化工进展 ›› 2019, Vol. 38 ›› Issue (05): 2164-2178.DOI: 10.16085/j.issn.1000-6613.2018-1844
糠醛的水解制备和应用研究进展
- 南开大学环境科学与工程学院,天津市生物质类固废资源化技术工程中心,天津 300350
-
收稿日期:2018-09-12修回日期:2018-11-30出版日期:2019-05-05发布日期:2019-05-05 -
通讯作者:鞠美庭 -
作者简介:<named-content content-type="corresp-name">聂一凡</named-content>(1996—),女,硕士研究生,研究方向为生物质固废资源化。E-mail:<email>2120170635@mail.nankai.edu.cn</email>。 -
基金资助:国家自然科学基金面上项目(21878163);天津市自然科学基金(17JCZDJC39500);国家重点研发计划(2018YFD080083-03);国家自然科学基金青年科学基金(51708301);天津市自然科学基金重点基金(17JCZDJC39500)
Advances in production furfural via hydrolysis and application of furfural
Yifan NIE( ),Qidong HOU,Weizun LI,Chuanyunlong BAI,Meiting JU(
),Qidong HOU,Weizun LI,Chuanyunlong BAI,Meiting JU( )
)
- College of Environmental Science & Engineering, Nankai University, Tianjin Engineering Research Center of Biomass Solid Waste Resources Technology, Tianjin 300350, China
-
Received:2018-09-12Revised:2018-11-30Online:2019-05-05Published:2019-05-05 -
Contact:Meiting JU
摘要:
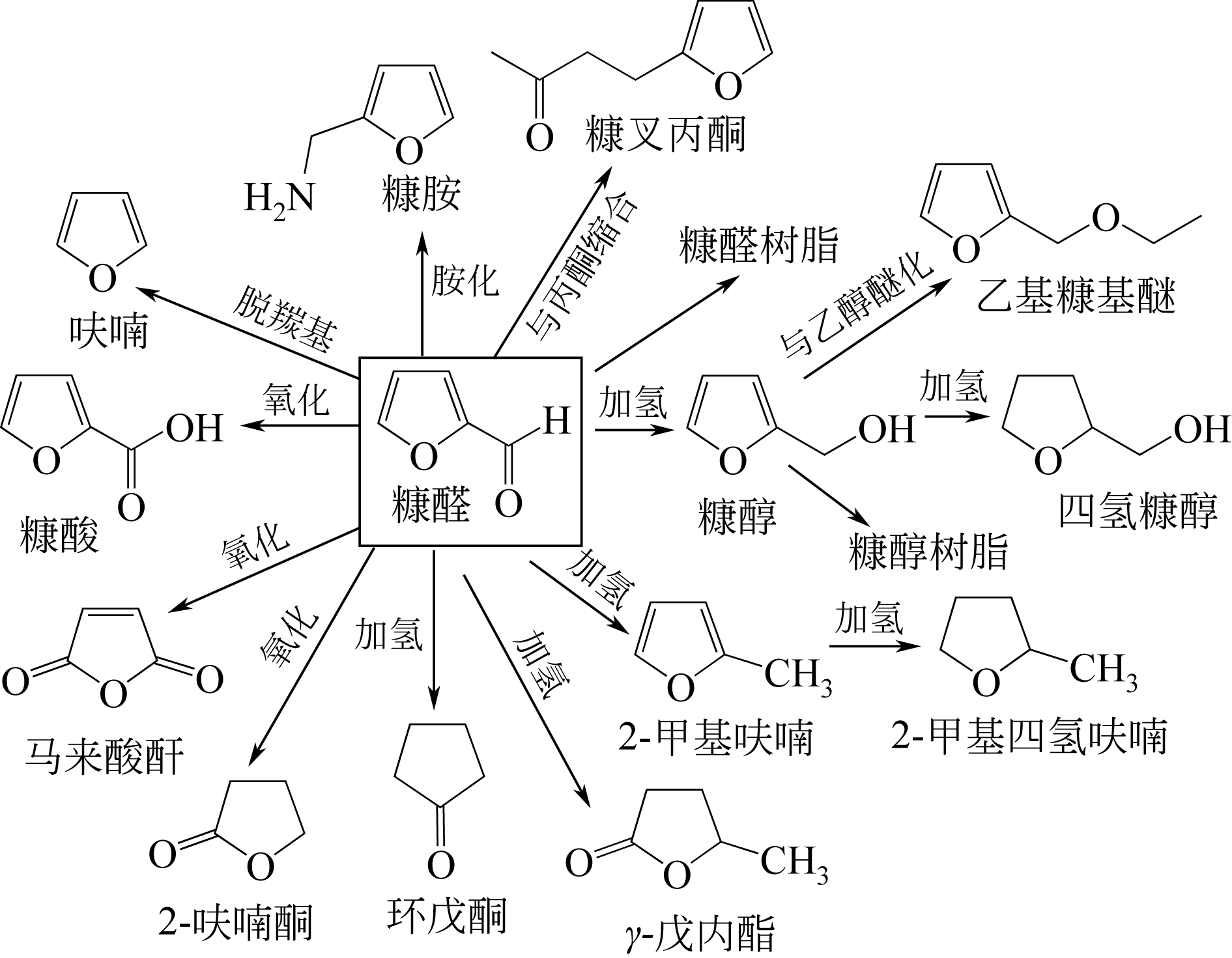
糠醛是由可再生的生物质为原料转化得到的高价值化工产品,具有广阔的应用前景。本文综述了近年来生物质催化水解制备糠醛的研究进展,同时总结了糠醛衍生产品的制备和应用。在对半纤维素水解产糖反应和木糖脱水反应进行机理分析的基础上,从反应原料、溶剂体系、催化剂和分离方法等方面归纳总结了生物质催化水解制备糠醛的最新研究进展,并提出当前生物质制备糠醛方法中存在的问题和应对方案。在此基础上,分析了糠醛经氢化、胺化、氧化、缩醛化、聚合等反应获得高价值衍生产品的研究进展。提出要实现糠醛绿色高效的生产和应用,应着力设计低成本、低能耗、低污染且高效率的催化反应体系,同时推进重要糠醛衍生产品的综合高效利用。
中图分类号:
引用本文
聂一凡, 候其东, 李维尊, 白川云龙, 鞠美庭. 糠醛的水解制备和应用研究进展[J]. 化工进展, 2019, 38(05): 2164-2178.
Yifan NIE, Qidong HOU, Weizun LI, Chuanyunlong BAI, Meiting JU. Advances in production furfural via hydrolysis and application of furfural[J]. Chemical Industry and Engineering Progress, 2019, 38(05): 2164-2178.
| 催化剂 | 溶剂(/萃取剂) | 温度/℃ | 时间/min | 底物负载/% | 转化率/% | 产率/% | 选择率/% | 参考文献 |
|---|---|---|---|---|---|---|---|---|
| 酸性离子液体 | ||||||||
| [Bmim]Cl-AlCl3 | GVL-水 | 120 | 120 | — | 99.70 | 79.80 | 80.0 | [ |
| [Sbmim]HSO4 | 水/MIBK | 150 | 25 | 12.7 | 95.30 | 91.5 | 96.0 | [ |
| [SbPy]BF4 | 水/THF | 150 | 60 | 3.6 | 90.00 | 75.0 | 83.3 | [ |
| [SbPy]MeSO3 | 水/THF | 150 | 60 | 3.6 | 45.00 | 36.0 | 80.0 | [ |
| [Sbe3N]BF4 | 水/THF | 150 | 60 | 3.6 | 50.00 | 40.0 | 80.0 | [ |
| [Sbe3N]MeSO3 | 水/THF | 150 | 60 | 3.6 | 32.00 | 28.0 | 87.5 | [ |
| [Ch-SO4H][CF3SO3] | 1,4-二氧杂环乙烷 | 120 | 600 | 4.0 | 99.20 | 95.1 | 95.9 | [ |
| [Bmim][HSO4] | [Bmim][HSO4] | 140 | 300 | 10.0 | >95.00 | 36.7 | 38.6 | [ |
| [Bmim][HSO4]/甲苯 | 140 | 240 | 2.3 | — | 73.8 | — | [ | |
| [Bmim][HSO4]/MIBK | 140 | 360 | 2.3 | — | 80.3 | — | [ | |
| [Bmim][HSO4]/二噁烷 | 140 | 240 | 2.3 | — | 82.2 | — | [ | |
| 金属氯盐 | ||||||||
| AlCl3 | [Bmim]Cl | 160 | 1.5 | 1.9 | 97.27 | 82.2 | 84.5 | [ |
| SnCl4 | 水 | 140 | 120 | 11.3 | 55.00 | 32.0 | 58.0 | [ |
| SnCl4 | 水/正丁醇 | 140 | 300 | 4.3 | 90.00 | 77.0 | 85.0 | [ |
| SnCl4+LiCl | 水/正丁醇 | 140 | 300 | 4.3 | 95.00 | 84.0 | 88.0 | [ |
| SnCl4 | 水-DMSO | 140 | 240 | 20.0 | 93.70 | 48.9 | 52.2 | [ |
| SnCl4+LiCl | 水-DMSO | 130 | 360 | 10.0 | — | 63.0 | — | [ |
| CrCl2+LiBr | DMA | 100 | 240 | 10.0 | — | 56.0 | — | [ |
| 固体酸催化剂 | ||||||||
| PEG-OSO3H | [Bmim]PF6 | 120 | 18 | 3.8 | 82.00 | 65.0 | 79.3 | [ |
| PEG-OSO3H+MnCl2 | [Bmim]PF6 | 120 | 18 | 3.8 | 99.00 | 75.0 | 75.8 | [ |
| H3PW12O40 | [Bmim]Cl | 160 | 7.5 | 19.0 | — | 84.2 | — | [ |
| 铌酸+磷酸铌 | 水 | 160 | 30 | 2.0 | 74.70 | 32.9 | 44.0 | [ |
| Nb2O5 | 水 | 120 | 180 | 1.5 | 93.00 | 44.6 | 48.0 | [ |
| Nb2O5 | 水/ 甲苯 | 120 | 180 | 1.6 | >99.00 | 71.3 | 72.0 | [ |
| 介孔磷酸锡-MIL-101(Cr) | 水/ 甲苯 | 150 | 180 | 3.0 | 94.00 | 86.7 | 92.3 | [ |
| SO3H-MCM-41 | 水/甲苯 | 155 | 120 | 2.1 | 95.40 | 68.6 | 71.9 | [ |
| SO42-/Sn-MMT | NaCl水溶液/2-MTHF | 160 | 120 | 1.1 | — | 79.6 | — | [ |
| Nafion NR50 | NaCl水溶液/CPME | 170 | 40 | 4.3 | 100.00 | 80.0 | 80.0 | [ |
| CHA-20沸石 | 水/甲苯 | 170 | 360 | 0.2 | 100.00 | 61.0 | 61.0 | [ |
| 磺化纳米金刚石粉 | NaCl水溶液/CPME | 200 | 50 | 4.4 | 100.00 | 76.0 | 76.0 | [ |
| 布朗斯特酸 | ||||||||
| H2SO4 | GVL-水 | 170 | 15 | 2.0 | 2.30 | 87.0 | — | [ |
| 甜菜碱+HCOOH | 水/CPME | 170 | 60 | 4.4 | 100 | 80.0 | 80.0 | [ |
| H2SO4 | [Bmim]Cl/甲苯 | 100 | 240 | — | 83.00 | 44.0 | 53.0 | [ |
表1 木糖制备糠醛的反应体系
| 催化剂 | 溶剂(/萃取剂) | 温度/℃ | 时间/min | 底物负载/% | 转化率/% | 产率/% | 选择率/% | 参考文献 |
|---|---|---|---|---|---|---|---|---|
| 酸性离子液体 | ||||||||
| [Bmim]Cl-AlCl3 | GVL-水 | 120 | 120 | — | 99.70 | 79.80 | 80.0 | [ |
| [Sbmim]HSO4 | 水/MIBK | 150 | 25 | 12.7 | 95.30 | 91.5 | 96.0 | [ |
| [SbPy]BF4 | 水/THF | 150 | 60 | 3.6 | 90.00 | 75.0 | 83.3 | [ |
| [SbPy]MeSO3 | 水/THF | 150 | 60 | 3.6 | 45.00 | 36.0 | 80.0 | [ |
| [Sbe3N]BF4 | 水/THF | 150 | 60 | 3.6 | 50.00 | 40.0 | 80.0 | [ |
| [Sbe3N]MeSO3 | 水/THF | 150 | 60 | 3.6 | 32.00 | 28.0 | 87.5 | [ |
| [Ch-SO4H][CF3SO3] | 1,4-二氧杂环乙烷 | 120 | 600 | 4.0 | 99.20 | 95.1 | 95.9 | [ |
| [Bmim][HSO4] | [Bmim][HSO4] | 140 | 300 | 10.0 | >95.00 | 36.7 | 38.6 | [ |
| [Bmim][HSO4]/甲苯 | 140 | 240 | 2.3 | — | 73.8 | — | [ | |
| [Bmim][HSO4]/MIBK | 140 | 360 | 2.3 | — | 80.3 | — | [ | |
| [Bmim][HSO4]/二噁烷 | 140 | 240 | 2.3 | — | 82.2 | — | [ | |
| 金属氯盐 | ||||||||
| AlCl3 | [Bmim]Cl | 160 | 1.5 | 1.9 | 97.27 | 82.2 | 84.5 | [ |
| SnCl4 | 水 | 140 | 120 | 11.3 | 55.00 | 32.0 | 58.0 | [ |
| SnCl4 | 水/正丁醇 | 140 | 300 | 4.3 | 90.00 | 77.0 | 85.0 | [ |
| SnCl4+LiCl | 水/正丁醇 | 140 | 300 | 4.3 | 95.00 | 84.0 | 88.0 | [ |
| SnCl4 | 水-DMSO | 140 | 240 | 20.0 | 93.70 | 48.9 | 52.2 | [ |
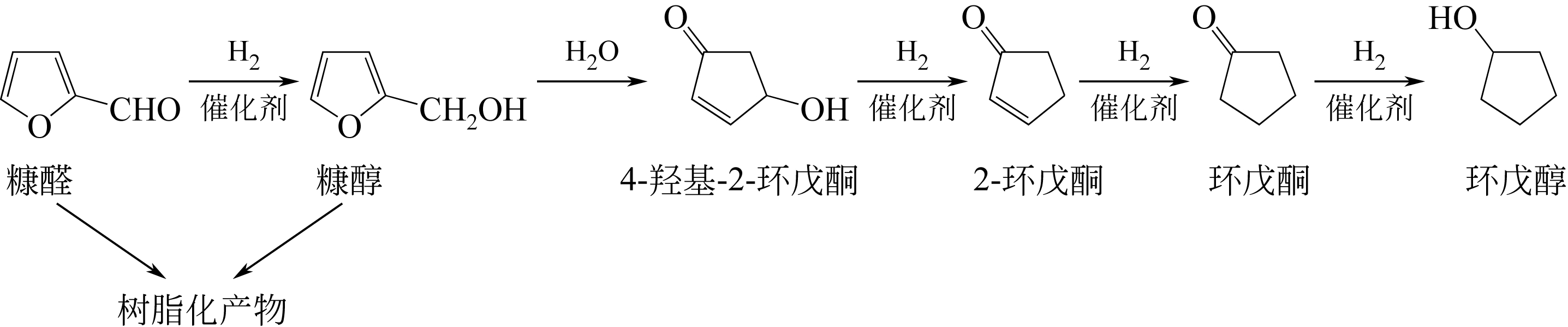
| SnCl4+LiCl | 水-DMSO | 130 | 360 | 10.0 | — | 63.0 | — | [ |
| CrCl2+LiBr | DMA | 100 | 240 | 10.0 | — | 56.0 | — | [ |
| 固体酸催化剂 | ||||||||
| PEG-OSO3H | [Bmim]PF6 | 120 | 18 | 3.8 | 82.00 | 65.0 | 79.3 | [ |
| PEG-OSO3H+MnCl2 | [Bmim]PF6 | 120 | 18 | 3.8 | 99.00 | 75.0 | 75.8 | [ |
| H3PW12O40 | [Bmim]Cl | 160 | 7.5 | 19.0 | — | 84.2 | — | [ |
| 铌酸+磷酸铌 | 水 | 160 | 30 | 2.0 | 74.70 | 32.9 | 44.0 | [ |
| Nb2O5 | 水 | 120 | 180 | 1.5 | 93.00 | 44.6 | 48.0 | [ |
| Nb2O5 | 水/ 甲苯 | 120 | 180 | 1.6 | >99.00 | 71.3 | 72.0 | [ |
| 介孔磷酸锡-MIL-101(Cr) | 水/ 甲苯 | 150 | 180 | 3.0 | 94.00 | 86.7 | 92.3 | [ |
| SO3H-MCM-41 | 水/甲苯 | 155 | 120 | 2.1 | 95.40 | 68.6 | 71.9 | [ |
| SO42-/Sn-MMT | NaCl水溶液/2-MTHF | 160 | 120 | 1.1 | — | 79.6 | — | [ |
| Nafion NR50 | NaCl水溶液/CPME | 170 | 40 | 4.3 | 100.00 | 80.0 | 80.0 | [ |
| CHA-20沸石 | 水/甲苯 | 170 | 360 | 0.2 | 100.00 | 61.0 | 61.0 | [ |
| 磺化纳米金刚石粉 | NaCl水溶液/CPME | 200 | 50 | 4.4 | 100.00 | 76.0 | 76.0 | [ |
| 布朗斯特酸 | ||||||||
| H2SO4 | GVL-水 | 170 | 15 | 2.0 | 2.30 | 87.0 | — | [ |
| 甜菜碱+HCOOH | 水/CPME | 170 | 60 | 4.4 | 100 | 80.0 | 80.0 | [ |
| H2SO4 | [Bmim]Cl/甲苯 | 100 | 240 | — | 83.00 | 44.0 | 53.0 | [ |
| 底物 | 催化剂 | 溶剂/(萃取剂) | 温度/℃ | 时间 /min | 底物负载/% 产率/% | 参考 文献 | |
|---|---|---|---|---|---|---|---|
| 木聚糖 | H2SO4 | 水 | 180 | 20 | 1.0 | 29.0 | [ |
| 木聚糖 | HCl | 水 | 180 | 20 | 1.0 | 34.3 | [ |
| 木聚糖 | H3PW12O40 | [Bmim]Cl | 160 | 10 | 1.9 | 93.7 | [ |
| 木聚糖 | Amberlyst-15 | [Bmim]Cl | 140 | 10 | 1.9 | 87.8 | [ |
| 木聚糖 | [Emim]HSO4 | [Emim]HSO4/甲苯 | 100 | 240 | 3.0 | 29.0 | [ |
| 木聚糖 | AlCl3 | [Bmim]Cl | 170 | 0.1 | 1.9 | 84.8 | [ |
| 木聚糖 | SO4 2-/Sn-MMT | 水/2-MTHF | 160 | 90 | 0.5 | 77.4 | [ |
| 木聚糖 | 甜菜碱+HCOOH | 水/CPME | 170 | 60 | 4.4 | 76.0 | [ |
| 木聚糖 | Nafion NR50 | NaCl水溶液/CPME | 190 | 60 | 3.8 | 55.0 | [ |
| 木聚糖 | HCl+CrCl2 | [Emim]Cl | 140 | 120 | 5.0 | 25.0 | [ |
| 木聚糖 | [Ch-SO4H][CF3SO3] | 1,4-二氧杂环乙烷 | 120 | 360 | 4.0 | 82.0 | [ |
| 未处理玉米芯 | AlCl3 | [Bmim]Cl | 160 | 3 | 2.5 | 19.1 | [ |
| 草 | AlCl3 | [Bmim]Cl | 160 | 3 | 2.5 | 31.4 | [ |
| 松木 | AlCl3 | [Bmim]Cl | 160 | 3 | 2.5 | 33.6 | [ |
| 玉米穗、柳枝稷、稻草、玉米秸秆、松木、白杨木 | PEG-OSO3H + MnCl2 | [Bmim]PF6 | 120 | 18 | 15.0 | 22.0~36.0 | [ |
| 玉米秸秆 | HCl + CrCl2 | [Emim]Cl | 140 | 120 | — | 22.0 | [ |
| 竹叶粉 | CHA-20沸石 | 水/甲苯 | 170 | 600 | 2.0 | 55.0 | [ |
| 玉米秸秆 | 炭质固体酸ISC-CCA | 水 | 190 | 240 | 2.2 | 43.1 | [ |
表2 半纤维素制备糠醛的反应体系
| 底物 | 催化剂 | 溶剂/(萃取剂) | 温度/℃ | 时间 /min | 底物负载/% 产率/% | 参考 文献 | |
|---|---|---|---|---|---|---|---|
| 木聚糖 | H2SO4 | 水 | 180 | 20 | 1.0 | 29.0 | [ |
| 木聚糖 | HCl | 水 | 180 | 20 | 1.0 | 34.3 | [ |
| 木聚糖 | H3PW12O40 | [Bmim]Cl | 160 | 10 | 1.9 | 93.7 | [ |
| 木聚糖 | Amberlyst-15 | [Bmim]Cl | 140 | 10 | 1.9 | 87.8 | [ |
| 木聚糖 | [Emim]HSO4 | [Emim]HSO4/甲苯 | 100 | 240 | 3.0 | 29.0 | [ |
| 木聚糖 | AlCl3 | [Bmim]Cl | 170 | 0.1 | 1.9 | 84.8 | [ |
| 木聚糖 | SO4 2-/Sn-MMT | 水/2-MTHF | 160 | 90 | 0.5 | 77.4 | [ |
| 木聚糖 | 甜菜碱+HCOOH | 水/CPME | 170 | 60 | 4.4 | 76.0 | [ |
| 木聚糖 | Nafion NR50 | NaCl水溶液/CPME | 190 | 60 | 3.8 | 55.0 | [ |
| 木聚糖 | HCl+CrCl2 | [Emim]Cl | 140 | 120 | 5.0 | 25.0 | [ |
| 木聚糖 | [Ch-SO4H][CF3SO3] | 1,4-二氧杂环乙烷 | 120 | 360 | 4.0 | 82.0 | [ |
| 未处理玉米芯 | AlCl3 | [Bmim]Cl | 160 | 3 | 2.5 | 19.1 | [ |
| 草 | AlCl3 | [Bmim]Cl | 160 | 3 | 2.5 | 31.4 | [ |
| 松木 | AlCl3 | [Bmim]Cl | 160 | 3 | 2.5 | 33.6 | [ |
| 玉米穗、柳枝稷、稻草、玉米秸秆、松木、白杨木 | PEG-OSO3H + MnCl2 | [Bmim]PF6 | 120 | 18 | 15.0 | 22.0~36.0 | [ |
| 玉米秸秆 | HCl + CrCl2 | [Emim]Cl | 140 | 120 | — | 22.0 | [ |
| 竹叶粉 | CHA-20沸石 | 水/甲苯 | 170 | 600 | 2.0 | 55.0 | [ |
| 玉米秸秆 | 炭质固体酸ISC-CCA | 水 | 190 | 240 | 2.2 | 43.1 | [ |
| 1 | PELETEIRO S , RIVAS S , ALONSO J L , et al . Furfural production using ionic liquids: a review[J]. Bioresource Technology, 2016, 202: 181-191. |
| 2 | MARISCAL R , MAIRELESTORRES P , OJEDA M , et al . Furfural: a renewable and versatile platform molecule for the synthesis of chemicals and fuels[J]. Energy & Environmental Science, 2016, 9(4): 1144-1189. |
| 3 | LIU Z , ZHANG FS . Effects of various solvents on the liquefaction of biomass to produce fuels and chemical feedstocks[J]. Energy Conversion and Management, 2008, 49(12): 3498-3504. |
| 4 | 张宏书 . 半纤维素及其结构研究[J]. 广州化学, 1990(1): 57-66. |
| ZHANG Hongshu . Study on hemicellulose and its structure[J]. Guangzhou Chemistry,1990(1):57-66. | |
| 5 | PETERSON A A , VOGEL F , LACHANCE R P , et al . Thermochemical biofuel production in hydrothermal media: a review of sub- and supercritical water technologies[J]. Energy & Environmental Science, 2008, 1(1): 32-65. |
| 6 | 徐燏, 肖传豪, 于英慧 . 糠醛生产工艺技术及展望[J]. 濮阳职业技术学院学报, 2010, 23(4): 150-152. |
| XU Yu , XIAO Chuanhao , YU Yinghui . Technology and prospect of furfural production[J]. Journal of Puyang Vocational and Technical College, 2010, 23(4): 150-152. | |
| 7 | 王文菊 . 氯化物催化木糖、半纤维素及木质纤维制备糠醛的研究[D].广州:华南理工大学, 2015. |
| WANG Wenju . Production of furfrual from xylose, hemicelluloses and lignocellulose using chlorides as catalysts[D]. Guangzhou:South China University of Technology, 2015. | |
| 8 | 张璐鑫, 于宏兵 . 糠醛生产工艺及制备方法研究进展[J]. 化工进展, 2013, 32(2): 425-432. |
| ZHANG Luxin , YU Hongbing . Research progress in the production and synthesis of furfural[J]. Chemical Industry and Engineering Progress, 2013, 32(2): 425-432. | |
| 9 | 刘菲, 郑明远, 王爱琴, 等 . 酸催化制备糠醛研究进展[J]. 化工进展, 2017, 36(1): 156-165. |
| LIU Fei , ZHENG Mingyuan , WANG Aiqin , et al . Research progresses in furfural production by acid catalysts[J]. Chemical Industry and Engineering Progress, 2017, 36(1): 156-165. | |
| 10 | GEBOERS J A , VYVER S V D , OOMS R , et al . Chemocatalytic conversion of cellulose: opportunities, advances and pitfalls[J]. Catalysis Science & Technology, 2011, 1(5): 714-726. |
| 11 | BUNNELL K , RICH A , LUCKETT C , et al . Plant maturity effects on the physicochemical properties and dilute acid hydrolysis of switchgrass (Panicum virgatum, L.) hemicelluloses[J]. ACS Sustainable Chemistry & Engineering, 2013, 1(6): 649-654. |
| 12 | 候其东, 鞠美庭, 李维尊, 等 . 基于离子液体的生物质组分分离研究进展[J]. 化工进展, 2016, 35(10): 3022-3031. |
| HOU Qidong , JU Meiting , LI Weizun , et al . Research progress on biomass fractionation using ionic liquids[J]. Chemical Industry and Engineering Progress, 2016, 35(10): 3022-3031. | |
| 13 | CHOI J H , JANG S K , KIM J H, et al . Simultaneous production of glucose, furfural, and ethanol organosolv lignin for total utilization of high recalcitrant biomass by organosolv pretreatment[J]. Renewable Energy, 2019, 130: 952-960. |
| 14 | MAMMAN A S , LEE J M, KIM Y C, et al . Furfural: hemicellulose/xylosederived biochemical[J]. Biofuels Bioproducts & Biorefining, 2008, 2(5): 438-454. |
| 15 | 金强, 张红漫, 严立石, 等 . 生物质半纤维素稀酸水解反应[J]. 化学进展, 2010, 22(4): 654-662. |
| JIN Qiang , ZHANG Hongman , YAN Lishi , et al . Dilute acid hydrolysis reaction of biomass hemicellulose[J]. Progress in Chemistry, 2010, 22(4): 654-662. | |
| 16 | NGUYEN Q A , TUCKER M P . Dilute acid/metal salt hydrolysis of lignocellulosics: US6423145[P]. 2002-01-01. |
| 17 | LU Y , MOSIER N S . Kinetic modeling analysis of maleic acid-catalyzed hemicellulose hydrolysis in corn stover[J]. Biotechnology & Bioengineering, 2010, 101(6): 1170-1181. |
| 18 | KUSEMA B T , NNOV T T , KI-ARVELA P M , et al . Acid hydrolysis of O-acetyl-galactoglucomannan[J]. Catalysis Science & Technology, 2013, 3(1): 116-122. |
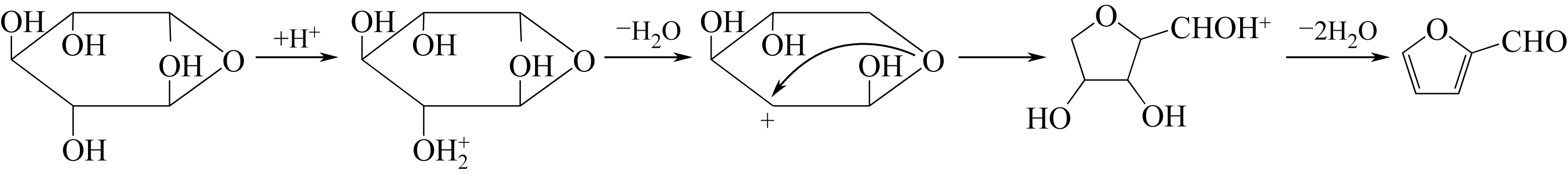
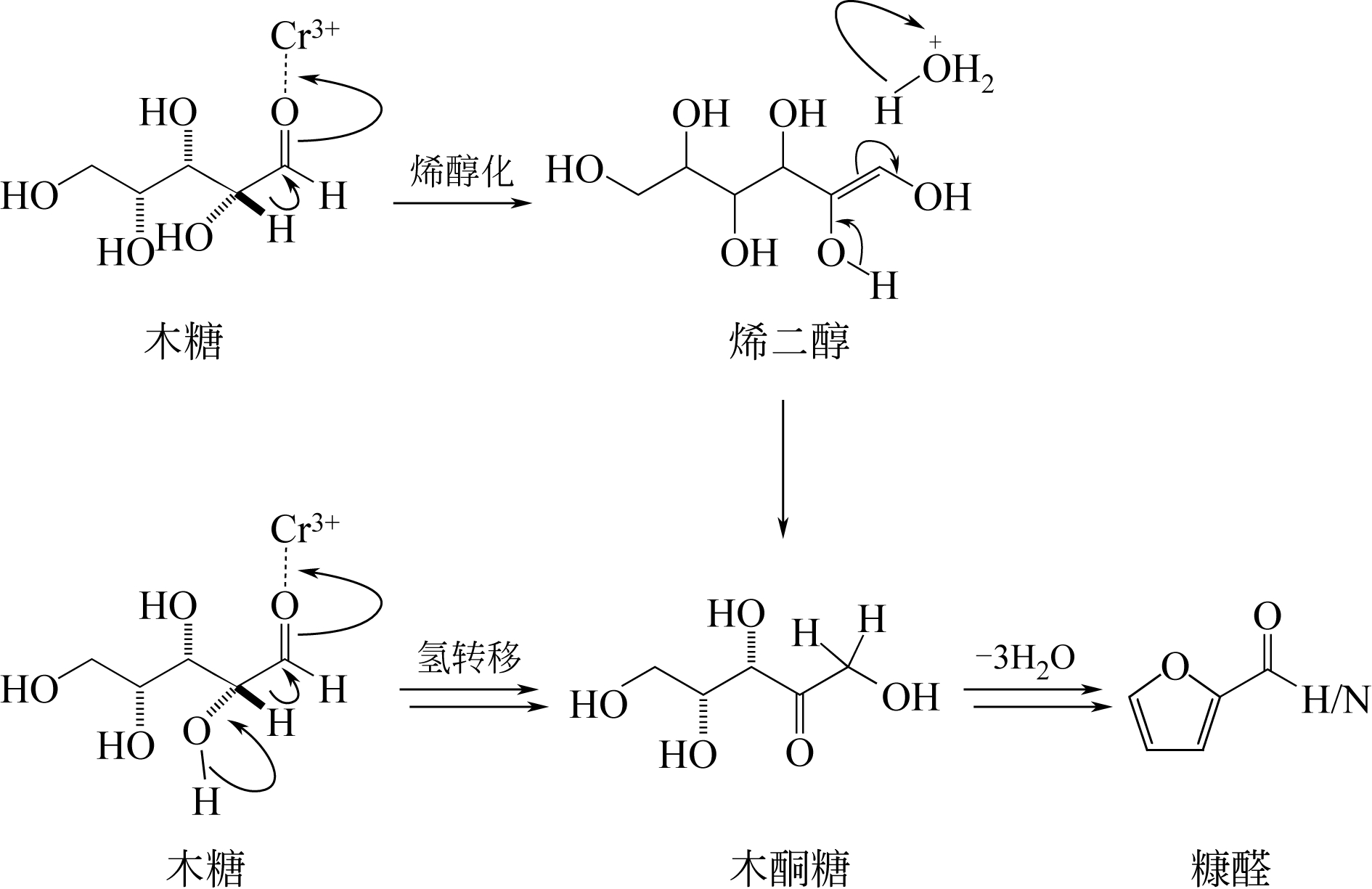
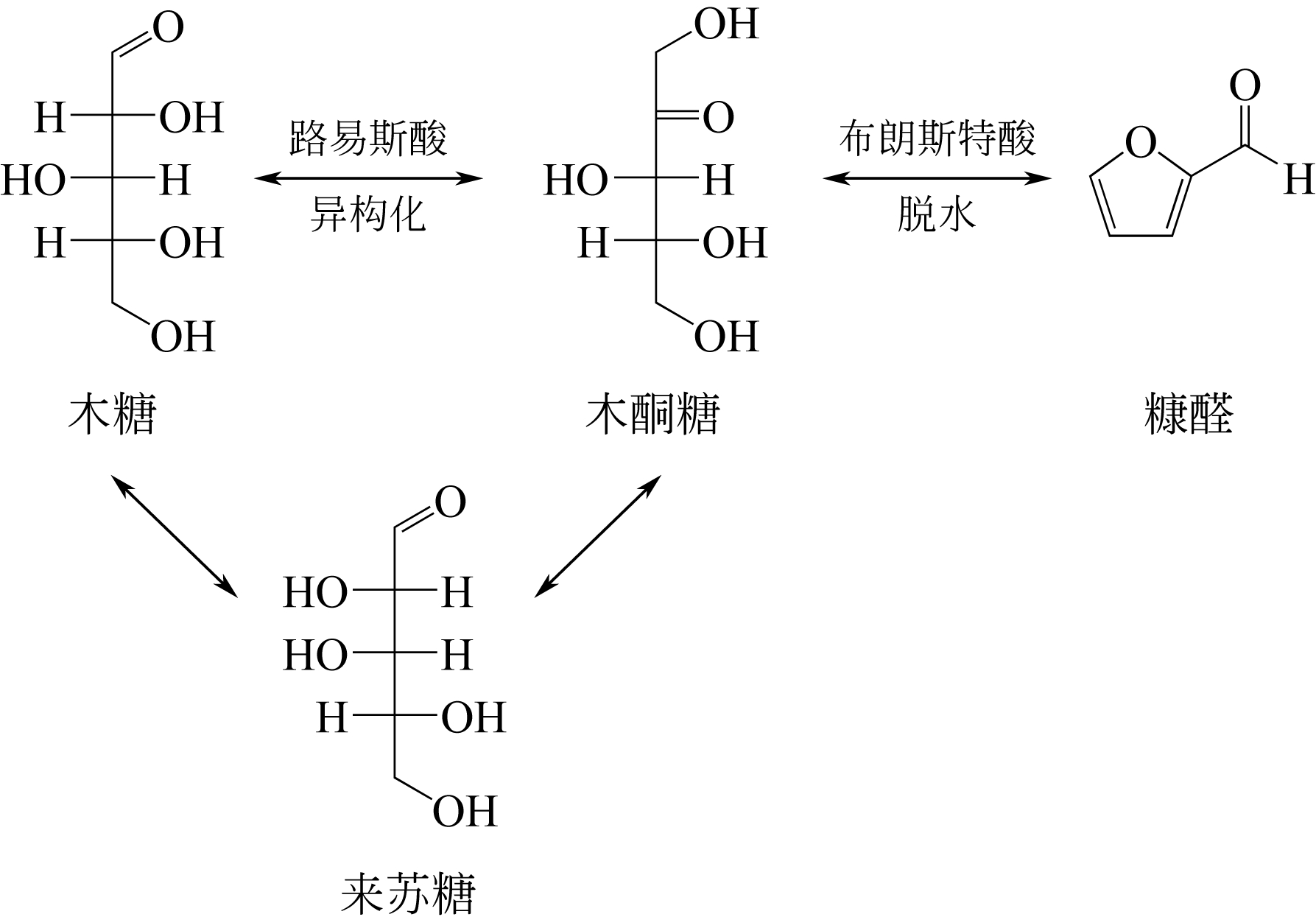
| 19 | ANTAL M J , RICHARDS M G N . Mechanism of formation of 2-furaldehyde from D-xylose[J]. Carbohydrate Research, 1991, 217(91): 71-85. |
| 20 | MIMLOS M R , QIAN X , DAVIS M , et al . Energetics of xylose decomposition as determined using quantum mechanics modeling[J]. Journal of Physical Chemistry A, 2006, 110(42): 11824-11838. |
| 21 | 王琼, 王闻, 亓伟, 等 . 木质纤维素酸催化制备糠醛的工艺及机理研究进展[J]. 农业工程学报, 2017, 33(15): 272-282. |
| WANG Qiong , WANG Wen , QI Wei , et al . Progress on technologies and mechanism of furfural production from lignocellulose catalyzed by acids[J]. Transactions of the Chinese Society of Agricultural Engineering, 2017, 33(15): 272-282. | |
| 22 | BINDER J B , BLANK J J , CEFALI A V , et al . Synthesis of furfural from xylose and xylan[J]. Chemsuschem, 2010, 3(11): 1268-1272. |
| 23 | CHOUDHARY V , SANDLER S I , VLACHOS D G .Conversion of xylose to furfural using Lewis and Bronsted acid catalysts in aqueous media [J].ACS Catalysis, 2012,2(9):2022-2088. |
| 24 | HU X , WESTERHOF R , DONG D , et al . Acid-catalyzed conversion of xylose in 20 solvents: insight into interactions of the solvents with xylose, furfural, and the acid catalyst[J]. ACS Sustainable Chemistry, 2014, 2(11): 2562-2575. |
| 25 | KIM Y C, LEE H S . Selective synthesis of furfural from xylose with supercritical carbon dioxide and solid acid catalyst[J]. Journal of Industrial & Engineering Chemistry, 2001, 7(6): 424-429. |
| 26 | WANG W , LI H , REN J , et al . An efficient process for dehydration of xylose to furfural catalyzed by inorganic salts in water/dimethyl sulfoxide system[J]. Chinese Journal of Catalysis, 2014, 35(5): 741-747. |
| 27 | WANG S , ZHAO Y , LIN H , et al . Conversion of C5 carbohydrates into furfural catalyzed by a lewis acidic ionic liquid in renewable γ-valerolactone[J]. Green Chemistry, 2017, 19(16):3869-3879. |
| 28 | OLIVIER-BOURBIGOU H , MAGNA L , MORVAN D . Ionic liquids and catalysis: recent progress from knowledge to applications[J]. Applied Catalysis A: General, 2010, 373(1): 1-56. |
| 29 | SHI C , ZHAO Y , XIN J , et al . Effects of cations and anions of ionic liquids on the production of 5-hydroxymethylfurfural from fructose[J]. Chemical Communications, 2012, 48(34): 4103-4105. |
| 30 | HOU Q , LI W , ZHEN M , et al . An ionic liquid-organic solvent biphasic system for efficient production of 5-hydroxymethyl furfural from carbohydrates at high concentrations[J]. RSC Advances, 2017, 7(75): 47288-47296. |
| 31 | 陈景平 . 离子液体催化半纤维素五碳糖制取糠醛研究[D]. 杭州:浙江大学, 2017. |
| CHEN Jingping . Catalytic conversion of hemicellulose-pentose into furfural by inonic-liquid[D]. Hangzhou: Zhejiang University, 2017. | |
| 32 | 张晔 . 无机盐催化半纤维素水解制备糠醛的研究[D]. 淮南:安徽理工大学, 2014. |
| ZHANG Ye . Conversion of hemicelluose into furfural using inorganic salt catalysts[D]. Huainan: Anhui University of Science and Technology, 2014. | |
| 33 | ENSLOW K R , BELL A T . SnCl4-catalyzed isomerization/dehydration of xylose and glucose to furanics in water[J]. Catalysis Science & Technology, 2015, 5(5): 2839-2847. |
| 34 | PELETEIRO S , LOPES A M D C , GARROTE G , et al . Simple and efficient furfural production from xylose in media containing 1-butyl-3-methylimidazolium hydrogen sulfate [J]. Industrial & Engineering Chemistry Research, 2015, 54:8368-8373. |
| 35 | GUPTA N K , FUKUOKA A , NAKAJIMA K . Amorphous Nb2O5 as a selective and reusable catalyst for furfural production from xylose in biphasic water and toluene[J]. ACS Catalysis, 2017, 7(4): 2430-2436. |
| 36 | TAO F T , SONG H S , CHOU L C . Efficient process for the conversion of xylose to furfural with acidic[J]. Can.J. Chem., 2011, 89(1): 83-87. |
| 37 | SERRANORUIZ J C , CAMPELO J M , FRANCAVILLA M , et al . Efficient microwave-assisted production of furfural from C5 sugars in aqueous media catalysed by Brönsted acidic ionic liquids[J]. Catalysis Science & Technology, 2012, 2(9): 1828-1832. |
| 38 | LE G S , GERGELA D , CEBALLOS C , et al . Furfural production from D-xylose and xylan by using stable nafion NR50 and NaCl in a microwave-assisted biphasic reaction[J]. Molecules, 2016, 21(8):1102-1112. |
| 39 | DELBECQ F , WANG Y , LEN C . Conversion of xylose, xylan and rice husk into furfural via betaine and formic acid mixture as novel homogeneous catalyst in biphasic system by microwave-assisted dehydration[J]. Journal of Molecular Catalysis A: Chemical, 2016, 423: 520-525. |
| 40 | LIN Q , LI H , WANG X , et al . SO4 2-/Sn-MMT solid acid catalyst for xylose and xylan conversion into furfural in the biphasic system[J]. Catalysts, 2017, 7(4): 118. |
| 41 | SUN Q , WANG S , AGUILA B , et al . Creating solvation environments in heterogeneous catalysts for efficient biomass conversion[J]. Nature Communications, 2018, 9(1):3236. |
| 42 | GUENIC S L , DELBECQ F , CEBALLOS C , et al . Microwave-assisted dehydration of D-xylose into furfural by diluted inexpensive inorganic salts solution in a biphasic system[J]. Journal of Molecular Catalysis A: Chemical, 2015, 410: 1-7. |
| 43 | PELETEIRO S , VELASCO G G , SANTOS V , et al . Conversion of hexoses and pentoses into furans in an ionic liquid[J]. Afinidad, 2014, 71(567): 202-206. |
| 44 | ZHANG L , YU H , WANG P , et al . Conversion of xylan, D-xylose and lignocellulosic biomass into furfural using AlCl3 as catalyst in ionic liquid[J]. Bioresource Technology, 2013, 130(2): 110-116. |
| 45 | 杨凤丽, 刘启顺, 白雪芳, 等 . 由生物质制备5-羟甲基糠醛的研究进展[J]. 现代化工, 2009, 29(5): 18-22. |
| YANG Fengli , LIU Qishun , BAI Xuefang , et al . Advances in production of 5-hydroxymethylfurfural from biomass[J]. Modern Chemical Industry, 2009, 29(5): 18-22. | |
| 46 | LAM E, CHONG J H , MAJID E , et al . Carbocatalytic dehydration of xylose to furfural in water[J]. Carbon, 2012, 50(3): 1033-1043. |
| 47 | AGIRREZABAL-TELLERIA I , HEMMANN F , JÄGER C , et al . Functionalized partially hydroxylated MgF2 as catalysts for the dehydration of D-xylose to furfural [J]. Journal of Catalysis, 2013, 305: 81-91. |
| 48 | CHATTERJEE A , HU X , LAM L Y . A dual acidic hydrothermally stable MOF-composite for upgrading xylose to furfural[J]. Applied Catalysis A General, 2018, 566: 130-139. |
| 49 | HRONEC M , FULAJTÁROVÁ K . Terephthalic acid from waste PET: an efficient and reusable catalyst for xylose conversion into furfural[J]. Catalysis Today, 2019,324:27-32. |
| 50 | CAI C M , ZHANG T Y , KUMAR R , et al . Integrated furfural production as a renewable fuel and chemical platform from lignocellulosic biomass[J]. Journal of Chemical Technology & Biotechnology, 2013, 89(1): 2-10. |
| 51 | SANGARUNLERT W , PIUMSOMBOON P , NGAMPRASERTSITH S . Furfural production by acid hydrolysis and supercritical carbon dioxide extraction from rice husk[J]. Korean Journal of Chemical Engineering, 2007, 24(6): 936-941. |
| 52 | AGIRREZABAL-TELLERIA I , LARREATEGUI A , REQUIES J , et al . Furfural production from xylose using sulfonic ion-exchange resins (Amberlyst) and simultaneous stripping with nitrogen[J]. Bioresource Technology, 2011, 102(16): 7478-7485. |
| 53 | JIN H , LIU X , BAN Y , et al . Conversion of xylose into furfural in a MOF-based mixed matrix membrane reactor[J]. Chemical Engineering Journal, 2016, 305: 12-18. |
| 54 | HUI W , ZHOU Y , DONG Y , et al . Efficient hydrolysis of hemicellulose to furfural by novel superacid SO4H- functionalized ionic liquids[J]. Green Energy & Environment, 2018, in press. |
| 55 | ZHANG Z , DU B , QUAN Z J , et al . Dehydration of biomass to furfural catalyzed by reusable polymer bound sulfonic acid (PEG-OSO3H) in ionic liquid[J]. Catalysis Science & Technology, 2014, 4(3): 633-638. |
| 56 | ZHANG L X , YU H B , PAN W . Solid acids as catalysts for the conversion of D-xylose, xylan and lignocellulosics into furfural in ionic liquid[J]. Bioresource Technology, 2013, 136(12): 515-521. |
| 57 | CARVALHO R S D , RODRIGUES F D A , MONTEIRO R S , et al . Optimization of furfural synthesis from xylose using niobic acid and niobium phosphate as catalysts[J]. Waste & Biomass Valorization, 2018(9): 1-8. |
| 58 | KAIPROMMARAT S , KONGPARAKUL S , REUBROYCHAROEN P , et al . Highly efficient sulfonic MCM-41 catalyst for furfural production: furan-based biofuel agent[J]. Fuel, 2016, 174: 189-196. |
| 59 | YOSHIDA K , NANAO H , KIYOZUMI Y , et al . Furfural production from xylose and bamboo powder over chabazite-type zeolite prepared by interzeolite conversion method[J]. Journal of the Taiwan Institute of Chemical Engineers, 2017,79:55-59. |
| 60 | DELBECQ F , TAKAHASHI Y , KONDO T , et al . Microwave assisted efficient furfural production using nano-sized surface-sulfonated diamond powder[J]. Catalysis Communications, 2018, 110: 74-78. |
| 61 | GALLO J M R , ALONSO D M , MELLMER M A , et al . Production of furfural from lignocellulosic biomass using beta zeolite and biomass-derived solvent[J]. Topics in Catalysis, 2013, 56(18/19/20): 1775-1781. |
| 62 | LIMA S , NEVES P , ANTUNES M M , et al . Conversion of mono/di/polysaccharides into furan compounds using 1-alkyl-3-methylimidazolium ionic liquids[J]. Applied Catalysis A: General, 2009, 363(1/2): 93-99. |
| 63 | WANG W , REN J , LI H , et al . Direct transformation of xylan-type hemicelluloses to furfural via SnCl₄ catalysts in aqueous and biphasic systems[J]. Bioresource Technology, 2015, 183:188-194. |
| 64 | ZHANG Z , ZHAO Z K . Microwave-assisted conversion of lignocellulosic biomass into furans in ionic liquid[J]. Bioresource Technology, 2010, 101(3): 1111-1114. |
| 65 | SEN S M, BINDER J B , RAINES R T , et al . Conversion of biomass to sugars via ionic liquid hydrolysis: process synthesis and economic evaluation[J]. Biofuels Bioproducts & Biorefining, 2012, 6(4): 444-452. |
| 66 | SWATLOSKI R P , SPEAR S K , HOLBREY J D , et al . Dissolution of cellulose with ionic liquids[J]. Journal of the American Chemical Society, 2002, 124(18): 4974-4975. |
| 67 | YEMIS O , MAZZA G . Acid-catalyzed conversion of xylose, xylan and straw into furfural by microwave-assisted reaction[J]. Bioresource Technology, 2011, 102(15): 7371-7378. |
| 68 | ZHANG S , LU J , LI M , et al . Efficient production of furfural from corncob by an integrated mineral-organic-Lewis acid catalytic process[J]. Bioresources, 2017, 12(2): 2965-2981. |
| 69 | YANG Y , HU C W , ABU-OMAR M M . Synthesis of furfural from xylose, xylan, and biomass using AlCl3·6H2O in biphasic media via xylose isomerization to xylulose[J]. Chemsuschem, 2012, 5(2): 405-410. |
| 70 | BESSON M , GALLEZOT P . Deactivation of metal catalysts in liquid phase organic reactions[J]. Catalysis Today, 2003, 81(4): 547-559. |
| 71 | LI W , ZHANG T , PEI G . Catalytic conversion of corn stover into furfural over carbon-based solid acids[J]. Bioresources, 2018, 13(1): 1425-1440. |
| 72 | HOYDONCKX H E , RHIJN W M V , RHIJN W V , et al . Ullmann's encyclopedia of industrial chemistry [M].Weinheim: Wiley-VCH,2007:1-29. |
| 73 | SITTHISA S , SOOKNOI T , MA Y, et al . Kinetics and mechanism of hydrogenation of furfural on Cu/SiO2 catalysis[J]. Journal of Catalysis, 2011, 277(1): 1-13. |
| 74 | NAKAGAWA Y , NAKAZAWA H , WATANABE H . Total hydrogenation of furfural over a silica-supported nickel catalyst prepared by the reduction of a nickel nitrate precursor[J]. ChemCatChem, 2012, 4(11): 1791-1797. |
| 75 | 崔静磊 . 糖类衍生物催化转化制备平台化合物的应用基础研究[D]. 北京: 中国科学院大学, 2016. |
| CUI Jinglei . Basic study on the application of carbohydrate derivatives in catalytic transformation to prepare platform compounds[D]. Beijing: University of Chinese Academy of Sciences, 2016. | |
| 76 | 黄玉辉, 任国卿, 孙蛟, 等 . Cu/ZnO催化糠醛气相加氢制2-甲基呋喃的研究[J]. 燃料化学学报, 2016, 44(11): 1349-1355. |
| HUANG Yuhui , REN Guoqing , SUN Jiao , et al . Study on the yapor phase hydrogenation of furfural to 2-methylfuran on Cu/ZnO catalyst[J].Journal of Fuel Chemistry and Technology, 2016, 44(11): 1349-1355. | |
| 77 | MARISCAL R , MAIRELES-TORRES P , OJEDA M , et al . Furfural: a renewable and versatile platform molecule for the synthesis of chemicals and fuels [J]. Energy and Environmental Science, 2016, 9(4): 1144-1189. |
| 78 | YANG W , PROF A S . One-step catalytic transformation of carbohydrates and cellulosic biomass to 2,5-dimethyltetrahydrofuran for liquid fuels[J]. ChemSusChem, 2010, 3(5): 597-603. |
| 79 | 郑睿, 沈健芬 . 新型溶剂2-甲基四氢呋喃的合成和应用研究进展[J]. 精细化工中间体, 2009, 39(2): 12-14. |
| ZHENG Rui , SHEN Jianfen . Progress in synthesis and application of 2-methyltetrahydrofuran[J]. Fine Chemical Intermediates, 2009, 39(2): 12-14. | |
| 80 | 王东 . 糠醛产业现状及其衍生物的生产与应用(二)[J]. 化工中间体, 2003(21): 19-22. |
| WANG Dong . Present situation of furfural industry and production and application of its derivatives(II)[J]. Chemical Intermediate, 2003(21): 19-22. | |
| 81 | 卢怡, 郑志锋, 黄元波, 等 . 半纤维素选择性催化制备糠醛及其衍生物的研究进展[J]. 林产化学与工业, 2018, 38(3): 1-16. |
| LU Yi , ZHENG Zhifeng , HUANG Yuanbo , et al . Research advances in preparation of furfural and its derivatives by selective catalytic conversion of hemicellulose[J]. Chemistry and Industry of Forest Products, 2018, 38(3): 1-16. | |
| 82 | YANG Y , DU Z , HUANG Y , et al . Conversion of furfural into cyclopentanone over Ni-Cu bimetallic catalysts[J]. Green Chemistry, 2013, 15(7): 1932-1940. |
| 83 | HRONEC M , FULAJTAROV K . Selective transformation of furfural to cyclopentanone[J]. Catalysis Communications, 2012, 24: 100-104. |
| 84 | HAAN R J . Gasoline composition and process for the preparation of alkylfurfuryl ether: EP2231832B1[P].2008-12-18. |
| 85 | LANGE J P , VAN D H E, VAN B J, et al . Furfural-a promising platform for lignocellulosic biofuels[J]. ChemSusChem, 2012, 5(1): 150-166. |
| 86 | NISHIMURA S , MIZUHORI K , EBITANI K . Reductive amination of furfural toward furfurylamine with aqueous ammonia under hydrogen over Ru-supported catalyst [J]. Research on Chemical Intermediates, 2016, 42(1): 1-12. |
| 87 | LOHBECK K , HAFERKORN H , FUHRMANN W , et al . Maleic and fumaric acids[J]. Analytical Chemistry, 2000(9): 1454-1459. |
| 88 | FELTHOUSE T R , BURNETT J C , MITCHELL S F , et al . Van nostrand's scientific encyclopedia. [M].Texas: Wiley & Sons,2005:1-58. |
| 89 | ZHANG W , ZHU Y , NIU S , et al . A study of furfural decarbonylation on K-doped Pd/Al2O3 catalysts[J]. Journal of Molecular Catalysis A: Chemical, 2011, 335(1): 71-81. |
| 90 | BUI L, LUO H , GUNTHER W R , et al . Innentitelbild: domino reaction catalyzed by zeolites with bronsted and lewis acid sites for the production of γ-valerolactone from furfural[J]. Angewandte Chemie, 2013, 25(31): 8022-8025. |
| 91 | ALONSO D M , WETTSTEIN S G , DUMESIC J A . Gamma-valerolactone, a sustainable platform molecule derived from lignocellulosic biomass[J]. Green Chemistry, 2013, 15(3): 584-595. |
| 92 | 颜廷良, 王德龙 . 糠醛的生产及应用[J]. 辽宁化工, 2002, 31(1): 36-37. |
| YAN Tingliang , WANG Delong . Production and application of furfural [J]. Liaoning Chemical Industry, 2002, 31(1): 36-37. | |
| 93 | 荣春光 . 糠醛生产工艺研究及糠醛废渣的综合利用[D]. 长春: 吉林大学, 2012. |
| RONG Chunguang . Studies on preation of furfural and comprehensive utilization of furfural residue[D]. Changchun: Jilin University, 2012. | |
| 94 | HE L , LI D , ZHANG G , et al . Synthesis of carbonaceous poly(furfuryl alcohol) membrane for water desalination[J]. Indengchemres, 2010, 49(9): 4175-4180. |
| 95 | OLIVEIRA F B , GARDRAT C , ENJALBAL C , et al . Phenol– furfural resins to elaborate composites reinforced with sisal fibers— molecular analysis of resin and properties of composites[J]. Journal of Applied Polymer Science, 2010, 109(4): 2291-2303. |
| 96 | 巫月亭 . 一种耐腐蚀高柔韧性改性糠醛丙酮树脂材料的制备方法: CN201510030281[P]. 2015-01-21. |
| WU Yueting . A preparation method of modified furfural acetone resin material with high corrosion resistance and flexibility: CN201510030281[P]. 2015-01-21. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 郑谦, 官修帅, 靳山彪, 张长明, 张小超. 铈锆固溶体Ce0.25Zr0.75O2光热协同催化CO2与甲醇合成DMC[J]. 化工进展, 2023, 42(S1): 319-327. |
| [6] | 王正坤, 黎四芳. 双子表面活性剂癸炔二醇的绿色合成[J]. 化工进展, 2023, 42(S1): 400-410. |
| [7] | 高雨飞, 鲁金凤. 非均相催化臭氧氧化作用机理研究进展[J]. 化工进展, 2023, 42(S1): 430-438. |
| [8] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [9] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [10] | 许友好, 王维, 鲁波娜, 徐惠, 何鸣元. 中国炼油创新技术MIP的开发策略及启示[J]. 化工进展, 2023, 42(9): 4465-4470. |
| [11] | 耿源泽, 周俊虎, 张天佑, 朱晓宇, 杨卫娟. 部分填充床燃烧器中庚烷均相/异相耦合燃烧[J]. 化工进展, 2023, 42(9): 4514-4521. |
| [12] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [13] | 王晋刚, 张剑波, 唐雪娇, 刘金鹏, 鞠美庭. 机动车尾气脱硝催化剂Cu-SSZ-13的改性研究进展[J]. 化工进展, 2023, 42(9): 4636-4648. |
| [14] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| [15] | 高彦静. 单原子催化技术国际研究态势分析[J]. 化工进展, 2023, 42(9): 4667-4676. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||