化工进展 ›› 2021, Vol. 40 ›› Issue (3): 1438-1448.DOI: 10.16085/j.issn.1000-6613.2020-0915
多孔超交联聚合物固载离子液体催化二氧化碳环加成反应的研究进展
赵朝阳( ), 罗小燕, 裴宝有, 项小燕, 李佳然, 马瑞勋, 张子姮, 林金清(
), 罗小燕, 裴宝有, 项小燕, 李佳然, 马瑞勋, 张子姮, 林金清( )
)
- 华侨大学材料科学与工程学院,福建 厦门 361021
-
收稿日期:2020-05-25出版日期:2021-03-05发布日期:2021-03-17 -
通讯作者:林金清 -
作者简介:赵朝阳(1995—),男,硕士研究生,研究方向为离子液体及绿色催化。E-mail:zzy18134405387@163.com 。 -
基金资助:国家自然科学基金(21803021);福建省自然科学基金(2020J01065)
Research progress on CO2 cycloaddition catalyzed by porous hyper-crosslinked polymers immobilized ionic liquids
ZHAO Zhaoyang( ), LUO Xiaoyan, PEI Baoyou, XIANG Xiaoyan, LI Jiaran, MA Ruixun, ZHANG Ziheng, LIN Jinqing(
), LUO Xiaoyan, PEI Baoyou, XIANG Xiaoyan, LI Jiaran, MA Ruixun, ZHANG Ziheng, LIN Jinqing( )
)
- College of Materials Sciences & Engineering, Huaqiao University, Xiamen 361021, Fujian, China
-
Received:2020-05-25Online:2021-03-05Published:2021-03-17 -
Contact:LIN Jinqing
摘要:
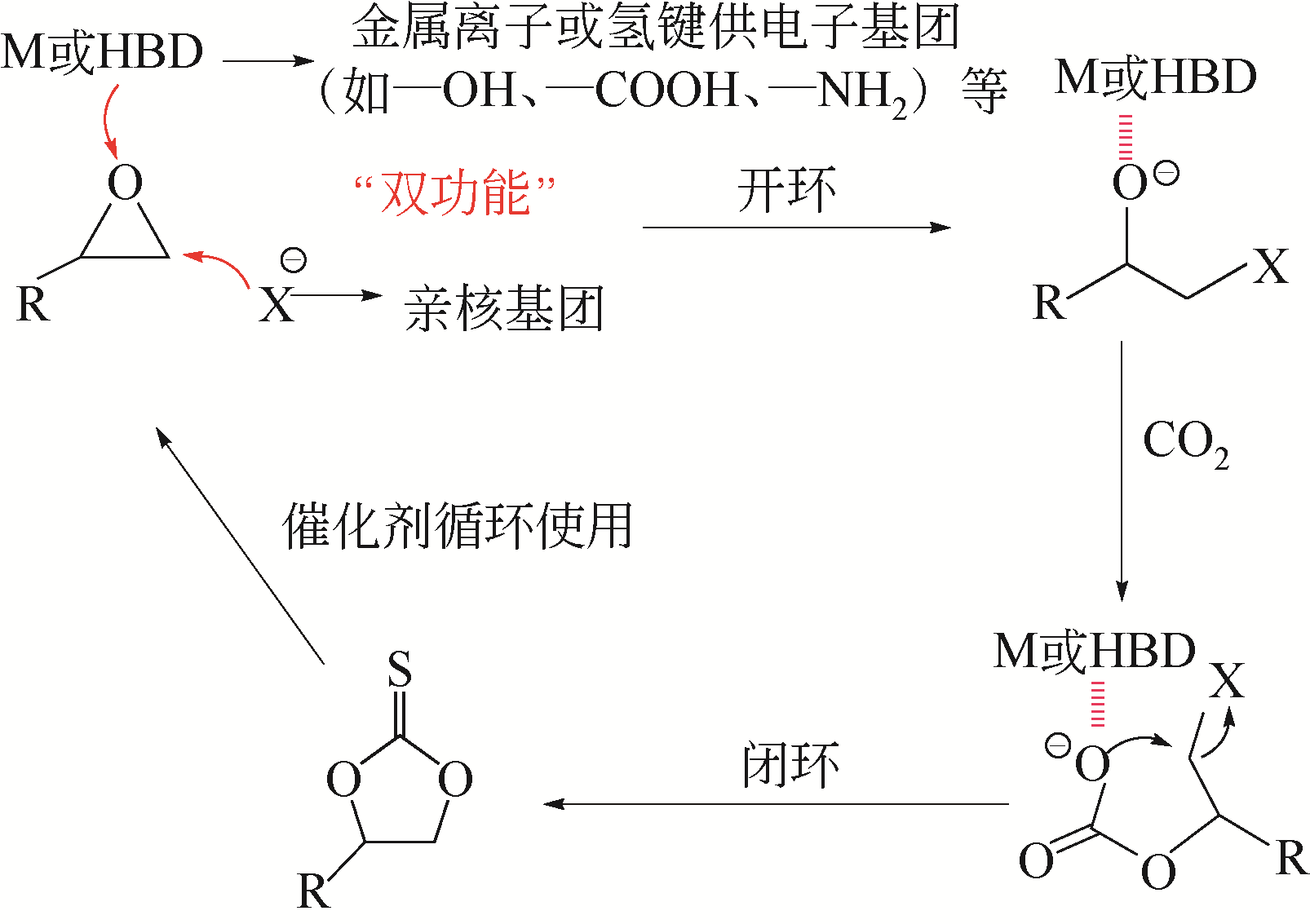
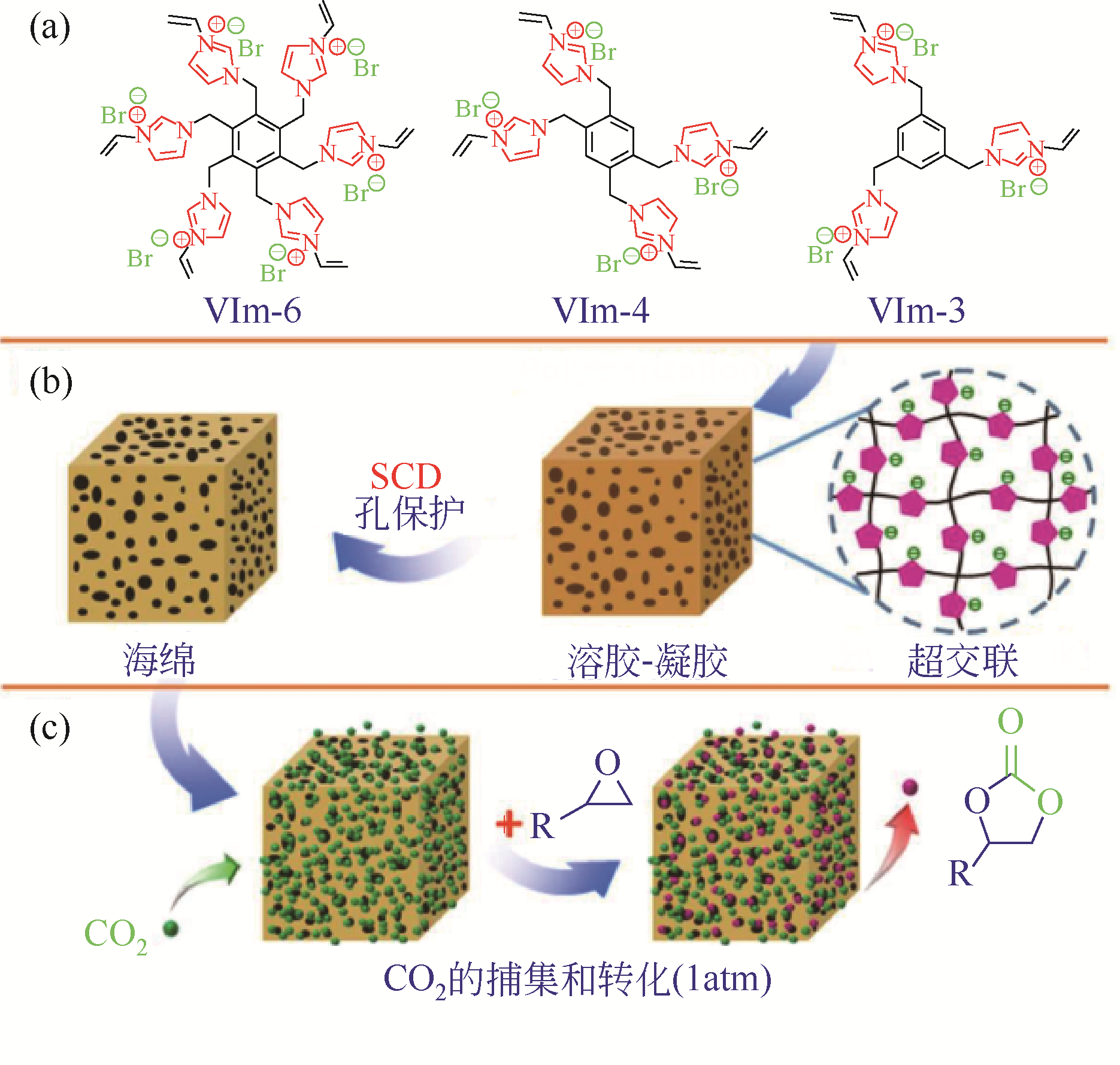
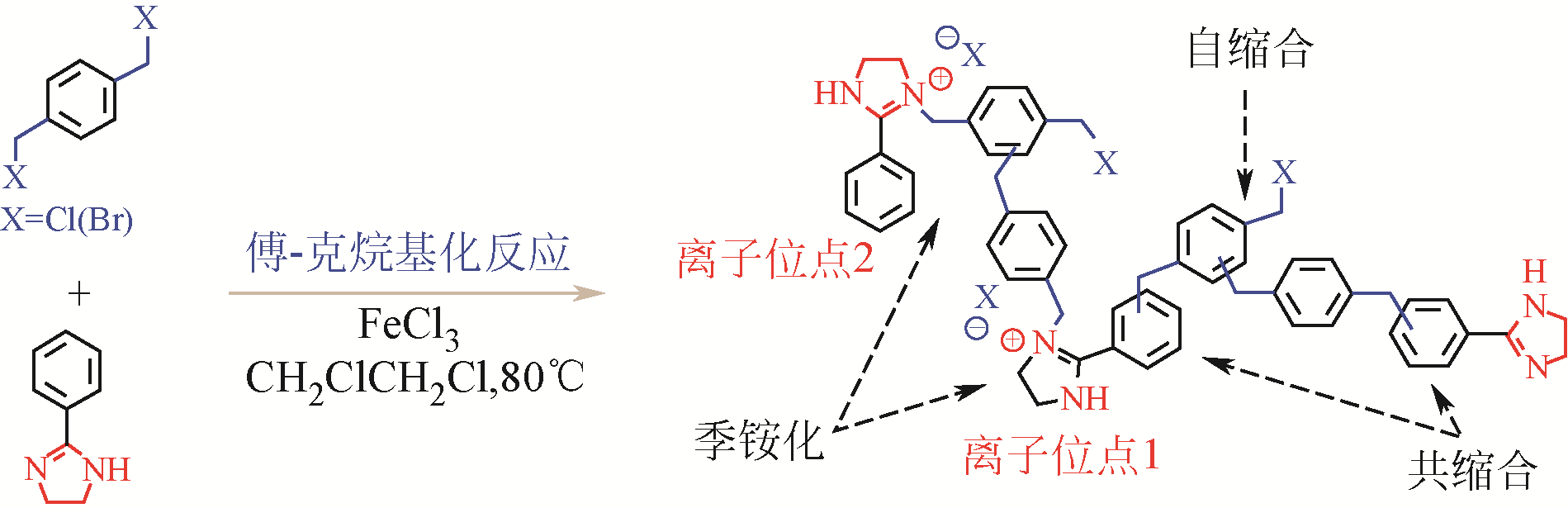
CO2是一种主要的温室气体,以CO2为原料与环氧化物发生环加成反应可以制备各种环状碳酸酯,是一种绿色可行的CO2捕集及利用途径。多孔超交联聚合物固载离子液体(hypercrosslinked polymers immobilized ionic liquids,HCP-ILs)催化CO2环加成反应具有无需溶剂、金属和助催化剂等优点。本文对其近年来的最新研究进展进行了综述,总结了离子单体自聚/共聚或交联法、离子与交联一步法以及交联后修饰法三种制备超交联聚合物固载离子液体方法的特点,分析了目前还存在离子密度偏低、催化效率不够高以及制备成本偏高等不利于“CO2化工”应用的问题,并指出为实现在常压下快速催化CO2与环氧化物的环加成反应,应从提高离子密度、调控表面活化功能基团和离子微环境以及降低制备成本等方向加强理论研究和技术攻关。
中图分类号:
引用本文
赵朝阳, 罗小燕, 裴宝有, 项小燕, 李佳然, 马瑞勋, 张子姮, 林金清. 多孔超交联聚合物固载离子液体催化二氧化碳环加成反应的研究进展[J]. 化工进展, 2021, 40(3): 1438-1448.
ZHAO Zhaoyang, LUO Xiaoyan, PEI Baoyou, XIANG Xiaoyan, LI Jiaran, MA Ruixun, ZHANG Ziheng, LIN Jinqing. Research progress on CO2 cycloaddition catalyzed by porous hyper-crosslinked polymers immobilized ionic liquids[J]. Chemical Industry and Engineering Progress, 2021, 40(3): 1438-1448.
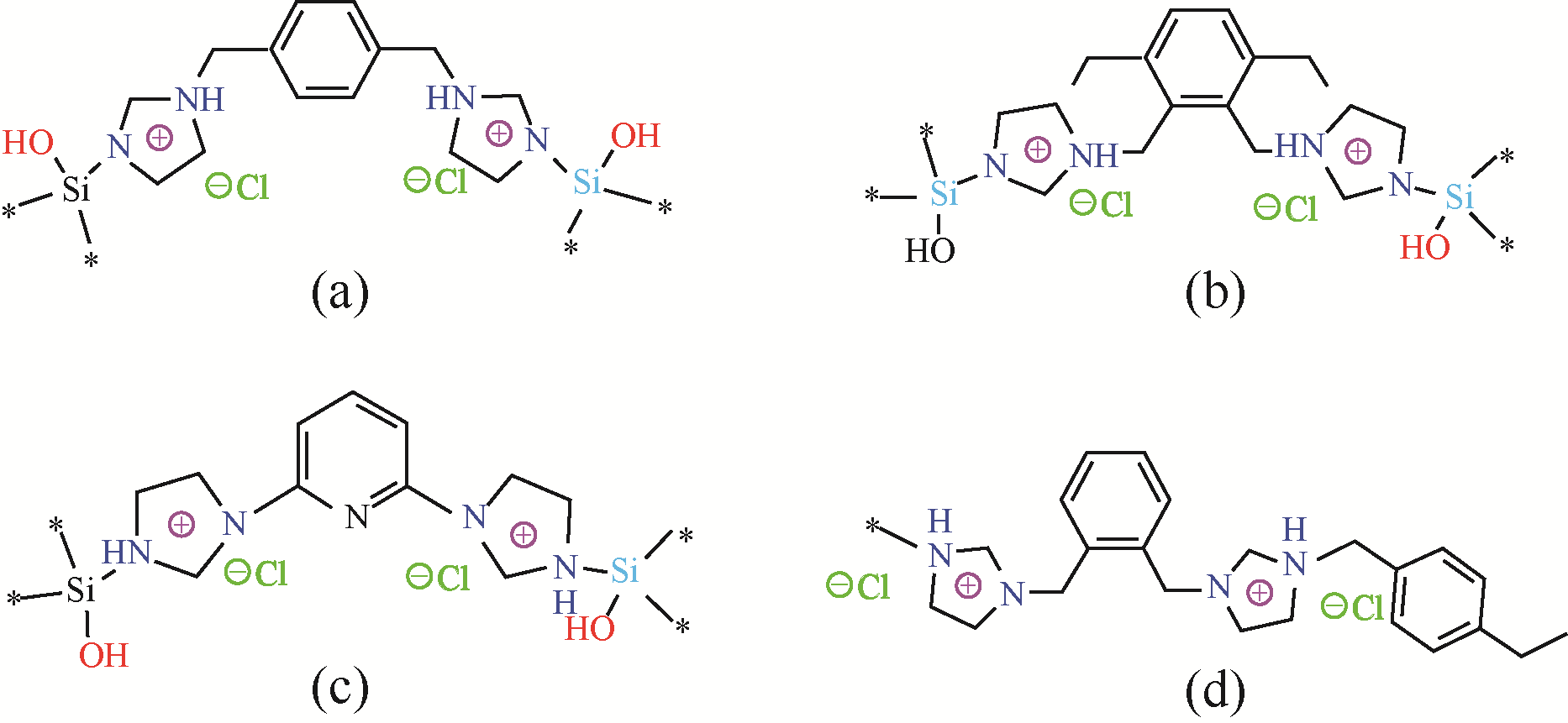
| 催化剂 | 单体 | 底物 | 压力 /MPa | 温度 /℃ | 时间 /h | 产率 /% | 参考文献 |
|---|---|---|---|---|---|---|---|
| AIMDFP | 双氰胺 | 环氧丙烷 | 2 | 130 | 3.5 | 99 | [ |
| P(DMAEMA-EtOH)Br | 甲基丙烯酸二甲氨基乙酯 | 环氧丙烷 | 2 | 110 | 3 | 96.2 | [ |
| PDMBr | 1-乙烯基咪唑 | 环氧丙基苯基醚 | 0.1 | 70 | 48 | 90 | [ |
| PIP-Bn-Cl | 乙烯基功能化的三苯基膦 | 环氧丙烷 | 0.1 | 50 | 24 | 93.2 | [ |
| Polymer 1 | 三苯基膦 | 氧化苯乙烯 | 1 | 130 | 12 | 96 | [ |
| PVIm-6-SCD | 1-乙烯基咪唑1,2,3,4,5,6-六苄溴苯 | 环氧丙烷 | 0.1 | 50 | 24 | 99.5 | [ |
| DVB@ISA | 1-乙烯基咪唑二乙烯基苯1,2-环己二胺 | 1,2-环氧己烷 | 1 | 60 | 24 | 99 | [ |
| AE-PIL-Cl | 1-乙烯基咪唑乙烯基苯 | 环氧丙烷 | 1 | 140 | 60 | 99 | [ |
| 1P+Br-&1PPh3@POPs | 乙烯基功能化的三苯基膦 | 环氧丙烷 | 3 | 120 | 5 | 90 | [ |
| IHCP-OH(1) | 二乙烯基苯 1-乙烯基咪唑等 | 环氧丙基苯基醚 | 1 | 120 | 2 | 90 | [ |
| [AlTMG]Br | 乙烯基苯功能化的季铵离子液体 | 环氧苯乙烯 | 0.1 | 100 | 6 | 99 | [ |
| [PAD][IDA] | 二乙烯基苯 1-乙烯基咪唑 | 环氧苯乙烯 | 0.1 | 65 | 12 | 99 | [ |
| Py-Im-6 | 1-乙烯基咪唑1,2,3,4,5,6-六苄溴 | 环氧丙烷 | 0.1 | 120 | 1 | 94.5 | [ |
| PIL-4 | 1-乙烯基咪唑二(2-氯乙基)醚 | 环氧氯丙烷 | 1 | 100 | 12 | 99 | [ |
| NHC-CAP-1(Zn2+) | 苯基咪唑等 | 环氧氯丙烷 | 2 | 100 | 3 | 97 | [ |
| IC2HCP-5b | 1-苄基咪唑等 | 环氧氯丙烷 | 3 | 120 | 20 | 99 | [ |
| IM-iPHP-2 | 1-苄基咪唑VPSOSS | 环氧氯丙烷 | 0.1 | 80 | 48 | 99 | [ |
| V-iPHP-1 | 4,4-联吡啶等 | 环氧氯丙烷 | 0.1 | 60 | 72 | 99 | [ |
| T-IM | 含咪唑的交联单体等 | 环氧氯丙烷 | 1 | 140 | 10 | 87(1H NMR) | [ |
| PP-Br | 三(4-氯苯基)膦 | 环氧丙基苯基醚 | 0.1 | 140 | 20 | 98.5 | [ |
| CPP-IL | 1,2-二甲基-3-(丙-2-炔基)-1H-咪唑-3-溴化铵等 | 环氧氯丙烷 | 0.1 | 100 | 24 | 99 | [ |
| PAM-PyMe(Cl) | 2,6-二氨基吡啶 | 环氧氯丙烷 | 0.1 | 100 | 20 | 77 | [ |
| UIIP | 含有咪唑的交联单体 | 环氧丙烷 | 1 | 110 | 2 | 97 | [ |
| HIP-Br-2 | 苯基咪唑等 | 环氧丙烷 | 0.1 | 55 | 96 | 99 | [ |
| HPILs-Cl | 苯基咪唑等 | 环氧丙烷 | 0.1 | 70 | 9 | 99(1H NMR) | [ |
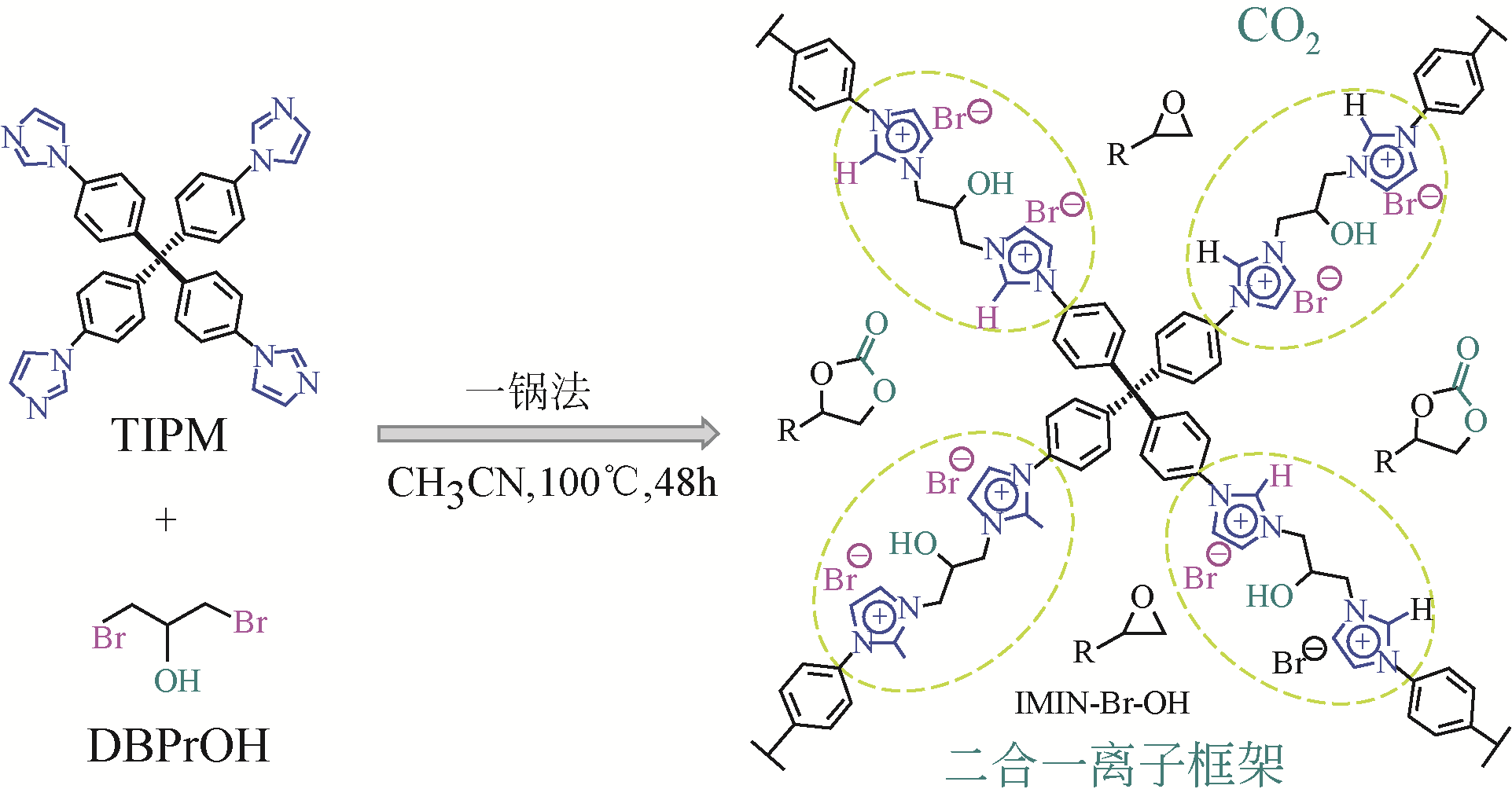
| IMIN-Br-OH | 咪唑修饰的四苯甲烷 | 环氧氯丙烷 | 0.1 | 40 | 72 | 99(1H NMR) | [ |
| HMIB | 1,4-对二氯苄咪唑 | 环氧丙烷 | 1 | 110 | 1.5 | 90 | [ |
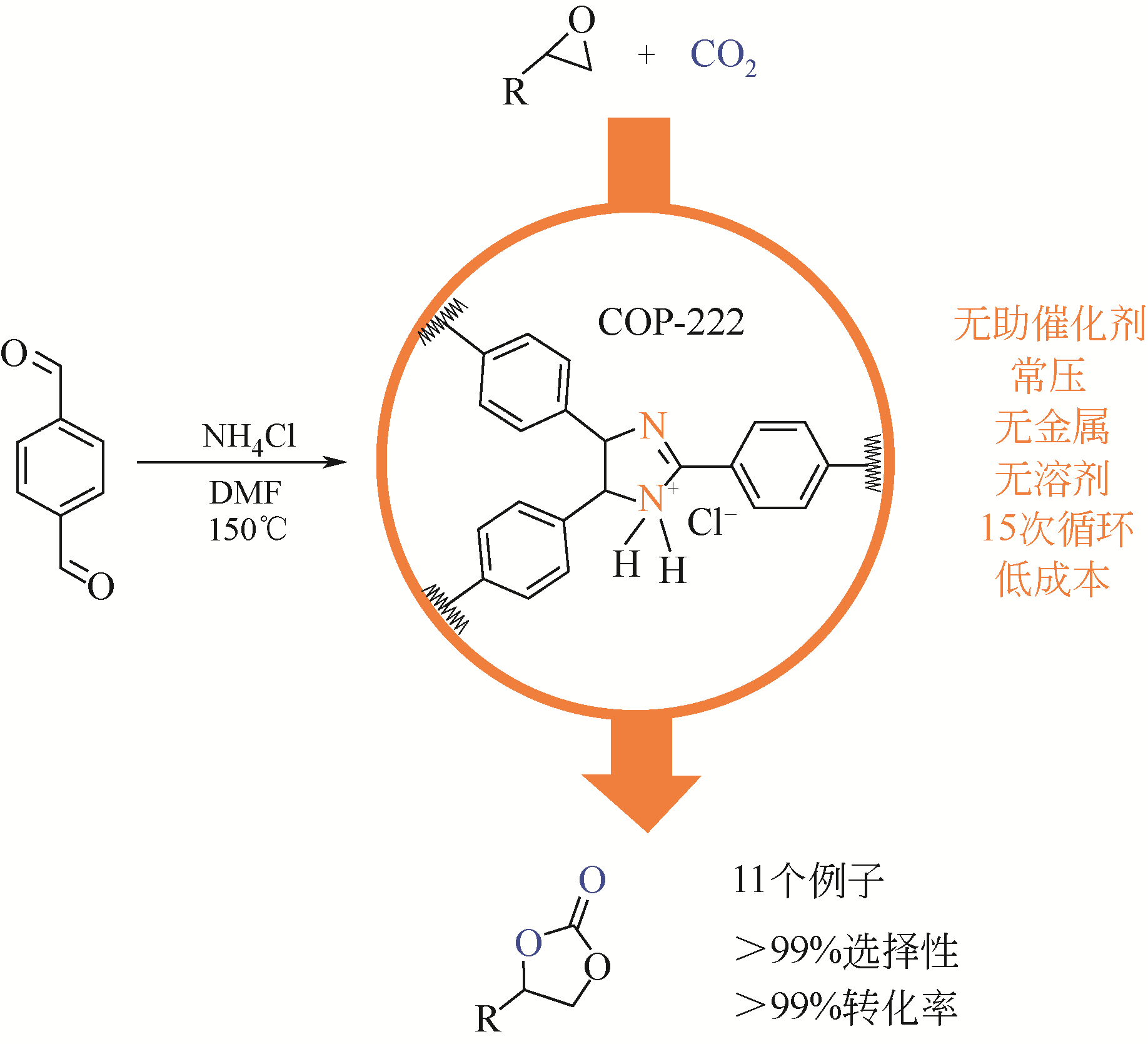
| COP-222 | 对苯二甲醛氯化铵 | 环氧氯丙烷 | 0.1 | 100 | 24 | 99(1H NMR) | [ |
| POM3-IM | 1-甲基咪唑等 | 环氧丙烷 | 1 | 120 | 4 | 78 | [ |
| PS-[CETBD]Br | 氯球(PS-Cl) | 环氧丙烷 | 2 | 140 | 2 | 96.3 | [ |
| PQA-NH2Py-Br | 2-氨基吡啶 | 1-丁基-2-苯基氮丙啶 | 2 | 70 | 4 | 99(1H NMR) | [ |
| 催化剂 7 | 4-乙烯基吡啶 | 环氧丙基苯醚 | 0.1 | 80 | 12 | 66(1H NMR) | [ |
表1 典型的超交联聚合物固载离子液体及其催化CO2环加成反应条件和产率
| 催化剂 | 单体 | 底物 | 压力 /MPa | 温度 /℃ | 时间 /h | 产率 /% | 参考文献 |
|---|---|---|---|---|---|---|---|
| AIMDFP | 双氰胺 | 环氧丙烷 | 2 | 130 | 3.5 | 99 | [ |
| P(DMAEMA-EtOH)Br | 甲基丙烯酸二甲氨基乙酯 | 环氧丙烷 | 2 | 110 | 3 | 96.2 | [ |
| PDMBr | 1-乙烯基咪唑 | 环氧丙基苯基醚 | 0.1 | 70 | 48 | 90 | [ |
| PIP-Bn-Cl | 乙烯基功能化的三苯基膦 | 环氧丙烷 | 0.1 | 50 | 24 | 93.2 | [ |
| Polymer 1 | 三苯基膦 | 氧化苯乙烯 | 1 | 130 | 12 | 96 | [ |
| PVIm-6-SCD | 1-乙烯基咪唑1,2,3,4,5,6-六苄溴苯 | 环氧丙烷 | 0.1 | 50 | 24 | 99.5 | [ |
| DVB@ISA | 1-乙烯基咪唑二乙烯基苯1,2-环己二胺 | 1,2-环氧己烷 | 1 | 60 | 24 | 99 | [ |
| AE-PIL-Cl | 1-乙烯基咪唑乙烯基苯 | 环氧丙烷 | 1 | 140 | 60 | 99 | [ |
| 1P+Br-&1PPh3@POPs | 乙烯基功能化的三苯基膦 | 环氧丙烷 | 3 | 120 | 5 | 90 | [ |
| IHCP-OH(1) | 二乙烯基苯 1-乙烯基咪唑等 | 环氧丙基苯基醚 | 1 | 120 | 2 | 90 | [ |
| [AlTMG]Br | 乙烯基苯功能化的季铵离子液体 | 环氧苯乙烯 | 0.1 | 100 | 6 | 99 | [ |
| [PAD][IDA] | 二乙烯基苯 1-乙烯基咪唑 | 环氧苯乙烯 | 0.1 | 65 | 12 | 99 | [ |
| Py-Im-6 | 1-乙烯基咪唑1,2,3,4,5,6-六苄溴 | 环氧丙烷 | 0.1 | 120 | 1 | 94.5 | [ |
| PIL-4 | 1-乙烯基咪唑二(2-氯乙基)醚 | 环氧氯丙烷 | 1 | 100 | 12 | 99 | [ |
| NHC-CAP-1(Zn2+) | 苯基咪唑等 | 环氧氯丙烷 | 2 | 100 | 3 | 97 | [ |
| IC2HCP-5b | 1-苄基咪唑等 | 环氧氯丙烷 | 3 | 120 | 20 | 99 | [ |
| IM-iPHP-2 | 1-苄基咪唑VPSOSS | 环氧氯丙烷 | 0.1 | 80 | 48 | 99 | [ |
| V-iPHP-1 | 4,4-联吡啶等 | 环氧氯丙烷 | 0.1 | 60 | 72 | 99 | [ |
| T-IM | 含咪唑的交联单体等 | 环氧氯丙烷 | 1 | 140 | 10 | 87(1H NMR) | [ |
| PP-Br | 三(4-氯苯基)膦 | 环氧丙基苯基醚 | 0.1 | 140 | 20 | 98.5 | [ |
| CPP-IL | 1,2-二甲基-3-(丙-2-炔基)-1H-咪唑-3-溴化铵等 | 环氧氯丙烷 | 0.1 | 100 | 24 | 99 | [ |
| PAM-PyMe(Cl) | 2,6-二氨基吡啶 | 环氧氯丙烷 | 0.1 | 100 | 20 | 77 | [ |
| UIIP | 含有咪唑的交联单体 | 环氧丙烷 | 1 | 110 | 2 | 97 | [ |
| HIP-Br-2 | 苯基咪唑等 | 环氧丙烷 | 0.1 | 55 | 96 | 99 | [ |
| HPILs-Cl | 苯基咪唑等 | 环氧丙烷 | 0.1 | 70 | 9 | 99(1H NMR) | [ |
| IMIN-Br-OH | 咪唑修饰的四苯甲烷 | 环氧氯丙烷 | 0.1 | 40 | 72 | 99(1H NMR) | [ |
| HMIB | 1,4-对二氯苄咪唑 | 环氧丙烷 | 1 | 110 | 1.5 | 90 | [ |
| COP-222 | 对苯二甲醛氯化铵 | 环氧氯丙烷 | 0.1 | 100 | 24 | 99(1H NMR) | [ |
| POM3-IM | 1-甲基咪唑等 | 环氧丙烷 | 1 | 120 | 4 | 78 | [ |
| PS-[CETBD]Br | 氯球(PS-Cl) | 环氧丙烷 | 2 | 140 | 2 | 96.3 | [ |
| PQA-NH2Py-Br | 2-氨基吡啶 | 1-丁基-2-苯基氮丙啶 | 2 | 70 | 4 | 99(1H NMR) | [ |
| 催化剂 7 | 4-乙烯基吡啶 | 环氧丙基苯醚 | 0.1 | 80 | 12 | 66(1H NMR) | [ |
| 1 | ZHANG X P, ZENG S J, FENG J Q, et al. CO2 chemical engineering: CO2 green conversion enhanced by ionic liquid microhabitat[J]. Science China Chemistry, 2020, 50: 282-298. |
| 2 | LIU Q, WU L P, JACKSTELL R, et al. Using carbon dioxide as a building block in organic synthesis[J]. Nature Communication, 2015, 6: 5933. |
| 3 | SUBRAMANIAN S, OPPENHEIM J, KIM D, et al. Catalytic non-redox carbon dioxide fixation in cyclic carbonates[J]. Chem., 2019, 5(12): 3232-3242. |
| 4 | SUN J, CHENG W G, YANG Z F, et al. Superbase/cellulose: an environmentally benign catalyst for chemical fixation of carbon dioxide into cyclic carbonates[J]. Green Chemistry, 2014, 16: 3071-3078. |
| 5 | KAMPHUIS A J, MILOCCO F, KOITER L, et al. Highly selective single-component formazanate ferrate(Ⅱ) catalysts for the conversion of CO2 into cyclic carbonates[J]. ChemSusChem, 2019, 12: 1-8. |
| 6 | HU J Y, LIU H Z, HAN B X. Basic ionic liquids promoted chemical transformation of CO2 to organic carbonates[J]. Science China Chemistry, 2018, 61(12): 1486-1493. |
| 7 | KAMPUIS A J, PICCHIONI F, PECARMONA P P. CO2-fixation into cyclic and polymeric carbonates: principles and applications[J]. Green Chemistry, 2019, 21: 406-448. |
| 8 | 谭亚南, 何霖, 王凯, 等. 环氧乙烷和二氧化碳合成碳酸乙烯酯催化剂研究进展[J]. 化工进展, 2017, 36(S1): 250-255. |
| TAN Y A, HE L, WANG K, et al. Research progress of catalyst for ethylene carbonate synthesis from ethylene oxide and carbon dioxide[J]. Chemical Industry and Engineering Progress, 2017, 36(S1): 250-255. | |
| 9 | LIU H X, ZENG R Q, HUA R M. 2,2’,2”-Terpyridine-catalyzed synthesis of cyclic carbonates from epoxides and carbon dioxide under solvent-free conditions[J]. International Journal of Molecular Sciences, 2014, 15(6): 9945-9951. |
| 10 | WHITEOAK C J, KIELLAND N, LASERNA V, et al. A powerful aluminum catalyst for the synthesis of highly functional organic carbonates[J]. Journal of the American Chemical Society, 2013, 135(4): 1228-1231. |
| 11 | XUAN K, PU Y F, LI F, et al. Direct synthesis of dimethyl carbonate from CO2 and methanol over trifluoroacetic acid modulated UiO-66[J]. Journal of CO2 Untilization, 2018, 27: 272-282. |
| 12 | HU L H, CHEN L, PENG X, et al. Bifunctional metal-doped ZIF-8: a highly efficient catalyst for the synthesis of cyclic carbonates from CO2 cycloaddition[J]. Microporous and Mesoporous Materials, 2020, 299: 110123. |
| 13 | QU R, REN Z J, LI N. Solvent-free cycloaddition of carbon dioxide and epichlorohydrin catalyzed by surface-attached imidazolium-type poly(ionic liquid) monolayers[J]. Journal of CO2 Untilization, 2020, 38: 168-176. |
| 14 | WANG L, LI P, JIN X F, et al. Mechanism of fixation of CO2 in the presence of hydroxyl-functionalized quaternary ammonium salts[J]. Journal of CO2 Utilization, 2015, 10: 113-119. |
| 15 | XIE Y, ZHANG Z F, JIANG T, et al. CO2 cycloaddition reactions catalyzed by an ionic liquid grafted onto a highly cross-linked polymer matrix[J]. Angewandte Chemie International Edition, 2007, 46: 7255-7258. |
| 16 | LIU D, LI G, LIU J X, et al. Organic-inorganic hybrid mesoporous titanium silica material as bi-functional heterogeneous catalyst for the CO2 cycloaddition[J]. Fuel, 2019, 244: 196-206. |
| 17 | DING M L, JIANG H L. Incorporation of imidazolium-based poly(ionic liquid)s into a metal-organic framework for CO2 capture and conversion[J]. ACS Catalysis, 2018, 8: 3194-3201. |
| 18 | DING L G, YAO B J, LI F, et al. Ionic liquid-decorated COF and its covalent composite aerogel for selective CO2 adsorption and catalytic conversion[J]. Journal of Materials Chemistry A, 2019, 7: 4689-4698. |
| 19 | XU D, GUO J N, YAN F. Porous ionic polymers: design, synthesis and applications[J]. Progress in Polymer Science, 2018, 79: 121-143. |
| 20 | 华凌. 新技术将二氧化碳变高值化“宝贝”[N]. 科技日报, 2019-10-11(4). |
| HUA L. Carbon dioxide becomes high value “Chemicals” with new technology[N]. Science and Technology Daily, 2019-10-11(4). | |
| 21 | 项小燕, 罗小燕, 裴宝有, 等. 超交联多孔离子聚合物的研究进展[J]. 化工进展, 2020, 39(8): 3120-3134. |
| XIANG X Y, LUO X Y, PEI B Y, et al. Research progresses on hyper-crosslinked porous ionic polymers[J]. Chemical Industry and Engineering Progress, 2020, 39(8): 3120-3134. | |
| 22 | TAN L X, TAN B E. Hyper-cross-linked porous polymer materials: design, synthesis, and applications[J]. Chemical Society Reviews, 2017, 46(11): 3322-3356. |
| 23 | STEPHENSON A, LI B Y, CHEN L J, et al. Efficient separation of propane and propene by a hypercrosslinked polymer doped with Ag(Ⅰ)[J]. Journal of Materials Chemistry A, 2019, 7(44): 25521-25525. |
| 24 | SHEN Y J, ZHENG Q S, ZHU H B, et al. Hierarchical porous organometallic polymers fabricated by direct knitting: recyclable single-site catalysts with enhanced activity[J]. Advanced Materials, 2020, 32: 1905950. |
| 25 | LU Y, LIANG J N, DENG S F, et al. Hypercrosslinked polymers enabled micropore-dominant N,S co-doped porous carbon for ultrafast electron/ion transport supercapacitors[J]. Nano Energy, 2019, 65: 103993. |
| 26 |
XIE Y Q, LIN J, LIANG J, et al. Hypercrosslinked mesoporous poly (ionic liquid)s with high density of ion pairs: efficient adsorbents for Cr( ) removal via ion-exchange[J]. Chemical Engineering Journal, 2019, 378: 122107. ) removal via ion-exchange[J]. Chemical Engineering Journal, 2019, 378: 122107.
|
| 27 | LIU M S, WANG X, JIA Y C, et al. Hydrogen bond activation strategy for cyclic carbonates synthesis from epoxides and CO2: current state-of-the art of catalyst development and reaction analysis[J]. Catalysis Reviews, 2018, 61(2): 214-269. |
| 28 | PEI Y C, RU J, YAO K S, et al. Nanoreactors stable up to 200℃: a class of high temperature microemulsions composed solely of ionic liquids[J]. Chemical Communications, 2018, 54: 6260-6263. |
| 29 | MENG X L, JU Z Y, ZHANG S J, et al. Efficient transformation of CO2 to cyclic carbonates using bifunctional protic ionic liquids under mild conditions[J]. Green Chemistry, 2019, 21: 3456-3463. |
| 30 | MENG X L, NIE Y, SUN J, et al. Functionalized dicyandiamide-formaldehyde polymers as efficient heterogeneous catalysts for conversion of CO2 into organic carbonates[J]. Green Chemistry, 2014, 16: 2771-2778. |
| 31 | SU Q, QI Y Q, YAO X Q, et al. Ionic liquids tailored and confined by one-step assembly with mesoporous silica for boosting the catalytic conversion of CO2 into cyclic carbonates[J]. Green Chemistry, 2018, 20: 3232-3241. |
| 32 | YING T, TAN X, SU Q, et al. Polymeric ionic liquids tailored by different chain groups for the efficient conversion of CO2 into cyclic carbonate[J]. Green Chemistry, 2019, 21: 2352-2361. |
| 33 | WANG X C, ZHOU Y, GUO Z J, et al. Heterogeneous conversion of CO2 into cyclic carbonates at ambient pressure catalyzed by ionothermal-derived meso-macroporous hierarchical poly(ionic liquid)s[J]. Chemical Science, 2015, 6: 6916-6924. |
| 34 | SUN Q, JIN Y Y, AGUILA B, et al. Porous ionic polymers as a robust and efficient platform for capture and chemical fixation of atmospheric CO2[J]. ChemSusChem, 2017, 10: 1160-1165. |
| 35 | WANG J Q, YANG G W, YI G S, et al. Phosphonium salt incorporated hypercrosslinked porous polymers for CO2 capture and conversion[J]. Chemical Communications, 2015, 51(86): 15708-15711. |
| 36 | XIE Y Q, LIANG J, FU Y W, et al. Hypercrosslinked mesoporous poly(ionic liquid)s with high ionic density for efficient CO2 capture and conversion into cyclic carbonates[J]. Journal of Materials Chemistry A, 2018, 6(15): 6660-6666. |
| 37 | LUO R C, CHEN Y J, HE Q, et al. Metallosalen-based ionic porous polymers as bifunctional catalysts for the conversion of CO2 into valuable chemicals[J]. ChemSusChem, 2016, 10: 1526-1533. |
| 38 | QIN L, WANG B S, ZHANG Y Y, et al. Anion exchange: a novel way of preparing hierarchical porous structure in poly(ionic liquid)s[J]. Chemical Communications, 2017, 53: 3785-3788. |
| 39 | LI C Y, WANG W L, YAN L, et al. Phosphonium salt and ZnX2-PPh3 integrated hierarchical POPs: tailorable synthesis and highly efficient cooperative catalysis in CO2 utilization[J]. Journal of Materials Chemistry A, 2016, 4: 16017-16027. |
| 40 | JIA D G, MA L, WANG Y, et al. Efficient CO2 enrichment and fixation by engineering micropores of multi-functional hypercrosslinked ionic polymers[J]. Chemical Engineering Journal, 2020, 390: 124652. |
| 41 | HUI W, HE X M, XU X Y, et al. Highly efficient cycloaddition of diluted and waste CO2 into cyclic carbonates catalyzed by porous ionic copolymers[J]. Journal of CO2 Utilization, 2020, 36: 169-176. |
| 42 | ZHOU Y, ZHANG W L, MA L, et al. Amino acid anion paired mesoporous poly (ionic liquids) as metal-/halogen-free heterogeneous catalysts for carbon dioxide fixation[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(10): 9387-9398. |
| 43 | XIE Y Q, LIANG J, FU Y W, et al. Poly(ionic liquid)s with high density of nucleophile /electrophile for CO2 fixation to cyclic carbonates at mild conditions[J]. Journal of CO2 Utilization, 2019, 32: 281-289. |
| 44 | SONG H B, WANG Y J, XIAO M, et al. Design of novel poly (ionic liquid)s for the conversion of CO2 to cyclic carbonates under mild conditions without solvent[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(10): 9489-9497. |
| 45 | PUTHIARAJ P, RAVI S, YU K S, et al. CO2 adsorption and conversion into cyclic carbonates over a porous ZnBr2-grafted N-heterocyclic carbene-based aromatic polymer[J]. Applied Catalysis B: Environmental, 2019, 251: 195-205. |
| 46 | XIA L, CUI Q, SUO X, et al. Efficient, selective, and reversible SO2 capture with highly crosslinked ionic microgels via a selective swelling mechanism[J]. Advanced Functional Materials, 2018, 28(13):1704292.1-1704292.10. |
| 47 | ZHANG W L, MA F P, MA L, et al. Imidazolium-functionalized ionic hypercrosslinked porous polymers for efficient synthesis of cyclic carbonates from simulated flue gas[J]. ChemSusChem, 2020, 13: 341-350. |
| 48 | CHEN G J, ZHANG Y D, XU J Y, et al. Imidazolium-based ionic porous hybrid polymers with POSS-derived silanols for efficient heterogeneous catalytic CO2 conversion under mild conditions[J]. Chemical Engineering Journal, 2020, 381: 122765. |
| 49 | ZAHNG Y D, LIU K, WU L, et al. Silanol-enriched viologen-based ionic porous hybrid polymers for efficient catalytic CO2 fixation into cyclic carbonates under mild conditions[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(19): 16907-16916. |
| 50 | CHO H C, LEE H S, CHUN J S, et al. Tubular microporous organic networks bearing imidazolium salts and their catalytic CO2 conversion to cyclic carbonates[J]. Chemical Communications, 2011, 47: 917-919. |
| 51 | ZHANG Q, ZHANG S B, LI S H. Novel functional organic network containing quaternary phosphonium and tertiary phosphorus[J]. Macromolecules, 2012, 45: 2981-2988. |
| 52 | CUI C Y, SA R J, HONG Z X, et al. Ionic-liquid-modified click-based porous organic polymers for controlling capture and catalytic conversion of CO2 [J]. ChemSusChem, 2020, 13: 180-187. |
| 53 | MAYA E M, VERDE-SESTO E, MANTONE D, et al. New poly (ionic liquid)s based on poly(azomethine-pyridinium) salts and its use as heterogeneous catalysts for CO2 conversion[J]. European Polymer Journal, 2019, 110: 107-113. |
| 54 | ZIAEE M A, TANG Y J, ZHONG H, et al. Urea-functionalized imidazolium-based ionic polymer for chemical conversion of CO2 into organic carbonates[J]. ACS Sustainable Chemistry & Engineering, 2019,7 (2): 2380-2387. |
| 55 | LI J, JIA D G, GUO Z J, et al. Imidazolinium based porous hypercrosslinked ionic polymers for efficient CO2 capture and fixation with epoxides[J]. Green Chemistry, 2017, 19(11): 2675-2686. |
| 56 | SANG Y F, HAUNG J H. Benzimidazole-based hyper-cross-linked poly(ionic liquid)s for efficient CO2 capture and conversion[J]. Chemical Engineering Journal, 2020, 385: 123973. |
| 57 | ZAHNG Y D, CHEN G J, WU L. Two-in-one: construction of hydroxyl and imidazolium-bifunctionalized ionic networks in one-pot toward synergistic catalytic CO2 fixation[J]. Chemical Communications, 2020,56: 3309-3312. |
| 58 | WANG J Q, LEONG J Y, ZHANG Y G. Efficient fixation of CO2 into cyclic carbonates catalysed by silicon-based main chain poly-imidazolium salts[J]. Green Chemistry, 2014, 16: 4515-4519. |
| 59 | WANG J Q, SNG W H, YI G S, et al. Imidazolium salt modified porous hypercrosslinked polymers for synergistic CO2 capture and conversion[J]. Chemical Communications, 2015, 51(60): 12076-12079. |
| 60 | DAI W L, MAO J, LIU Y, et al. Commercial polymer microsphere grafted TBD-based ionic liquids as efficient and low-cost catalyst for the cycloaddition of CO2 with epoxides[J]. Catalysis Letter, 2019, 149: 699-712. |
| 61 | SONG Y P, SUN Q, AGUILA B, et al. Optimizing the performance of porous pyridinium frameworks for carbon dioxide transformation[J]. Catalysis Today, 2020, 356: 557-562. |
| 62 | OCHIAI B, ENDO T. Polymer-supported pyridinium catalysts for synthesis of cyclic carbonate by reaction of carbon dioxide and oxirane[J]. Journal of Polymer Science A: Polymer Chemistry, 2007, 45(23): 5673-5678. |
| 63 | JIN T, DONG F, LIU Y, et al. Novel and effective strategy of dual bis(trifluoromethylsulfonyl)imide imidazolium ionic liquid immobilized on periodic mesoporous organosilica for greener cycloaddition of carbon dioxide to epoxides[J]. New Journal Chemistry, 2019, 43(6): 2583-2590. |
| [1] | 杨寒月, 孔令真, 陈家庆, 孙欢, 宋家恺, 王思诚, 孔标. 微气泡型下向流管式气液接触器脱碳性能[J]. 化工进展, 2023, 42(S1): 197-204. |
| [2] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [3] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [4] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [5] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [6] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [7] | 郑谦, 官修帅, 靳山彪, 张长明, 张小超. 铈锆固溶体Ce0.25Zr0.75O2光热协同催化CO2与甲醇合成DMC[J]. 化工进展, 2023, 42(S1): 319-327. |
| [8] | 戴欢涛, 曹苓玉, 游新秀, 徐浩亮, 汪涛, 项玮, 张学杨. 木质素浸渍柚子皮生物炭吸附CO2特性[J]. 化工进展, 2023, 42(S1): 356-363. |
| [9] | 王正坤, 黎四芳. 双子表面活性剂癸炔二醇的绿色合成[J]. 化工进展, 2023, 42(S1): 400-410. |
| [10] | 高雨飞, 鲁金凤. 非均相催化臭氧氧化作用机理研究进展[J]. 化工进展, 2023, 42(S1): 430-438. |
| [11] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [12] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [13] | 孙玉玉, 蔡鑫磊, 汤吉海, 黄晶晶, 黄益平, 刘杰. 反应精馏合成甲基丙烯酸甲酯工艺优化及节能[J]. 化工进展, 2023, 42(S1): 56-63. |
| [14] | 许友好, 王维, 鲁波娜, 徐惠, 何鸣元. 中国炼油创新技术MIP的开发策略及启示[J]. 化工进展, 2023, 42(9): 4465-4470. |
| [15] | 耿源泽, 周俊虎, 张天佑, 朱晓宇, 杨卫娟. 部分填充床燃烧器中庚烷均相/异相耦合燃烧[J]. 化工进展, 2023, 42(9): 4514-4521. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||