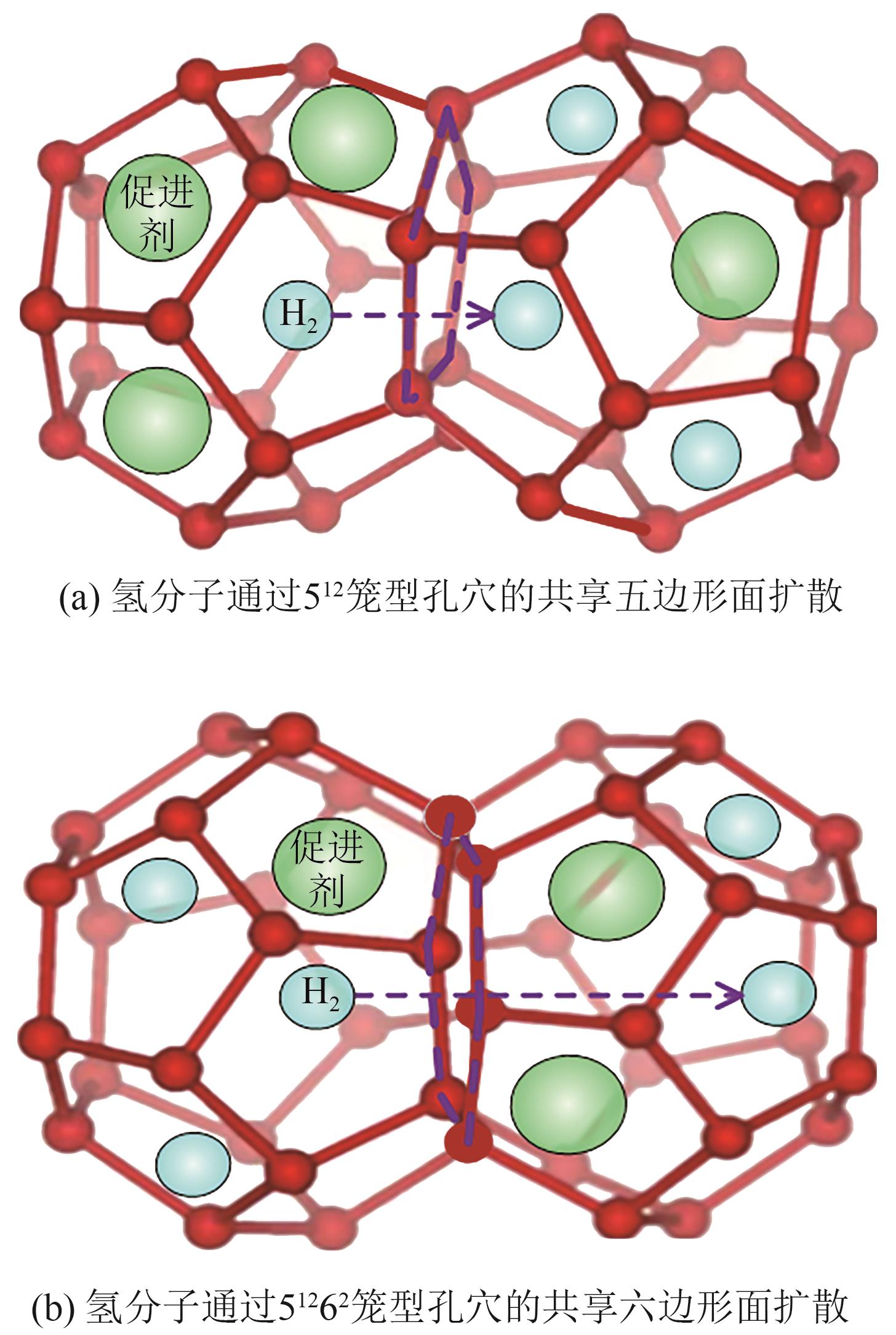
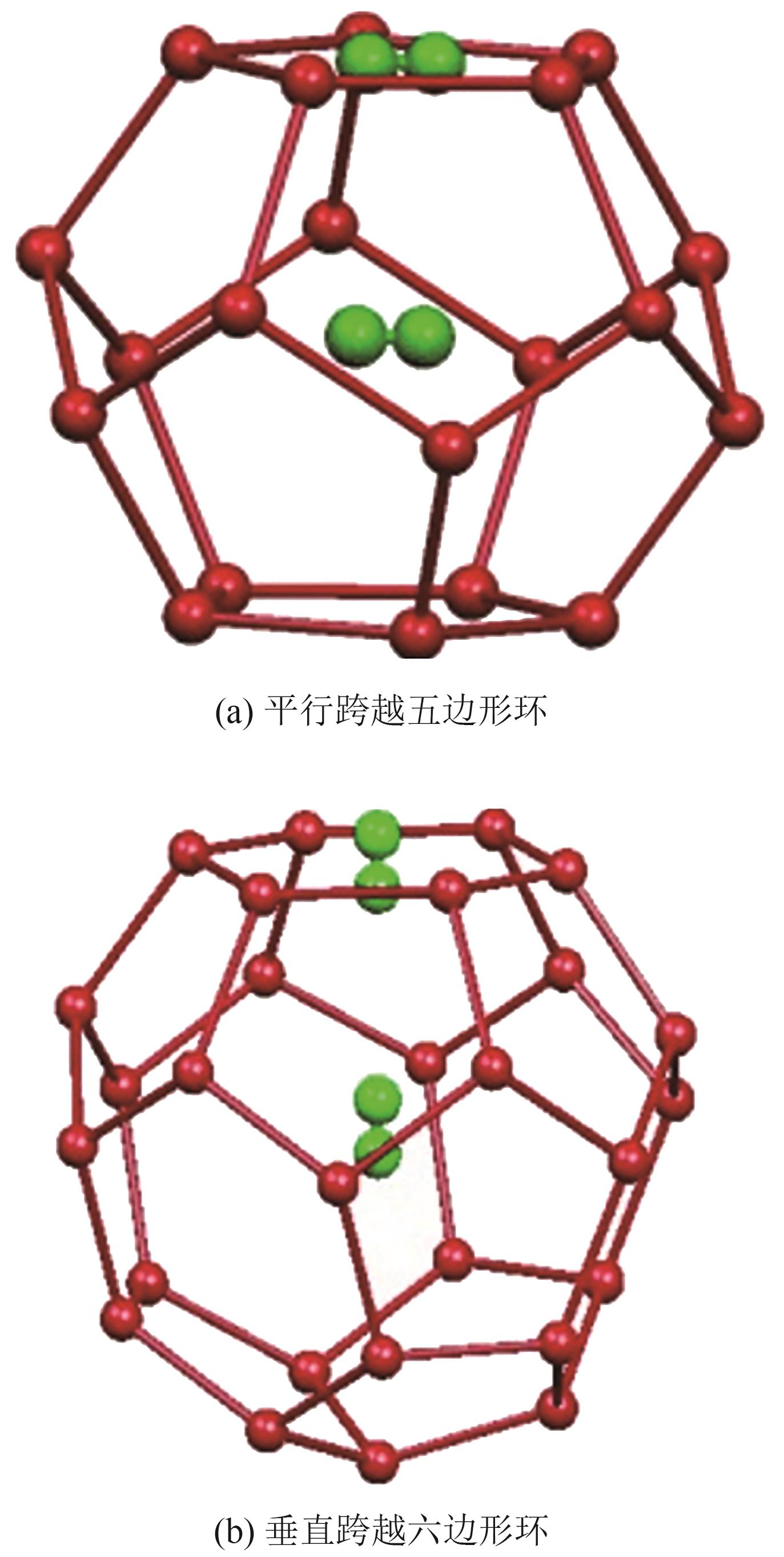
化工进展 ›› 2025, Vol. 44 ›› Issue (S1): 112-123.DOI: 10.16085/j.issn.1000-6613.2025-0770
• 能源加工与技术 • 上一篇
水合物储氢分子动力学行为研究进展
秦菲( ), 张志, 宋光春(
), 张志, 宋光春( ), 王武昌, 李玉星, 王世鑫, 何思成, 王江妍
), 王武昌, 李玉星, 王世鑫, 何思成, 王江妍
- 山东省油气与新能源储运安全重点实验室,中国石油大学(华东)储运与建筑工程学院,山东 青岛 266580
-
收稿日期:2025-05-28修回日期:2025-08-02出版日期:2025-10-25发布日期:2025-11-24 -
通讯作者:宋光春 -
作者简介:秦菲(2001—),女,硕士研究生,研究方向为水合物储氢。E-mail:qf_165@163.com。 -
基金资助:山东省自然科学基金面上项目(ZR2024ME016);山东省自然科学基金(ZR2021QE169);国家重大专项(2025ZD1408103)
Advances in research on the molecular dynamics behaviors of hydrate-based hydrogen storage
QIN Fei( ), ZHANG Zhi, SONG Guangchun(
), ZHANG Zhi, SONG Guangchun( ), WANG Wuchang, LI Yuxing, WANG Shixin, HE Sicheng, WANG Jiangyan
), WANG Wuchang, LI Yuxing, WANG Shixin, HE Sicheng, WANG Jiangyan
- Shandong Provincial Key Laboratory of Oil, Gas and New Energy Storage and Transportation Safety, College of Pipeline and Architectural Engineering, China University of Petroleum (East China), Qingdao 266580, Shandong, China
-
Received:2025-05-28Revised:2025-08-02Online:2025-10-25Published:2025-11-24 -
Contact:SONG Guangchun
摘要:
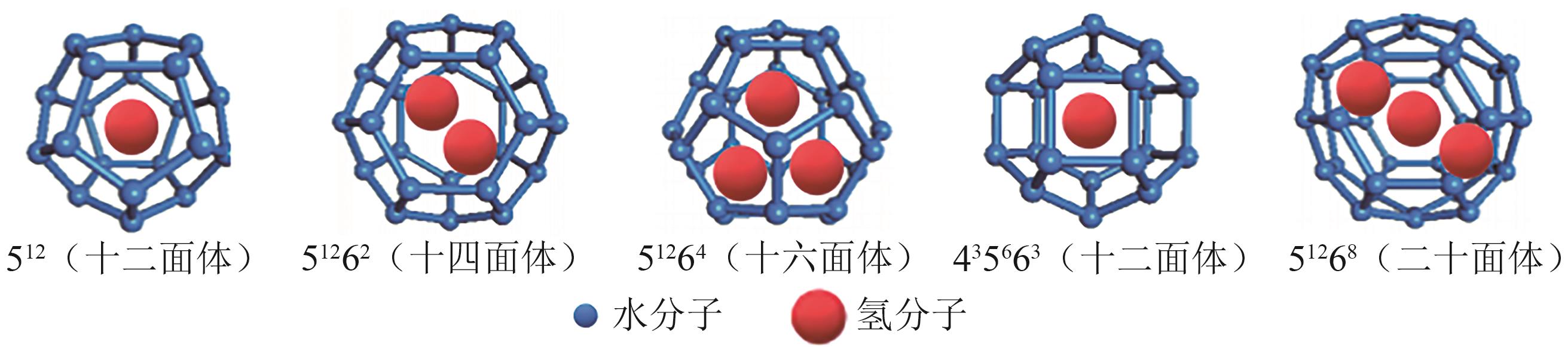
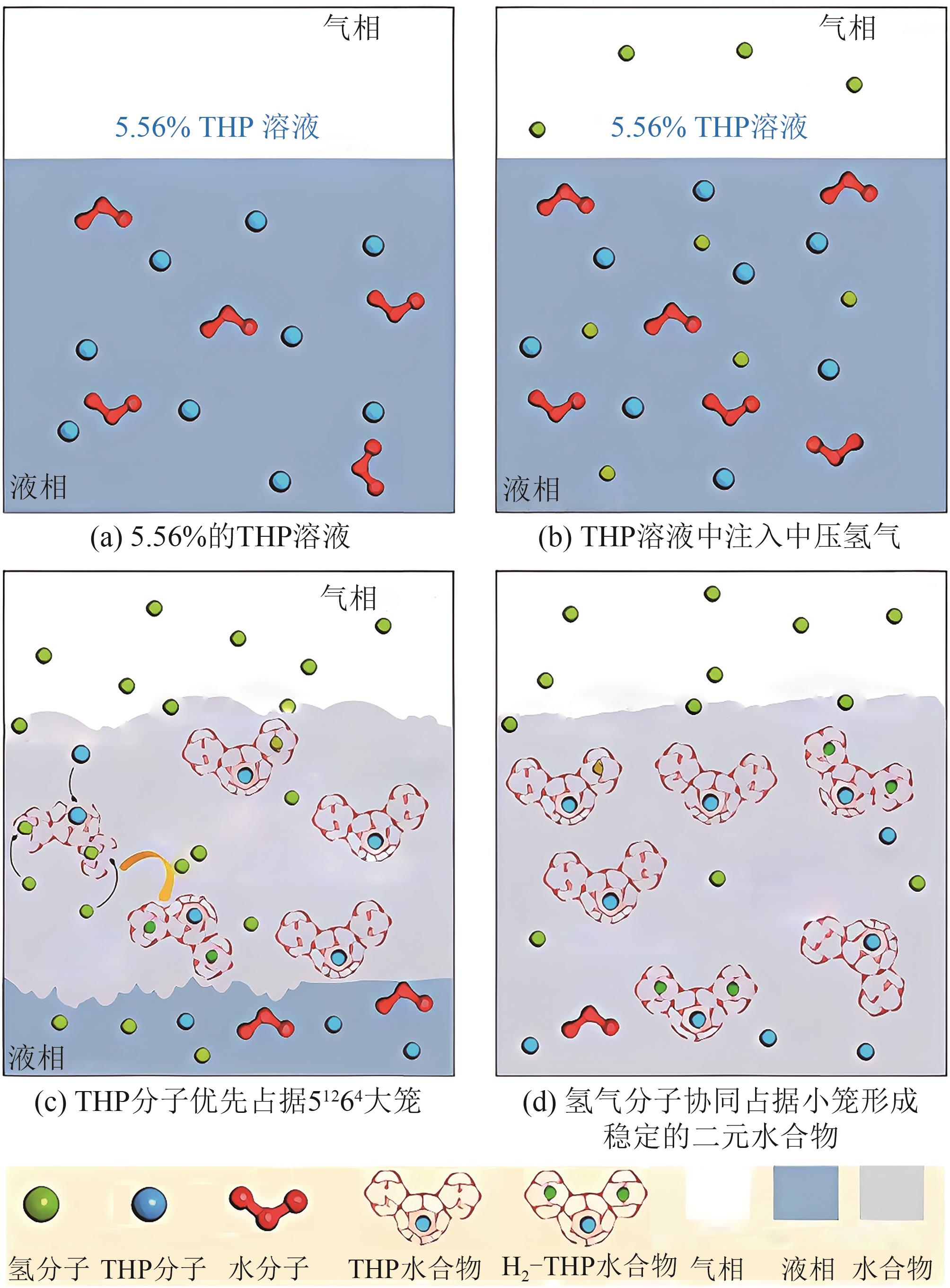
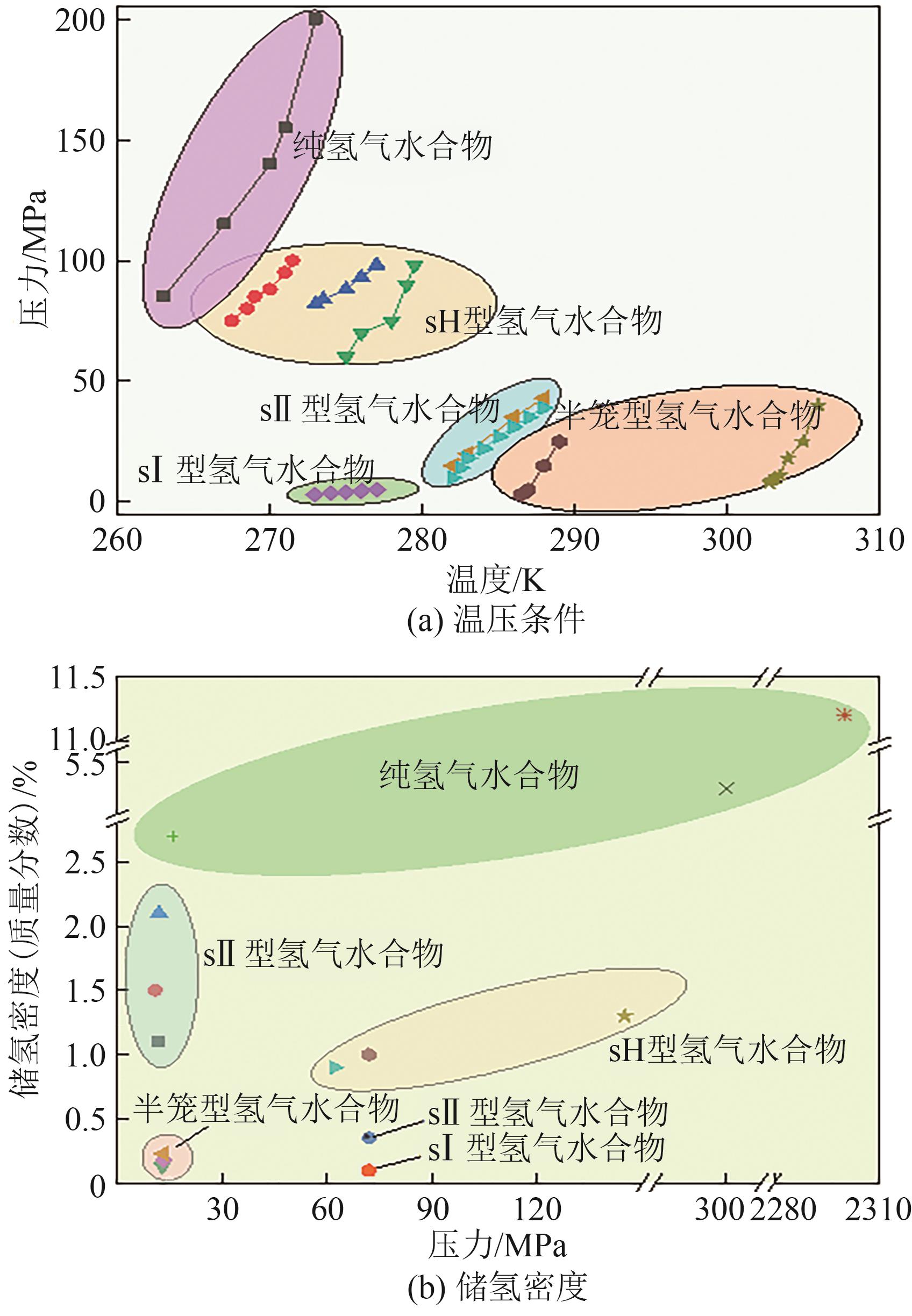
在“双碳”目标迫近及能源结构绿色转型的背景下,氢能因其来源丰富、燃烧热值高、绿色低碳及应用广泛的特点而受到广泛关注。水合物储氢作为一种新兴的固态储氢技术,表现出储氢安全性高且储氢密度大的特点,具有巨大的发展前景及应用价值。然而,目前水合物储氢技术的发展受困于氢气水合物形成条件严苛、生长速率低及储氢密度不稳定等问题。上述问题存在的根本原因在于水合物储氢过程中氢分子、水分子和促进剂分子间的相关动力学行为及机制尚不明确。基于此,本文以水合物储氢过程的分子动力学行为及机制为研究对象,阐述了促进剂作用下氢气水合物的动力学生长机制,研究了促进剂作用下氢气水合物笼形孔穴的稳定填充以及水合物中的分子笼间扩散行为。本文研究结果可为促进剂作用下氢气水合物形成热力学及动力学理论体系的完善提供分子层面的成果支持,助力水合物储氢技术的发展与应用。
中图分类号:
引用本文
秦菲, 张志, 宋光春, 王武昌, 李玉星, 王世鑫, 何思成, 王江妍. 水合物储氢分子动力学行为研究进展[J]. 化工进展, 2025, 44(S1): 112-123.
QIN Fei, ZHANG Zhi, SONG Guangchun, WANG Wuchang, LI Yuxing, WANG Shixin, HE Sicheng, WANG Jiangyan. Advances in research on the molecular dynamics behaviors of hydrate-based hydrogen storage[J]. Chemical Industry and Engineering Progress, 2025, 44(S1): 112-123.
| 水合物结构 | 典型促进剂 |
|---|---|
| sⅠ型 | 甲烷、二氧化碳、乙烷、环氧乙烷(EO)和环丙烷(CPA) |
| sⅡ型 | 四氢呋喃、环戊烷(CP)、环己酮、四氢噻吩(THT)、1,3-二氧戊环(DIOX)、叔丁醇(TBA)、三氯甲烷(CHCl3)、呋喃、乙烷、丙烷和丁烷 |
| sH型 | 甲基环己烷(MCH)、甲基叔丁基醚(MTBE)、2,2,3-三甲基丁烷(2,2,3-TMB)、1,1-二甲基环己烷(1,1-DMCH) |
| sc型 | 四丁基溴化铵、四丁基氟化铵(TBAF)、四丁基氯化铵(TBACI)、四丁基溴化磷(TBPB)、四丁基硼氢化铵(TBABH)、四丁基硝酸铵(TBANO3)和1,1-二氯-1-氟乙烷(HCFC-141B) |
| sⅥ型 | 叔丁胺(tert-butylamine) |
表1 典型促进剂及其对应形成的水合物类型
| 水合物结构 | 典型促进剂 |
|---|---|
| sⅠ型 | 甲烷、二氧化碳、乙烷、环氧乙烷(EO)和环丙烷(CPA) |
| sⅡ型 | 四氢呋喃、环戊烷(CP)、环己酮、四氢噻吩(THT)、1,3-二氧戊环(DIOX)、叔丁醇(TBA)、三氯甲烷(CHCl3)、呋喃、乙烷、丙烷和丁烷 |
| sH型 | 甲基环己烷(MCH)、甲基叔丁基醚(MTBE)、2,2,3-三甲基丁烷(2,2,3-TMB)、1,1-二甲基环己烷(1,1-DMCH) |
| sc型 | 四丁基溴化铵、四丁基氟化铵(TBAF)、四丁基氯化铵(TBACI)、四丁基溴化磷(TBPB)、四丁基硼氢化铵(TBABH)、四丁基硝酸铵(TBANO3)和1,1-二氯-1-氟乙烷(HCFC-141B) |
| sⅥ型 | 叔丁胺(tert-butylamine) |
| 研究人员 | 系统 | 稳定填充方式(H2) | 研究方法 |
|---|---|---|---|
| Alavi等[ | 纯H2体系(sⅡ) | 512笼单占据,51264笼四占据 | 分子动力学模拟、DFT计算 |
| Liu等[ | 纯H2体系(sⅡ) | 512笼双占据,51264笼三占据 | 从头计算、从头分子动力学模拟 |
| Liu等[ | H2+THF体系(sⅡ) | 512笼单占据,51264笼单个H2与THF共占据 | 从头计算、从头分子动力学模拟 |
| Lu等[ | 纯H2体系(sⅠ、sⅡ) | 512笼单占据、51262与51264笼双占据 | 从头计算 |
| Zheng等[ | H2+THF体系(sⅡ) | 512笼双占据、51264笼H2与THF共占据 | 分子动力学模拟 |
| Jafari Daghalian Sahar等[ | 纯H2体系(sⅡ) | 512笼双占据、51264笼六占据 | DFT计算 |
| Brumby等[ | 纯H2体系(sⅡ) | 512笼单占据,51264笼四占据 | 等压等温Gibbs系综蒙特卡罗模拟 |
| Katsumasa等[ | 纯H2体系(sⅡ) | 512笼单占据,51264笼四占据 | 蒙特卡罗模拟 |
| Omran等[ | CH4-H2、CO2-H2体系(sⅠ) | 512笼双占据,51262笼四占据 | DFT计算 |
| Matsumoto等[ | H2 + CH4体系(sⅠ) | 512笼单占据,51262笼双占据 | 拉曼光谱分析 |
| Moon等[ | 甲烷/乙烷/氢气混合物(sⅡ) | 512笼双占据, 51264笼四占据 | 拉曼光谱分析 |
| 张宏淑等[ | CO2/N2+H2体系(sⅠ) | 512笼三占据,51262笼单占据 | DFT计算 |
| Krishnan等[ | H2/D2体系(sⅡ) | 512笼单占据,51264笼双占据 | 经典分子动力学模拟 |
表2 部分关于氢气水合物中笼填充率的研究
| 研究人员 | 系统 | 稳定填充方式(H2) | 研究方法 |
|---|---|---|---|
| Alavi等[ | 纯H2体系(sⅡ) | 512笼单占据,51264笼四占据 | 分子动力学模拟、DFT计算 |
| Liu等[ | 纯H2体系(sⅡ) | 512笼双占据,51264笼三占据 | 从头计算、从头分子动力学模拟 |
| Liu等[ | H2+THF体系(sⅡ) | 512笼单占据,51264笼单个H2与THF共占据 | 从头计算、从头分子动力学模拟 |
| Lu等[ | 纯H2体系(sⅠ、sⅡ) | 512笼单占据、51262与51264笼双占据 | 从头计算 |
| Zheng等[ | H2+THF体系(sⅡ) | 512笼双占据、51264笼H2与THF共占据 | 分子动力学模拟 |
| Jafari Daghalian Sahar等[ | 纯H2体系(sⅡ) | 512笼双占据、51264笼六占据 | DFT计算 |
| Brumby等[ | 纯H2体系(sⅡ) | 512笼单占据,51264笼四占据 | 等压等温Gibbs系综蒙特卡罗模拟 |
| Katsumasa等[ | 纯H2体系(sⅡ) | 512笼单占据,51264笼四占据 | 蒙特卡罗模拟 |
| Omran等[ | CH4-H2、CO2-H2体系(sⅠ) | 512笼双占据,51262笼四占据 | DFT计算 |
| Matsumoto等[ | H2 + CH4体系(sⅠ) | 512笼单占据,51262笼双占据 | 拉曼光谱分析 |
| Moon等[ | 甲烷/乙烷/氢气混合物(sⅡ) | 512笼双占据, 51264笼四占据 | 拉曼光谱分析 |
| 张宏淑等[ | CO2/N2+H2体系(sⅠ) | 512笼三占据,51262笼单占据 | DFT计算 |
| Krishnan等[ | H2/D2体系(sⅡ) | 512笼单占据,51264笼双占据 | 经典分子动力学模拟 |
| 因素 | 对稳定性的影响 | 关键机制 | 实际应用考量 |
|---|---|---|---|
| 温度 | 低温增强稳定性,高温导致分解 | 热运动破坏晶格结构 | 需要低温设备,能耗高 |
| 压力 | 高压促进形成,低压引发分解 | 压缩氢气分子进入晶格空腔 | 高压容器成本与安全性 |
| 协同 | 遵循温度-压力相图平衡 | 相变临界点的动态调控 | 需要优化条件以降低能耗和成本 |
表3 温压条件对水合物稳定性的影响
| 因素 | 对稳定性的影响 | 关键机制 | 实际应用考量 |
|---|---|---|---|
| 温度 | 低温增强稳定性,高温导致分解 | 热运动破坏晶格结构 | 需要低温设备,能耗高 |
| 压力 | 高压促进形成,低压引发分解 | 压缩氢气分子进入晶格空腔 | 高压容器成本与安全性 |
| 协同 | 遵循温度-压力相图平衡 | 相变临界点的动态调控 | 需要优化条件以降低能耗和成本 |
| 特征 | 自扩散 | 隧穿 |
|---|---|---|
| 机制 | 经典热力学(热激活) | 量子力学(波函数穿透势垒) |
| 温度依赖性 | 高温更显著(扩散系数Dself随温度升高而增大) | 低温更显著(热运动减弱,量子效应凸显) |
| 是否需要热能 | 需要热能克服势垒 | 不需要热能,依赖量子概率 |
| 主导条件 | 常规温度或高压环境 | 极低温或高势垒窄宽度条件 |
表4 氢气水合物中扩散机制对比
| 特征 | 自扩散 | 隧穿 |
|---|---|---|
| 机制 | 经典热力学(热激活) | 量子力学(波函数穿透势垒) |
| 温度依赖性 | 高温更显著(扩散系数Dself随温度升高而增大) | 低温更显著(热运动减弱,量子效应凸显) |
| 是否需要热能 | 需要热能克服势垒 | 不需要热能,依赖量子概率 |
| 主导条件 | 常规温度或高压环境 | 极低温或高势垒窄宽度条件 |
| 体系 | 跨越五元环能垒 | 跨越六元环能垒 | 计算方式 |
|---|---|---|---|
| H2+THF水合物 | 48kJ/mol | 5kJ/mol | Trinh等[ |
| 纯H2水合物 | 105~121kJ/mol | 0~25kJ/mol | Alavi等[ |
| H2+THF水合物 | 在皮秒时间尺度内未观测到笼间扩散 | Pefoute等[ | |
| H2+THF水合物 | 20~34kJ/mol | — | Cao等[ |
| H2+THF水合物 | 21~26kJ/mol | — | Gorman等[ |
| sⅠ型氢气水合物 | 0.55eV | 0.14eV | Lu等[ |
| sⅡ型氢气水合物 | 200kJ/mol | 30kJ/mol | Frankcombe等[ |
表5 氢气分子扩散能垒的部分研究
| 体系 | 跨越五元环能垒 | 跨越六元环能垒 | 计算方式 |
|---|---|---|---|
| H2+THF水合物 | 48kJ/mol | 5kJ/mol | Trinh等[ |
| 纯H2水合物 | 105~121kJ/mol | 0~25kJ/mol | Alavi等[ |
| H2+THF水合物 | 在皮秒时间尺度内未观测到笼间扩散 | Pefoute等[ | |
| H2+THF水合物 | 20~34kJ/mol | — | Cao等[ |
| H2+THF水合物 | 21~26kJ/mol | — | Gorman等[ |
| sⅠ型氢气水合物 | 0.55eV | 0.14eV | Lu等[ |
| sⅡ型氢气水合物 | 200kJ/mol | 30kJ/mol | Frankcombe等[ |
| 系统 | 初始笼占据 | 扩散后笼占据 | 扩散能垒/eV |
|---|---|---|---|
| LL | (H2)L6L(空) | L6L(H2)(空) | 0.281 |
| (H2)(CH4)L6L | (CH4)L6L(CH4)(H2) | 0.189 | |
| (H2)(CO2)L6L | (CO2)L6L(CO2)(H2) | 0.181 | |
| (H2)(THF)L6L | (THF)L6L(H2) | 0.32 | |
| (H2)(THF)L6L | (THF)L6L(H2) | 0.30 | |
| LS | (H2)L5S | S5L(H2) | 0.717 |
| (H2)(CH4)L5S | (CH4)S5L(H2) | 0.82 | |
| (H2)(N2)L5S | (N2)S5(H2) | 1.13 | |
| (H2)(CO2)L5S | (CO2)S5L(H2) | 0.742 |
表6 不同促进剂作用下跨越水合物笼的扩散能垒[70-92]
| 系统 | 初始笼占据 | 扩散后笼占据 | 扩散能垒/eV |
|---|---|---|---|
| LL | (H2)L6L(空) | L6L(H2)(空) | 0.281 |
| (H2)(CH4)L6L | (CH4)L6L(CH4)(H2) | 0.189 | |
| (H2)(CO2)L6L | (CO2)L6L(CO2)(H2) | 0.181 | |
| (H2)(THF)L6L | (THF)L6L(H2) | 0.32 | |
| (H2)(THF)L6L | (THF)L6L(H2) | 0.30 | |
| LS | (H2)L5S | S5L(H2) | 0.717 |
| (H2)(CH4)L5S | (CH4)S5L(H2) | 0.82 | |
| (H2)(N2)L5S | (N2)S5(H2) | 1.13 | |
| (H2)(CO2)L5S | (CO2)S5L(H2) | 0.742 |
| 模拟编号 | 晶胞中THF分子的数量 | 晶胞中H2的数量 | 时间/ps | 晶胞长度/Å | 扩散系数/m2·s-1 |
|---|---|---|---|---|---|
| 1 | 8 | 16 | 1500 | 17.31 | 无扩散 |
| 2 | 7 | 20 | 1500 | 17.32 | 4.50×10-11 |
| 3 | 6 | 24 | 1500 | 17.34 | 1.17×10-10 |
| 4 | 5 | 28 | 1500 | 17.37 | 1.93×10-10 |
| 5 | 4 | 32 | 100 | — | 补水失败 |
表7 模拟中计算得到的300K下氢分子的扩散系数[71]
| 模拟编号 | 晶胞中THF分子的数量 | 晶胞中H2的数量 | 时间/ps | 晶胞长度/Å | 扩散系数/m2·s-1 |
|---|---|---|---|---|---|
| 1 | 8 | 16 | 1500 | 17.31 | 无扩散 |
| 2 | 7 | 20 | 1500 | 17.32 | 4.50×10-11 |
| 3 | 6 | 24 | 1500 | 17.34 | 1.17×10-10 |
| 4 | 5 | 28 | 1500 | 17.37 | 1.93×10-10 |
| 5 | 4 | 32 | 100 | — | 补水失败 |
| [1] | 黄维和, 李玉星, 陈朋超. 碳中和愿景下中国二氧化碳管道发展战略[J]. 天然气工业, 2023, 43(7): 1-9. |
| HUANG Weihe, LI Yuxing, CHEN Pengchao. China’s CO2 pipeline development strategy under the strategy of carbon neutrality[J]. Natural Gas Industry, 2023, 43(7): 1-9. | |
| [2] | 刘翠伟, 裴业斌, 韩辉, 等. 氢能产业链及储运技术研究现状与发展趋势[J]. 油气储运, 2022, 41(5): 498-514. |
| LIU Cuiwei, PEI Yebin, HAN Hui, et al. Research status and development trend of hydrogen energy industry chain and the storage and transportation technologies[J]. Oil & Gas Storage and Transportation, 2022, 41(5): 498-514. | |
| [3] | ZHANG Ye, BHATTACHARJEE Gaurav, KUMAR Rajnish, et al. Solidified hydrogen storage (solid-HyStore) via clathrate hydrates[J]. Chemical Engineering Journal, 2022, 431: 133702. |
| [4] | 赵予生, 徐洪武, 于晓辉, 等. 笼形水合物的科学与技术研究以及在能源和环境领域中的应用[J]. 物理, 2009, 38(2): 92-99. |
| ZHAO Yusheng, XU Hongwu, YU Xiaohui, et al. Clathrate hydrate science and technology with energy and environmental applications[J]. Physics, 2009, 38(2): 92-99. | |
| [5] | 李昊阳, 张炜, 李小森, 等. 水合物储氢的研究进展[J]. 化工进展, 2022, 41(12): 6285-6294. |
| LI Haoyang, ZHANG Wei, LI Xiaosen, et al. Research process of hydrate-based hydrogen storage[J]. Chemical Industry and Engineering Progress, 2022, 41(12): 6285-6294. | |
| [6] | RIPMEESTER John A, John S TSE, RATCLIFFE Christopher I, et al. A new clathrate hydrate structure[J]. Nature, 1987, 325(6100): 135-136. |
| [7] | 李新颖. TBAB耦合sⅡ型水合物制备高储氢材料及机理研究[D]. 广州: 华南理工大学, 2024. |
| LI Xinying. Study on preparation and mechanism of high hydrogen storage materials by coupling TBAB with sⅡ hydrate[D]. Guangzhou: South China University of Technology, 2024. | |
| [8] | Willem L VOS, FINGER Larry W, HEMLEY Russell J, et al. Novel H2-H2O clathrates at high pressures[J]. Physical Review Letters, 1993, 71(19): 3150-3153. |
| [9] | ZHENG Ruyi, MOHAMMED Sohaib, JIA Yang, et al. The effect of H2 occupancy modes in small and large cages of H2-tetrahydrofuran hydrates on the hydrates’ stability and H2 storage capacity[J]. Physical Chemistry Chemical Physics, 2025, 27(13): 6532-6545. |
| [10] | BAO Wancheng, TENG Ying, WANG Pengfei, et al. Molecular analysis of hydrogen-propane hydrate formation mechanism and its influencing factors for hydrogen storage[J]. International Journal of Hydrogen Energy, 2024, 50: 697-708. |
| [11] | Yun-Ho AHN, MOON Seokyoon, Dong-Yeun KOH, et al. One-step formation of hydrogen clusters in clathrate hydrates stabilized via natural gas blending[J]. Energy Storage Materials, 2020, 24: 655-661. |
| [12] | DI PROFIO Pietro, CANALE Valentino, GERMANI Raimondo, et al. Reverse micelles enhance the formation of clathrate hydrates of hydrogen[J]. Journal of Colloid and Interface Science, 2018, 516: 224-231. |
| [13] | 谢应明, 龚金明, 刘道平, 等. 一种新型储氢方法——水合物储氢的研究概况与发展方向[J]. 化工进展, 2010, 29(5): 796-800, 806. |
| XIE Yingming, GONG Jinming, LIU Daoping, et al. Hydrogen stored in hydrates—A novel hydrogen storage method[J]. Chemical Industry and Engineering Progress, 2010, 29(5): 796-800, 806. | |
| [14] | GUO Yuanyuan, WU Wanqing, HAO Benhao, et al. Formation of hydrogen hydrate in the presence of thermodynamic promoters: A review and prospects[J]. International Journal of Hydrogen Energy, 2024, 60: 1462-1480. |
| [15] | YU Honglin, ZHANG Peng, LIU Mengqi, et al. CTAB serves as the best kinetic promoters of H2/DIOX mixed hydrates for moderate solidified hydrogen storage via clathrates[J]. Chemical Engineering Journal, 2025, 506: 159818. |
| [16] | MAO Wendy L, MAO Ho-Kwang, GONCHAROV Alexander F, et al. Hydrogen clusters in clathrate hydrate[J]. Science, 2002, 297(5590): 2247-2249. |
| [17] | MAO Wendy L, MAO Ho-Kwang. Hydrogen storage in molecular compounds[J]. Proceedings of the National Academy of Sciences of the United States of America, 2004, 101(3): 708-710. |
| [18] | WANG Xiaohui, QIN Huibo, DANDEKAR Abhijit, et al. Hydrate phase equilibrium of H2/CH4/CO2 ternary gas mixtures and cage occupancy percentage of hydrogen molecules[J]. Fluid Phase Equilibria, 2015, 403: 160-166. |
| [19] | 高佳佳, 米媛媛, 周洋, 等. 新型储氢材料研究进展[J]. 化工进展, 2021, 40(6): 2962-2971. |
| GAO Jiajia, MI Yuanyuan, ZHOU Yang, et al. Recent developments in new hydrogen storage materials[J]. Chemical Industry and Engineering Progress, 2021, 40(6): 2962-2971. | |
| [20] | Naveed KHAN M. Hydrogen as future sustainable energy resource: An insight into technological advancements inhydrate-based hydrogen storage[J]. International Journal of Hydrogen Energy, 2025, 97: 1386-1398. |
| [21] | KIM Min-Kyung, Yun-Ho AHN. Gas hydrates for hydrogen storage: A comprehensive review and future prospects[J]. Korean Journal of Chemical Engineering, 2024, 41(1): 73-94. |
| [22] | SAIKIA Tinku, PATIL Shirish, SULTAN Abdullah. Hydrogen hydrate promoters for gas storage—A review[J]. Energies, 2023, 16(6): 2667. |
| [23] | 张景, 张宏淑. sⅡ型氢气水合物促进剂的研究进展[J]. 山东化工, 2024, 53(7): 69-72. |
| ZHANG Jing, ZHANG Hongshu. Research progress on sⅡ type hydrogen hydrate promoter[J]. Shandong Chemical Industry, 2024, 53(7): 69-72. | |
| [24] | BABU Ponnivalavan, NAMBIAR Abhishek, HE Tianbiao, et al. A review of clathrate hydrate based desalination to strengthen energy-water nexus[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(7): 8093-8107. |
| [25] | WALSH Matthew R, Carolyn A KOH, SLOAN E Dendy, et al. Microsecond simulations of spontaneous methane hydrate nucleation and growth[J]. Science, 2009, 326(5956): 1095-1098. |
| [26] | WOLF Joachim. Liquid-hydrogen technology for vehicles[J]. MRS Bulletin, 2002, 27(9): 684-687. |
| [27] | DYADIN Yuri A, LARIONOV Eduard G, MANAKOV Andrei Yu, et al. Clathrate hydrates of hydrogen and neon[J]. Mendeleev Communications, 1999, 9(5): 209-210. |
| [28] | HASHIMOTO Shunsuke, MURAYAMA Shu, SUGAHARA Takeshi, et al. Thermodynamic and Raman spectroscopic studies on H2+tetrahydrofuran+water and H2+tetra-n-butyl ammonium bromide+water mixtures containing gas hydrates[J]. Chemical Engineering Science, 2006, 61(24): 7884-7888. |
| [29] | TSUDA Takaaki, OGATA Kyohei, HASHIMOTO Shunsuke, et al. Storage capacity of hydrogen in tetrahydrothiophene and furan clathrate hydrates[J]. Chemical Engineering Science, 2009, 64(19): 4150-4154. |
| [30] | KUMAR Asheesh, DARABOINA Nagu, KUMAR Rajnish, et al. Experimental investigation to elucidate why tetrahydrofuran rapidly promotes methane hydrate formation kinetics: Applicable to energy storage[J]. The Journal of Physical Chemistry C, 2016, 120(51): 29062-29068. |
| [31] | ZHANG Jibao, LI Yan, YIN Zhenyuan, et al. Coupling amino acidL-Val with THF for superior hydrogen hydrate kinetics: Implication for hydrate-based hydrogen storage[J]. Chemical Engineering Journal, 2023, 467: 143459. |
| [32] | ZHANG Jibao, LI Yan, RAO Yizhi, et al. Probing the pathway of H2-THF and H2-DIOX sⅡ hydrates formation: Implication on hydrate-based H2 storage[J]. Applied Energy, 2024, 376: 124289. |
| [33] | YAGASAKI Takuma, MATSUMOTO Masakazu, TANAKA Hideki. Formation of clathrate hydrates of water-soluble guest molecules[J]. The Journal of Physical Chemistry C, 2016, 120(38): 21512-21521. |
| [34] | YAGASAKI Takuma, MATSUMOTO Masakazu, TANAKA Hideki. Mechanism of slow crystal growth of tetrahydrofuran clathrate hydrate[J]. The Journal of Physical Chemistry C, 2016, 120(6): 3305-3313. |
| [35] | Dong-Yeun KOH, KANG Hyery, LEE Huen. Multiple guest occupancy in clathrate hydrates and its significance in hydrogen storage[J]. Chemical Communications, 2013, 49(60): 6782-6784. |
| [36] | LEE Huen, LEE Jong-Won, KIM Do Youn, et al. Tuning clathrate hydrates for hydrogen storage[J]. Nature, 2005, 434(7034): 743-746. |
| [37] | KANG Dong Woo, LEE Wonhyeong, Yun-Ho AHN, et al. Exploring tuning phenomena of THF-H2 hydrates via molecular dynamics simulations[J]. Journal of Molecular Liquids, 2022, 349: 118490. |
| [38] | LEE Wonhyeong, KIM Min-Kyung, MOON Seokyoon, et al. Rapid hydrogen enclathration and unprecedented tuning phenomenon within superabsorbent polymers[J]. Applied Energy, 2025, 377: 124367. |
| [39] | ALAVI Saman, RIPMEESTER J A, KLUG D D. Molecular-dynamics study of structure Ⅱ hydrogen clathrates[J]. The Journal of Chemical Physics, 2005, 123(2): 24507. |
| [40] | LIU Jinxiang, HOU Jian, XU Jiafang, et al. Ab initio study of the molecular hydrogen occupancy in pure H2 and binary H2-THF clathrate hydrates[J]. International Journal of Hydrogen Energy, 2017, 42(27): 17136-17143. |
| [41] | LU Hailong, WANG Jianwei, LIU Changling, et al. Multiple H2 occupancy of cages of clathrate hydrate under mild conditions[J]. Journal of the American Chemical Society, 2012, 134(22): 9160-9162. |
| [42] | JAFARI DAGHALIAN SOFLA Sahar, Alejandro D REY, SERVIO Phillip. Atomistic Investigation of the occupancy limits and stability of hydrogen hydrates as a hydrogen storage medium[J]. International Journal of Hydrogen Energy, 2024, 51: 184-192. |
| [43] | JAFARI DAGHALIAN SOFLA Sahar, Alejandro D REY, SERVIO Phillip. Impact of hydrogen cage occupancy on the mechanical properties and elastic anisotropies of sII hydrates[J]. Fluid Phase Equilibria, 2024, 585: 114172. |
| [44] | BRUMBY Paul E, YUHARA Daisuke, HASEGAWA Tomohiro, et al. Cage occupancies, lattice constants, and guest chemical potentials for structure Ⅱ hydrogen clathrate hydrate from Gibbs ensemble Monte Carlo simulations[J]. The Journal of Chemical Physics, 2019, 150(13): 134503. |
| [45] | KATSUMASA Keisuke, KOGA Kenichiro, TANAKA Hideki. On the thermodynamic stability of hydrogen clathrate hydrates[J]. The Journal of Chemical Physics, 2007, 127(4): 044509. |
| [46] | OMRAN Ahmed, NESTERENKO Nikolai, VALTCHEV Valentin. Ab initio mechanistic insights into the stability, diffusion and storage capacity of sⅠ clathrate hydrate containing hydrogen[J]. International Journal of Hydrogen Energy, 2022, 47(13): 8419-8433. |
| [47] | MATSUMOTO Yuuki, Gary GRIM R, KHAN Naveed M, et al. Investigating the thermodynamic stabilities of hydrogen and methane binary gas hydrates[J]. The Journal of Physical Chemistry C, 2014, 118(7): 3783-3788. |
| [48] | MOON Seokyoon, LEE Yunseok, SEO Dongju, et al. Critical hydrogen concentration of hydrogen-natural gas blends in clathrate hydrates for blue hydrogen storage[J]. Renewable and Sustainable Energy Reviews, 2021, 141: 110789. |
| [49] | 张宏淑, 梁攀, 薛颖颖, 等. 二元包合水合物储氢及促进剂作用的理论研究[J]. 高等学校化学学报, 2024, 45(1): 127-136. |
| ZHANG Hongshu, LIANG Pan, XUE Yingying, et al. Theoretical study on hydrogen storage and promoter effect of binary clathrate hydrates[J]. Chemical Journal of Chinese Universities, 2024, 45(1): 127-136. | |
| [50] | KRISHNAN Yogeshwaran, GHAANI Mohammad Reza, DESMEDT Arnaud, et al. Hydrogen inter-cage hopping and cage occupancies inside hydrogen hydrate: Molecular-dynamics analysis[J]. Applied Sciences, 2021, 11(1): 282. |
| [51] | RASOOLZADEH Ali, SHARIATI Alireza. Hydrogen hydrate cage occupancy: A key parameter for hydrogen storage and transport[J]. Fluid Phase Equilibria, 2019, 494: 8-20. |
| [52] | 王芳, 穆金池, 张政, 等. 温和条件下甲烷提高水合物储氢稳定特性机理[J]. 天然气工业, 2024, 44(2): 188-196. |
| WANG Fang, MU Jinchi, ZHANG Zheng, et al. Mechanism of methane in improving the stability of hydrate hydrogen storage under mild conditions[J]. Natural Gas Industry, 2024, 44(2): 188-196. | |
| [53] | GENG Chunyu, HAN Qingzhen, WEN Hao, et al. Molecular dynamics simulation on the decomposition of type SⅡ hydrogen hydrate and the performance of tetrahydrofuran as a stabiliser[J]. Molecular Simulation, 2010, 36(6): 474-483. |
| [54] | TRUEBA Alondra Torres, ROVETTO Laura J, FLORUSSE Louw J, et al. Phase equilibrium measurements of structure Ⅱ clathrate hydrates of hydrogen with various promoters[J]. Fluid Phase Equilibria, 2011, 307(1): 6-10. |
| [55] | VELUSWAMY Hari Prakash, KUMAR Rajnish, LINGA Praveen. Hydrogen storage in clathrate hydrates: Current state of the art and future directions[J]. Applied Energy, 2014, 122: 112-132. |
| [56] | ZHANG Jiudan, SCHÄFER Sylva Mareike, KABISCH Stefan, et al. Implication of sugar, protein and incretins in excessive glucagon secretion in type 2 diabetes after mixed meals[J]. Clinical Nutrition, 2023, 42(4): 467-476. |
| [57] | Naveed KHAN M, WARRIER Pramod, PETERS Cor J, et al. Advancements in hydrate phase equilibria and modeling of gas hydrates systems[J]. Fluid Phase Equilibria, 2018, 463: 48-61. |
| [58] | LIU Jinxiang, YAN Yujie, CHEN Gang, et al. Prediction of efficient promoter molecules of sH hydrogen hydrate: An abinitio study[J]. Chemical Physics, 2019, 516: 15-21. |
| [59] | ALAVI Saman, RIPMEESTER John A. Hydrogen-gas migration through clathrate hydrate cages[J]. Angewandte Chemie International Edition, 2007, 46(32): 6102-6105. |
| [60] | CHOI Yong Nam, SUNGIL PARK J M, Thierry STRÄSSLE, et al. Dynamics of hydrogen molecules in the channels of binary THF-H2 clathrate hydrate and its physicochemical significance on hydrogen storage[J]. International Journal of Hydrogen Energy, 2010, 35(23): 13068-13072. |
| [61] | KRISHNAN Yogeshwaran, GHAANI Mohammad Reza, ENGLISH Niall J. Hydrogen and deuterium molecular escape from clathrate hydrates: “Leaky” microsecond-molecular-dynamics predictions[J]. The Journal of Physical Chemistry C, 2021, 125(15): 8430-8439. |
| [62] | KLOTZ S, BESSON J M, HAMEL G, et al. Metastable ice Ⅶ at low temperature and ambient pressure[J]. Nature, 1999, 398(6729): 681-684. |
| [63] | TRINH Thuat T, WAAGE Magnus H, VAN ERP Titus S, et al. Low barriers for hydrogen diffusion in sⅡ clathrate[J]. Physical Chemistry Chemical Physics, 2015, 17(21): 13808-13812. |
| [64] | PEFOUTE E, KEMNER E, SOETENS J C, et al. Diffusive motions of molecular hydrogen confined in THF clathrate hydrate[J]. The Journal of Physical Chemistry C, 2012, 116(32): 16823-16829. |
| [65] | CAO Huayu, ENGLISH Niall J, MACELROY J M D. Diffusive hydrogen inter-cage migration in hydrogen and hydrogen-tetrahydrofuran clathrate hydrates[J]. The Journal of Chemical Physics, 2013, 138(9): 094507. |
| [66] | GORMAN Paul D, ENGLISH Niall J, MACELROY J M D. Dynamical cage behaviour and hydrogen migration in hydrogen and hydrogen-tetrahydrofuran clathrate hydrates[J]. The Journal of Chemical Physics, 2012, 136(4): 044506. |
| [67] | FRANKCOMBE Terry J, KROES Geert-Jan. Molecular dynamics simulations of type-sⅡ hydrogen clathrate hydrate close to equilibrium conditions[J]. The Journal of Physical Chemistry C, 2007, 111(35): 13044-13052. |
| [68] | BURNHAM Christian J, FUTERA Zdenek, ENGLISH Niall J. Study of hydrogen-molecule guests in type Ⅱ clathrate hydrates using a force-matched potential model parameterised from ab initio molecular dynamics[J]. The Journal of Chemical Physics, 2018, 148(10): 102323. |
| [69] | 颜克凤, 李小森, 孙丽华, 等. 储氢笼型水合物生成促进机理的分子动力学模拟研究[J]. 物理学报, 2011, 60(12): 645-652. |
| YAN Kefeng, LI Xiaosen, SUN Lihua, et al. Molecular dynamics simulation of promotion mechanism of store hydrogen of clathrate hydrate[J]. Acta Physica Sinica, 2011, 60(12): 645-652. | |
| [70] | CHEN Siyuan, WANG Yanhong, FAN Shuanshi, et al. Intriguing phenomenon of hydrogen molecules occupancy in clathrate hydrate cages: Implications for hydrogen storage[J]. Chemical Engineering Journal, 2024, 499: 156089. |
| [71] | ENGLISH Niall J, GORMAN Paul D, MACELROY J M D. Mechanisms for thermal conduction in hydrogen hydrate[J]. The Journal of Chemical Physics, 2012, 136(4): 044501. |
| [72] | CENDAGORTA Joseph R, SHEN Hengyuan, Zlatko BAČIĆ, et al. Enhanced sampling path integral methods using neural network potential energy surfaces with application to diffusion in hydrogen hydrates[J]. Advanced Theory and Simulations, 2021, 4(4): 2000258. |
| [73] | LU Qiangna, HE Xiao, HU Wenxin, et al. Stability, vibrations, and diffusion of hydrogen gas in clathrate hydrates: Insights from ab initio calculations on condensed-phase crystalline structures[J]. The Journal of Physical Chemistry C, 2019, 123(19): 12052-12061. |
| [74] | ZHANG Mingmin, NI Dongdong. Evaluating the gas storage capacity of 1,3-dioxolane-hydrogen binary hydrates via molecular simulations[J]. Journal of Molecular Liquids, 2024, 393: 123542. |
| [75] | LIU Shengli, ZHANG Wenxiu, WU Huanhua, et al. Molecular hydrogen storage in binary H2-CH4 clathrate hydrates[J]. Journal of Molecular Liquids, 2023, 376: 121496. |
| [76] | STRUZHKIN Viktor V, MILITZER Burkhard, MAO Wendy L, et al. Hydrogen storage in molecular clathrates[J]. Chemical Reviews, 2007, 107(10): 4133-4151. |
| [77] | LEE Wonhyeong, KANG Dong Woo, Yun-Ho AHN, et al. Blended hydrate seed and liquid promoter for the acceleration of hydrogen hydrate formation[J]. Renewable and Sustainable Energy Reviews, 2023, 177: 113217. |
| [78] | FLORUSSE Louw J, PETERS Cor J, SCHOONMAN Joop, et al. Stable low-pressure hydrogen clusters stored in a binary clathrate hydrate[J]. Science, 2004, 306(5695): 469-471. |
| [79] | ZHANG Shixi, CHEN Guangjin, MA Changfeng, et al. Hydrate formation of hydrogen + hydrocarbon gas mixtures[J]. Journal of Chemical & Engineering Data, 2000, 45(5): 908-911. |
| [80] | WANG Yu, GLAZYRIN Konstantin, ROIZEN Valery, et al. Novel hydrogen clathrate hydrate[J]. Physical Review Letters, 2020, 125(25): 255702. |
| [81] | 岳子瀚, 龙臻, 周雪冰, 等. sⅡ型水合物储氢研究进展[J]. 化工进展, 2023, 42(10): 5121-5134. |
| YUE Zihan, LONG Zhen, ZHOU Xuebing, et al. State of the art on hydrogen storage of sⅡ clathrate hydrate[J]. Chemical Industry and Engineering Progress, 2023, 42(10): 5121-5134. | |
| [82] | 陈玉凤, 周雪冰, 梁德青, 等. TBAB-CO2水合物形成过程的微观实验[J]. 光谱学与光谱分析, 2019, 39(9): 2889. |
| CHEN Yufeng, ZHOU Xuebing, LIANG Deqing, et al. Microscopic experimental study on the crystallization of TBAB-CO2 hydrate[J]. Spectroscopy and Spectral Analysis, 2019, 39(9): 2889. | |
| [83] | STROBEL Timothy A, TAYLOR Craig J, HESTER Keith C, et al. Molecular hydrogen storage in binary THF-H2 clathrate hydrates[J]. The Journal of Physical Chemistry B, 2006, 110(34): 17121-17125. |
| [84] | TRUEBA Alondra Torres, RADOVIĆ Ivona R, ZEVENBERGEN John F, et al. Kinetic measurements and in situ Raman spectroscopy study of the formation of TBAF semi-hydrates with hydrogen and carbon dioxide[J]. International Journal of Hydrogen Energy, 2013, 38(18): 7326-7334. |
| [85] | PRASAD Pinnelli S R, SUGAHARA Takeshi, Amadeu K SUM, et al. Hydrogen storage in double clathrates with tert-butylamine[J]. The Journal of Physical Chemistry A, 2009, 113(24): 6540-6543. |
| [86] | OGATA Kyohei, HASHIMOTO Shunsuke, SUGAHARA Takeshi, et al. Storage capacity of hydrogen in tetrahydrofuran hydrate[J]. Chemical Engineering Science, 2008, 63(23): 5714-5718. |
| [87] | SUGAHARA Takeshi, HAAG Joanna C, PRASAD Pinnelli S R, et al. Increasing hydrogen storage capacity using tetrahydrofuran[J]. Journal of the American Chemical Society, 2009, 131(41): 14616-14617. |
| [88] | HASHIMOTO Shunsuke, SUGAHARA Takeshi, SATO Hiroshi, et al. Thermodynamic stability of H2 + tetrahydrofuran mixed gas hydrate in nonstoichiometric aqueous solutions[J]. Journal of Chemical & Engineering Data, 2007, 52(2): 517-520. |
| [89] | CHAPOY Antonin, ANDERSON Ross, TOHIDI Bahman. Low-pressure molecular hydrogen storage in semi-clathrate hydrates of quaternary ammonium compounds[J]. Journal of the American Chemical Society, 2007, 129(4): 746-747. |
| [90] | STROBEL Timothy A, Carolyn A KOH, SLOAN E Dendy. Water cavities of sH clathrate hydrate stabilized by molecular hydrogen[J]. The Journal of Physical Chemistry B, 2008, 112(7): 1885-1887. |
| [91] | VELUSWAMY Hari Prakash, CHEN Jianyu, LINGA Praveen. Surfactant effect on the kinetics of mixed hydrogen/propane hydrate formation for hydrogen storage as clathrates[J]. Chemical Engineering Science, 2015, 126: 488-499. |
| [92] | PARK Jeasung, LEE Huen. Spectroscopic evidences of the double hydrogen hydrates stabilized with ethane and propane[J]. Korean Journal of Chemical Engineering, 2007, 24(4): 624-627. |
| [93] | Gary GRIM R, KERKAR Prasad B, SHEBOWICH Michele, et al. Synthesis and characterization of sⅠ clathrate hydrates containing hydrogen[J]. The Journal of Physical Chemistry C, 2012, 116(34): 18557-18563. |
| [94] | LUIS D P, ROMERO-RAMIREZ I E, GONZÁLEZ-CALDERÓN A, et al. The coexistence temperature of hydrogen clathrates: A molecular dynamics study[J]. The Journal of Chemical Physics, 2018, 148(11): 114503. |
| [95] | WANG Pengfei, LI Kehan, YANG Jianyu, et al. Experimental and theoretical study on dissociation thermodynamics and kinetics of hydrogen-propane hydrate[J]. Chemical Engineering Journal, 2021, 426: 131279. |
| [96] | HASEGAWA Tomohiro, BRUMBY Paul E, YASUOKA Kenji, et al. Mechanism for H2 diffusion in sⅡ hydrates by molecular dynamics simulations[J]. The Journal of Chemical Physics, 2020, 153(5): 054706. |
| [1] | 田小革, 李光耀, 高凯, 吴清浩, 黄思丹, 谢振. 干法工艺中废胶粉与沥青-集料界面的相互作用行为[J]. 化工进展, 2025, 44(9): 5174-5183. |
| [2] | 黄可儿, 刘佳豪, 李昊明, 周天航, 高金森, 蓝兴英. 基于分子动力学模拟的胺溶剂碳捕集过程自扩散系数[J]. 化工进展, 2025, 44(8): 4352-4364. |
| [3] | 齐妍, 常昊, 张磊. 基于分子动力学模拟的结构性产品配方设计方法[J]. 化工进展, 2025, 44(8): 4341-4351. |
| [4] | 李艳平, 杨涛, 王洪勋, 张城, 温国胜, 韩治成, 蓝公家, 严大洲. 三氯氢硅在氢气氛中的热分解及还原体系的反应分子动力学模拟[J]. 化工进展, 2025, 44(8): 4322-4330. |
| [5] | 戴月明, 周梅芳, 沈建华, 姜海波, 李春忠. TiO2纳米颗粒烧结机制分子动力学模拟[J]. 化工进展, 2025, 44(4): 2202-2214. |
| [6] | 冯鹏, 徐东海, 何冰, 刘欢腾, 杨立杰, 王攀, 刘青山. 亚/超临界水中典型硫酸盐Na2SO4和K2SO4的溶解特性及机理[J]. 化工进展, 2025, 44(3): 1706-1715. |
| [7] | 林梅, 雷雨, 李萍, 张强. 石墨烯/橡胶复合改性沥青-集料界面黏附性能及机理[J]. 化工进展, 2025, 44(2): 991-1002. |
| [8] | 白依冉, 翟玉玲, 戴晶慧, 李舟航. 微纳尺度池沸腾表面润湿性的气泡成核及强化传热机制[J]. 化工进展, 2025, 44(2): 743-751. |
| [9] | 张强, 孙楠, 郑俊杰, 吴强, 刘传海, 李元吉. 混合热力学促进剂对水合物法分离回收瓦斯的影响[J]. 化工进展, 2025, 44(1): 192-201. |
| [10] | 谢娟, 贺文, 赵勖丞, 李帅辉, 卢真真, 丁哲宇. 分子动力学模拟在沥青体系中的应用研究进展[J]. 化工进展, 2024, 43(8): 4432-4449. |
| [11] | 黄淄博, 周文静, 魏进家. 基于ReaxFF MD模拟的低阶煤热解产物演化规律及反应机理[J]. 化工进展, 2024, 43(5): 2409-2419. |
| [12] | 黄文荻, 周国兵, 曹保鑫. 三水醋酸钠/石墨烯复合相变材料界面热阻的分子动力学模拟[J]. 化工进展, 2024, 43(12): 6820-6827. |
| [13] | 史柯, 马峰, 宋瑞萌, 傅珍. 基于分子模拟的废大豆油再生沥青扩散行为[J]. 化工进展, 2024, 43(12): 6794-6803. |
| [14] | 吴艳, 李彬, 鞠明东, 向伟, 王海, 王贞涛, 王军锋, 王振波. 纳米限域条件下油滴驱替强化机理的分子动力学模拟[J]. 化工进展, 2024, 43(10): 5393-5402. |
| [15] | 高江雨, 张耀君, 贺攀阳, 刘礼才, 张枫烨. 磷酸基地质聚合物的制备及其性能研究进展[J]. 化工进展, 2023, 42(3): 1411-1425. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||