化工进展 ›› 2019, Vol. 38 ›› Issue (03): 1259-1268.DOI: 10.16085/j.issn.1000-6613.2018-1053
生物质转化制备5-乙氧基甲基糠醛液体燃料研究进展
徐桂转1,2( ),陈炳霖1,2,张少浩1,2,郑张斌1,2,杨雨青1,王晨1,2
),陈炳霖1,2,张少浩1,2,郑张斌1,2,杨雨青1,王晨1,2
- 1. 河南农业大学机电工程学院,河南 郑州 450002
2. 生物质能源河南省协同创新中心,河南 郑州 450002
-
收稿日期:2018-05-22修回日期:2018-07-17出版日期:2019-03-05发布日期:2019-03-05 -
作者简介:徐桂转 (1972—),女,教授,主要从事生物质能源转换及利用技术研究。E-mail:xuguizhuan@126.com 。 -
基金资助:河南省基础与前沿技术研究项目(162300410007)
A review: research progress in production of 5-ethoxymethylfurfural
Guizhuan XU1,2( ),Binglin CHEN1,2,Shaohao ZHANG1,2,Zhangbin ZHENG1,2,Yuqing YANG1,Chen WANG1,2
),Binglin CHEN1,2,Shaohao ZHANG1,2,Zhangbin ZHENG1,2,Yuqing YANG1,Chen WANG1,2
- 1. College of Mechanical and Electrical Engineering, Henan Agricultural University, Zhengzhou 450002, Henan, China
2. Collaborative Innovation Center of Biomass Energy, Henan Province, Zhengzhou 450002, Henan, China
-
Received:2018-05-22Revised:2018-07-17Online:2019-03-05Published:2019-03-05
摘要:
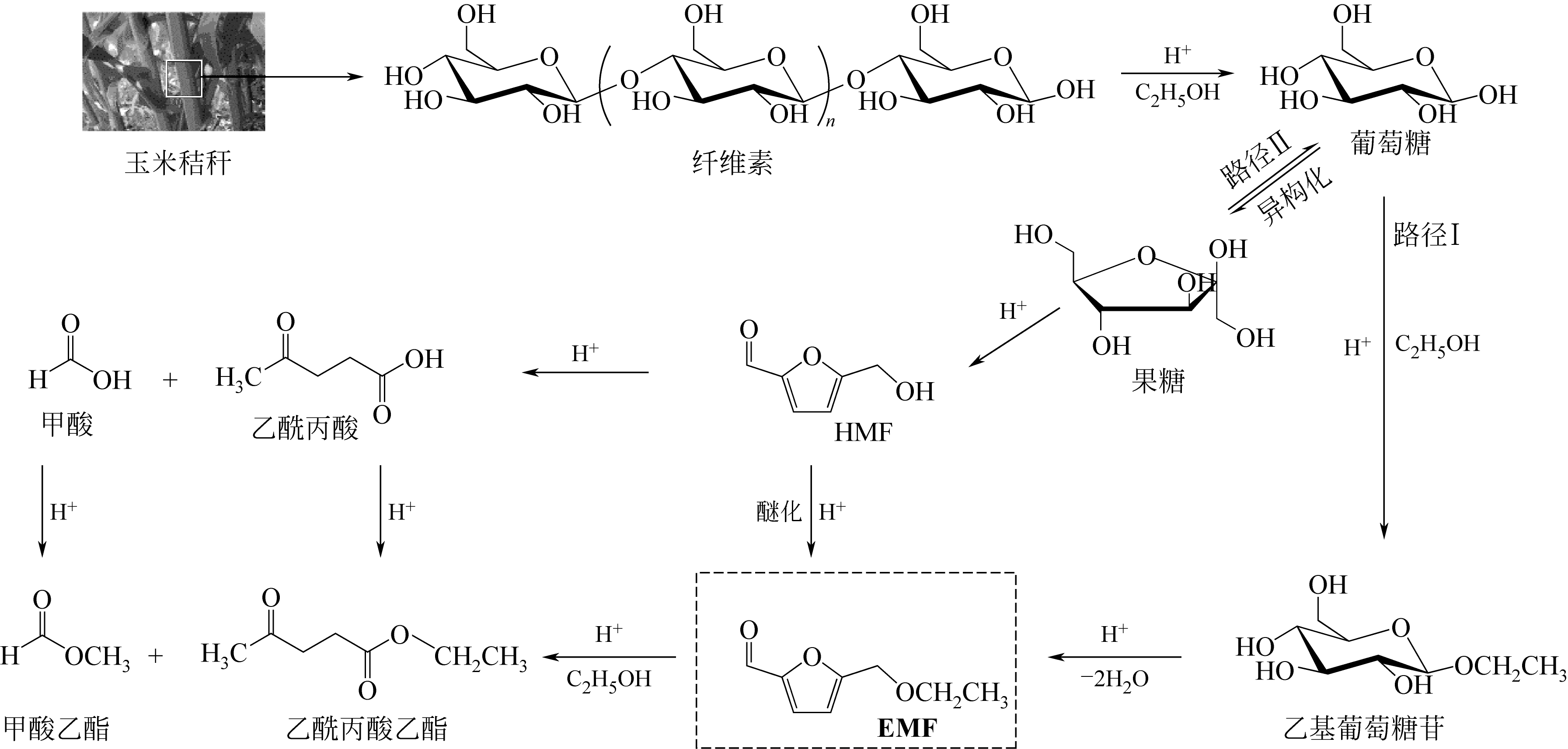
5-乙氧基甲基糠醛(5-ethoxymethylfurfural,简称EMF)是一种具有成为新一代生物燃料或者燃料添加剂潜力的高附加值化学品,具有优良的能量密度和燃烧性能。通过醇解制备EMF是生物质资源化利用的重要途径之一,也是农业废弃物生物质资源化利用的重要方式。本文介绍了EMF制取原料、催化剂及催化体系的特性,综述了糖类、农林废弃生物质等不同种类原料制取EMF的研究现状,虽然农林废弃生物质转化率较低,但具有原料成本低、来源丰富的优势。在此基础上,分类综述了无机酸、固体酸、离子液体等不同催化体系制取EMF的现状、优势和不足,无机酸存在易腐蚀、不易回收等问题;离子液体目前成本较高,固体酸则催化效率较高、易回收,极具发展潜力。提出开发以低廉生物质资源为原料直接合成EMF的技术将是未来研究的重要方向,反应高效、环境友好、经济可行的EMF生产路径是实现工业化生产的基础。
中图分类号:
引用本文
徐桂转,陈炳霖,张少浩,郑张斌,杨雨青,王晨. 生物质转化制备5-乙氧基甲基糠醛液体燃料研究进展[J]. 化工进展, 2019, 38(03): 1259-1268.
Guizhuan XU,Binglin CHEN,Shaohao ZHANG,Zhangbin ZHENG,Yuqing YANG,Chen WANG. A review: research progress in production of 5-ethoxymethylfurfural[J]. Chemical Industry and Engineering Progress, 2019, 38(03): 1259-1268.
| 序号 | 底物 | 催化剂 | 反应介质 | 温度/℃ | 时间 | 产率/% | 文献 |
|---|---|---|---|---|---|---|---|
| 1 | 菊粉 | 7.7%H2SO4 | 乙醇 | 130 | 30min | 29 | [ |
| 2 | 果糖 | 0.1%H2SO4 | 乙醇 | 120 | 1h | 79.33 | [ |
| 3 | 葡萄糖 | 0.1%H2SO4 | 乙醇 | 210 | 14min | 21.78 | [ |
| 4 | 纤维素 | 0.1%H2SO4 | 乙醇 | 200 | 80min | 14.35 | [ |
| 5 | 木薯 | 0.52%H2SO4 | 乙醇 | 200 | 6h | 2.05 | [ |
| 6 | HMF | 0.14%H3PO4 | 乙醇 | 90 | 2h | 1.7 | [ |
| 7 | HMF | 0.18%H2SO4 | 乙醇 | 90 | 2h | 79 | [ |
| 8 | 果糖 | 0.1%H2SO4 | 乙醇 | 120 | 3h | 66.29 | [ |
| 9 | HMF | 10.9%H2SO4 | 乙醇 | 70 | 18h | 79.8 | [ |
表1 无机酸催化体系制备EMF的研究结果
| 序号 | 底物 | 催化剂 | 反应介质 | 温度/℃ | 时间 | 产率/% | 文献 |
|---|---|---|---|---|---|---|---|
| 1 | 菊粉 | 7.7%H2SO4 | 乙醇 | 130 | 30min | 29 | [ |
| 2 | 果糖 | 0.1%H2SO4 | 乙醇 | 120 | 1h | 79.33 | [ |
| 3 | 葡萄糖 | 0.1%H2SO4 | 乙醇 | 210 | 14min | 21.78 | [ |
| 4 | 纤维素 | 0.1%H2SO4 | 乙醇 | 200 | 80min | 14.35 | [ |
| 5 | 木薯 | 0.52%H2SO4 | 乙醇 | 200 | 6h | 2.05 | [ |
| 6 | HMF | 0.14%H3PO4 | 乙醇 | 90 | 2h | 1.7 | [ |
| 7 | HMF | 0.18%H2SO4 | 乙醇 | 90 | 2h | 79 | [ |
| 8 | 果糖 | 0.1%H2SO4 | 乙醇 | 120 | 3h | 66.29 | [ |
| 9 | HMF | 10.9%H2SO4 | 乙醇 | 70 | 18h | 79.8 | [ |
| 序号 | 底物 | 催化剂 | 反应介质 | 温度/℃ | 时间 | 产物 | 产率/% | 文献 |
|---|---|---|---|---|---|---|---|---|
| 1 | HMF | AlCl3 | 乙醇 | 100 | 5h | EMF | 92.9 | [ |
| 2 | 果糖 | AlCl3 | 乙醇 | 100 | 11h | EMF | 71.2 | [ |
| 3 | 葡萄糖 | AlCl3 | 乙醇 | 100 | 11h | EMF | 38.4 | [ |
| 4 | 菊粉 | AlCl3 | 乙醇 | 100 | 11h | EMF | 58.2 | [ |
| 5 | 淀粉 | AlCl3 | 乙醇 | 100 | 11h | EMF | 27.2 | [ |
| 6 | 木薯 | Al2(SO4)3 | 乙醇 | 200 | 6h | EMF | 0.36 | [ |
| 7 | 木薯 | Fe2(SO4)3 | 乙醇 | 200 | 6h | EMF | 3.01 | [ |
| 8 | 木薯 | NaHSO4 | 乙醇 | 200 | 6h | EMF | 4.43 | [ |
| 9 | 木薯 | MgSO4 | 乙醇 | 200 | 6h | EMF | 0.09 | [ |
| 10 | 木薯 | ZnSO4 | 乙醇 | 200 | 6h | EMF | 3.25 | [ |
| 11 | 木薯 | NiSO4 | 乙醇 | 200 | 6h | EMF | 11.4 | [ |
| 12 | 葡萄糖 | AlCl3 | 乙醇/水 | 160 | 15min | HMF和EMF | 57 | [ |
| 13 | 果糖 | FeCl3 | 乙醇/[Bmim]Cl | 100 | 4h | EMF | 30.1 | [ |
表2 金属盐类催化体系制备EMF研究结果
| 序号 | 底物 | 催化剂 | 反应介质 | 温度/℃ | 时间 | 产物 | 产率/% | 文献 |
|---|---|---|---|---|---|---|---|---|
| 1 | HMF | AlCl3 | 乙醇 | 100 | 5h | EMF | 92.9 | [ |
| 2 | 果糖 | AlCl3 | 乙醇 | 100 | 11h | EMF | 71.2 | [ |
| 3 | 葡萄糖 | AlCl3 | 乙醇 | 100 | 11h | EMF | 38.4 | [ |
| 4 | 菊粉 | AlCl3 | 乙醇 | 100 | 11h | EMF | 58.2 | [ |
| 5 | 淀粉 | AlCl3 | 乙醇 | 100 | 11h | EMF | 27.2 | [ |
| 6 | 木薯 | Al2(SO4)3 | 乙醇 | 200 | 6h | EMF | 0.36 | [ |
| 7 | 木薯 | Fe2(SO4)3 | 乙醇 | 200 | 6h | EMF | 3.01 | [ |
| 8 | 木薯 | NaHSO4 | 乙醇 | 200 | 6h | EMF | 4.43 | [ |
| 9 | 木薯 | MgSO4 | 乙醇 | 200 | 6h | EMF | 0.09 | [ |
| 10 | 木薯 | ZnSO4 | 乙醇 | 200 | 6h | EMF | 3.25 | [ |
| 11 | 木薯 | NiSO4 | 乙醇 | 200 | 6h | EMF | 11.4 | [ |
| 12 | 葡萄糖 | AlCl3 | 乙醇/水 | 160 | 15min | HMF和EMF | 57 | [ |
| 13 | 果糖 | FeCl3 | 乙醇/[Bmim]Cl | 100 | 4h | EMF | 30.1 | [ |
| 序号 | 底物 | 催化剂 | 催化剂性质 | 反应介质 | 温度/℃ | 时间 | 产率/% | 文献 |
|---|---|---|---|---|---|---|---|---|
| 1 | HMF | 30% K-10 clay-HPW | 比表面积117m2/g | 乙醇 | 100 | 10h | 91.5 | [ |
| 2 | 果糖 | 30% K-10 clay-HPW | 乙醇 | 100 | 10h | 61.5 | [ | |
| 3 | HMF | HPW | 乙醇 | 100 | 6h | 83.1 | [ | |
| 4 | HMF | HPW(0.051mmol H+) | 比表面积926m2/g;总孔容1.47cm3/g | 乙醇 | 90 | 2h | 80.9 | [ |
| 5 | HMF | HSiW(0.051mmol H+) | 乙醇 | 90 | 2h | 85.3 | [ | |
| 6 | HMF | HSiW(0.028mmol H+) | 乙醇 | 90 | 2h | 86.5 | [ | |
| 7 | HMF | 20%HSiW/M-Ns | 乙醇 | 90 | 2h | 82.7 | [ | |
| 8 | HMF | 40%HSiW/M-Ns | 乙醇 | 90 | 2h | 85.8 | [ | |
| 9 | HMF | 40%HSiW/M-Ns | 乙醇 | 90 | 4h | 84.1 | [ | |
| 10 | HMF | 60%HSiW/M-Ns | 乙醇 | 90 | 2h | 83.2 | [ | |
| 11 | HMF | [MIMBS]3PW12O40 | 乙醇 | 70 | 24h | 90.7 | [ | |
| 12 | 果糖 | [MIMBS]3PW12O40 | 乙醇 | 90 | 24h | 90.5 | [ | |
| 13 | 果糖 | HPW | 乙醇/DMSO① | 140 | 130min | 64 | [ | |
| 14 | 蔗糖 | HPW | 乙醇/DMSO | 140 | 130min | 28 | [ | |
| 15 | 菊粉 | HPW | 乙醇/DMSO | 140 | 130min | 54 | [ | |
| 16 | HMF | Fe3O4@SiO2-HPW | 比表面积27.6m2/g;孔容0.064cm3/g;平均孔径1.85 nm | 乙醇 | 100 | 11h | 84 | [ |
| 17 | HMF | Fe3O4@SiO2-HPW | 乙醇 | 100 | 24h | 55 | [ | |
| 18 | HMF | Ag1H2PW | 乙醇 | 100 | 10h | 88.7 | [ | |
| 19 | HMF | 40%MCM-41-HPW | 比表面积998.2m2/g;总孔容0.87cm3/g | 乙醇 | 100 | 12h | 83.4 | [ |
| 20 | 果糖 | 40%MCM-41-HPW | 乙醇 | 100 | 12h | 42.9 | [ | |
| 21 | 果糖 | H3PW12O40 | 乙醇/THF② | 130 | 30min | 76 | [ | |
| 22 | 蔗糖 | H3PW12O40 | 乙醇/THF | 130 | 30min | 33 | [ | |
| 23 | 菊粉 | H3PW12O40 | 乙醇/THF | 130 | 30min | 62 | [ |
表3 杂多酸类催化体系制备EMF研究结果
| 序号 | 底物 | 催化剂 | 催化剂性质 | 反应介质 | 温度/℃ | 时间 | 产率/% | 文献 |
|---|---|---|---|---|---|---|---|---|
| 1 | HMF | 30% K-10 clay-HPW | 比表面积117m2/g | 乙醇 | 100 | 10h | 91.5 | [ |
| 2 | 果糖 | 30% K-10 clay-HPW | 乙醇 | 100 | 10h | 61.5 | [ | |
| 3 | HMF | HPW | 乙醇 | 100 | 6h | 83.1 | [ | |
| 4 | HMF | HPW(0.051mmol H+) | 比表面积926m2/g;总孔容1.47cm3/g | 乙醇 | 90 | 2h | 80.9 | [ |
| 5 | HMF | HSiW(0.051mmol H+) | 乙醇 | 90 | 2h | 85.3 | [ | |
| 6 | HMF | HSiW(0.028mmol H+) | 乙醇 | 90 | 2h | 86.5 | [ | |
| 7 | HMF | 20%HSiW/M-Ns | 乙醇 | 90 | 2h | 82.7 | [ | |
| 8 | HMF | 40%HSiW/M-Ns | 乙醇 | 90 | 2h | 85.8 | [ | |
| 9 | HMF | 40%HSiW/M-Ns | 乙醇 | 90 | 4h | 84.1 | [ | |
| 10 | HMF | 60%HSiW/M-Ns | 乙醇 | 90 | 2h | 83.2 | [ | |
| 11 | HMF | [MIMBS]3PW12O40 | 乙醇 | 70 | 24h | 90.7 | [ | |
| 12 | 果糖 | [MIMBS]3PW12O40 | 乙醇 | 90 | 24h | 90.5 | [ | |
| 13 | 果糖 | HPW | 乙醇/DMSO① | 140 | 130min | 64 | [ | |
| 14 | 蔗糖 | HPW | 乙醇/DMSO | 140 | 130min | 28 | [ | |
| 15 | 菊粉 | HPW | 乙醇/DMSO | 140 | 130min | 54 | [ | |
| 16 | HMF | Fe3O4@SiO2-HPW | 比表面积27.6m2/g;孔容0.064cm3/g;平均孔径1.85 nm | 乙醇 | 100 | 11h | 84 | [ |
| 17 | HMF | Fe3O4@SiO2-HPW | 乙醇 | 100 | 24h | 55 | [ | |
| 18 | HMF | Ag1H2PW | 乙醇 | 100 | 10h | 88.7 | [ | |
| 19 | HMF | 40%MCM-41-HPW | 比表面积998.2m2/g;总孔容0.87cm3/g | 乙醇 | 100 | 12h | 83.4 | [ |
| 20 | 果糖 | 40%MCM-41-HPW | 乙醇 | 100 | 12h | 42.9 | [ | |
| 21 | 果糖 | H3PW12O40 | 乙醇/THF② | 130 | 30min | 76 | [ | |
| 22 | 蔗糖 | H3PW12O40 | 乙醇/THF | 130 | 30min | 33 | [ | |
| 23 | 菊粉 | H3PW12O40 | 乙醇/THF | 130 | 30min | 62 | [ |
| 序号 | 底物 | 催化剂 | 催化剂性质 | 反应介质 | 温度/℃ | 时间/h | 产率/% | 文献 |
|---|---|---|---|---|---|---|---|---|
| 1 | HMF | Silica-SO3H | 乙醇 | 100 | 10 | 83.8 | [ | |
| 2 | 果糖 | Silica-SO3H | 乙醇 | 100 | 24 | 63.1 | [ | |
| 3 | HMF | 磺酸纤维素 | 硫含量0.56mmol/g | 乙醇 | 100 | 10 | 84.4 | [ |
| 4 | 果糖 | 磺酸纤维素 | 乙醇 | 100 | 12 | 72.5 | [ | |
| 5 | 果糖 | OMC-SO3H | [H+]含量为1.31mmol/g,比表面积515m2/g,孔容0.98cm3/g | 乙醇 | 140 | 24 | 55.7 | [ |
| 6 | 菊粉 | OMC-SO3H | 乙醇 | 140 | 24 | 53.6 | [ | |
| 7 | 蔗糖 | OMC-SO3H | 乙醇 | 140 | 24 | 26.8 | [ | |
| 8 | HMF | 10% Glu-Fe3O4-SO3H | 乙醇 | 80 | 12 | 28 | [ | |
| 9 | HMF | 20% Glu-Fe3O4-SO3H | 乙醇 | 80 | 8 | 70 | [ | |
| 10 | HMF | 30% Glu-Fe3O4-SO3H | 乙醇 | 80 | 2 | 92 | [ | |
| 11 | HMF | 50% Glu-Fe3O4-SO3H | 乙醇 | 80 | 2 | 92 | [ | |
| 12 | 果糖 | 30% Glu-Fe3O4-SO3H | 乙醇 | 80 | 24 | 55 | [ | |
| 13 | 果糖 | 50% Glu-Fe3O4-SO3H | 乙醇 | 80 | 24 | 81 | [ | |
| 14 | 葡萄糖 | 50% Glu-Fe3O4-SO3H | 乙醇/DMSO (体积比2∶8) | 140 | 48 | 27 | [ | |
| 15 | 菊粉 | 50% Glu-Fe3O4-SO3H | 乙醇/DMSO (体积比2∶8) | 100 | 24 | 85 | [ | |
| 16 | HMF | Fe3O4@C-SO3H | 硫含量1.38mmol/g,[H+]含量为1.40mmol/g,比表面积 29.9m2/g;孔容0.07cm3/g,孔径9.4nm | 乙醇 | 100 | 12 | 88.4 | [ |
| 17 | 果糖 | Fe3O4@C-SO3H | 乙醇 | 140 | 24 | 67.8 | [ | |
| 18 | 菊粉 | Fe3O4@C-SO3H | 乙醇 | 140 | 24 | 58.4 | [ | |
| 19 | 蔗糖 | Fe3O4@C-SO3H | 乙醇 | 140 | 24 | 33.2 | [ | |
| 20 | 果糖 | MIL-101-SO3H (100) | 乙醇 | 130 | 15 | 67.7 | [ | |
| 21 | 菊粉 | MIL-101-SO3H (100) | 乙醇 | 130 | 15 | 54.2 | [ |
表4 磺酸类固体酸催化转化制备EMF研究结果
| 序号 | 底物 | 催化剂 | 催化剂性质 | 反应介质 | 温度/℃ | 时间/h | 产率/% | 文献 |
|---|---|---|---|---|---|---|---|---|
| 1 | HMF | Silica-SO3H | 乙醇 | 100 | 10 | 83.8 | [ | |
| 2 | 果糖 | Silica-SO3H | 乙醇 | 100 | 24 | 63.1 | [ | |
| 3 | HMF | 磺酸纤维素 | 硫含量0.56mmol/g | 乙醇 | 100 | 10 | 84.4 | [ |
| 4 | 果糖 | 磺酸纤维素 | 乙醇 | 100 | 12 | 72.5 | [ | |
| 5 | 果糖 | OMC-SO3H | [H+]含量为1.31mmol/g,比表面积515m2/g,孔容0.98cm3/g | 乙醇 | 140 | 24 | 55.7 | [ |
| 6 | 菊粉 | OMC-SO3H | 乙醇 | 140 | 24 | 53.6 | [ | |
| 7 | 蔗糖 | OMC-SO3H | 乙醇 | 140 | 24 | 26.8 | [ | |
| 8 | HMF | 10% Glu-Fe3O4-SO3H | 乙醇 | 80 | 12 | 28 | [ | |
| 9 | HMF | 20% Glu-Fe3O4-SO3H | 乙醇 | 80 | 8 | 70 | [ | |
| 10 | HMF | 30% Glu-Fe3O4-SO3H | 乙醇 | 80 | 2 | 92 | [ | |
| 11 | HMF | 50% Glu-Fe3O4-SO3H | 乙醇 | 80 | 2 | 92 | [ | |
| 12 | 果糖 | 30% Glu-Fe3O4-SO3H | 乙醇 | 80 | 24 | 55 | [ | |
| 13 | 果糖 | 50% Glu-Fe3O4-SO3H | 乙醇 | 80 | 24 | 81 | [ | |
| 14 | 葡萄糖 | 50% Glu-Fe3O4-SO3H | 乙醇/DMSO (体积比2∶8) | 140 | 48 | 27 | [ | |
| 15 | 菊粉 | 50% Glu-Fe3O4-SO3H | 乙醇/DMSO (体积比2∶8) | 100 | 24 | 85 | [ | |
| 16 | HMF | Fe3O4@C-SO3H | 硫含量1.38mmol/g,[H+]含量为1.40mmol/g,比表面积 29.9m2/g;孔容0.07cm3/g,孔径9.4nm | 乙醇 | 100 | 12 | 88.4 | [ |
| 17 | 果糖 | Fe3O4@C-SO3H | 乙醇 | 140 | 24 | 67.8 | [ | |
| 18 | 菊粉 | Fe3O4@C-SO3H | 乙醇 | 140 | 24 | 58.4 | [ | |
| 19 | 蔗糖 | Fe3O4@C-SO3H | 乙醇 | 140 | 24 | 33.2 | [ | |
| 20 | 果糖 | MIL-101-SO3H (100) | 乙醇 | 130 | 15 | 67.7 | [ | |
| 21 | 菊粉 | MIL-101-SO3H (100) | 乙醇 | 130 | 15 | 54.2 | [ |
| 序号 | 底物 | 催化剂 | 反应介质 | 温度/℃ | 时间 | 产率/% | 文献 |
|---|---|---|---|---|---|---|---|
| 1 | 0.5mmol果糖 | 1g [C4mim][HSO4] | 乙醇(2.5mL) | 130 | 20min | 79 | [ |
| 2 | 0.5mmol果糖 | 1g [C1im][HSO4] | 乙醇(2.5mL) | 130 | 20min | 73 | [ |
| 3 | 0.5mmol果糖 | 1g [C2mim][HSO4] | 乙醇(2.5mL) | 130 | 20min | 54 | [ |
| 4 | 0.5mmol果糖 | 1g [C4mim][Cl] | 乙醇(2.5mL) | 130 | 20min | 0.2 | [ |
| 5 | 0.5mmol果糖 | 1g [C4mim][DMP] | 乙醇(2.5mL) | 130 | 20min | 2 | [ |
| 6 | 0.5mmol果糖 | 1g [C4mim][DEP] | 乙醇(2.5mL) | 130 | 20min | 4 | [ |
| 7 | 0.5mmol果糖 | 1g [C4mim][AC] | 乙醇(2.5mL) | 130 | 20min | 0 | [ |
| 8 | 0.5mmol果糖 | 1g [C4mim][AC] | 乙醇(2.5mL) | 130 | 20min | 0 | [ |
| 9 | 0.5mmol果糖 | 1g [C4mim][HSO4] | 乙醇(2.5mL) | 130 | 20min | 83 | [ |
| 10 | 0.5mmol果糖 | 1g [C4im][HSO4] | 乙醇(2.5mL) | 130 | 15min | 77 | [ |
| 11 | 0.5mmol果糖 | 1g [C2mim][HSO4] | 乙醇(2.5mL) | 130 | 30min | 81 | [ |
| 12 | HMF | Fe3O4@SiO2-SH-Im-HSO4 | 乙醇 | 100 | 12h | 89.6 | [ |
| 13 | 果糖 | Fe3O4@SiO2-SH-Im-HSO4 | 乙醇 | 120 | 24h | 60.4 | [ |
| 14 | 蔗糖 | Fe3O4@SiO2-SH-Im-HSO4 | 乙醇 | 120 | 24h | 34.4 | [ |
| 15 | 菊粉 | Fe3O4@SiO2-SH-Im-HSO4 | 乙醇 | 120 | 24h | 56.1 | [ |
| 16 | 0.09g 菊粉 | 1g [BMIM][HSO4] | 乙醇/水(2.5mL/0.02mL) | 130 | 30min | 77 | [ |
| 17 | 0.09g 菊粉 | 1g [EMIM][HSO4] | 乙醇/水(2.5mL/0.02mL) | 130 | 30min | 51 | [ |
| 18 | 0.09g 菊粉 | 1g [BMIM][HSO4] | 乙醇/水(2.5mL/0.02mL) | 130 | 30min | 63 | [ |
| 19 | 0.09g 菊粉 | 2.5g [BMIM][HSO4] | 乙醇(2.5g) | 130 | 30min | 77 | [ |
| 20 | 蔗糖 | 1g [EMIM][HSO4] | 乙醇/水(2.5mL/0.02mL) | 130 | 30min | 43 | [ |
表5 离子液体合成EMF的研究结果
| 序号 | 底物 | 催化剂 | 反应介质 | 温度/℃ | 时间 | 产率/% | 文献 |
|---|---|---|---|---|---|---|---|
| 1 | 0.5mmol果糖 | 1g [C4mim][HSO4] | 乙醇(2.5mL) | 130 | 20min | 79 | [ |
| 2 | 0.5mmol果糖 | 1g [C1im][HSO4] | 乙醇(2.5mL) | 130 | 20min | 73 | [ |
| 3 | 0.5mmol果糖 | 1g [C2mim][HSO4] | 乙醇(2.5mL) | 130 | 20min | 54 | [ |
| 4 | 0.5mmol果糖 | 1g [C4mim][Cl] | 乙醇(2.5mL) | 130 | 20min | 0.2 | [ |
| 5 | 0.5mmol果糖 | 1g [C4mim][DMP] | 乙醇(2.5mL) | 130 | 20min | 2 | [ |
| 6 | 0.5mmol果糖 | 1g [C4mim][DEP] | 乙醇(2.5mL) | 130 | 20min | 4 | [ |
| 7 | 0.5mmol果糖 | 1g [C4mim][AC] | 乙醇(2.5mL) | 130 | 20min | 0 | [ |
| 8 | 0.5mmol果糖 | 1g [C4mim][AC] | 乙醇(2.5mL) | 130 | 20min | 0 | [ |
| 9 | 0.5mmol果糖 | 1g [C4mim][HSO4] | 乙醇(2.5mL) | 130 | 20min | 83 | [ |
| 10 | 0.5mmol果糖 | 1g [C4im][HSO4] | 乙醇(2.5mL) | 130 | 15min | 77 | [ |
| 11 | 0.5mmol果糖 | 1g [C2mim][HSO4] | 乙醇(2.5mL) | 130 | 30min | 81 | [ |
| 12 | HMF | Fe3O4@SiO2-SH-Im-HSO4 | 乙醇 | 100 | 12h | 89.6 | [ |
| 13 | 果糖 | Fe3O4@SiO2-SH-Im-HSO4 | 乙醇 | 120 | 24h | 60.4 | [ |
| 14 | 蔗糖 | Fe3O4@SiO2-SH-Im-HSO4 | 乙醇 | 120 | 24h | 34.4 | [ |
| 15 | 菊粉 | Fe3O4@SiO2-SH-Im-HSO4 | 乙醇 | 120 | 24h | 56.1 | [ |
| 16 | 0.09g 菊粉 | 1g [BMIM][HSO4] | 乙醇/水(2.5mL/0.02mL) | 130 | 30min | 77 | [ |
| 17 | 0.09g 菊粉 | 1g [EMIM][HSO4] | 乙醇/水(2.5mL/0.02mL) | 130 | 30min | 51 | [ |
| 18 | 0.09g 菊粉 | 1g [BMIM][HSO4] | 乙醇/水(2.5mL/0.02mL) | 130 | 30min | 63 | [ |
| 19 | 0.09g 菊粉 | 2.5g [BMIM][HSO4] | 乙醇(2.5g) | 130 | 30min | 77 | [ |
| 20 | 蔗糖 | 1g [EMIM][HSO4] | 乙醇/水(2.5mL/0.02mL) | 130 | 30min | 43 | [ |
| 1 | LI H , FANG Z , SMITH R L , et al . Efficient valorization of biomass to biofuels with bifunctional solid catalytic materials[J]. Progress in Energy & Combustion Science, 2016, 55:98-194. |
| 2 | RAGAUSKAS A J , WILLIAMS C K , DAVISON B H , et al . The path forward for biofuels and biomaterials[J]. Science, 2006, 311(5760):484-489. |
| 3 | CORMA A , IBORRA S , VELTY A . Chemical routes for the transformation of biomass into chemicals[J]. Chemical Reviews, 2007, 38(36):2411-2502. |
| 4 | LIU B , GOU Z Z , LIU A Q , et al . Synthesis of furan compounds from HMF and fructose catalyzed by aluminum-exchanged K-10 clay[J]. Journal of Industrial & Engineering Chemistry, 2015, 21(1):338-339. |
| 5 | VIIL I , BREDIHHIN A , MÄEORG U , et al . Preparation of potential biofuel 5-ethoxymethylfurfural and other 5-alkoxymethylfurfurals in the presence of oil shale ash[J]. RSC Advances, 2014, 4(11):5689-5693. |
| 6 | 刘波, 张岩, 马明, 等 . 平台化合物5-乙氧甲基糠醛的最新研究进展[J]. 山东化工, 2015, 44(9): 49-52. |
| LIU B , ZHANG Y , MA M, et al . Advances in bio-based platform chemical 5-ethoxymethylfurfural[J]. Shandong Chemical Industry, 2015, 44(9): 49-52. | |
| 7 | 陈涛, 彭林才 . 新型生物燃料5-乙氧基甲基糠醛的合成进展[J]. 化学通报, 2018, 81(1): 45-51. |
| CHEN T , PENG L C . Advances in the synthesis of novel biofuel 5-ethoxymethylfurfural[J]. Chemistry, 2018, 81(1): 45-51. | |
| 8 | HU L , LIN L , WU Z , et al . Recent advances in catalytic transformation of biomass-derived 5-hydroxymethylfurfural into the innovative fuels and chemicals[J]. Renewable & Sustainable Energy Reviews, 2017, 74:230-257. |
| 9 | KRAUS G A , GUNEY T . A direct synthesis of 5-alkoxymethylfurfural ethers from fructose via sulfonic acid-functionalized ionic liquids[J]. Green Chemistry, 2012, 14(6):1593-1596. |
| 10 | GUO H X , DUEREH A , HIRAGA Y , et al . Perfect recycle and mechanistic role of hydrogen sulfate ionic liquids as additive in ethanol for efficient conversion of carbohydrates into 5-ethoxymethylfurfural[J]. Chemical Engineering Journal, 2017,323(9):287-294. |
| 11 | LI H , FANG Z , SMITH R L , et al . Efficient valorization of biomass to biofuels with bifunctional solid catalytic materials[J]. Progress in Energy & Combustion Science, 2016, 55:98-194. |
| 12 | LIU A Q , LIU B , WANG Y M , et al . Efficient one-pot synthesis of 5-ethoxymethylfurfural from fructose catalyzed by heteropolyacid supported on K-10 clay[J]. Fuel, 2014, 117(1):68-73. |
| 13 | KUMARI P K , RAO B S , PADMAKAR D , et al . Lewis acidity induced heteropoly tungustate catalysts for the synthesis of 5-ethoxymethyl furfural from fructose and 5-hydroxymethylfurfural[J]. Molecular Catalysis, 2018, 448:108-115. |
| 14 | LIU B , ZHANG Z H , HUANG K C . Cellulose sulfuric acid as a bio-supported and recyclable solid acid catalyst for the synthesis of 5-hydroxymethylfurfural and 5-ethoxymethylfurfural from fructose[J]. Cellulose, 2013, 20(4):2081-2089. |
| 15 | BALAKRISHNAN M , SACIA E R , BELL A . Etherification and reductive etherification of 5-(hydroxymethyl)furfural: 5-(alkoxymethyl)furfurals and 2,5-bis(alkoxymethyl)furans as potential bio-diesel candidates[J]. Green Chemistry, 2012, 14(6):1626-1634. |
| 16 | LEW C M, RAJABBEIGI N , TSAPATSIS M . One-pot synthesis of 5-(ethoxymethyl)furfural from glucose using Sn-BEA and Amberlyst catalysts[J]. Industrial & Engineering Chemistry Research, 2012, 51(14): 5364- 5366. |
| 17 | LIU B , ZHANG Z H , HUANG K C , et al . Efficient conversion of carbohydrates into 5-ethoxymethylfurfural in ethanol catalyzed by AlCl3 [J]. Fuel, 2013, 113(2): 625-631. |
| 18 | XU G Z , CHANG C , FANG S Q , et al . Cellulose reactivity in ethanol at elevate temperature and the kinetics of one-pot preparation of ethyl levulinate from cellulose[J]. Renewable Energy, 2015, 78: 583-589. |
| 19 | YIN S S , SUN J , LIU B , et al . Magnetic material grafted cross-linked imidazolium based polyionic liquids: an efficient acid catalyst for the synthesis of promising liquid fuel 5-ethoxymethylfurfural from carbohydrates[J]. Journal of Materials Chemistry A, 2015, 3(9): 4992-4999. |
| 20 | CHEN T , PENG L C , YU X , et al . Magnetically recyclable cellulose-derived carbonaceous solid acid catalyzed the biofuel 5-ethoxymethylfurfural synthesis from renewable carbohydrates[J]. Fuel, 2018, 219: 344-352. |
| 21 | BREDIHHIN A , MÄEORG U , VARES L . Evaluation of carbohydrates and lignocellulosic biomass from different wood species as raw material for the synthesis of 5-bromomethyfurfural[J]. Carbohydrate Research, 2013, 375(12): 63-67. |
| 22 | MASCAL M , NIKITIN E B . Direct, high-yield conversion of cellulose into biofuel[J]. Angewandte Chemie: International Edition, 2008,47(41): 7924-7926. |
| 23 | XU G Z , CHANG C , ZHU W N , et al . A comparative study on direct production of ethyl levulinate from glucose in ethanol media catalysed by different acid catalysts[J]. Chemical Papers, 2013, 67(11):1355-1363. |
| 24 | LI H , ZHANG Q Y , RIISAGER A , et al . Catalytic valorization of cellulose and cellobiose with nanoparticles[J]. Current Nanoscience, 2015, 11(1):1-14. |
| 25 | 李凯 . 一锅法催化生物质制取5-乙氧基甲基糠醛试验研究[D]. 郑州: 河南农业大学, 2016. |
| LI K . One-pot synthesis of 5-ethoxymethylfurfural from carbohydrate catalyzed[D]. Zhangzhou: Henan Agricultural University, 2016. | |
| 26 | DUTTA S , DE S, ALAM M I , et al . Direct conversion of cellulose and lignocellulosic biomass into chemicals and biofuel with metal chloride catalysts[J]. Journal of Catalysis, 2012, 288(2): 8-15. |
| 27 | TAN J , LIU Q Y , CHEN L G , et al . Efficient production of ethyl levulinate from cassava over Al2(SO4)3 catalyst in ethanol-water system[J]. Journal of Energy Chemistry, 2017, 26(1): 115-120. |
| 28 | CHANG C , XU G X , JIANG X X . Production of ethyl levulinate by direct conversion of wheat straw in ethanol media[J]. Bioresource Technology, 2012, 121(10): 93-99. |
| 29 | SUN Y N , ZHANG Q Q , ZHANG P P , et al . Nitrogen-doped carbon-based acidic ionic liquid hollow nanospheres for efficient and selective conversion of fructose to 5-ethoxymethylfurfural and ethyl levulinate[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(5): 6771-6782. |
| 30 | TARABANKO V . SMIRNOVA M A, CHERNYAK M. Investigation of acid-catalytic conversion of carbohydrates in the presence of aliphatic alcohols at mild temperatures[J]. Chemistry for Sustainable Development, 2005, 13: 551-558. |
| 31 | CHE P H , LU F , ZHANG J J , et al . Catalytic selective etherification of hydroxyl groups in 5-hydroxymethylfurfural over H4SiW12O40/MCM-41 nanospheres for liquid fuel production[J]. Bioresource Technology, 2012, 119(3): 433-436. |
| 32 | XU G Z , CHEN B L , ZHENG Z B , et al . One-pot ethanolysis of carbohydrates to promising biofuels: 5-ethoxymethylfurfural and ethyl levulinate: One-pot ethanolysis of carbohydrates to biofuels[J]. Asia-Pacific Journal of Chemical Engineering, 2017, 12(4): 527-535. |
| 33 | LIU B , ZHANG Z H , DENG K J . Efficient one-pot synthesis of 5-(ethoxymethyl)furfural from fructose catalyzed by a novel solid catalyst[J]. Industrial & Engineering Chemistry Research, 2012, 51(47):15331-15336. |
| 34 | FLANNELLY T , DOOLEY S , LEAHY J J . Reaction pathway analysis of ethyl levulinate and 5-ethoxymethylfurfural from D-fructose acid hydrolysis in ethanol[J]. Energy & Fuels, 2015, 29(11): 7554-7565. |
| 35 | XIANG B , WANG Y , QI T , et al . Promotion catalytic role of ethanol on brønsted acid for the sequential dehydration-etherification of fructose to 5-ethoxymethylfurfural[J]. Journal of Catalysis, 2017, 352: 586-598. |
| 36 | GARVES K . Acid catalyzed degradation of cellulose in alcohols[J]. Journal of Wood Chemistry & Technology, 1988, 8(1): 121-134. |
| 37 | HISHIKAWA Y , YAMAGUCHI M , KUBO S , et al . Direct preparation of butyl levulinate by a single solvolysis process of cellulose[J]. Journal of Wood Science, 2013, 59(2): 179-182. |
| 38 | LI H , PENG L C , LIN L , et al . Synthesis, isolation and characterization of methyl levulinate from cellulose catalyzed by extremely low concentration acid[J]. Journal of Energy Chemistry, 2013, 22(6):895-901. |
| 39 | CHANG J L , BAI J , CHANG C , et al . Products distribution of glucose through ethanolysis reaction catalyzed by extremely low acid under high temperature[J]. Chemistry & Industry of Forest Products, 2015, 35(6): 8-14. |
| 40 | ZHANG Z H , HUBER G W . Catalytic oxidation of carbohydrates into organic acids and furan chemicals[J]. Chemical Society Reviews, 2018, 47(4): 1351-1390. |
| 41 | YANG Y , HU C W , ABU-OMAR M M . Conversion of glucose into furans in the presence of AlCl3 in an ethanol–water solvent system[J]. Bioresource Technology, 2012, 116(7): 190-194. |
| 42 | ZHOU X M , ZHANG Z H , LIU B , et al . Catalytic conversion of fructose into furans using FeCl3 as catalyst[J]. Journal of Industrial & Engineering Chemistry, 2014, 20(2): 644-649. |
| 43 | WANG H L , DENG T S , WANG Y X , et al . Efficient catalytic system for the conversion of fructose into 5-ethoxymethylfurfural[J]. Bioresource Technology, 2013, 136(5): 394-400. |
| 44 | WANG S G , ZHANG Z H , LIU B , al et , Silica coated magnetic Fe 3O 4 nanoparticles supported phosphotungstic acid: a novel environmentally friendly catalyst for the synthesis of 5-ethoxymethylfurfural from 5-hydroxymethylfurfural and fructose[J]. Catalysis Science & Technology, 2013, 3(8): 2104-2112. |
| 45 | REN Y S , LIU B , ZHANG Z H , et al . Silver-exchanged heteropolyacid catalyst (Ag1H2PW): an efficient heterogeneous catalyst for the synthesis of 5-ethoxymethylfurfural from 5-hydroxymethylfurfural and fructose[J]. Journal of Industrial & Engineering Chemistry, 2015, 21(1):1127-1131. |
| 46 | LIU A Q , ZHANG Z H , FANG Z F , et al . Synthesis of 5-ethoxymethylfurfural from 5-hydroxymethylfurfural and fructose in ethanol catalyzed by MCM-41 supported phosphotungstic acid[J]. Journal of Industrial & Engineering Chemistry, 2014, 20(4):1977-1984. |
| 47 | YANG Y , ABU-OMAR M M , HU C W . Heteropolyacid catalyzed conversion of fructose, sucrose, and inulin to 5-ethoxymethylfurfural, a liquid biofuel candidate[J]. Applied Energy, 2012, 99(2): 80-84. |
| 48 | LIU B , ZHANG Z H . One-pot conversion of carbohydrates into 5-ethoxymethylfurfural and ethyl D-glucopyranoside in ethanol catalyzed by a silica supported sulfonic acid catalyst[J]. RSC Advances, 2013, 3(30): 12313-12319. |
| 49 | LIU B , ZHANG Z H , HUANG K C . Cellulose sulfuric acid as bio-supported and recyclable solid acid catalyst for synthesis of 5-hydroxymethylfurfural and 5-ethoxymethylfurfural from fructose[J]. Cellulose, 2013, 20(4): 2081-2089. |
| 50 | LIU X F , LI H , PAN H , et al . Efficient catalytic conversion of carbohydrates into 5-ethoxymethylfurfural over MIL-101-based sulfated porous coordination polymers[J]. Journal of Energy Chemistry, 2016, 25(3): 523-530. |
| 51 | ZHONG R Y , YU F , SCHUTYSER W , et al . Acidic mesostructured silica-carbon nanocomposite catalysts for biofuels and chemicals synthesis from sugars in alcoholic solutions[J]. Applied Catalysis B:Environmental, 2016, 206: 74-88. |
| 52 | WANG J M , ZHANG Z H , JIN S W , et al . Efficient conversion of carbohydrates into 5-hydroxylmethylfurfan and 5-ethoxymethylfurfural over sulfonic acid-functionalized mesoporous carbon catalyst[J]. Fuel, 2017, 192: 102-107. |
| 53 | THOMBAL R S , JADHAV V H . Application of glucose derived magnetic solid acid for etherification of 5-HMF to 5-EMF, dehydration of sorbitol to isosorbide, and esterification of fatty acids[J]. Tetrahedron Letters, 2016, 57(39): 4398-4400. |
| 54 | YUAN Z L , ZHANG Z H , ZHENG J D , et al . Efficient synthesis of promising liquid fuels 5-ethoxymethylfurfural from carbohydrates[J]. Fuel, 2015, 150: 236-242. |
| 55 | LIU X F , LI H , PAN H , et al . Efficient catalytic conversion of carbohydrates into 5-ethoxymethylfurfural over MIL-101-based sulfated porous coordination polymers[J]. Journal of Energy Chemistry, 2016, 25(3): 523-530. |
| 56 | MORALES G , PANIAGUA M , MELERO J A , et al . Efficient production of 5-ethoxymethylfurfural from fructose by sulfonic mesostructured silica using DMSO as co-solvent[J]. Catalysis Today, 2017, 279: 305-316. |
| 57 | LANZAFAME P , TEMI D M , PERATHONER S , et al . Etherification of 5-hydroxymethyl-2-furfural(HMF) with ethanol to biodiesel components using mesoporous solid acidic catalysts[J]. Catalysis Today, 2011, 175(1): 435-441. |
| 58 | 张秋云, 蔡杰, 张玉涛, 等 . 基于生物质转化制备5-乙氧基甲基糠醛研究进展[J]. 精细石油化工, 2015, 32(1): 42-47. |
| ZHANG Q Y , CAI J , ZHANG Y T , et al . Recent advances in conversion of biomass to novel platform chemical 5-ethoxymethylfurfural[J]. Speciality Petrochemicals, 2015, 32(1): 42-47. | |
| 59 | TAKAGAKI A , OHARA M , NISHIMURA S , et al . A one-pot reaction for biorefinery: combination of solid acid and base catalysts for direct production of 5-hydroxymethylfurfural from saccharides[J]. Cheminform, 2010, 41(10): 6276-6278. |
| 60 | LI H , GOVIND K S , KOTNI R , et al . Direct catalytic transformation of carbohydrates into 5-ethoxymethylfurfural with acid-base bifunctional hybrid nanospheres.[J]. Energy Conversion & Management, 2014, 88:1245-1251. |
| 61 | POLIAKOOFF M , FITZPATRICK J M , FARREN T R , et al . Green chemistry: science and politics of change[J]. Science, 2002, 297(5582):807-810. |
| 62 | WANG H , GURAU G , ROGERS R D . Ionic liquid processing of cellulose[J]. Chemical Society Reviews, 2012, 41(4): 1519-1537. |
| 63 | QI X H , WATANABE M , AIDA T M , et al . Efficient one-pot production of 5-hydroxymethylfurfural from inulin in ionic liquids[J]. Green Chemistry, 2010, 12(10): 1855-1860. |
| 64 | COLE A C , JENSEN J L , NTAI I , et al . Novel brønsted acidic ionic liquids and their use as dual solvent-catalysts[J]. Journal of the American Chemical Society, 2002, 124(21): 5962-5963. |
| 65 | GUO H X , QI X H , HIRAGA Y , et al . Efficient conversion of fructose into 5-ethoxymethylfurfural with hydrogen sulfate ionic liquids as co-solvent and catalyst[J]. Chemical Engineering Journal, 2017, 314: 508-514. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [6] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [7] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [8] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| [9] | 张启, 赵红, 荣峻峰. 质子交换膜燃料电池中氧还原反应抗毒性电催化剂研究进展[J]. 化工进展, 2023, 42(9): 4677-4691. |
| [10] | 王伟涛, 鲍婷玉, 姜旭禄, 何珍红, 王宽, 杨阳, 刘昭铁. 醛酮树脂基非金属催化剂催化氧气氧化苯制备苯酚[J]. 化工进展, 2023, 42(9): 4706-4715. |
| [11] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [12] | 向阳, 黄寻, 魏子栋. 电催化有机合成反应的活性和选择性调控研究进展[J]. 化工进展, 2023, 42(8): 4005-4014. |
| [13] | 王耀刚, 韩子姗, 高嘉辰, 王新宇, 李思琪, 杨全红, 翁哲. 铜基催化剂电还原二氧化碳选择性的调控策略[J]. 化工进展, 2023, 42(8): 4043-4057. |
| [14] | 刘毅, 房强, 钟达忠, 赵强, 李晋平. Ag/Cu耦合催化剂的Cu晶面调控用于电催化二氧化碳还原[J]. 化工进展, 2023, 42(8): 4136-4142. |
| [15] | 王兰江, 梁瑜, 汤琼, 唐明兴, 李学宽, 刘雷, 董晋湘. 快速热解铂前体合成高分散的Pt/HY催化剂及其萘深度加氢性能[J]. 化工进展, 2023, 42(8): 4159-4166. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||