化工进展 ›› 2019, Vol. 38 ›› Issue (01): 334-343.DOI: 10.16085/j.issn.1000-6613.2018-1280
丁烯氧化脱氢钼铋系催化剂:晶相之间的协同效应
张思泽1( ),万超1,曾亮2,程党国1(
),万超1,曾亮2,程党国1( ),陈丰秋1,巩金龙2(
),陈丰秋1,巩金龙2( )
)
- 1. 浙江大学化学工程与生物工程学院,浙江 杭州 310027
2. 天津大学化工学院,天津化学化工协同创新中心,天津300072
-
收稿日期:2018-06-21修回日期:2018-10-12出版日期:2019-01-05发布日期:2019-01-05 -
通讯作者:程党国,巩金龙 -
作者简介:张思泽(1994—),男,硕士研究生。E-mail:<email>21628025@zju.edu.cn</email>。|程党国,教授。E-mail:<email>dgcheng@zju.edu.cn</email>|巩金龙,教授。E-mail:<email>jlgong@tju.edu.cn</email> -
基金资助:国家自然科学基金(21622606);国家自然科学基金(21622606)。
Oxidative dehydrogenation of butene over bismuth molybdate catalysts: synergetic effect between different crystalline phases
Size ZHANG1( ),Chao WAN1,Liang ZENG2,Dangguo CHENG1(
),Chao WAN1,Liang ZENG2,Dangguo CHENG1( ),Fengqiu CHEN1,Jinlong GONG2(
),Fengqiu CHEN1,Jinlong GONG2( )
)
- 1. College of Chemical and Biological Engineering,Zhejiang University,Hangzhou 310027,Zhejiang,China
2. Collaborative Innovation Centre of Chemical Science and Engineering, School of Chemical Engineering and Technology, Tianjin University, Tianjin 300072, China
-
Received:2018-06-21Revised:2018-10-12Online:2019-01-05Published:2019-01-05 -
Contact:Dangguo CHENG,Jinlong GONG
摘要:
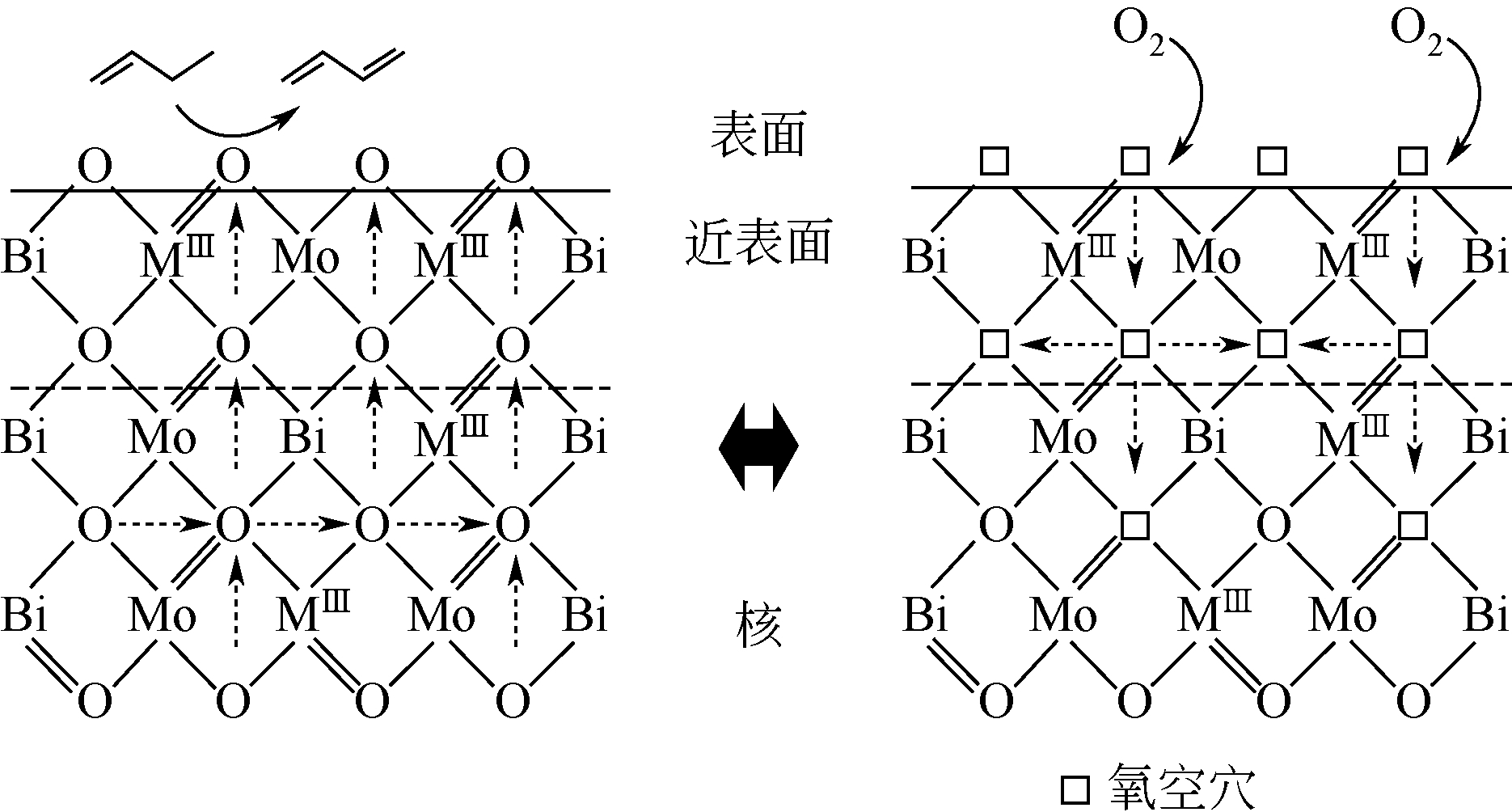
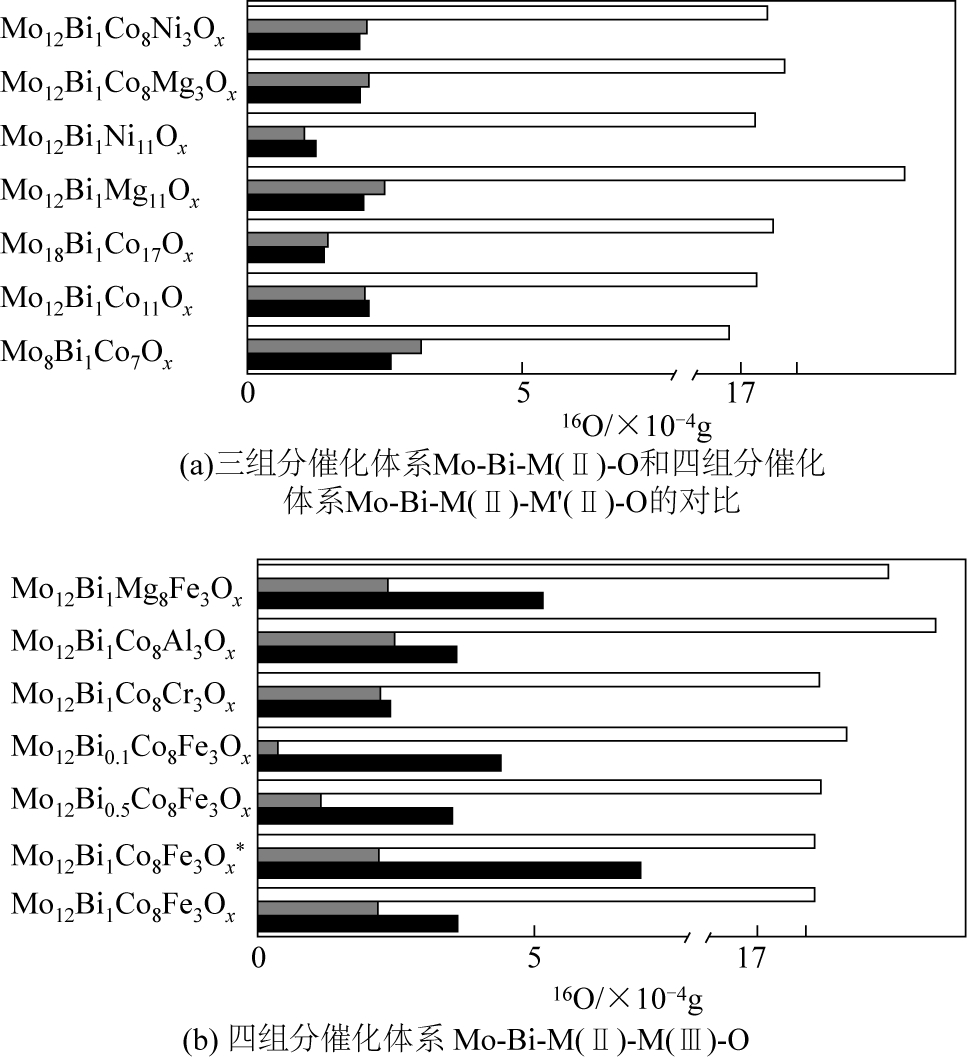
钼铋系催化剂以其优良的性能一直以来都是丁烯氧化脱氢研究和应用的热点。本文简述了已有研究中对钼铋系催化剂及改性后的多组分催化剂的晶相结构及其与反应性能间关系的研究进展。指出在钼铋催化剂中,有较多晶格缺陷的α-Bi2(MoO4)3提供吸附位,氧流动性较强的γ-Bi2MoO6提供晶格氧,二者的协同作用提高了催化剂的活性。而在改性后的多组分钼铋系催化剂中,添加的组分与钼铋元素结合生成新的晶相,产生了更多的晶格缺陷及氧供体,从而提升了催化性能。对于钼铋系催化剂进一步改进的方向,本文认为在添加组分的方法基础上,还可以从催化剂表面结构方面入手,进行进一步的深入探究。
中图分类号:
引用本文
张思泽, 万超, 曾亮, 程党国, 陈丰秋, 巩金龙. 丁烯氧化脱氢钼铋系催化剂:晶相之间的协同效应[J]. 化工进展, 2019, 38(01): 334-343.
Size ZHANG, Chao WAN, Liang ZENG, Dangguo CHENG, Fengqiu CHEN, Jinlong GONG. Oxidative dehydrogenation of butene over bismuth molybdate catalysts: synergetic effect between different crystalline phases[J]. Chemical Industry and Engineering Progress, 2019, 38(01): 334-343.
| 催化剂 | 反应温度/℃ | 实验变量 | 最优活性① | ||
|---|---|---|---|---|---|
| S/% | Y/% | TOS/h | |||
| α-Bi2(MoO4)3 [ | 420 | 单相 | 74 | 27 | 48 |
| β-Bi2Mo2O9 [ | 420 | 单相(不稳定) | 92 | 60 | 100 |
| γ-Bi2MoO6 [ | 420 | 制备pH = 3 | 90 | 46 | 12 |
| 440 | 丁烯∶氧气 = 1∶0.75 | 91 | 47 | 48 | |
| β-Bi2Mo2O9 + γ-Bi2MoO6 [ | 420 | β + 32% γ 混合相(摩尔分数) | 73 | 29 | null |
| α-Bi2(MoO4)3 + γ-Bi2MoO6 [ | 420 | α + 90% γ 混合相(质量分数) | 90 | 59 | null |
表1 单一和混合晶相钼酸盐(α、β、γ)在丁烯氧化脱氢中的反应活性
| 催化剂 | 反应温度/℃ | 实验变量 | 最优活性① | ||
|---|---|---|---|---|---|
| S/% | Y/% | TOS/h | |||
| α-Bi2(MoO4)3 [ | 420 | 单相 | 74 | 27 | 48 |
| β-Bi2Mo2O9 [ | 420 | 单相(不稳定) | 92 | 60 | 100 |
| γ-Bi2MoO6 [ | 420 | 制备pH = 3 | 90 | 46 | 12 |
| 440 | 丁烯∶氧气 = 1∶0.75 | 91 | 47 | 48 | |
| β-Bi2Mo2O9 + γ-Bi2MoO6 [ | 420 | β + 32% γ 混合相(摩尔分数) | 73 | 29 | null |
| α-Bi2(MoO4)3 + γ-Bi2MoO6 [ | 420 | α + 90% γ 混合相(质量分数) | 90 | 59 | null |
| 项目 | 金属 | |||||||
|---|---|---|---|---|---|---|---|---|
| Ni | Co | Fe | Mg | Mn | Cd | Ca | Pb | |
| 离子半径 /? | 0.69 | 0.72 | 0.74 | 0.66 | 0.80 | 0.97 | 0.99 | 1.20 |
| 稳定性 结构 | α-CoMoO4型(单斜晶系) M(Ⅱ):6-配位; M6+:6-配位 | CaWO4型(白钨矿,四方晶系) M(Ⅱ)∶8-配位;M6+∶4-配位 | ||||||
| α-MnMoO4型 M(Ⅱ):6-配位; M6+:4-配位 | ||||||||
表2 二价金属钼酸盐的晶体结构[10]
| 项目 | 金属 | |||||||
|---|---|---|---|---|---|---|---|---|
| Ni | Co | Fe | Mg | Mn | Cd | Ca | Pb | |
| 离子半径 /? | 0.69 | 0.72 | 0.74 | 0.66 | 0.80 | 0.97 | 0.99 | 1.20 |
| 稳定性 结构 | α-CoMoO4型(单斜晶系) M(Ⅱ):6-配位; M6+:6-配位 | CaWO4型(白钨矿,四方晶系) M(Ⅱ)∶8-配位;M6+∶4-配位 | ||||||
| α-MnMoO4型 M(Ⅱ):6-配位; M6+:4-配位 | ||||||||
| 催化剂 | 晶相 | 反应温度/℃ | 最优活性① | TPRO峰/℃ | ||
|---|---|---|---|---|---|---|
| S/% | Y/% | TOS/h | ||||
| BiMoCe0.2 [ | Ce(MoO4)2, α-Bi2(MoO4)3, β-Bi2Mo2O9, γ-Bi2MoO6 | 440 | 96 | 70 | 2 | 183 |
| BiMoLa0.2 [ | La2(MoO4)3, α-Bi2(MoO4)3, β-Bi2Mo2O9, γ-Bi2MoO6 | 440 | 95 | 71 | 100 | 174 |
| BiMoZr0.4 [ | Zr(MoO4)2, α-Bi2(MoO4)3, β-Bi2Mo2O9, γ-Bi2MoO6 | 440 | 91 | 68 | 2 | 171 |
| BiV0.6Mo0.4 [ | Bi0.93Mo0.21V0.79O4, γ-Bi2MoO6 | 420 | 89 | 64 | 8 | 181 |
| BiMoV0.15 [ | BiVO4, α-Bi2(MoO4)3, β-Bi2Mo2O9, γ-Bi2MoO6 | 440 | 96 | 73 | 2 | 180 |
| BiMoFe0.65 [ | Fe2(MoO4)3, α-Bi2(MoO4)3, β-Bi2Mo2O9, γ-Bi2MoO6 | 420 | 91 | 63 | 6 | 155 |
表3 三组分钼铋系催化剂在丁烯氧化脱氢中的组成晶相和反应活性
| 催化剂 | 晶相 | 反应温度/℃ | 最优活性① | TPRO峰/℃ | ||
|---|---|---|---|---|---|---|
| S/% | Y/% | TOS/h | ||||
| BiMoCe0.2 [ | Ce(MoO4)2, α-Bi2(MoO4)3, β-Bi2Mo2O9, γ-Bi2MoO6 | 440 | 96 | 70 | 2 | 183 |
| BiMoLa0.2 [ | La2(MoO4)3, α-Bi2(MoO4)3, β-Bi2Mo2O9, γ-Bi2MoO6 | 440 | 95 | 71 | 100 | 174 |
| BiMoZr0.4 [ | Zr(MoO4)2, α-Bi2(MoO4)3, β-Bi2Mo2O9, γ-Bi2MoO6 | 440 | 91 | 68 | 2 | 171 |
| BiV0.6Mo0.4 [ | Bi0.93Mo0.21V0.79O4, γ-Bi2MoO6 | 420 | 89 | 64 | 8 | 181 |
| BiMoV0.15 [ | BiVO4, α-Bi2(MoO4)3, β-Bi2Mo2O9, γ-Bi2MoO6 | 440 | 96 | 73 | 2 | 180 |
| BiMoFe0.65 [ | Fe2(MoO4)3, α-Bi2(MoO4)3, β-Bi2Mo2O9, γ-Bi2MoO6 | 420 | 91 | 63 | 6 | 155 |
| 催化剂 | 晶相 | 最优活性① | ||
|---|---|---|---|---|
| S/% | Y/% | TOS/h | ||
| Co9Fe3Bi1Mo12O51 [ | α-CoMoO4, β-CoMoO4, α-Bi2(MoO4)3, γ-Bi2MoO6, Fe2(MoO4)3 | 93 | 61 | 6 |
| Ni9Fe3Bi1Mo12O51 [ | α-NiMoO4, β-NiMoO4, α-Bi2(MoO4)3, γ-Bi2MoO6, Fe2(MoO4)3 | 90 | 53 | 6 |
| Mn9Fe3Bi1Mo12O51 [ | α-MnMoO4, α-Bi2(MoO4)3, γ-Bi2MoO6, Fe2(MoO4)3 | 90 | 48 | 6 |
| Mg9Fe3Bi1Mo12O51 [ | β-MgMoO4,α-Bi2(MoO4)3, γ-Bi2MoO6, Fe2(MoO4)3 | 91 | 47 | 6 |
| Zn9Fe3Bi1Mo12O51 [ | α-ZnMoO4, α-Bi2(MoO4)3, γ-Bi2MoO6, Fe2(MoO4)3 | 92 | 40 | 6 |
| BiFe0.65Ni0.05Mo[ | Bi3Mo2Fe1O12[derived α-Bi2(MoO4)3], Fe2(MoO4)3, NiMoO4 | 84 | 72 | 14 |
| BiFe0.65Co0.05Mo[ | Bi3Mo2Fe1O12[derived α-Bi2(MoO4)3], Fe2(MoO4)3, CoMoO4 | 82 | 68 | 14 |
| BiFe0.65Zn0.05Mo[ | Bi3Mo2Fe1O12[derived α-Bi2(MoO4)3], Fe2(MoO4)3, ZnMoO4 | 86 | 67 | 14 |
| BiFe0.65Mn0.05Mo[ | Bi3Mo2Fe1O12[derived α-Bi2(MoO4)3], Fe2(MoO4)3, MnMoO4 | 85 | 64 | 14 |
| BiFe0.65Cu0.05Mo[ | Bi3Mo2Fe1O12[derived α-Bi2(MoO4)3], Fe2(MoO4)3, CuMoO4 | 78 | 46 | 14 |
表4 四组分钼铋系催化剂的晶相及其在420℃条件下丁烯氧化脱氢中的反应活性
| 催化剂 | 晶相 | 最优活性① | ||
|---|---|---|---|---|
| S/% | Y/% | TOS/h | ||
| Co9Fe3Bi1Mo12O51 [ | α-CoMoO4, β-CoMoO4, α-Bi2(MoO4)3, γ-Bi2MoO6, Fe2(MoO4)3 | 93 | 61 | 6 |
| Ni9Fe3Bi1Mo12O51 [ | α-NiMoO4, β-NiMoO4, α-Bi2(MoO4)3, γ-Bi2MoO6, Fe2(MoO4)3 | 90 | 53 | 6 |
| Mn9Fe3Bi1Mo12O51 [ | α-MnMoO4, α-Bi2(MoO4)3, γ-Bi2MoO6, Fe2(MoO4)3 | 90 | 48 | 6 |
| Mg9Fe3Bi1Mo12O51 [ | β-MgMoO4,α-Bi2(MoO4)3, γ-Bi2MoO6, Fe2(MoO4)3 | 91 | 47 | 6 |
| Zn9Fe3Bi1Mo12O51 [ | α-ZnMoO4, α-Bi2(MoO4)3, γ-Bi2MoO6, Fe2(MoO4)3 | 92 | 40 | 6 |
| BiFe0.65Ni0.05Mo[ | Bi3Mo2Fe1O12[derived α-Bi2(MoO4)3], Fe2(MoO4)3, NiMoO4 | 84 | 72 | 14 |
| BiFe0.65Co0.05Mo[ | Bi3Mo2Fe1O12[derived α-Bi2(MoO4)3], Fe2(MoO4)3, CoMoO4 | 82 | 68 | 14 |
| BiFe0.65Zn0.05Mo[ | Bi3Mo2Fe1O12[derived α-Bi2(MoO4)3], Fe2(MoO4)3, ZnMoO4 | 86 | 67 | 14 |
| BiFe0.65Mn0.05Mo[ | Bi3Mo2Fe1O12[derived α-Bi2(MoO4)3], Fe2(MoO4)3, MnMoO4 | 85 | 64 | 14 |
| BiFe0.65Cu0.05Mo[ | Bi3Mo2Fe1O12[derived α-Bi2(MoO4)3], Fe2(MoO4)3, CuMoO4 | 78 | 46 | 14 |
| 1 | 杨为民 . 碳四烃转化与利用技术研究进展及发展前景[J]. 化工进展, 2015, 34(1): 1-9. |
| YANG W M . Progress and perspectives on conversion and utilization of C4 hydrocarbons[J]. Chemical Industry and Engineering Progress, 2015, 34(1):1-9. | |
| 2 | JUNG J C , LEE H , SONG I K . Production of 1,3-butadiene from C-4 raffinate-3 through oxidative dehydrogenation of n-butene over bismuth molybdate catalysts[J]. Catalysis Surveys from Asia, 2009, 13(2): 78-93. |
| 3 | JUNG J C , KIM H , CHUNG Y , et al . Unusual catalytic behavior of beta-Bi2Mo2O9 in the oxidative dehydrogenation of n-butene to 1,3-butadiene[J]. Journal of Molecular Catalysis A: Chemical, 2007, 264(1/2): 237-240. |
| 4 | JUNG J C , KIM H , CHOI A S , et al . Preparation, characterization, and catalytic activity of bismuth molybdate catalysts for the oxidative dehydrogenation of n-butene into 1,3-butadiene[J]. ournal of Molecular Catalysis A: Chemical, 2006, 259(1/2): 166-170. |
| 5 | JUNG J C , LEE H , KIM H , et al . A synergistic effect of alpha-Bi2Mo3O12 and gamma-Bi2MoO6 catalysts in the oxidative dehydrogenation of C-4 raffinate-3 to 1,3-butadiene[J]. Journal of Molecular Catalysis A: Chemical, 2007, 271(1/2): 261-265. |
| 6 | SOARES A P V , DIMITROV L D , DE OLIVEIRA M C R A , et al . Synergy effects between beta and gamma phases of bismuth molybdates in the selective catalytic oxidation of 1-butene[J]. Applied Catalysis A: General, 2003, 253(1): 191-200. |
| 7 | WAN C , CHENG D G , CHEN F Q , et al . The role of active phase in Ce modified BiMo catalysts for oxidative dehydrogenation of 1-butene[J]. Catalysis Today, 2016, 264: 180-184. |
| 8 | WAN C , CHENG D G , CHEN F Q , et al . Characterization and kinetic study of BiMoLa x oxide catalysts for oxidative dehydrogenation of 1-butene to 1,3-butadiene[J]. Chemical Engineering Science, 2015, 135: 553-558. |
| 9 | JUNG J C , LEE H , SEO J G , et al . Oxidative dehydrogenation of n-butene to 1,3-butadiene over multicomponent bismuth molybdate (MII 9Fe3Bi1Mo12O51) catalysts: effect of divalent metal (MII)[J]. Catalysis Today, 2009, 141(3/4): 325-329. |
| 10 | MORO-OKA Y , UEDA W . Multicomponent bismuth molybdate catalyst: a highly functionalized catalyst system for the selective oxidation of olefin[J]. Advances in Catalysis, 1994, 40: 233-273. |
| 11 | WAN C , CHENG D G , CHEN F Q , et al . Effects of zirconium content on the catalytic performance of BiMoZr x in the oxidative dehydrogenation of 1-butene to 1,3-butadiene[J]. Journal of Chemical Technology & Biotechnology, 2016, 91(2): 353-358. |
| 12 | PARK J H , SHIN C H . Influence of the catalyst composition in the oxidativedehydrogenation of 1-butene over BiV x Mo1− x oxide catalysts[J]. Applied Catalysis A: General, 2015, 495: 1-7. |
| 13 | PARK J H , SHIN C H . Oxidative dehydrogenation of butenes to butadiene over Bi-Fe-Me(Me=Ni, Co, Zn, Mn and Cu)-Mo oxide catalysts[J]. Journal of Industrial and Engineering Chemistry, 2015, 21: 683-688. |
| 14 | WAN C , CHENG D G , CHEN F Q , et al . Oxidative dehydrogenation of 1-butene over vanadium modified bismuth molybdate catalyst: an insight into mechanism[J]. RSC Advances, 2015, 5(53): 42609-42615. |
| 15 | PARK J H , SHIN C H . Influence of phosphorous addition on Bi3Mo2Fe1 oxide catalysts for the oxidative dehydrogenation of 1-butene[J]. Korean Journal of Chemical Engineering, 2016, 33(3): 823-830. |
| 16 | MARS P , VAN KREVELEN D W . Oxidations carried out by means of vanadium oxide catalysts[J]. Chemical Engineering Science, 1954, 3: 41-59. |
| 17 | ZHAI Z , WANG X , LICHT R , et al . Selective oxidation and oxidative dehydrogenation of hydrocarbons on bismuth vanadium molybdenum oxide[J]. Journal of Catalysis, 2015, 325: 87-100. |
| 18 | GOLUNSKI S E , WALKER A P . Mechanism of low-temperature oxydehydrogenation of 1-butene to 1,3-butadiene over a novel Pd-Fe-O catalyst[J]. Journal of Catalysis, 2001, 204(1): 209-218. |
| 19 | CENTI G , TRIFIRO F . Some aspects of the control of selectivity in catalytic-oxidation on mixed oxides: a review[J]. Applied Catalysis, 1984, 12(1): 1-21. |
| 20 | GRASSELLI R K . Fundamental principles of selective heterogeneous oxidation atalysis[J]. Topics in Catalysis, 2002, 21(1/2/3): 79-88. |
| 21 | ROYER S , DUPREZ D , KALIAGUINE S . Oxygen mobility in LaCoO3 perovskites[J]. Catalysis Today, 2006, 112(1/2/3/4): 99-102. |
| 22 | CAVANI F , TRIFIRO F . Some aspects that affect the selective oxidation of paraffins[J]. Catalysis Today, 1997, 36: 431-439. |
| 23 | GRABOWSKI R . Kinetics of oxidative dehydrogenation of C2-C3 alkanes on oxide catalysts[J]. Catalysis Reviews, 2006, 48: 199. |
| 24 | VEDRINE J C . Acid-base characterization of heterogeneous catalysts: an up-to-date overview[J]. Research on Chemical Intermediates, 2015, 41(12): 9387-9423. |
| 25 | RAO T S R P , KRISHNAMURTHY K R . Role of iron in multicomponent molybdate catalysts for selective oxidation of propylene[J]. Journal of Catalysis, 1985, 95(1): 209-219. |
| 26 | KEULKS G W . Mechanism of oxygen atom incorporation into products of propylene oxidation over bismuth molybdate[J]. Journal of Catalysis, 1970, 19(2): 232-235. |
| 27 | BATIST P , LIPPENS B , SCHUIT G . Catalytic oxidation of 1-butene over bismuth molybdate catalysts: II. Dependence of activity and selectivity on catalyst composition[J]. Journal of Catalysis, 1966, 5(1): 55-64. |
| 28 | MATSUURA I , SCHUT R , HIRAKAWA K . The surface-structure of the active bismuth molybdate catalyst[J]. Journal of Catalysis, 1980, 63(1): 152-166. |
| 29 | BATIST P A , DER KINDEREN A H W M , LEEUWENBURGH Y , et al . Catalytic oxidation of 1-butene over bismuth molybdate catalysts: Ⅳ. Dependence of activity on structures of catalysts[J]. Journal of Catalysis, 1968, 12(1): 45-60. |
| 30 | JUNG J C , KIM H , CHOI A S , et al . Effect of pH in the preparation of gamma-Bi2MoO6 for oxidative dehydrogenation of n-butene to 1,3-butadiene: correlation between catalytic performance and oxygen mobility of gamma-Bi2MoO6 [J]. Catalysis Communications, 2007, 8(3): 625-628. |
| 31 | JUNG J C , LEE H , PARK D R , et al . Effect of calcination temperature on the catalytic performance of gamma-Bi2MoO6 in the oxidative dehydrogenation of n-butene to 1,3-butadiene[J]. Catalysis Letters, 2009, 131(3/4): 401-405. |
| 32 | JUNG J C , KIM H , KIM Y S , et al . Catalytic performance of bismuth molybdate catalysts in the oxidative dehydrogenation of C-4 raffinate-3 to 1,3-butadiene[J]. Applied Catalysis A: General, 2007, 317(2): 244-249. |
| 33 | WRAGG R D , ASHMORE P G , HOCKEY J A . Selective oxidation of propene over bismuth molybdate catalysts: the oxidation of propene using 18O labeled oxygen and catalyst[J]. Journal of Catalysis, 1971, 22(1): 49-53. |
| 34 | BRAZDIL J F , SURESH D D , GRASSELLI R K . Redox kinetics of bismuth molybdate ammoxidation catalysts[J]. Journal of Catalysis, 1980, 66(2): 347-367. |
| 35 | ERTL G , KNOZINGER H , WEITKAMP J . Handbook of heterogeneous catalysis[M]. VCH, 1997: 290. |
| 36 | VEJUX A , COURTINE P . Interfacial reactions between V2O5 and TiO2 (anatase): role of structural-properties[J]. Journal of Solid State Chemistry, 1978, 23(1/2): 93-103. |
| 37 | VAN OEFFELEN D A G , VAN HOOFF J H C , SCHUIT G C A . In situ measurements of the electrical-conductivity of bismuth molybdate catalysts in operation for oxidative dehydrogenation of butene[J]. Journal of Catalysis, 1985, 95(1): 84-100. |
| 38 | BRAZDIL J F , GLAESER L C , GRASSELLI R K . An investigation of the role of bismuth and defect cation vacancies in selective oxidation and ammoxidation catalysis[J]. Journal of Catalysis, 1983, 81(1): 142-146. |
| 39 | NOTERMANN T , KEULKS G W , SKLIAROV A , et al . Physicochemical properties of bismuth iron molybdate system[J]. Journal of Catalysis, 1975, 39(2): 286-293. |
| 40 | AYKAN K , HALVORSON D , SLEIGHT A W , et al . Olefin oxidation and ammoxidation studies over molybdate, tungstate, and vanadate catalysts having point-defects[J]. Journal of Catalysis, 1974, 35(3): 401-406. |
| 41 | BRAZDIL J F , GRASSELLI R K . Relationship between solid-state structure and catalytic activity of rare-earth and bismuth-containing molybdate ammoxidation catalysts[J]. Journal of Catalysis, 1983, 79(1): 104-117. |
| 42 | SMITH G W , IBERS J A . Crystal structure of cobalt molybdate CoMoO4 [J]. Acta Crystallographica, 1965, 19: 269-275. |
| 43 | ABRAHAMS S C , REDDY J M . Crystal structure of the transition-metal molybdates: Ⅰ. Paramagnetic alpha-MnMoO4 [J]. Journal of Chemical Physics, 1965, 43(7): 2533-2543. |
| 44 | LECIEJEWICZ J . A neutron crystallographic investigation of lead molybdenum oxide PbMoO4 [J]. Zeitschrift Fur Kristallographie, 1965, 121(2/3/4): 158-164. |
| 45 | WENG L , DELMON B . Phase cooperation and remote-control effects in selective oxidation catalysts[J]. Applied Catalysis A: General, 1992, 81(2): 141-213. |
| 46 | SCHUH K , KLEIST W , HOJ M , et al . Bismuth molybdate catalysts prepared by mild hydrothermal synthesis: influence of pH on the selective oxidation of propylene[J]. Catalysts, 2015, 5(3): 1554-1573. |
| 47 | BRAZDIL J F , TOFT M A , LIN S S Y , et al . Characterization of bismuth-cerium-molybdate selective propylene ammoxidation catalysts[J]. Applied Catalysis A: General, 2015, 495: 115-123. |
| 48 | PARK J H , NOH H , PARK J W , et al . Effects of iron content on bismuth molybdate for the oxidative dehydrogenation of n-butenes to 1,3-butadiene[J]. Applied Catalysis A: General, 2012, 431/432: 137-143. |
| 49 | PONCEBLANC H . Study of multiphasic molybdate-based catalysts: Ⅰ. Electrical-conductivity study of valence states and solubility limits in mixed iron and cobalt molybdates[J]. Journal of Catalysis, 1993, 142(2): 373-380. |
| 50 | MILLET J . Study of multiphasic molybdate-based catalysts: Ⅱ. Synergy effect between bismuth molybdates and mixed iron and cobalt molybdates in mild oxidation of propene[J]. Journal of Catalysis, 1993, 142(2): 381-391. |
| 51 | SUN Y N , TAO L , YOU T Z , et al . Effect of sulfation on the performance of Fe2O3/Al2O3 catalyst in catalytic dehydrogenation of propane to propylene[J]. Chemical Engineering Journal, 2014, 244: 145-151. |
| 52 | UEDA W , MORO-OKA Y , IKAWA T . Study of ternary-component bismuth molybdate catalysts by 18O2 tracer in the oxidation of propylene to acrolein[J]. Journal of Catalysis, 1981, 70: 409-417. |
| 53 | PARK J H , ROW K , SHIN C H . Oxidative dehydrogenation of 1-butene to 1,3-butadiene over BiFe0.65Ni x Mo oxide catalysts: effect of nickel content[J]. Catalysis Communications, 2013, 31: 76-80. |
| 54 | UEDA W , CHEN C L , ASAKAWA K , et al . Catalytic properties of tricomponent metal oxides having the scheelite structure: Ⅱ. Structural stability in the reduction oxidation cycle[J]. Journal of Catalysis, 1986, 101: 369-375. |
| 55 | UEDA W , ASAKAWA K , CHEN C L , et al . Catalytic properties of tricomponent metal oxides having the scheelite structure: Ⅰ. Role of bulk diffusion of lattice oxide ions in the oxidation of propylene[J]. Journal of Catalysis, 1986, 101: 360-368. |
| 56 | HE D , UEDA W , MORO-OKA Y . Promotion effect of molybdate support on Bi2Mo3O12 catalyst in the selective oxidative of propylene[J]. Catalysis Letters, 1992, 12(1/2/3): 35-44. |
| 57 | MATSUURA I . Active bismuth molybdate on Me2+-molybdate based catalysts[J]. Studies in Surface Science & Catalysis, 1981, 7: 1099-1112. |
| 58 | PONCEBLANC H , MILLET J M M , COUDURIER G , et al . Solid solid-phase equilibria in the binary system CoMoO4-FeMoO4 and effect of FeIII on the phase equilibria[J]. Journal of Physical Chemistry, 1992, 96(23): 9462-9465. |
| 59 | KRYLOV O V , MAKSIMOV Y V , MARGOLIS L Y . In situ study of ferric molybdate rearrangement in partial propylene oxidation[J]. Journal of Catalysis, 1985, 95(1): 289-292. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [6] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [7] | 许友好, 王维, 鲁波娜, 徐惠, 何鸣元. 中国炼油创新技术MIP的开发策略及启示[J]. 化工进展, 2023, 42(9): 4465-4470. |
| [8] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [9] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| [10] | 张启, 赵红, 荣峻峰. 质子交换膜燃料电池中氧还原反应抗毒性电催化剂研究进展[J]. 化工进展, 2023, 42(9): 4677-4691. |
| [11] | 王伟涛, 鲍婷玉, 姜旭禄, 何珍红, 王宽, 杨阳, 刘昭铁. 醛酮树脂基非金属催化剂催化氧气氧化苯制备苯酚[J]. 化工进展, 2023, 42(9): 4706-4715. |
| [12] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [13] | 常印龙, 周启民, 王青月, 王文俊, 李伯耿, 刘平伟. 废弃聚烯烃的高值化学回收研究进展[J]. 化工进展, 2023, 42(8): 3965-3978. |
| [14] | 吴海波, 王希仑, 方岩雄, 纪红兵. 3D打印催化材料开发与应用进展[J]. 化工进展, 2023, 42(8): 3956-3964. |
| [15] | 向阳, 黄寻, 魏子栋. 电催化有机合成反应的活性和选择性调控研究进展[J]. 化工进展, 2023, 42(8): 4005-4014. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||