化工进展 ›› 2021, Vol. 40 ›› Issue (7): 3772-3784.DOI: 10.16085/j.issn.1000-6613.2020-1733
Ni与Co催化乙醇制氢研究进展
郭俊艳( ), 张海军(
), 张海军( ), 段红娟, 李孝建, 刘鑫, 韩磊
), 段红娟, 李孝建, 刘鑫, 韩磊
- 武汉科技大学省部共建耐火材料与冶金国家重点实验室,湖北 武汉 430081
-
收稿日期:2020-08-28修回日期:2020-11-23出版日期:2021-07-06发布日期:2021-07-19 -
通讯作者:张海军 -
作者简介:郭俊艳(1995—),女,硕士研究生,研究方向为能源催化。E-mail:1134279328@qq.com 。 -
基金资助:国家自然科学基金(51872210);湖北自然科学基金创新群体项目(2017CFA004);湖北省教育厅高等学校优秀中青年科技创新团队计划(T201602)
Recently progress of Ni and Co-based catalysts for hydrogen production from ethanol
GUO Junyan( ), ZHANG Haijun(
), ZHANG Haijun( ), DUAN Hongjuan, LI Xiaojian, LIU Xin, HAN Lei
), DUAN Hongjuan, LI Xiaojian, LIU Xin, HAN Lei
- The State Key Laboratory of Refractories and Metallurgy, Wuhan University of Science and Technology, Wuhan 430081, Hubei, China
-
Received:2020-08-28Revised:2020-11-23Online:2021-07-06Published:2021-07-19 -
Contact:ZHANG Haijun
摘要:
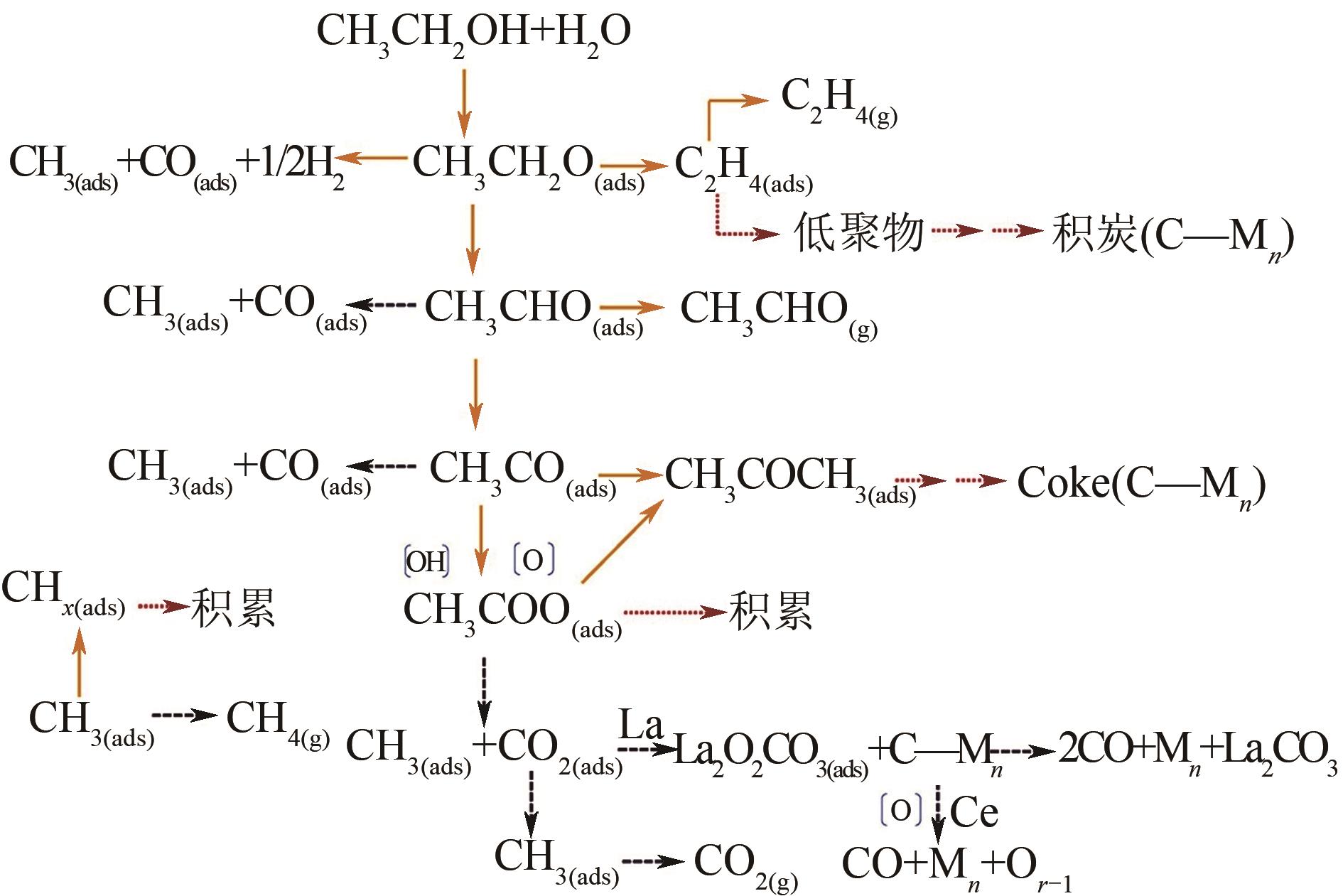
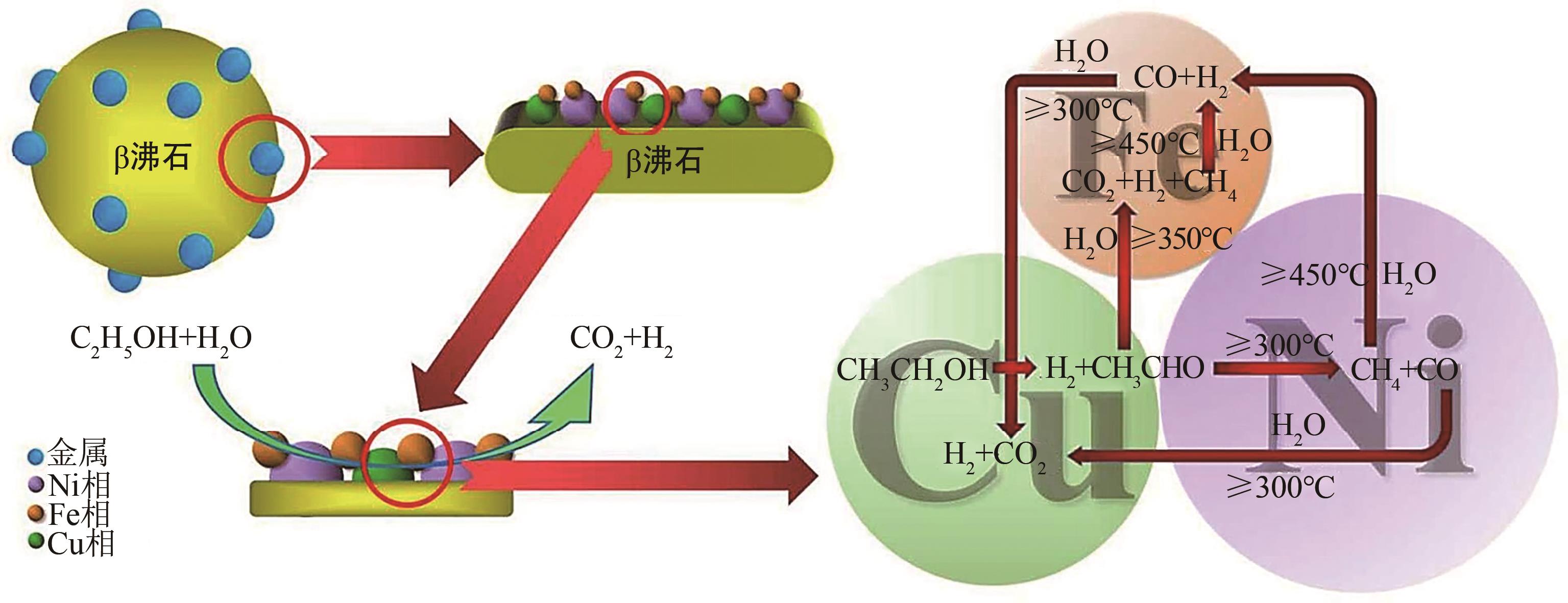
乙醇制氢具有环保、清洁、可持续等优点,其关键在于选择合适的催化剂。相较于贵金属催化剂而言,Ni与Co基催化剂因其具有成本低且活性高的优点,近年来在乙醇催化制氢中逐渐成为研究热点。然而Ni与Co基催化剂在催化乙醇水蒸气重整制氢的过程中仍存在着稳定性较差、低温催化活性低和使用寿命较短等问题。本文介绍了乙醇制氢的机理,围绕载体的类型及催化剂活性纳米颗粒的合金化改性,综述了近年来Ni与Co基催化剂催化乙醇水蒸气重整制氢的研究现状,总结了提高催化剂性能的方法及措施,并展望了Ni与Co基催化剂未来的发展方向,为颗粒尺寸小且低温催化活性高、稳定性好的Ni与Co基催化剂的制备提供思路。
中图分类号:
引用本文
郭俊艳, 张海军, 段红娟, 李孝建, 刘鑫, 韩磊. Ni与Co催化乙醇制氢研究进展[J]. 化工进展, 2021, 40(7): 3772-3784.
GUO Junyan, ZHANG Haijun, DUAN Hongjuan, LI Xiaojian, LIU Xin, HAN Lei. Recently progress of Ni and Co-based catalysts for hydrogen production from ethanol[J]. Chemical Industry and Engineering Progress, 2021, 40(7): 3772-3784.
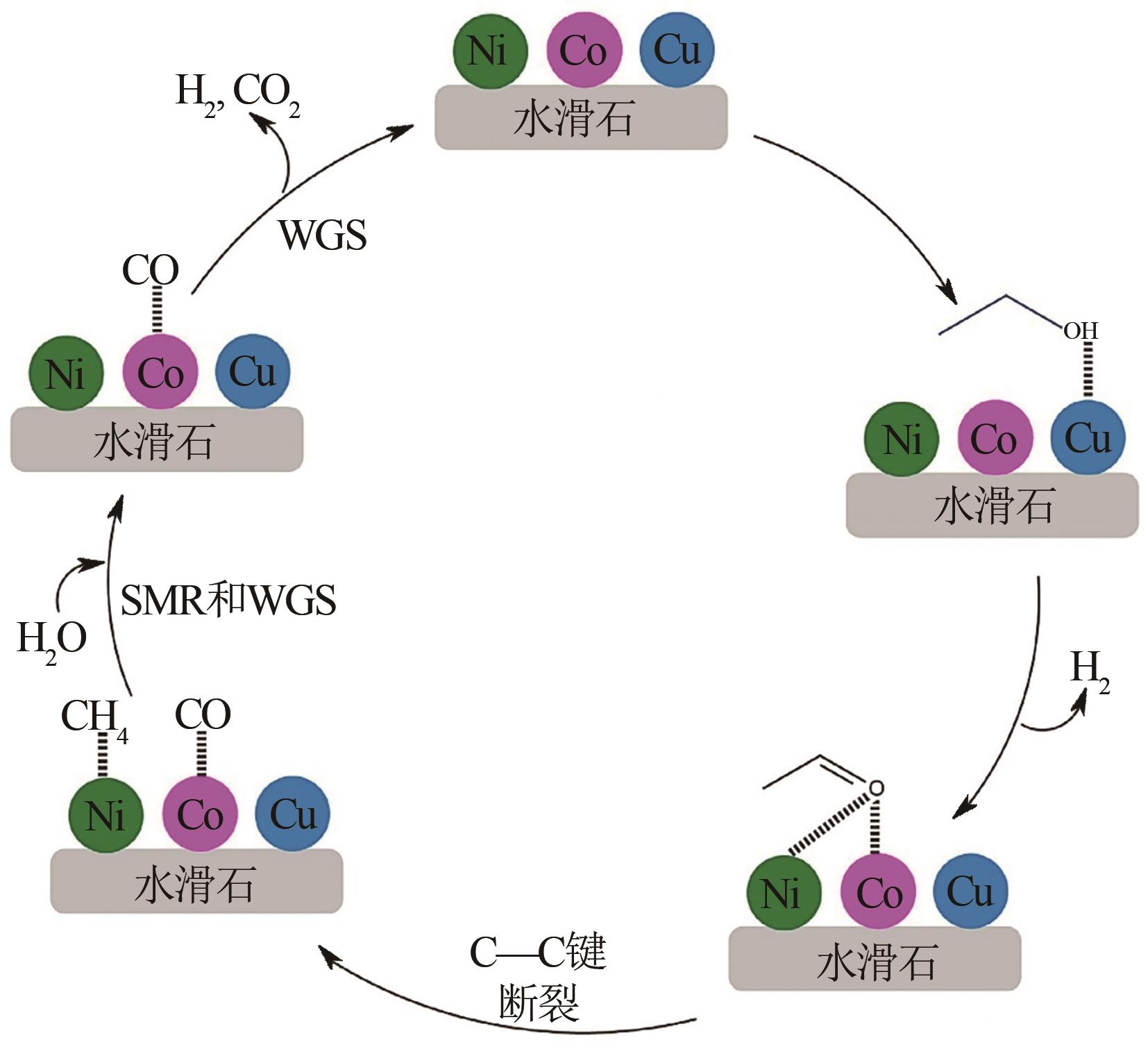
| 化学反应 | 反应方程式 | 编号 | |
|---|---|---|---|
| 乙醇脱氢反应 | C2H5OH | ? | (4) |
| 乙醇分解反应 | C2H5OH | ? | (5) |
| 乙醛水蒸气重整 | CH3CHO+H2O | ? | (6) |
| 水煤气变换反应 | CO+H2O | ? | (7) |
| 甲烷水蒸气重整 | CH4+H2O | ? | (8) |
| 乙醇脱水反应 | C2H5OH | ? | (9) |
| 乙醛分解反应 | CH3CHO | ? | (10) |
| 乙醛羟醛缩合反应 | 2CH3CHO | ? | (11) |
| 一氧化碳甲烷化反应 | CO+3H2 | ? | (12) |
| 二氧化碳甲烷化反应 | CO2+4H2 | ? | (13) |
| 乙烯分解反应 | C2H4 | ? | (14) |
| 甲烷分解反应 | CH4 | ? | (15) |
| 一氧化碳歧化反应 | 2CO | ? | (16) |
表1 乙醇制氢反应可能发生的副反应
| 化学反应 | 反应方程式 | 编号 | |
|---|---|---|---|
| 乙醇脱氢反应 | C2H5OH | ? | (4) |
| 乙醇分解反应 | C2H5OH | ? | (5) |
| 乙醛水蒸气重整 | CH3CHO+H2O | ? | (6) |
| 水煤气变换反应 | CO+H2O | ? | (7) |
| 甲烷水蒸气重整 | CH4+H2O | ? | (8) |
| 乙醇脱水反应 | C2H5OH | ? | (9) |
| 乙醛分解反应 | CH3CHO | ? | (10) |
| 乙醛羟醛缩合反应 | 2CH3CHO | ? | (11) |
| 一氧化碳甲烷化反应 | CO+3H2 | ? | (12) |
| 二氧化碳甲烷化反应 | CO2+4H2 | ? | (13) |
| 乙烯分解反应 | C2H4 | ? | (14) |
| 甲烷分解反应 | CH4 | ? | (15) |
| 一氧化碳歧化反应 | 2CO | ? | (16) |
| 催化剂 | 制备方法 | 金属负载量 (质量分数)/% | 乙醇转化率/% | H2产率/% | 反应条件 | 参考文献 |
|---|---|---|---|---|---|---|
| Ni/多孔SiO2 | 浸渍法 | 10 | 95 | 47 | T=873K、S/E①=9、 W/F②=18g·h·mol-1 | [ |
| Ni/SiO2纤维 | 浸渍法 | 10 | 100 | 55 | ||
| Ni/SiO2纤维 | 共沉淀法 | 10 | 100 | 60 | ||
| Ni/SiO2纤维 | 静电吸附法 | 10 | 100 | 65 | ||
| Ni/SBA-15 | 浸渍法 | 6 | 100 | 82.5 | T=823K、S/E=3、 | [ |
| Ni/β沸石 | 溶胶-凝胶等体积 浸渍法 | 15 | 100 | 78 | T=823K、S/E = 6、 WHSV④=30h-1 | [ |
| Ni/Al2O3-TiO2 | 一步法 | 10 | 93 | 88 | T=773K、S/E=3、 WHSV=2773h-1 | [ |
| Ni/La2O3-α-Al2O3 | 浸渍法 | 10 | 100 | 82 | T=873K、S/E=6、 W/F=0.35g·h·g-1 | [ |
| Ni/La2O3-α-Al2O3 | 浸渍法 | 15 | 100 | 62 | T=723K、S/E=3、 GHSV⑤=23140mL·g-1·h-1 | [ |
| Ni/La2O3-CeO2 | 浸渍法 | 8 | 100 | 70 | T=823K、S/E=6、 | [ |
| Ni/La2O3-CeO2-ZrO2 | 浸渍法 | 10 | 90 | 85 | T=773K、S/E =3、 GHSV=64.8h-1 | [ |
| Ni/凹凸棒石 | 共沉淀法 | 20 | 95 | 78 | T=873K、S/E = 1.5、 WHSV=12.6h-1 | [ |
| Ni/Mg-凹凸棒石 | 共沉淀法 | 20 | 100 | 96.7 | T=973K、S/E=1.5、 GHSV=19200~19400h-1 | [ |
| Au-Ni/CaO-CeO2 | 浸渍法 | 10 | 100 | 75 | T=773K、S/E=3、 | [ |
| Ni/水滑石 | 浸渍法 | 10 | 75 | 68 | T=673K、S/E=9、 GHSV=2700mL·g-1·h-1 | [ |
| Cu-Ni-Co/水滑石 | 浸渍法 | 10 | 85 | 83 | ||
| Ni/CeO2 | 浸渍法 | 10 | 100 | 60 | T=873K、S/E=3、 | [ |
| Ni/CeO2 | 浸渍法 | 10 | 100 | 68 | T=693K、S/E=12、 GHSV=60000mL·g-1·h-1 | [ |
| K-Ni/CeO2 | 100 | 85 | ||||
| Ni/CeO2 | 浸渍法 | 13 | 89 | 71 | T=873K、S/E=6、 GHSV=20000mL·g-1·h-1 | [ |
| 4Cu-Ni/CeO2 | 99 | 73 | ||||
| 4Co-Ni/CeO2 | 91 | 73 | ||||
| 4Mg-Ni/CeO2 | 96 | 74 | ||||
| Ni/La2O3-CeO2 | 浸渍法 | 10 | 100 | 88 | T=733K、S/E=12、 GHSV=60000mL·g-1·h-1 | [ |
| Ni/Ce0.5Zr0.5O2 | 浸渍法 | 10 | 100 | 73 | T=723K、S/E=13、 GHSV=22000h-1 | [ |
| Ni/Ce0.5Zr0.5O2 | 共沉淀法 | 10 | 100 | 89 | ||
| B-Ni/Ce0.5Zr0.5O2 | 共沉淀法 | 10 | 100 | 92 | ||
| Ni/MgO-CaO | 共沉淀法 | 5 | 100 | 97.2 | T=873K、S/E=4、 GHSV=2620mL·g-1·h-1 | [ |
| Ni/脱铝β沸石 | 浸渍法 | 10 | 100 | 75 | T=773K、S/E = 12、 WHSV=9.5h-1 | [ |
| Ce-Ni/SBA-15 | 表面活性剂辅助 等容浸渍法 | 3 | 100 | 97 | T=923K、S/E=4、 W/F=1.7g·h·mol-1 | [ |
| Ce-Ni/蒙脱石 | 超声波辅助阳离子交换浸渍法 | 10 | 97.3 | 88.5 | T=923K、S/E=4、 | [ |
| Fe-Cu-Ni/β沸石 | 浸渍法 | 10 | 100 | 72.1 | T=773K、S/E=8、 WHSV=7.35h -1 | [ |
| Rh-Ni/La2O3-CeO2-Al2O3 | 浸渍法 | 10 | 100 | 69 | T=773K、S/E=3、 GHSV=26000h -1 | [ |
表2 Ni基催化剂催化乙醇水蒸气重整的活性
| 催化剂 | 制备方法 | 金属负载量 (质量分数)/% | 乙醇转化率/% | H2产率/% | 反应条件 | 参考文献 |
|---|---|---|---|---|---|---|
| Ni/多孔SiO2 | 浸渍法 | 10 | 95 | 47 | T=873K、S/E①=9、 W/F②=18g·h·mol-1 | [ |
| Ni/SiO2纤维 | 浸渍法 | 10 | 100 | 55 | ||
| Ni/SiO2纤维 | 共沉淀法 | 10 | 100 | 60 | ||
| Ni/SiO2纤维 | 静电吸附法 | 10 | 100 | 65 | ||
| Ni/SBA-15 | 浸渍法 | 6 | 100 | 82.5 | T=823K、S/E=3、 | [ |
| Ni/β沸石 | 溶胶-凝胶等体积 浸渍法 | 15 | 100 | 78 | T=823K、S/E = 6、 WHSV④=30h-1 | [ |
| Ni/Al2O3-TiO2 | 一步法 | 10 | 93 | 88 | T=773K、S/E=3、 WHSV=2773h-1 | [ |
| Ni/La2O3-α-Al2O3 | 浸渍法 | 10 | 100 | 82 | T=873K、S/E=6、 W/F=0.35g·h·g-1 | [ |
| Ni/La2O3-α-Al2O3 | 浸渍法 | 15 | 100 | 62 | T=723K、S/E=3、 GHSV⑤=23140mL·g-1·h-1 | [ |
| Ni/La2O3-CeO2 | 浸渍法 | 8 | 100 | 70 | T=823K、S/E=6、 | [ |
| Ni/La2O3-CeO2-ZrO2 | 浸渍法 | 10 | 90 | 85 | T=773K、S/E =3、 GHSV=64.8h-1 | [ |
| Ni/凹凸棒石 | 共沉淀法 | 20 | 95 | 78 | T=873K、S/E = 1.5、 WHSV=12.6h-1 | [ |
| Ni/Mg-凹凸棒石 | 共沉淀法 | 20 | 100 | 96.7 | T=973K、S/E=1.5、 GHSV=19200~19400h-1 | [ |
| Au-Ni/CaO-CeO2 | 浸渍法 | 10 | 100 | 75 | T=773K、S/E=3、 | [ |
| Ni/水滑石 | 浸渍法 | 10 | 75 | 68 | T=673K、S/E=9、 GHSV=2700mL·g-1·h-1 | [ |
| Cu-Ni-Co/水滑石 | 浸渍法 | 10 | 85 | 83 | ||
| Ni/CeO2 | 浸渍法 | 10 | 100 | 60 | T=873K、S/E=3、 | [ |
| Ni/CeO2 | 浸渍法 | 10 | 100 | 68 | T=693K、S/E=12、 GHSV=60000mL·g-1·h-1 | [ |
| K-Ni/CeO2 | 100 | 85 | ||||
| Ni/CeO2 | 浸渍法 | 13 | 89 | 71 | T=873K、S/E=6、 GHSV=20000mL·g-1·h-1 | [ |
| 4Cu-Ni/CeO2 | 99 | 73 | ||||
| 4Co-Ni/CeO2 | 91 | 73 | ||||
| 4Mg-Ni/CeO2 | 96 | 74 | ||||
| Ni/La2O3-CeO2 | 浸渍法 | 10 | 100 | 88 | T=733K、S/E=12、 GHSV=60000mL·g-1·h-1 | [ |
| Ni/Ce0.5Zr0.5O2 | 浸渍法 | 10 | 100 | 73 | T=723K、S/E=13、 GHSV=22000h-1 | [ |
| Ni/Ce0.5Zr0.5O2 | 共沉淀法 | 10 | 100 | 89 | ||
| B-Ni/Ce0.5Zr0.5O2 | 共沉淀法 | 10 | 100 | 92 | ||
| Ni/MgO-CaO | 共沉淀法 | 5 | 100 | 97.2 | T=873K、S/E=4、 GHSV=2620mL·g-1·h-1 | [ |
| Ni/脱铝β沸石 | 浸渍法 | 10 | 100 | 75 | T=773K、S/E = 12、 WHSV=9.5h-1 | [ |
| Ce-Ni/SBA-15 | 表面活性剂辅助 等容浸渍法 | 3 | 100 | 97 | T=923K、S/E=4、 W/F=1.7g·h·mol-1 | [ |
| Ce-Ni/蒙脱石 | 超声波辅助阳离子交换浸渍法 | 10 | 97.3 | 88.5 | T=923K、S/E=4、 | [ |
| Fe-Cu-Ni/β沸石 | 浸渍法 | 10 | 100 | 72.1 | T=773K、S/E=8、 WHSV=7.35h -1 | [ |
| Rh-Ni/La2O3-CeO2-Al2O3 | 浸渍法 | 10 | 100 | 69 | T=773K、S/E=3、 GHSV=26000h -1 | [ |
| 催化剂 | 制备方法 | 金属负载量 (质量分数)/% | 乙醇转化率/% | H2产率/% | 反应条件 | 参考文献 |
|---|---|---|---|---|---|---|
| Co/SiO2 | 浸渍法 | 20 | 100 | 75 | T=873K、S/E=6、 GHSV=51700h-1 | [ |
| Co/CeO2 | 浸渍法 | 10 | 97.1 | 93.3 | T=723K、S/E=10、 WHSV=0.19~2.88g·g-1·h-1 | [ |
| Co/Al2O3 | 浸渍法 | 15 | 100 | 80 | T=873K、S/E=5、 | [ |
| Na-Co/Al2O3 | 100 | 90 | ||||
| K-Co/Al2O3 | 100 | 90 | ||||
| Ce-Co/Al2O3 | 100 | 86 | ||||
| Ni-Co/Al2O3 | 15Co 5Ni | 100 | 88 | |||
| Co/CaO-Al2O3 | 浸渍法 | 15 | 100 | 74.6 | T=723K、S/E=3、 GHSV=23139mL·g-1·h-1 | [ |
| Co-Ce/海泡石 | 表面活性剂辅助 共沉淀法 | 10 | 96.2 | 77.9 | T=873K、S/E=3、 WHSV=21h-1 | [ |
| Co-Ce/海泡石 | 共沉淀法 | 10 | 90.8 | 69.1 | T=873K、S/E=3、 WHSV=21.5h-1 | [ |
| Ru-Co/活性炭 | 一步法 | 1 | 99 | 77 | T=823K、S/E=3、 GHSV=89.4h-1 | [ |
| Ni-Co/CeO2 | 浸渍法 | 10Ni 10Co | 95 | 59 | T=773K、S/E=6、 W/F=0.12g·h·mol-1 | [ |
| 反相微乳液 共沉淀法 | 85 | 56 | ||||
| Ni-Co/MCM-41 | 浸渍法 | 9Ni 1Co | 90 | 80 | T=763K、S/E=5、 | [ |
| MC-Ni-Co/Zr | 软模板水热法及 浸渍法 | — | 95 | 65 | T=648K、S/E=12、 GHSV=15L·g-1·h-1 | [ |
| OMC-Ni-Co/Zr | 软模板水热法及 浸渍法 | — | 83 | 68.2 | T=648K、S/E=12、 GHSV=15L·g-1·h-1 | [ |
| Co/Al2O3 | 浸渍法 | 10 | 99.5 | 86.1 | T=823K、S/E=3、 WHSV=8.0h-1 | [ |
| Co/CeO2 | 共沉淀法 | 29 | 100 | 96 | T=773K、S/E=12、 GHSV=60000mL·g-1·h-1 | [ |
| Co/CeO2 | 浸渍法 | 10 | 100 | 84.6 | T=693K、S/E=3、 | [ |
| K-Co/CeO2 | 100 | 98.5 | ||||
| Ce-Co/CeO2 | 浸渍法 | 10 | 100 | 86 | T=813K、S/E=4、 GHSV=60000mL·g-1·h-1 | [ |
| Co/La2O3–CeO2 | 浸渍法 | 10 | 100 | 94 | T=773K、S/E=12、 GHSV=60000mL·g-1·h-1 | [ |
| Co/Y2O3-ZrO2 | 浸渍法 | 3 | 100 | 70 | T=773K、S/E=13、 GHSV=4500h-1 | [ |
| La-Co/Y2O3-ZrO2 | 99 | 73 | ||||
| Co/Zn-Al水滑石 衍生物 | 共沉淀法 | 20 | 100 | 73.7 | T=773K、S/E=10、 GHSV=4700h-1 | [ |
| La-Co/Zn-Al水滑石衍生物 | 100 | 75.5 | ||||
| Ni-Co/Al2O3 | 浸渍法 | 7.5Ni 7.5Co | 97.2 | 88.9 | T=823K、S/E=13、 LHSV①=7.5h -1 | [ |
| Ni-Co/SBA-15 | 共沉淀法 | 8Ni 2Co | 85.6 | 62.4 | T=773K、S/E=3.7、 | [ |
| LaNi0.7Co0.3O3/ZrO2 | 柠檬酸盐络合法 | — | 100 | 75 | T=673K、S/E=3、 GHSV=66000mL·g-1·h-1 | [ |
表3 Co基催化剂催化乙醇水蒸气重整的活性
| 催化剂 | 制备方法 | 金属负载量 (质量分数)/% | 乙醇转化率/% | H2产率/% | 反应条件 | 参考文献 |
|---|---|---|---|---|---|---|
| Co/SiO2 | 浸渍法 | 20 | 100 | 75 | T=873K、S/E=6、 GHSV=51700h-1 | [ |
| Co/CeO2 | 浸渍法 | 10 | 97.1 | 93.3 | T=723K、S/E=10、 WHSV=0.19~2.88g·g-1·h-1 | [ |
| Co/Al2O3 | 浸渍法 | 15 | 100 | 80 | T=873K、S/E=5、 | [ |
| Na-Co/Al2O3 | 100 | 90 | ||||
| K-Co/Al2O3 | 100 | 90 | ||||
| Ce-Co/Al2O3 | 100 | 86 | ||||
| Ni-Co/Al2O3 | 15Co 5Ni | 100 | 88 | |||
| Co/CaO-Al2O3 | 浸渍法 | 15 | 100 | 74.6 | T=723K、S/E=3、 GHSV=23139mL·g-1·h-1 | [ |
| Co-Ce/海泡石 | 表面活性剂辅助 共沉淀法 | 10 | 96.2 | 77.9 | T=873K、S/E=3、 WHSV=21h-1 | [ |
| Co-Ce/海泡石 | 共沉淀法 | 10 | 90.8 | 69.1 | T=873K、S/E=3、 WHSV=21.5h-1 | [ |
| Ru-Co/活性炭 | 一步法 | 1 | 99 | 77 | T=823K、S/E=3、 GHSV=89.4h-1 | [ |
| Ni-Co/CeO2 | 浸渍法 | 10Ni 10Co | 95 | 59 | T=773K、S/E=6、 W/F=0.12g·h·mol-1 | [ |
| 反相微乳液 共沉淀法 | 85 | 56 | ||||
| Ni-Co/MCM-41 | 浸渍法 | 9Ni 1Co | 90 | 80 | T=763K、S/E=5、 | [ |
| MC-Ni-Co/Zr | 软模板水热法及 浸渍法 | — | 95 | 65 | T=648K、S/E=12、 GHSV=15L·g-1·h-1 | [ |
| OMC-Ni-Co/Zr | 软模板水热法及 浸渍法 | — | 83 | 68.2 | T=648K、S/E=12、 GHSV=15L·g-1·h-1 | [ |
| Co/Al2O3 | 浸渍法 | 10 | 99.5 | 86.1 | T=823K、S/E=3、 WHSV=8.0h-1 | [ |
| Co/CeO2 | 共沉淀法 | 29 | 100 | 96 | T=773K、S/E=12、 GHSV=60000mL·g-1·h-1 | [ |
| Co/CeO2 | 浸渍法 | 10 | 100 | 84.6 | T=693K、S/E=3、 | [ |
| K-Co/CeO2 | 100 | 98.5 | ||||
| Ce-Co/CeO2 | 浸渍法 | 10 | 100 | 86 | T=813K、S/E=4、 GHSV=60000mL·g-1·h-1 | [ |
| Co/La2O3–CeO2 | 浸渍法 | 10 | 100 | 94 | T=773K、S/E=12、 GHSV=60000mL·g-1·h-1 | [ |
| Co/Y2O3-ZrO2 | 浸渍法 | 3 | 100 | 70 | T=773K、S/E=13、 GHSV=4500h-1 | [ |
| La-Co/Y2O3-ZrO2 | 99 | 73 | ||||
| Co/Zn-Al水滑石 衍生物 | 共沉淀法 | 20 | 100 | 73.7 | T=773K、S/E=10、 GHSV=4700h-1 | [ |
| La-Co/Zn-Al水滑石衍生物 | 100 | 75.5 | ||||
| Ni-Co/Al2O3 | 浸渍法 | 7.5Ni 7.5Co | 97.2 | 88.9 | T=823K、S/E=13、 LHSV①=7.5h -1 | [ |
| Ni-Co/SBA-15 | 共沉淀法 | 8Ni 2Co | 85.6 | 62.4 | T=773K、S/E=3.7、 | [ |
| LaNi0.7Co0.3O3/ZrO2 | 柠檬酸盐络合法 | — | 100 | 75 | T=673K、S/E=3、 GHSV=66000mL·g-1·h-1 | [ |
| 1 | 黄格省, 李锦山, 魏寿祥, 等. 化石原料制氢技术发展现状与经济性分析[J]. 化工进展, 2019, 38(12): 5217-5224. |
| HUANG G S, LI J S, WEI S X, et al. Status and economic analysis of hydrogen production technology from fossil raw materials[J]. Chemical Industry and Engineering Progress, 2019, 38(12): 5217-5224. | |
| 2 | 赵永志, 蒙波, 陈霖新, 等. 氢能源的利用现状分析[J]. 化工进展, 2015, 34(9): 3248-3255. |
| ZHAO Y Z, MENG B, CHEN L X, et al. Utilization status of hydrogen energy[J]. Chemical Industry and Engineering Progress, 2015, 34(9): 3248-3255. | |
| 3 | 谢欣烁, 杨卫娟, 施伟, 等. 制氢技术的生命周期评价研究进展[J]. 化工进展, 2018, 37(6): 2147-2158. |
| XIE X S, YANG W J, SHI W, et al. Life cycle assessment of technologies for hydrogen production—A review[J]. Chemical Industry and Engineering Progress, 2018, 37(6): 2147-2158. | |
| 4 | ZANCHET D, SANTOS J B O, DAMYANOVA S, et al. Toward understanding metal-catalyzed ethanol reforming[J]. ACS Catalysis, 2015, 5(6): 3841-3863. |
| 5 | HUANG L, CHOONG C, CHEN L, et al. Oxide-supported Rh catalysts for H2 generation from low-temperature ethanol steam reforming: effects of support, Rh precursor and Rh loading on catalytic performance[J]. RSC Advances, 2015, 5(120): 99461-99482. |
| 6 | SENGODAN S, LAN R, HUMPHREYS J, et al. Advances in reforming and partial oxidation of hydrocarbons for hydrogen production and fuel cell applications[J]. Renewable and Sustainable Energy Reviews, 2018, 82(1): 761-780. |
| 7 | VIZCAÍNO A J, CARRERO A, CALLES J A. Comparison of ethanol steam reforming using Co and Ni catalysts supported on SBA-15 modified by Ca and Mg[J]. Fuel Processing Technology, 2016, 146(1): 99-109. |
| 8 | OGO S, SEKINE Y. Recent progress in ethanol steam reforming using non-noble transition metal catalysts: a review[J]. Fuel Processing Technology, 2020, 199: 106238. |
| 9 | PIREZ C, FANG W, CAPRON M, et al. Steam reforming, partial oxidation and oxidative steam reforming for hydrogen production from ethanol over cerium nickel based oxyhydride catalyst[J]. Applied Catalysis A, 2016, 518: 78-86. |
| 10 | FANG W, ROMANI Y, WEI Y, et al. Steam reforming and oxidative steam reforming for hydrogen production from bioethanol over Mg2AlNixHzOy nano-oxyhydride catalysts[J]. International Journal of Hydrogen Energy, 2018, 43(37): 17643-17655. |
| 11 | NANDA S, RANA R, ZHENG Y, et al. Insights on pathways for hydrogen generation from ethanol[J]. Sustainable Energy & Fuels, 2017, 1(6): 1232-1245. |
| 12 | 陈俊, 舒红飞, 阮祝华, 等. 乙醇催化重整产氢催化剂及催化反应机制[J]. 化工进展, 2019, 38(5): 2320-2328. |
| CHEN J, SHU H F, RUAN Z H, et al. Catalysts and catalytic mechanism for hydrogen production from ethanol steam reforming (ESR)[J]. Chemical Industry and Engineering Progress, 2019, 38(5): 2320-2328. | |
| 13 | 赵华博, 李民, 罗刚. 镍基乙醇水汽重整催化剂:机理、失活与构效关系[J]. 化工进展, 2018, 37(2): 419-428. |
| ZHAO H B, LI M, LUO G. Nickel based ethanol steam reforming catalysts: mechanism, deactivation and structure-activity relationship[J]. Chemical Industry and Engineering Progress, 2018, 37(2): 419-428. | |
| 14 | MHADMHAN S, NATEWONG P, PRASONGTHUM N, et al. Investigation of Ni/SiO2 fiber catalysts prepared by different methods on hydrogen production from ethanol steam reforming[J]. Catalysts, 2018, 8(8): 319. |
| 15 | HE S, MEI Z, LIU N, et al. Ni/SBA-15 catalysts for hydrogen production by ethanol steam reforming: effect of nickel precursor[J]. International Journal of Hydrogen Energy, 2017, 42(21): 14429-14438. |
| 16 | WANG S, HE B, TIAN R, et al. Ni-hierarchical beta zeolite catalysts were applied to ethanol steam reforming: effect of sol-gel method on loading Ni and the role of hierarchical structure[J]. Molecular Catalysis, 2018, 453: 64-73. |
| 17 | GONÇALVES A A S, FAUSTINO P B, ASSAF J M, et al. One-pot synthesis of mesoporous Ni-Ti-Al ternary oxides: highly active and selective catalysts for steam reforming of ethanol[J]. ACS Applied Materials & Interfaces, 2017, 9(7): 6079-6092. |
| 18 | MONTERO C, REMIRO A, BENITO P L, et al. Optimum operating conditions in ethanol steam reforming over a Ni/La2O3-α-Al2O3 catalyst in a fluidized bed reactor[J]. Fuel Processing Technology, 2018, 169: 207-216. |
| 19 | SONG J H, YOO S, YOO J, et al. Hydrogen production by steam reforming of ethanol over Ni/Al2O3-La2O3 xerogel catalysts[J]. Molecular Catalysis, 2017, 434: 123-133. |
| 20 | PIZZOLITTO C, MENEGAZZO F, GHEDINI E, et al. Increase of ceria redox ability by lanthanum addition on Ni based catalysts for hydrogen production[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(11): 13867-13876. |
| 21 | ZHURKA M D, LEMONIDOU A A, KECHAGIOPOULOS P N. Elucidation of metal and support effects during ethanol steam reforming over Ni and Rh based catalysts supported on (CeO2)-ZrO2-La2O3[J]. Catalysis Today, 2020, 20: 12733. |
| 22 | WANG Y, WANG C, CHEN M, et al. Hydrogen production from steam reforming ethanol over Ni/attapulgite catalysts — Part Ⅰ: Effect of nickel content[J]. Fuel Processing Technology, 2019, 192: 227-238. |
| 23 | CHEN M, WANG Y, YANG Z, et al. Effect of Mg-modified mesoporous Ni/Attapulgite catalysts on catalytic performance and resistance to carbon deposition for ethanol steam reforming[J]. Fuel, 2018, 220: 32-46. |
| 24 | MENEGAZZO F, PIZZOLITTO C, ZANARDO D, et al. Hydrogen production by ethanol steam reforming on Ni-based catalysts: effect of the support and of CaO and Au doping[J]. Chemistryselect, 2017, 2(29): 9523-9531. |
| 25 | SHEJALE A D, YADAV G D. Cu promoted Ni-Co/hydrotalcite catalyst for improved hydrogen production in comparison with several modified Ni-based catalysts via steam reforming of ethanol[J]. International Journal of Hydrogen Energy, 2017, 42(16): 11321-11332. |
| 26 | ARAIZA D G, GÓMEZ-CORTÉS A, DÍAZ G. Effect of ceria morphology on the carbon deposition during steam reforming of ethanol over Ni/CeO2 catalysts[J]. Catalysis Today, 2020, 349: 235-243. |
| 27 | SŁOWIK G, GRELUK M, ROTKO M, et al. Evolution of the structure of unpromoted and potassium-promoted ceria-supported nickel catalysts in the steam reforming of ethanol[J]. Applied Catalysis B, 2018, 221: 490-509. |
| 28 | NIAZI Z, IRANKHAH A, WANG Y, et al. Cu, Mg and Co effect on nickel-ceria supported catalysts for ethanol steam reforming reaction[J]. International Journal of Hydrogen Energy, 2020, 45(41): 21512-21522. |
| 29 | GRELUK M, ROTKO M, TURCZYNIAK-SURDACKA S. Enhanced catalytic performance of La2O3 promoted Co/CeO2 and Ni/CeO2 catalysts for effective hydrogen production by ethanol steam reforming[J]. Renewable Energy, 2020, 155: 378-395. |
| 30 | WU R C, TANG C W, HUANG H H, et al. Effect of boron doping and preparation method of Ni/Ce0.5Zr0.5O2 catalysts on the performance for steam reforming of ethanol[J]. International Journal of Hydrogen Energy, 2019, 44(28): 14279-14289. |
| 31 | SANG S, ZHAO Z J, TIAN H, et al. Promotional role of MgO on sorption‐enhanced steam reforming of ethanol over Ni/CaO catalysts[J]. AIChE Journal, 2019, 66(4): 16877. |
| 32 | GAC W, GRELUK M, SŁOWIK G, et al. Effects of dealumination on the performance of Ni-containing BEA catalysts in bioethanol steam reforming[J]. Applied Catalysis B, 2018, 237: 94-109. |
| 33 | LI D, ZENG L, LI X, et al. Ceria-promoted Ni/SBA-15 catalysts for ethanol steam reforming with enhanced activity and resistance to deactivation[J]. Applied Catalysis B, 2015, 176/177: 532-541. |
| 34 | LI L, TANG D, SONG Y, et al. Hydrogen production from ethanol steam reforming on Ni-Ce/MMT catalysts[J]. Energy, 2018, 149: 937-943. |
| 35 | ZHENG Z, SUN C, DAI R, et al. Ethanol steam reforming on Ni-based catalysts: effect of Cu and Fe addition on the catalytic activity and resistance to deactivation[J]. Energy & Fuels, 2017, 31(3): 3091-3100. |
| 36 | CAMPOS C H, PECCHI G, FIERRO J L G, et al. Enhanced bimetallic Rh-Ni supported catalysts on alumina doped with mixed lanthanum-cerium oxides for ethanol steam reforming[J]. Molecular Catalysis, 2019, 469: 87-97. |
| 37 | PRASONGTHUM N, XIAO R, ZHANG H, et al. Highly active and stable Ni supported on CNTs-SiO2 fiber catalysts for steam reforming of ethanol[J]. Fuel Processing Technology, 2017, 160: 185-195. |
| 38 | WU C, DUPONT V, NAHIL M A, et al. Investigation of Ni/SiO2 catalysts prepared at different conditions for hydrogen production from ethanol steam reforming[J]. Journal of the Energy Institute, 2017, 90(2): 276-284. |
| 39 | PRASONGTHUM N, CHAIYA C, SAMART C, et al. Co-production of hydrogen and carbon nanotube-silica fiber composites from ethanol steam reforming over an Ni-silica fiber catalyst[J]. Monatshefte für Chemie: Chemical Monthly, 2017, 148(7): 1311-1321. |
| 40 | MULEWA W, TAHIR M, AMIN N A S. MMT-supported Ni/TiO2 nanocomposite for low temperature ethanol steam reforming toward hydrogen production[J]. Chemical Engineering Journal, 2017, 326: 956-969. |
| 41 | LI T, ZHANG J, XIE X, et al. Montmorillonite-supported Ni nanoparticles for efficient hydrogen production from ethanol steam reforming[J]. Fuel, 2015, 143: 55-62. |
| 42 | MOOGI S, LEE I G, PARK J Y. Effect of La2O3 and CeO2 loadings on formation of nickel-phyllosilicate precursor during preparation of Ni/SBA-15 for hydrogen-rich gas production from ethanol steam reforming[J]. International Journal of Hydrogen Energy, 2019, 44(56): 29537-29546. |
| 43 | MONTERO C, REMIRO A, ARANDIA A, et al. Reproducible performance of a Ni/La2O3-α-Al2O3 catalyst in ethanol steam reforming under reaction-regeneration cycles[J]. Fuel Processing Technology, 2016, 152: 215-222. |
| 44 | MONDAL T, PANT K K, DALAI A K. Catalytic oxidative steam reforming of bio-ethanol for hydrogen production over Rh promoted Ni/CeO2-ZrO2 catalyst[J]. International Journal of Hydrogen Energy, 2015, 40(6): 2529-2544. |
| 45 | RUOCCO C, PALMA V, RICCA A. Hydrogen production by oxidative reforming of ethanol in a fluidized bed reactor using a Pt-Ni/CeO2-SiO2 catalyst[J]. International Journal of Hydrogen Energy, 2019, 44(25): 12661-12670. |
| 46 | RUOCCO C, PALMA V, RICCA A. Experimental and kinetic study of oxidative steam reforming of ethanol over fresh and spent bimetallic catalysts[J]. Chemical Engineering Journal, 2019, 377: 119778. |
| 47 | 赵燕凌, 代蓉, 郑子良, 等. Cu/Ni负载型Beta分子筛催化剂的乙醇水蒸气重整制氢催化性能研究[J]. 燃料化学学报, 2017, 45(11): 1392-1400. |
| ZHAO Y L, DAI R, ZHENG Z L, et al. Beta zeolite supported Cu/Ni catalyst for hydrogen production through ethanol steam reforming[J]. Journal of Fuel Chemistry and Technology, 2017, 45(11): 1392-1400. | |
| 48 | 连晨帅, 代蓉, 田韧, 等. Ni-Cu双金属催化剂上乙醇水蒸气重整制氢研究——制备方法对催化性能的影响[J]. 分子催化, 2019, 33(4): 297-308. |
| LIAN C S, DAI R, TIAN R, et al. The effect of preparation method on catalytic properties over Ni-Cu bimetallic catalysts for steam reforming of ethanol[J]. Journal of Molecular Catalysis, 2019, 33(4): 297-308. | |
| 49 | RIANI P, GARBARINO G, CAVATTONI T, et al. CO2 hydrogenation and ethanol steam reforming over Co/SiO2 catalysts: deactivation and selectivity switches[J]. Catalysis Today, 2020. DOI: 10.1016/j.cattod. 2020.05.002. |
| 50 | SOHN H, CELIK G, GUNDUZ S, et al. Oxygen mobility in pre-reduced nano- and macro-ceria with CO loading: an AP-XPS, in-situ DRIFTS and TPR study[J]. Catalysis Letters, 2017, 147(11): 2863-2876. |
| 51 | YUHANG L, ZHANMING Z, PENG J, et al. Ethanol steam reforming over cobalt catalysts: effect of a range of additives on the catalytic behaviors[J]. Journal of the Energy Institute, 2020, 93(1): 165-184. |
| 52 | HAN S J, SONG J H, YOO J, et al. Sorption-enhanced hydrogen production by steam reforming of ethanol over mesoporous Co/CaO-Al2O3 xerogel catalysts: effect of Ca/Al molar ratio[J]. International Journal of Hydrogen Energy, 2017, 42(9): 5886-5898. |
| 53 | WANG C, WANG Y, CHEN M, et al. Hydrogen production from ethanol steam reforming over Co-Ce/sepiolite catalysts prepared by a surfactant assisted coprecipitation method[J]. International Journal of Hydrogen Energy, 2019, 44(49): 26888-26904. |
| 54 | CHEN M, WANG C, WANG Y, et al. Hydrogen production from ethanol steam reforming: effect of Ce content on catalytic performance of Co/sepiolite catalyst[J]. Fuel, 2019, 247: 344-355. |
| 55 | EFIMOV M N, MIRONOVA E Y, PAVLOV A A, et al. Novel polyacrylonitrile-based C/Co-Ru metal-carbon nanocomposites as effective catalysts for ethanol steam reforming[J]. International Journal of Nanoscience, 2020, 19(4): 1950031. |
| 56 | PINTON N, VIDAL M V, SIGNORETTO M, et al. Ethanol steam reforming on nanostructured catalysts of Ni, Co and CeO2: influence of synthesis method on activity, deactivation and regenerability[J]. Catalysis Today, 2017, 296: 135-143. |
| 57 | NEJAT T, JALALINEZHAD P, HORMOZI F, et al. Hydrogen production from steam reforming of ethanol over Ni-Co bimetallic catalysts and MCM-41 as support[J]. Journal of the Taiwan Institute of Chemical Engineers, 2019, 97: 216-226. |
| 58 | SHAHED GHARAHSHIRAN V, YOUSEFPOUR M, AMINI V. A comparative study of zirconia and yttria promoted mesoporous carbon-nickel-cobalt catalysts in steam reforming of ethanol for hydrogen production[J]. Molecular Catalysis, 2020, 484: 110767. |
| 59 | GHARAHSHIRAN V S, YOUSEFPOUR M. Synthesis and characterization of Zr-promoted Ni-Co bimetallic catalyst supported OMC and investigation of its catalytic performance in steam reforming of ethanol[J]. International Journal of Hydrogen Energy, 2018, 43(14): 7020-7037. |
| 60 | FERENCZ Z, VARGA E, PUSKÁS R, et al. Reforming of ethanol on Co/Al2O3 catalysts reduced at different temperatures[J]. Journal of Catalysis, 2018, 358: 118-130. |
| 61 | GRELUK M, ROTKO M, SŁOWIK G, et al. Hydrogen production by steam reforming of ethanol over Co/CeO2 catalysts: effect of cobalt content[J]. Journal of the Energy Institute, 2019, 92(2): 222-238. |
| 62 | TURCZYNIAK S, GRELUK M, SŁOWIK G, et al. Surface state and catalytic performance of ceria-supported cobalt catalysts in the steam reforming of ethanol[J]. ChemCatChem, 2017, 9(5): 782-797. |
| 63 | GRELUK M, ROTKO M, TURCZYNIAK-SURDACKA S. Comparison of catalytic performance and coking resistant behaviors of cobalt- and nickel based catalyst with different Co/Ce and Ni/Ce molar ratio under SRE conditions[J]. Applied Catalysis A, 2020, 590: 117334. |
| 64 | GAUDILLERE C, GONZÁLEZ J J, CHICA A, et al. YSZ monoliths promoted with Co as catalysts for the production of H2 by steam reforming of ethanol[J]. Applied Catalysis A, 2017, 538: 165-173. |
| 65 | CERDÁ-MORENO C, COSTA-SERRA J F DA, CHICA A. Co and La supported on Zn-hydrotalcite-derived material as efficient catalyst for ethanol steam reforming[J]. International Journal of Hydrogen Energy, 2019, 44(25): 12685-12692. |
| 66 | ZHAO X, LU G. Modulating and controlling active species dispersion over Ni-Co bimetallic catalysts for enhancement of hydrogen production of ethanol steam reforming[J]. International Journal of Hydrogen Energy, 2016, 41(5): 3349-3362. |
| 67 | RODRIGUEZ-GOMEZ A, CABALLERO A. Bimetallic Ni-Co/SBA-15 catalysts for reforming of ethanol: how cobalt modifies the nickel metal phase and product distribution[J]. Molecular Catalysis, 2018, 449: 122-130. |
| 68 | ZHAO L, HAN T, WANG H, et al. Ni-Co alloy catalyst from LaNi1-xCoxO3 perovskite supported on zirconia for steam reforming of ethanol[J]. Applied Catalysis B, 2016, 187: 19-29. |
| 69 | SOHN H, OZKAN U S. Cobalt-based catalysts for ethanol steam reforming: an overview[J]. Energy & Fuels, 2016, 30(7): 5309-5322. |
| 70 | OGO S, SHIMIZU T, NAKAZAWA Y, et al. Steam reforming of ethanol over K promoted Co catalyst[J]. Applied Catalysis A, 2015, 495: 30-38. |
| 71 | 王云珠, 泮子恒, 赵燚, 等. 吸附强化蒸汽重整制氢中CO2固体吸附剂的研究进展[J]. 化工进展, 2019, 38(11): 5103-5113. |
| WANG Y Z, PAN Z H, ZHAO Y, et al. Research progress in CO2 solid sorbents for hydrogen production by sorption-enhanced steam reforming: a review[J]. Chemical Industry and Engineering Progress, 2019, 38(11): 5103-5113. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [6] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [7] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [8] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| [9] | 高彦静. 单原子催化技术国际研究态势分析[J]. 化工进展, 2023, 42(9): 4667-4676. |
| [10] | 张启, 赵红, 荣峻峰. 质子交换膜燃料电池中氧还原反应抗毒性电催化剂研究进展[J]. 化工进展, 2023, 42(9): 4677-4691. |
| [11] | 王伟涛, 鲍婷玉, 姜旭禄, 何珍红, 王宽, 杨阳, 刘昭铁. 醛酮树脂基非金属催化剂催化氧气氧化苯制备苯酚[J]. 化工进展, 2023, 42(9): 4706-4715. |
| [12] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [13] | 吴海波, 王希仑, 方岩雄, 纪红兵. 3D打印催化材料开发与应用进展[J]. 化工进展, 2023, 42(8): 3956-3964. |
| [14] | 王报英, 王皝莹, 闫军营, 汪耀明, 徐铜文. 聚合物包覆膜在金属分离回收中的研究进展[J]. 化工进展, 2023, 42(8): 3990-4004. |
| [15] | 向阳, 黄寻, 魏子栋. 电催化有机合成反应的活性和选择性调控研究进展[J]. 化工进展, 2023, 42(8): 4005-4014. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||