化工进展 ›› 2022, Vol. 41 ›› Issue (2): 619-627.DOI: 10.16085/j.issn.1000-6613.2021-0517
离子液体萃取分离烃类化合物的研究进展
- 中国石化石油化工科学研究院重油加工研究室,北京 100083
-
收稿日期:2021-03-15修回日期:2021-08-06出版日期:2022-02-05发布日期:2022-02-23 -
通讯作者:管翠诗 -
作者简介:倪清(1995—),男,博士研究生,研究方向为石油馏分溶剂萃取分离。E-mail:niqing.ripp@sinopec.com 。 -
基金资助:中国石油化工股份有限公司课题(119028-1)
Progress in extraction separation of hydrocarbons by ionic liquids
NI Qing( ), LAI Jinbo, PENG Dongyue, GUAN Cuishi(
), LAI Jinbo, PENG Dongyue, GUAN Cuishi( ), LONG Jun
), LONG Jun
- Heavy Oil Processing Department, Sinopec Research Institute of Petroleum Processing, Beijing 100083, China
-
Received:2021-03-15Revised:2021-08-06Online:2022-02-05Published:2022-02-23 -
Contact:GUAN Cuishi
摘要:
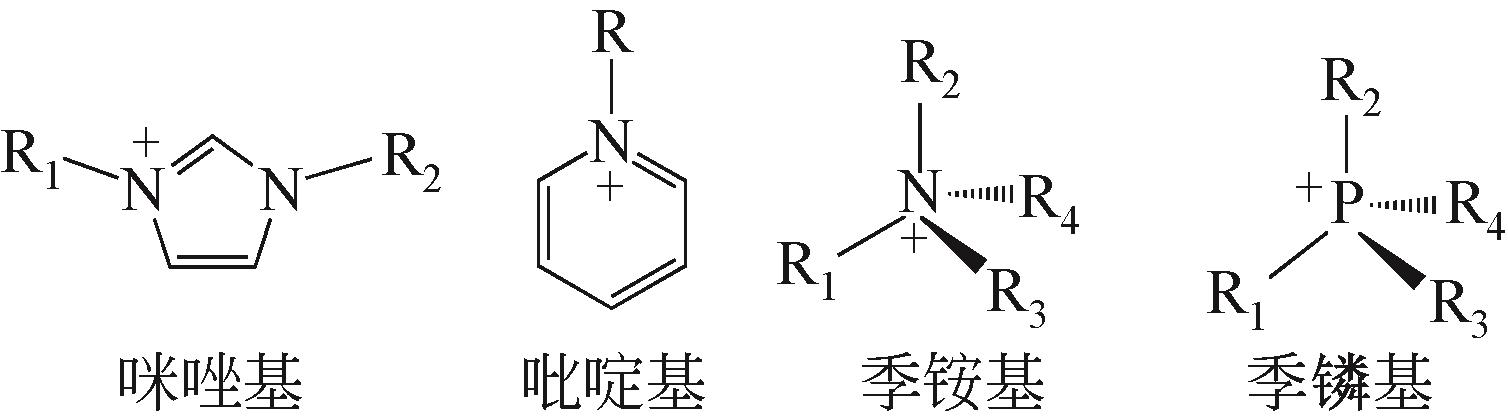
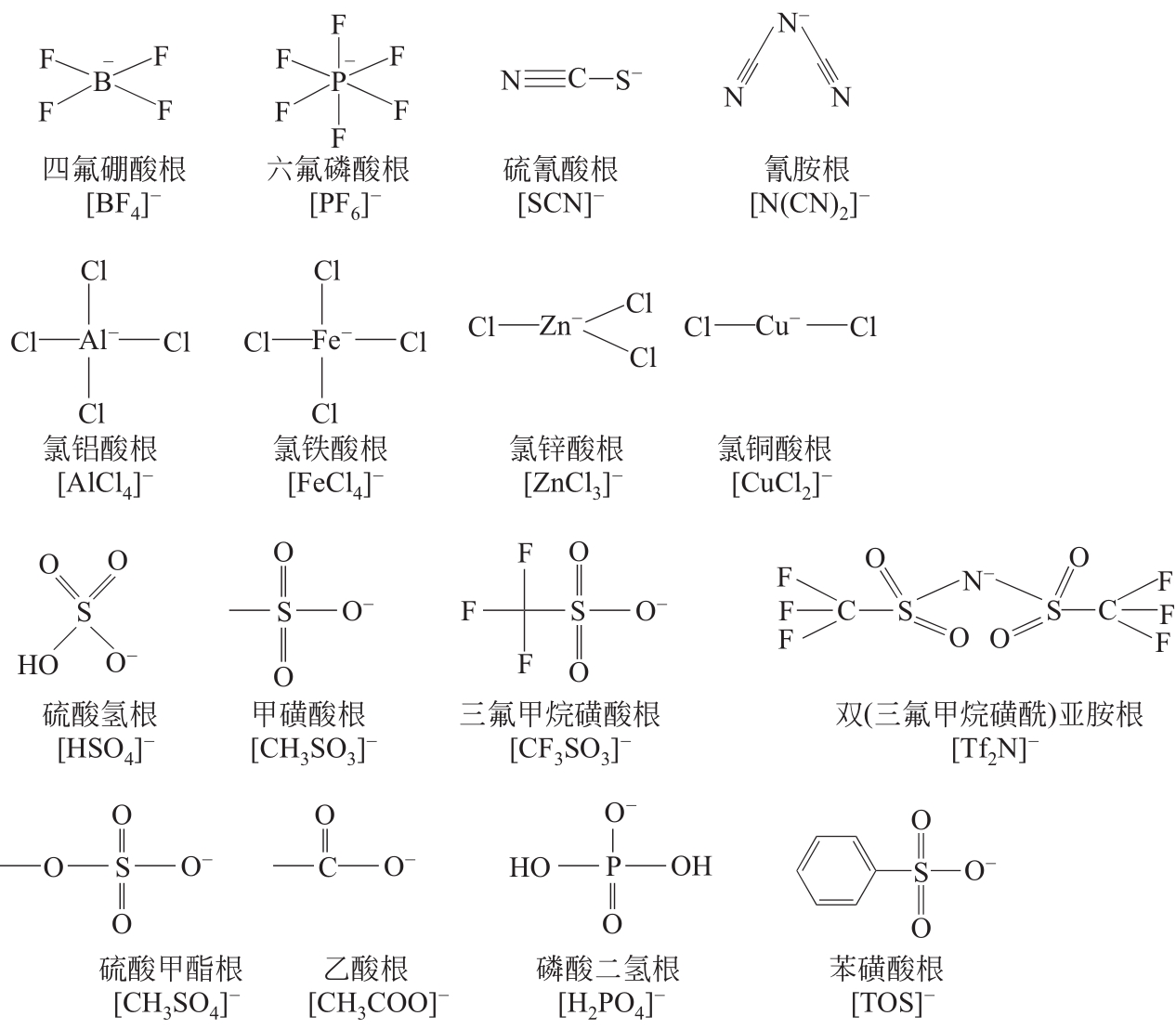
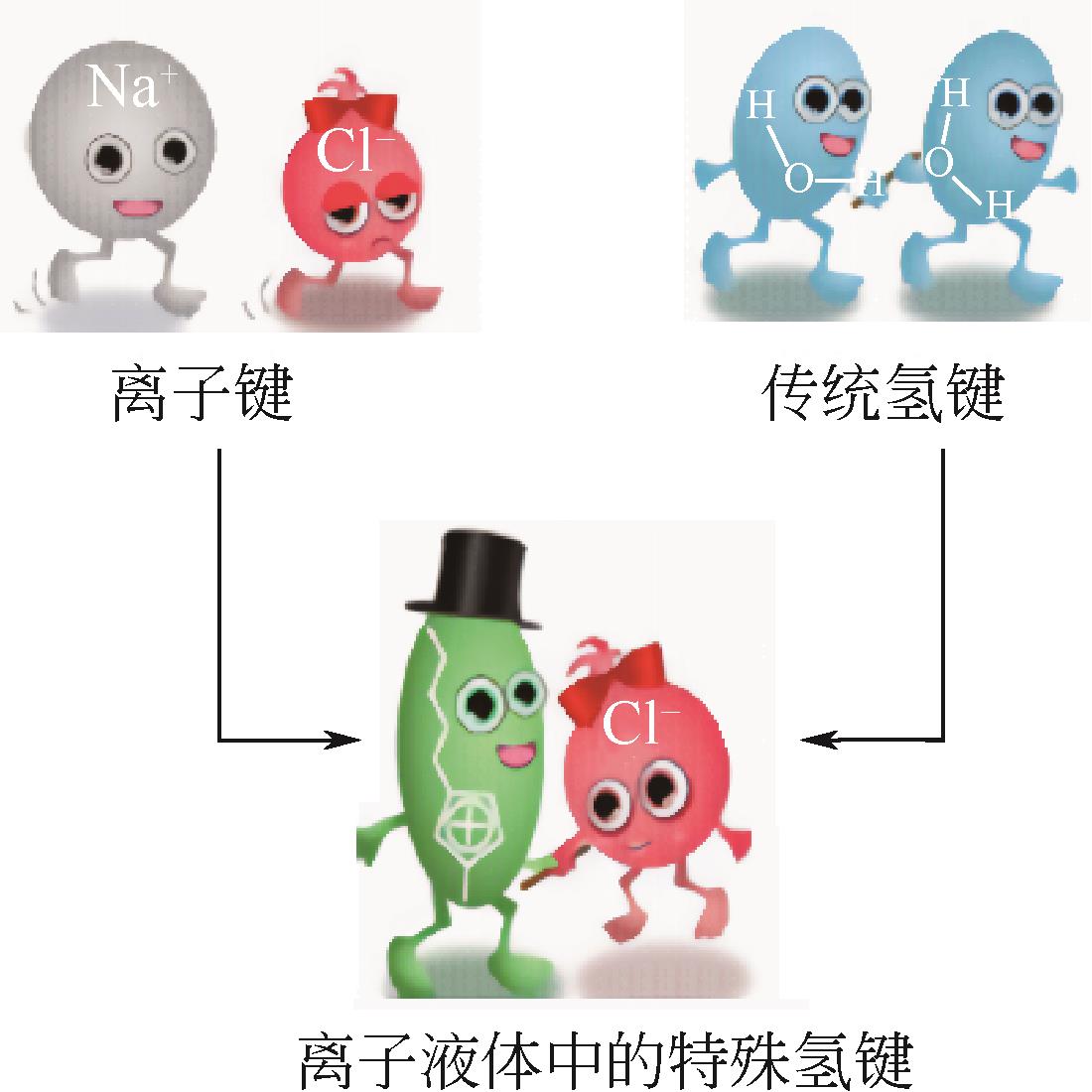
溶剂萃取分离技术广泛应用于石油化工领域,为石油石化产品的分离和提纯生产提供了有力的技术支撑。离子液体的结构可设计性、化学稳定性和热稳定性、极低的蒸气压等优点,使其在烃类化合物分离领域受到了研究者广泛关注。本文首先介绍了离子液体的性质及分类,根据分离目标不同,归纳了离子液体在芳烃-饱和烃分离、脱硫脱氮、烯烃-烷烃分离领域取得的最新进展,探讨了离子液体在油品分离领域研究中存在的问题和未来发展方向。文中指出:阳离子烷基侧链和极性是影响其对芳烃萃取效果的关键因素,然而对实际体系芳烃-饱和烃分离还有待进一步研究;离子液体对杂原子含硫含氮化合物均表现出较强的分离能力,但是碱性氮化物和非碱性氮化物不易通过一种离子液体同时脱除;氢键碱性是影响离子液体分离烯烃的关键因素,然而大部分离子液体对烯烃选择性仍然不高。根据不同的分离任务,从分子水平上认识离子液体结构与分离效果的关系,进而设计出兼具高效分离能力和低环境影响的新型离子液体,对提升油品中关键组分的高附加值转化利用具有重要意义。
中图分类号:
引用本文
倪清, 来锦波, 彭东岳, 管翠诗, 龙军. 离子液体萃取分离烃类化合物的研究进展[J]. 化工进展, 2022, 41(2): 619-627.
NI Qing, LAI Jinbo, PENG Dongyue, GUAN Cuishi, LONG Jun. Progress in extraction separation of hydrocarbons by ionic liquids[J]. Chemical Industry and Engineering Progress, 2022, 41(2): 619-627.
| 离子液体 | 温度/K | 分离体系 | 分配系数 | 选择性 | 参考文献 |
|---|---|---|---|---|---|
| 1-丁基吡啶四氟硼酸盐 | 313.2 | 庚烷+甲苯 | 0.3~0.4 | 23.4~74.4 | [ |
| 1-庚基吡啶四氟硼酸盐 | 313.2 | 庚烷+甲苯 | 0.53~0.67 | 7.4~25.6 | |
| 1-乙基-3甲基咪唑双三氟甲磺酰亚胺盐 | 313.2 | 庚烷+甲苯 | 0.66~0.88 | 9.3~37.7 | [ |
| 1-丁基-3甲基咪唑双三氟甲磺酰亚胺盐 | 313.2 | 庚烷+甲苯 | 0.76~1.26 | 5.9~25.0 | |
| 1-乙基-3-甲基咪唑甲磺酸盐 | 313.2 | 庚烷+甲苯 | 0.13~0.16 | 22.0~79.7 | [ |
| 1-乙基-3-甲基咪唑三氟甲烷磺酸盐 | 313.2 | 庚烷+甲苯 | 0.28~0.39 | 14.5~42.9 | |
| 1-乙基-3-甲基咪唑四氟乙烷磺酸盐 | 313.2 | 庚烷+甲苯 | 0.25~0.31 | 16.8~53.8 | |
| 双(1-乙基-3-甲基咪唑)硫氰钴酸盐 | 313.2 | 庚烷+甲苯 | 0.81~1.28 | 23.7~68.7 | [ |
| 1-乙基-3-甲基咪唑硫酸氢盐 | 313.2 | 庚烷+甲苯 | 0.03~0.05 | 23.1~75.4 | [ |
| 1-乙基-3-甲基咪唑硫酸甲酯盐 | 313.2 | 庚烷+甲苯 | 0.2~0.26 | 17.6~68.1 | |
| 1,4-双(1-乙基咪唑)己基二双三氟甲磺酰亚胺盐 | 303.15 | 环己烷+苯 | 24.9 | 2.25 | [ |
| 1-丁基-3-甲基咪唑氯铁酸盐 | 298.15 | 环己烷+苯 | 2.16 | 11.55 | [ |
| 1-庚基-3-甲基吡啶四氟硼酸盐 | 313.15 | 癸烷+丁苯 | 0.45 | 49.4 | [ |
表1 离子液体分离芳烃-饱和烃的比较
| 离子液体 | 温度/K | 分离体系 | 分配系数 | 选择性 | 参考文献 |
|---|---|---|---|---|---|
| 1-丁基吡啶四氟硼酸盐 | 313.2 | 庚烷+甲苯 | 0.3~0.4 | 23.4~74.4 | [ |
| 1-庚基吡啶四氟硼酸盐 | 313.2 | 庚烷+甲苯 | 0.53~0.67 | 7.4~25.6 | |
| 1-乙基-3甲基咪唑双三氟甲磺酰亚胺盐 | 313.2 | 庚烷+甲苯 | 0.66~0.88 | 9.3~37.7 | [ |
| 1-丁基-3甲基咪唑双三氟甲磺酰亚胺盐 | 313.2 | 庚烷+甲苯 | 0.76~1.26 | 5.9~25.0 | |
| 1-乙基-3-甲基咪唑甲磺酸盐 | 313.2 | 庚烷+甲苯 | 0.13~0.16 | 22.0~79.7 | [ |
| 1-乙基-3-甲基咪唑三氟甲烷磺酸盐 | 313.2 | 庚烷+甲苯 | 0.28~0.39 | 14.5~42.9 | |
| 1-乙基-3-甲基咪唑四氟乙烷磺酸盐 | 313.2 | 庚烷+甲苯 | 0.25~0.31 | 16.8~53.8 | |
| 双(1-乙基-3-甲基咪唑)硫氰钴酸盐 | 313.2 | 庚烷+甲苯 | 0.81~1.28 | 23.7~68.7 | [ |
| 1-乙基-3-甲基咪唑硫酸氢盐 | 313.2 | 庚烷+甲苯 | 0.03~0.05 | 23.1~75.4 | [ |
| 1-乙基-3-甲基咪唑硫酸甲酯盐 | 313.2 | 庚烷+甲苯 | 0.2~0.26 | 17.6~68.1 | |
| 1,4-双(1-乙基咪唑)己基二双三氟甲磺酰亚胺盐 | 303.15 | 环己烷+苯 | 24.9 | 2.25 | [ |
| 1-丁基-3-甲基咪唑氯铁酸盐 | 298.15 | 环己烷+苯 | 2.16 | 11.55 | [ |
| 1-庚基-3-甲基吡啶四氟硼酸盐 | 313.15 | 癸烷+丁苯 | 0.45 | 49.4 | [ |
| 离子液体 | 体系 | 温度/K | 脱除率 | 参考文献 |
|---|---|---|---|---|
| 1-丁基-3-甲基咪唑氯盐 | 直馏柴油 | 333.15 | 含氮量>50%,含硫量<5% | [ |
| 1-辛基吡啶氯盐 | 直馏柴油 | 333.15 | ||
| 1-丁基-3-甲基咪唑四氟硼酸盐 | 正十二烷+苯并噻吩+吡啶+哌啶 | 303.15 | 苯并噻吩12%,吡啶45%,哌啶9% | [ |
| 1-丁基-3-甲基咪唑溴盐-氯化锌 | 正十二烷+甲苯+喹啉+吲哚 | 313.15 | 喹啉94%,吲哚82% | [ |
| 页岩油柴油馏分 | 343.15 | 总氮77% | ||
| 1-甲基-3-甲基咪唑磷酸二甲酯盐 | 煤焦油柴油馏分 | 313.15 | 碱氮37%,非碱氮50% | [ |
| 1-丁基-3-甲基咪唑磷酸二乙酯盐 | 煤焦油柴油馏分 | 313.15 | 碱氮36.1%,非碱氮59.7% | |
| 1-丁基-3-甲基咪唑磷酸二丁酯盐 | 煤焦油柴油馏分 | 313.15 | 碱氮32.5%,非碱氮58.4% | |
| 1-乙基-3-甲基咪唑磷酸二氢盐 | 煤焦油柴油馏分 | 313.15 | 碱氮86.7%,非碱氮60.1% | |
| 1-乙基-3-甲基咪唑二氰胺盐 | 己烷+甲苯+吡啶+咔唑+噻吩+二苯并噻吩 | 303.15 | 吡啶69.13%,咔唑100%,噻吩49.2%,二苯并噻吩55.6% | [ |
| 1-戊基-3-甲基咪唑双三氟甲磺酰亚胺盐 | 正十二烷+正十六烷+甲苯+吡啶+噻吩+苯并噻吩 | 303.15 | 吡啶87.8%,噻吩53.58%,苯并噻吩66.29% | [ |
表2 离子液体脱硫脱氮的比较
| 离子液体 | 体系 | 温度/K | 脱除率 | 参考文献 |
|---|---|---|---|---|
| 1-丁基-3-甲基咪唑氯盐 | 直馏柴油 | 333.15 | 含氮量>50%,含硫量<5% | [ |
| 1-辛基吡啶氯盐 | 直馏柴油 | 333.15 | ||
| 1-丁基-3-甲基咪唑四氟硼酸盐 | 正十二烷+苯并噻吩+吡啶+哌啶 | 303.15 | 苯并噻吩12%,吡啶45%,哌啶9% | [ |
| 1-丁基-3-甲基咪唑溴盐-氯化锌 | 正十二烷+甲苯+喹啉+吲哚 | 313.15 | 喹啉94%,吲哚82% | [ |
| 页岩油柴油馏分 | 343.15 | 总氮77% | ||
| 1-甲基-3-甲基咪唑磷酸二甲酯盐 | 煤焦油柴油馏分 | 313.15 | 碱氮37%,非碱氮50% | [ |
| 1-丁基-3-甲基咪唑磷酸二乙酯盐 | 煤焦油柴油馏分 | 313.15 | 碱氮36.1%,非碱氮59.7% | |
| 1-丁基-3-甲基咪唑磷酸二丁酯盐 | 煤焦油柴油馏分 | 313.15 | 碱氮32.5%,非碱氮58.4% | |
| 1-乙基-3-甲基咪唑磷酸二氢盐 | 煤焦油柴油馏分 | 313.15 | 碱氮86.7%,非碱氮60.1% | |
| 1-乙基-3-甲基咪唑二氰胺盐 | 己烷+甲苯+吡啶+咔唑+噻吩+二苯并噻吩 | 303.15 | 吡啶69.13%,咔唑100%,噻吩49.2%,二苯并噻吩55.6% | [ |
| 1-戊基-3-甲基咪唑双三氟甲磺酰亚胺盐 | 正十二烷+正十六烷+甲苯+吡啶+噻吩+苯并噻吩 | 303.15 | 吡啶87.8%,噻吩53.58%,苯并噻吩66.29% | [ |
| 离子液体 | 温度/K | 压力/kPa | 体系 | 选择性 | 参考文献 |
|---|---|---|---|---|---|
| 1-丁基-3-甲基咪唑三氟甲烷磺酸盐 | 313 | 常压 | 己烯/己烷 | 1.95 | [ |
| 1-丁基-3-甲基咪唑四氟硼酸盐 | 313 | 常压 | 己烯/己烷 | 2.6 | |
| 1-丁基-3-甲基咪唑醋酸盐 | 313 | 常压 | 己烯/己烷 | 1.86 | |
| 1-乙基-3-甲基咪唑二氰胺盐 | 313 | 常压 | 己烯/己烷 | 2.58 | |
| 1-炔乙基-3-甲基咪唑双三氟甲磺酰亚胺盐 | 313 | 60~100 | 乙烯/乙烷 | 0.65 | [ |
| 1-氰丙基-3-甲基咪唑二氰胺盐 | 313 | 60~100 | 乙烯/乙烷 | 0.67 | |
| 1-丁基-3-甲基咪唑磷酸甲酯盐 | 313 | 60~100 | 乙烯/乙烷 | 1.45 | |
| 1-乙基-3-甲基咪唑双三氟甲磺酰亚胺盐 | 298 | 常压 | 环己烯/环己烷 | 1.9 | [ |
| 1-乙基-3-甲基咪唑三氰基甲烷盐 | 298 | 常压 | 环己烯/环己烷 | 3 | |
| 1-苄基-3-甲基咪唑双三氟甲磺酰亚胺盐 | 298 | 常压 | 环己烯/环己烷 | 1.76 | |
| 四丁基膦正己酸盐 | 298 | 20~160 | 乙烯/乙炔 | 21.4 | [ |
| 三己基十四烷基膦醋酸盐 | 298 | 20~160 | 乙烯/乙炔 | 9.4 | |
| 二甲基苯甲酰胺双三氟甲磺酰亚胺银盐 | 298 | 100 | 庚烯/庚烷 | 5.07 | [ |
| 298 | 100 | 辛烯/辛烷 | 3.39 | ||
| 盐酸三乙胺-氯化亚铜 | 303 | 250 | 乙烯/乙烷 | 8.78 | [ |
表3 离子液体分离烯烃-烷烃混合物的比较
| 离子液体 | 温度/K | 压力/kPa | 体系 | 选择性 | 参考文献 |
|---|---|---|---|---|---|
| 1-丁基-3-甲基咪唑三氟甲烷磺酸盐 | 313 | 常压 | 己烯/己烷 | 1.95 | [ |
| 1-丁基-3-甲基咪唑四氟硼酸盐 | 313 | 常压 | 己烯/己烷 | 2.6 | |
| 1-丁基-3-甲基咪唑醋酸盐 | 313 | 常压 | 己烯/己烷 | 1.86 | |
| 1-乙基-3-甲基咪唑二氰胺盐 | 313 | 常压 | 己烯/己烷 | 2.58 | |
| 1-炔乙基-3-甲基咪唑双三氟甲磺酰亚胺盐 | 313 | 60~100 | 乙烯/乙烷 | 0.65 | [ |
| 1-氰丙基-3-甲基咪唑二氰胺盐 | 313 | 60~100 | 乙烯/乙烷 | 0.67 | |
| 1-丁基-3-甲基咪唑磷酸甲酯盐 | 313 | 60~100 | 乙烯/乙烷 | 1.45 | |
| 1-乙基-3-甲基咪唑双三氟甲磺酰亚胺盐 | 298 | 常压 | 环己烯/环己烷 | 1.9 | [ |
| 1-乙基-3-甲基咪唑三氰基甲烷盐 | 298 | 常压 | 环己烯/环己烷 | 3 | |
| 1-苄基-3-甲基咪唑双三氟甲磺酰亚胺盐 | 298 | 常压 | 环己烯/环己烷 | 1.76 | |
| 四丁基膦正己酸盐 | 298 | 20~160 | 乙烯/乙炔 | 21.4 | [ |
| 三己基十四烷基膦醋酸盐 | 298 | 20~160 | 乙烯/乙炔 | 9.4 | |
| 二甲基苯甲酰胺双三氟甲磺酰亚胺银盐 | 298 | 100 | 庚烯/庚烷 | 5.07 | [ |
| 298 | 100 | 辛烯/辛烷 | 3.39 | ||
| 盐酸三乙胺-氯化亚铜 | 303 | 250 | 乙烯/乙烷 | 8.78 | [ |
| 1 | 张锁江, 徐春明, 吕兴梅. 离子液体与绿色化学[M]. 北京: 科学出版社, 2009. |
| ZHANG S J, XU C M, LYU X M. Ionic liquids and green chemistry[M]. Beijing: Science Press, 2009. | |
| 2 | ROGERS R D, SEDDON K R. Ionic liquids—Solvents of the future?[J]. Science, 2003, 302(5646): 792-793. |
| 3 | EARLE M J, SEDDON K R. Ionic liquids. Green solvents for the future[J]. Pure and Applied Chemistry, 2000, 72(7): 1391-1398. |
| 4 | GALIŃSKI M, LEWANDOWSKI A, STĘPNIAK I. Ionic liquids as electrolytes[J]. Electrochimica Acta, 2006, 51(26): 5567-5580. |
| 5 | SHELDON R A, LAU R M, SORGEDRAGER M J, et al. Biocatalysis in ionic liquids[J]. Green Chemistry, 2002, 4(2): 147-151. |
| 6 | 易兰, 李文英, 冯杰. 离子液体/低共熔溶剂在煤基液体分离中的应用[J]. 化工进展, 2020, 39(6): 2066-2078. |
| YI L, LI W Y, FENG J. Application of ionic liquids and deep eutectic solvents in the separation of coal-based liquids[J]. Chemical Industry and Engineering Progress, 2020, 39(6): 2066-2078. | |
| 7 | REBELO L P N, LOPES J N C, ESPERANÇA J M S S, et al. Accounting for the unique, doubly dual nature of ionic liquids from a molecular thermodynamic and modeling standpoint[J]. Accounts of Chemical Research, 2007, 40(11): 1114-1121. |
| 8 | HEIMER N E, SESTO R E DEL, MENG Z Z, et al. Vibrational spectra of imidazolium tetrafluoroborate ionic liquids[J]. Journal of Molecular Liquids, 2006, 124(1/2/3): 84-95. |
| 9 | TALATY E R, RAJA S, STORHAUG V J, et al. Raman and infrared spectra and ab initio calculations of C2~4mim imidazolium hexafluorophosphate ionic liquids[J]. The Journal of Physical Chemistry B, 2004, 108(35): 13177-13184. |
| 10 | RIBEIRO M C C. Strong anion-anion hydrogen bond in the ionic liquid 1-ethyl-3-methylimidazolium hydrogen sulfate[J]. Journal of Molecular Liquids, 2020, 310: 113178. |
| 11 | FEDOROVA I V, SAFONOVA L P. Ion pair structures and hydrogen bonding in RnNH4-n alkylammonium ionic liquids with hydrogen sulfate and mesylate anions by DFT computations[J]. The Journal of Physical Chemistry A, 2020, 124(16): 3170-3179. |
| 12 | DONG K, ZHANG S J, WANG J J. Understanding the hydrogen bonds in ionic liquids and their roles in properties and reactions[J]. Chemical Communications, 2016, 52(41): 6744-6764. |
| 13 | DUPONT J, SUAREZ P A D, DE SOUZA R F, et al. C—H-π interactions in 1-n-butyl-3-methylimidazolium tetraphenylborate molten salt: solid and solution structures[J]. Chemistry-a European Journal, 2000, 6(13): 2377-2381. |
| 14 | DONG K, SONG Y T, LIU X M, et al. Understanding structures and hydrogen bonds of ionic liquids at the electronic level[J]. The Journal of Physical Chemistry B, 2012, 116(3): 1007-1017. |
| 15 | DONG K, ZHANG S J. Hydrogen bonds: a structural insight into ionic liquids[J]. Chemistry -a European Journal, 2012, 18(10): 2748-2761. |
| 16 | DONG K, ZHANG S J, WANG D X, et al. Hydrogen bonds in imidazolium ionic liquids[J]. The Journal of Physical Chemistry A, 2006, 110(31): 9775-9782. |
| 17 | GARCIA S, LARRIBA M, GARCIA J, et al. 1-Alkyl-2,3-dimethylimidazolium bis(trifluoromethylsulfonyl)imide ionic liquids for the liquid-liquid extraction of toluene from heptane[J]. Journal of Chemical & Engineering Data, 2011, 56(8): 3468-3474. |
| 18 | LUBBEN M J, CANALES R I, LYU Y Y, et al. Promising thiolanium ionic liquid for extraction of aromatics from aliphatics: experiments and modeling[J]. Industrial & Engineering Chemistry Research, 2020, 59(35): 15707-15717. |
| 19 | GARCIA S, LARRIBA M, GARCIA J, et al. Separation of toluene from n-heptane by liquid-liquid extraction using binary mixtures of [bpy][BF4] and [4bmpy][Tf2N] ionic liquids as solvent[J]. The Journal of Chemical Thermodynamics, 2012, 53(1): 119-124. |
| 20 | GARCÍA S, GARCÍA J, LARRIBA M, et al. Sulfonate-based ionic liquids in the liquid-liquid extraction of aromatic hydrocarbons[J]. Journal of Chemical & Engineering Data, 2011, 56(7): 3188-3193. |
| 21 | LARRIBA M, NAVARRO P, DELGADO-MELLADO N, et al. Extraction of aromatic hydrocarbons from pyrolysis gasoline using tetrathiocyanatocobaltate-based ionic liquids: experimental study and simulation[J]. Fuel Processing Technology, 2017, 159: 96-110. |
| 22 | TAN L, ZHU J M, HE X D, et al. The mechanism of toluene absorption by phosphonium ionic liquids with multiple sites[J]. Journal of Molecular Liquids, 2021, 331: 115501. |
| 23 | TAN L, ZHU J M, ZHOU M, et al. The effect of imidazolium and phosphonium ionic liquids on toluene absorption studied by a molecular simulation[J]. Journal of Molecular Liquids, 2020, 298(15): 112054-112061. |
| 24 | LYU Y Y, BRENNECKE J F, STADTHERR M A. Review of recent aromatic-aliphatic-ionic liquid ternary liquid-liquid equilibria and their modeling by COSMO-RS[J]. Industrial & Engineering Chemistry Research, 2020, 59(19): 8871-8893. |
| 25 | YAO C F, HOU Y C, WU W Z, et al. Imidazolium-based dicationic ionic liquids: highly efficient extractants for separating aromatics from aliphatics[J]. Green Chemistry, 2018, 20(13): 3101-3111. |
| 26 | YAO C F, HOU Y C, REN S H, et al. Selective extraction of aromatics from aliphatics using dicationic ionic liquid-solvent composite extractants[J]. Journal of Molecular Liquids, 2019, 291: 111267. |
| 27 | ZHANG F, LI Y, ZHANG L, et al. Benzyl- and vinyl-functionalized imidazoium ionic liquids for selective separating aromatic hydrocarbons from alkanes[J]. Industrial & Engineering Chemistry Research, 2016, 55(3): 747-756. |
| 28 | GARCIA S, GARCIA J, LARRIBA M, et al. Liquid-liquid extraction of toluene from heptane by {[4bmpy][Tf2N] + [emim][CHF2CF2SO3]} ionic liquid mixed solvents[J]. Fluid Phase Equilibria, 2013, 337(15): 47-52. |
| 29 | GARCIA S, LARRIBA M, CASAS A, et al. Separation of toluene and heptane by liquid liquid extraction using binary mixtures of the ionic liquids 1-butyl-4-methylpyridinium bis(trifluoromethylsulfonyl)imide and 1-ethyl-3-methylimidazolium ethylsulfate[J]. Journal of Chemical & Engineering Data, 2012, 57(9): 2472-2478. |
| 30 | GARCIA S, LARRIBA M, GARCIA J, et al. Liquid-liquid extraction of toluene from n-heptane using binary mixtures of N-butylpyridinium tetrafluoroborate and N-butylpyridinium bis(trifluoromethylsulfonyl)imide ionic liquids[J]. Chemical Engineering Journal, 2012, 180: 210-215. |
| 31 | DELGADO-MELLADO N, GARCÍA J, RODRÍGUEZ F, et al. Insights into ionic liquid/aromatic systems from NMR spectroscopy: how water affects solubility and intermolecular interactions[J]. ChemPlusChem, 2019, 84(7): 872-881. |
| 32 | YAO C F, HOU Y C, SUN Y, et al. Extraction of aromatics from aliphatics using a hydrophobic dicationic ionic liquid adjusted with small-content water[J]. Separation and Purification Technology, 2020, 236: 116287. |
| 33 | 史军军, 吴巍, 葸雷. 离子液体1-己基-4-甲基吡啶四氟硼酸盐与烃类的相互作用规律[J]. 石油学报(石油加工), 2021, 37(3): 541-548. |
| SHI J J, WU W, XI L. Interaction mechanism between [C6MPy][BF4] ionic liquid and hydrocarbon[J]. Acta Petrolei Sinica (Petroleum Processing Section), 2021, 37(3): 541-548. | |
| 34 | BÖSMANN A, DATSEVICH L, JESS A, et al. Deep desulfurization of diesel fuel by extraction with ionic liquids[J]. Chemical Communications, 2002, 23(23): 2494-2495. |
| 35 | ZHANG S G, ZHANG Q L, ZHANG Z C. Extractive desulfurization and denitrogenation of fuels using ionic liquids[J]. Industrial & Engineering Chemistry Research, 2004, 43(2): 614-622. |
| 36 | CHEN X C, YUAN S, ABDELTAWAB A A, et al. Extractive desulfurization and denitrogenation of fuels using functional acidic ionic liquids[J]. Separation and Purification Technology, 2014, 133: 187-193. |
| 37 | ZHANG S G, CONRAD ZHANG Z. Novel properties of ionic liquids in selective sulfur removal from fuels at room temperature[J]. Green Chemistry, 2002, 4(4): 376-379. |
| 38 | ALONSO L, ARCE A, FRANCISCO M, et al. Phase behaviour of 1-methyl-3-octylimidazolium bis[trifluoromethylsulfonyl] imide with thiophene and aliphatic hydrocarbons: the influence of n-alkane chain length[J]. Fluid Phase Equilibria, 2008, 263(2): 176-181. |
| 39 | RODRÍGUEZ-CABO B, SOTO A, ARCE A. Desulfurization of fuel-oils with [C2mim][NTf2]: a comparative study[J]. The Journal of Chemical Thermodynamics, 2013, 57(1): 248-255. |
| 40 | MANZANILLA B, DOMÍNGUEZ Z, DOMÍNGUEZ-ESQUIVEL J M, et al. Study on the interactions between [BMIM][SCN] and naphtalene/ dibenzothiophene: a theory-experiment comparison[J]. Journal of Molecular Structure, 2020, 1207:127846. |
| 41 | WANG J W, SONG Z, LI X X, et al. Toward rational functionalization of ionic liquids for enhanced extractive desulfurization: computer-aided solvent design and molecular dynamics simulation[J]. Industrial & Engineering Chemistry Research, 2020, 59(5): 2093-2103. |
| 42 | NEJAD N F, SOOLARI E S, ADIBI M, et al. Imidazolium-based alkylsulfate ionic liquids and removal of sulfur content from model of gasoline[J]. Petroleum Science and Technology, 2013, 31(5): 472-480. |
| 43 | NIE Y, LI C X, SUN A J, et al. Extractive desulfurization of gasoline using imidazolium-based phosphoric ionic liquids[J]. Energy & Fuels, 2006, 20(5): 2083-2087. |
| 44 | 林赛燕, 刘丹, 王红, 等. 酸性离子液体萃取脱除焦化柴油中碱性氮化物[J]. 石油化工高等学校学报, 2012, 25(1): 8-12. |
| LIN S Y, LIU D, WANG H, et al. Removing basic nitrogen compounds from coker diesel by extraction with acidic ionic liquid[J]. Journal of Petrochemical Universities, 2012, 25(1): 8-12. | |
| 45 | WANG H, XIE C X, YU S T, et al. Denitrification of simulated oil by extraction with H2PO4-based ionic liquids[J]. Chemical Engineering Journal, 2014, 237: 286-290. |
| 46 | HIZADDIN H F, HADJ-KALI M K, RAMALINGAM A, et al. Extraction of nitrogen compounds from diesel fuel using imidazolium- and pyridinium-based ionic liquids: experiments, COSMO-RS prediction and NRTL correlation[J]. Fluid Phase Equilibria, 2015, 405: 55-67. |
| 47 | 冯锦锋. 离子液体的合成、表征及其对柴油中碱性氮的脱除研究[D]. 武汉: 武汉工程大学, 2012. |
| FENG J F. Synthesis and characterization of ionic liquid and removal basic nitrogen from diesel fuel[D]. Wuhan: Wuhan Institute of Technology, 2012. | |
| 48 | 孙汉文, 冯波, 吴远远, 等. 室温离子液体在原子光谱分析中的应用研究进展[J]. 河北大学学报(自然科学版), 2010, 30(2): 216-224. |
| SUN H W, FENG B, WU Y Y, et al. Research advancement for applications of room-temperature ionic liquid in atomic spectrometry[J]. Journal of Hebei University (Natural Science Edition), 2010, 30(2): 216-224. | |
| 49 | XIE L L, FAVRE-REGUILLON A, PELLET-ROSTAING S, et al. Selective extraction and identification of neutral nitrogen compounds contained in straight-run diesel feed using chloride based ionic liquid[J]. Industrial & Engineering Chemistry Research, 2008, 47(22): 8801-8807. |
| 50 | 周兆骞, 李文深, 刘洁. [C4mim]Br/ZnCl2离子液体脱除油品中的氮化物[J]. 石油学报(石油加工), 2017, 33(5): 934-940. |
| ZHOU Z Q, LI W S, LIU J. Removal of nitrogen compounds from fuel oil with [C4mim]Br/ZnCl2 ionic liquid[J]. Acta Petrolei Sinica (Petroleum Processing Section), 2017, 33(5): 934-940. | |
| 51 | 苏晓琳, 宋军, 杨敬一, 等. 磷酸基咪唑离子液体脱除煤焦油柴油馏分中的氮化物[J]. 化工进展, 2016, 35(4): 1081-1086. |
| SU X L, SONG J, YANG J Y, et al. Extractive denitrification of coal tar diesel fraction using phosphate-based alkylimidazolium ionic liquids[J]. Chemical Industry and Engineering Progress, 2016, 35(4): 1081-1086. | |
| 52 | ASUMANA C, YU G R, GUAN Y W, et al. Extractive denitrogenation of fuel oils with dicyanamide-based ionic liquids[J]. Green Chemistry, 2011, 13(11): 3300-3305. |
| 53 | GABRIĆ B, SANDER A, CVJETKO BUBALO M, et al. Extraction of S- and N-compounds from the mixture of hydrocarbons by ionic liquids as selective solvents[J]. The Scientific World Journal, 2013, 2013: 512953. |
| 54 | FALLANZA M, GONZÁLEZ-MIQUEL M, RUIZ E, et al. Screening of RTILs for propane/propylene separation using COSMO-RS methodology[J]. Chemical Engineering Journal, 2013, 220(15): 284-293. |
| 55 | LI R, XING H, YANG Q, et al. Selective extraction of 1-hexene against n-hexane in ionic liquids with or without silver salt[J]. Industrial & Engineering Chemistry Research, 2012, 51(25): 8588-8597. |
| 56 | MOURA L, DARWICH W, SANTINI C C, et al. Imidazolium-based ionic liquids with cyano groups for the selective absorption of ethane and ethylene[J]. Chemical Engineering Journal, 2015, 280: 755-762. |
| 57 | DOMAŃSKA U, KARPIŃSKA M, WLAZŁO M. Separation of hex-1-ene/hexane and cyclohexene/cyclohexane compounds with [EMIM]-based ionic liquids[J]. Fluid Phase Equilibria, 2016, 427: 421-428. |
| 58 | NAVARRO P, OVEJERO-PÉREZ A, AYUSO M, et al. Cyclohexane/cyclohexene separation by extractive distillation with cyano-based ionic liquids[J]. Journal of Molecular Liquids, 2019, 289(1): 111120-111130. |
| 59 | XING H B, ZHAO X, LI R L, et al. Improved efficiency of ethylene/ethane separation using a symmetrical dual nitrile-functionalized ionic liquid[J]. ACS Sustainable Chemistry & Engineering, 2013, 1(11): 1357-1363. |
| 60 | ZHAO X, XING H B, YANG Q, et al. Differential solubility of ethylene and acetylene in room-temperature ionic liquids: a theoretical study[J]. The Journal of Physical Chemistry B, 2012, 116(13): 3944-3953. |
| 61 | XING H B, ZHAO X, YANG Q W, et al. Molecular dynamics simulation study on the absorption of ethylene and acetylene in ionic liquids[J]. Industrial & Engineering Chemistry Research, 2013, 52(26): 9308-9316. |
| 62 | ZHAO X, YANG Q W, XU D, et al. Design and screening of ionic liquids for C2H2/C2H4 separation by COSMO-RS and experiments[J]. AIChE Journal, 2015, 61(6): 2016-2027. |
| 63 | AYUSO M, OVEJERO-PÉREZ A, DELGADO-MELLADO N D, et al. Tetrathiocyanatocobaltate and bis(trifluoromethylsulfonyl)imide-based ionic liquids as mass agents in the separation of cyclohexane and cyclohexene mixtures by homogeneous extractive distillation[J]. The Journal of Chemical Thermodynamics, 2021, 157: 106403-106413. |
| 64 | WANG Y, THOMPSON J, ZHOU J J, et al. Use of water in aiding olefin/paraffin (liquid+liquid) extraction via complexation with a silver bis(trifluoromethylsulfonyl)imide salt[J]. The Journal of Chemical Thermodynamics, 2014, 77: 230-240. |
| 65 | WANG Y, HAO W Y, JACQUEMIN J, et al. Enhancing liquid-phase olefin-paraffin separations using novel silver-based ionic liquids[J]. Journal of Chemical & Engineering Data, 2015, 60(1): 28-36. |
| 66 | 张睿, 董淑媛, 伍洛, 等. 小分子烷烃与烯烃在离子液体中的溶解性能[J]. 化工学报, 2020, 71(10): 4674-4687. |
| ZHANG R, DONG S Y, WU L, et al. Solubility of light alkanes and alkenes in ionic liquids[J]. CIESC Journal, 2020, 71(10): 4674-4687. |
| [1] | 贺美晋. 分子管理在炼油领域分离技术中的应用和发展趋势[J]. 化工进展, 2023, 42(S1): 260-266. |
| [2] | 崔守成, 徐洪波, 彭楠. 两种MOFs材料用于O2/He吸附分离的模拟分析[J]. 化工进展, 2023, 42(S1): 382-390. |
| [3] | 李世霖, 胡景泽, 王毅霖, 王庆吉, 邵磊. 电渗析分离提取高值组分的研究进展[J]. 化工进展, 2023, 42(S1): 420-429. |
| [4] | 郭强, 赵文凯, 肖永厚. 增强流体扰动强化变压吸附甲硫醚/氮气分离的数值模拟[J]. 化工进展, 2023, 42(S1): 64-72. |
| [5] | 许友好, 王维, 鲁波娜, 徐惠, 何鸣元. 中国炼油创新技术MIP的开发策略及启示[J]. 化工进展, 2023, 42(9): 4465-4470. |
| [6] | 廖志新, 罗涛, 王红, 孔佳骏, 申海平, 管翠诗, 王翠红, 佘玉成. 溶剂脱沥青技术应用与进展[J]. 化工进展, 2023, 42(9): 4573-4586. |
| [7] | 钱思甜, 彭文俊, 张先明. PET熔融缩聚与溶液解聚形成环状低聚物的对比分析[J]. 化工进展, 2023, 42(9): 4808-4816. |
| [8] | 潘宜昌, 周荣飞, 邢卫红. 高效分离同碳数烃的先进微孔膜:现状与挑战[J]. 化工进展, 2023, 42(8): 3926-3942. |
| [9] | 常印龙, 周启民, 王青月, 王文俊, 李伯耿, 刘平伟. 废弃聚烯烃的高值化学回收研究进展[J]. 化工进展, 2023, 42(8): 3965-3978. |
| [10] | 杨静, 李博, 李文军, 刘晓娜, 汤刘元, 刘月, 钱天伟. 焦化污染场地中萘降解菌的分离及降解特性[J]. 化工进展, 2023, 42(8): 4351-4361. |
| [11] | 吴亚, 赵丹, 方荣苗, 李婧瑶, 常娜娜, 杜春保, 王文珍, 史俊. 用于复杂原油乳液的高效破乳剂开发及应用研究进展[J]. 化工进展, 2023, 42(8): 4398-4413. |
| [12] | 刘柏成, 李法云, 赵琦慧, 吝美霞. 禾本科植物修复多环芳烃污染土壤研究进展[J]. 化工进展, 2023, 42(7): 3736-3748. |
| [13] | 谭利鹏, 申峻, 王玉高, 刘刚, 徐青柏. 煤沥青和石油沥青共混改性的研究进展[J]. 化工进展, 2023, 42(7): 3749-3759. |
| [14] | 娄宝辉, 吴贤豪, 张驰, 陈臻, 冯向东. 纳米流体用于二氧化碳吸收分离研究进展[J]. 化工进展, 2023, 42(7): 3802-3815. |
| [15] | 索寒生, 贾梦达, 宋光, 刘东庆. 数字孪生技术助力石化智能工厂[J]. 化工进展, 2023, 42(7): 3365-3373. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||