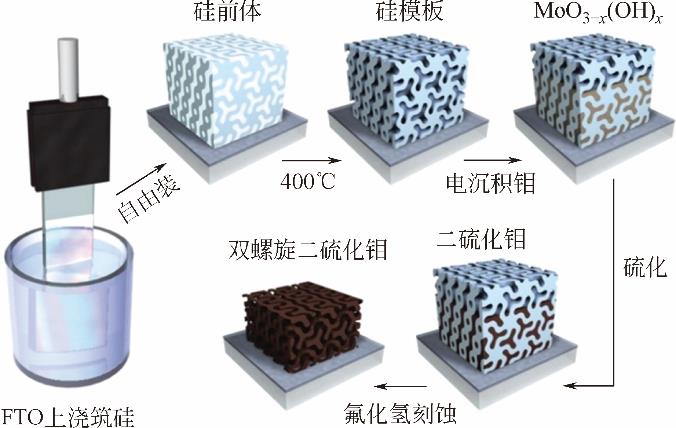
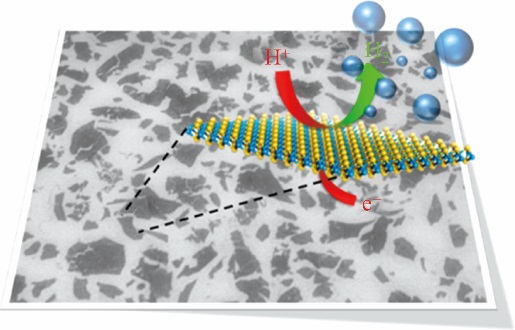
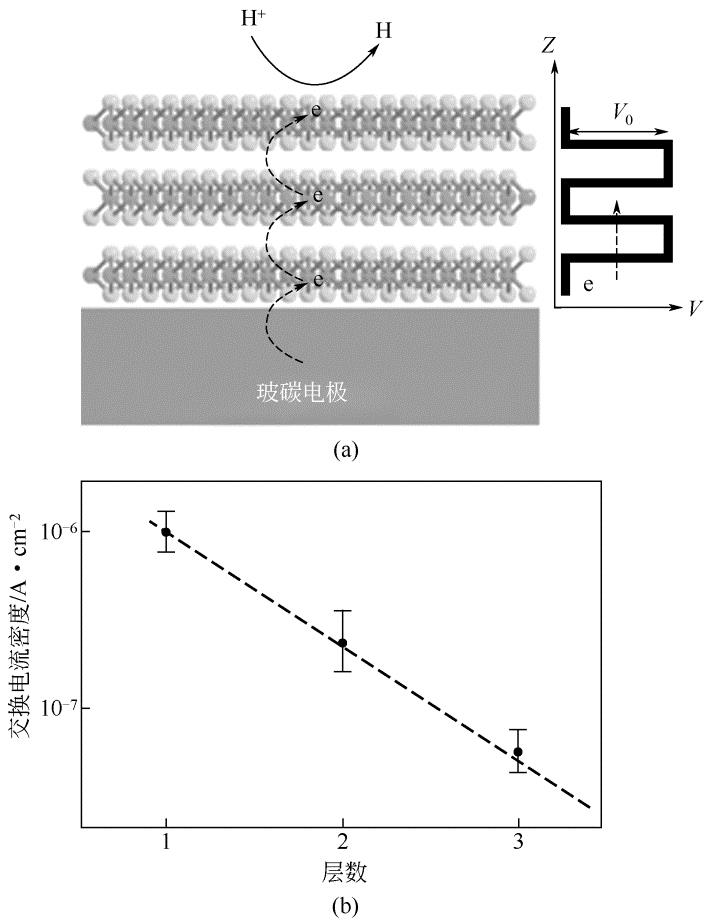
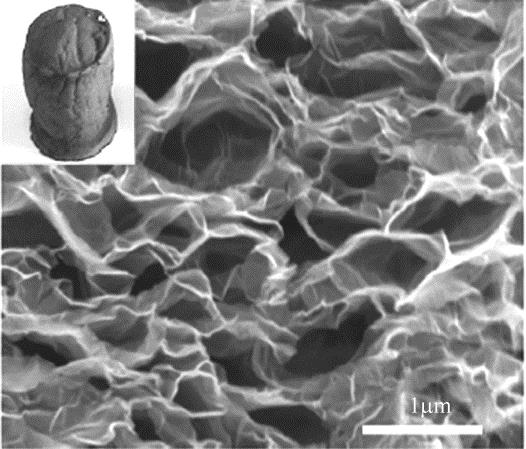
化工进展 ›› 2019, Vol. 38 ›› Issue (01): 278-291.DOI: 10.16085/j.issn.1000-6613.2018-1107
电解水制氢MoS2催化剂研究与氢能技术展望
- 清华大学化学工程系,北京 100084
-
收稿日期:2018-05-29修回日期:2018-11-02出版日期:2019-01-05发布日期:2019-01-05 -
通讯作者:王保国 -
作者简介:王培灿(1993—),男,博士研究生。E-mail:<email>wangpcleo@gmail.com</email>。|王保国,博士,教授,从事膜分离技术、储能科学与技术研究。E-mail:<email>bgwang@tsinghua.edu.cn</email>。 -
基金资助:国家自然科学基金(21776154);国家高技术研究发展计划(2012AA051203);国家自然科学基金(21776154);国家高技术研究发展计划(2012AA051203)。
MoS2-based electrocatalysts for hydrogen evolution and the prospect of hydrogen energy technology
Peican WANG( ),Qing LEI,Shuai LIU,Baoguo WANG(
),Qing LEI,Shuai LIU,Baoguo WANG( )
)
- Department of Chemical Engineering, Tsinghua University, Beijing 100084, China
-
Received:2018-05-29Revised:2018-11-02Online:2019-01-05Published:2019-01-05 -
Contact:Baoguo WANG
摘要:
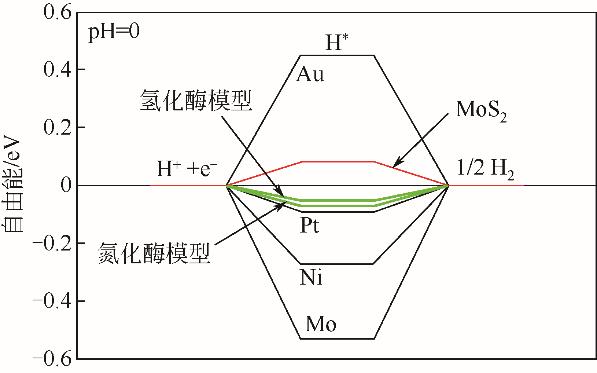
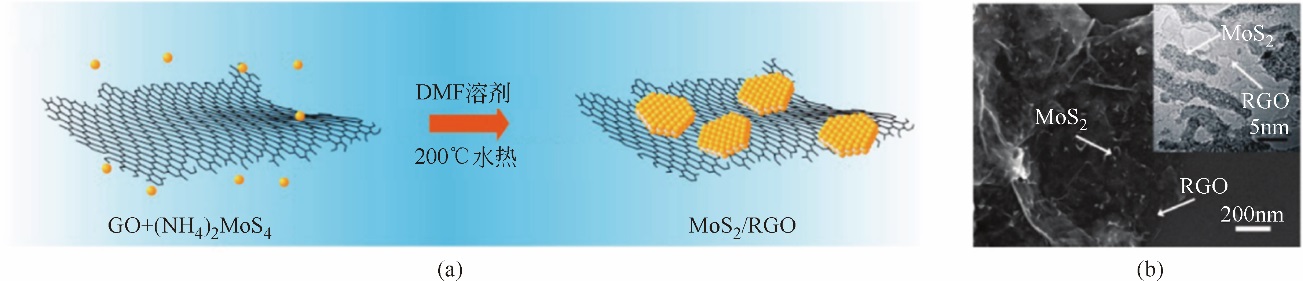
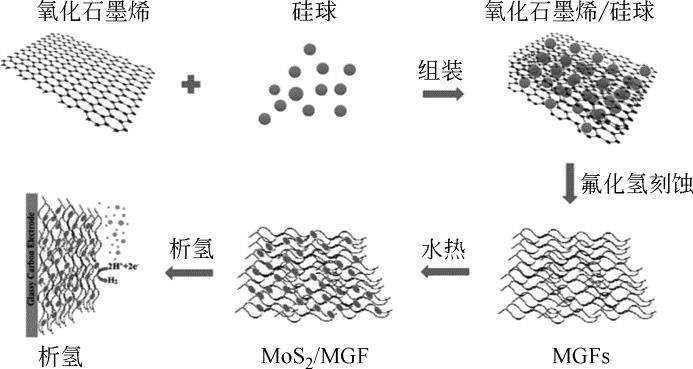
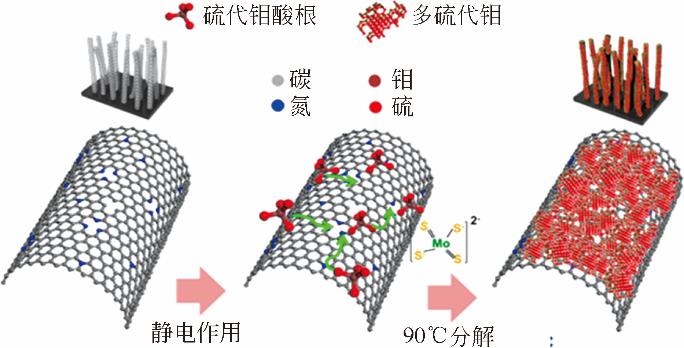
氢气具有质量轻、热值高、燃烧产物清洁等优点,被认为是理想的能源载体。氢气既能作为燃料电池的燃料,又能作为储能介质调节风能、太阳能发电系统的随机性、间歇性,正在成为未来能源的重要组成部分。为了促进电解水制氢技术与装备发展,研究高效电催化剂十分重要。本文围绕“粉末型”与“自支撑型”电催化剂结构特征,讨论基于二硫化钼(MoS2)的析氢电催化剂的研究现状,阐述了催化活性位点调控策略与提高导电性两条技术途径,并以析氢过电位和塔菲尔曲线斜率为依据,比较不同方法制备的二硫化钼电催化剂的催化活性。表明提高二硫化钼晶相稳定性、调节其电子结构和优化催化电极结构等方法,将进一步提高基于二硫化钼的析氢催化电极性能。
中图分类号:
引用本文
王培灿, 雷青, 刘帅, 王保国. 电解水制氢MoS2催化剂研究与氢能技术展望[J]. 化工进展, 2019, 38(01): 278-291.
Peican WANG, Qing LEI, Shuai LIU, Baoguo WANG. MoS2-based electrocatalysts for hydrogen evolution and the prospect of hydrogen energy technology[J]. Chemical Industry and Engineering Progress, 2019, 38(01): 278-291.
| 种类 | 催化剂 | 起始过电位/mV | 10mA·cm-2相应过电位/mV | 塔菲尔斜率/mV·dec-1 | 参考文献 |
|---|---|---|---|---|---|
| 纯二硫化钼型 | 二硫化钼纳米带 | 170 | 约180 | 70 | [ |
| 富缺陷二硫化钼 | 120 | 约180 | 50 | [ | |
| 1T相二硫化钼 | 100 | 约200 | 40 | [ | |
| 氧掺杂二硫化钼 | 120 | 约180 | 55 | [ | |
| 双螺旋二硫化钼 | — | 206 | 50 | [ | |
| 镍-磷/多硫化钼 | — | 140 | 64 | [ | |
| 量子点二硫化钼 | 约120 | 约250 | 69 | [ | |
| 基底型 | 石墨烯基二硫化钼 | 约100 | 150 | 41 | [ |
| 氮杂石墨烯二硫化钼 | 100 | 146 | 105 | [ | |
| 二硫化钼/还原性石墨烯 | 约140 | — | 46 | [ | |
| 介孔石墨烯基二硫化钼 | 100 | — | 42 | [ | |
| 二硫化钼/氮杂还原性石墨烯 | 5 | 56 | 41.3 | [ | |
| 低结晶度二硫化钼 | 90 | 约170 | 40 | [ | |
| 多硫化钼/氧化石墨烯 | — | 约180 | 47.7 | [ | |
| 自支撑型 | 二硫化钼/碳布 | 100 | 150 | 50 | [ |
| 二硫化钼/碳布 | 100 | — | 39 | [ | |
| 二氧化钼/还原性氧化石墨烯 | 190 | — | 95 | [ | |
| 二硫化钼/氧化石墨烯膜 | 70 | 100 | 41 | [ | |
| 二硫化钼/玻碳 | 约200 | 约400 | 约105 | [ | |
| 二硫化钼/二氧化钼/碳布 | 142 | 约200 | 35.6 | [ | |
| 二硫化钼/二硫化钴/碳布 | — | 87 | 73.4 | [ | |
| 镍杂硫化钼/碳布 | 130 | 约200 | 85.4 | [ | |
| 二硫化钼/石墨烯骨架 | 107 | — | 86.3 | [ |
表1 不同类型二硫化钼基析氢催化电极性能比较
| 种类 | 催化剂 | 起始过电位/mV | 10mA·cm-2相应过电位/mV | 塔菲尔斜率/mV·dec-1 | 参考文献 |
|---|---|---|---|---|---|
| 纯二硫化钼型 | 二硫化钼纳米带 | 170 | 约180 | 70 | [ |
| 富缺陷二硫化钼 | 120 | 约180 | 50 | [ | |
| 1T相二硫化钼 | 100 | 约200 | 40 | [ | |
| 氧掺杂二硫化钼 | 120 | 约180 | 55 | [ | |
| 双螺旋二硫化钼 | — | 206 | 50 | [ | |
| 镍-磷/多硫化钼 | — | 140 | 64 | [ | |
| 量子点二硫化钼 | 约120 | 约250 | 69 | [ | |
| 基底型 | 石墨烯基二硫化钼 | 约100 | 150 | 41 | [ |
| 氮杂石墨烯二硫化钼 | 100 | 146 | 105 | [ | |
| 二硫化钼/还原性石墨烯 | 约140 | — | 46 | [ | |
| 介孔石墨烯基二硫化钼 | 100 | — | 42 | [ | |
| 二硫化钼/氮杂还原性石墨烯 | 5 | 56 | 41.3 | [ | |
| 低结晶度二硫化钼 | 90 | 约170 | 40 | [ | |
| 多硫化钼/氧化石墨烯 | — | 约180 | 47.7 | [ | |
| 自支撑型 | 二硫化钼/碳布 | 100 | 150 | 50 | [ |
| 二硫化钼/碳布 | 100 | — | 39 | [ | |
| 二氧化钼/还原性氧化石墨烯 | 190 | — | 95 | [ | |
| 二硫化钼/氧化石墨烯膜 | 70 | 100 | 41 | [ | |
| 二硫化钼/玻碳 | 约200 | 约400 | 约105 | [ | |
| 二硫化钼/二氧化钼/碳布 | 142 | 约200 | 35.6 | [ | |
| 二硫化钼/二硫化钴/碳布 | — | 87 | 73.4 | [ | |
| 镍杂硫化钼/碳布 | 130 | 约200 | 85.4 | [ | |
| 二硫化钼/石墨烯骨架 | 107 | — | 86.3 | [ |
| 1 | DINCER I , ACAR C . Review and evaluation of hydrogen production methods for better sustainability[J]. International Journal of Hydrogen Energy, 2015, 40(34): 11094-11111. |
| 2 | WANG L , LI Y , YIN X , et al . Comparison of three nickel-based carbon composite catalysts for hydrogen evolution reaction in alkaline solution[J]. International Journal of Hydrogen Energy, 2017, 42(36): 22655-22662. |
| 3 | COOK T R , DOGUTAN D K , REECE S Y , et al . Solar energy supply and storage for the legacy and nonlegacy worlds[J]. Chemical reviews, 2010, 110(11): 6474-6502. |
| 4 | ZENG K , ZHANG D K . Recent progress in alkaline water electrolysis for hydrogen production and applications[J]. Progress in Energy & Combustion Science, 2010, 36(3): 307-326. |
| 5 | PAUL B , ANDREWS J . PEM unitised reversible/regenerative hydrogen fuel cell systems: state of the art and technical challenges[J]. Renewable & Sustainable Energy Reviews, 2017, 79: 585-599. |
| 6 | 王保国, 王培灿, 雷青, 等 . 一种电解水制氢气的方法及装置:CN105483747A[P]. 2016-04-13. |
| WANG B G , WANG P C , LEI Q , et al .Hydrogen production method and device through electrolysis of water: CN105483747A[P]. 2016-04-13. | |
| 7 | MA L, XU L , XU X , et al . Cobalt-doped edge-rich MoS2/nitrogenated graphene composite as an electrocatalyst for hydrogen evolution reaction[J]. Materials Science & Engineering B, 2016, 212: 30-38. |
| 8 | TAN Y , YU K , YANG T , et al . The combinations of hollow MoS2 micro@nano-spheres: one-step synthesis, excellent photocatalytic and humidity sensing properties[J]. Journal of Materials Chemistry C, 2014, 2(27): 5422-5430. |
| 9 | TIAN J , LIU Q , ASIRI A M , et al . Self-supported nanoporous cobalt phosphide nanowire arrays: an efficient 3D hydrogen-evolving cathode over the wide range of pH 0-14[J]. Journal of the American Chemical Society, 2014, 136(21): 7587-7590. |
| 10 | TANG C , GAN L , ZHANG R , et al . Ternary Fe x Co1– x P nanowire array as a robust hydrogen evolution reaction electrocatalyst with Pt-like activity: experimental and theoretical insight[J]. Nano letters, 2016, 16(10): 6617-6621. |
| 11 | WANG H , KONG D , JOHANES P , et al . MoSe2 and WSe2 nanofilms with vertically aligned molecular layers on curved and rough surfaces[J]. Nano Letters, 2013, 13(7): 3426-3433. |
| 12 | DUAN J , CHEN S , JARONIEC M , et al . Porous C3N4 nanolayers@N-graphene films as catalyst electrodes for highly efficient hydrogen evolution[J]. ACS Nano, 2015, 9(1): 931-940. |
| 13 | CONWAY B E , TILAK B V . Interfacial processes involving electrocatalytic evolution and oxidation of H2, and the role of chemisorbed H[J]. Electrochimica Acta, 2002, 47(22): 3571-3594. |
| 14 | MCCRORY C C , JUNG S , FERRER I M , et al . Benchmarking hydrogen evolving reaction and oxygen evolving reaction electrocatalysts for solar water splitting devices[J].J.Am.Chem.Soc., 2015, 137(13): 4347-4357. |
| 15 | KIBSGAARD J , JARAMILLO T F , BESENBACHER F . Building an appropriate active-site motif into a hydrogen-evolution catalyst with thiomolybdate [Mo3S13]2- clusters[J]. Nature Chemistry, 2014, 6(3): 248-253. |
| 16 | HU J , HUANG B , ZHANG C , et al . Engineering stepped edge surface structures of MoS2 sheet stacks to accelerate the hydrogen evolution reaction[J]. Energy & Environmental Science, 2017, 10(2): 593-603. |
| 17 | KONG D , WANG H , CHA J J , et al . Synthesis of MoS2 and MoSe2 films with vertically aligned layers[J]. Nano Lett., 2013, 13(3): 1341-1347. |
| 18 | HINNEMANN B , MOSES P G , BONDE J , et al . Biomimetic hydrogen evolution: MoS2 nanoparticles as catalyst for hydrogen evolution[J].AmJ. Chem. Soc., 2005, 36(25): 5308-5309. |
| 19 | JARAMILLO T F , JΦGENSEN K P , BONDE J , et al . Identification of active edge sites for electrochemical H2 evolution from MoS2 nanocatalysts[J]. Science, 2007, 317(5834): 100-102. |
| 20 | KARUNADASA H I , MONTALVO E , SUN Y , et al . A molecular MoS2 edge site mimic for catalytic hydrogen generation[J]. Science, 2012, 335(6069): 698-702. |
| 21 | SUN Y , ALIMOHAMMADI F , ZHANG D , et al . Enabling colloidal synthesis of edge-oriented MoS2 with expanded interlayer spacing for enhanced HER catalysis[J]. Nano Letters, 2017, 17(3): 1963-1969. |
| 22 | KUMAR S , SAHOO P K , SATPATI A K . Electrochemical and SECM investigation of MoS2/GO and MoS2/rGO nanocomposite materials for HER electrocatalysis[J]. ACS Omega, 2017, 2(11): 7532-7545. |
| 23 | JIANG W J , LIN G , LI L , et al . Understanding the high activity of Fe-N-C electrocatalysts in oxygen reduction: Fe/Fe3C nanoparticles boost the activity of Fe-N x [J].J.Am.Chem.Soc., 2016, 138(10): 3570-3578. |
| 24 | LI Y G , WANG H L , XIE L M , et al . MoS2 nanoparticles grown on graphene: an advanced catalyst for the hydrogen evolution reaction[J].AmJ. Chem. Soc., 2011, 133(19): 7296-7299. |
| 25 | ZHENG X , XU J , YAN K , et al . Space-confined growth of MoS2 nanosheets within graphite: the layered hybrid of MoS2 and graphene as an active catalyst for hydrogen evolution reaction[J]. Chemistry of Materials, 2014, 26(7): 2344-2353. |
| 26 | YAN Y , GE X , LIU Z , et al . Facile synthesis of low crystalline MoS2 nanosheet-coated CNTs for enhanced hydrogen evolution reaction[J]. Nanoscale, 2013, 5(17): 7768-7771. |
| 27 | LIAO L , ZHU J , BIAN X , et al . MoS2 formed on mesoporous graphene as a highly active catalyst for hydrogen evolution[J]. Advanced Functional Materials, 2013, 23(42): 5326-5333. |
| 28 | RHEEM Y , HAN Y , LEE K H , et al . Synthesis of hierarchical MoO2/MoS2 nanofibers for electrocatalytic hydrogen evolution[J]. Nanotechnology, 2017, 28(10): 105605. |
| 29 | GUO Y , GAN L , SHANG C , et al . A cake-style CoS2@MoS2/RGO hybrid catalyst for efficient hydrogen evolution[J]. Advanced Functional Materials, 2017, 27(5): 1602699. |
| 30 | LI D J , MAITI U N , LIM J , et al . Molybdenum sulfide/N-doped CNT forest hybrid catalysts for high-performance hydrogen evolution reaction[J]. Nano Lett., 2014, 14(3): 1228-1233. |
| 31 | TANG Y J , WANG Y , WANG X L , et al . Molybdenum disulfide/nitrogen-doped reduced graphene oxide nanocomposite with enlarged interlayer spacing for electrocatalytic hydrogen evolution[J]. Advanced Energy Materials, 2016, 6(12): 1600116. |
| 32 | DAI X , DU K , LI Z , et al . Co-doped MoS2 nanosheets with the dominant CoMoS phase coated on carbon as an excellent electrocatalyst for hydrogen evolution[J]. ACS Appl. Mater. Interfaces, 2015, 7(49): 27242-27253. |
| 33 | WU Z , FANG B , WANG Z , et al . MoS2 nanosheets: a designed structure with high active site density for the hydrogen evolution reaction[J]. ACS Catalysis, 2013, 3(9): 2101-2107. |
| 34 | XU S , LI D , WU P . One-pot, facile, and versatile synthesis of monolayer MoS2/WS2 quantum dots as bioimaging probes and efficient electrocatalysts for hydrogen evolution reaction[J]. Advanced Functional Materials, 2015, 25(7): 1127-1136. |
| 35 | HUANG L B , ZHAO L , ZHANG Y , et al . Self-limited on-site conversion of MoO3 nanodots into vertically aligned ultrasmall monolayer MoS2 for efficient hydrogen evolution[J]. Advanced Energy Materials, 2018, 8(21): 1800734. |
| 36 | KIBSGAARD J , CHEN Z , REINECKE B N , et al . Engineering the surface structure of MoS2 to preferentially expose active edge sites for electrocatalysis[J]. Nat. Mater., 2012, 11(11): 963-969. |
| 37 | ZHANG L , WU H B , YAN Y , et al . Hierarchical MoS2 microboxes constructed by nanosheets with enhanced electrochemical properties for lithium storage and water splitting[J]. Energy Environ. Sci., 2014, 7(10): 3302-3306. |
| 38 | SHANG X , HU W H , LI X , et al . Oriented stacking along vertical (002) planes of MoS2: a novel assembling style to enhance activity for hydrogen evolution[J]. Electrochimica Acta, 2017, 224∶ 25-31. |
| 39 | XIE J , ZHANG H , LI S , et al . Defect-rich MoS2 ultrathin nanosheets with additional active edge sites for enhanced electrocatalytic hydrogen evolution[J]. Adv. Mater., 2013, 25(40): 5807-5813. |
| 40 | LI H , TSAI C , KOH A L , et al . Activating and optimizing MoS2 basal planes for hydrogen evolution through the formation of strained sulphur vacancies[J]. Nature Materials, 2016, 15(1): 48-53. |
| 41 | BONDE J , MOSES P G , JARAMILLO T F , et al . Hydrogen evolution on nano-particulate transition metal sulfides[J]. Faraday Discussions, 2008, 140(1): 219-231. |
| 42 | XIE J , ZHANG J , LI S , et al . Controllable disorder engineering in oxygen-incorporated MoS2 ultrathin nanosheets for efficient hydrogen evolution[J].AmJ. Chem. Soc., 2013, 135(47): 17881-17888. |
| 43 | YU X Y , FENG Y , JEON Y , et al . Formation of Ni-Co-MoS2 nanoboxes with enhanced electrocatalytic activity for hydrogen evolution[J]. Adv. Mater., 2016, 28(40): 9006-9011. |
| 44 | XIAO W , LIU P , ZHANG J , et al . Dual-functional N dopants in edges and basal plane of MoS2 nanosheets toward efficient and durable hydrogen evolution[J]. Advanced Energy Materials, 2017, 7(7): 1602086. |
| 45 | HUANG X , LENG M , XIAO W , et al . Activating basal planes and S-terminated edges of MoS2 toward more efficient hydrogen evolution[J]. Advanced Functional Materials, 2016, 27(6): 1604943. |
| 46 | XIONG Q , WANG Y , LIU P F , et al . Cobalt covalent doping in MoS2 to induce bifunctionality of overall water splitting[J]. Adv. Mater., 2018, 30(29): 1801450. |
| 47 | LUKOWSKI M A , DANIEL A S , MENG F , et al . Enhanced hydrogen evolution catalysis from chemically exfoliated metallic MoS2 nanosheets[J]. Journal of the American Chemical Society, 2013, 135(28): 10274-10277. |
| 48 | WANG H , LU Z , XU S , et al . Electrochemical tuning of vertically aligned MoS2 nanofilms and its application in improving hydrogen evolution reaction[J]. Proc. Natl. Acad. Sci. USA, 2013, 110(49): 19701-19706. |
| 49 | VOIRY D , SALEHI M , SILVA R , et al . Conducting MoS2 nanosheets as catalysts for hydrogen evolution reaction[J]. Nano Letters, 2013, 13(12): 6222-6227. |
| 50 | ZHANG J , WU J , GUO H , et al . Unveiling active sites for the hydrogen evolution reaction on monolayer MoS2 [J]. Advanced Materials, 2017, 29(42): 1701955. |
| 51 | WANG S , ZHANG D , LI B , et al . Ultrastable in-plane 1T-2H MoS2 heterostructures for enhanced hydrogen evolution reaction[J]. Advanced Energy Materials, 2018, 8(25): 1801345. |
| 52 | LI H , CHEN S , JIA X , et al . Amorphous nickel-cobalt complexes hybridized with 1T-phase molybdenum disulfide via hydrazine-induced phase transformation for water splitting[J]. Nat. Commun., 2017, 8: 15377. |
| 53 | YU Y , HUANG S Y , LI Y , et al . Layer-dependent electrocatalysis of MoS2 for hydrogen evolution[J]. Nano Lett., 2014, 14(2): 553-558. |
| 54 | YAN Y , XIA B , LI N , et al . Vertically oriented MoS2 and WS2 nanosheets directly grown on carbon cloth as efficient and stable 3-dimensional hydrogen-evolving cathodes[J]. Journal of Materials Chemistry A, 2015, 3(1): 131-135. |
| 55 | YU H , YU X , CHEN Y , et al . A strategy to synergistically increase the number of active edge sites and the conductivity of MoS2 nanosheets for hydrogen evolution[J]. Nanoscale, 2015, 7(19): 8731-8738. |
| 56 | ZHANG Z , LI W , YUEN M F , et al . Hierarchical composite structure of few-layers MoS2 nanosheets supported by vertical graphene on carbon cloth for high-performance hydrogen evolution reaction[J]. Nano Energy, 2015, 18: 196-204. |
| 57 | ZHANG Z , LI W , YUEN M F , et al . Hierarchical composite structure of few-layers MoS2 nanosheets supported by vertical graphene on carbon cloth for high-performance hydrogen evolution reaction[J]. Nano Energy, 2015, 18: 196-204. |
| 58 | NIKAM R D , LU A Y , SONAWANE P A , et al . Three-dimensional heterostructures of MoS2 nanosheets on conducting MoO2 as an efficient electrocatalyst to enhance hydrogen evolution reaction[J]. ACS Appl. Mater. Interfaces, 2015, 7(41): 23328-23335. |
| 59 | SU C , XIANG J , WEN F , et al . Microwave synthesized three-dimensional hierarchical nanostructure CoS2/MoS2 growth on carbon fiber cloth: a bifunctional electrode for hydrogen evolution reaction and supercapacitor[J]. Electrochimica Acta, 2016, 212:941-949. |
| 60 | MIAO J , XIAO F X , YANG H B , et al . Hierarchical Ni-Mo-S nanosheets on carbon fiber cloth: a flexible electrode for efficient hydrogen generation in neutral electrolyte[J]. Science Advances, 2015, 1(7): e1500259. |
| 61 | ZHU H , LYU F , DU M , et al . Design of two-dimensional, ultrathin MoS2 nanoplates fabricated within one-dimensional carbon nanofibers with thermosensitive morphology: high-performance electrocatalysts for the hydrogen evolution reaction[J]. ACS Appl. Mater. Interfaces, 2014, 6(24): 22126-22137. |
| 62 | OUYANG C , FENG S , HUO J , et al . Three-dimensional hierarchical MoS2 /CoS2 heterostructure arrays for highly efficient electrocatalytic hydrogen evolution[J]. Green Energy & Environment, 2017, 2(2): 134-141. |
| 63 | ZHOU W , ZHOU K , HOU D , et al . Three-dimensional hierarchical frameworks based on MoS2 nanosheets self-assembled on graphene oxide for efficient electrocatalytic hydrogen evolution[J]. ACS Appl. Mater. Interfaces, 2014, 6(23): 21534-21540. |
| 64 | YANG Y , FEI H , RUAN G , et al . Edge-oriented MoS2 nanoporous films as flexible electrodes for hydrogen evolution reactions and supercapacitor devices[J]. Adv. Mater., 2014, 26(48): 8163-8168. |
| 65 | HAN G-Q , LI X , XUE J , et al . Electrodeposited hybrid Ni-P/MoS x film as efficient electrocatalyst for hydrogen evolution in alkaline media[J]. International Journal of Hydrogen Energy, 2017, 42(5): 2952-2960. |
| 66 | CHEN S , DUAN J , TANG Y , et al . Molybdenum sulfide clusters-nitrogen-doped graphene hybrid hydrogel film as an efficient three-dimensional hydrogen evolution electrocatalyst[J]. Nano Energy, 2015, 11: 11-18. |
| 67 | HU W H , SHANG X , HAN G Q , et al . MoS x supported graphene oxides with different degree of oxidation as efficient electrocatalysts for hydrogen evolution[J]. Carbon, 2016, 100: 236-242. |
| 68 | ZHANG N , GAN S , WU T , et al . Growth control of MoS2 nanosheets on carbon cloth for maximum active edges exposed: an excellent hydrogen evolution 3D cathode[J]. ACS Appl. Mater. Interfaces, 2015, 7(22): 12193-12202. |
| 69 | MA C B, QI X , CHEN B , et al . MoS2 nanoflower-decorated reduced graphene oxide paper for high-performance hydrogen evolution reaction[J]. Nanoscale, 2014, 6(11): 5624-5629. |
| 70 | BEHRANGINIA A , ASADI M , LIU C , et al . Highly efficient hydrogen evolution reaction using crystalline layered three-dimensional molybdenum disulfides grown on graphene film[J]. Chemistry of Materials, 2016, 28(2): 549-555. |
| [1] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [2] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [3] | 杨建平. 降低HPPO装置反应系统原料消耗的PSE[J]. 化工进展, 2023, 42(S1): 21-32. |
| [4] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [5] | 葛全倩, 徐迈, 梁铣, 王凤武. MOFs材料在光电催化领域应用的研究进展[J]. 化工进展, 2023, 42(9): 4692-4705. |
| [6] | 史柯柯, 刘木子, 赵强, 李晋平, 刘光. 镁基储氢材料的性能及研究进展[J]. 化工进展, 2023, 42(9): 4731-4745. |
| [7] | 刘木子, 史柯柯, 赵强, 李晋平, 刘光. 固体储氢材料的研究进展[J]. 化工进展, 2023, 42(9): 4746-4769. |
| [8] | 毛善俊, 王哲, 王勇. 基团辨识加氢:从概念到应用[J]. 化工进展, 2023, 42(8): 3917-3922. |
| [9] | 王兰江, 梁瑜, 汤琼, 唐明兴, 李学宽, 刘雷, 董晋湘. 快速热解铂前体合成高分散的Pt/HY催化剂及其萘深度加氢性能[J]. 化工进展, 2023, 42(8): 4159-4166. |
| [10] | 郭晋, 张耕, 陈国华, 朱鸣, 谭粤, 李蔚, 夏莉, 胡昆. 车载液氢气瓶设计技术的研究进展[J]. 化工进展, 2023, 42(8): 4221-4229. |
| [11] | 张亚娟, 徐惠, 胡贝, 史星伟. 化学镀法制备NiCoP/rGO/NF高效电解水析氢催化剂[J]. 化工进展, 2023, 42(8): 4275-4282. |
| [12] | 王晓晗, 周亚松, 于志庆, 魏强, 孙劲晓, 姜鹏. 不同晶粒尺寸Y分子筛的合成及其加氢裂化反应性能[J]. 化工进展, 2023, 42(8): 4283-4295. |
| [13] | 储甜甜, 刘润竹, 杜高华, 马嘉浩, 张孝阿, 王成忠, 张军营. 有机胍催化脱氢型RTV硅橡胶的制备和可降解性能[J]. 化工进展, 2023, 42(7): 3664-3673. |
| [14] | 于姗, 段元刚, 张怡欣, 唐春, 付梦瑶, 黄靖元, 周莹. 分步法分解硫化氢制氢和硫黄催化剂研究进展[J]. 化工进展, 2023, 42(7): 3780-3790. |
| [15] | 孙旭东, 赵玉莹, 李诗睿, 王琦, 李晓健, 张博. 我国地方性氢能发展政策的文本量化分析[J]. 化工进展, 2023, 42(7): 3478-3488. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||