化工进展 ›› 2023, Vol. 42 ›› Issue (7): 3780-3790.DOI: 10.16085/j.issn.1000-6613.2022-1633
分步法分解硫化氢制氢和硫黄催化剂研究进展
于姗1,2( ), 段元刚1,2, 张怡欣2, 唐春1,2, 付梦瑶2, 黄靖元2, 周莹1,2(
), 段元刚1,2, 张怡欣2, 唐春1,2, 付梦瑶2, 黄靖元2, 周莹1,2( )
)
- 1.油气藏地质及开发工程国家重点实验室,四川 成都 610500
2.西南石油大学新能源与材料学院,四川 成都 610500
-
收稿日期:2022-09-05修回日期:2022-11-08出版日期:2023-07-15发布日期:2023-08-14 -
通讯作者:于姗,周莹 -
作者简介:于姗(1986—),女,博士研究生,副教授,研究方向为天然气资源的清洁利用。E-mail:yushan@swpu.edu.cn。 -
基金资助:国家自然科学基金(22211530070);2021年成都市鼓励校地校企合作培养产业发展人才项目
Research progress of catalysts for two-step hydrogen sulfide decomposition to produce hydrogen and sulfur
YU Shan1,2( ), DUAN Yuangang1,2, ZHANG Yixin2, TANG Chun1,2, FU Mengyao2, HUANG Jinyuan2, ZHOU Ying1,2(
), DUAN Yuangang1,2, ZHANG Yixin2, TANG Chun1,2, FU Mengyao2, HUANG Jinyuan2, ZHOU Ying1,2( )
)
- 1.State Key Laboratory of Oil and Gas Reservoir Geology and Exploitation, Chengdu 610500, Sichuan, China
2.School of New Energy and Materials, Southwest Petroleum University, Chengdu 610500, Sichuan, China
-
Received:2022-09-05Revised:2022-11-08Online:2023-07-15Published:2023-08-14 -
Contact:YU Shan, ZHOU Ying
摘要:
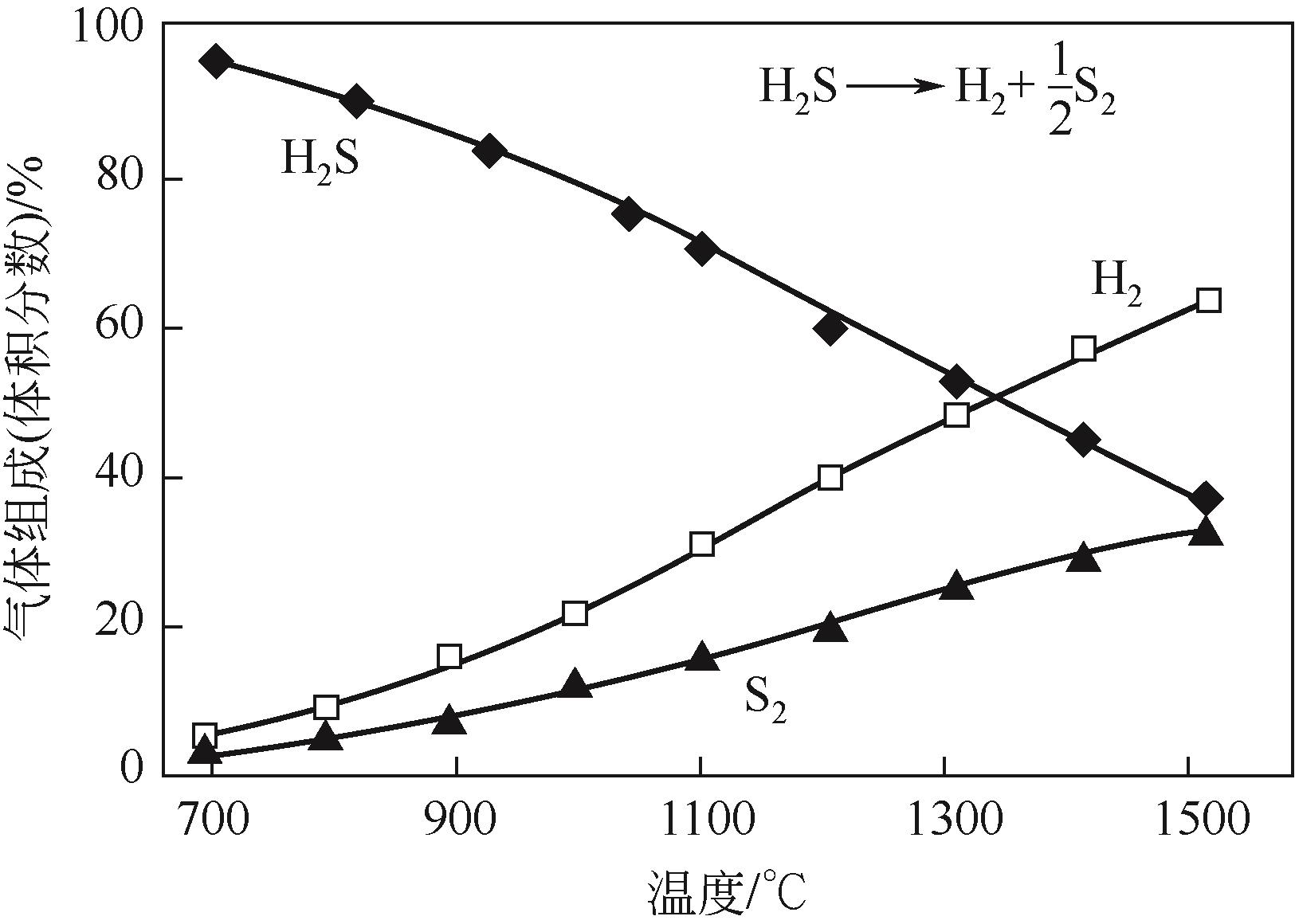
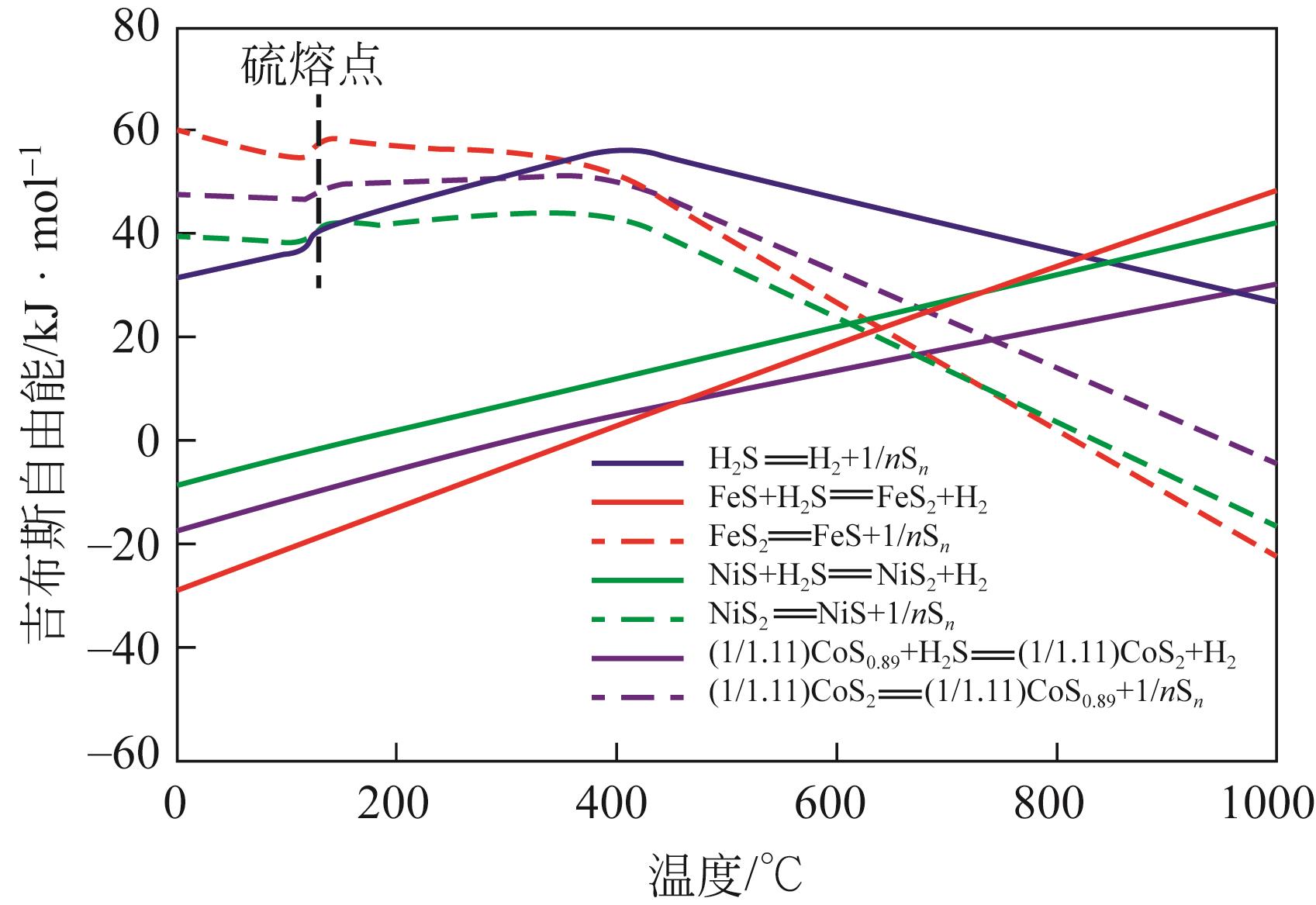
硫化氢(H2S)不仅是一种剧毒且高腐蚀性气体,更是蕴含丰富氢能和硫元素的宝贵资源。在国家能源战略需求和双碳目标下,实现天然气藏中H2S的低碳高值利用是天然气开发的必然发展趋势。分步法是一种可在较低温度区间将H2S高值转化为H2和硫黄的方法,具有H2S分解效率高、能耗低的优点,但存在循环效率差、硫黄回收困难、反应过程机理认识浅薄等问题。为了深入了解分步法分解H2S的研究现状和面临问题,本文主要综述了分步法的反应原理、发展历程、活性催化剂的研究进展,重点概述了金属硫化物和金属这两类催化剂的活性测试结果及具体反应过程,并对分步法分解H2S所面临的关键问题进行了总结与展望。本文指出分步法最大问题在于催化剂硫化产氢和分解脱硫过程机理尚不明晰,使得催化剂的优化设计缺乏指导,从而导致分步法循环效率低。未来应当借助更多原位表征技术和理论模拟计算认识转化过程机理,同时加强相关实验设计以衡量不同催化剂优劣,开发出适用于分步法高效循环过程的催化剂。
中图分类号:
引用本文
于姗, 段元刚, 张怡欣, 唐春, 付梦瑶, 黄靖元, 周莹. 分步法分解硫化氢制氢和硫黄催化剂研究进展[J]. 化工进展, 2023, 42(7): 3780-3790.
YU Shan, DUAN Yuangang, ZHANG Yixin, TANG Chun, FU Mengyao, HUANG Jinyuan, ZHOU Ying. Research progress of catalysts for two-step hydrogen sulfide decomposition to produce hydrogen and sulfur[J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3780-3790.
| 金属硫化物 | 产氢温度 /℃ | 脱硫温度 /℃ | H2S质量分数 /% | H2产率 /% | 年份 | 文献 |
|---|---|---|---|---|---|---|
| Li2S | 400~800 | — | 99.5 | 0~7 | 1985 | [ |
| Na2S | 400~650 | — | 99.5 | 0~14 | ||
| Na2S2 | 400~800 | — | 99.5 | 0~4 | ||
| Na2S3 | 400~800 | — | 99.5 | 0~2 | ||
| Na2S4 | 400~800 | — | 99.5 | 0~1.8 | ||
| K2S | 400~700 | — | 99.5 | 0~18 | ||
| K2S2 | 400~800 | — | 99.5 | 0~7.5 | ||
| K2S3 | 400~800 | — | 99.5 | 0~4.5 | ||
| K2S4 | 400~800 | — | 99.5 | 0~2.5 |
表1 ⅠA族金属元素组成的硫化物用于分步法的研究
| 金属硫化物 | 产氢温度 /℃ | 脱硫温度 /℃ | H2S质量分数 /% | H2产率 /% | 年份 | 文献 |
|---|---|---|---|---|---|---|
| Li2S | 400~800 | — | 99.5 | 0~7 | 1985 | [ |
| Na2S | 400~650 | — | 99.5 | 0~14 | ||
| Na2S2 | 400~800 | — | 99.5 | 0~4 | ||
| Na2S3 | 400~800 | — | 99.5 | 0~2 | ||
| Na2S4 | 400~800 | — | 99.5 | 0~1.8 | ||
| K2S | 400~700 | — | 99.5 | 0~18 | ||
| K2S2 | 400~800 | — | 99.5 | 0~7.5 | ||
| K2S3 | 400~800 | — | 99.5 | 0~4.5 | ||
| K2S4 | 400~800 | — | 99.5 | 0~2.5 |
| 金属硫化物 | 产氢温度 /℃ | 脱硫温度 /℃ | H2S质量分数 /% | H2产率 /% | 年份 | 文献 |
|---|---|---|---|---|---|---|
| ZrS2 | 100~500 | 650 | 0.01 | 0~99 | 2022 | [ |
| VS | 530~750 | 530~750 | 99.5 | 0~94 | 1987 | [ |
| V2S3 | 500~800 | 500~800 | 99.5 | 3.9~98 | 1987 | [ |
| V2S3/Al2O3 | 400~800 | 400~800 | 99.5 | 40 | 1987 | [ |
| V2S3/FeS | 400~800 | 400~800 | 99.5 | 0~8 | 1987 | [ |
| V2S3/Cu9S5 | 400~800 | 400~800 | 99.5 | 9~80 | 1987 | [ |
| V2S3/Cu9S5/Al2O3 | 400~800 | 400~800 | 99.5 | 40 | 1987 | [ |
| V2S3/ZnS | 400~800 | 400~800 | 99.5 | 0~8.5 | 1987 | [ |
| 5%V2S3/Al2O3 | 450~600 | 450~600 | — | 0~61 | 1990 | [ |
| 5%V2O5/Al2O3 | 450~600 | 450~600 | — | 0~68 | 1990 | [ |
| 10%V2O5/Al2O3 | 450~600 | 450~600 | — | 0~69 | 1990 | [ |
| 50%V2O5/Al2O3 | 450~600 | 450~600 | — | 0~66 | 1990 | [ |
| Cr2S3 | 400~800 | 400~800 | 99.5 | 1.5~7.5 | 1980 | [ |
| MoS2 | 400~800 | 400~800 | 99.5 | 0.5~9 | 1980 | [ |
| MoS2 | 530~800 | 530~800 | 99.5 | 1.5~80 | 1987 | [ |
| WS2 | 400~800 | 400~800 | 99.5 | 0.5~8.5 | 1980 | [ |
表2 ⅣB-ⅥB族金属元素组成的硫化物用于分步法的研究
| 金属硫化物 | 产氢温度 /℃ | 脱硫温度 /℃ | H2S质量分数 /% | H2产率 /% | 年份 | 文献 |
|---|---|---|---|---|---|---|
| ZrS2 | 100~500 | 650 | 0.01 | 0~99 | 2022 | [ |
| VS | 530~750 | 530~750 | 99.5 | 0~94 | 1987 | [ |
| V2S3 | 500~800 | 500~800 | 99.5 | 3.9~98 | 1987 | [ |
| V2S3/Al2O3 | 400~800 | 400~800 | 99.5 | 40 | 1987 | [ |
| V2S3/FeS | 400~800 | 400~800 | 99.5 | 0~8 | 1987 | [ |
| V2S3/Cu9S5 | 400~800 | 400~800 | 99.5 | 9~80 | 1987 | [ |
| V2S3/Cu9S5/Al2O3 | 400~800 | 400~800 | 99.5 | 40 | 1987 | [ |
| V2S3/ZnS | 400~800 | 400~800 | 99.5 | 0~8.5 | 1987 | [ |
| 5%V2S3/Al2O3 | 450~600 | 450~600 | — | 0~61 | 1990 | [ |
| 5%V2O5/Al2O3 | 450~600 | 450~600 | — | 0~68 | 1990 | [ |
| 10%V2O5/Al2O3 | 450~600 | 450~600 | — | 0~69 | 1990 | [ |
| 50%V2O5/Al2O3 | 450~600 | 450~600 | — | 0~66 | 1990 | [ |
| Cr2S3 | 400~800 | 400~800 | 99.5 | 1.5~7.5 | 1980 | [ |
| MoS2 | 400~800 | 400~800 | 99.5 | 0.5~9 | 1980 | [ |
| MoS2 | 530~800 | 530~800 | 99.5 | 1.5~80 | 1987 | [ |
| WS2 | 400~800 | 400~800 | 99.5 | 0.5~8.5 | 1980 | [ |
| 金属硫化物 | 实验温度/℃ | 脱硫温度/℃ | H2S质量分数/% | H2S转化率/% | H2产率/% | 年份 | 文献 |
|---|---|---|---|---|---|---|---|
| FeS | 400~800 | 400~800 | 99.5 | — | 2~8.3 | 1980 | [ |
| FeS | 550~600 | 750 | — | — | 3.5~33 | 1982 | [ |
| FeS | 250~590 | 600 | 1.2~19.5 | 16.3① | 34① | 2021 | [ |
| FeS | 200~400 | 900 | 0.9 | 38~100 | — | 2021 | [ |
| FeS2 | 400~800 | 400~800 | 99.5 | — | 0.5~8 | 1980 | [ |
| Fe7S8 | 400~800 | 400~800 | 99.5 | — | 0.5~9 | 1980 | [ |
| FeS/Al2O3 | 22 | 600 | 1.04 | 0~100 | — | 2021 | [ |
| 2%Mo-FeS | 200~400 | 900 | 0.9 | 52~100 | — | 2021 | [ |
| CoS | 400~800 | 400~800 | 99.5 | — | 2.5~7 | 1980 | [ |
| CoS | 20 | 600 | 7.5 | 10.6① | 26① | 2021 | [ |
| CoS2 | 400~800 | 400~800 | 99.5 | — | 0.5~6.5 | 1980 | [ |
| CoS1.13-1.2 | 400~800 | 400~800 | 99.5 | — | 2~7.5 | 1980 | [ |
| Co9S8 | 550~850 | 850 | — | — | 0~55 | 1982 | [ |
| CoS/Al2O3 | 22~150 | 600 | 0.9 | 0~100 | — | 2021 | [ |
| CoMoS/Al2O3 | 21 | 600 | 1.05~19.7 | 0~100 | 0~0.6 | 2021 | [ |
| NiS | 400~800 | 400~800 | 99.5 | — | 2.7~9.8 | 1980 | [ |
| NiS | 20~400 | 600 | 1.2~19.5 | 2.8① | 32① | 2021 | [ |
| NiS2 | 400~800 | 400~800 | 99.5 | — | 0.5~7 | 1980 | [ |
| NiS1.2 | 400~800 | 400~800 | 99.5 | — | 0.3~7.6 | 1980 | [ |
| Ni3S2 | 550 | 750 | — | — | 35~97 | 1982 | [ |
| Ni3S2 | 300~700 | 800 | — | — | 0~50 | 1983 | [ |
| Ni3S2 | 200~500 | 500~800 | 0.01 | 80 | — | 2022 | [ |
| Ni3S2/MoS2 | 400~500 | 800 | — | — | 40~85 | 1982 | [ |
| Ni3S2/MoS2 | 300~700 | 800 | — | — | 0~79 | 1983 | [ |
| NiS/Al2O3 | 19~150 | 600 | 0.9 | 0~100 | — | 2021 | [ |
| Ni3S2/Al2O3 | 300~700 | 800 | — | — | 0~82 | 1983 | [ |
| Ni3S2/Al2O3 | 500~800 | 500~800 | — | — | 0~85 | 1984 | [ |
| NiMoS4/Al2O3 | 22-300 | 400 | 0.9 | 0~100 | 0.3~15.7 | 2021 | [ |
表3 第Ⅷ族金属元素组成的硫化物用于分步法的研究
| 金属硫化物 | 实验温度/℃ | 脱硫温度/℃ | H2S质量分数/% | H2S转化率/% | H2产率/% | 年份 | 文献 |
|---|---|---|---|---|---|---|---|
| FeS | 400~800 | 400~800 | 99.5 | — | 2~8.3 | 1980 | [ |
| FeS | 550~600 | 750 | — | — | 3.5~33 | 1982 | [ |
| FeS | 250~590 | 600 | 1.2~19.5 | 16.3① | 34① | 2021 | [ |
| FeS | 200~400 | 900 | 0.9 | 38~100 | — | 2021 | [ |
| FeS2 | 400~800 | 400~800 | 99.5 | — | 0.5~8 | 1980 | [ |
| Fe7S8 | 400~800 | 400~800 | 99.5 | — | 0.5~9 | 1980 | [ |
| FeS/Al2O3 | 22 | 600 | 1.04 | 0~100 | — | 2021 | [ |
| 2%Mo-FeS | 200~400 | 900 | 0.9 | 52~100 | — | 2021 | [ |
| CoS | 400~800 | 400~800 | 99.5 | — | 2.5~7 | 1980 | [ |
| CoS | 20 | 600 | 7.5 | 10.6① | 26① | 2021 | [ |
| CoS2 | 400~800 | 400~800 | 99.5 | — | 0.5~6.5 | 1980 | [ |
| CoS1.13-1.2 | 400~800 | 400~800 | 99.5 | — | 2~7.5 | 1980 | [ |
| Co9S8 | 550~850 | 850 | — | — | 0~55 | 1982 | [ |
| CoS/Al2O3 | 22~150 | 600 | 0.9 | 0~100 | — | 2021 | [ |
| CoMoS/Al2O3 | 21 | 600 | 1.05~19.7 | 0~100 | 0~0.6 | 2021 | [ |
| NiS | 400~800 | 400~800 | 99.5 | — | 2.7~9.8 | 1980 | [ |
| NiS | 20~400 | 600 | 1.2~19.5 | 2.8① | 32① | 2021 | [ |
| NiS2 | 400~800 | 400~800 | 99.5 | — | 0.5~7 | 1980 | [ |
| NiS1.2 | 400~800 | 400~800 | 99.5 | — | 0.3~7.6 | 1980 | [ |
| Ni3S2 | 550 | 750 | — | — | 35~97 | 1982 | [ |
| Ni3S2 | 300~700 | 800 | — | — | 0~50 | 1983 | [ |
| Ni3S2 | 200~500 | 500~800 | 0.01 | 80 | — | 2022 | [ |
| Ni3S2/MoS2 | 400~500 | 800 | — | — | 40~85 | 1982 | [ |
| Ni3S2/MoS2 | 300~700 | 800 | — | — | 0~79 | 1983 | [ |
| NiS/Al2O3 | 19~150 | 600 | 0.9 | 0~100 | — | 2021 | [ |
| Ni3S2/Al2O3 | 300~700 | 800 | — | — | 0~82 | 1983 | [ |
| Ni3S2/Al2O3 | 500~800 | 500~800 | — | — | 0~85 | 1984 | [ |
| NiMoS4/Al2O3 | 22-300 | 400 | 0.9 | 0~100 | 0.3~15.7 | 2021 | [ |
| 金属硫化物 | 产氢温度 /℃ | 脱硫温度 /℃ | H2S质量分数 /% | H2产率 /% | 年份 | 文献 |
|---|---|---|---|---|---|---|
| Cu2S | 400~800 | 400~800 | 99.5 | 6.9 | 1980 | [ |
| Cu9S5 | 400~800 | 400~800 | 99.5 | 6.7 | 1980 | [ |
| CuS | 400~800 | 400~800 | 99.5 | 6.5 | 1980 | [ |
表4 ⅠB族金属元素组成的硫化物用于分步法的研究
| 金属硫化物 | 产氢温度 /℃ | 脱硫温度 /℃ | H2S质量分数 /% | H2产率 /% | 年份 | 文献 |
|---|---|---|---|---|---|---|
| Cu2S | 400~800 | 400~800 | 99.5 | 6.9 | 1980 | [ |
| Cu9S5 | 400~800 | 400~800 | 99.5 | 6.7 | 1980 | [ |
| CuS | 400~800 | 400~800 | 99.5 | 6.5 | 1980 | [ |
| 金属 | 产氢温度/℃ | 脱硫温度/℃ | H2产率/% | 年份 | 文献 |
|---|---|---|---|---|---|
| Cu | 250~550 | — | — | 1991 | [ |
| Ag | 500~700 | — | 0~56 | 1982 | [ |
| Pb | 600 | — | — | 1982 | [ |
| Bi | 271 | 927 | 47.3 | 1975 | [ |
表5 金属用于分步法分解H2S的研究
| 金属 | 产氢温度/℃ | 脱硫温度/℃ | H2产率/% | 年份 | 文献 |
|---|---|---|---|---|---|
| Cu | 250~550 | — | — | 1991 | [ |
| Ag | 500~700 | — | 0~56 | 1982 | [ |
| Pb | 600 | — | — | 1982 | [ |
| Bi | 271 | 927 | 47.3 | 1975 | [ |
| 1 | 王震, 孔盈皓, 李伟. “碳中和”背景下中国天然气产业发展综述[J]. 天然气工业, 2021, 41(8): 194-202. |
| WANG Zhen, KONG Yinghao, LI Wei. Review on the development of China’s natural gas industry in the background of “carbon neutrality”[J]. Natural Gas Industry, 2021, 41(8): 194-202. | |
| 2 | 张超, 宋鹏飞, 侯建国, 等. 碳中和进程中天然气与氢能产业深度融合的新发展模式探讨[J]. 现代化工, 2022, 42(9): 7-12. |
| ZHANG Chao, SONG Pengfei, HOU Jianguo, et al. A new development mode for deep integration between natural gas and hydrogen energy industries in carbon neutralization progress[J]. Modern Chemical Industry, 2022, 42(9): 7-12. | |
| 3 | 周淑慧, 孙慧, 梁严, 等. “双碳”目标下“十四五”天然气发展机遇与挑战[J]. 油气与新能源, 2021, 33(3): 27-36. |
| ZHOU Shuhui, SUN Hui, LIANG Yan, et al. Carbon neutrality oriented 14th Five-Year opportunities and challenges of natural gas development[J]. Petroleum and New Energy, 2021, 33(3): 27-36. | |
| 4 | 周淑慧, 王军, 梁严. 碳中和背景下中国“十四五”天然气行业发展[J]. 天然气工业, 2021, 41(2): 171-182. |
| ZHOU Shuhui, WANG Jun, LIANG Yan. Development of China’s natural gas industry during the 14th Five-Year Plan in the background of carbon neutrality[J]. Natural Gas Industry, 2021, 41(2): 171-182. | |
| 5 | 吴亚军, 王宁, 张广东, 等. 高含硫气藏单质硫溶解度测试方法及预测模型[J]. 特种油气藏, 2022, 29(2): 104-109. |
| WU Yajun, WANG Ning, ZHANG Guangdong, et al. Testing method and prediction model of elemental sulfur solubility in sour gas reservoirs[J]. Special Oil & Gas Reservoirs, 2022, 29(2): 104-109. | |
| 6 | ZHANG Xin, TANG Yuyin, QU Siqiu, et al. H2S-selective catalytic oxidation: Catalysts and processes[J]. ACS catalysis, 2015, 5(2): 1053-1067. |
| 7 | MONNERY W D, SVRCEK W Y, BEHIE L A. Modelling the modified Claus process reaction furnace and the implications on plant design and recovery[J]. The Canadian Journal of Chemical Engineering, 1993, 71(5): 711-724. |
| 8 | PIÉPLU A, SAUR O, LAVALLEY J C, et al. Claus catalysis and H2S selective oxidation[J]. Catalysis Reviews, 1998, 40(4): 409-450. |
| 9 | 段超, 唐春, 吴梦南, 等. 电催化分解硫化氢制氢脱硫研究进展[J]. 天然气化工(C1化学与化工), 2021, 46(S1): 24-30. |
| DUAN Chao, TANG Chun, WU Mengnan, et al. Progress in electrocatalytic decomposition of hydrogen sulfide to hydrogen and sulfur[J]. Natural Gas Chemical Industry, 2021, 46(S1): 24-30. | |
| 10 | RUBAN P, SELLAPPA K. Concurrent hydrogen production and hydrogen sulfide decomposition by solar photocatalysis[J]. Clean: Soil, Air, Water, 2016, 44(8): 1023-1035. |
| 11 | KHANCHANDANI S, KUMAR S, GANGULI A K. Comparative study of TiO2/CuS core/shell and composite nanostructures for efficient visible light photocatalysis[J]. ACS Sustainable Chemistry & Engineering, 2016, 4(3): 1487-1499. |
| 12 | DAN Meng, YU Shan, LI Yi, et al. Hydrogen sulfide conversion: how to capture hydrogen and sulfur by photocatalysis[J]. Journal of Photochemistry Photobiology C: Photochemistry Reviews, 2020, 42: 100339. |
| 13 | PORTELA R, SUÁREZ S, RASMUSSEN S B, et al. Photocatalytic-based strategies for H2S elimination[J]. Catalysis Today, 2010, 151(1/2): 64-70. |
| 14 | NAVAKOTESWARA RAO V, LAKSHMANA REDDY N, MAMATHA KUMARI M, et al. Photocatalytic recovery of H2 from H2S containing wastewater: Surface and interface control of photo-excitons in Cu2S@TiO2 core-shell nanostructures[J]. Applied Catalysis B: Environmental, 2019, 254: 174-185. |
| 15 | 张婧, 张铁, 孙峰, 等. 硫化氢直接分解制取氢气和硫黄研究进展[J]. 化工进展, 2017, 36(4): 1448-1459. |
| ZHANG Jing, ZHANG Tie, SUN Feng, et al. Research progress on hydrogen and sulfur production from direct decomposition of hydrogen sulfide[J]. Chemical Industry and Engineering Progress, 2017, 36(4): 1448-1459. | |
| 16 | MATROS Y S, NOSKOV A S, ZAGDRUIKO A N, et al. Comparison of technological design of the catalytic processes under unsteady-state conditions[J]. Theoretical Foundations of Chemical Engineering, 1994, 28(2): 120-125. |
| 17 | MATROS Y S. Performance of catalytic processes under unsteady conditions[J]. Chemical Engineering Science, 1990, 45(8): 2097-2102. |
| 18 | ZAGORUIKO A N, BOBROVA L, VERNIKOVSKAYA N, et al. Unsteady-state operation of reactors with fixed catalyst beds[J]. Reviews in Chemical Engineering, 2021, 37(1): 193-225. |
| 19 | ZAGORUIKO A. Low-temperature chemisorption-enhanced catalytic decomposition of hydrogen sulfide: Thermodynamic analysis and process concept[J]. Catalysis Today, 2019, 329: 171-176. |
| 20 | BANDERMANN F, HARDER K. Production of H2 via thermal decomposition of H2S and separation of H2 and H2S by pressure swing adsorption[J]. International Journal of Hydrogen Energy, 1982, 7(6): 471-475. |
| 21 | FUKUDA K, DOKIYA M, KAMEYAMA T, et al. Catalytic decomposition of hydrogen sulfide[J]. Industrial & Engineering Chemistry Fundamentals, 1978, 17(4): 243-248. |
| 22 | JEMNI A. Study of two-dimensional heat and mass transfer during absorption in a metal-hydrogen reactor[J]. International Journal of Hydrogen Energy, 1995, 20(1): 43-52. |
| 23 | KAMEYAMA T, DOKIYA M, FUKUDA K, et al. Differential permeation of hydrogen sulfide through a microporous vycor-type glass membrane in the separation system of hydrogen and hydrogen sulfide[J]. Separation Science and Technology, 1979, 14(10): 953-957. |
| 24 | SADRZADEH M, MOHAMMADI T, IVAKPOUR J, et al. Separation of lead ions from wastewater using electrodialysis: Comparing mathematical and neural network modeling[J]. Chemical Engineering Journal, 2008, 144(3): 431-441. |
| 25 | SUNLEY G J, WATSON D J. High productivity methanol carbonylation catalysis using iridium: The CativaTM process for the manufacture of acetic acid[J]. Catalysis Today, 2000, 58(4): 293-307. |
| 26 | ZAMAN J, CHAKMA A. A simulation study on the thermal decomposition of hydrogen sulfide in a membrane reactor[J]. International Journal of Hydrogen Energy, 1995, 20(1): 21-28. |
| 27 | ADANEZ J, ABAD A, GARCIA-LABIANO F, et al. Progress in chemical-looping combustion and reforming technologies[J]. Progress in Energy and Combustion Science, 2012, 38(2): 215-282. |
| 28 | TANG Mingchen, XU Long, FAN Maohong. Progress in oxygen carrier development of methane-based chemical-looping reforming: A review[J]. Applied Energy, 2015, 151: 143-156. |
| 29 | CHEN Liangyong, BAO Jinhua, KONG Liang, et al. The direct solid-solid reaction between coal char and iron-based oxygen carrier and its contribution to solid-fueled chemical looping combustion[J]. Applied Energy, 2016, 184: 9-18. |
| 30 | ZENG Liang, CHENG Zhuo, FAN J A, et al. Metal oxide redox chemistry for chemical looping processes[J]. Nature Reviews Chemistry, 2018, 2(11): 349-364. |
| 31 | ZHU Xing, ZHANG Mingyue, LI Kongzhai, et al. Chemical-looping water splitting over ceria-modified iron oxide: Performance evolution and element migration during redox cycling[J]. Chemical Engineering Science, 2018, 179: 92-103. |
| 32 | 杨杰, 常辉, 隋志军, 等. 化学链催化甲烷氧化反应研究进展[J]. 化工进展, 2021, 40(4): 1928-1947. |
| YANG Jie, CHANG Hui, SUI Zhijun, et al. Advances in chemical looping methane oxidation[J]. Chemical Industry and Engineering Progress, 2021, 40(4): 1928-1947. | |
| 33 | GOGOLEV I, PIKKARAINEN T, KAUPPINEN J, et al. Investigation of biomass alkali release in a dual circulating fluidized bed chemical looping combustion system[J]. Fuel, 2021, 297: 120743. |
| 34 | 周怀荣, 马迎文, 王可, 等. 化学链空分联合化学链制氢的煤制甲醇过程参数优化与分析[J]. 化工进展, 2022, 41(10): 5332-5341. |
| ZHOU Huairong, MA Yingwen, WANG Ke, et al. Optimization and analysis of coal-to-methanol process by integrating chemical looping air separation and hydrogen technology[J]. Chemical Industry and Engineering Progress, 2022, 41(10): 5332-5341. | |
| 35 | CHIVERS T, HYNE J B, LAU C. The thermal decomposition of hydrogen sulfide over transition metal sulfides[J]. International Journal of Hydrogen Energy, 1980, 5(5): 499-506. |
| 36 | KIUCHI H, NAKAMURA T, FUNAKI K, et al. Recovery of hydrogen from hydrogen sulfide with metals or metal sulfides[J]. International Journal of Hydrogen Energy, 1982, 7(6): 477-482. |
| 37 | ZAGORUIKO A, MIKENIN P. Decomposition of hydrogen sulfide into elements in the cyclic chemisorption-catalytic regime[J]. Catalysis Today, 2021, 378: 176-188. |
| 38 | WEINER J G, LEGGETT C W. Process for production of hydrogen and sulfur: US2979384A[P]. 1961-04-11. |
| 39 | CHIVERS T, LAU C. The thermal decomposition of hydrogen sulfide over alkali metal sulfides and polysulfides[J]. International Journal of Hydrogen Energy, 1985, 10(1): 21-25. |
| 40 | CHIVERS T, LAU C. The thermal decomposition of hydrogen sulfide over vanadium and molybdenum sulfides and mixed sulfide catalysts in quartz and thermal diffusion column reactors[J]. International Journal of Hydrogen Energy, 1987, 12(4): 235-243. |
| 41 | AL-SHAMMA L M, NAMAN S A. Kinetic study for thermal production of hydrogen from H2S by heterogeneous catalysis of vanadium sulfide in a flow system[J]. International Journal of Hydrogen Energy, 1989, 14(3): 173-179. |
| 42 | AL-SHAMMA L M, NAMAN S A. The production and separation of hydrogen and sulfur from thermal decomposition of hydrogen sulfide over vanadium oxide/sulfide catalysts[J]. International Journal of Hydrogen Energy, 1990, 15(1): 1-5. |
| 43 | KIUCHI H, FUNAKI K, NAKAI Y, et al. Thermochemical decomposition cycle of H2S with nickel sulfide[J]. International Journal of Hydrogen Energy, 1984, 9(8): 701-705. |
| 44 | KIUCHI H, FUNAKI K, TANAKA T. Thermochemical decomposition of hydrogen sulfide with nickel sulfide[J]. Metallurgical Transactions B, 1983, 14(3): 347-352. |
| 45 | REDDY S, NADGOUDA S G, TONG A, et al. Metal sulfide-based process analysis for hydrogen generation from hydrogen sulfide conversion[J]. International Journal of Hydrogen Energy, 2019, 44(39): 21336-21350. |
| 46 | JANGAM K, CHEN Y Y, QIN Lang, et al. Mo-doped FeS mediated H2 production from H2S via an in situ cyclic sulfur looping scheme[J]. ACS Sustainable Chemistry & Engineering, 2021, 9(33): 11204-11211. |
| 47 | OSASUYI O, QUANG D V, BASINA G, et al. Reversible metal sulfide transition in a two-step thermochemical H2S splitting[J]. Industrial & Engineering Chemistry Research, 2022, 61(18): 6135-6145. |
| 48 | CHEN Qiyuan, LI Lili, HEPLER L G. Kinetics of desulfurization of hydrogen sulfide using metallic copper as a desulfurizer[J]. The Canadian Journal of Chemical Engineering, 1991, 69(5): 1160-1165. |
| 49 | SOLIMAN M A, CARTY R H, CONGER W L, et al. New thermochemical cycles for hydrogen production[J]. The Canadian Journal of Chemical Engineering, 1975, 53(2): 164-169. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 高雨飞, 鲁金凤. 非均相催化臭氧氧化作用机理研究进展[J]. 化工进展, 2023, 42(S1): 430-438. |
| [6] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [7] | 赵景超, 谭明. 表面活性剂对电渗析减量化工业含盐废水的影响[J]. 化工进展, 2023, 42(S1): 529-535. |
| [8] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [9] | 杨建平. 降低HPPO装置反应系统原料消耗的PSE[J]. 化工进展, 2023, 42(S1): 21-32. |
| [10] | 王谨航, 何勇, 史伶俐, 龙臻, 梁德青. 气体水合物阻聚剂研究进展[J]. 化工进展, 2023, 42(9): 4587-4602. |
| [11] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [12] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| [13] | 张启, 赵红, 荣峻峰. 质子交换膜燃料电池中氧还原反应抗毒性电催化剂研究进展[J]. 化工进展, 2023, 42(9): 4677-4691. |
| [14] | 葛全倩, 徐迈, 梁铣, 王凤武. MOFs材料在光电催化领域应用的研究进展[J]. 化工进展, 2023, 42(9): 4692-4705. |
| [15] | 王伟涛, 鲍婷玉, 姜旭禄, 何珍红, 王宽, 杨阳, 刘昭铁. 醛酮树脂基非金属催化剂催化氧气氧化苯制备苯酚[J]. 化工进展, 2023, 42(9): 4706-4715. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||