化工进展 ›› 2021, Vol. 40 ›› Issue (6): 3203-3214.DOI: 10.16085/j.issn.1000-6613.2020-1318
非金属氮掺杂活性炭催化剂制备及其催化CH4-CO2重整反应
秦晓伟1,2( ), 张国杰1,2(
), 张国杰1,2( ), 李晟1,2, 郭晓菲1,2, 阎煌煜1,2, 徐英1,2, 刘俊1,2
), 李晟1,2, 郭晓菲1,2, 阎煌煜1,2, 徐英1,2, 刘俊1,2
- 1.太原理工大学省部共建煤基能源清洁高效利用国家重点实验室,山西 太原 030024
2.太原理工大学煤科学与 技术教育部重点实验室,山西 太原 030024
-
收稿日期:2020-07-13修回日期:2020-11-11出版日期:2021-06-06发布日期:2021-06-22 -
通讯作者:张国杰 -
作者简介:秦晓伟(1995—),男,硕士研究生,研究方向为甲烷-二氧化碳重整催化剂的制备。E-mail:229413119@qq.com 。 -
基金资助:国家自然科学基金(21878200)
Preparation of metal-free nitrogen-doped activated carbon as catalysts for carbon dioxide reforming of methane
QIN Xiaowei1,2( ), ZHANG Guojie1,2(
), ZHANG Guojie1,2( ), LI Sheng1,2, GUO Xiaofei1,2, YAN Huangyu1,2, XU Ying1,2, LIU Jun1,2
), LI Sheng1,2, GUO Xiaofei1,2, YAN Huangyu1,2, XU Ying1,2, LIU Jun1,2
- 1.State Key Laboratory of Clean and Efficient Coal Utilization, Taiyuan University of Technology, Taiyuan 030024, Shanxi, China
2.Key Laboratory of Coal Science and Technology, Ministry of Education, Taiyuan University of Technology, Taiyuan 030024, Shanxi, China
-
Received:2020-07-13Revised:2020-11-11Online:2021-06-06Published:2021-06-22 -
Contact:ZHANG Guojie
摘要:
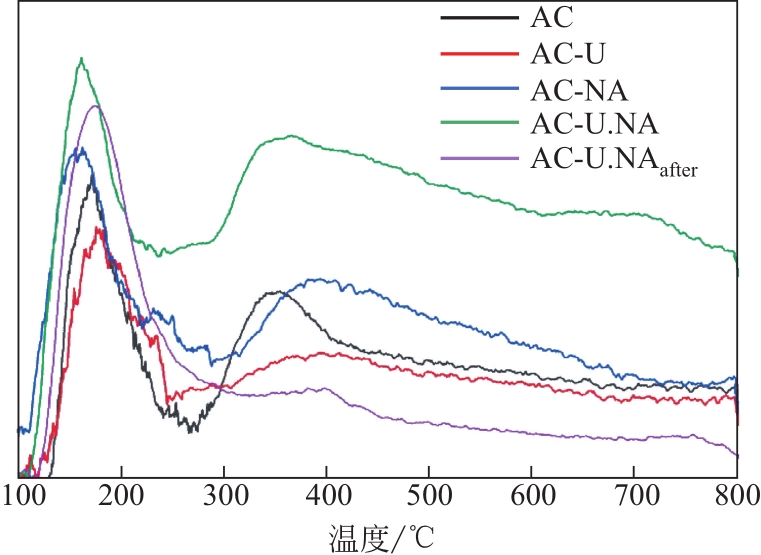
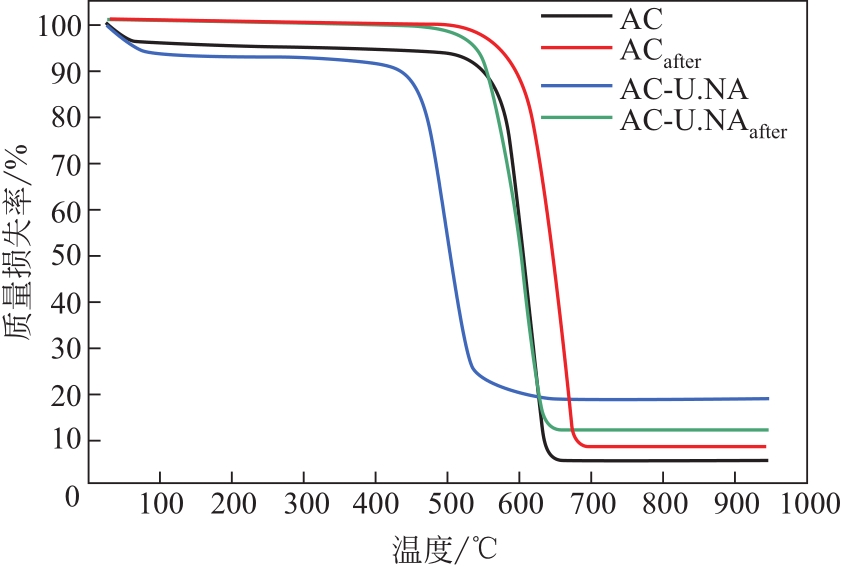
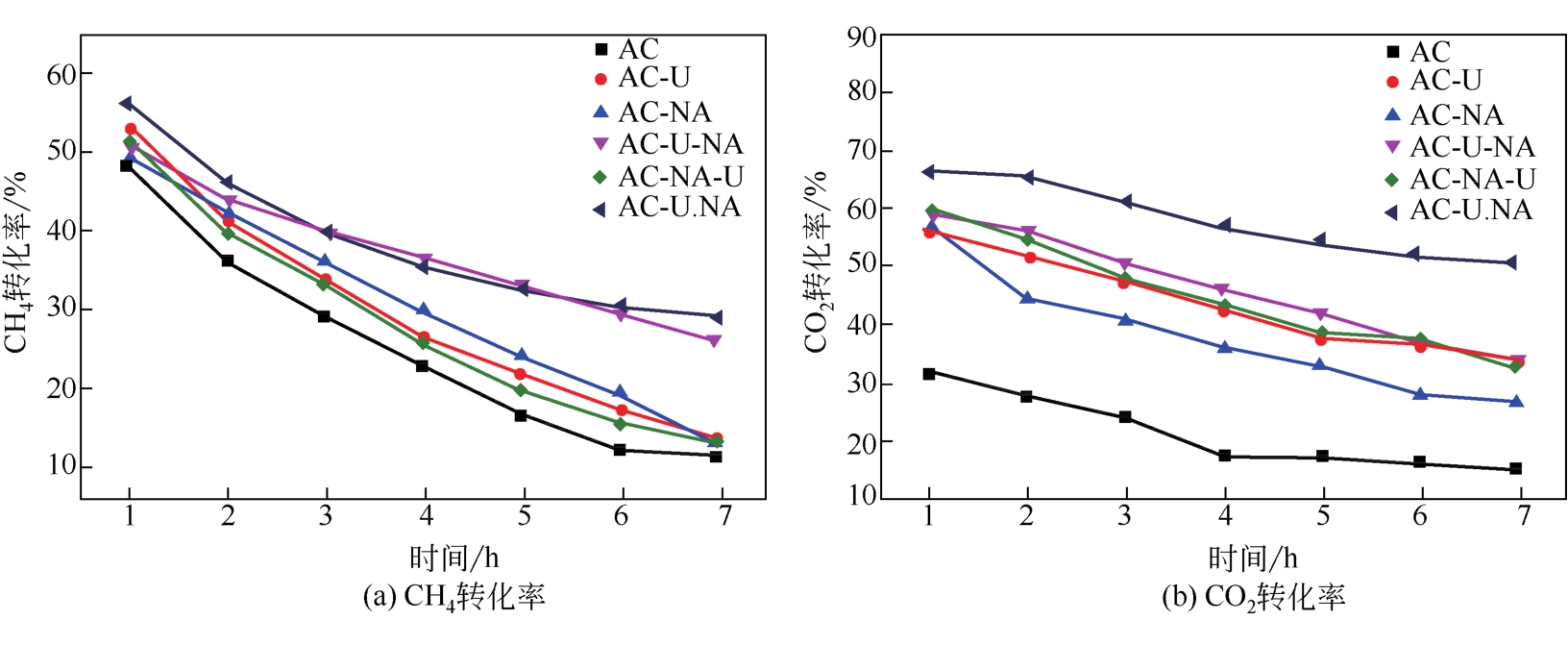
采用硝酸和尿素联合对活性炭进行改性,制备了富含氮元素的氮掺杂活性炭,考察了孔结构、氮含量和氮种类(吡啶氮、吡咯氮和石墨氮)对CH4-CO2重整反应催化性能的影响。采用BET、SEM、EA、FTIR、XPS、CO2-TPD和TG表征手段对反应前后催化剂的物理化学性质进行了表征,对引入活性炭表面的含氮官能团的种类及其在重整过程中所起的作用进行了分析。相比于未改性的原活性炭,硝酸和尿素同时改性制备的氮掺杂活性炭(AC-U.NA)引入了更多的羟基官能团和含氮官能团。特别是通过两者共同改性后,所制备的氮掺杂活性炭引入的吡啶氮官能团比例明显提高,为CH4-CO2重整反应提供了更多的活性位点,初始CH4和CO2转化率达到55.94%和66.46%。同时经过两者联合改性后,所制备的AC-U.NA材料表面具有极性,不仅有利于酸性CO2分子的吸附和活化,而且有利于CO2消碳反应,减少了积炭的生成,对所制备的非金属重整催化剂的活性和抗积炭性具有重要的意义。
中图分类号:
引用本文
秦晓伟, 张国杰, 李晟, 郭晓菲, 阎煌煜, 徐英, 刘俊. 非金属氮掺杂活性炭催化剂制备及其催化CH4-CO2重整反应[J]. 化工进展, 2021, 40(6): 3203-3214.
QIN Xiaowei, ZHANG Guojie, LI Sheng, GUO Xiaofei, YAN Huangyu, XU Ying, LIU Jun. Preparation of metal-free nitrogen-doped activated carbon as catalysts for carbon dioxide reforming of methane[J]. Chemical Industry and Engineering Progress, 2021, 40(6): 3203-3214.
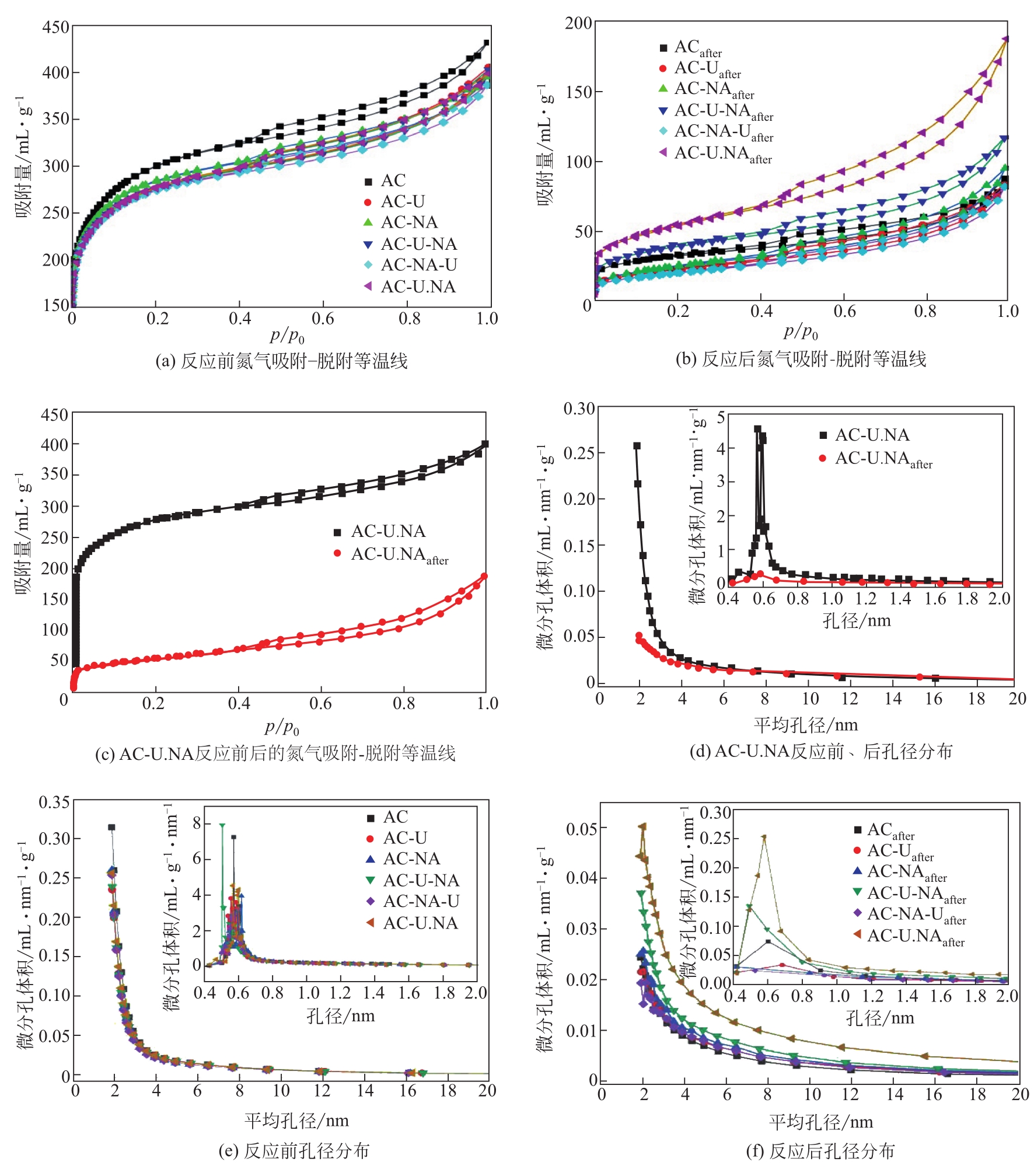
| 样品 | 比表面积/m2·g-1 | 总孔体积/mL·g-1 | 平均孔径/nm | |||
|---|---|---|---|---|---|---|
| 反应前 | 反应后① | 反应前 | 反应后① | 反应前 | 反应后① | |
| AC | 981.42 | 110.22 | 0.67 | 0.14 | 2.72 | 4.94 |
| AC-U | 886.97 | 82.83 | 0.63 | 0.13 | 2.83 | 6.26 |
| AC-NA | 919.60 | 91.39 | 0.61 | 0.15 | 2.66 | 6.45 |
| AC-U-NA | 890.36 | 136.36 | 0.62 | 0.18 | 2.80 | 5.31 |
| AC-NA-U | 887.71 | 74.46 | 0.60 | 0.13 | 2.69 | 6.86 |
| AC-U.NA | 899.46 | 189.63 | 0.62 | 0.29 | 2.74 | 6.12 |
表1 反应前后催化剂孔结构参数
| 样品 | 比表面积/m2·g-1 | 总孔体积/mL·g-1 | 平均孔径/nm | |||
|---|---|---|---|---|---|---|
| 反应前 | 反应后① | 反应前 | 反应后① | 反应前 | 反应后① | |
| AC | 981.42 | 110.22 | 0.67 | 0.14 | 2.72 | 4.94 |
| AC-U | 886.97 | 82.83 | 0.63 | 0.13 | 2.83 | 6.26 |
| AC-NA | 919.60 | 91.39 | 0.61 | 0.15 | 2.66 | 6.45 |
| AC-U-NA | 890.36 | 136.36 | 0.62 | 0.18 | 2.80 | 5.31 |
| AC-NA-U | 887.71 | 74.46 | 0.60 | 0.13 | 2.69 | 6.86 |
| AC-U.NA | 899.46 | 189.63 | 0.62 | 0.29 | 2.74 | 6.12 |
| 样品 | 氮含量(质量分数)/% | 碳含量(质量分数)/% | 氢含量(质量分数)/% | 氧含量(质量分数)/% |
|---|---|---|---|---|
| AC | 0.19 | 88.38 | 3.69 | 7.60 |
| AC-U | 1.82 | 84.27 | 1.14 | 12.63 |
| AC-NA | 0.45 | 84.84 | 1.28 | 12.06 |
| AC-U-NA | 1.39 | 84.24 | 1.43 | 12.80 |
| AC-NA-U | 2.39 | 84.03 | 1.26 | 12.18 |
| AC-U.NA | 1.97 | 84.74 | 1.07 | 12.08 |
| AC-U.NAafter | 0.47 | 89.93 | 0.88 | 8.58 |
表2 不同催化剂的元素含量
| 样品 | 氮含量(质量分数)/% | 碳含量(质量分数)/% | 氢含量(质量分数)/% | 氧含量(质量分数)/% |
|---|---|---|---|---|
| AC | 0.19 | 88.38 | 3.69 | 7.60 |
| AC-U | 1.82 | 84.27 | 1.14 | 12.63 |
| AC-NA | 0.45 | 84.84 | 1.28 | 12.06 |
| AC-U-NA | 1.39 | 84.24 | 1.43 | 12.80 |
| AC-NA-U | 2.39 | 84.03 | 1.26 | 12.18 |
| AC-U.NA | 1.97 | 84.74 | 1.07 | 12.08 |
| AC-U.NAafter | 0.47 | 89.93 | 0.88 | 8.58 |
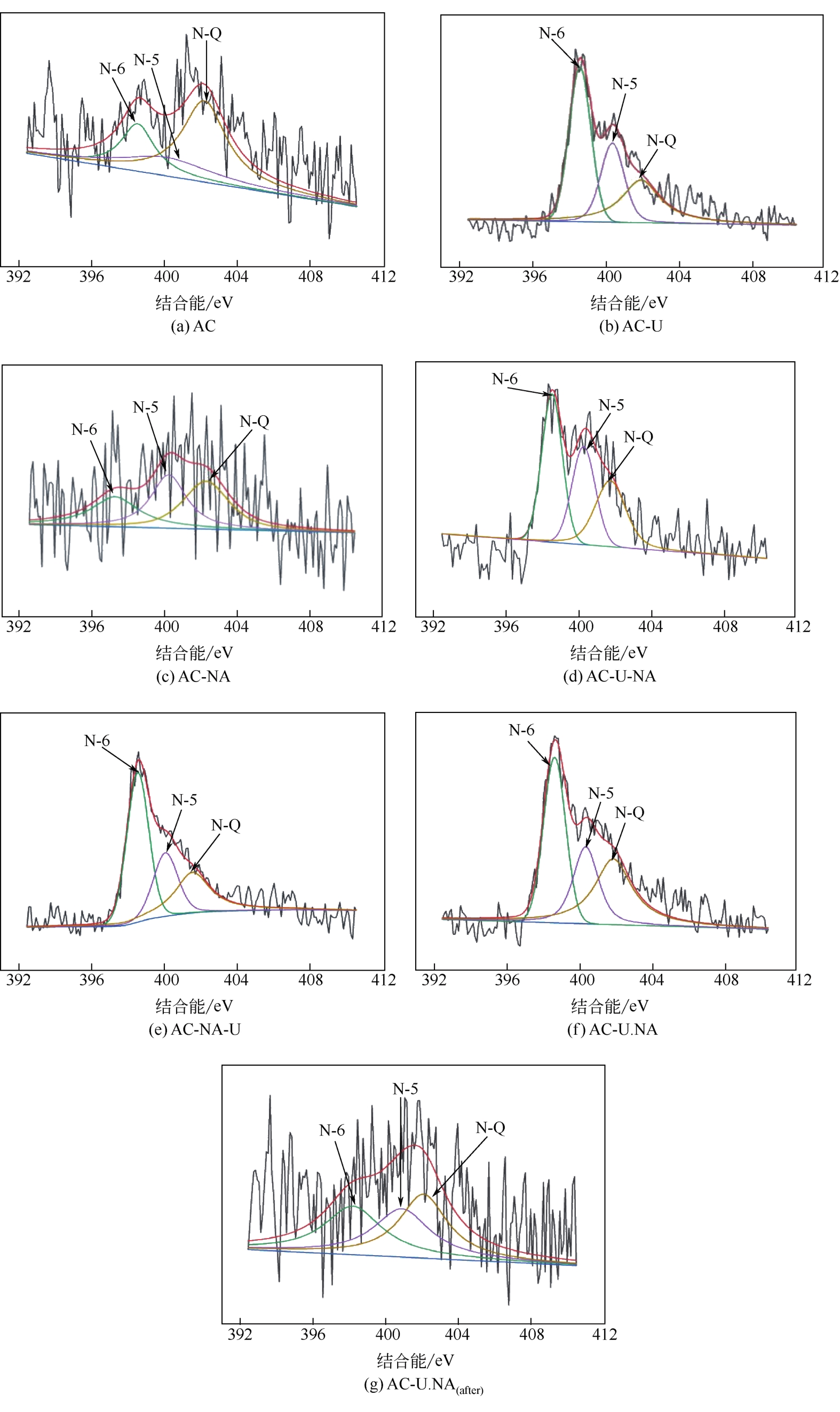
| 样品 | 氮含量(质量分数) /% | 氮物种占比 | 吡啶氮含量 (质量分数)/% | 吡咯氮含量 (质量分数)/% | 石墨氮含量 (质量分数)/% | ||
|---|---|---|---|---|---|---|---|
| 吡啶氮占比/% | 吡咯氮占比/% | 石墨氮占比/% | |||||
| AC | 0.21 | 22.95 | 24.69 | 52.36 | 0.05 | 0.05 | 0.11 |
| AC-U | 2.04 | 42.74 | 25.99 | 31.27 | 0.87 | 0.53 | 0.64 |
| AC-NA | 0.36 | 27.76 | 35.31 | 36.93 | 0.10 | 0.13 | 0.13 |
| AC-U-NA | 1.08 | 39.84 | 29.93 | 30.23 | 0.43 | 0.32 | 0.33 |
| AC-NA-U | 2.43 | 47.00 | 23.96 | 29.04 | 1.14 | 0.58 | 0.71 |
| AC-U.NA | 1.77 | 41.16 | 27.89 | 30.99 | 0.73 | 0.49 | 0.55 |
| AC-U.NAafter | 0.31 | 32.81 | 31.61 | 35.58 | 0.10 | 0.10 | 0.11 |
表3 不同催化剂反应前后的XPS N 1s分析数据
| 样品 | 氮含量(质量分数) /% | 氮物种占比 | 吡啶氮含量 (质量分数)/% | 吡咯氮含量 (质量分数)/% | 石墨氮含量 (质量分数)/% | ||
|---|---|---|---|---|---|---|---|
| 吡啶氮占比/% | 吡咯氮占比/% | 石墨氮占比/% | |||||
| AC | 0.21 | 22.95 | 24.69 | 52.36 | 0.05 | 0.05 | 0.11 |
| AC-U | 2.04 | 42.74 | 25.99 | 31.27 | 0.87 | 0.53 | 0.64 |
| AC-NA | 0.36 | 27.76 | 35.31 | 36.93 | 0.10 | 0.13 | 0.13 |
| AC-U-NA | 1.08 | 39.84 | 29.93 | 30.23 | 0.43 | 0.32 | 0.33 |
| AC-NA-U | 2.43 | 47.00 | 23.96 | 29.04 | 1.14 | 0.58 | 0.71 |
| AC-U.NA | 1.77 | 41.16 | 27.89 | 30.99 | 0.73 | 0.49 | 0.55 |
| AC-U.NAafter | 0.31 | 32.81 | 31.61 | 35.58 | 0.10 | 0.10 | 0.11 |
| 1 | ZHANG Meng, ZHANG Junfeng, ZHOU Zeling, et al. Effects of the surface adsorbed oxygen species tuned by rare-earth metal doping on dry reforming of methane over Ni/ZrO2 catalyst[J]. Applied Catalysis B: Environmental, 2020, 264: 118522. |
| 2 | ZHANG Meng, ZHANG Junfeng, WU Yingquan, et al. Insight into the effects of the oxygen species over Ni/ZrO2 catalyst surface on methane reforming with carbon dioxide[J]. Applied Catalysis B: Environmental, 2019, 244: 427-437. |
| 3 | 王明智, 张秋林, 张腾飞, 等. Ni基甲烷二氧化碳重整催化剂研究进展[J]. 化工进展, 2015, 34(8): 3027-3033, 3039. |
| WANG Mingzhi, ZHANG Qiulin, ZHANG Tengfei, et al. Advance in Ni-based catalysts for the carbon dioxide reforming of methane[J]. Chemical Industry and Engineering Progress, 2015, 34(8): 3027-3033, 3039. | |
| 4 | 曹军, 张莉, 徐宏, 等. 甲烷二氧化碳重整制合成气中积炭效应的数值模拟[J]. 化工进展, 2015, 34(10): 3630-3635, 3655. |
| CAO Jun, ZHANG Li, XU Hong, et al. Numerical simulation on the carbon deposition effect in methane carbon dioxide reforming[J]. Chemical Industry and Engineering Progress, 2015, 34(10): 3630-3635, 3655. | |
| 5 | ARAMOUNI Nicolas Abdel Karim, TOUMA Jad G, TARBOUSH Belal Abu, et al. Catalyst design for dry reforming of methane: analysis review[J]. Renewable and Sustainable Energy Reviews, 2018, 82: 2570-2585. |
| 6 | ZHANG Xiaoping, WANG Feng, SONG Zhengwei, et al. Comparison of carbon deposition features between Ni/ZrO2 and Ni/SBA-15 for the dry reforming of methane[J]. Reaction Kinetics, Mechanisms and Catalysis, 2020, 129(1): 457-470. |
| 7 | GENG Jiancheng, XUE Dingming, LIU Xiaoqin, et al. N-doped porous carbons for CO2 capture: rational choice of N-containing polymer with high phenyl density as precursor[J]. AIChE Journal, 2017, 63(5): 1648-1658. |
| 8 | YANG Jie, YUE Limin, LIN Binbin, et al. CO2 adsorption of nitrogen-doped carbons prepared from nitric acid preoxidized petroleum coke[J]. Energy & Fuels, 2017, 31(10): 11060-11068. |
| 9 | TIAN Ke, WU Zhengchen, XIE Feifei, et al. Nitrogen-doped porous carbons derived from triarylisocyanurate-cored polymers with high CO2 adsorption properties[J]. Energy & Fuels, 2017, 31(11): 12477-12486. |
| 10 | FIDALGO B, ARENILLAS A, MENÉNDÉZ J A. Influence of porosity and surface groups on the catalytic activity of carbon materials for the microwave-assisted CO2 reforming of CH4[J]. Fuel, 2010, 89: 4002-4007. |
| 11 | GUO Fengbo, ZHANG Yongfa, ZHANG Guojie, et al. Syngas production by carbon dioxide reforming of methane over different semi-cokes[J]. Journal of Power Sources, 2013, 231: 82-90. |
| 12 | JIANG Xu, LAN Xinzhe, SONG Yonghui, et al. Adsorption of COD in coking wastewater on nitric acid-modified blue coke activated carbon[J]. Journal of Chemistry, 2019, 11: 8593742. |
| 13 | GOKCE Yavuz, AKTAS Zeki. Nitric acid modification of activated carbon produced from waste tea and adsorption of methylene blue and phenol[J]. Applied Surface Science, 2014, 313: 352-359. |
| 14 | WANG Zean, JIN Hu, WANG Kun, et al. A two-step method for the integrated removal of HCl, SO2 and NO at low temperature using viscose-based activated carbon fibers modified by nitric acid[J]. Fuel, 2019, 239: 272-281. |
| 15 | XU Long, LIU Yanan, LI Yanjun, et al. Catalytic CH4 reforming with CO2 over activated carbon based catalysts[J]. Applied Catalysis A: General, 2014, 469: 387-397. |
| 16 | WANG Haoqiang, ZHAO Zongbin, CHEN Meng, et al. Nitrogen-doped mesoporous carbon nanosheets from coal tar as high performance anode materials for lithium ion batteries[J]. New Carbon Materials, 2014, 29(4): 280-286. |
| 17 | KIM Ji Hyun, Seho CHO, Tae Sung BAE, et al. Enzyme biosensor based on an N-doped activated carbon fiber electrode prepared by a thermal solid-state reaction[J]. Sensors and Actuators B: Chemical, 2014, 197: 20-27. |
| 18 | LIU Ningning, YIN Longwei, WANG Chengxiaong, et al. Adjusting the texture and nitrogen content of ordered mesoporous nitrogen-doped carbon materials prepared using SBA-15 silica as a template[J]. Carbon, 2010, 48: 3579-3591. |
| 19 | ZHANG Bao, YUAN Xinbo, LI Hui, et al. Nitrogen-doped-carbon coated lithium iron phosphate cathode material with high performance for lithium-ion batteries[J]. Journal of Alloys and Compounds, 2015, 627: 13-19. |
| 20 | FAN Yanru, ZHAO Zongbin, ZHOU Quan, et al. Nitrogen-doped carbon microfibers with porous textures[J]. Carbon, 2013, 58: 128-133. |
| 21 | LI Mingming, XU Fan, LI Haoran, et al. Nitrogen-doped porous carbon materials: promising catalysts or catalyst supports for heterogeneous hydrogenation and oxidation[J]. Catalysis Science & Technology, 2016, 6(11): 3670-3693. |
| 22 | ZHAN Yunfeng, YU Xiang, CAO Linmin, et al. The influence of nitrogen source and doping sequence on the electrocatalytic activity for oxygen reduction reaction of nitrogen doped carbon materials[J]. International Journal of Hydrogen Energy, 2016, 41(31): 13493-13503. |
| 23 | SHAO Yuyan, Jiehe SUI, YIN Geping, et al. Nitrogen-doped carbon nanostructures and their composites as catalytic materials for proton exchange membrane fuel cell[J]. Applied Catalysis B: Environmental, 2008, 79(1): 89-99. |
| 24 | WEI Qiliang, TONG Xin, ZHANG Gaixia, et al. Nitrogen-doped carbon nanotube and graphene materials for oxygen reduction reactions[J]. Catalysts, 2015, 5(3): 1574-1602. |
| 25 | SINGH Gurwinder, KIM In Young, LAKHI Kripal S, et al. Heteroatom functionalized activated porous biocarbons and their excellent performance for CO2 capture at high pressure[J]. Journal of Materials Chemistry A, 2017, 5: 21196-21204. |
| 26 | WANG Lifeng, YANG Ralph T. Significantly increased CO2 adsorption performance of nanostructured templated carbon by tuning surface area and nitrogen doping[J]. The Journal of Physical Chemistry C, 2012, 116(1): 1099-1106. |
| 27 | WICKRAMARATNE Nilantha P, XU Jiantie, WANG Min, et al. Nitrogen enriched porous carbon spheres: attractive materials for supercapacitor electrodes and CO2 adsorption[J]. Chemistry of Materials, 2014, 26(9): 2820-2828. |
| 28 | HE Jiajun, John TO, MEI Jianguo, et al. Facile synthesis of nitrogen-doped porous carbon for selective CO2 capture[J]. Energy Procedia, 2014, 63: 2144-2151. |
| 29 | SEVILLA Marta, Patricia VALLE-VIGÓN, FUERTES ANTONIO B. N-doped polypyrrole-based porous carbons for CO2 capture[J]. Advanced Functional Materials, 2011, 21(14): 2781-2787. |
| 30 | ZHANG Guojie, SUN Yinghui, XU Ying, et al. Catalytic performance of N-doped activated carbon supported cobalt catalyst for carbon dioxide reforming of methane to synthesis gas[J]. Journal of the Taiwan Institute of Chemical Engineers, 2018, 93: 234-244. |
| 31 | WANG Changzhen, SUN Nannan, ZHAO Ning, et al. The properties of individual carbon residuals and their influence on the deactivation of Ni-CaO-ZrO2 catalysts in CH4 dry reforming[J]. ChemCatChem, 2014, 6: 640-648. |
| 32 | Dong Yeon RYU, SHIMOHARA Takaaki, NAKABAYASHI Koji, et al. Urea/nitric acid co-impregnated pitch-based activated carbon fiber for the effective removal of formaldehyde[J]. Journal of Industrial and Engineering Chemistry, 2019, 80: 98-105. |
| 33 | CHEN Jie, YANG Jie, HU Gengshen, et al. Enhanced CO2 capture capacity of nitrogen-doped biomass-derived porous carbons[J]. ACS Sustainable Chemistry & Engineering, 2016, 4: 1439-1445. |
| 34 | SING K S W, EVERETT D H, HAUL R A W, et al. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity[J]. Pure and Applied Chemistry, 1985, 57(4): 603-619. |
| 35 | 张伟庆, 黄滨, 余小岚, 等. 对BJH方法计算孔径分布过程的解读[J]. 大学化学, 2020, 35(2): 98-106. |
| ZHANG Weiqing, HUANG Bin, YU Xiaolan, et al. Interpretation of BJH method for calculating aperture distribution process[J]. University Chemistry, 2020, 35(2): 98-106. | |
| 36 | YUE Limin, XIA Qiongzhang, WANG Liwei, et al. CO2 adsorption at nitrogen-doped carbons prepared by K2CO3 activation of urea-modified coconut shell[J]. Journal of Colloid & Interface Science, 2018, 511: 259-267. |
| 37 | 张登峰, 鹿雯, 王盼盼, 等. 活性炭纤维湿氧化改性表面含氧官能团的变化规律[J]. 煤炭学报, 2008, 33(4): 439-443. |
| ZHANG Dengfeng, LU Wen, Wang Panpan, et al. Effect of wet oxidized modification on oxygen-containing functional groups of activated carbon fibers[J]. Journal of China Coal Society, 2008, 33(4): 439-443. | |
| 38 | 张译方. 硝酸介质中尿素与HNO2的反应动力学[J]. 广东化工, 2017, 44(7): 117-119. |
| ZHANG Yifang. Reaction kinetics of urea with nitrous acid in nitric acid medium[J]. Guangdong Chemical Industry, 2017, 44(7): 117-119. | |
| 39 | 吴珧萍, 陈天朗, 肖慎修. 尿素-甲醛法测定硝酸根[J]. 理化检验-化学分册, 2005, 41(10): 746-747. |
| WU Yaoping, CHEN Tianlang, XIAO Shenxiu. Urea formol titration for determination of large amount of nitrate[J]. Physical Testing and Chemical Analysis Part B: Chemical Analysis, 2005, 41(10): 746-747. | |
| 40 | 师兆忠. 硝酸磷肥中氮含量测定方法的研究[J]. 中国土壤与肥料, 2007(6): 88-90. |
| SHI Zhaozhong. The research of determination of nitrogen content in nitrophosphate[J]. Soil and Fertilizer Sciences in China, 2007(6): 88-90. | |
| 41 | 苏爱廷. 活性炭改性对CH4-CO2重整反应催化性能的研究[D]. 太原: 太原理工大学, 2016. |
| SU Aiting. Catalytic performance of modified activated carbon for CO2 reforming of CH4[D]. Taiyuan: Taiyuan University of Technology, 2016. | |
| 42 | SONG Jian, SHEN Wenzhong, WANG Jianguo, et al. Superior carbon-based CO2 adsorbents prepared from poplar anthers[J]. Carbon, 2014, 69: 255-263. |
| 43 | LI Dawei, ZHOU Jiaojiao, ZHANG Zongbo, et al. Improving low-pressure CO2 capture performance of N-doped active carbons by adjusting flow rate of protective gas during alkali activation[J]. Carbon, 2017, 114: 496-503. |
| 44 | KOU Jiahui, SUN Linbing. Nitrogen-doped porous carbons derived from carbonization of a nitrogen-containing polymer: efficient adsorbents for selective CO2 capture[J]. Industrial & Engineering Chemistry Research, 2016, 55(41): 10916-10925. |
| 45 | TIAN Wenjie, ZHANG Huayang, SUN Hongqi, et al. Heteroatom (N or N-S)-doping induced layered and honeycomb microstructures of porous carbons for CO2 capture and energy applications[J]. Advanced Functional Materials, 2016, 26: 8651-8661. |
| 46 | SHANGGUAN Ju, LI Chunhu, GUO Hanxian. Hydrolysis of carbonyl sulfide and carbon disulfide over alumina based catalysis Ⅱ: Study on CO2-TPD of catalysis[J]. Journal of Natural Gas Chemistry, 1998(1): 24-30. |
| 47 | WANG Xueqian, WANG Fei, CHEN Wei, et al. Adsorption of carbon disulfide on Cu/CoSPc/Ce modified activated carbon under microtherm and micro-oxygen conditions[J]. Industrial & Engineering Chemistry Research, 2014, 53(35): 13626-13634. |
| 48 | 张子靖, 刘畅, 李如会, 等. 硅烷化多壁碳纳米管/硅橡胶复合材料的制备和介电性能[J]. 复合材料学报, 2020, 37(7): 1676-1683. |
| ZHANG Zijing, LIU Chang, LI Ruhui, et al. Preparation and dielectric properties of silanized multi-walled carbon nanotubes/silicone rubber composites[J]. Acta Materiae Compositae Sinica, 2020, 37(7): 1676-1683. | |
| 49 | ZHANG Fangbai, WANG Ning, YANG Lu, et al. Ni-Co bimetallic MgO-based catalysts for hydrogen production via steam reforming of acetic acid from bio-oil[J]. International Journal of Hydrogen Energy, 2014, 39(32): 18688-18694. |
| 50 | MA Hongyan, ZENG Liang TIAN Hao, et al. Efficient hydrogen production from ethanol steam reforming over La-modified ordered mesoporous Ni-based catalysts[J]. Applied Catalysis B: Environmental, 2016, 181: 321-331. |
| 51 | 舒新前, 王祖讷, 徐精求, 等. 神府煤煤岩组分的结构特征及其差异[J]. 燃料化学学报, 1996, 24(5): 426-433. |
| SHU Xinqian, WANG Zuna, XU Jingqiu, et al. Structural characteristics and differences among lithotypes[J]. Journal of Fuel Chemistry and Technology, 1996, 24(5): 426-433. | |
| 52 | ZHANG Guojie, QU Jiangwen, SU Aiting, et al. Towards understanding the carbon catalyzed CO2 reforming of methane to syngas[J]. Journal of Industrial & Engineering Chemistry, 2015, 21: 311-317. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 张杰, 王放放, 夏忠林, 赵光金, 马双忱. “双碳”目标下SF6排放现状、减排手段分析及未来展望[J]. 化工进展, 2023, 42(S1): 447-460. |
| [6] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [7] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [8] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [9] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| [10] | 张启, 赵红, 荣峻峰. 质子交换膜燃料电池中氧还原反应抗毒性电催化剂研究进展[J]. 化工进展, 2023, 42(9): 4677-4691. |
| [11] | 王伟涛, 鲍婷玉, 姜旭禄, 何珍红, 王宽, 杨阳, 刘昭铁. 醛酮树脂基非金属催化剂催化氧气氧化苯制备苯酚[J]. 化工进展, 2023, 42(9): 4706-4715. |
| [12] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [13] | 张丽宏, 金要茹, 程芳琴. 煤气化渣资源化利用[J]. 化工进展, 2023, 42(8): 4447-4457. |
| [14] | 吴海波, 王希仑, 方岩雄, 纪红兵. 3D打印催化材料开发与应用进展[J]. 化工进展, 2023, 42(8): 3956-3964. |
| [15] | 向阳, 黄寻, 魏子栋. 电催化有机合成反应的活性和选择性调控研究进展[J]. 化工进展, 2023, 42(8): 4005-4014. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||