气体发酵 | 0.01 | 示范 | 钢厂烟气 | 乙醇 | 台湾,中国/2014年 | 运行 |
| LanzaTech | 气体发酵 | 17~34 | 商业化 | 钢厂烟气 | 乙醇,汽油添加剂 | 台湾,中国 | 计划 |
| 公司名称 | 过程 | 容量 /百万加仑·年-1 | 系统规模 | 气体 | 产物 | 地点/年份 | 状态 |
|---|---|---|---|---|---|---|---|
| BRI | 生物质气化/气体发酵 | — | 试用 | 气化合成气 | 乙醇 | 费耶特维尔,美国/2003年 | 运行 |
| INEOS Bio | 生物质气化/气体发酵 | 8 | 商业化 | 气化合成气 | 乙醇 | 佛罗里达,美国/2013年 | 运行 |
| INEOS Bio | 生物质气化/气体发酵 | 7.9 | 商业化 | 气化合成气 | 乙醇 | 蒂赛德,英国 | 运行 |
| Coskata Inc. | 生物质气化/气体发酵 | 0.04 | 示范 | 气化合成气 | 乙醇 | 麦迪逊,美国/2009年 | 运行 |
| LanzaTech | 气体发酵 | 0.015 | 试用 | 钢厂烟气 | 乙醇 | 格兰布,新西兰/2008年 | 运行 |
| LanzaTech | 气体发酵 | 0.1 | 示范 | 钢厂烟气 | 乙醇 | 上海,中国,宝山钢铁厂/2012年 | 运行 |
| LanzaTech | 气体发酵 | 50 | 商业化 | 钢厂烟气 | 乙醇 | 上海,中国,宝山钢铁厂/2013年 | 计划 |
| LanzaTech | 气体发酵 | 0.1 | 示范 | 钢厂烟气 | 乙醇 | 北京,中国,首都钢铁厂/2013年 | 运行 |
| LanzaTech | 气体发酵 | 0.01 | 示范 | 钢厂烟气 | 乙醇 | 台湾,中国/2014年 | 运行 |
| LanzaTech | 气体发酵 | 17~34 | 商业化 | 钢厂烟气 | 乙醇,汽油添加剂 | 台湾,中国 | 计划 |
| 1 | 刘朝全 . 2017年国内外油气行业发展报告[M]. 北京:石油工业出版社, 2018. |
| LIU Chaoquan . Report on development of domestic and foreign oil and gas industry in 2017 [M]. Beijing: Petroleum Industry Press, 2018. | |
| 2 | 袁振宏 .能源微生物学[M].北京:化学工业出版社,2012. |
| YUAN Zhenhong . Energy microbiology[M]. Beijing: Chemical Industry Press, 2012. | |
| 3 | 袁振宏 .生物质能高效利用技术[M]. 北京:化学工业出版社,2014. |
| YUAN Zhenhong . Technologies on bioenergy high efficiency utilization[M]. Beijing: Chemical Industry Press, 2014. | |
| 4 | 徐惠娟 .合成气梭菌发酵乙醇及其代谢途径分析[D]. 北京:中国科学院大学,2016. |
| XU Huijuan . Syngas fermentation to ethanol with Clostridium and its metabolic pathway[D]. Beijing: University of Chinese Academy of Sciences,2016. | |
| 5 | MUNASINGHE P C , KHANAL S K . Biomass-derived syngas fermentation into biofuels: opportunities and challenges[J]. Bioresource Technology , 2010, 101: 5013-5022. |
| 6 | WU C , TU X . Biological and fermentative conversion of syngas[M] |
| //RAFAEL L , CAROL S K L , KAREN W , et al . Handbook of biofuels production. Duxford: Woodhead Publishing, 2011: 335-357. | |
| 7 | BRADLEY E S , RYAN A B , DILA R B , et al . Syngas fermentation to biofuels: effects of hydrogen partial pressure on hydrogenase efficiency[J]. Biomass and Bioenergy,2013,55:156-162. |
| 8 | DEBABOV V G . Bioethanol from synthesis gas[J]. Applied Biochemistry and Microbiology, 2013, 49(7): 619-628. |
| 9 | PHILLIPS J R CLAUSEN E C , GADDY J L ,et a1 . Biological production of ethanol from coal synthesis gas[J].Applied Biochemistry and Biotechnology, 1993, 39/40(1):559-571. |
| 10 | HENSTRA A M , SIPMA J , RINZEMA A , et a1 . Microbiology of synthesis gas fermentation for biofuel production[J]. Current Opinion in Biotechnology, 2007, 18: 200-206. |
| 11 | ABUBACKAR H N , VEIGA M C , KENNES C . Biological conversion of carbon monoxide: rich syngas or waste gases to bioethanol[J]. Biofuels, Bioproducts and Biorefining, 2011, 5 (1): 93-114. |
| 12 | MOHAMMADI M , NAJAFPOUR G D , YOUNESI H , et al . Bioconversion of synthesis gas to second generation biofuels: a review[J]. Renewable and Sustainable Energy Reviews, 2011, 15 (9), 4255-4273. |
| 13 | LIEW F M , KÖPKE M , SIMPSON S D . Gas fermentation for commercial biofuels production[M]// FANG Z, editor. Biofuel Prod Dev Prospect. Rijeka: InTech, 2013: 125-74. |
| 14 | GADDY J L , CLAUSEN E C . Clostridiumm ljungdahlii, an anaerobic ethanol and acetate producing microorganism:US5173429 [P]. 1992-12-22. |
| 15 | KLASSON K T , ACKERSON C M D , CLAUSEN E C , et al . Biological conversion of synthesis gas into fuels[J]. International Journal of Hydrogen Energy, 1992, 17 (4): 281-288. |
| 16 | TANNER R S , MILLER L M , YANG D . Clostridium ljungdahlii sp. nov., an acetogenic species in clostridial rRNA homology group I[J]. International Journal of Systematic Bacteriology, 1993, 43: 232-236. |
| 17 | RAJAGOPALAN S , DATAR R P , LEWIS R S . Formation of ethanol from carbon monoxide via a new microbial catalyst[J]. Biomass and Bioenergy, 2002, 23: 487-493. |
| 18 | DATAR R P , SHENKMAN R M , CATENI B G , et al . Fermentation of biomass-generated producer gas to ethanol[J]. Biotechnology and Bioengineering, 2004, 86: 587-594. |
| 19 | ABRINI J , NAVEAU H , NYNS E J . autoethanogenum Clostridium , sp . nov., an anerobic bacterium that produces ethanol from carbon monoxide[J]. Archives of Microbiology, 1994,161: 345-351. |
| 20 | 郭颖 . 合成气乙醇发酵培养基及工艺优化研究[D].北京:中国科学院大学, 2010. |
| GUO Ying . Medium and process optimization for the biological production of ethanol from syngas[D]. Beijing: University of Chinese Academy of Sciences,2010. | |
| 21 | XU H , LIANG C , YUAN Z , et al . A study of CO/syngas bioconversion by Clostridium autoethanogenum with a flexible gas-cultivation system[J]. Enzyme and Microbial Technology, 2017, 101: 24-29. |
| 22 | 徐惠娟,梁翠谊,许敬亮,等 . CO 一步法C. autoethanogenum 发酵产乙醇的工艺研究[J]. 农业工程学报,2017,33(23): 246-251. |
| XU Huijuan , LIANG Cuiyi , XU Jingliang , et al . Study on one-step ethanol production from CO by C. autoethanogenum [J]. Transactions of the Chinese Society of Agricultural Engineering, 2017, 33(23): 246-251. | |
| 23 | HURST K M , LEWIS R S . Carbon monoxide partial pressure effects on the metabolic process of syngas fermentation[J]. Biochemical Engineering Journal, 2010, 48: 159-165. |
| 24 | 朱小飞, 谭相石 . 金属组学:Wood-Ljungdahl通路中的金属蛋白/金属酶[J]. 中国科学B辑(化学), 2009, 39 (7): 607-619. |
| ZHU Xiaofei , TAN Xiangshi . Metalloproteins/metalloenzymes for the synthesis of acetyl-CoA in the Wood-Ljungdahl pathway[J].Science in China Series B(Chemistry), 2009, 39 (7): 607-619. | |
| 25 | RAGSDALE S W . Life with carbon monoxide[J]. Critical Reviews in Biochemistry and Molecular Biology, 2004, 39:165-195. |
| 26 | KIM Y K , PARK S E , LEE H , et al . Enhancement of bioethanol production in syngas fermentation with Clostridium ljungdahlii using nanoparticles[J]. Bioresource Technology, 2014, 159: 446-450. |
| 27 | SUN X , ATIYEH H K , KUMAR A , et al . Enhanced ethanol production by Clostridium ragsdalei from syngas by incorporating biochar in the fermentation medium[J]. Bioresource Technology, 2018, 247: 291-301. |
| 28 | ABUBACKAR N H , VEIGA M C , KENNES C . Biological conversion of carbon monoxide to ethanol: effect of pH, gas pressure, reducing agent and yeast extract[J]. Bioresource Technology, 2012, 114: 518-522. |
| 29 | SIM J H , KAMARUDDIN A H . Optimization of acetic acid production from synthesis gas by chemolithotrophic bacterium—Clostridium aceticum using statistical approach[J]. Bioresource Technology, 2008, 99: 2724-2735. |
| 30 | KIMURA Z I , KITA A , IWASAKI Y , et al . Glycerol acts as alternative electron sink during syngas fermentation by thermophilic anaerobe Moorella thermoacetica [J]. Journal of Bioscience and Bioengineering, 2016, 121(3): 268-273. |
| 31 | TISSERA S D , KÖPKE M , SIMPSON S D , et al . Syngas biorefinery and syngas utilization[J]. Advances in Biochemical Engineering/Biotechnology, 2017, 5:1-34. |
| 32 | AHMED A , CATENI B G , HUHNKE R L , et al . Effects of biomass-generated producer gas constituents on cell growth, product distribution and hydrogenase activity of Clostridium carboxidivorans P7T[J]. Biomass Bioenergy, 2006, 30: 665-672. |
| 33 | AHMED A , LEWIS R L . Fermentation of biomass generated synthesis gas: effects of nitric oxide[J]. Biotechnology and Bioengineering, 2007, 97 (5): 1080-1086. |
| 34 | ESQUIVEL-ELIZONDO S , DELGADO A G , RITTMANN B E , et al . The effects of CO2 and H2 on CO metabolism by pure and mixed microbial cultures[J]. Biotechnology for Biofuels, 2017, 10: 220. |
| 35 | ZHANG J , TAYLOR S , WANG Y . Effects of end products on fermentation profiles in Clostridium carboxidivorans P7 for syngas fermentation[J]. Bioresource Technology, 2016, 218: 1055-1063. |
| 36 | MADDIPATI P , ATIYEH H K , BELLMER D D , et al . Ethanol production from syngas by Clostridium strain P11 using corn steep liquor as a nutrient replacement to yeast extract[J]. Bioresource Technology, 2011, 102: 6494-6501. |
| 37 | GUO Y , XU J L , ZHANG Y , et al . Medium optimization for ethanol production with Clostridium autoethanogenum with carbon monoxide as sole carbon source[J]. Bioresource Technology, 2010, 101: 8784-8789. |
| 38 | KUNDIYANA D K , HUHNKE R L , WILKINS M R . Effect of nutrient limitation and two-stage continuous fermentor design on productivities during "Clostridium ragsdalei" syngas fermentation[J]. Bioresource Technology, 2011, 102: 6058-6064. |
| 39 | DIENDER M , STAMS A J M , SOUSA D Z . Production of medium-chain fatty acids and higher alcohols by a synthetic co-culture grown on carbon monoxide or syngas[J]. Biotechnology for Biofuels, 2016, 9, 82: 1-11. |
| 40 | MUNASINGHE P C , KHANAL S K . Syngas fermentation to biofuel: evaluation of carbon monoxide mass transfer coefficient (kLa) in different reactor configurations[J]. Biotechnology Progress,2010, 26 (6): 1616-1621. |
| 41 | LANE J . Coskata’s technology re-emerges as Synata Bio : biofuels digest[EB/OL]. [2016-01-24]. . |
| 42 | HEIJSTRA B D , LEANG C , JUMINAGA A . Gas fermentation: cellular engineering possibilities and scale up[J]. Microbial Cell Factories, 2017, 16: 60. |
| 43 | FUNGMIN L , ANNE M H , MICHAEL K , et al . Metabolic engineering of Clostridium autoethanogenum for selective alcohol production[J]. Metabolic Engineering, 2017, 40: 104-114. |
| 44 | MUELLER A , KOEPKE M , NAGARAJU S . Recombinant microorganisms and uses therefor: US9890384[P]. 2018-02-13. |
| 45 | FENG X , ZHUANG W Q , COLLETTI P , et al . Metabolic pathway determination and flux analysis in nonmodel microorganisms through 13C-isotope labeling[J]. Microbial Systems Biology, 2012,96(4): 309-330. |
| [1] | SHI Yongxing, LIN Gang, SUN Xiaohang, JIANG Weigeng, QIAO Dawei, YAN Binhang. Research progress on active sites in Cu-based catalysts for CO2 hydrogenation to methanol [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 287-298. |
| [2] | YANG Xiazhen, PENG Yifan, LIU Huazhang, HUO Chao. Regulation of active phase of fused iron catalyst and its catalytic performance of Fischer-Tropsch synthesis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 310-318. |
| [3] | XU Jiaheng, LI Yongsheng, LUO Chunhuan, SU Qingquan. Optimization of methanol steam reforming process [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 41-46. |
| [4] | SHU Bin, CHEN Jianhong, XIONG Jian, WU Qirong, YU Jiangtao, YANG Ping. Necessity analysis of promoting the development of green methanol under the goal of carbon neutrality [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4471-4478. |
| [5] | WANG Xueting, GU Xia, XU Xianbao, ZHAO Lei, XUE Gang, LI Xiang. Effectiveness of hydrothermal pretreatment on valeric acid production during food waste fermentation [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4994-5002. |
| [6] | LU Yang, ZHOU Jinsong, ZHOU Qixin, WANG Tang, LIU Zhuang, LI Bohao, ZHOU Lingtao. Leaching mechanism of Hg-absorption products on CeO2/TiO2 sorbentsin syngas [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3875-3883. |
| [7] | LU Shaojie, LIU Jia, JI Qianzhu, LI Ping, HAN Yueyang, TAO Min, LIANG Wenjun. Preparation of diatomaceous earth-based composite filler and its xylene removal performance by a biotrickling filter [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3884-3892. |
| [8] | QIN Kai, YANG Shilin, LI Jun, CHU Zhenyu, BO Cuimei. A Kalman filter algorithm-based high precision detection method for glucoamylase biosensors [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 3177-3186. |
| [9] | WANG Zijian, KE Ming, SONG Zhaozheng, LI Jiahan, TONG Yanbing, SUN Jinru. Progress in alkylation of gasoline with molecular sieve catalyst for benzene reduction [J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2371-2389. |
| [10] | ZHAO Yao, ZHOU Zhihui, WU Hongdan, HU Chuanzhi, ZHANG Guochun, WU Ruipeng. Response surface analysis and optimization of membrane permeation vaporization by Silicalite-1 molecular sieve [J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2586-2594. |
| [11] | HUANG Yue, ZHAO Lixin, YAO Zonglu, YU Jiadong, LI Zaixing, SHEN Ruixia, AN Kemeng, HUANG Yali. Research progress in directed bioconversion of lactic acid and acetic acid from wood lignocellulosic wastes [J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2691-2701. |
| [12] | RUAN Peng, YANG Runnong, LIN Zirong, SUN Yongming. Advances in catalysts for catalytic partial oxidation of methane to syngas [J]. Chemical Industry and Engineering Progress, 2023, 42(4): 1832-1846. |
| [13] | TIAN Yuan, LOU Shujie, MENG Shanru, YAN Jingru, XIAO Haicheng. Recent progress of Co-based catalysts for higher alcohols synthesis form syngas [J]. Chemical Industry and Engineering Progress, 2023, 42(4): 1869-1876. |
| [14] | XIAO Yaoxin, ZHANG Jun, HU Sheng, SHAN Rui, YUAN Haoran, CHEN Yong. Cu-Zn catalyzed hydrogenation of furfural with methanol as hydrogen donor [J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1341-1352. |
| [15] | HUANG Qizhong, LIU Bing, MA Hongpeng, LYU Wenjie. Methanol to olefin wastewater treatment based on a novel microchannel separation technology [J]. Chemical Industry and Engineering Progress, 2023, 42(2): 669-676. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||