化工进展 ›› 2019, Vol. 38 ›› Issue (03): 1244-1258.DOI: 10.16085/j.issn.1000-6613.2018-0248
CO2 混合物热物性在CCS研究中的作用:实验数据、理论模型和典型应用
王珺瑶1,2( ),张月1,邓帅1,赵军1(
),张月1,邓帅1,赵军1( ),孙太尉1,李恺翔1,徐耀锋1
),孙太尉1,李恺翔1,徐耀锋1
- 1. 天津大学中低温热能高效利用教育部重点实验室,天津 300350
2. 天津大学环境科学与工程学院,天津 300350
-
收稿日期:2018-01-28修回日期:2018-12-07出版日期:2019-03-05发布日期:2019-03-05 -
通讯作者:赵军 -
作者简介:<named-content content-type="corresp-name">王珺瑶</named-content>(1990—),女,博士研究生,研究方向为化学吸收法碳捕集。E-mail:<email>wangjunyao_hkust@126.com</email>。|赵军,教授,主要从事碳捕集和中低温热能高效利用研究。E-mail: <email>zhaojun@tju.edu.cn</email>。 -
基金资助:国家自然科学基金面上项目(51876134)
Role of thermodynamic properties of CO2 mixtures in CCS: data, models and typical applications
Junyao WANG1,2( ),Yue ZHANG1,Shuai DENG1,Jun ZHAO1(
),Yue ZHANG1,Shuai DENG1,Jun ZHAO1( ),Taiwei SUN1,Kaixiang LI1,Yaofeng XU1
),Taiwei SUN1,Kaixiang LI1,Yaofeng XU1
- 1. Key Laboratory of Efficient Utilization of Low and Medium Grade Energy, Tianjin University, Tianjin 300350, China
2. School of Environmental Science and Engineering, Tianjin University, Tianjin 300350, China
-
Received:2018-01-28Revised:2018-12-07Online:2019-03-05Published:2019-03-05 -
Contact:Jun ZHAO
摘要:
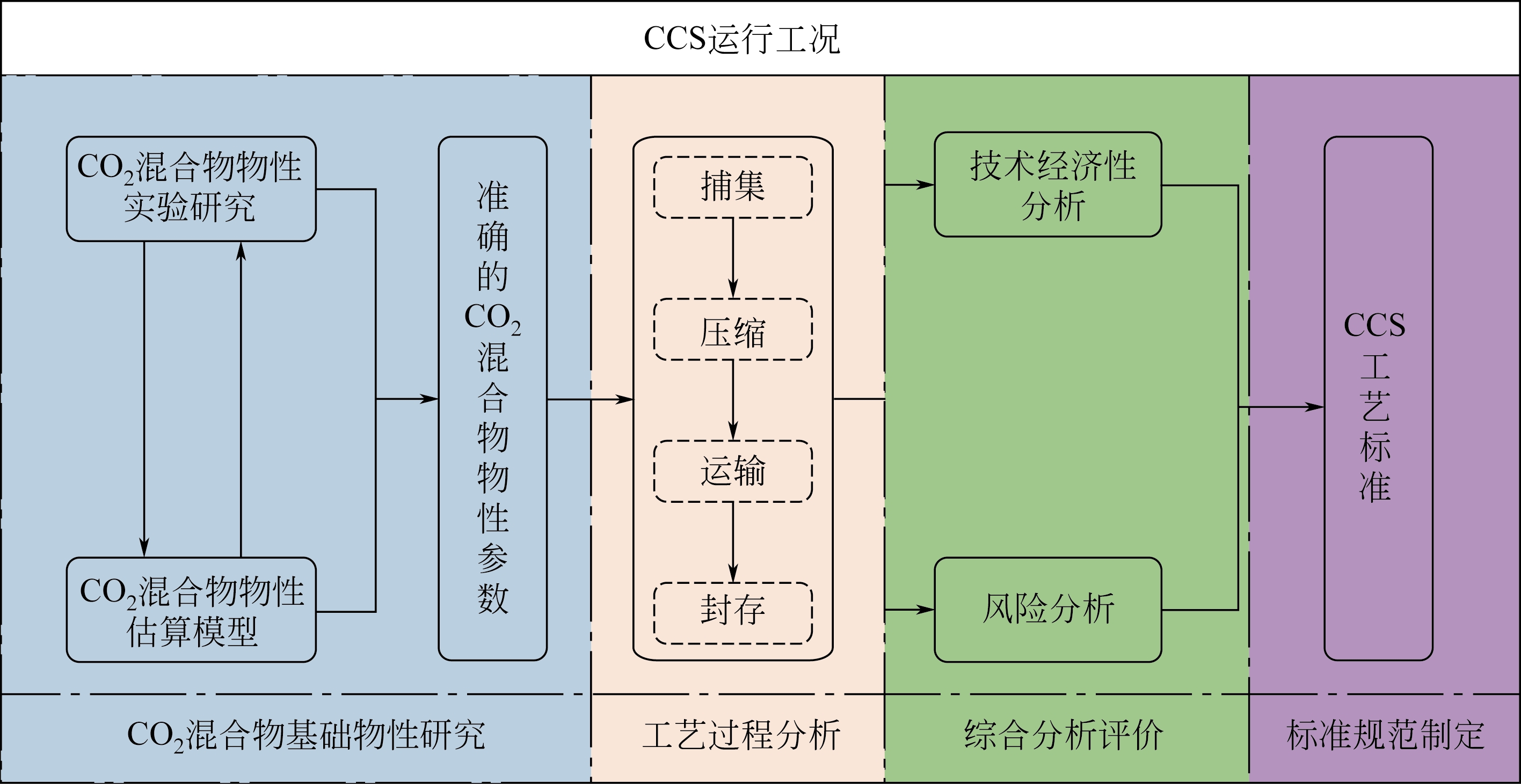
二氧化碳捕集与封存(CCS)各工艺过程的设计、运行都依赖于对CO2及其混合物热物理性质的深入理解。同时,CCS的规模化发展和商业化进程,对CO2混合物及其热物性的准确性提出了更高的要求。本文从实验数据、理论模型和典型应用3个方面综述了CO2及其混合物热物性的发展现状,并尝试对发展趋势进行归纳。在实验研究方面,CO2混合体系的研究进展视组分不同,差异较大,其中CO2-N2、CO2-CH4、CO2-H2O和CO2-H2二元体系已形成较完善的物性数据库,而CO2-NH3、CO2-NO x 和CO2-CO体系的物性数据还比较欠缺;在物性估算方面,面向CCS的物性估算模型研究自2008年开始活跃,基于不同理论构架,目前已逐步形成面向CCS的多元化的物性估算体系。物性研究在CCS中的应用主要体现在物性是支撑CCS过程研究的基础,其不准确性在过程模拟或计算中会被“放大”,从而影响过程评估的准确性,本文从物性在循环构建和能效分析中的作用以及CO2水合物的形成3个方面入手做了说明。文章最后对面向CCS的物性研究趋势进行了梳理,对分子模拟技术、通用性强的物性估算模型和物性在过程设计和循环分析中的角色进行了展望。
中图分类号:
引用本文
王珺瑶, 张月, 邓帅, 赵军, 孙太尉, 李恺翔, 徐耀锋. CO2 混合物热物性在CCS研究中的作用:实验数据、理论模型和典型应用[J]. 化工进展, 2019, 38(03): 1244-1258.
Junyao WANG, Yue ZHANG, Shuai DENG, Jun ZHAO, Taiwei SUN, Kaixiang LI, Yaofeng XU. Role of thermodynamic properties of CO2 mixtures in CCS: data, models and typical applications[J]. Chemical Industry and Engineering Progress, 2019, 38(03): 1244-1258.
| 项目名称 | 国别/地区 | 研究对象 | 研究过程 | 研究方法 | 来源 |
|---|---|---|---|---|---|
| CO2 QUEST | 国际合作(英国、瑞典、法国、中国、比利时、希腊、加拿大、以色列) | CO2混合物 | CO2运输和封存过程 | 物理模型和实验研究 | [4] |
| CO2 Interface-Transport-Interface-Storage (CO2 IT IS) | 挪威 | CO2混合物 | CO2运输和封存过程 | 物理模型和实验研究 | [5] |
| CO2 Dynamic | 挪威 | CO2混合物 | CO2运输和封存过程 | 物理模型 | [6] |
| CO2 MIX Project | 挪威/德国 | CO2混合物 | CO2压缩液化和运输过程 | 物理模型和实验研究 | [7] |
| The Impact Project | 国际合作(挪威、英国、荷兰、中国等) | CO2混合物 | CO2运输和封存过程 | 物理模型和实验研究 | [8] |
| Gas Annexes Project | 法国 | CO2混合物 | CO2封存过程 | 物理模型和实验研究 | [9] |
表1 涉及CO2物性研究的代表性项目总结
| 项目名称 | 国别/地区 | 研究对象 | 研究过程 | 研究方法 | 来源 |
|---|---|---|---|---|---|
| CO2 QUEST | 国际合作(英国、瑞典、法国、中国、比利时、希腊、加拿大、以色列) | CO2混合物 | CO2运输和封存过程 | 物理模型和实验研究 | [4] |
| CO2 Interface-Transport-Interface-Storage (CO2 IT IS) | 挪威 | CO2混合物 | CO2运输和封存过程 | 物理模型和实验研究 | [5] |
| CO2 Dynamic | 挪威 | CO2混合物 | CO2运输和封存过程 | 物理模型 | [6] |
| CO2 MIX Project | 挪威/德国 | CO2混合物 | CO2压缩液化和运输过程 | 物理模型和实验研究 | [7] |
| The Impact Project | 国际合作(挪威、英国、荷兰、中国等) | CO2混合物 | CO2运输和封存过程 | 物理模型和实验研究 | [8] |
| Gas Annexes Project | 法国 | CO2混合物 | CO2封存过程 | 物理模型和实验研究 | [9] |
| 气体种类 | 摩尔分数最小值/% | 摩尔分数最大值/% |
|---|---|---|
| CO2 | 75 | 99 |
| N2 | 0.02 | 10 |
| H2O | 0.005 | 6.5 |
| O2 | 0.04 | 5 |
| H2 | 0.06 | 4 |
| CH4 | 0.7 | 4 |
| Ar | 0.005 | 3.5 |
| NH3 | <10?3 | 3 |
| SO2 | <10?3 | 1.5 |
| H2S/COS | 0.01 | 1.5 |
| NO x | <0.002 | 0.3 |
| CO | <10?3 | 0.2 |
| 胺 | <10?3 | 0.01 |
表2 捕集后的CO2混合气成分[19,21]
| 气体种类 | 摩尔分数最小值/% | 摩尔分数最大值/% |
|---|---|---|
| CO2 | 75 | 99 |
| N2 | 0.02 | 10 |
| H2O | 0.005 | 6.5 |
| O2 | 0.04 | 5 |
| H2 | 0.06 | 4 |
| CH4 | 0.7 | 4 |
| Ar | 0.005 | 3.5 |
| NH3 | <10?3 | 3 |
| SO2 | <10?3 | 1.5 |
| H2S/COS | 0.01 | 1.5 |
| NO x | <0.002 | 0.3 |
| CO | <10?3 | 0.2 |
| 胺 | <10?3 | 0.01 |
| 混合物 | 文献数量 | 相关文献 | 数据范围 | 研究现状 | ||||
|---|---|---|---|---|---|---|---|---|
| 温度/K | 压力/MPa | X(CO2) | CCS相关文献 | 实验数据点 | ||||
| 注: √表示实验数据点较多, 已开展基于CCS过程的实验; △表示实验数据点有局限性, 已开展基于CCS过程的实验; ?表示实验数据点较少, 且实验数据年份较早。 | ||||||||
| CO2-N2 | 31 | [ | 208~303 | 0.6~21.4 | 0.15~0.999 | [ | √ | |
| CO2-O2 | 13 | [ | 218~298 | 0.9~20 | 0.15~0.99 | [ | △ | |
| CO2-H2 | 8 | [ | 218~303 | 0.9~172 | 0.07~0.999 | [ | √ | |
| CO2-CH4 | 19 | [ | 152~320 | 0.6~48 | 0.026~0.99 | [ | √ | |
| CO2-Ar | 6 | [ | 223~299 | 1.5~20 | 0.24~0.99 | [ | △ | |
| CO2-NH3 | 2 | [ | 413~513 | 4.3~81.7 | 0.023~0.33 | — | ? | |
| CO2-SO2 | 4 | [ | 273~418 | 1.1~29 | 0.03~0.98 | [ | ? | |
| CO2-H2S | 8 | [ | 248~365 | 0.3~41 | 0.01~0.97 | [ | ? | |
| CO2-N2O | 1 | [ | 293~307 | 5.3~7.2 | 0.28~0.88 | — | ? | |
| CO2-NO/N2O4 | 2 | [ | 262~328 | 0.17~9.0 | 0.005~0.88 | — | ? | |
| CO2-CO | 5 | [ | 223~343 | 0.1~20 | 0.20~0.996 | [ | ? | |
| CO2-H2O | >50 | [ | 251~623 | 0.1~350 | 0.08~1 | [ | √ | |
| CO2-N2-O2 | 3 | [ | 218~273 | 5.1~13 | 0.15~0.96 | — | ? | |
| CO2-N2-H2 | 1 | [ | 253~302 | 2.1~8.7 | 0.93~0.95 | [ | △ | |
| CO2-N2-Ar | 1 | [ | 268~303 | 3.52~7.66 | 0.90~0.98 | [ | △ | |
| CO2-N2-CH4 | 6 | [ | 220~293 | 6~11 | 0.27~0.99 | — | ? | |
| CO2-Ar-H2 | 1 | [ | 268~301 | 3.26~8.91 | 0.90~0.98 | [ | △ | |
| CO2-CO-H2 | 3 | [ | 233~303 | 4~20 | 0.17~0.99 | — | ? | |
| CO2-CH4-H2S | 1 | [ | 222~239 | 2.1~4.8 | 0.024~0.78 | — | ? | |
| CO2-CH4-H2O | 5 | [ | 243~432 | 0.1~100 | 0.001~0.83 | — | ? | |
| CO2-O2-Ar-N2 | 1 | [ | 253~293 | 7.1~9.0 | 0.90 | [ | ? | |
表3 CO2混合物pVTxy实验数据
| 混合物 | 文献数量 | 相关文献 | 数据范围 | 研究现状 | ||||
|---|---|---|---|---|---|---|---|---|
| 温度/K | 压力/MPa | X(CO2) | CCS相关文献 | 实验数据点 | ||||
| 注: √表示实验数据点较多, 已开展基于CCS过程的实验; △表示实验数据点有局限性, 已开展基于CCS过程的实验; ?表示实验数据点较少, 且实验数据年份较早。 | ||||||||
| CO2-N2 | 31 | [ | 208~303 | 0.6~21.4 | 0.15~0.999 | [ | √ | |
| CO2-O2 | 13 | [ | 218~298 | 0.9~20 | 0.15~0.99 | [ | △ | |
| CO2-H2 | 8 | [ | 218~303 | 0.9~172 | 0.07~0.999 | [ | √ | |
| CO2-CH4 | 19 | [ | 152~320 | 0.6~48 | 0.026~0.99 | [ | √ | |
| CO2-Ar | 6 | [ | 223~299 | 1.5~20 | 0.24~0.99 | [ | △ | |
| CO2-NH3 | 2 | [ | 413~513 | 4.3~81.7 | 0.023~0.33 | — | ? | |
| CO2-SO2 | 4 | [ | 273~418 | 1.1~29 | 0.03~0.98 | [ | ? | |
| CO2-H2S | 8 | [ | 248~365 | 0.3~41 | 0.01~0.97 | [ | ? | |
| CO2-N2O | 1 | [ | 293~307 | 5.3~7.2 | 0.28~0.88 | — | ? | |
| CO2-NO/N2O4 | 2 | [ | 262~328 | 0.17~9.0 | 0.005~0.88 | — | ? | |
| CO2-CO | 5 | [ | 223~343 | 0.1~20 | 0.20~0.996 | [ | ? | |
| CO2-H2O | >50 | [ | 251~623 | 0.1~350 | 0.08~1 | [ | √ | |
| CO2-N2-O2 | 3 | [ | 218~273 | 5.1~13 | 0.15~0.96 | — | ? | |
| CO2-N2-H2 | 1 | [ | 253~302 | 2.1~8.7 | 0.93~0.95 | [ | △ | |
| CO2-N2-Ar | 1 | [ | 268~303 | 3.52~7.66 | 0.90~0.98 | [ | △ | |
| CO2-N2-CH4 | 6 | [ | 220~293 | 6~11 | 0.27~0.99 | — | ? | |
| CO2-Ar-H2 | 1 | [ | 268~301 | 3.26~8.91 | 0.90~0.98 | [ | △ | |
| CO2-CO-H2 | 3 | [ | 233~303 | 4~20 | 0.17~0.99 | — | ? | |
| CO2-CH4-H2S | 1 | [ | 222~239 | 2.1~4.8 | 0.024~0.78 | — | ? | |
| CO2-CH4-H2O | 5 | [ | 243~432 | 0.1~100 | 0.001~0.83 | — | ? | |
| CO2-O2-Ar-N2 | 1 | [ | 253~293 | 7.1~9.0 | 0.90 | [ | ? | |
| 序号 | 研究机构 | 物性 | CO2混合物体系 | 状态方程 | 主要结论 | 参考文献 | 年份 |
|---|---|---|---|---|---|---|---|
| 1 | 挪威科技大学;挪威科技工业研究院 | VLE pρT | CO2-N2; CO2-O2; CO2-CH4; CO2-N2-O2 | SRK SRK-Peneloux PR Lee-Kesler SPUNG/SRK GERG-2004 | GERG-2004的估算精度最高(除了在估算CO2-O2体系VLE性质时误差达到20%); SPUNG可能成为较好估算精度和较短估算时间的折中选择 | [ | 2012 |
| VLE pρT | CO2-H2O | SPUNG-vdW SRK- vdW ; SRK-HV | SPUNG在估算密度性质时比SRK更精确; SRK-HV在估算VLE性质时优于SPUNG; 参比流体和参比状态方程的选取对SPUNG的估算精度有较大影响 | [ | 2014 | ||
| VLE pρT | CO2-H2O | ECS-HV SPUNG- vdW | 提出一种新的扩展对比态状态方程ECS; 新的状态方程在估算CO2-H2O体系的VLE和密度性质时都具有较高的精度; ECS估算精度较SPUNG有了很大的提高; ECS估算时间与SPUNG在相同数量级 | [ | 2015 | ||
| 2 | 德国波鸿大学 | VLE | CO2-N2 CO2-O2 CO2-Ar CO2-CH4 | SRK-vdW GERG-2008 EOS-CG | 实验测量了CO2-N2/O2/Ar/CH4在278~298K之间的相平衡性质; 当杂质含量较低时,SRK-vdW、GERG-2008和EOS-CG都有较好的估算精度 | [ | 2014 |
| VLE pρT | CO2-H2O/N2/O2/Ar/CO H2O-N2/O2/Ar/CO N2-O2/Ar/CO O2-Ar/CO Ar-CO | EOS-CG,GERG-2008 LKP SRK-vdW | 在GEGR-2008的基础上, 提出了EOS-CG模型,该模型以CCS为背景过程; 相比于GERG-2008,EOS-CG模型很大程度上提高了针对CCS混合流体的热物性估算精度 | [ | 2016 | ||
| 3 | 法国洛林大学 | VLE pρT 黏度 | CO2- N2O CO2+ NO/N2O2 | PR- vdW SRK- vdW | 应用蒙特卡洛分子模拟获得了CO2-NO/N2O2体系缺失的相平衡实验数据; PR-vdW和SRK-vdW在估算CO2-NO x 体系VLE性质时都具有较好的精度 | [ | 2012 |
| VLE 混合焓变 | 含有CO2、N2、H2O、Ar、SO2、O2、N2和碳氢化合物的二元体系 | E-PPR78-vdW | 将E-PPR78扩展应用到包含SO2、O2和NO的二元体系 | [ | 2015 | ||
| VLE | 含有CO2、SO2、O2、NO、H2O和碳氢化合物的二元体系 | E-PPR78 PC-SAFT | E-PPR78和PC-SAFT都能够较好的估算大多数二元体系的VLE性质; 对大多数二元体系,E-PPR78比PC-SAFT的估算精度高; 应用蒙特卡洛分子分子模拟或得了实验缺失的相平衡数据 | [ | 2017 | ||
| VLE 混合焓变 | 含有CO2、CH4、N2、O2、Ar、H2、H2S、COS、SO2、NH3、NO、NO2、N2O4、N2O、CO和H2O的二元体系; 含有CO2、CH4、CO、H2S、N2和H2的三元体系 | E-PPR78-vdW | 将E-PPR78扩展到包含COS、NH3、NO/N2O2、N2O的二元体系;E-PPR78适用于多数CCS混合流体二元和三元体系的VLE和混合焓变的物性估算 | [ | 2017 | ||
| 4 | 丹麦技术大学;希腊塞萨洛尼基亚里士多德大学 | VLE LLE pρT VLE pρT | CO2和水, 醇类, 二醇类,碳氢化合物的二元体系 | CPA | CPA对上述二元体系的VLE,LLE和密度性质估算具有较好精度 | [ | 2010 |
| CPA SRK-HV | CPA对上述二元体系的VLE和密度性质估算具有较好精度 | [ | 2014 | ||||
表4 CO2混合物物性估算研究现状(2008—2017)
| 序号 | 研究机构 | 物性 | CO2混合物体系 | 状态方程 | 主要结论 | 参考文献 | 年份 |
|---|---|---|---|---|---|---|---|
| 1 | 挪威科技大学;挪威科技工业研究院 | VLE pρT | CO2-N2; CO2-O2; CO2-CH4; CO2-N2-O2 | SRK SRK-Peneloux PR Lee-Kesler SPUNG/SRK GERG-2004 | GERG-2004的估算精度最高(除了在估算CO2-O2体系VLE性质时误差达到20%); SPUNG可能成为较好估算精度和较短估算时间的折中选择 | [ | 2012 |
| VLE pρT | CO2-H2O | SPUNG-vdW SRK- vdW ; SRK-HV | SPUNG在估算密度性质时比SRK更精确; SRK-HV在估算VLE性质时优于SPUNG; 参比流体和参比状态方程的选取对SPUNG的估算精度有较大影响 | [ | 2014 | ||
| VLE pρT | CO2-H2O | ECS-HV SPUNG- vdW | 提出一种新的扩展对比态状态方程ECS; 新的状态方程在估算CO2-H2O体系的VLE和密度性质时都具有较高的精度; ECS估算精度较SPUNG有了很大的提高; ECS估算时间与SPUNG在相同数量级 | [ | 2015 | ||
| 2 | 德国波鸿大学 | VLE | CO2-N2 CO2-O2 CO2-Ar CO2-CH4 | SRK-vdW GERG-2008 EOS-CG | 实验测量了CO2-N2/O2/Ar/CH4在278~298K之间的相平衡性质; 当杂质含量较低时,SRK-vdW、GERG-2008和EOS-CG都有较好的估算精度 | [ | 2014 |
| VLE pρT | CO2-H2O/N2/O2/Ar/CO H2O-N2/O2/Ar/CO N2-O2/Ar/CO O2-Ar/CO Ar-CO | EOS-CG,GERG-2008 LKP SRK-vdW | 在GEGR-2008的基础上, 提出了EOS-CG模型,该模型以CCS为背景过程; 相比于GERG-2008,EOS-CG模型很大程度上提高了针对CCS混合流体的热物性估算精度 | [ | 2016 | ||
| 3 | 法国洛林大学 | VLE pρT 黏度 | CO2- N2O CO2+ NO/N2O2 | PR- vdW SRK- vdW | 应用蒙特卡洛分子模拟获得了CO2-NO/N2O2体系缺失的相平衡实验数据; PR-vdW和SRK-vdW在估算CO2-NO x 体系VLE性质时都具有较好的精度 | [ | 2012 |
| VLE 混合焓变 | 含有CO2、N2、H2O、Ar、SO2、O2、N2和碳氢化合物的二元体系 | E-PPR78-vdW | 将E-PPR78扩展应用到包含SO2、O2和NO的二元体系 | [ | 2015 | ||
| VLE | 含有CO2、SO2、O2、NO、H2O和碳氢化合物的二元体系 | E-PPR78 PC-SAFT | E-PPR78和PC-SAFT都能够较好的估算大多数二元体系的VLE性质; 对大多数二元体系,E-PPR78比PC-SAFT的估算精度高; 应用蒙特卡洛分子分子模拟或得了实验缺失的相平衡数据 | [ | 2017 | ||
| VLE 混合焓变 | 含有CO2、CH4、N2、O2、Ar、H2、H2S、COS、SO2、NH3、NO、NO2、N2O4、N2O、CO和H2O的二元体系; 含有CO2、CH4、CO、H2S、N2和H2的三元体系 | E-PPR78-vdW | 将E-PPR78扩展到包含COS、NH3、NO/N2O2、N2O的二元体系;E-PPR78适用于多数CCS混合流体二元和三元体系的VLE和混合焓变的物性估算 | [ | 2017 | ||
| 4 | 丹麦技术大学;希腊塞萨洛尼基亚里士多德大学 | VLE LLE pρT VLE pρT | CO2和水, 醇类, 二醇类,碳氢化合物的二元体系 | CPA | CPA对上述二元体系的VLE,LLE和密度性质估算具有较好精度 | [ | 2010 |
| CPA SRK-HV | CPA对上述二元体系的VLE和密度性质估算具有较好精度 | [ | 2014 | ||||
| 序号 | 研究机构 | 物性 | CO2混合物体系 | 状态方程 | 主要结论 | 参考文献 | 2012 | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| VLE pρT | CO2和烷烃类二元体系 | CPA SRK PR | 将CPA的应用范围由n-eicosane(n-C20)扩展到了hexatriacontane (n-C36); CPA能够应用于CO2和烷烃类二元体系的VLE和密度性质估算 | [ | 2015 | ||||||
| VLE VLLE | 含CO2、醇类和水的三元和四元体系 | CPA | CPA能够较精确地估算前述多元体系的VLE和VLLE性质 | [ | 2015 | ||||||
| VLE VLLE | CO2和烷烃类、水、二醇类的多元体系 | CPA | CPA能够较精确地估算前述多元体系的VLE和VLLE性质 | [ | 2016 | ||||||
| VLE VLLE | CO2-烷烃类 CO2-二醇类 CO2-H2O | CPA qCPA | 在CPA的基础上引入了电四极矩校正项,进而提出了qCPA qCPA极大地提高了CO2-n-alkane体系的VLE和VLLE估算精度 | [ | 2016 | ||||||
| 5 | 瑞典皇家理工学院;瑞典梅拉达伦大学 | VLE | CO2-N2; CO2-O2; CO2-Ar; CO2-CH4; CO2-H2S; CO2-SO2 | PR/PT/RK/S RK/3P1T/-vdW | CO2-N2/O2/Ar体系建议用PT; CO2-CH4/H2S体系建议用PR; CO2-SO2体系建议用3P1T; 利用实验数据回归二元交互系数能够很大程度提高立方型状态方程的估算精度 | [ | 2009 | ||||
| 6 | 法国石油与新能源研究院 | VLE pρT 黏度 | CO2-N2O; CO2-NO/N2O2 | PR SRK | 采用蒙特卡洛分子模拟的方法回归了CO2-NO x 体系缺失的实验数据并拟合了PR、SRK状态方程二元交互系数; PR和SRK对VLE性质的估算结果都较好; PR对两相区的液相密度估算较SRK更接近蒙特卡洛分子模拟的结果 | [ | 2012 | ||||
| 7 | 意大利米兰理工大学 | VLE | 含有CO2,H2,N2,O2,Ar,CO,CH4, | PR+EOS / | PR+EOS/ | [ | 2016 | ||||
表4 CO2混合物物性估算研究现状(2008~2017)
| 序号 | 研究机构 | 物性 | CO2混合物体系 | 状态方程 | 主要结论 | 参考文献 | 2012 | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| VLE pρT | CO2和烷烃类二元体系 | CPA SRK PR | 将CPA的应用范围由n-eicosane(n-C20)扩展到了hexatriacontane (n-C36); CPA能够应用于CO2和烷烃类二元体系的VLE和密度性质估算 | [ | 2015 | ||||||
| VLE VLLE | 含CO2、醇类和水的三元和四元体系 | CPA | CPA能够较精确地估算前述多元体系的VLE和VLLE性质 | [ | 2015 | ||||||
| VLE VLLE | CO2和烷烃类、水、二醇类的多元体系 | CPA | CPA能够较精确地估算前述多元体系的VLE和VLLE性质 | [ | 2016 | ||||||
| VLE VLLE | CO2-烷烃类 CO2-二醇类 CO2-H2O | CPA qCPA | 在CPA的基础上引入了电四极矩校正项,进而提出了qCPA qCPA极大地提高了CO2-n-alkane体系的VLE和VLLE估算精度 | [ | 2016 | ||||||
| 5 | 瑞典皇家理工学院;瑞典梅拉达伦大学 | VLE | CO2-N2; CO2-O2; CO2-Ar; CO2-CH4; CO2-H2S; CO2-SO2 | PR/PT/RK/S RK/3P1T/-vdW | CO2-N2/O2/Ar体系建议用PT; CO2-CH4/H2S体系建议用PR; CO2-SO2体系建议用3P1T; 利用实验数据回归二元交互系数能够很大程度提高立方型状态方程的估算精度 | [ | 2009 | ||||
| 6 | 法国石油与新能源研究院 | VLE pρT 黏度 | CO2-N2O; CO2-NO/N2O2 | PR SRK | 采用蒙特卡洛分子模拟的方法回归了CO2-NO x 体系缺失的实验数据并拟合了PR、SRK状态方程二元交互系数; PR和SRK对VLE性质的估算结果都较好; PR对两相区的液相密度估算较SRK更接近蒙特卡洛分子模拟的结果 | [ | 2012 | ||||
| 7 | 意大利米兰理工大学 | VLE | 含有CO2,H2,N2,O2,Ar,CO,CH4, | PR+EOS / | PR+EOS/ | [ | 2016 | ||||
| 1 | 全浩, 温雪峰, 郭琳琳 . CO2捕集和地下封存技术的现状及发展趋势(一)[J]. 煤炭工程, 2007(12): 75-79. |
| QUAN H , WEN X F , GUO L L . Present status and development tendency of CO2 collection and underground sealed storage technology[J]. Coal Engineering, 2007(12): 75-79. | |
| 2 | CCS Institute Global . Large-scale CCS facilities [EB/OL]. 2017-09-10. http: // . |
| 3 | TAN Y T , NOOKUEA W , LI H L , et al . Property impacts on carbon capture and storage (CCS) processes: a review[J]. Energy Conversion & Management, 2016, 118: 204-222. |
| 4 | CO2 QUEST . Impact of the quality of CO2 on storage and transport[EB/OL]. 2017-12-02. http: // . |
| 5 | KOEIJER G D , BORCH J H , DRESCHER M , et al . CO2 transport – depressurization, heat transfer and impurities[J]. Energy Procedia, 2011, 4(22): 3008-3015. |
| 6 | SINTEF . CO2 dynamics [EB/OL]. 2017-12-02. https: // . |
| 7 | LOVSETH S W , SKAUGEN G , STANG H G J , et al . CO2 mix project: experimental determination of thermo physical properties of CO2-rich mixtures[J]. Energy Procedia, 2013, 37: 2888-2896. |
| 8 | BRUNSVOLD A , JAKOBSEN J P , MAZZETTI M J , et al . Key findings and recommendations from the IMPACTS project[J]. International Journal of Greenhouse Gas Control, 2016, 54: 588-598. |
| 9 | STERPENICH J , DUBESSY J , PIRONON J , et al . Role of impurities on CO2 injection: experimental and numerical simulations of thermodynamic properties of water-salt-gas mixtures (CO2 + co-injected gases) under geological storage conditions[J]. Energy Procedia, 2013, 37(4): 3638-3645. |
| 10 | 李政, 徐兆丰, 张东杰, 等 . 中国实施CO2捕集与封存的参考意见[M]. 北京: 清华大学出版社, 2012: 7-14. |
| LI Z , XU Z F , ZHANG D J , et al . Guidelines for carbon dioxide capture and storage in China[M]. Beijing: Tsinghua University Press, 2012: 7-14. | |
| 11 | KLER R , NEELE F , NIENOORD M , et al . Transportation and unloading of CO2 by ship——a comparative assessment[R]. WP9 Final Report, 2015. |
| 12 | RUSIN A . Advances in carbon dioxide compression and pipeline transportation processes[M]. Berlin: Springer International Publishing, 2015. |
| 13 | LI H L . Thermodynamic properties of CO2 mixtures and their applications in advanced power cycles with CO2 capture processes [D]. Swedish: KTH Royal Institute of Technology, 2008. |
| 14 | ZHANG F Z , JIANG P X , XU R N . System thermodynamic performance comparison of CO2-EGS and water-EGS systems[J]. Applied Thermal Engineering, 2013, 61(2): 236-244. |
| 15 | XU G , LI L , YANG Y P , et al . A novel CO2 cryogenic liquefaction and separation system[J]. Energy, 2012, 42(1): 522-529. |
| 16 | SONG C F , KITAMURA Y , LI S H , et al . Design of a cryogenic CO2 capture system based on stirling coolers[J]. International Journal of Greenhouse Gas Control, 2012, 7(2): 107-114. |
| 17 | XU J X , LIN W S . A CO2 cryogenic capture system for flue gas of an LNG-fired power plant[J]. International Journal of Hydrogen Energy, 2017, 42(29): 18674-18680. |
| 18 | LI H L , JAKOBSEN J P , Ø WILHELMSEN , et al . pVTxy properties of CO2 mixtures relevant for CO2 capture, transport and storage: review of available experimental data and theoretical models[J]. Applied Energy, 2011, 88(11): 3567-3579. |
| 19 | Intergovernmental Panel on Climate Change (IPCC) . IPCC special report on carbon dioxide capture and storage [R]. London: Cambridge University Press, 2005. |
| 20 | WANG J S , RYAN D , ANTHONY E J , et al . The effect of impurities in oxyfuel flue gas on CO2 storage capacity[J]. International Journal of Greenhouse Gas Control, 2012, 11(6): 158-162. |
| 21 | MUNKEJORD S T , HAMMER M , LØVSETH S W . CO2 transport: data and models - a review[J]. Applied Energy, 2016, 169: 499-523. |
| 22 | LIANG Z W , FU K Y , IDEM R , et al . Review on current advances, future challenges and consideration issues for post-combustion CO2 capture using amine-based absorbents[J]. Chinese Journal of Chemical Engineering, 2016, 24(2): 278-288. |
| 23 | RAYER A V . Solvent chemistry: solubility of CO2 in reactive solvents for post-combustion CO2 [J]. Carbon Management, 2012, 3(5): 467-484. |
| 24 | MUHAMMAD A , GADELHAK Y . Simulation based improvement techniques for acid gases sweetening by chemical absorption: a review[J]. International Journal of Greenhouse Gas Control, 2015, 37: 481-491. |
| 25 | KUMAR S , CHO J H, MOON I . Ionic liquid-amine blends and CO2 BOLs: prospective solvents for natural gas sweetening and CO2 capture technology——a review[J]. International Journal of Greenhouse Gas Control, 2014, 20: 87-116. |
| 26 | GERNERT J , SPAN R . EOS-CG: A Helmholtz energy mixture model for humid gases and CCS mixtures[J]. Journal of Chemical Thermodynamics, 2016, 93: 274-293. |
| 27 | TENORIO M J , PARROTT A J , CALLADINE J A , et al . Measurement of the vapour-liquid equilibrium of binary and ternary mixtures of CO2, N2 and H2, systems which are of relevance to CCS technology[J]. International Journal of Greenhouse Gas Control, 2015, 41: 68-81. |
| 28 | KE J , SULEIMAN N , SANCHEZ-VICENTE Y , et al . The phase equilibrium and density studies of the ternary mixtures of CO2+Ar+N2 and CO2+Ar+H2, systems relevance to CCS technology[J]. International Journal of Greenhouse Gas Control, 2017, 56: 55-66. |
| 29 | CHAPOY A , NAZERI M , KAPATEH M , et al . Effect of impurities on thermophysical properties and phase behaviour of a CO2-rich system in CCS[J]. International Journal of Greenhouse Gas Control, 2013, 19(2): 92-100. |
| 30 | LACHET V , BRUIN T D , UNGERER P , et al . Thermodynamic behavior of the CO2+SO2 mixture: experimental and Monte Carlo simulation studies[J]. Energy Procedia, 2009, 1(1): 1641-1647. |
| 31 | NAZERI M , CHAPOY A , VALTZ A , et al . Densities and derived thermophysical properties of the 0.9505 CO2+0.0495 H2S mixture from 273K to 353K and pressures up to 41MPa[J]. Fluid Phase Equilibria, 2016, 423: 156-171. |
| 32 | NAZERI M , CHAPOY A , VALTZ A , et al . New experimental density data and derived thermophysical properties of carbon dioxide-Sulphur dioxide binary mixture (CO2-SO2) in gas, liquid and supercritical phases from 273K to 353K and at pressures up to 42MPa[J]. Fluid Phase Equilibria, 2017, 454: 64-77. |
| 33 | LASALA S , CHIESA P , PRIVAT R , et al . VLE properties of CO2-based binary systems containing N2, O2 and Ar: experimental measurements and modelling results with advanced cubic equations of state[J]. Fluid Phase Equilibria, 2016, 428: 18-31. |
| 34 | AHMAD M , GERNERT J , WILBERS E . Effect of impurities in captured CO2 on liquid-vapor equilibrium[J]. Fluid Phase Equilibria, 2014, 363(4): 149-155. |
| 35 | MAZZOCCOLI M , BOSIO B , ARATO E . Pressure-density-temperature measurements of binary mixtures rich in CO2 for pipeline transportation in the CCS Process[J]. Journal of Chemical & Engineering Data, 2012, 57(10): 2774-2783. |
| 36 | WESTMAN S F , STANG H G J , LØVSETH S W , et al . Vapor-liquid equilibrium data for the carbon dioxide and oxygen (CO2 +O2) system at the temperatures 218, 233, 253, 273, 288 and 298K and pressures up to 14MPa[J]. Fluid Phase Equilibria, 2016, 421: 67-87. |
| 37 | BLANCO S T , RIVAS C , BRAVO R , et al . Discussion of the influence of CO and CH4 in CO2 transport, injection, and storage for CCS technology[J]. Environmental Science & Technology, 2014, 48(18): 10984. |
| 38 | MA Z W, ZHANG P , BAO H S , et al . Review of fundamental properties of CO2 hydrates and CO2 capture and separation using hydration method[J]. Renewable & Sustainable Energy Reviews, 2016, 53: 1273-1302. |
| 39 | MUIRBROOK N K , PRAUSNITZ J M . Multicomponent vapor-liquid equilibria at high pressures: Part I. Experimental study of the nitrogen-oxygen-carbon dioxide system at 0℃[J]. AIChE Journal, 1965, 11(6): 1092-1096. |
| 40 | SARASHINA E , ARAI Y , SAITO S . Vapor-liquid equilibria for the nitrogen-methane-carbon dioxide system[J]. Journal of Chemical Engineering of Japan, 1971, 4(4): 377-378. |
| 41 | SOMAIT F A , KIDNAY A J . Liquid-vapor equilibriums at 270.00K for systems containing nitrogen, methane, and carbon dioxide[J]. Journal of Chemical & Engineering Data, 1978, 23(4): 301-305. |
| 42 | YOKOYAMA C , ARAI K , SAITO S , et al . Bubble-point pressures of the H2-CO- CO2 system[J]. Fluid Phase Equilibria, 1988, 39(1): 101-110. |
| 43 | KE J , HAN B X , MICHAEL W G , et al . How does the critical point change during a chemical reaction in supercritical fluids? A study of the hydro formylation of propene in supercritical CO2 [J]. Journal of the American Chemical Society, 2001, 123(16): 3661-3670. |
| 44 | SPAN R , WAGNER W . A new equation of state for carbon dioxide covering the fluid region from the triple point temperature to 1100K at pressures up to 800MPa[J]. Journal of Physical & Chemical Reference Data, 1996, 25(1): 151-159. |
| 45 | SANCHEZ-VICENTE Y , DRAGE T C , POLIAKOFF M , et al . Densities of the carbon dioxide+hydrogen, a system of relevance to carbon capture and storage[J]. International Journal of Greenhouse Gas Control, 2013, 13(3): 78-86. |
| 46 | SPAN R , GERNERT J , JÄGER A . Accurate thermodynamic-property models for CO2 -rich mixtures[J]. Energy Procedia, 2013, 37: 2914-2922. |
| 47 | GOOS E , RIEDEL U , ZHAO L , et al . Phase diagrams of CO2 and CO2-N2 gas mixtures and their application in compression processes[J]. Energy Procedia, 2011, 4(1): 3778-3785. |
| 48 | MAZZOCCOLI M , BOSIO B , ARATO E , et al . Comparison of equations-of-state with p-ρ-T experimental data of binary mixtures rich in CO2 under the conditions of pipeline transport[J]. Journal of Supercritical Fluids, 2014, 95: 474-490. |
| 49 | DEMETRIADES T A , DRAGE T C , GRAHAM R S . Developing a new equation of state for carbon capture and storage pipeline transport[J]. Proceedings of Institution of Mechanical Engineers Part E: Journal of Process Mechanical Engineering, 2013, 227(2): 117-124. |
| 50 | Ø WILHELMSEN , SKAUGEN G , JØRSTAD O , et al . Evaluation of SPUNG and other equations of state for use in carbon capture and storage modelling[J]. Energy Procedia, 2012, 23(23): 236-245. |
| 51 | LI H L , YAN J Y . Evaluating cubic equations of state for calculation of vapor-liquid equilibrium of CO2 and CO2-mixtures for CO2 capture and storage processes[J]. Applied Energy, 2009, 86(6): 826-836. |
| 52 | JØRSTAD O . Equations of state for hydro carbon mixtures [D]. Trondheim: Norwegian Institute of Technology, 1993. |
| 53 | IBRAHIM M , SKAUGEN G , ERTESVÅG I S , et al . Modeling CO2-water mixture thermodynamics using various equations of state (EoSs) with emphasis on the potential of the SPUNG EoS[J]. Chemical Engineering Science, 2014, 113(3): 22-34. |
| 54 | IBRAHIM M , SKAUGEN G , ERTESVÅG I S . An extended corresponding states equation of state (EoS) for CCS industry[J]. Chemical Engineering Science, 2015, 137: 572-582. |
| 55 | GERNERT J . A new Helmholtz energy model for humid gases and CCS mixtures [D]. Bochum: Ruhr University, 2013. |
| 56 | KUNZ O , WAGNER W . The GERG-2008 wide-range equation of state for natural gases and other mixtures: an expansion of GERG-2004[J]. Journal of Chemical & Engineering Data, 2012, 57(11): 3032-3091. |
| 57 | GERNERT J , SPAN R . EOS-CG: a Helmholtz energy mixture model for humid gases and CCS mixtures[J]. Journal of Chemical Thermodynamics, 2016, 93: 274-293. |
| 58 | JAUBERT J N , MUTELET F . VLE predictions with the Peng-Robinson equation of state and temperature dependent kij calculated through a group contribution method[J]. Fluid Phase Equilibria, 2004, 224(2): 285-304. |
| 59 | QIAN J W . Développement du modèle E-PPR78 pour prédire les équilibres de phases et les grandeurs de mélange de systèmes complexes d’intérêt pétrolier sur de larges gammes de températures et de pressions [D]. Vandoeuvre-les-Nancy: INPL, 2011. |
| 60 | XU X C , PRIVAT R , JAUBERT J N . Addition of the sulfur dioxide group (SO2), the oxygen group (O2), and the nitric oxide group (NO) to the E-PPR78 model[J]. Industrial & Engineering Chemistry Research, 2015, 54(38): 1273722626. |
| 61 | XU X C , LASALA S , PRIVAT R , et al . E -PPR78: A proper cubic EoS for modelling fluids involved in the design and operation of carbon dioxide capture and storage (CCS) processes[J]. International Journal of Greenhouse Gas Control, 2017, 56: 126-154. |
| 62 | XU X C , PRIVAT R , JAUBERT J N , et al . Phase equilibrium of CCS mixtures: equation of state modeling and Monte Carlo simulation[J]. Journal of Supercritical Fluids, 2016, 119: 169-202. |
| 63 | TSIVINTZELIS I , KONTOGEORGIS G M , MICHELSEN M L , et al . Modeling phase equilibria for acid gas mixtures using the CPA equation of state. I. Mixtures with H2S[J]. AIChE Journal, 2010, 56(11): 2965-2982. |
| 64 | TSIVINTZELIS I , KONTOGEORGIS G M , MICHELSEN M L , et al . Modeling phase equilibria for acid gas mixtures using the CPA equation of state. Part II: Binary mixtures with CO2 [J]. Fluid Phase Equilibria, 2011, 306(1): 38-56. |
| 65 | TSIVINTZELIS I , ALI S, KONTOGEORGIS G M . Modeling phase equilibria for acid gas mixtures using the cubic-plus-association equation of state 3. Applications relevant to liquid or supercritical CO2 transport[J]. Journal of Chemical & Engineering Data, 2014, 59(10): 2955-2972. |
| 66 | TSIVINTZELIS I , ALI S, KONTOGEORGIS G M . Modeling phase equilibria for acid gas mixtures using the CPA equation of state. Part Ⅳ. Applications to mixtures of CO2 with alkanes[J]. Fluid Phase Equilibria, 2015, 397: 1-17. |
| 67 | TSIVINTZELIS I , KONTOGEORGIS G M . Modelling phase equilibria for acid gas mixtures using the CPA equation of state. Part Ⅴ.Multicomponent mixtures containing CO2 and alcohols[J]. The Journal of Supercritical Fluids, 2015, 104: 29-39. |
| 68 | TSIVINTZELIS I , KONTOGEORGIS G M . Modelling phase equilibria for acid gas mixtures using the CPA equation of state. Part Ⅵ. Multicomponent mixtures with glycols relevant to oil and gas and to liquid or supercritical CO2 transport applications[J]. Journal of Chemical Thermodynamics, 2015, 93: 305-319. |
| 69 | BJØRNER M G , KONTOGEORGIS G M . Modeling derivative properties and binary mixtures with CO2 using the CPA and the quadrupolar CPA equations of state[J]. Fluid Phase Equilibria, 2016, 421: 104. |
| 70 | LASALA S , CHIESA P , PRIVAT R , et al . Modeling the thermodynamics of fluids treated by CO2 capture processes with Peng-Robinson+Residual Helmholtz energy-based mixing rules[J]. Industrial & Engineering Chemistry Research, 2017, 56(8): 2259-2276. |
| 71 | LACHET V , CRETON B , BRUIN T , et al . Equilibrium and transport properties of CO2+N2O and CO2+NO mixtures: molecular simulation and equation of state modelling study[J]. Fluid Phase Equilibria, 2012, 322-323: 66-78. |
| 72 | AHMAD M , GERNERT J , WILBERS E . Effect of impurities in captured CO2 on liquid-vapor equilibrium[J]. Fluid Phase Equilibria, 2014, 363(4): 149-155. |
| 73 | TAN Y , NOOKUEA W , LI H , et al . Property impacts on performance of CO2 pipeline transport[J]. Energy Procedia, 2015, 75: 2261-2267. |
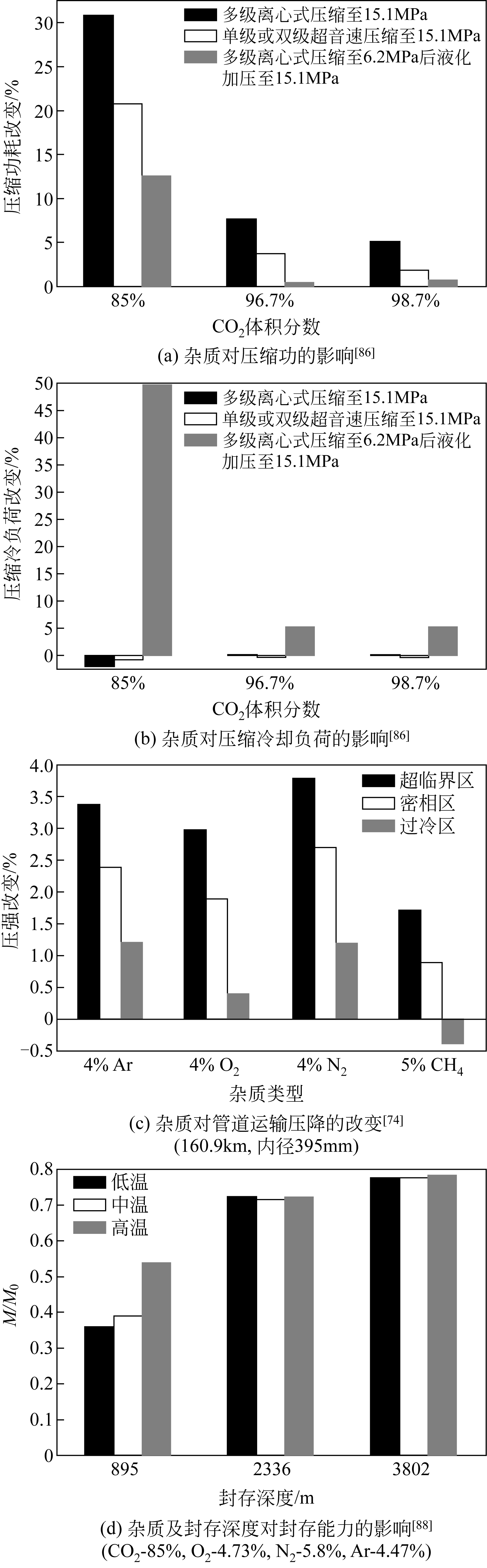
| 74 | MAZZOCCOLI M , GUIDO G D , BOSIO B , et al . CO2-mixture properties for pipeline transportation in the CCS process[J]. Chemical Engineering, 2013, 32: 1861-1866. |
| 75 | CHO M I, HUH C, KANG S G , et al . Evaluation of the two phase pressure drop during the CO2-N2 mixture pipeline transport[J]. Energy Procedia, 2014, 63(1): 2710-2714. |
| 76 | HUH C, CHO M I, HONG S , et al . Effect of impurities on depressurization of CO2 pipeline transport[J]. Energy Procedia, 2014, 63: 2583-2588. |
| 77 | CHO M I, HUH C, JUNG J Y , et al . Experimental study of N2 impurity effect on the steady and unsteady CO2 pipeline flow[J]. Energy Procedia, 2013, 37: 3039-3046. |
| 78 | HUH C, KANG S G , CHO M I, et al . Effect of water and nitrogen impurities on CO2 pipeline transport for geological storage[J]. Energy Procedia, 2011, 4: 2214-2221. |
| 79 | KOEIJER G D , BORCH J H , JAKOBSENB J , et al . Experiments and modeling of two-phase transient flow during CO2 pipeline depressurization[J]. Energy Procedia, 2009, 1: 1683-1689. |
| 80 | AURSAND P , HAMMER M , MUNKEJORD S T , et al . Pipeline transport of CO2 mixtures: models for transient simulation[J]. International Journal of Greenhouse Gas Control, 2013, 15: 174-185. |
| 81 | ZHAO Q , LI Y X . The influence of impurities on the transportation safety of an anthropogenic CO2 pipeline[J]. Process Safety & Environmental Protection, 2014, 92(1): 80-92. |
| 82 | VISSER E D , HENDRIKS C , BARRIO M , et al . Dynamics CO2 quality recommendations[J]. International Journal of Greenhouse Gas Control, 2008, 2(4): 478-484. |
| 83 | WALSPURGER S , DIJK H A J VAN . EDGAR CO2 purity: type and quantities of impurities related to CO2 point source and capture technology: a literature study [R]. Petten, The Netherlands: ECN, 2012. |
| 84 | WETENHALL B , AGHAJANI H , CHALMERS H , et al . Impact of CO2 impurity on CO2 compression, liquefaction and transportation[J]. Energy Procedia, 2014, 63(2): 2764-2778. |
| 85 | GOOS E , RIEDEL U , ZHAO L , et al . Phase diagrams of CO2 and CO2-N2 gas mixtures and their application in compression processes[J]. Energy Procedia, 2011, 4(1): 3778-3785. |
| 86 | MARTYNOV S B , DAUD N K , MAHGEREFTEH H , et al . Impact of stream impurities on compressor power requirements for CO2 pipeline transportation[J]. International Journal of Greenhouse Gas Control, 2016, 54: 652-661. |
| 87 | LI H L , YAN J Y , YAN J Y , et al . Impurity impacts on the purification process in oxy-fuel combustion based CO2 capture and storage system[J]. Applied Energy, 2009, 86(2): 202-213. |
| 88 | ZIABAKHSH-GANJI Z , KOOI H . Sensitivity of the CO2 storage capacity of underground geological structures to the presence of SO2 and other impurities[J]. Applied Energy, 2014, 135: 43-52. |
| 89 | WANG J S , RYAN D , ANTHONY E J , et al . Effects of impurities on CO2 transport, injection and storage[J]. Energy Procedia, 2011, 4(1): 3071-3078. |
| 90 | WANG J S , WANG Z Y , RYAN D , et al . A study of the effect of impurities on CO2 storage capacity in geological formations[J]. International Journal of Greenhouse Gas Control, 2015, 42: 132-137. |
| 91 | ZHAO R K , DENG S , WANG S P , et al . Thermodynamic research of adsorbent materials on energy efficiency of vacuum-pressure swing adsorption cycle for CO2 capture[J]. Applied Thermal Engineering, 2018, 128: 818-829. |
| 92 | SONG C F , KITAMURA Y , LI S H . Energy analysis of the cryogenic CO2 capture process based on stirling coolers[J]. Energy, 2014, 65(4): 580-589. |
| 93 | ALABDULKAREM A , HWANG Y , RADERMACHER R . Development of CO2 liquefaction cycles for CO2 sequestration[J]. Applied Thermal Engineering, 2012, 33(1): 144-156. |
| 94 | JEON S H , KIM M S . Effects of impurities on re-liquefaction system of liquefied CO2 transport ship for CCS[J]. International Journal of Greenhouse Gas Control, 2015, 43(2): 225-232. |
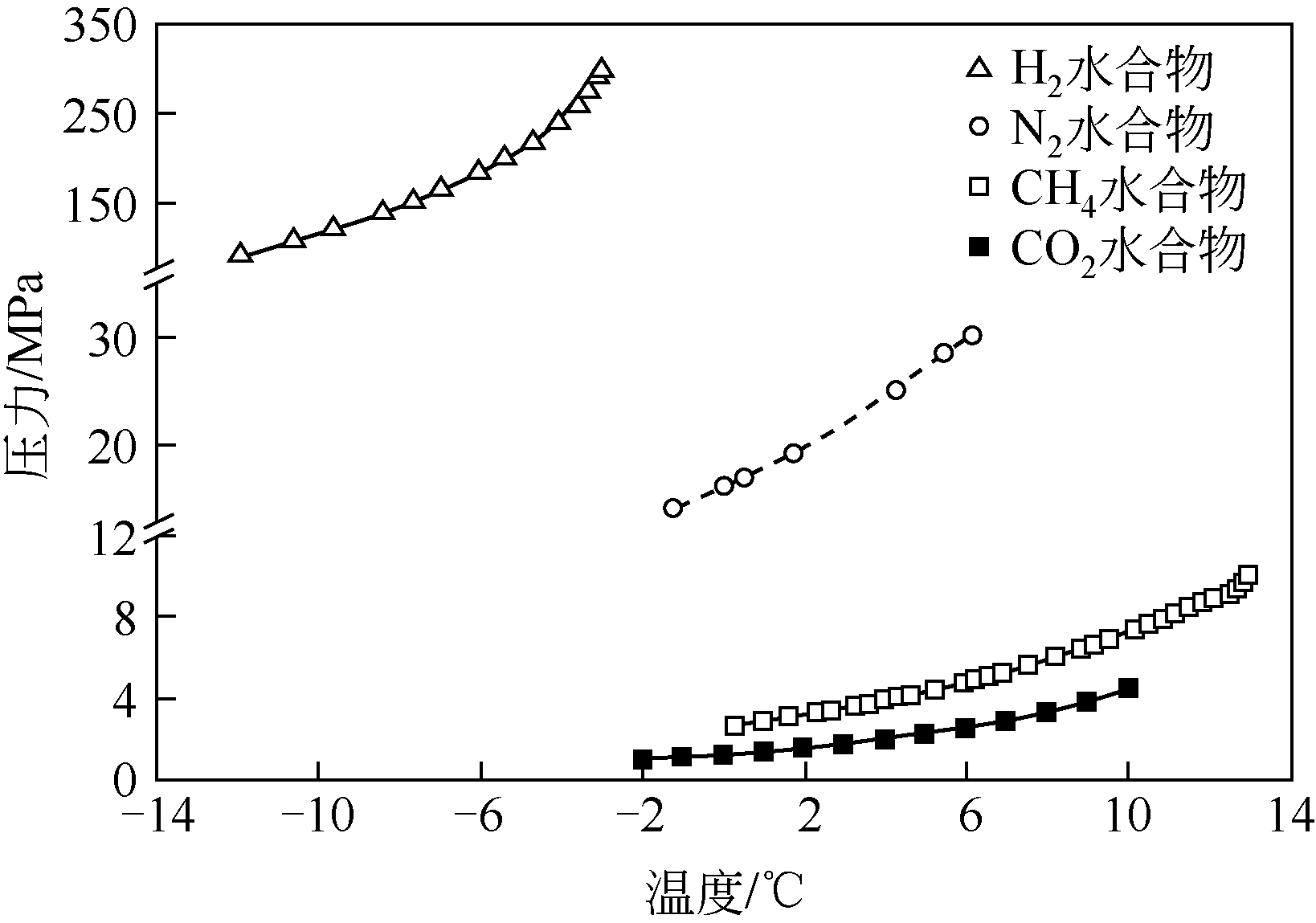
| 95 | DYADIN Y A , LARIONOV E G , MANAKOV A Y , et al . Clathrate hydrates of hydrogen and neon[J]. Mendeleev Communications, 1999, 9(5): 209-210. |
| 96 | MA Z W, ZHANG P , BAO H S , et al . Review of fundamental properties of CO2 hydrates and CO2 capture and separation using hydration method[J]. Renewable & Sustainable Energy Reviews, 2016, 53: 1273-1302. |
| 97 | UDACHIN K A , AND C I R, RIPMEESTER J A . Structure, composition, and thermal expansion of CO2 hydrate from single crystal X-ray diffraction measurements[J]. Journal of Physical Chemistry B, 2001, 105(19): 4200-4204. |
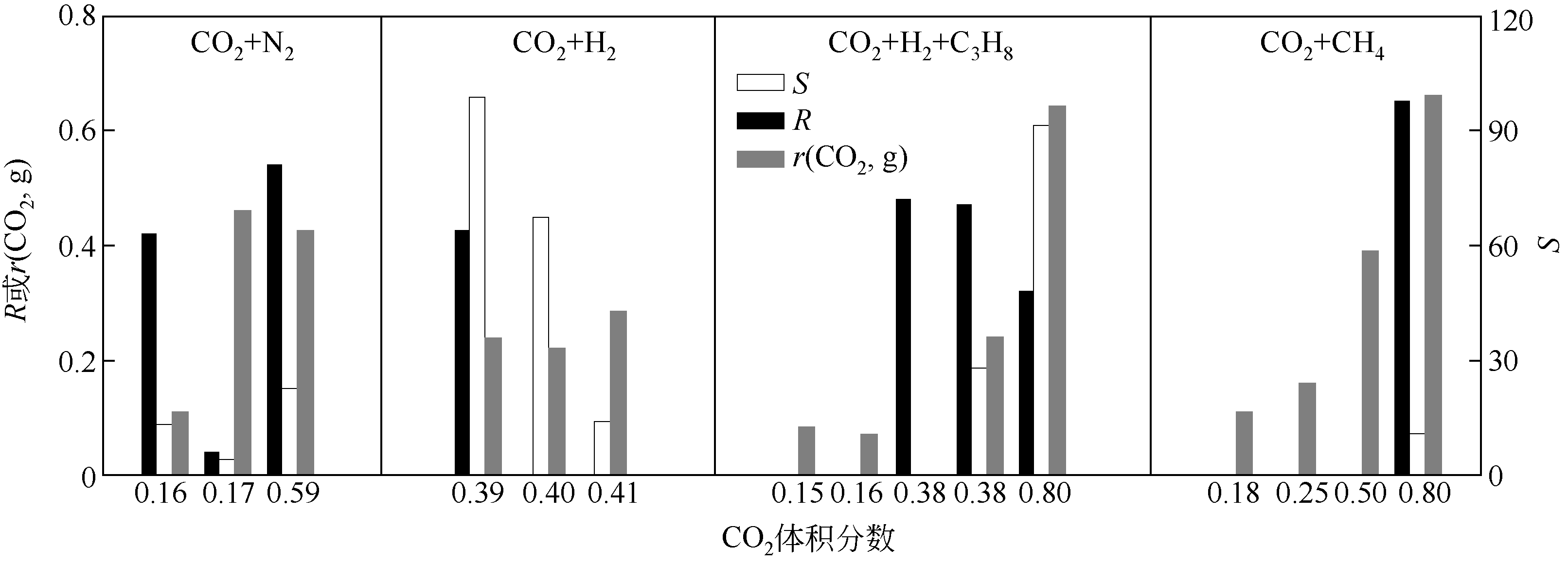
| 98 | LINGA P , KUMAR R , ENGLEZOS P . Gas hydrate formation from hydrogen/carbon dioxide and nitrogen/carbon dioxide gas mixtures[J]. Chemical Engineering Science, 2007, 62(16): 4268-4276. |
| 99 | LINGA P , KUMAR R , JU D L , et al . A new apparatus to enhance the rate of gas hydrate formation: application to capture of carbon dioxide[J]. International Journal of Greenhouse Gas Control, 2010, 4(4): 630-637. |
| 100 | TANG J F , ZENG D L , WANG C L , et al . Study on the influence of SDS and THF on hydrate-based gas separation performance[J]. Chemical Engineering Research & Design, 2013, 91(9): 1777-1782. |
| 101 | DARABOINA N , RIPMEESTER J , ENGLEZOS P . The impact of SO2 on post combustion carbon dioxide capture in bed of silica sand through hydrate formation[J]. International Journal of Greenhouse Gas Control, 2013, 15(5): 97-103. |
| 102 | KUMAR R , LINGA P , RIPMEESTER J A , et al . Two-stage clathrate hydrate/membrane process for pre-combustion capture of carbon dioxide and hydrogen[J]. Journal of Environmental Engineering, 2009, 135(6): 411. |
| 103 | SEO Y, KANG S P . Enhancing CO2 separation for pre-combustion capture with hydrate formation in silica gel pore structure[J]. Chemical Engineering Journal, 2010, 161(1/2): 308-312. |
| 104 | PARK S , LEE S, LEE Y, et al . Hydrate-based pre-combustion capture of carbon dioxide in the presence of a thermodynamic promoter and porous silica gels[J]. International Journal of Greenhouse Gas Control, 2013, 14(2): 193-199. |
| 105 | SUROVTSEVA D , AMIN R , BARIFCANI A . Design and operation of pilot plant for CO2 capture from IGCC flue gases by combined cryogenic and hydrate method[J]. Chemical Engineering Research & Design, 2011, 89(9): 1752-1757. |
| 106 | DENDEREN M V , INEKE E , GOLOMBOK M . CO2 removal from contaminated natural gas mixtures by hydrate formation[J]. Industrial & Engineering Chemistry Research, 2012, 48(12): 5802-5807. |
| 107 | 李玉星, 刘梦诗, 张建 . 气体杂质对CO2管道输送系统安全的影响[J]. 天然气工业, 2014, 34(1): 17. |
| LI Y X , LIU M S , ZHANG J . Impacts of gas impurities on the security of CO2 pipelines[J]. Natural Gas Industry, 2014, 34(1): 17 | |
| 108 | CHAPOY A , BURGASS R , TOHIDI B , et al . Hydrate and phase behavior modeling in CO2-rich pipelines[J]. Journal of Chemical & Engineering Data, 2014, 60(2): 447-453. |
| 109 | BURGASS R , CHAPOY A , DUCHET-SUCHAUX P , et al . Experimental water content measurements of carbon dioxide in equilibrium with hydrates at (223.15 to 263.15)K and (1.0 to 10.0)MPa[J]. Journal of Chemical Thermodynamics, 2014, 69: 1-5. |
| 110 | KIM S H, HUH C, KANG S-G , et al . Phase equilibria containing gas hydrate of carbon dioxide, sulfur dioxide, and water mixtures[J]. Journal of Chemical & Engineering Data, 2013, 58(6): 1879-1882. |
| 111 | AASEN A , HAMMER M , SKAUGEN G , et al . Thermodynamic models to accurately describe the pVTxy-behavior of water / carbon dioxide mixtures[J]. Fluid Phase Equilibria, 2017, 442: 125-139. |
| 112 | DIAMANTONIS N I , BOULOUGOURIS G C , MANSOOR E , et al . Evaluation of cubic, SAFT, and PC-SAFT equations of state for the vapor-liquid equilibrium modeling of CO2 mixtures with other gases[J]. Industrial & Engineering Chemistry Research, 2013, 52(10): 3933-3942. |
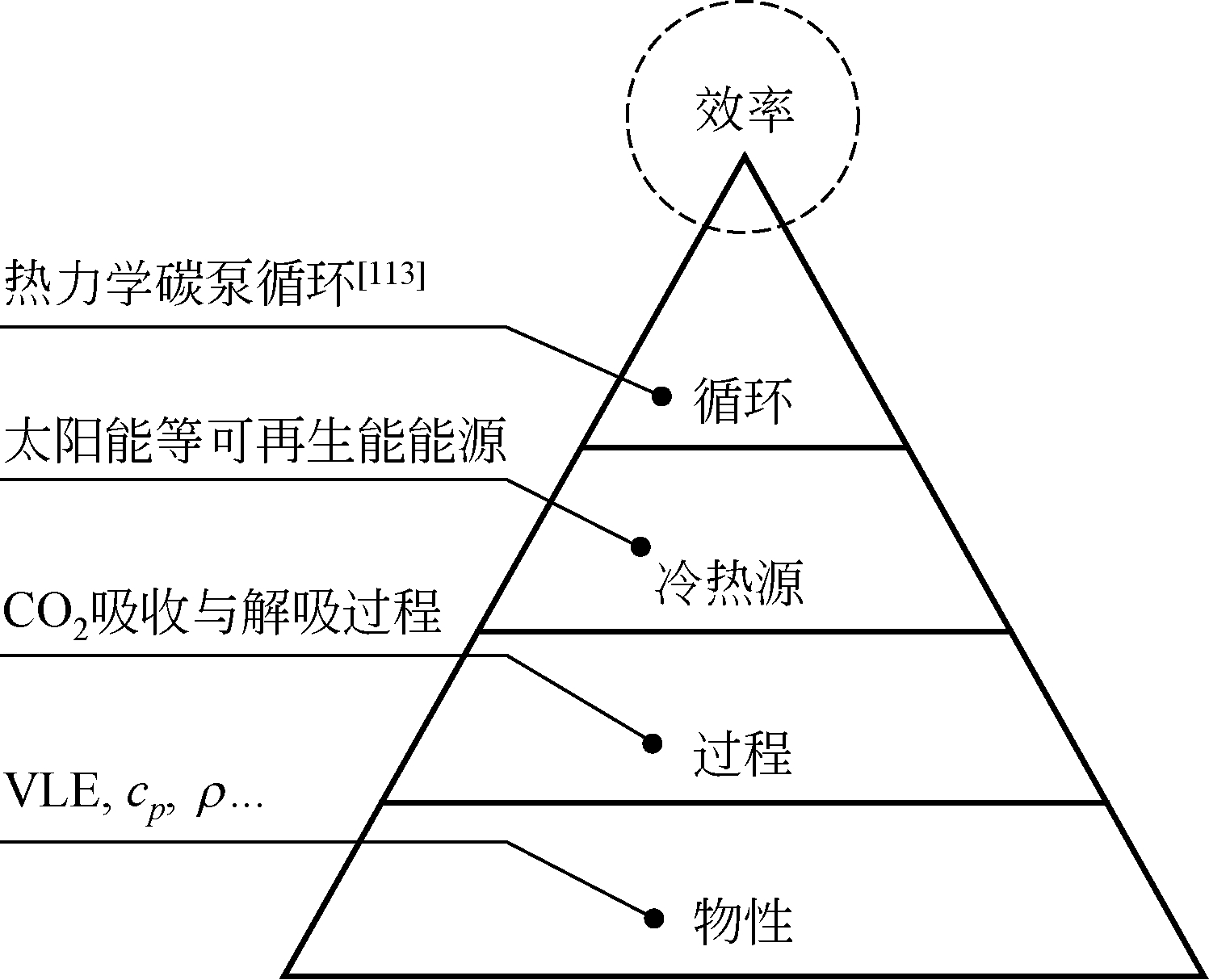
| 113 | ZHAO R K , DENG S , LIU Y N , et al . Carbon pump: fundamental theory and applications[J]. Energy, 2016, 119: 1131-1143. |
| [1] | 杨寒月, 孔令真, 陈家庆, 孙欢, 宋家恺, 王思诚, 孔标. 微气泡型下向流管式气液接触器脱碳性能[J]. 化工进展, 2023, 42(S1): 197-204. |
| [2] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [3] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [4] | 郑谦, 官修帅, 靳山彪, 张长明, 张小超. 铈锆固溶体Ce0.25Zr0.75O2光热协同催化CO2与甲醇合成DMC[J]. 化工进展, 2023, 42(S1): 319-327. |
| [5] | 戴欢涛, 曹苓玉, 游新秀, 徐浩亮, 汪涛, 项玮, 张学杨. 木质素浸渍柚子皮生物炭吸附CO2特性[J]. 化工进展, 2023, 42(S1): 356-363. |
| [6] | 孙玉玉, 蔡鑫磊, 汤吉海, 黄晶晶, 黄益平, 刘杰. 反应精馏合成甲基丙烯酸甲酯工艺优化及节能[J]. 化工进展, 2023, 42(S1): 56-63. |
| [7] | 王耀刚, 韩子姗, 高嘉辰, 王新宇, 李思琪, 杨全红, 翁哲. 铜基催化剂电还原二氧化碳选择性的调控策略[J]. 化工进展, 2023, 42(8): 4043-4057. |
| [8] | 刘毅, 房强, 钟达忠, 赵强, 李晋平. Ag/Cu耦合催化剂的Cu晶面调控用于电催化二氧化碳还原[J]. 化工进展, 2023, 42(8): 4136-4142. |
| [9] | 黄玉飞, 李子怡, 黄杨强, 金波, 罗潇, 梁志武. 光催化CO2和CH4重整催化剂研究进展[J]. 化工进展, 2023, 42(8): 4247-4263. |
| [10] | 娄宝辉, 吴贤豪, 张驰, 陈臻, 冯向东. 纳米流体用于二氧化碳吸收分离研究进展[J]. 化工进展, 2023, 42(7): 3802-3815. |
| [11] | 白亚迪, 邓帅, 赵睿恺, 赵力, 杨英霞. 变温吸附碳捕集机组标准化测试方案探讨及性能实验[J]. 化工进展, 2023, 42(7): 3834-3846. |
| [12] | 顾诗亚, 董亚超, 刘琳琳, 张磊, 庄钰, 都健. 考虑中间节点的碳捕集管路系统设计与优化[J]. 化工进展, 2023, 42(6): 2799-2808. |
| [13] | 杨许召, 李庆, 袁康康, 张盈盈, 韩敬莉, 吴诗德. 含Gemini离子液体低共熔溶剂热力学性质[J]. 化工进展, 2023, 42(6): 3123-3129. |
| [14] | 吕超, 张习文, 金理健, 杨林军. 新型两相吸收剂-离子液体系统高效捕获CO2[J]. 化工进展, 2023, 42(6): 3226-3232. |
| [15] | 王科菊, 赵成, 胡晓玫, 云军阁, 魏凝涵, 姜雪迎, 邹昀, 陈志航. 金属氧化物低温催化氧化VOCs的研究进展[J]. 化工进展, 2023, 42(5): 2402-2412. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||