化工进展 ›› 2019, Vol. 38 ›› Issue (05): 2434-2440.DOI: 10.16085/j.issn.1000-6613.2018-1503
固体生物聚合硫酸铁的制备及其絮凝性能
徐小惠1,2( ),鲁敏1,杨柳1,王悦3,刘雅琪4,关晓辉1(
),鲁敏1,杨柳1,王悦3,刘雅琪4,关晓辉1( )
)
- 1. 东北电力大学化学工程学院,吉林 吉林 132012
2. 东北大学资源与土木工程学院,辽宁 沈阳 110819
3. 广东省特种设备检测研究院珠海检测院,广东 珠海 519000
4. 内蒙古国华呼伦贝尔发电有限公司,内蒙古 呼伦贝尔 021025
-
收稿日期:2018-07-22修回日期:2018-11-03出版日期:2019-05-05发布日期:2019-05-05 -
通讯作者:关晓辉 -
作者简介:<named-content content-type="corresp-name">徐小惠</named-content>(1986—),女,博士研究生,研究方向为水处理。E-mail:<email>xuxiaohui06@126.com</email>。 -
基金资助:吉林省科技发展计划(20130206059GX)
Preparation of solid biopolymerized ferric sulfate and its flocculation performance
Xiaohui XU1,2( ),Min LU1,Liu YANG1,Yue WANG3,Yaqi LIU4,Xiaohui GUAN1(
),Min LU1,Liu YANG1,Yue WANG3,Yaqi LIU4,Xiaohui GUAN1( )
)
- 1. School of Chemical Engineering, Northeast Electric Power University, Jilin 132012, Jilin, China
2. School of Resources and Civil Engineering, Northeastern University, Shenyang 110819, Liaoning, China
3. Guangdong Institute of Special Equipment Testing Zhuhai Inspection Institute, Zhuhai 519000, Guangdong, China
4. Inner Mongolia Guohua Hulunbeier Power Generation Co. , Ltd. ,Hulun Buir 021025, Inner Mongolia, China
-
Received:2018-07-22Revised:2018-11-03Online:2019-05-05Published:2019-05-05 -
Contact:Xiaohui GUAN
摘要:
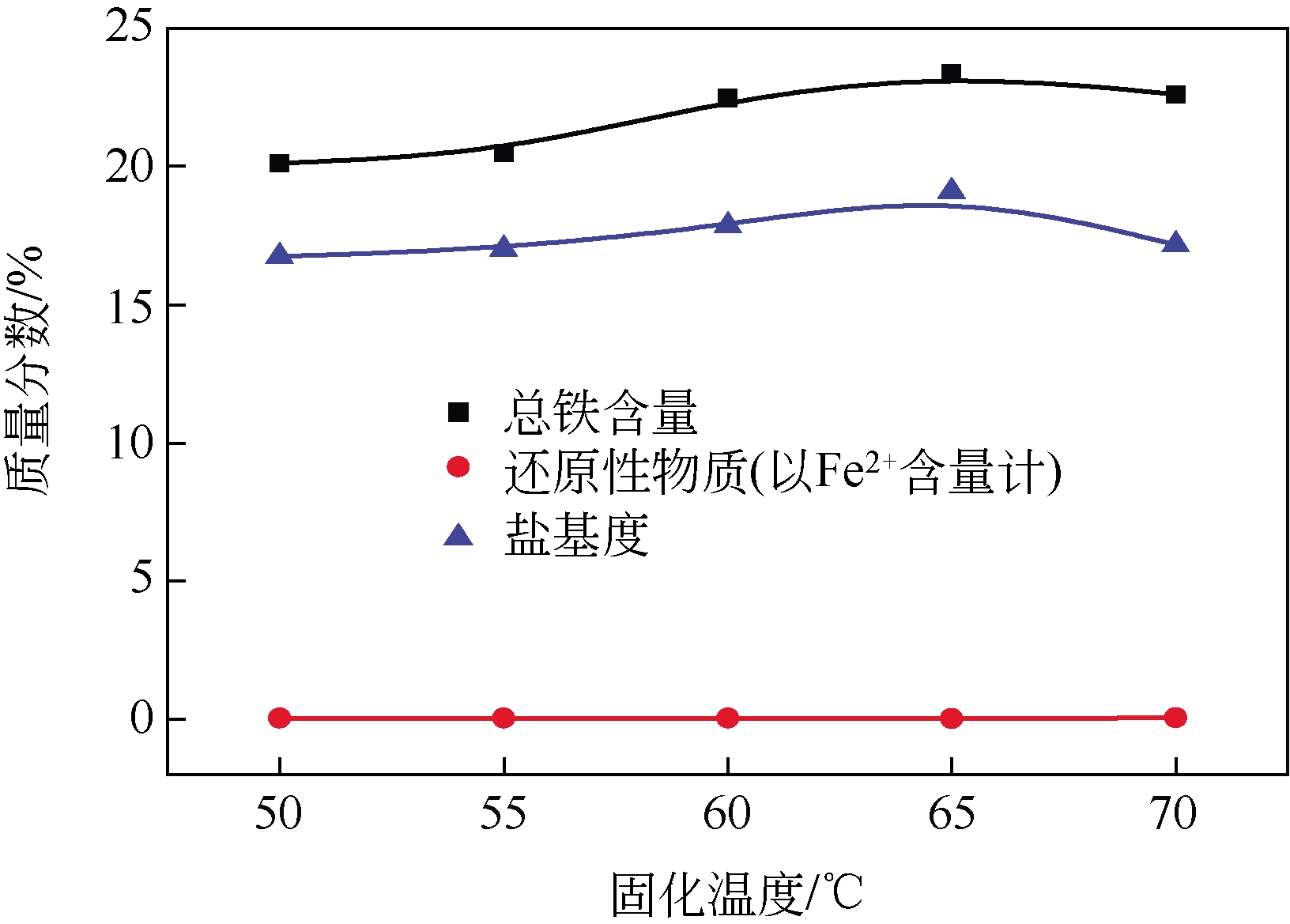
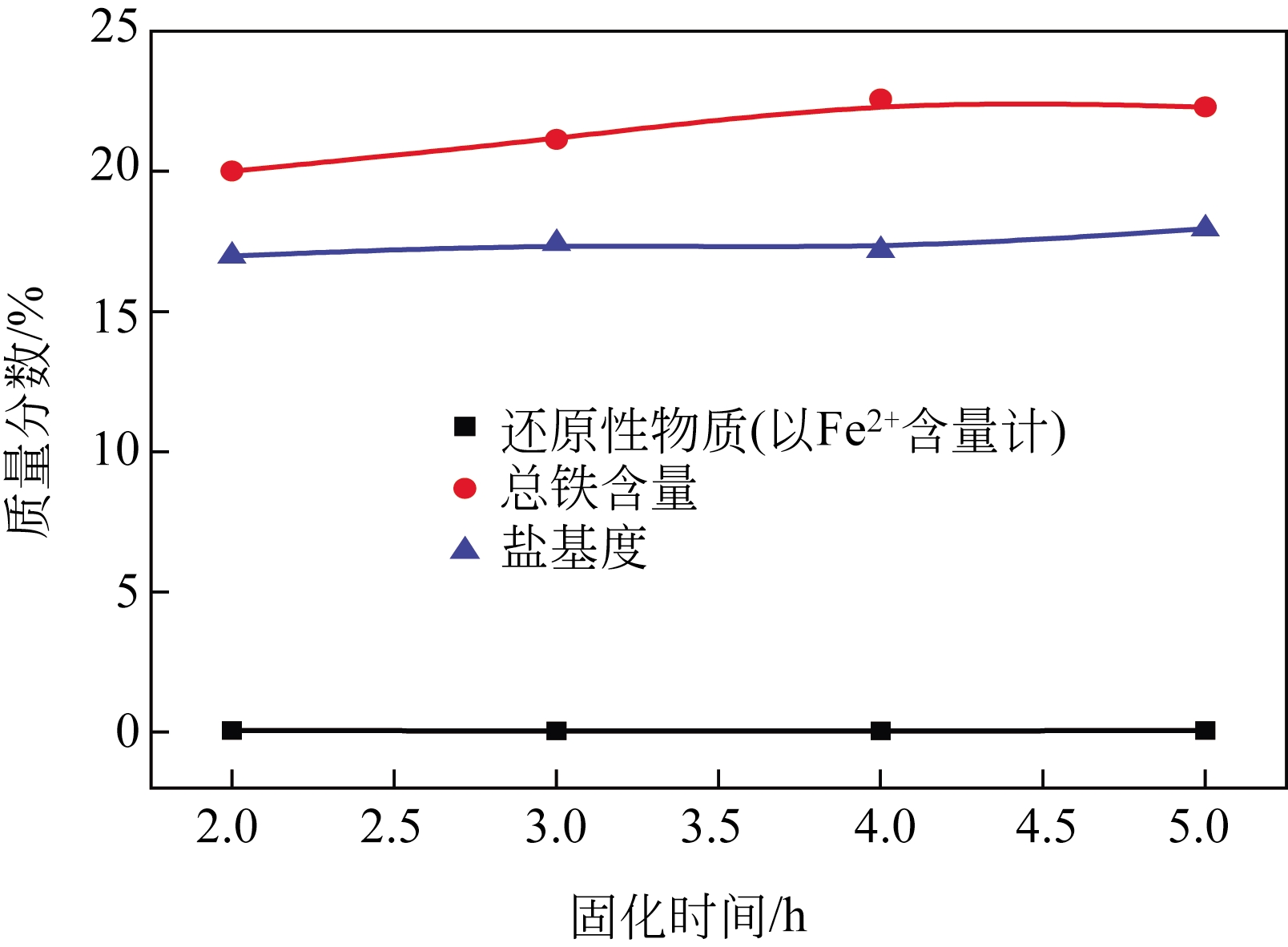
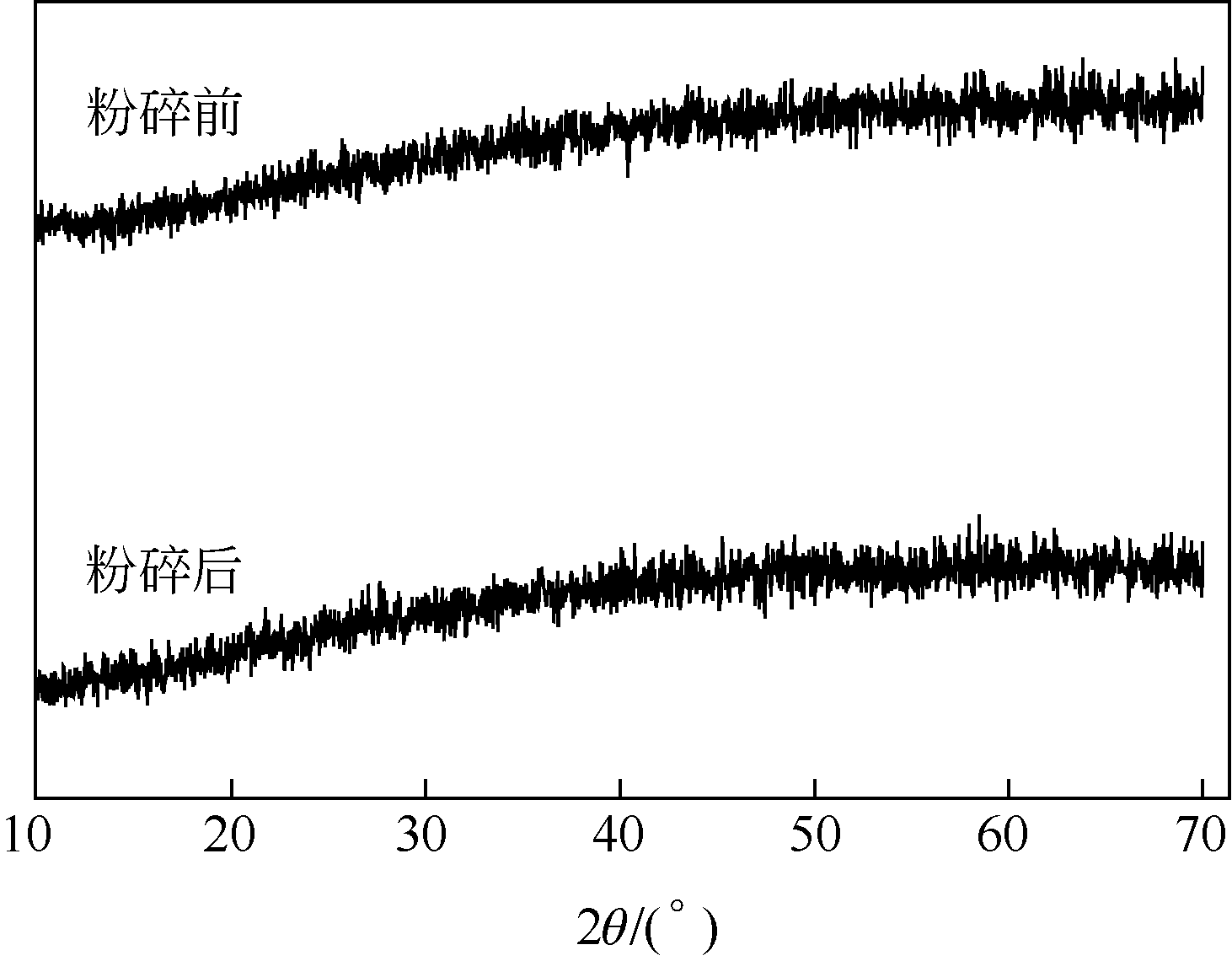
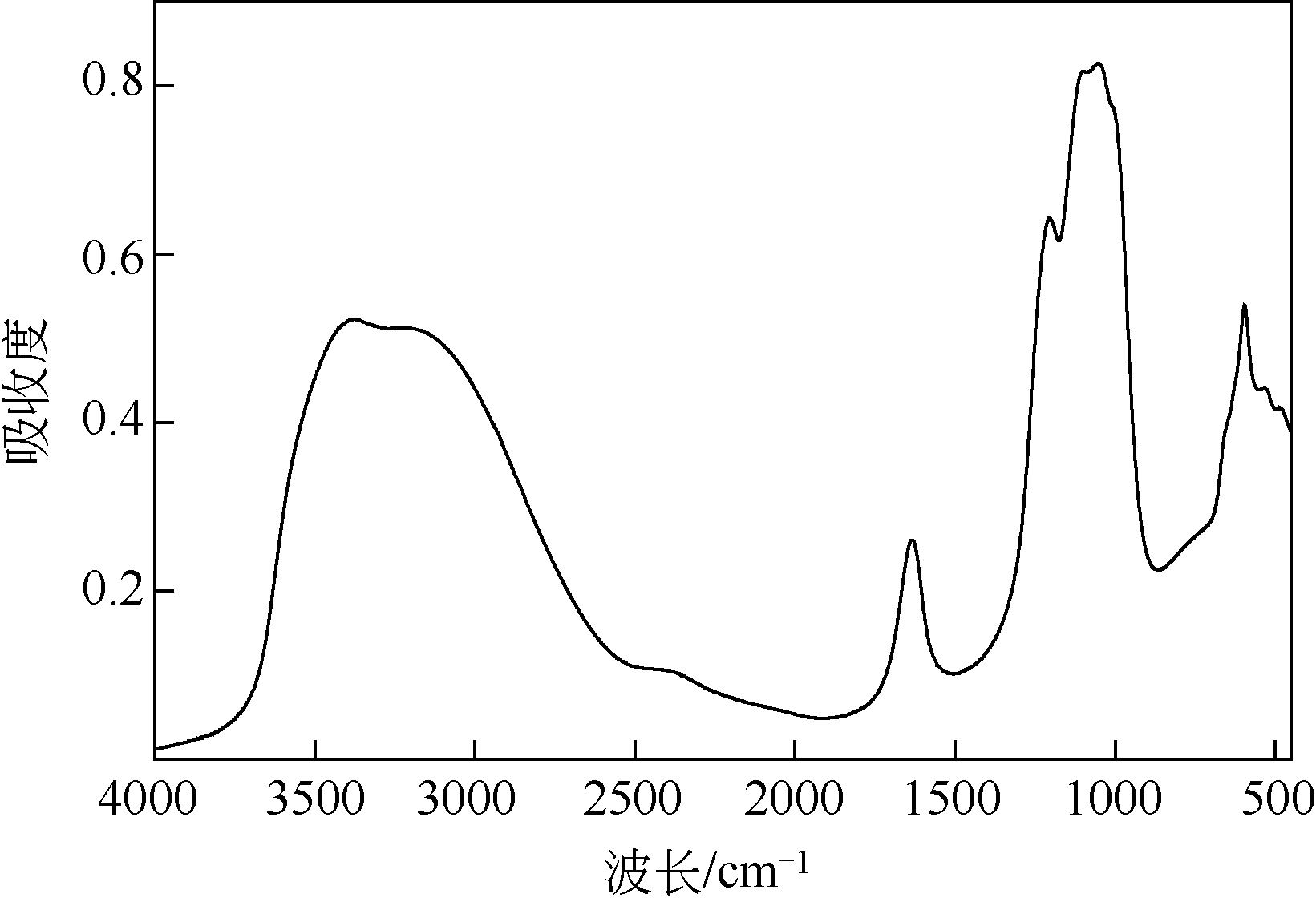
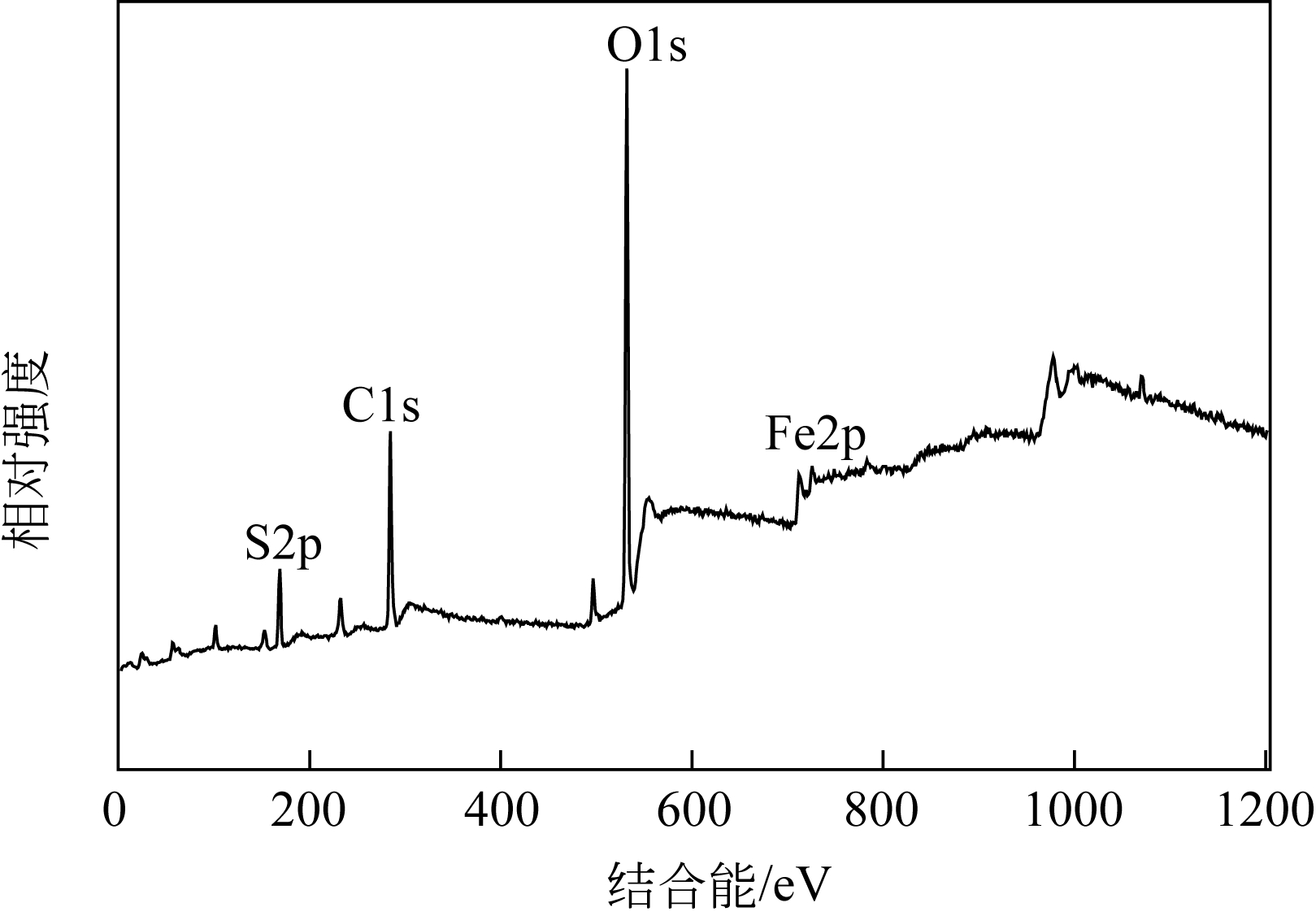
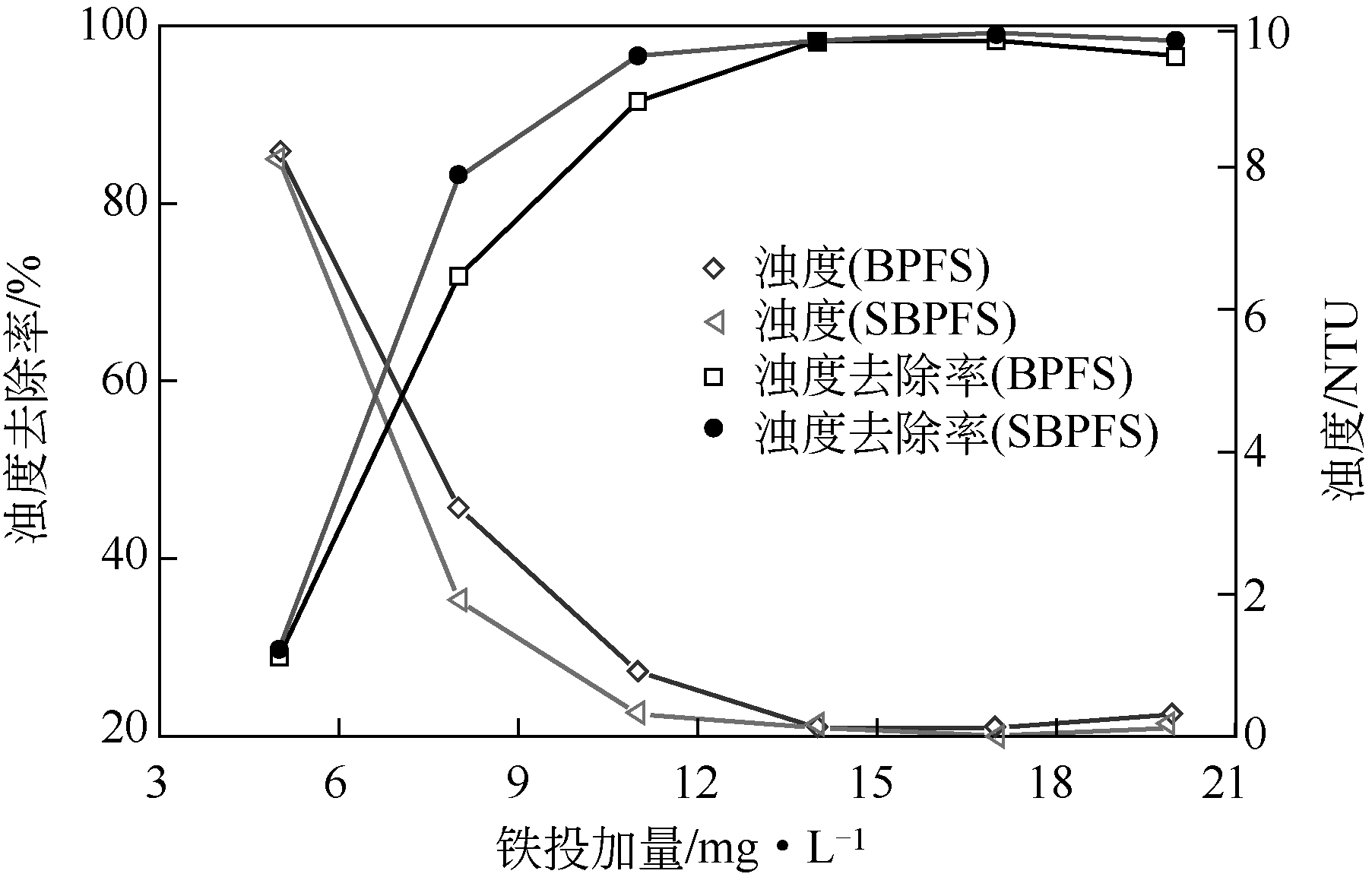
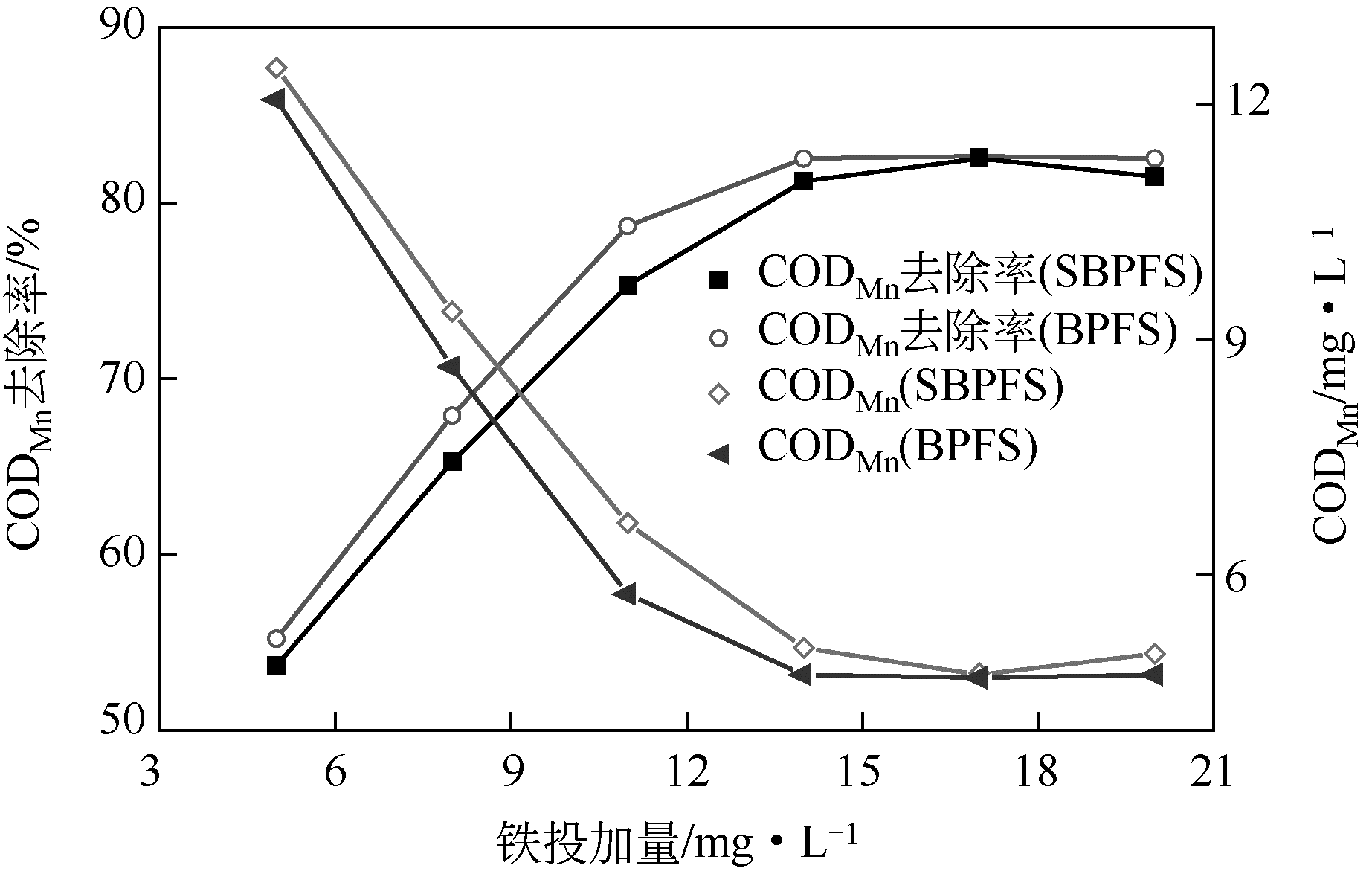
以工业FeSO4·7H2O为原料,制备生物聚合硫酸铁(bio-polymeric ferric sulfate,BPFS),采用减压蒸发技术制备固体生物聚合硫酸铁(solid bio-polymeric ferric sulfate,SBPFS),以松花江水为处理对象研究其絮凝性。研究内容包括固化温度、固化时间和添加剂的确定,通过扫描电子显微镜(SEM)、X射线衍射(XRD)、红外光谱分析(FTIR)和X射线光电子能谱分析(XPS)对其结构进行表征。研究结果表明:当固化温度55℃、固化时间4h、添加剂为KAl(SO4)2时,絮凝剂的絮凝性强。SEM表明产物质地膨松、比表面积大;XRD表明产物属于非晶态物质,固体粉末颗粒没有特定的形状和结构;FTIR表明了产物的结构;XPS表明产品的主要成分。将制备的BPFS和SBPFS用于处理松花江水时,SBPFS对松花江水浊度和CODMn的去除率略优于BPFS,除浊率和CODMn去除率分别可达96%和80%以上,絮凝效果佳。
中图分类号:
引用本文
徐小惠, 鲁敏, 杨柳, 王悦, 刘雅琪, 关晓辉. 固体生物聚合硫酸铁的制备及其絮凝性能[J]. 化工进展, 2019, 38(05): 2434-2440.
Xiaohui XU, Min LU, Liu YANG, Yue WANG, Yaqi LIU, Xiaohui GUAN. Preparation of solid biopolymerized ferric sulfate and its flocculation performance[J]. Chemical Industry and Engineering Progress, 2019, 38(05): 2434-2440.
| 固化温度/℃ | 产品外观 | 溶解性 |
|---|---|---|
| 50 | 软,深黄 | 不易 |
| 55 | 脆,黄色 | 易溶 |
| 60 | 脆,黄色 | 易溶 |
| 65 | 脆,深黄 | 易溶 |
| 70 | 硬,发黑 | 不易 |
表1 固化温度对SBPFS质量的影响
| 固化温度/℃ | 产品外观 | 溶解性 |
|---|---|---|
| 50 | 软,深黄 | 不易 |
| 55 | 脆,黄色 | 易溶 |
| 60 | 脆,黄色 | 易溶 |
| 65 | 脆,深黄 | 易溶 |
| 70 | 硬,发黑 | 不易 |
| 固化时间/h | 产品外观 | 溶解性 |
|---|---|---|
| 2 | 软黏,深黄 | 不易 |
| 3 | 软黏,深黄 | 不易 |
| 4 | 脆,浅黄 | 易溶 |
| 5 | 脆,黄色 | 易溶 |
表2 固化时间对SBPFS质量的影响
| 固化时间/h | 产品外观 | 溶解性 |
|---|---|---|
| 2 | 软黏,深黄 | 不易 |
| 3 | 软黏,深黄 | 不易 |
| 4 | 脆,浅黄 | 易溶 |
| 5 | 脆,黄色 | 易溶 |
| 添加剂 | 投加比例(mTFe:m膨化剂) | 固化时间/h | 还原性物质质量分数 (以Fe2+含量计)/% | 全铁质量分数/% | 盐基度/% | 产品外观 | 溶解性 |
|---|---|---|---|---|---|---|---|
| — | — | 5 | 0.062 | 22.46 | 17.86 | 脆,黄色 | 易溶 |
| KAl(SO4)2 | 10∶1 | 3 | 0.016 | 20.33 | 19.23 | 脆,浅黄 | 易溶 |
| SPFS | 10∶1 | 4 | 0.034 | 21.70 | 16.78 | 脆,深黄 | 易溶 |
| 混合 | 10∶1 | 4 | 0.028 | 21.18 | 16.28 | 脆,深黄 | 易溶 |
表3 添加剂对SBPFS质量的影响
| 添加剂 | 投加比例(mTFe:m膨化剂) | 固化时间/h | 还原性物质质量分数 (以Fe2+含量计)/% | 全铁质量分数/% | 盐基度/% | 产品外观 | 溶解性 |
|---|---|---|---|---|---|---|---|
| — | — | 5 | 0.062 | 22.46 | 17.86 | 脆,黄色 | 易溶 |
| KAl(SO4)2 | 10∶1 | 3 | 0.016 | 20.33 | 19.23 | 脆,浅黄 | 易溶 |
| SPFS | 10∶1 | 4 | 0.034 | 21.70 | 16.78 | 脆,深黄 | 易溶 |
| 混合 | 10∶1 | 4 | 0.028 | 21.18 | 16.28 | 脆,深黄 | 易溶 |
| 水平 | A/℃ | B/h | C |
|---|---|---|---|
| 1 | 55 | 4 | 不添加 |
| 2 | 60 | 5 | KAl(SO4)2 |
| 3 | 65 | 6 | SPFS粉末 |
表4 正交因素水平表
| 水平 | A/℃ | B/h | C |
|---|---|---|---|
| 1 | 55 | 4 | 不添加 |
| 2 | 60 | 5 | KAl(SO4)2 |
| 3 | 65 | 6 | SPFS粉末 |
| 实验号 | A | 空列 | B | C | 盐基度/% | 总铁/% | |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 1 | 17.02 | 21.42 | |
| 2 | 1 | 2 | 2 | 2 | 18.58 | 20.94 | |
| 3 | 1 | 3 | 3 | 3 | 16.72 | 21.15 | |
| 4 | 2 | 1 | 2 | 3 | 16.21 | 21.32 | |
| 5 | 2 | 2 | 3 | 1 | 16.54 | 21.34 | |
| 6 | 2 | 3 | 1 | 2 | 19.23 | 21.56 | |
| 7 | 3 | 1 | 3 | 2 | 18.30 | 20.48 | |
| 8 | 3 | 2 | 1 | 3 | 16.13 | 21.15 | |
| 9 | 3 | 3 | 2 | 1 | 16.47 | 21.15 | |
| 均值k1 | 17.44 | 17.18 | 17.46 | 16.68 | |||
| 均值k2 | 17.33 | 17.08 | 17.09 | 18.70 | |||
| 均值k3 | 16.97 | 17.47 | 17.19 | 16.35 | |||
| 极差R | 0.47 | 0.39 | 0.37 | 2.35 | |||
| 因素主次 | C添加剂>A固化温度>B固化时间 | ||||||
| 最佳水平 | C2A1B1 | ||||||
表5 正交实验结果
| 实验号 | A | 空列 | B | C | 盐基度/% | 总铁/% | |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 1 | 17.02 | 21.42 | |
| 2 | 1 | 2 | 2 | 2 | 18.58 | 20.94 | |
| 3 | 1 | 3 | 3 | 3 | 16.72 | 21.15 | |
| 4 | 2 | 1 | 2 | 3 | 16.21 | 21.32 | |
| 5 | 2 | 2 | 3 | 1 | 16.54 | 21.34 | |
| 6 | 2 | 3 | 1 | 2 | 19.23 | 21.56 | |
| 7 | 3 | 1 | 3 | 2 | 18.30 | 20.48 | |
| 8 | 3 | 2 | 1 | 3 | 16.13 | 21.15 | |
| 9 | 3 | 3 | 2 | 1 | 16.47 | 21.15 | |
| 均值k1 | 17.44 | 17.18 | 17.46 | 16.68 | |||
| 均值k2 | 17.33 | 17.08 | 17.09 | 18.70 | |||
| 均值k3 | 16.97 | 17.47 | 17.19 | 16.35 | |||
| 极差R | 0.47 | 0.39 | 0.37 | 2.35 | |||
| 因素主次 | C添加剂>A固化温度>B固化时间 | ||||||
| 最佳水平 | C2A1B1 | ||||||
| 1 | 刘维, 吴冰, 曹江林, 等. 钛对聚合硫酸铁制备与性能的影响[J]. 环境化学, 2012, 31(4): 437-442 |
| LIUWei, WUBing, CAOJianglin, et al. Effect of titanium on the preparation and coagulation efficiency of polyferric sulphate[J]. Environmental Chemistry, 2012, 31(4): 437-442. | |
| 2 | 万俐, 赵君凤, 付永胜, 等. 不同絮凝剂对活性污泥特性及除污效能的影响研究[J]. 环境工程, 2017, 35(2): 49-52. |
| WANLi, ZHAOJunfeng, FUYongsheng, et al. Effect of flocculants on sludge characteristics and pollutant removal efficiency[J]. Environmental Engineering, 2017, 35(2): 49-52. | |
| 3 | WANGH M, MINX B, CHAIL Y, et al. Biological preparation and application of poly-ferric sulfate flocculant[J]. Transactions of Nonferrous Metals Society of China, 2011, 21(11): 2542-2547. |
| 4 | 关晓辉, 王立刚, 鲁敏, 等. 基于生物催化氧化技术的生物聚合硫酸铁制备及性能研究综述[J]. 东北电力大学学报, 2013, 33(4): 15-18. |
| GUANXiaohui, WANGLigang, LUMin, et al. Progress in the preparation and properties of bio-polymeric ferric sulfate based on the bio-catalysis and oxidation technology[J]. Journal of Northeast Dianli University, 2013, 33(4): 15-18. | |
| 5 | 喻文超, 郭亚丹, 陈锦全, 等. 生物聚合硫酸铁絮凝剂去除废水中氟氯的实验研究[J]. 科学技术与工程, 2017,17(34): 166-173. |
| YUWenchao, GUOYadan, CHENJinquan, et al. Experimental investigation for removal of fluoride and chloride from waste water with bio-polymeric ferric sulphate[J]. Science Technology and Engineering, 2017,17(34): 166-173. | |
| 6 | 周云, 刘英, 张志强, 等. 微生物絮凝剂制备的研究新进展[J]. 环境污染与防治, 2014, 36(4): 80-85. |
| ZHOUYun,LIUYing, ZHANGZhiqiang,et al.Recent research progress on the preparation of microbial flocculcants[J].Environmental pollution & Control,2014,36(4):80-85. | |
| 7 | 皮姗姗, 李昂, 魏薇, 等. 微生物絮凝剂在水污染控制中的应用研究进展[J]. 中国给水排水, 2017, 33(16): 27-31. |
| PIShanshan, LIAng, WEIWei, et al. Research progress on application of microbial flocculants in water pollution control[J]. China Water & Wastewater , 2017, 33(16): 27-31. | |
| 8 | 叶有权, 贺晶晶, 吕向红. 固体钙铁复合絮凝剂的制备及应用研究[J]. 水处理技术, 2012, 38(4): 58-61. |
| YEYouquan, HEJingjing, XianghongLÜ. Experimental research on the preparation and application of Ca-Fe composite flocculant[J]. Technology of Water Treatment, 2012, 38(4): 58-61. | |
| 9 | 郑怀礼, 房慧丽, 蒋绍阶, 等. 负载硅藻土的固体聚合硫酸铁的制备及结构表征[J]. 光谱学与光谱分析, 2011, 31(7): 1917-1921. |
| ZHENGHuaili, FANGHuili, JIANGShaojie, et al. Preparation and structural analysis of diatomite-supported SPF flocculant[J]. Spectroscopy and Spectral Analysis, 2011, 31(7): 1917-1921. | |
| 10 | 郑雅杰, 彭超. 膨化法制备固体聚合硫酸铁技术[J]. 环境科学学报, 2009, 29(9): 1939-1943. |
| ZHENGYajie, PENGChao. A new technology for preparation of solid polyferric sulphate by gas expansion[J]. Acta Scientiae Circumstantiae, 2009, 29(9): 1939-1943. | |
| 11 | 关晓辉, 王维雪, 刘雅琪, 等. 固体生物聚合硫酸铁的制备及其结构和性能研究[J]. 东北电力大学学报, 2015, 35(4): 26-32. |
| GUANXiaohui, WANGWeixue, LIUYaqi, et al. The preparation structure and performance study of solid bio-polymeric ferric sulphate[J]. Journal of Northeast Dianli University, 2015, 35(4): 26-32. | |
| 12 | CHENGW.Hydrolysis characteris of polyferric sulfate coagulant and its optimal condition of preparation[J]. Colloids and Surfaces, A:Physicochemical and Engineering Aspects, 2001, 182(1/2/3): 57-63. |
| 13 | 关晓辉, 王磊, 马啸飞, 等. 改性生物聚合硫酸铁的制备及其性能研究[J]. 中国电机工程学报, 2010, 30(32): 58-62. |
| GUANXiaohui, WANGLei, XiaofeiMA, et al. Preparation and performance research of the modified bio-polymeric ferric sulphate[J]. Proceedings of the CSEE, 2010, 30(32): 58-62. | |
| 14 | HONGH, CHANGJ H, HSU H S, et al. Metal removal by Acidithiobacillus ferrooxidans through cells and extra-cellular culture supernatant in biomachining[J]. CIRP Journal of Manufacturing Science and Technology, 2012, 5: 137-141. |
| 15 | CHANDRAPRABHAM N, NATARAJANK A. Role of outer membrane exopolymers of Acidithiobacillus ferrooxidans in adsorption of cells onto pyrite and chalcopyrite[J]. International Journal of Mineral Processing, 2013, 123: 152-157. |
| 16 | LIAOZ, ZHUB, XUEX, et al. Study on fermentation kinetics of yeast cells immobilized of Chinese ceramics[J]. Journal of Shaoguan University(Social Science Edition), 2001, 22(9): 131-138. |
| 17 | 彭超. 膨化固化法制备固体聚合硫酸铁新工艺[D]. 长沙:中南大学, 2009. |
| PENGChao. A new technology for preparation of solid polyferric sulphate by gas expansion[D]. Changsha:Central South University, 2009. | |
| 18 | 张良佺, 陈纪忠, 祝巨, 等. 固体聚合硫酸铁的制取及工艺优化研究[J]. 高校化学工程学报, 2004, 18(4): 494-500. |
| ZHANGLiangquan, CHENJizhong, ZHUJu, et al. Study on manufacture and technology optimization of solid polymerized ferric sulphate[J]. Journal of Chemical Engineering of Chinese Universities, 2004, 18(4): 494-500. | |
| 19 | 晏井春, 朱富坤, 闫永胜, 等. 纳米聚合硫酸铁的制备、表征及应用研究[J]. 无机盐工业, 2007, 39(11): 27-29. |
| YANJingchun, ZHUFukun, YANYongsheng, et al. Study on preparation, characterization and application of nano-sized poly ferric sulphate coagulant[J]. Norganic Chemicals Industry, 2007, 39(11): 27-29. | |
| 20 | ZHUAG, ZHENGH, ZHANGZ, et al. Characterization and coagulation-flocculation behaviour of polymeric aluminium ferric sulphate (PAFS)[J]. Chemical Engineering Journal, 2011(178): 50-59. |
| 21 | MOUSSASP A, ZOUBOULISA I. A study on the properties and coagulation behaviour of modified inorganic polymeric coagulant——polyferric silicate sulphate(PFSiS)[J]. Separation and Purification Technology, 2008(63):475-483. |
| 22 | 张鹏, 王雨露, 张文, 等. 聚合硫酸铝钛的制备与结构表征[J]. 化工进展, 2018, 37(7): 2740-2747 |
| ZHANGPeng, WANGYulu, ZHANGWen, et al. Synthesis and characterization of an inorganic composite polymer coagulant of poly aluminum titanium sulfate[J]. Chemical Industry and Engineering Progress, 2018, 37(7): 2740-2747. | |
| 23 | 唐海, 冯玲, 颜酉斌, 等. 粉煤灰制备聚硅酸铁铝的结构特征与混凝机理[J]. 高效化学工程学报, 2013, 27(6): 1045-1050. |
| 24 | TANGHai, FENGLing, YANYoubin, et al. Structural characteristics and coagulation mechanisms of PSFA prepared by fly ash[J]. Journal of Chemical Engineering of Chinese Universities, 2013, 27(6): 1045-1050. |
| 25 | 陈伟, 郑怀礼, 翟俊, 等. 聚合硫酸铁钛混凝剂的制备及红外、紫外-可见光谱分析[J]. 光谱学与光谱分析, 2016, 36(4): 1038-1042. |
| CHENWei, ZHENGHuaili, ZHAIJun, et al. Study on the preparation of a new composite coagulant: poly-ferric-titanium-sulfate and analysis of FTIR spectrum and UV-vis spectrum[J]. Spectroscopy and Spectral Analysis, 2016, 36(4): 1038-1042. | |
| 26 | 赵湘骥, 马荣骏, 杨镜全. 固体聚合硫酸铁的制备及其特性研究[J]. 矿冶工程, 1995, 15(2): 33-38. |
| ZHAOXiangji, RongjunMA, YANGJingquan. Preparion and characteristics of solid polyferric sulfate[J]. Ming and Metallurgical Engineering, 1995, 15(2): 33-38. | |
| 27 | SADRI MOGHADDAS, ALAVI MOGHADDAMM, ARAMIM. Response surface optimization of acid red 119 dye from simulated wastewater using Al based waterworks sludge and polyaluminium chloride as coagulant[J]. J. Environ. Manage, 2011, 92(4): 1284-1291. |
| 28 | 李文朴, 卢静芳, 柳美乐, 等. 高岭土对氢氧化镁混凝去除活性橙染料效果的影响[J]. 化工进展, 2017, 36(11): 4286-4292. |
| LIWenpu, LUJingfang, LIUMeile, et al. Effect of Kaolin on the removal of reactive orange by magnesium hydroxide coagulation process[J]. Chemical Industry and Engineering Progress, 2017, 36(11): 4286-4292. | |
| 29 | 高倩, 张大为, 徐慧, 等. 水体中Cr(VI)对不同混凝剂混凝过程的影响[J]. 环境科学, 2018, 39(8): 3704-3712. |
| GAOQian, ZHANGDawei, XUHui, et al. Effect of Cr(Ⅵ) on coagulation process of different coagulants[J]. Environmental Science, 2018, 39(8): 3704-3712. | |
| 30 | ZHENGH, ZHUG, JIANGS, et al. Investigations of coagulation-flocculation process by performance optimization, model prediction and fractal structure of flocs[J]. Desalination, 2011, 269(1/2/3):148-156. |
| 31 | HAMIDN A A, ISMAILA A F, MATSUURAT, et al. Morphological and separation performance study of polysulfone/titanium dioxide (PSF/TiO2) ultrafiltration membranes for humic acid removal[J]. Desalination, 2011, 273(1): 85-92. |
| 32 | 郑怀礼, 余炳宏, 阳春, 等.聚磷氯化铝的制备新方法及其性能研究[J].土木建筑与环境工程, 2011, 33(5): 125-131 |
| ZHENGHuaili, YUBinghong, YANGChun, et al. On novel synthetic method and performance of polymeric phosphate-aluminum chloride(PPAC)[J]. Journal of Civil , Architectural & Environmental Engineering, 2011, 33(5): 125-131. | |
| 33 | 郑怀礼, 冯力, 蒋贞贞, 等. 聚合硫酸铁铝处理印染废水[J]. 土木建 筑与环境工程, 2013, 35(5): 1-6 |
| ZHENGHuaili, FENGLi, JIANGZhenzhen, et al. Analysis on polymeric aluminum ferric sulfate(PFAS) in printing and dyeing wastewater treatment[J]. Journal of Civil , Architectural & Environmental Engineering, 2013, 35(5): 1-6. | |
| 34 | 郑怀礼, 陈文源, 张智, 等. 无机高分子复合混凝剂聚合硫酸铝铁的制备与应用[J]. 重庆大学学报, 2013, 36(7): 114-120. |
| ZHENGHuaili, CHENWenyuan, ZHANGZhi, et al. Preparation and applications of inorganic polymer composite flocculant poly aluminum ferric sulfate[J]. Journal of Chongqing University, 2013, 36(7): 114-120. | |
| 35 | ZHUG, ZHENGH , CHENW, et al. Preparation of a composite coagulant: polymeric aluminum ferric sulfate (PAFS) for wastewater treatment[J]. Desanlination, 2012, 285: 315-323. |
| [1] | 高彦静. 单原子催化技术国际研究态势分析[J]. 化工进展, 2023, 42(9): 4667-4676. |
| [2] | 吴海波, 王希仑, 方岩雄, 纪红兵. 3D打印催化材料开发与应用进展[J]. 化工进展, 2023, 42(8): 3956-3964. |
| [3] | 李润蕾, 王子彦, 王志苗, 李芳, 薛伟, 赵新强, 王延吉. CuO-CeO2/TiO 2 高效催化CO低温氧化反应性能[J]. 化工进展, 2023, 42(8): 4264-4274. |
| [4] | 储甜甜, 刘润竹, 杜高华, 马嘉浩, 张孝阿, 王成忠, 张军营. 有机胍催化脱氢型RTV硅橡胶的制备和可降解性能[J]. 化工进展, 2023, 42(7): 3664-3673. |
| [5] | 俞俊楠, 俞建峰, 程洋, 齐一搏, 化春键, 蒋毅. 基于深度学习的变宽度浓度梯度芯片性能预测[J]. 化工进展, 2023, 42(7): 3383-3393. |
| [6] | 余希希, 张金帅, 雷文, 刘承果. 基于动态共价键自修复的光固化高分子材料研究进展[J]. 化工进展, 2023, 42(7): 3589-3599. |
| [7] | 杨家添, 唐金铭, 梁恣荣, 黎胤宏, 胡华宇, 陈渊. 新型淀粉基高吸水树脂抑尘剂的制备及其应用[J]. 化工进展, 2023, 42(6): 3187-3196. |
| [8] | 陈怡欣, 甄摇摇, 陈瑞浩, 吴继伟, 潘丽美, 姚翀, 罗杰, 卢春山, 丰枫, 王清涛, 张群峰, 李小年. 铂基纳米催化剂的制备及在加氢领域的进展[J]. 化工进展, 2023, 42(6): 2904-2915. |
| [9] | 陈明星, 王新亚, 张威, 肖长发. 纤维基耐高温空气过滤材料研究进展[J]. 化工进展, 2023, 42(5): 2439-2453. |
| [10] | 于捷, 张文龙. 锂离子电池隔膜的发展现状与进展[J]. 化工进展, 2023, 42(4): 1760-1768. |
| [11] | 高江雨, 张耀君, 贺攀阳, 刘礼才, 张枫烨. 磷酸基地质聚合物的制备及其性能研究进展[J]. 化工进展, 2023, 42(3): 1411-1425. |
| [12] | 张育新, 王灿, 舒文祥. 二氧化碳的还原及其利用研究进展[J]. 化工进展, 2023, 42(2): 944-956. |
| [13] | 王晓亮, 于振秋, 常雷明, 赵浩男, 宋晓琦, 高靖淞, 张一波, 黄传辉, 刘忆, 杨绍斌. 电沉积法制备氢氧化物/氧化物超级电容器电极的研究进展[J]. 化工进展, 2023, 42(10): 5272-5285. |
| [14] | 杨凯璐, 陈明星, 王新亚, 张威, 肖长发. 染料废水处理用纳滤膜制备及改性研究进展[J]. 化工进展, 2023, 42(10): 5470-5486. |
| [15] | 李钊铭, 沈伯雄, 封硕, 边瑶. 锰基催化剂结构形貌对催化剂抗硫抗水性能影响[J]. 化工进展, 2023, 42(1): 226-235. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||