化工进展 ›› 2019, Vol. 38 ›› Issue (10): 4437-4443.DOI: 10.16085/j.issn.1000-6613.2019-0007
草酸强化三价铁离子的反萃性能
- 1. 中南大学冶金与环境学院,湖南 长沙 410083
2. 稀有金属冶金与材料制备湖南省重点实验室,湖南 长沙 410083
-
收稿日期:2019-01-02出版日期:2019-10-05发布日期:2019-10-05 -
通讯作者:袁飞刚 -
作者简介:袁飞刚(1986—),男,博士研究生,研究方向为有色金属复杂资源高效提取、相似元素分离和冶金过程强化等。E-mail:fgyuan@csu.edu.cn 。 -
基金资助:国家重点研发计划(2018YFC1901603)
Intensification of the stripping characteristics of iron (Ⅲ) using oxalic acid
- 1. School of Metallurgy and Environment, Central South University, Changsha 410083, Hunan, China
2. Key Laboratory of Hunan Province for Metallurgy and Material Processing of Rare Metals, Changsha 410083, Hunan, China
-
Received:2019-01-02Online:2019-10-05Published:2019-10-05 -
Contact:Feigang YUAN
摘要:
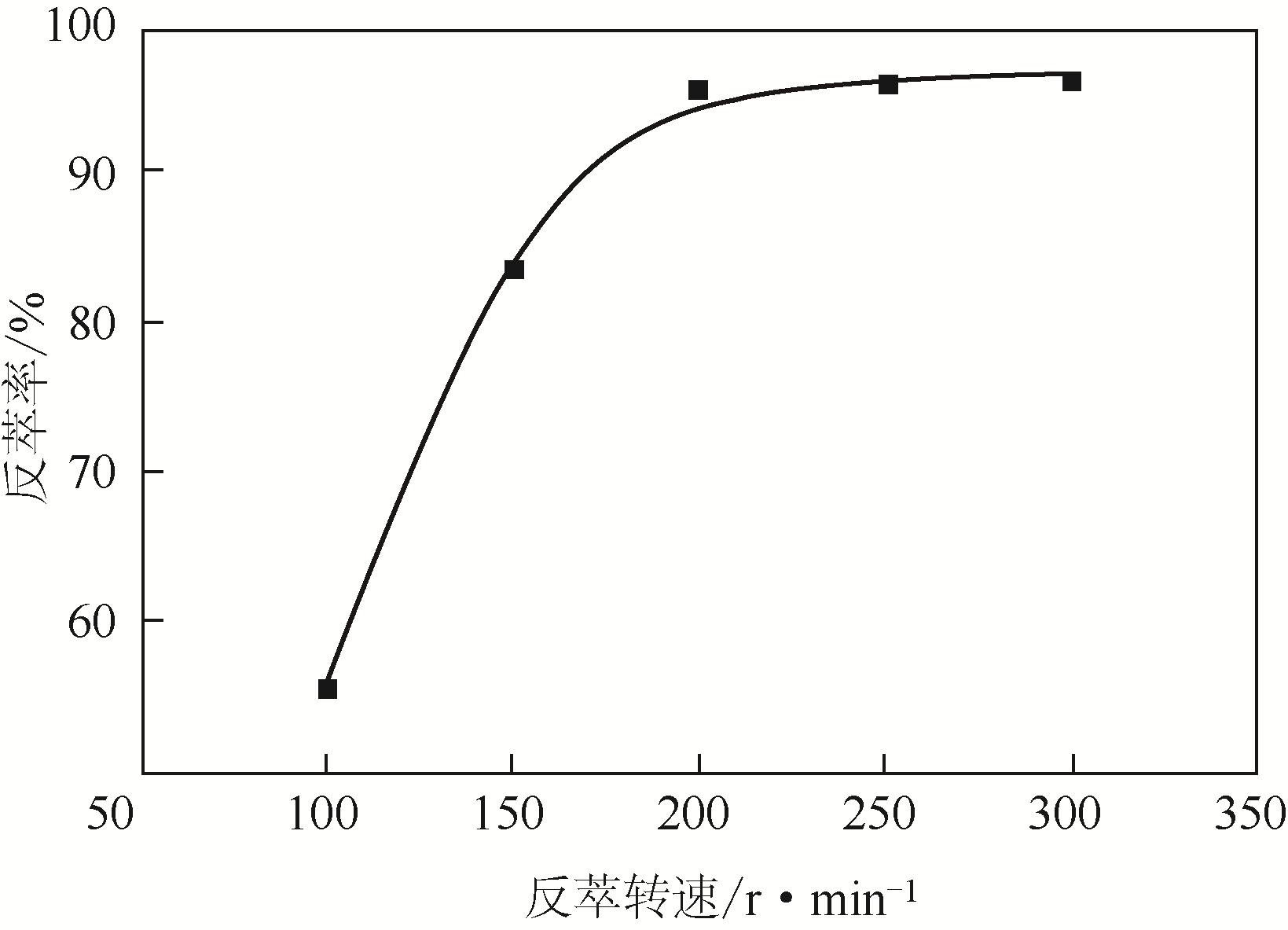
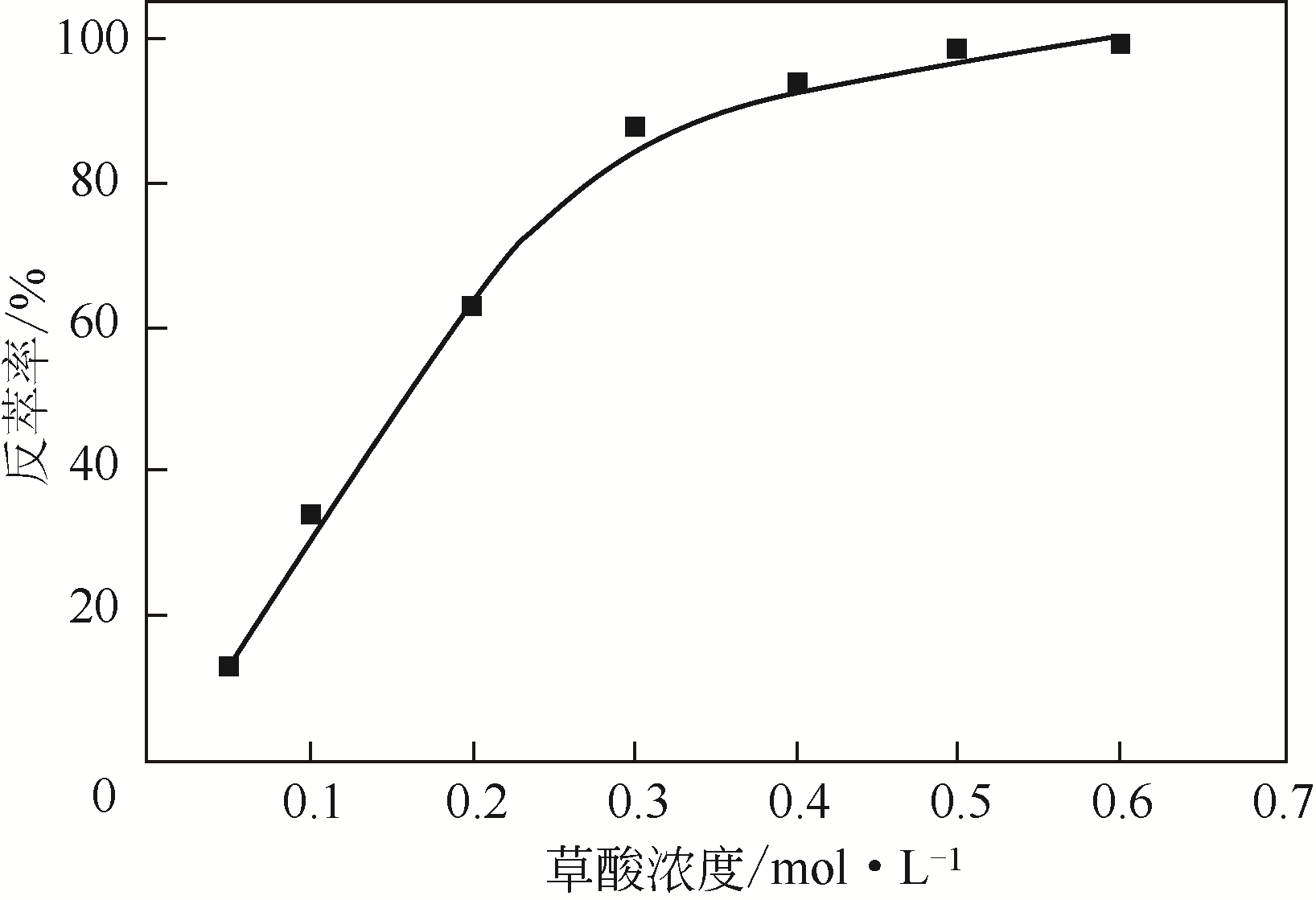
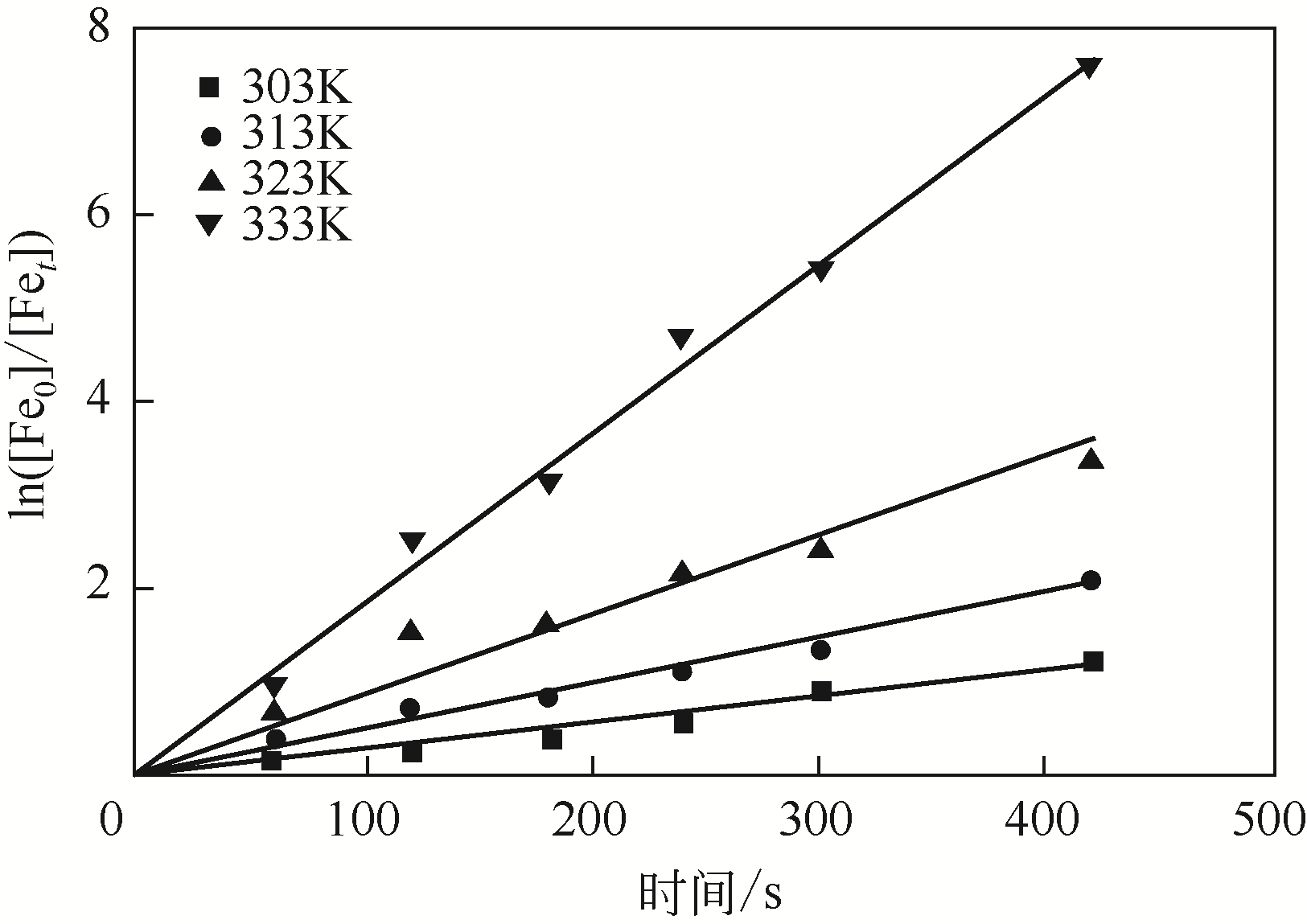
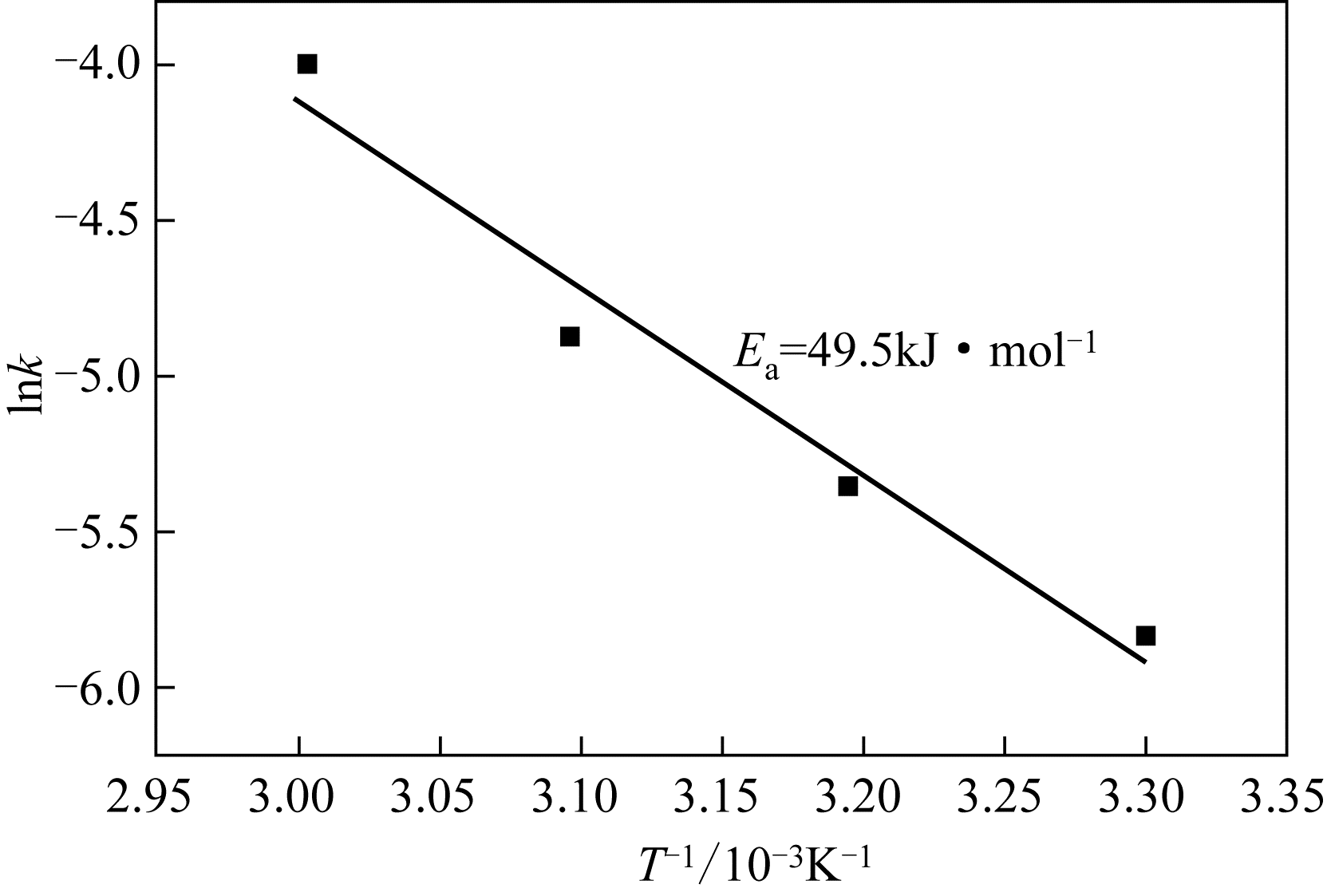
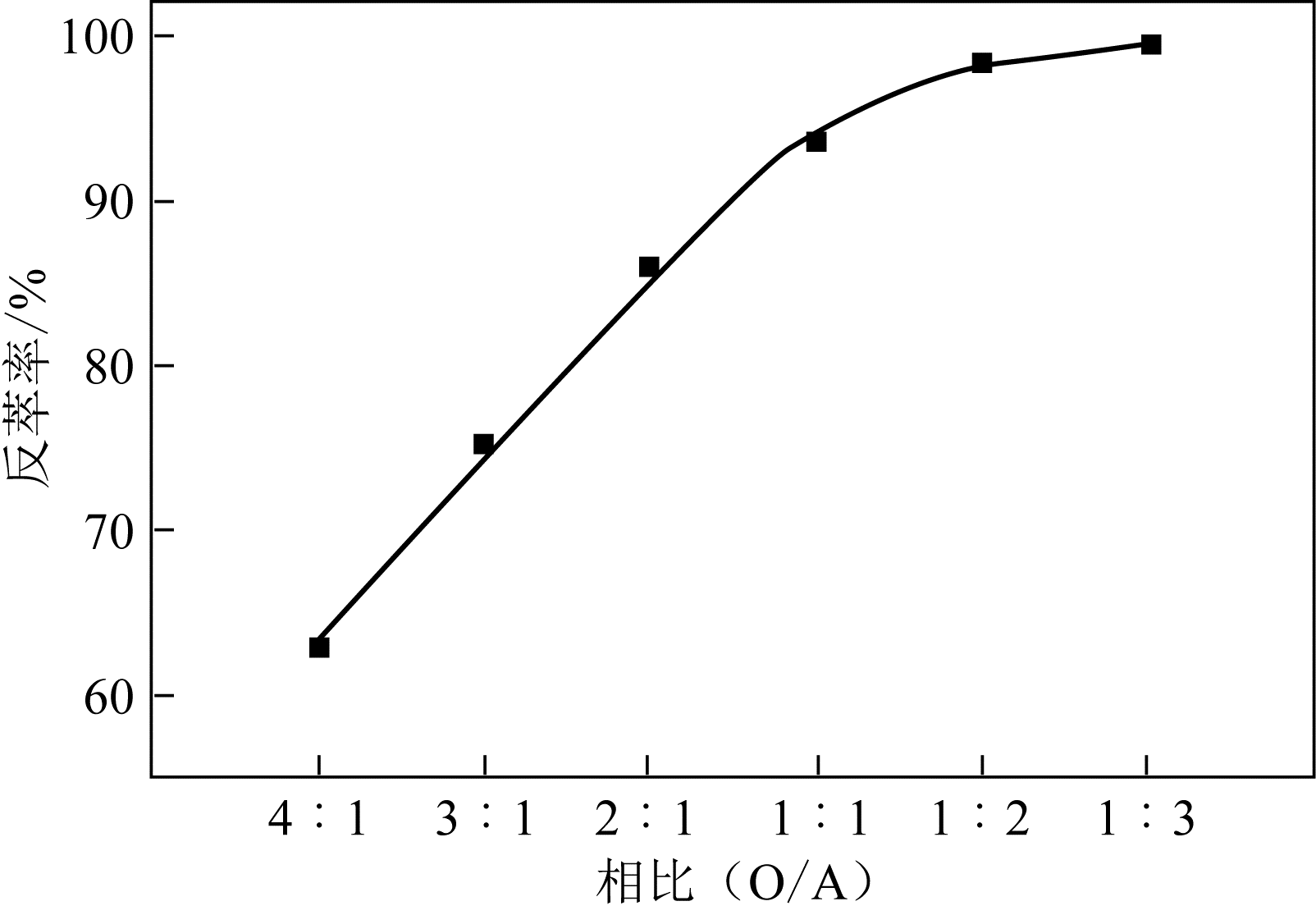
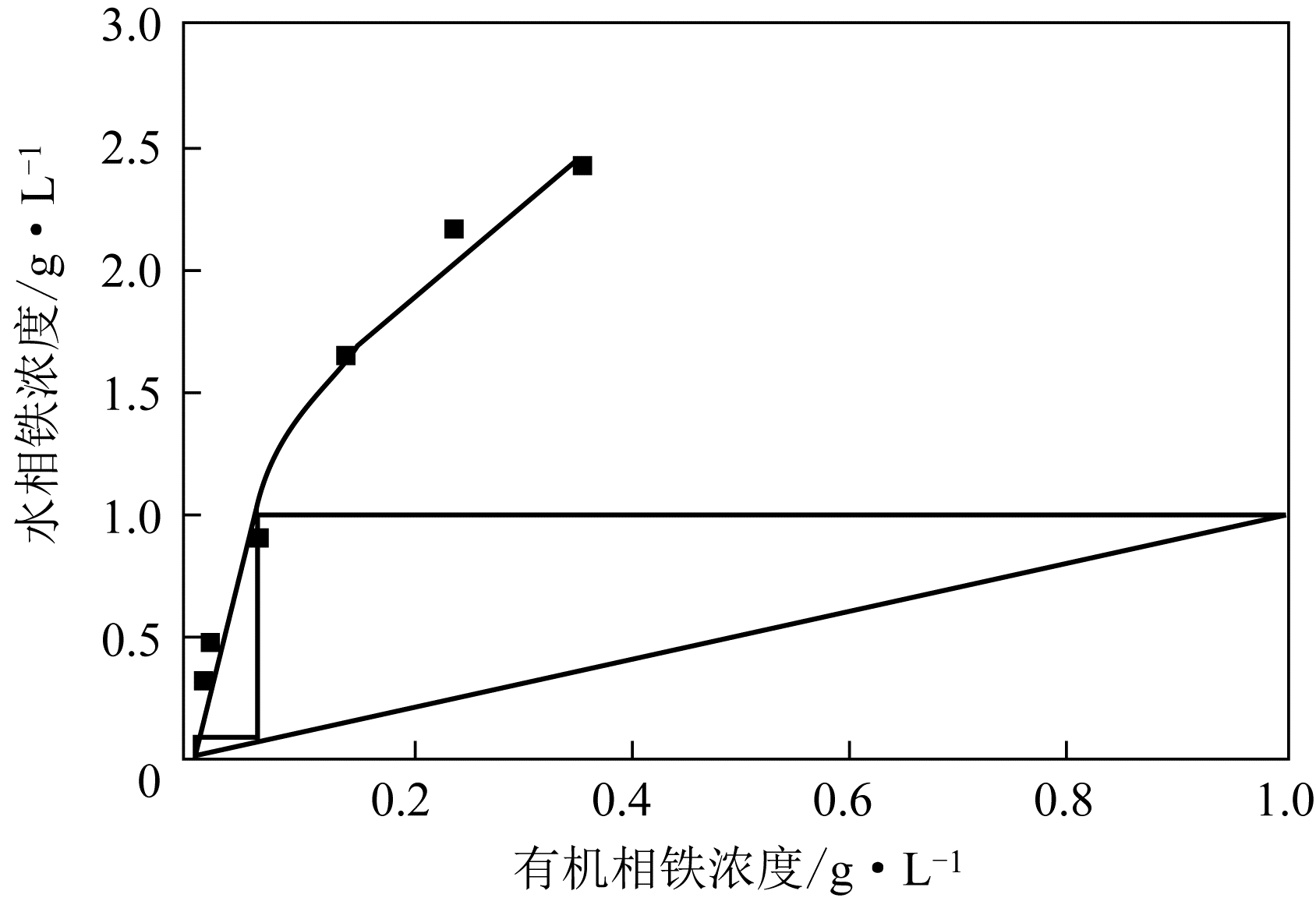
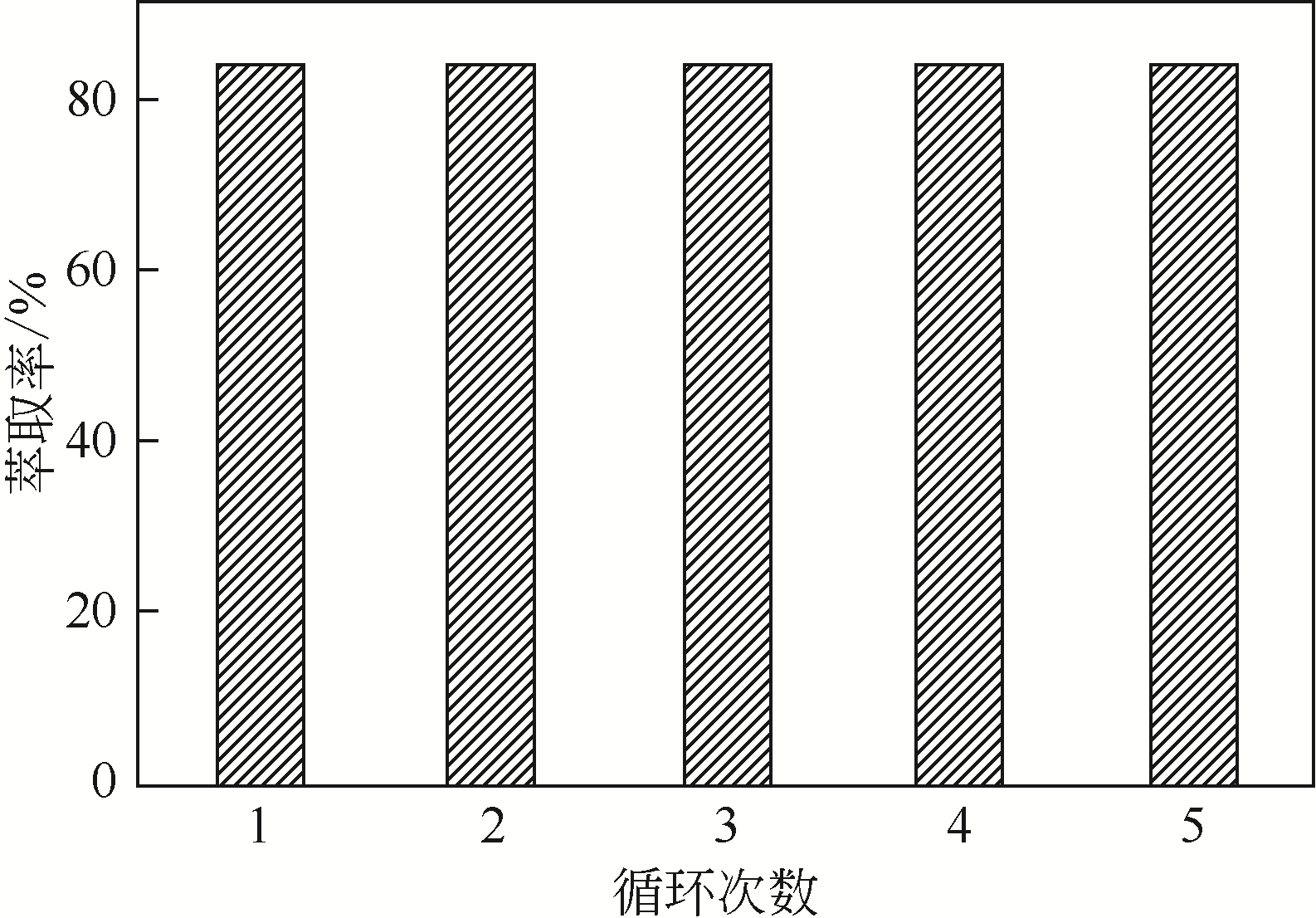
二(2-乙基己基)磷酸(P204)常作为溶液净化除铁的萃取剂,P204-磺化煤油体系中Fe3+与有机相形成络合能力较强的萃合物,使得Fe3+反萃比较困难,需采用较高浓度的酸作为反萃剂,但高浓度的酸会破坏有机分子的结构,影响萃取剂循环利用。针对P204-磺化煤油负铁有机相反萃困难的问题,提出利用草酸为反萃剂对负载1g/L铁的P204-磺化煤油有机相的反萃行为进行研究,考察了反萃转速、草酸浓度、反萃温度、反萃时间和相比对Fe3+反萃率的影响。结果表明:以反萃转速200r/min,草酸0.4mol/L,反萃时间10min,反萃温度40℃,反萃相比1∶1,采用二级逆流萃取方式,铁的反萃率可以达到99%以上;Fe3+反萃过程是吸热反应,其反应的焓变为81.58kJ/mol,反萃过程符合准一级反应动力学方程,对应活化能为49.5kJ/mol。进一步研究了反萃后P204-磺化煤油有机相对Fe3+的萃取性能。结果表明:经5次草酸反萃后的P204-磺化煤油有机相萃铁性能几乎不变,对比于高浓度的酸反萃,草酸反萃简化了反萃流程,降低了萃取剂的消耗。
中图分类号:
引用本文
袁飞刚. 草酸强化三价铁离子的反萃性能[J]. 化工进展, 2019, 38(10): 4437-4443.
Feigang YUAN. Intensification of the stripping characteristics of iron (Ⅲ) using oxalic acid[J]. Chemical Industry and Engineering Progress, 2019, 38(10): 4437-4443.
| 项目 | 有机相 | 一级 | 二级 |
|---|---|---|---|
| 浓度/mg·L-1 | 1000 | 43.82 | 1.08 |
| 每级反萃率/% | — | 95.62 | 97.53 |
| 总反萃率/% | 99.89 | ||
表1 二级逆流反萃Fe3+结果
| 项目 | 有机相 | 一级 | 二级 |
|---|---|---|---|
| 浓度/mg·L-1 | 1000 | 43.82 | 1.08 |
| 每级反萃率/% | — | 95.62 | 97.53 |
| 总反萃率/% | 99.89 | ||
| 30 | MUSADAIDZWA J M , TSHININGAYAMWE E I . Skorpion zinc solvent extraction: the upset conditions[J]. Journal of the Southern African Institute of Mining and Metallurgy, 2009, 109: 691-695. |
| 31 | WEERT G V , SANDWIJK T VAN , HOGEWEG P . Solvent extraction of ferric iron from zinc sulfate solutions with DEHPA-investigation of nitric acid as stripping agent[C]//MISHRA B. Extraction and processing division congress. Warrendale: The Minerals Metals and Materials Society, 1998: 245-266. |
| 32 | YU S Q , CHEN J Y , CHEN C Y . Stripping of Fe (Ⅲ) extracted by di-2-ethylhexyl phosphoric acid from sulfate solutions with sulfuric acid[J]. Hydrometallurgy, 1989, 22(1/2): 267-272. |
| 33 | WATANABE T , HOSHINO M , UCHINO K . et al . A new recovery process for stainless steel pickling waste liquor with an iron removal process using solvent extraction[C]//DUTRIZAC J E, MONHEMIUS A J. Iron control in hydrometallurgy. Chichester: Ellis Horwood, 1986: 549-570. |
| 34 | CHEN J Y , YU S Q , LIU H Z , et al . New mixed solvent systems for the extraction and separation of ferric iron in sulfate solutions[J]. Hydrometallurgy, 1992, 30(1/2/3): 401-416. |
| 35 | YU S Q , CHEN J Y , CHEN C Y . Synergistic extraction of ferric iron in sulfate solutions by tertiary amine and 2-ethylhexyl 2-ethylhexylphosphonic acid (HEHEHP) or dialkylphosphonic acid[J]. Hydrometallurgy, 1989, 22(1/2): 183-192. |
| 36 | MAJIMA H, IZAKI T, SANUKI S, Reductive stripping of Fe(Ⅲ)-loaded D2EHPA with the aqueous solutions containing sulfur dioxide[J]. Metallurgical Transactions B, 1985, 16(2): 187-194. |
| 37 | DEMOPOULOS G P , GEFVERT D L . Iron (Ⅲ) removal from base-metal electrolyte solutions by solvent extraction[J]. Hydrometallurgy, 1984, 12(3): 299-315. |
| 38 | LUPI C , PILONE D . Reductive stripping in vacuum of Fe(Ⅲ) from D2EHPA[J]. Hydrometallurgy, 2000, 57(3): 201-207. |
| 39 | MOATS M S , O’KEEFE T J . Optimization of ferric ion reduction in di-ethylhexyl phosphoric acid by separate galvanic stripping[C]//RAMACHANDRAN V, NESBITT C C. Second International Symposium on Extraction and Processing for the Treatment and Minimization of Wastes. Warrendale: The Minerals Metals and Materials Society, 1996: 673-684. |
| 40 | CHIA L M , NEIRA M P , FLORES C , et al . Overview of galvanic stripping of organic solvents in waste materials treatment[C]//HAGER J, HANSEN B. Extraction and processing for the treatment and minimization of wastes. Warrendale: The Minerals Metals and Materials Society, 1994: 279-291. |
| 41 | BELEW B , HARLAMOVS J R , O’KEEFE T J , et al . Reductive stripping of iron Ⅲ from di(2-ethylhexyl) phosphoric acid[C]//HISKEY J B, WARREN G W. Hydrometallurgy fundamentals: technology and innovation. Littleton: American Society of Mechanical Engineers, 1993: 817-830. |
| 1 | 陈家镛, 于淑秋, 伍志春 . 湿法冶金中铁的分离与利用[M]. 北京: 冶金工业出版社, 1991: 3. |
| CHEN J Y , YU S Q , WU Z C . Separation and utilization of iron in hydrometallurgy[M]. Beijing: Metallurgical Industry Press, 1991: 3. | |
| 42 | BONNEY C F . Removal of iron from Kaolin and quartz: dissolution with organic acids[C]//Institution of Mining & Metallurgy. International Symposium “Hydrometallurgy 94”. Dordrecht: Springer, 1994: 313-323. |
| 43 | AMBIKADEVI V R , LALITHAMBIKA M . Effect of organic acids on ferric iron removal from iron-stained kaolinite[J]. Applied Clay Science, 2000, 16(3/4): 133-145. |
| 44 | VEGLIO F , PASSARIELLIO B , TORO L , et al . Development of a bleaching process for a Kaolin of industrial interest by oxalic, ascorbic, and sulfuric acids: preliminary study using statistical methods of experimental design[J]. Industrial & Engineering Chemistry Research, 1996, 35(5): 1680-1687. |
| 45 | WORTHINGTON R E , MAGDICS A . Process for stripping uranium from an alkyl pyrophosphoric acid: US4490336[P]. 1984-12-25. |
| 2 | 何静, 罗超, 唐谟堂, 等 . 采用铅黄铁矾去除硫酸体系中的铁[J]. 中国有色金属学报, 2012, 22(10): 2890-2895. |
| HE J , LUO C , TANG M T , et al . Technique of ferrum-removal by lead jarosite from sulfuric acid solution[J]. The Chinese Journal of Nonferrous Metals, 2012, 22(10): 2890-2895. | |
| 3 | 陈红彬 . 用针铁矿法从钴白合金酸浸液中除铁研究[J]. 金属材料与冶金工程, 2012, 40(4): 27-29. |
| CHEN H B . Iron removal from acidic leaching solution of Co white alloy by goethite process[J]. Metal Materials and Metallurgy Engineering, 2012, 40(4): 27-29. | |
| 4 | 杨凡, 邓志敢, 魏昶, 等 . 采用赤铁矿去除高铁闪锌矿浸出液中的铁[J]. 中国有色金属学报, 2014, 24(9): 2387-2392. |
| YANG F , DENG Z G , WEI C , et al . Iron-removal by hematite from leaching liquor of high iron sphalerite[J]. The Chinese Journal of Nonferrous Metals, 2014, 24(9): 2387-2392. | |
| 5 | 李勇刚, 马蕾, 伍铁斌, 等 . 多反应器级联的沉铁过程pH优化控制[J]. 化工学报, 2018, 69(6): 2586-2593. |
| LI Y G , MA L , WU T B , et al . pH optimization and control in iron removal process of multi-reactor cascade[J]. CIESC Journal, 2018, 69(6): 2586-2593. | |
| 6 | 陈国宝, 杨洪英, 周立杰, 等 . 针铁矿法从铜钴矿生物浸出液中除铁的研究[J]. 有色金属(冶炼部分), 2013(3): 1-3. |
| CHEN G B , YANG H Y , ZHOU L J , et al . Study on goethite deironization from bioleaching solution of Cu-Co ore[J]. Nonferrous Metals (Extractive Metallurgy), 2013(3): 1-3. | |
| 7 | AGRAWAL A , KUMARI S , SAHU K K . Iron and copper recovery/removal from industrial waste: a review[J]. Industrial & Engineering Chemistry Research, 2009, 48(13): 6145-6161. |
| 8 | SAHU K K . DAS R P. Synergistic extraction of iron (Ⅲ) at higher concentrations in D2EHPA-TBP mixed solvent systems [J]. Metallurgical and Materials Transactions B, 1997, 28(2): 181-189. |
| 9 | BISWAS R K , BEGUM D A . Solvent extraction of Fe3+ from chloride solution by D2EHPA in kerosene[J]. Hydrometallurgy, 1998, 50(2): 153-168. |
| 10 | SATO T , NAKAMURA T , IKENO M . The extraction of iron(Ⅲ) from aqueous acid solutions by di(2-ethylhexyl)phosphoric acid[J]. Hydrometallurgy, 1985, 15(2): 209-217. |
| 11 | 刘铭, 周雍茂 . 用N235-TBP混合体系从硫酸盐溶液中协同萃取除铁[J]. 中国有色金属学报, 2005, 15(10): 1648-1654. |
| LIU M , ZHOU Y M . Removal of Fe(Ⅲ) from sulphate solutions by synergistic extraction using N235-TBP mixedsolvent systems[J]. The Chinese Journal of Nonferrous Metals, 2005, 15(10): 1648-1654 | |
| 46 | 李以圭 . 金属溶剂萃取热力学[M]. 北京: 清华大学出版社, 1988: 100-111. |
| LI Y G . Thermodynamics of metal solvent extraction[M]. Beijing: Tsinghua University Press, 1988: 100-111. | |
| 12 | SILVA G C DA , CUNHA J W S D D , DWECK J , et al . Liquid-liquid extraction (LLE) of iron and titanium by bis-(2-ethyl-hexyl)phosphoric acid (D2EHPA)[J]. Minerals Engineering, 2008, 21(5): 416-419. |
| 13 | MISHRA R K , ROUT P C , SARANGI K , et al . A comparative study on extraction of Fe (Ⅲ) from chloride leach liquor using TBP, Cyanex 921 and Cyanex 923[J]. Hydrometallurgy, 2010, 104(2): 298-303. |
| 14 | 李际嵩,刘代俊,陈建钧,等 .从磷酸中萃取铁离子的研究[J]. 无机盐工业, 2015, 47(3): 12-15. |
| LI J S , LIU D J , CHEN J J , et al . Study on extraction of iron ion from phosphoric acid[J]. Inorganic Chemicals Industry, 2015, 47(3): 12-15. | |
| 15 | NAIK M T , DHADKE P M . Extraction of iron (Ⅲ) with bis(2-ethylhexyl) phosphinic acid and bis(2-ethylhexyl) phosphoric acid: experimental equilibrium study[J]. Journal of Chemical & Engineering Data, 1999, 44(5): 1037-1040. |
| 16 | PRINCIPE F , DEMOPOULOS G P . Comparative study of iron(Ⅲ) separation from zinc sulphate-sulphuric acid solutions using the organophosphorus extractants, OPAP and D2EHPA. Part Ⅰ: extraction[J]. Hydrometallurgy, 2005, 74: 93-102. |
| 17 | 张魁芳, 刘志强, 曹洪杨, 等 . 用N235从富铁高酸度硫酸浸出液中萃取除铁[J]. 中国有色金属学报 2015, 25(5): 1370-1377. |
| ZHANG K F , LIU Z Q , CAO H Y , et al . Removal of Fe3+ from iron rich and high acidity sulfuric acid leaching liquid by extraction of N235[J]. The Chinese Journal of Nonferrous Metals, 2015, 25(5): 1370-1377. | |
| 18 | EL-NADI Y A , EL-HEFNY N E . Removal of iron from Cr-electroplating solution by extraction with di(2-ethylhexyl)phosphoric acid in kerosene[J]. Chemical Engineering and Processing: Process Intensification, 2010, 49(2): 159-164. |
| 19 | AGRAWAL A. KUMARI S. RAY B C, et al. Extraction of acid and iron values from sulphate waste pickle liquor of a steel industry by solvent extraction route[J]. Hydrometallurgy, 2007, 88(1/2/3/4): 58-66. |
| 20 | YADAV S K , SINGH O V , TANDON S N . Extraction and separation of some 3d transition elements using Cyanex 925: application to zinc ores[J]. Hydrometallurgy,1994, 36(1): 53-59. |
| 21 | JAYACHANDRAN J , DHADKE P M . Liquid-liquid extraction separation of iron (Ⅲ) with 2-ethyl hexyl phosphonic acid mono 2-ethyl hexyl ester[J]. Talanta, 1997, 44(7): 1285-1290. |
| 22 | OREN J J , GOUGH K M , GESSER H D . The solvent extraction of Fe (Ⅲ) from acidic chloride solutions by open cell polyurethane foam sponge (OCPUFS)[J]. Canadian Journal of Chemistry, 2011, 57(15): 2032-2036. |
| 23 | JIN Y , MA Y J , WENG, Y L, et al . Solvent extraction of Fe3+ from the hydrochloric acid route phosphoric acid by D2EHPA in kerosene[J]. Journal of Industrial and Engineering Chemistry, 2014, 20(5): 3446-3452. |
| 24 | MELEŠ S , PROŠTENIK M V . Solvent extraction of Fe (Ⅲ) by di-(2-ethylhexyl) phosphoric acid from phosphoric acid solutions[J]. Polyhedron, 1984, 3(5): 615-617. |
| 25 | AGATZINI S , KONTOPOULOS A , MARABOUTIS P , et al . Removal of iron from iron-nickel-cobalt solutions by precipitation and solvent extraction techniques[C]//DUTRIZAC J E, MONHEMIUS A J. Iron control in hydrometallurgy. Chichester: Ellis Horwood, 1986: 353-373. |
| 26 | SATO T , NAKAMURA T , IKENO M . The extraction of iron(Ⅲ) from aqueous acid solutions by di(2-ethylhexyl)phosphoric acid[J]. Hydrometallurgy, 1985, 15(2): 209-217. |
| 27 | NISHIMURA S , WATANABE M . Process for the production of high-purity metallic iron: EP0046974[P]. 1982-03-10. |
| 28 | AKHLAGHI M , RASHCHI F , VAHIDI E . Stripping of Fe (Ⅲ) from D2EHPA using different reagents[C]// Proceedings of the ⅹ International Mineral Processing Congress. Brisbane: Australasian Institute for Mining and Metallurgy, 2010: 255-262. |
| 29 | HIRATO T , WU Z C , YAMADA Y , et al . Improvement of the stripping characteristics of Fe(Ⅲ) utilizing a mixture of di(2-ethylhexyl) phosphoric acid and tri-n-butyl phosphate[J]. Hydrometallurgy, 1992, 28(1): 81-93. |
| [1] | 汪鹏, 张洋, 范兵强, 何登波, 申长帅, 张贺东, 郑诗礼, 邹兴. 高碳铬铁盐酸浸出过程工艺及动力学[J]. 化工进展, 2023, 42(S1): 510-517. |
| [2] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [3] | 史天茜, 石永辉, 武新颖, 张益豪, 秦哲, 赵春霞, 路达. Fe2+对厌氧氨氧化EGSB反应器运行性能的影响[J]. 化工进展, 2023, 42(9): 5003-5010. |
| [4] | 许中硕, 周盼盼, 王宇晖, 黄威, 宋新山. 硫铁矿介导的自养反硝化研究进展[J]. 化工进展, 2023, 42(9): 4863-4871. |
| [5] | 钱思甜, 彭文俊, 张先明. PET熔融缩聚与溶液解聚形成环状低聚物的对比分析[J]. 化工进展, 2023, 42(9): 4808-4816. |
| [6] | 徐沛瑶, 陈标奇, KANKALA Ranjith Kumar, 王士斌, 陈爱政. 纳米材料用于铁死亡联合治疗的研究进展[J]. 化工进展, 2023, 42(7): 3684-3694. |
| [7] | 李海东, 杨远坤, 郭姝姝, 汪本金, 岳婷婷, 傅开彬, 王哲, 何守琴, 姚俊, 谌书. 炭化与焙烧温度对植物基铁碳微电解材料去除As(Ⅲ)性能的影响[J]. 化工进展, 2023, 42(7): 3652-3663. |
| [8] | 王俊杰, 潘艳秋, 牛亚宾, 俞路. 分子水平催化重整装置模型构建及应用[J]. 化工进展, 2023, 42(7): 3404-3412. |
| [9] | 龚鹏程, 严群, 陈锦富, 温俊宇, 苏晓洁. 铁酸钴复合碳纳米管活化过硫酸盐降解铬黑T的性能及机理[J]. 化工进展, 2023, 42(7): 3572-3581. |
| [10] | 杨竞莹, 施万胜, 黄振兴, 谢利娟, 赵明星, 阮文权. 改性纳米零价铁材料制备的研究进展[J]. 化工进展, 2023, 42(6): 2975-2986. |
| [11] | 吴锋振, 刘志炜, 谢文杰, 游雅婷, 赖柔琼, 陈燕丹, 林冠烽, 卢贝丽. 生物质基铁/氮共掺杂多孔炭的制备及其活化过一硫酸盐催化降解罗丹明B[J]. 化工进展, 2023, 42(6): 3292-3301. |
| [12] | 杨红梅, 高涛, 鱼涛, 屈撑囤, 高家朋. 高铁酸盐处理难降解有机物磺化酚醛树脂[J]. 化工进展, 2023, 42(6): 3302-3308. |
| [13] | 李瑞东, 黄辉, 同国虎, 王跃社. 原油精馏塔中铵盐吸湿特性及其腐蚀行为[J]. 化工进展, 2023, 42(6): 2809-2818. |
| [14] | 葛伟童, 廖亚龙, 李明原, 嵇广雄, 郗家俊. Pd-Fe/MWCNTs双金属催化剂制备及其脱氯动力学[J]. 化工进展, 2023, 42(4): 1885-1894. |
| [15] | 贺山明, 潘界昌, 徐国钻, 李文君, 梁勇. 粗钨酸钠溶液亚铁盐沉淀法除铬、钒的热力学分析及实验验证[J]. 化工进展, 2023, 42(4): 2171-2179. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||