化工进展 ›› 2021, Vol. 40 ›› Issue (2): 778-788.DOI: 10.16085/j.issn.1000-6613.2020-0609
掺杂多孔碳材料催化硝基苯还原反应的研究进展
- 福建农林大学材料工程学院,福建 福州 350108
-
收稿日期:2002-04-17修回日期:2020-06-04出版日期:2021-02-05发布日期:2021-02-09 -
通讯作者:卢贝丽 -
作者简介:卢贝丽(1984—),女,博士,副教授,主要从事生物质资源高值化利用与新材料的研究。E-mail:lubl@fafu.edu.cn 。 -
基金资助:国家自然科学基金(31770611);福建省自然科学基金(2019J01388);福建农林大学科技创新专项基金(CXZX2018005)
Recent development on doped porous carbon materials for catalytic reduction of nitrobenzene
Beili LU( ), Xing LIU, Zhu YIN, Biao HUANG
), Xing LIU, Zhu YIN, Biao HUANG
- College of Material Engineering, Fujian Agriculture and Forestry University, Fuzhou 350108, Fujian, China
-
Received:2002-04-17Revised:2020-06-04Online:2021-02-05Published:2021-02-09 -
Contact:Beili LU
摘要:
苯胺是重要的化工原料和合成中间体,通过硝基苯的催化还原反应可以方便地制备苯胺类化合物。多孔碳材料因其高比表面积、发达的孔隙结构和容易回收等特点在催化领域越来越受到重视,然而其应用受到自身活性位点缺乏和化学惰性的限制。杂原子掺杂可以增强碳材料的表面极性,调节电子结构,改善其催化性能,可作为硝基苯催化还原反应的有效催化剂。本文对近年来掺杂多孔碳材料在硝基苯催化还原反应中的研究进展进行了总结。本文概述了氮掺杂型多孔碳材料、共掺杂型多孔碳材料、负载贵金属的掺杂多孔碳材料和负载廉价金属的掺杂多孔碳材料这4种主要的掺杂多孔碳材料的制备方法,并详细介绍了不同掺杂多孔碳材料在催化硝基苯催化还原反应时的催化性能、可能的催化活性位点以及催化机理。最后,指出目前掺杂多孔碳材料催化硝基苯还原还需要解决反应选择性、催化剂催化活性和生产成本等问题,以生物质为前体,开发共掺杂型和二元双金属负载的掺杂多孔碳材料是未来的重要发展方向之一。
中图分类号:
引用本文
卢贝丽, 刘杏, 尹铸, 黄彪. 掺杂多孔碳材料催化硝基苯还原反应的研究进展[J]. 化工进展, 2021, 40(2): 778-788.
Beili LU, Xing LIU, Zhu YIN, Biao HUANG. Recent development on doped porous carbon materials for catalytic reduction of nitrobenzene[J]. Chemical Industry and Engineering Progress, 2021, 40(2): 778-788.
| 催化剂 | 第一步HNO3 | 第二步KOH | 第三步H2O2 | 转化率/% | 选择性/% |
|---|---|---|---|---|---|
| non-C | no | no | no | 42.1 | 100 |
| PC | no | 活化(1h) | no | 62.7 | 100 |
| OPC | no | 活化(1h) | 24 | 83.2 | 100 |
| ONPC | 氧化(16h) | 活化(1h)① | no | 100 | 100 |
表1 不同碳材料催化硝基苯的还原制备苯胺
| 催化剂 | 第一步HNO3 | 第二步KOH | 第三步H2O2 | 转化率/% | 选择性/% |
|---|---|---|---|---|---|
| non-C | no | no | no | 42.1 | 100 |
| PC | no | 活化(1h) | no | 62.7 | 100 |
| OPC | no | 活化(1h) | 24 | 83.2 | 100 |
| ONPC | 氧化(16h) | 活化(1h)① | no | 100 | 100 |
| 催化剂 | 4-硝基苯酚的转化率 /% | (N/B) /% | 表观活化能 /kJ·mol-1 |
|---|---|---|---|
| NPC | 16 | 11.3/0 | 48.2 |
| B-NPC-800 | 32 | 8.5/0 | 39.1 |
| B-NPC-1000 | 77 | 4.2/0.9 | 30.2 |
| B-NPC-1200 | 94 | 1.9/1.5 | 27.0 |
表2 4-硝基苯酚还原反应中共掺杂原子的协同效应
| 催化剂 | 4-硝基苯酚的转化率 /% | (N/B) /% | 表观活化能 /kJ·mol-1 |
|---|---|---|---|
| NPC | 16 | 11.3/0 | 48.2 |
| B-NPC-800 | 32 | 8.5/0 | 39.1 |
| B-NPC-1000 | 77 | 4.2/0.9 | 30.2 |
| B-NPC-1200 | 94 | 1.9/1.5 | 27.0 |
| 底物 | 反应时间/h | 转化率/% | 选择性/% |
|---|---|---|---|
 | 0.5 | 100 | 99.5 |
 | 0.5 | 100 | 98.8 |
 | 0.5 | 100 | 95.5 |
 | 2 | 100 | 100 |
 | 2 | 100 | 99.2 |
 | 2 | 100 | 100 |
 | 2 | 100 | 100 |
 | 2 | 100 | 85.4 |
 | 2 | 100 | 70 |
表3 Pt/NOMC催化带有不同取代基的硝基苯的氢化反应
| 底物 | 反应时间/h | 转化率/% | 选择性/% |
|---|---|---|---|
 | 0.5 | 100 | 99.5 |
 | 0.5 | 100 | 98.8 |
 | 0.5 | 100 | 95.5 |
 | 2 | 100 | 100 |
 | 2 | 100 | 99.2 |
 | 2 | 100 | 100 |
 | 2 | 100 | 100 |
 | 2 | 100 | 85.4 |
 | 2 | 100 | 70 |
| 1 | CORMA A, SERNA P. Chemoselective hydrogenation of nitro compounds with supported gold catalysts[J]. Science, 2006, 313(5785): 332-334. |
| 2 | MANTHA R, TAYLOR K E, BISWAS N, et al. A continuous system for Fe0 reduction of nitrobenzene in synthetic wastewater[J]. Environmental Science and Technology, 2001, 35(15): 3231-3236. |
| 3 | 王同洲, 王鸿. 多孔碳材料的研究进展[J]. 中国科学(化学), 2019, 49(5): 729-740. |
| WANG T Z, WANG H. Research progress on porous carbon materials[J]. Scientia Sinica Chimica, 2019, 49(5): 729-740. | |
| 4 | ZHANG L H, SHI Y M, WANG Y, et al. Nanocarbon catalysts: recent understanding regarding the active sites[J]. Advanced Science, 2020, 7(5): 1902126. |
| 5 | SONG J, HUANG Z F, PAN L, et al. Review on selective hydrogenation of nitroarene by catalytic, photocatalytic and electrocatalytic reactions[J]. Applied Catalysis B: Environmental, 2018, 227: 386-408. |
| 6 | NASEEM K, BEGUM R, FAROOQI Z H. Catalytic reduction of 2-nitroaniline: a review[J]. Environmental Science & Pollution Research, 2017, 24(7): 1-15. |
| 7 | YANG Y, GU L, GUO S W, et al. N-doped mesoporous carbons: from synthesis to applications as metal-free reduction catalysts and energy storage materials[J]. Frontiers in Chemistry, 2019, 7: 761. |
| 8 | JEON I Y, NOH H J, BAEK J B. Nitrogen-doped carbon nanomaterials: synthesis, characteristics and applications[J]. Chemistry: an Asian Journal, 2020, 15: 2282-2293. |
| 9 | LIAO C J, LIU B, CHI Q, et al. Nitrogen-doped carbon materials for the metal-free reduction of nitro compounds[J]. ACS Applied Materials & Interfaces, 2018, 10(51): 44421-44429. |
| 10 | 王嘉, 李福伟. 氮掺杂碳包覆金属催化剂的制备及其在多相催化反应中的应用[J]. 中国科学(化学), 2018, 48(12): 1587-1602. |
| WANG J, LI F W. Synthesis of a N-doped carbon coating metal catalyst and its application in the heterogeneously catalytic reaction[J]. Scientia Sinica Chimica, 2018, 48(12): 1587-1602. | |
| 11 | 李晓微, 许海芬, 周晋, 等. 氮掺杂碳材料负载Pd纳米催化剂在有机反应中的最新研究进展[J]. 有机化学, 2018, 38(8): 74-86. |
| LI X W, XU H F, ZHOU J, et al. Recent progress of N-doped carbon materials supported Pd nanocatalysts in organic reactions[J]. Chinese Journal of Organic Chemistry, 2018, 38(8): 74-86. | |
| 12 | HE L, WENIGER F, NEUMANN H, et al. Synthesis, characterization, and application of metal nanoparticles supported on nitrogen-doped carbon: catalysis beyond electrochemistry[J]. Angewandte Chemie International Edition, 2016, 55(41): 12582-12594. |
| 13 | SHAN J X, SUN X Q, ZHENG S Y, et al. Graphitic N-dominated nitrogen-doped carbon nanotubes as efficient metal-free catalysts for hydrogenation of nitroarenes[J]. Carbon, 2019, 146: 60-69. |
| 14 | 余正发, 王旭珍, 刘宁, 等. N掺杂多孔碳材料研究进展[J]. 化工进展, 2013, 32(4): 824-831. |
| YU Z F, WANG X Z, LIU N, et al. Recent progress of N-doped porous carbon materials[J]. Chemical Industry and Engineering Progress, 2013, 32(4): 824-831. | |
| 15 | ZHANG W, WU W, LONG Y, et al. Co-Ag alloy protected by nitrogen doped carbon as highly efficient and chemoselective catalysts for the hydrogenation of halogenated nitrobenzenes[J]. Journal of Colloid and Interface Science, 2018, 522: 217-227. |
| 16 | LIU C, TANG P, CHEN A. et al. One-step assembly of N-doped partially graphitic mesoporous carbon for nitrobenzene reduction[J]. Materials Letters, 2013, 108: 285-288. |
| 17 | YANG Y, ZHANG W, MA X H, et al. Facile construction of mesoporous N-doped carbons as highly efficient 4-nitrophenol reduction catalysts[J]. ChemCatChem, 2015, 7(21): 3454-3459. |
| 18 | LIU N, DING L, LI H, et al. N-doped nanoporous carbon as efficient catalyst for nitrobenzene reduction in sulfide-containing aqueous solutions[J]. Journal of Colloid & Interface Science, 2017, 490: 677-684. |
| 19 | FUJITA S I, WATANABE H, KATAGIRI A, et al. Nitrogen and oxygen-doped metal-free carbon catalysts for chemoselective transfer hydrogenation of nitrobenzene, styrene, and 3-nitrostyrene with hydrazine[J]. Journal of Molecular Catalysis A: Chemical, 2014, 393: 257-262. |
| 20 | WEI Q H, QIN F F, MA Q X, et al. Coal tar-and residual oil-derived porous carbon as metal-free catalyst for nitroarene reduction to aminoarene[J]. Carbon, 2019, 141: 542-552. |
| 21 | LI L Y, LI L, CUI C Y, et al. Hierarchical hollow covalent organic frameworks-derived heteroatom-doped carbon spheres for metal-free catalysis[J]. ChemSusChem, 2017, 10(24): 4921-4926. |
| 22 | NGUYEN C V, LEE S, CHUNG Y G, et al. Synergistic effect of metal-organic framework-derived boron and nitrogen heteroatom-doped three-dimensional porous carbons for precious-metal-free catalytic reduction of nitroarenes[J]. Applied Catalysis B: Environmental, 2019, 257: 117888. |
| 23 | LIU S, CUI L, PENG Z, et al. Eco-friendly synthesis of N, S co-doped hierarchical nanocarbon as a highly efficient metal-free catalyst for the reduction of nitroarenes[J]. Nanoscale, 2018, 10(46): 21764-21771. |
| 24 | BEGUM R, REHAN R, FAEQQAI Z H, et al. Physical chemistry of catalytic reduction of nitroarenes using various nanocatalytic systems: past, present, and future[J]. Journal of Nanoparticle Research, 2016, 18(8): 231. |
| 25 | CHEN L, ZHANG L, CHEN Z, et al. A covalent organic framework-based route to the in situ encapsulation of metal nanoparticles in N-rich hollow carbon spheres[J]. Chemical Science, 2016, 7(9): 6015-6020. |
| 26 | ZUO P P, DUAN J Q, FAN H L, et al. Facile synthesis high nitrogen-doped porous carbon nanosheet from pomelo peel and as catalyst support for nitrobenzene hydrogenation[J]. Applied Surface Science, 2018, 435: 1020-1028. |
| 27 | LU C S, WANG M J, FENG Z L, et al. A phosphorus-carbon framework over activated carbon supported palladium nanoparticles for the chemoselective hydrogenation of para-chloronitrobenzene[J]. Catalysis Science & Technology, 2017, 7(7): 1581-1589. |
| 28 | ZHANG Q F, LI K, XIANG Y Z, et al. Sulfur-doped porous carbon supported palladium catalyst for high selective O-chloro-nitrobenzene hydrogenation[J]. Applied Catalysis A: General, 2019, 581: 74-81. |
| 29 | LIANG J, ZHANG X, JING L, et al. N-doped ordered mesoporous carbon as a multifunctional support of ultrafine Pt nanoparticles for hydrogenation of nitroarenes[J]. Chinese Journal of Catalysis, 2017, 38(7): 1252-1260. |
| 30 | SHOKOUHIMEHR M, KIM T, JUN S W, et al. Magnetically separable carbon nanocomposite catalysts for efficient nitroarene reduction and Suzuki reactions[J]. Applied Catalysis A: General, 2014, 476: 133-139. |
| 31 | ZHONG H, GONG Y Q, LIU W H, et al. Robust ultrafine ruthenium nanoparticles enabled by covalent organic gel precursor for selective reduction of nitrobenzene in water[J]. Dalton Transactions, 2019, 48(7): 2345-2351. |
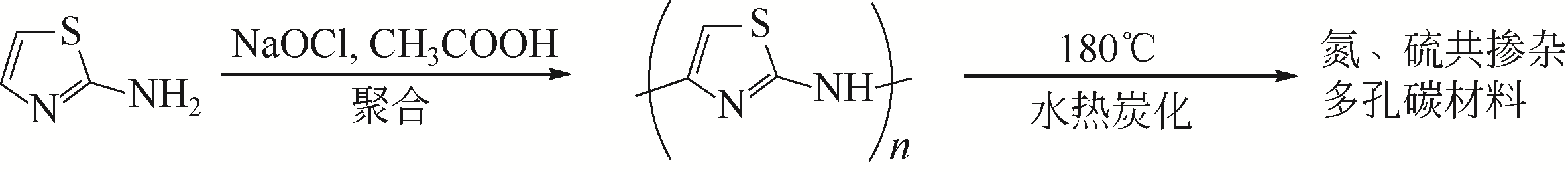
| 32 | BIDE Y, NABID M R, DASTAR F. Poly(2-aminothiazole) as a unique precursor for nitrogen and sulfur co-doped porous carbon: immobilization of very small gold nanoparticles and its catalytic application[J]. RSC Advances, 2015, 5(78): 63421-63428. |
| 33 | WESTERHAUS F A, JAGADEESH R V, WIENHÇFER G, et al. Heterogenized cobalt oxide catalysts for nitroarene reduction by pyrolysis of molecularly defined complexes[J]. Nature Chemistry, 2013, 5: 537-543. |
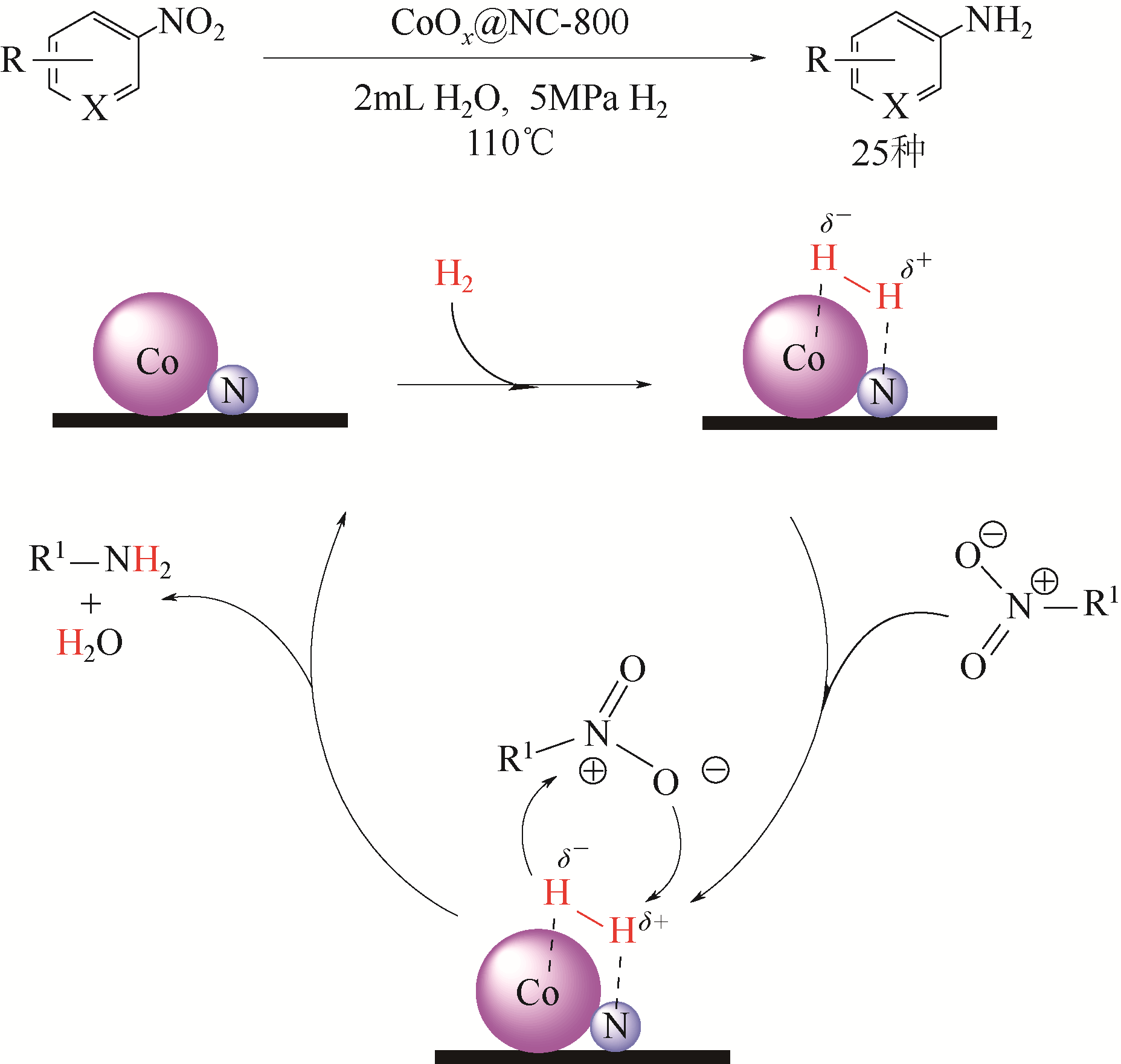
| 34 | HU A, LU X, CAI D, et al. Selective hydrogenation of nitroarenes over MOF-derived Co@CN catalysts at mild conditions[J]. Molecular Catalysis, 2019, 472: 27-36. |
| 35 | SUN X H, OLIVOS-SUAREZ A I, OSADCHII D, et al. Single cobalt sites in mesoporous N-doped carbon matrix for selective catalytic hydrogenation of nitroarenes[J]. Journal of Catalysis, 2018, 357: 20-28. |
| 36 | SONG T, REN P, DUAN Y N, et al. Cobalt nanocomposites on N-doped hierarchical porous carbon for highly selective formation of anilines and imines from nitroarenes[J]. Green Chemistry, 2018, 20(20): 4629-4637. |
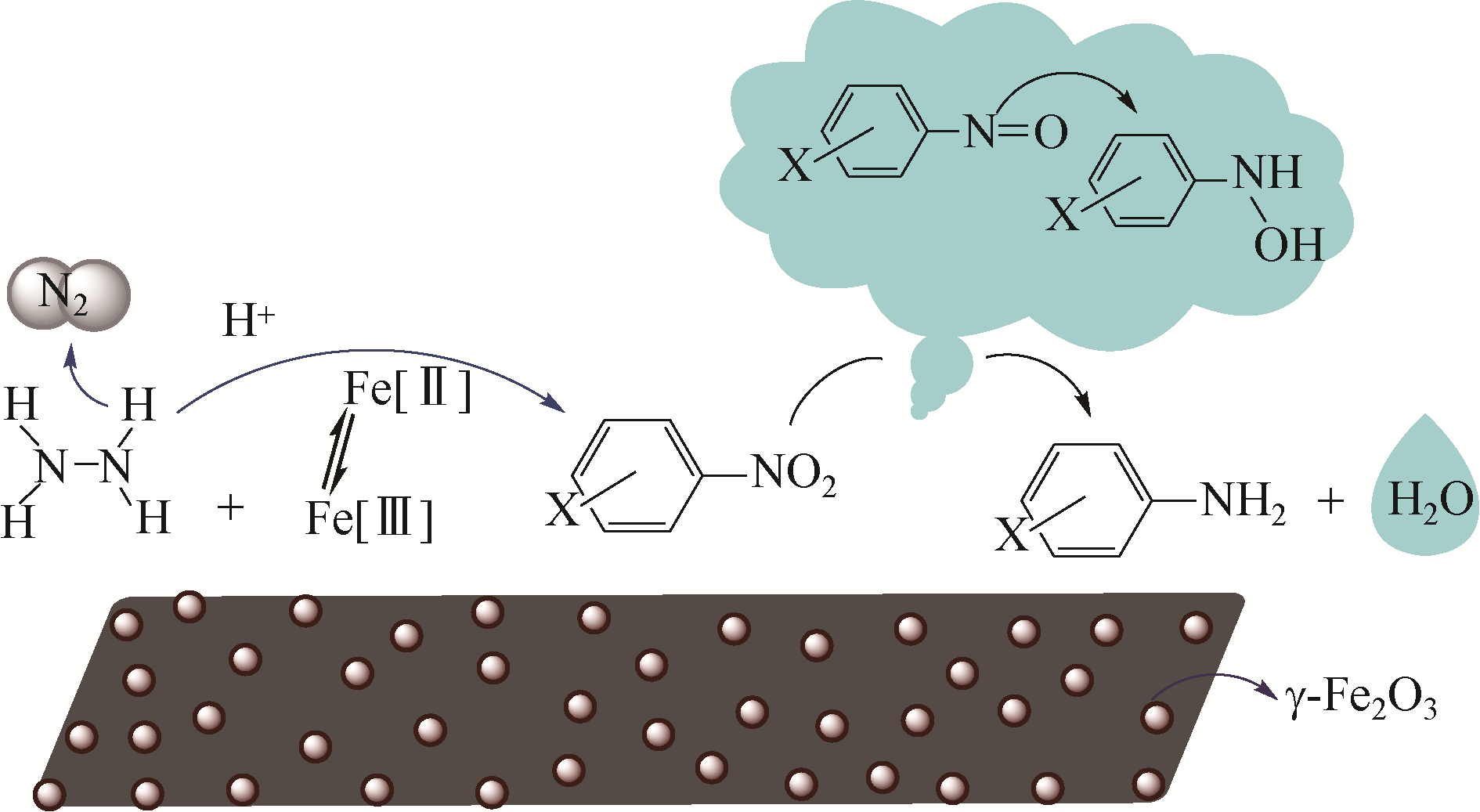
| 37 | CUI X L, ZHANG Q L, TIAN M, et al. Facile fabrication of γ-Fe2O3-nanoparticle modified N-doped porous carbon materials for the efficient hydrogenation of nitroaromatic compounds[J]. New Journal of Chemistry, 2017, 41(18): 10165-10173. |
| 38 | TIAN M, CUI X L, YUAN M, et al. Efficient chemoselective hydrogenation of halogenated nitrobenzenes over an easily prepared γ-Fe2O3-modified mesoporous carbon catalyst[J]. Green Chemistry, 2017, 19(6): 1548-1554. |
| 39 | WANG H, LIU X, XU G, et al. In situ synthesis of Fe-N-C catalysts from cellulose for hydrogenation of nitrobenzene to aniline[J]. Chinese Journal of Catalysis, 2019, 40: 1557-1565. |
| 40 | YANG F, WANG M J, LIU W, et al. Atomically dispersed Ni as the active site towards selective hydrogenation of nitroarenes[J]. Green Chemistry, 2019, 21(3): 704-711. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 高雨飞, 鲁金凤. 非均相催化臭氧氧化作用机理研究进展[J]. 化工进展, 2023, 42(S1): 430-438. |
| [6] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [7] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [8] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [9] | 王晋刚, 张剑波, 唐雪娇, 刘金鹏, 鞠美庭. 机动车尾气脱硝催化剂Cu-SSZ-13的改性研究进展[J]. 化工进展, 2023, 42(9): 4636-4648. |
| [10] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| [11] | 高彦静. 单原子催化技术国际研究态势分析[J]. 化工进展, 2023, 42(9): 4667-4676. |
| [12] | 张启, 赵红, 荣峻峰. 质子交换膜燃料电池中氧还原反应抗毒性电催化剂研究进展[J]. 化工进展, 2023, 42(9): 4677-4691. |
| [13] | 葛全倩, 徐迈, 梁铣, 王凤武. MOFs材料在光电催化领域应用的研究进展[J]. 化工进展, 2023, 42(9): 4692-4705. |
| [14] | 王伟涛, 鲍婷玉, 姜旭禄, 何珍红, 王宽, 杨阳, 刘昭铁. 醛酮树脂基非金属催化剂催化氧气氧化苯制备苯酚[J]. 化工进展, 2023, 42(9): 4706-4715. |
| [15] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||