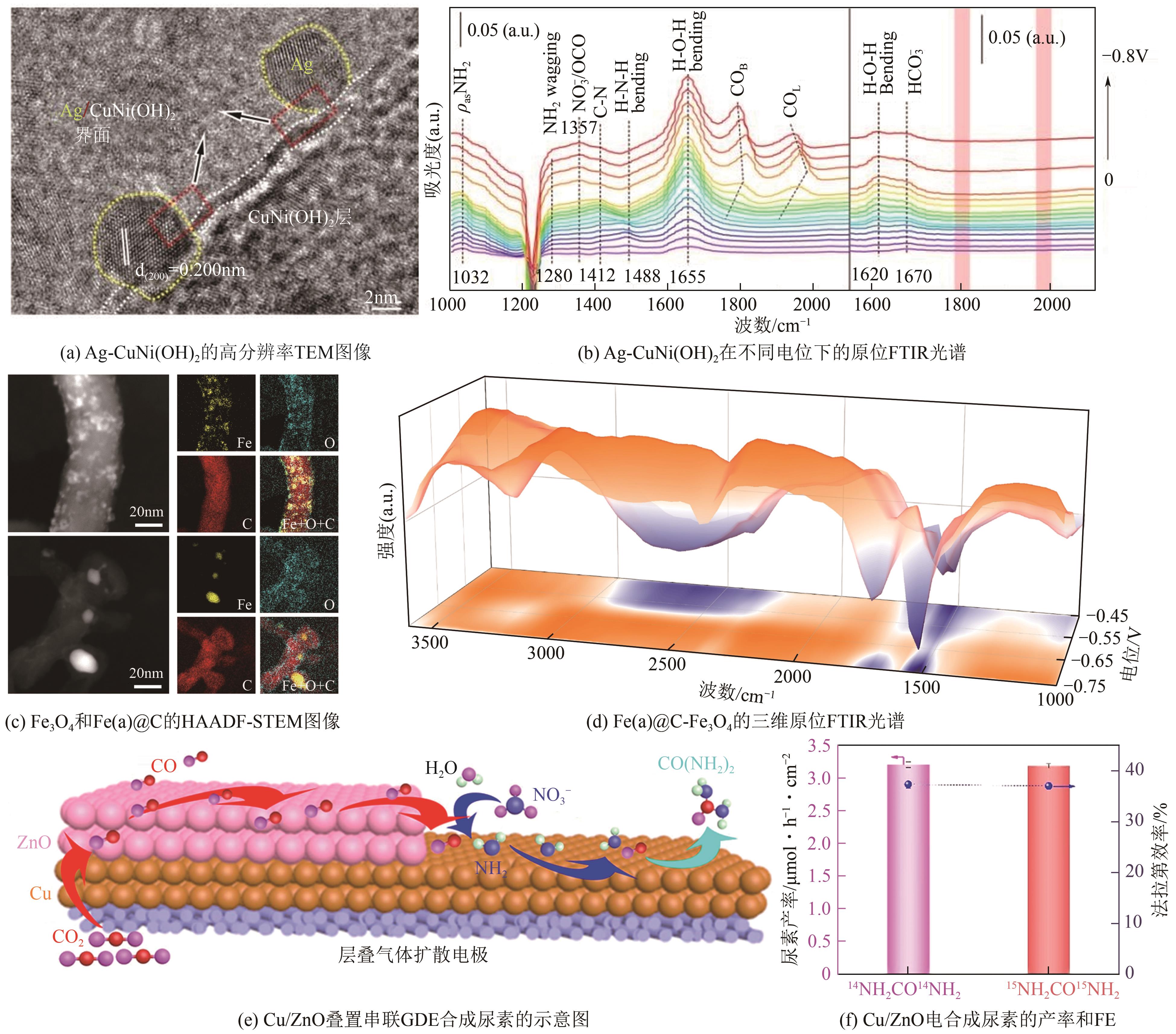
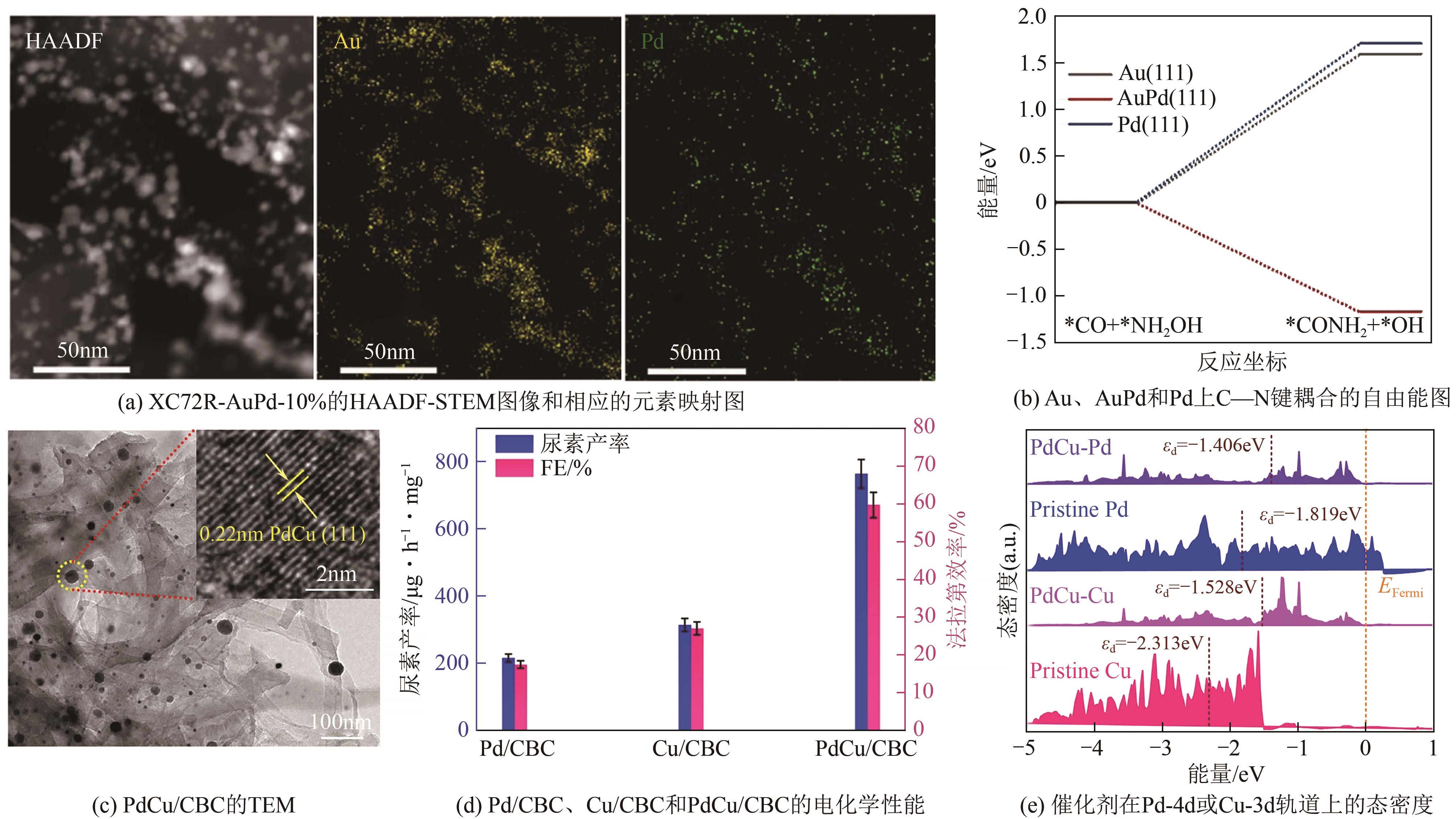
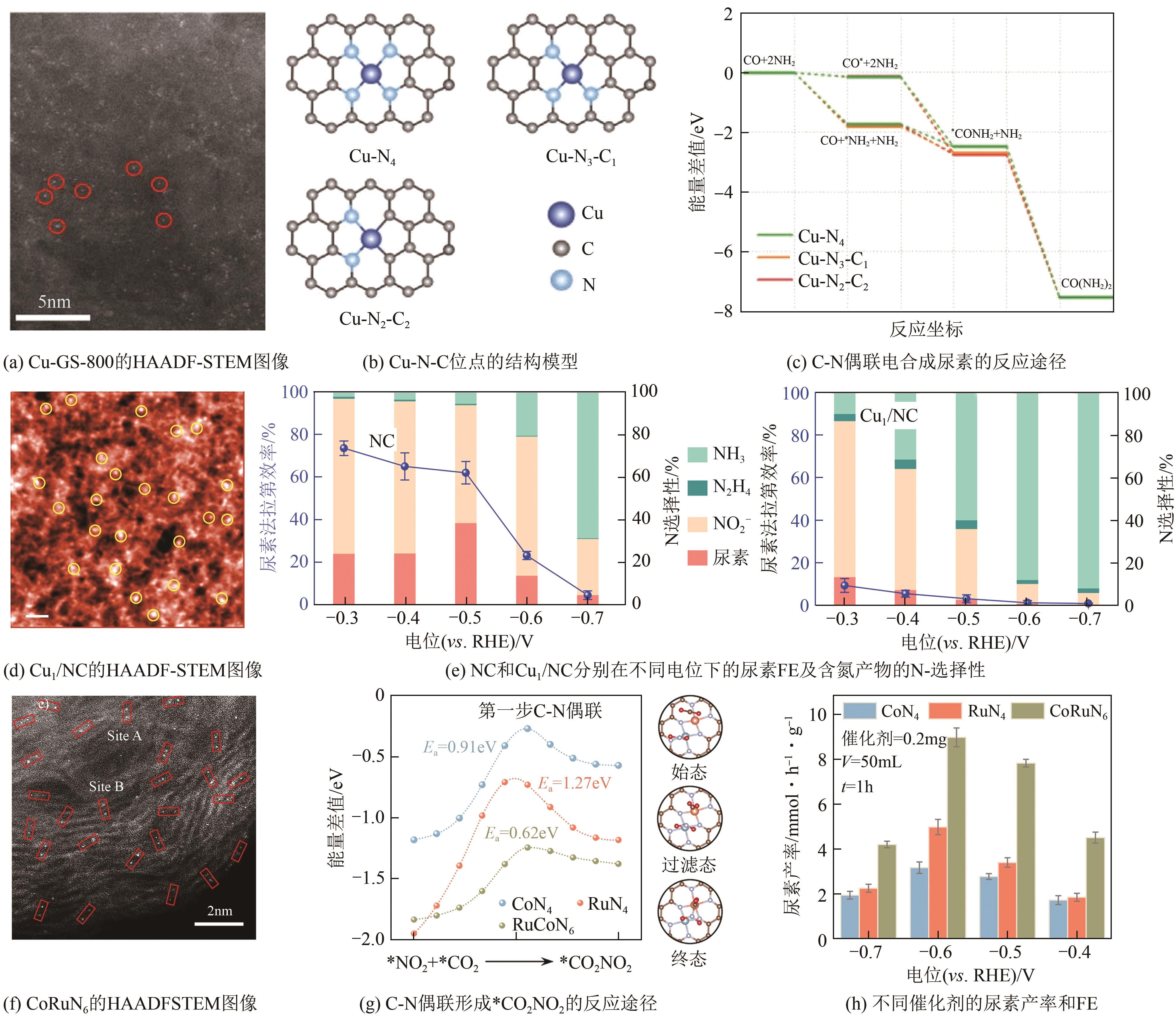
化工进展 ›› 2025, Vol. 44 ›› Issue (5): 2856-2869.DOI: 10.16085/j.issn.1000-6613.2024-1800
• CO2减排利用 • 上一篇
电催化二氧化碳和硝酸根共还原合成尿素研究进展
- 1.成都理工大学材料与化学化工学院,四川 成都 610059
2.四川大学化学工程学院,四川 成都 610065
-
收稿日期:2024-11-06修回日期:2025-02-08出版日期:2025-05-25发布日期:2025-05-20 -
通讯作者:彭强 -
作者简介:范晓娅(1994—),女,博士,讲师,研究方向为电催化小分子合成。E-mail:xyfan@cdut.edu.cn。 -
基金资助:国家自然科学基金(22379101);四川省自然科学基金(2025ZNSFSC0897)
Review on electrocatalytic co-reduction of carbon dioxide and nitrate for urea synthesis
FAN Xiaoya1( ), ZHAO Zhen1, PENG Qiang1,2(
), ZHAO Zhen1, PENG Qiang1,2( )
)
- 1.College of Materials and Chemistry & Chemical Engineering, Chengdu University of Technology, Chengdu 610059, Sichuan, China
2.School of Chemical Engineering, Sichuan University, Chengdu 610065, Sichuan, China
-
Received:2024-11-06Revised:2025-02-08Online:2025-05-25Published:2025-05-20 -
Contact:PENG Qiang
摘要:
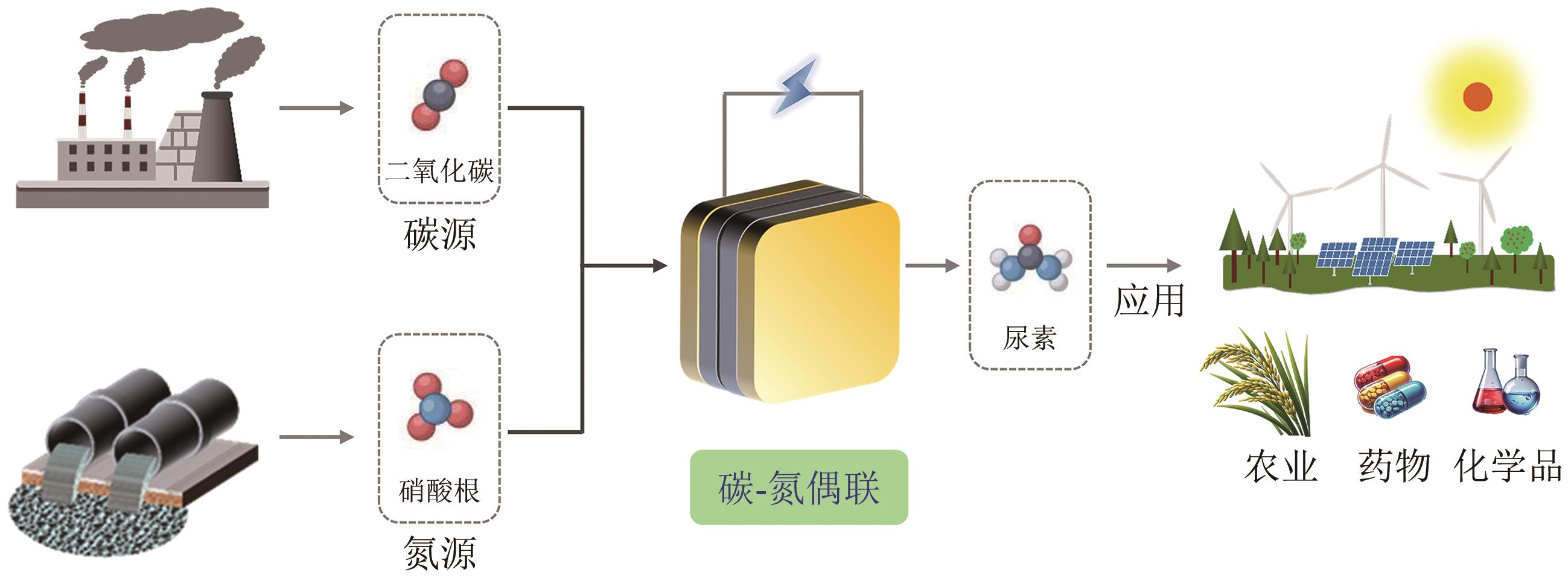
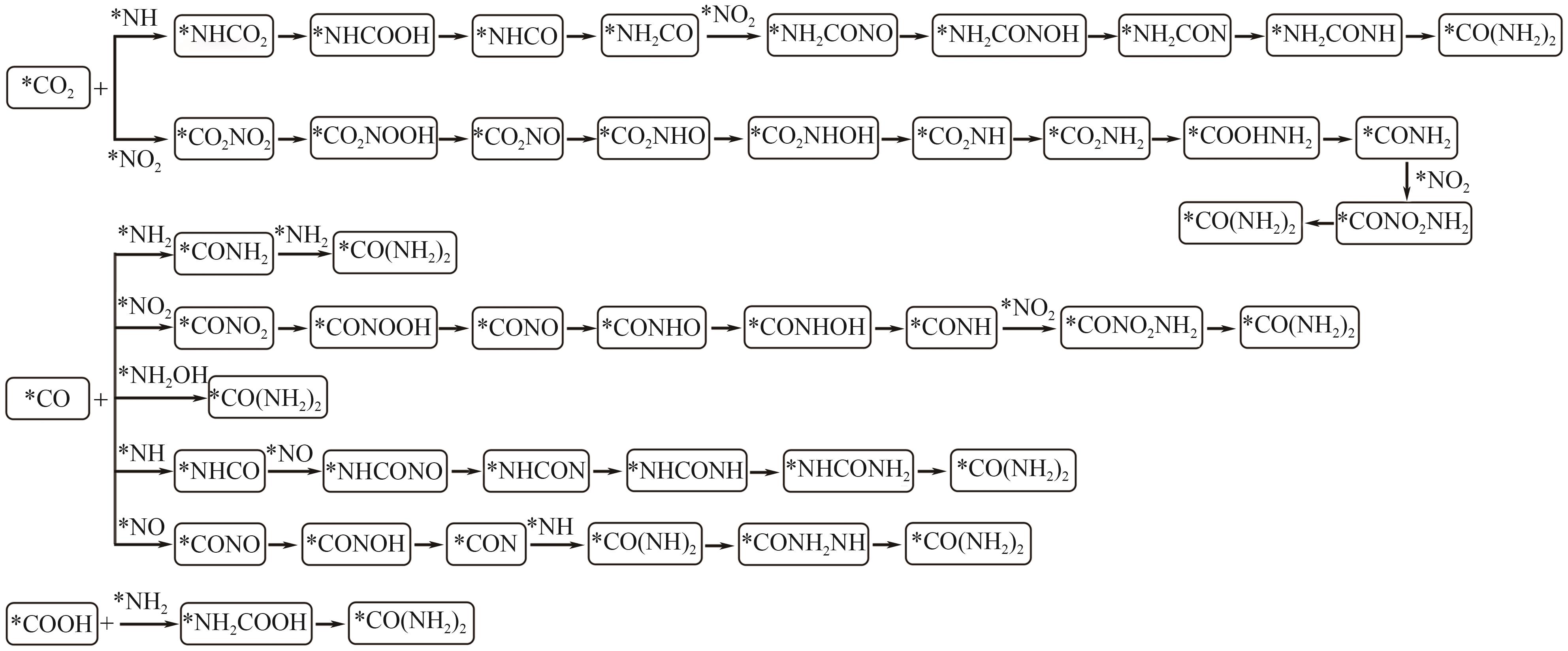
尿素是一种关键的农业氮肥,是作物生长不可或缺的原料。电催化CO2和NO3-共还原的C-N偶联反应制尿素被认为是实现清洁和可持续生产的一种有前途的策略,引起了广泛关注。相比于传统的Bosch-Meiser工艺,C-N偶联反应具有降低能耗和减少碳排放的潜力。本文综述了电催化CO2和NO3-共还原合成尿素的最新研究进展,深入探讨了该反应的机理,结合原位表征和密度泛函理论计算,揭示了促进C-N偶联和提升尿素合成效率的微观机制。本文还总结了提高尿素产率的关键催化剂设计策略,包括杂原子掺杂、缺陷工程、异质结构构建、合金化和原子尺度等调控策略。最后,本文提出了未来研究及工业化应用的挑战与展望,特别关注如何在大规模生产中实现高效、低能耗的尿素合成,同时分析了催化剂的结构设计和精细调控,为实现可持续尿素生产提供理论和实践支持。
中图分类号:
引用本文
范晓娅, 赵镇, 彭强. 电催化二氧化碳和硝酸根共还原合成尿素研究进展[J]. 化工进展, 2025, 44(5): 2856-2869.
FAN Xiaoya, ZHAO Zhen, PENG Qiang. Review on electrocatalytic co-reduction of carbon dioxide and nitrate for urea synthesis[J]. Chemical Industry and Engineering Progress, 2025, 44(5): 2856-2869.
| 催化剂 | 电解质 | 过电位(vs. RHE)/V | 尿素产率 | 法拉第效率/% | 参考文献 |
|---|---|---|---|---|---|
| Bi:10%In/C NPs(铟掺杂铋纳米颗粒) | 0.1mol/L KHCO3+0.1mol/L KNO3 | -0.45 | 606.38μg/(h·mg) | 20.31 | [ |
| VB12-CNTs(维生素B负载碳纳米管) | 0.1mol/L KNO3 | -0.5 | 164.04μg/(h·mg) | 26.04 | [ |
| Ru-Cu9Bi/CNT(钌掺杂铜铋碳纳米管) | 0.1mol/L KHCO3+0.1mol/L KNO3 | -0.4 | 2928μg/(h·mg) | 65.7 | [ |
| CuSiO x (硅酸铜) | 0.1mol/L KHCO3+0.1mol/L KNO3 | -0.2 | 1606.1μg/(h·mg) | 79.01 | [ |
| Vo-In-TiO2(富氧空位铟掺杂二氧化钛) | 0.1mol/L KHCO3+0.1mol/L KNO3 | -0.65 | 759.8μg/(h·mg) | 9.06 | [ |
| Co-O-C(氧配位钴单原子) | 0.1mol/L KHCO3+0.05mol/L KNO3 | -1.5 | 2704.2μg/(h·mg) | 31.4 | [ |
| Ti-DHTP(钛基MOF) | 0.5mol/L K2SO4+0.1mol/L KNO3 | -0.6 | 696.0μg/(h·mg) | 21.75 | [ |
| Cu1Au8@CeO2(氧化铈负载铜金单原子) | 0.1mol/L KNO3 | -0.74 | 813.6μg/(h·mg) | 45.2 | [ |
| Co@C(碳负载钴金属催化剂) | 0.2mol/L NaHCO3+0.05mol/L NaNO3 | -0.5 | 2217.5μg/(h·mg) | 54.3 | [ |
| Mo-PCN-222(Co)(钼钴串联催化剂) | 0.1mol/L KHCO3+0.05mol/L KNO3 | -0.4 | 844.11μg/(h·mg) | 33.9 | [ |
| CoPc-COF@TiO2(二氧化钛复合钴酞菁) | 0.3mol/L KHCO3+0.2mol/L KNO3 | -0.6 | 753.1μg/(h·mg) | 49 | [ |
| CuWO4(钨酸铜) | 0.1mol/L KNO3 | -0.2 | 98.5μg/(h·mg) | 70.1 | [ |
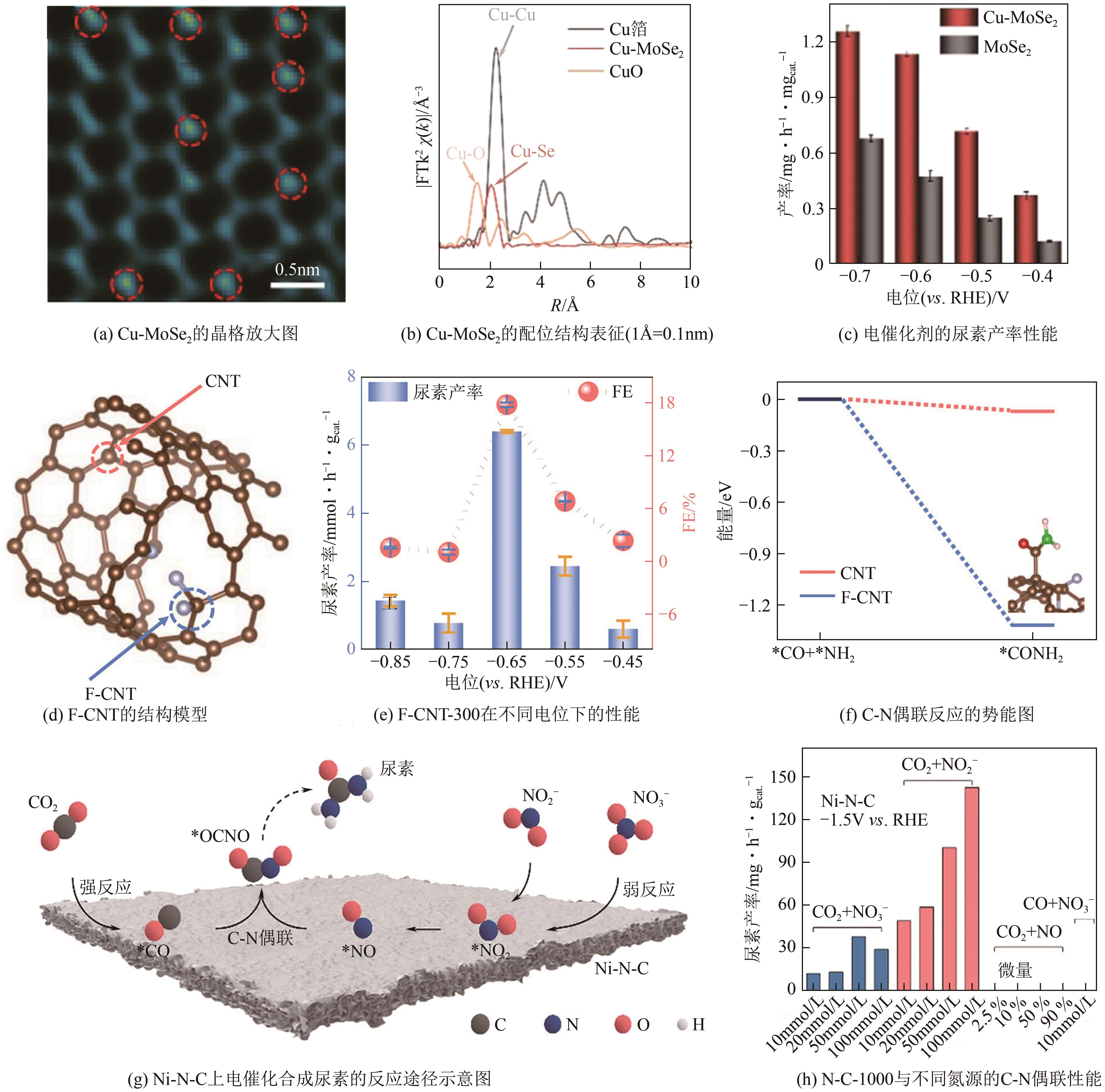
| F-doped CNTs(氟掺杂碳纳米管) | 0.1mol/L KHCO3+0.1mol/L KNO3 | -0.65 | 381.6μg/(h·mg) | 18 | [ |
| 6Å-Cu2O(原子级间距氧化亚铜) | 1mol/L KOH+0.1mol/L KNO3 | -0.53 | 7541.9μg/(h·mg) | 51.97 | [ |
| Cu@Zn(铜锌核壳纳米线) | 0.1mol/L KHCO3+0.1mol/L KNO3 | -1.02 | 7.29μmol/(h·cm2) | 9.28 | [ |
| O-PdZn/C(有序金属间钯锌催化剂) | 0.2mol/L KHCO3+0.1mol/L KNO3 | -0.4 | 1274.42μg/(h·mg) | 62.78 | [ |
| Cu97In3-C(碳负载铜铟催化剂) | 0.1mol/L KHCO3+0.1mol/L KNO3 | -1.4 | 786μg/(h·mg) | — | [ |
| Bi2Se3(硒化铋纳米片) | 0.1mol/L KNO3 | -0.4 | 276000μg/(h·mg) | 32 | [ |
表1 CO2和NO3-共还原电合成尿素催化剂及其性能
| 催化剂 | 电解质 | 过电位(vs. RHE)/V | 尿素产率 | 法拉第效率/% | 参考文献 |
|---|---|---|---|---|---|
| Bi:10%In/C NPs(铟掺杂铋纳米颗粒) | 0.1mol/L KHCO3+0.1mol/L KNO3 | -0.45 | 606.38μg/(h·mg) | 20.31 | [ |
| VB12-CNTs(维生素B负载碳纳米管) | 0.1mol/L KNO3 | -0.5 | 164.04μg/(h·mg) | 26.04 | [ |
| Ru-Cu9Bi/CNT(钌掺杂铜铋碳纳米管) | 0.1mol/L KHCO3+0.1mol/L KNO3 | -0.4 | 2928μg/(h·mg) | 65.7 | [ |
| CuSiO x (硅酸铜) | 0.1mol/L KHCO3+0.1mol/L KNO3 | -0.2 | 1606.1μg/(h·mg) | 79.01 | [ |
| Vo-In-TiO2(富氧空位铟掺杂二氧化钛) | 0.1mol/L KHCO3+0.1mol/L KNO3 | -0.65 | 759.8μg/(h·mg) | 9.06 | [ |
| Co-O-C(氧配位钴单原子) | 0.1mol/L KHCO3+0.05mol/L KNO3 | -1.5 | 2704.2μg/(h·mg) | 31.4 | [ |
| Ti-DHTP(钛基MOF) | 0.5mol/L K2SO4+0.1mol/L KNO3 | -0.6 | 696.0μg/(h·mg) | 21.75 | [ |
| Cu1Au8@CeO2(氧化铈负载铜金单原子) | 0.1mol/L KNO3 | -0.74 | 813.6μg/(h·mg) | 45.2 | [ |
| Co@C(碳负载钴金属催化剂) | 0.2mol/L NaHCO3+0.05mol/L NaNO3 | -0.5 | 2217.5μg/(h·mg) | 54.3 | [ |
| Mo-PCN-222(Co)(钼钴串联催化剂) | 0.1mol/L KHCO3+0.05mol/L KNO3 | -0.4 | 844.11μg/(h·mg) | 33.9 | [ |
| CoPc-COF@TiO2(二氧化钛复合钴酞菁) | 0.3mol/L KHCO3+0.2mol/L KNO3 | -0.6 | 753.1μg/(h·mg) | 49 | [ |
| CuWO4(钨酸铜) | 0.1mol/L KNO3 | -0.2 | 98.5μg/(h·mg) | 70.1 | [ |
| F-doped CNTs(氟掺杂碳纳米管) | 0.1mol/L KHCO3+0.1mol/L KNO3 | -0.65 | 381.6μg/(h·mg) | 18 | [ |
| 6Å-Cu2O(原子级间距氧化亚铜) | 1mol/L KOH+0.1mol/L KNO3 | -0.53 | 7541.9μg/(h·mg) | 51.97 | [ |
| Cu@Zn(铜锌核壳纳米线) | 0.1mol/L KHCO3+0.1mol/L KNO3 | -1.02 | 7.29μmol/(h·cm2) | 9.28 | [ |
| O-PdZn/C(有序金属间钯锌催化剂) | 0.2mol/L KHCO3+0.1mol/L KNO3 | -0.4 | 1274.42μg/(h·mg) | 62.78 | [ |
| Cu97In3-C(碳负载铜铟催化剂) | 0.1mol/L KHCO3+0.1mol/L KNO3 | -1.4 | 786μg/(h·mg) | — | [ |
| Bi2Se3(硒化铋纳米片) | 0.1mol/L KNO3 | -0.4 | 276000μg/(h·mg) | 32 | [ |
| 1 | 葛睿, 胡旭, 董灵玉, 等. 电化学耦合阴极二氧化碳还原与阳极氧化合成[J]. 化工进展, 2021, 40(9): 5132-5144. |
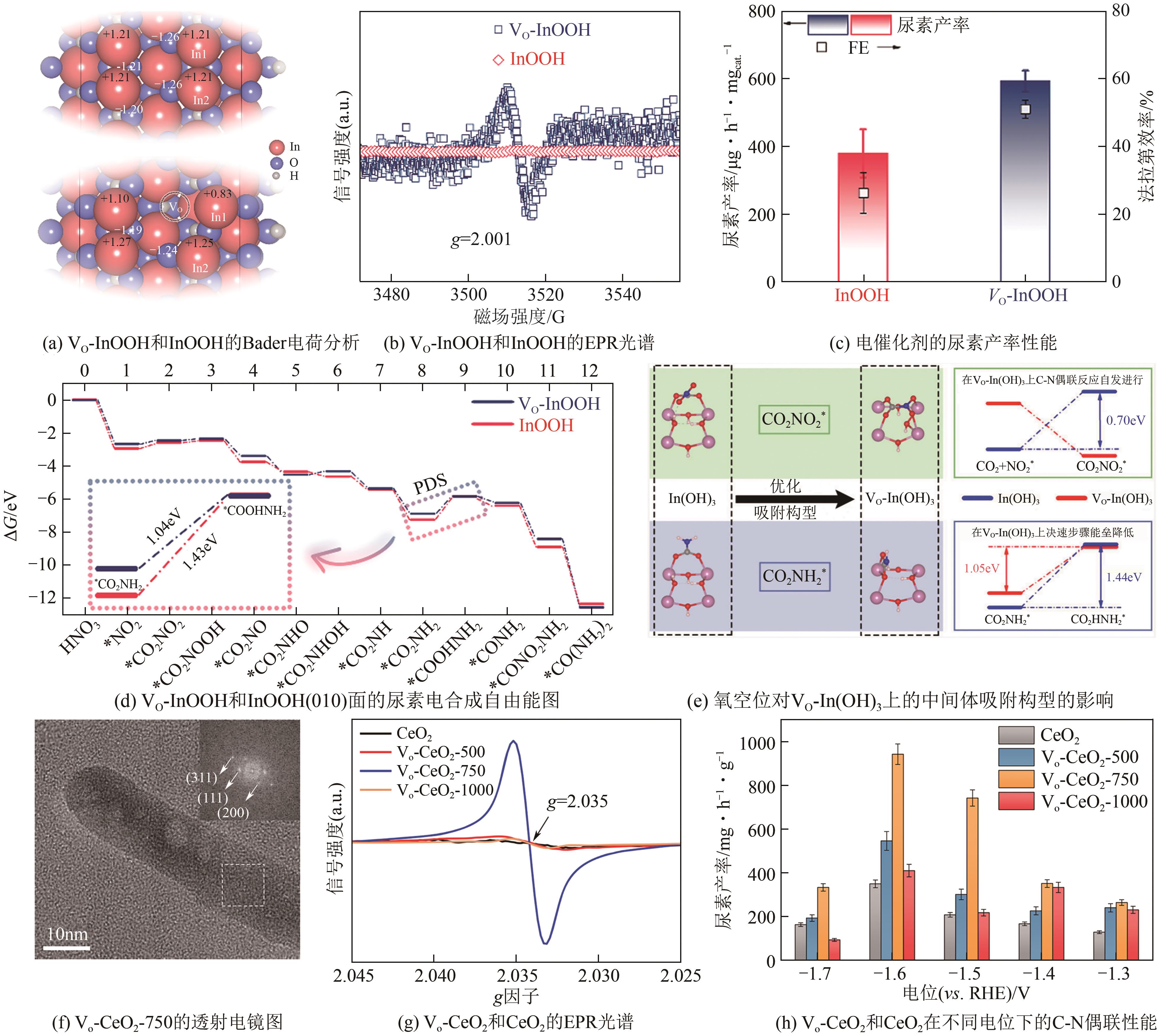
| GE Rui, HU Xu, DONG Lingyu, et al. Electrochemical coupling between cathodic carbon dioxide reduction and anodic oxidation synthesis[J]. Chemical Industry and Engineering Progress, 2021, 40(9): 5132-5144. | |
| 2 | Jeonghoon LIM, FERNÁNDEZ Carlos A, LEE Seung Woo, et al. Ammonia and nitric acid demands for fertilizer use in 2050[J]. ACS Energy Letters, 2021, 6(10): 3676-3685. |
| 3 | GRUBER Nicolas, GALLOWAY James N. An Earth-system perspective of the global nitrogen cycle[J]. Nature, 2008, 451(7176): 293-296. |
| 4 | KOHLHAAS Yannik, TSCHAUDER Yannick S, PLISCHKA Wenzel, et al. Electrochemical urea synthesis[J]. Joule, 2024, 8(6): 1579-1600. |
| 5 | LUO Yuting, XIE Ke, Pengfei OU, et al. Selective electrochemical synthesis of urea from nitrate and CO2 via relay catalysis on hybrid catalysts[J]. Nature Catalysis, 2023, 6(10): 939-948. |
| 6 | MA Lingjia, YUAN Jiongliang, LIU Zhaotao, et al. Mesoporous electrocatalysts with p-n heterojunctions for efficient electroreduction of CO2 and N2 to urea[J]. ACS Applied Materials & Interfaces, 2024, 16(20): 26015-26024. |
| 7 | YU Yaodong, Zheng LYU, LIU Ziyi, et al. Activation of Ga liquid catalyst with continuously exposed active sites for electrocatalytic C-N coupling[J]. Angewandte Chemie International Edition, 2024, 63(18): e202402236. |
| 8 | ZHANG Yangyang, ZHAO Yajun, SENDEKU Marshet Getaye, et al. Tuning intermediates adsorption and C-N coupling for efficient urea electrosynthesis via doping Ni into Cu[J]. Small Methods, 2024, 8(3): 2300811. |
| 9 | ZHANG Xiaoran, ZHU Xiaorong, BO Shuowen, et al. Identifying and tailoring C-N coupling site for efficient urea synthesis over diatomic Fe-Ni catalyst[J]. Nature Communications, 2022, 13(1): 5337. |
| 10 | YU Yaodong, SUN Yuyao, HAN Jiani, et al. Achieving efficient urea electrosynthesis through improving the coverage of a crucial intermediate across a broad range of nitrate concentrations[J]. Energy & Environmental Science, 2024, 17(14): 5183-5190. |
| 11 | WANG Jing, CAI Chao, WANG Yian, et al. Electrocatalytic reduction of nitrate to ammonia on low-cost ultrathin CoO x nanosheets[J]. ACS Catalysis, 2021, 11(24): 15135-15140. |
| 12 | HU Qi, ZHOU Weiliang, QI Shuai, et al. Pulsed co-electrolysis of carbon dioxide and nitrate for sustainable urea synthesis[J]. Nature Sustainability, 2024, 7(4): 442-451. |
| 13 | Chade LYU, ZHONG Lixiang, LIU Hengjie, et al. Selective electrocatalytic synthesis of urea with nitrate and carbon dioxide[J]. Nature Sustainability, 2021, 4(10): 868-876. |
| 14 | GAO Wantao, WU Qinyue, FAN Xinfei, et al. Promoting electrocatalytic reduction of CO2 and nitrate to urea on N‑doped porous hollow carbon spheres[J]. ACS Applied Materials & Interfaces, 2024, 16(38): 50726-50735. |
| 15 | ZHAO Jiamin, YUAN Ying, ZHAO Fei, et al. Identifying the facet-dependent active sites of Cu2O for selective C-N coupling toward electrocatalytic urea synthesis[J]. Applied Catalysis B: Environmental, 2024, 340: 123265. |
| 16 | LI Zhengyi, ZHOU Peng, ZHOU Min, et al. Synergistic electrocatalysis of crystal facet and O-vacancy for enhancive urea synthesis from nitrate and CO2 [J]. Applied Catalysis B: Environmental, 2023, 338: 122962. |
| 17 | ZHAO Qinglan, LU Xinxin, WANG Yinuo, et al. Sustainable and high-rate electrosynthesis of nitrogen fertilizer[J]. Angewandte Chemie International Edition, 2023, 62(33): e202307123. |
| 18 | MENG Nannan, HUANG Yanmei, LIU Yang, et al. Electrosynthesis of urea from nitrite and CO2 over oxygen vacancy-rich ZnO porous nanosheets[J]. Cell Reports Physical Science, 2021, 2(3): 100378. |
| 19 | LIU Xin, JIAO Yan, ZHENG Yao, et al. Mechanism of C-N bonds formation in electrocatalytic urea production revealed by ab initio molecular dynamics simulation[J]. Nature Communications, 2022, 13(1): 5471. |
| 20 | CAO Yongyong, MENG Yuxiao, WU Yuting, et al. Metal-free boron nanosheet as “buffer electron pool” for urea and ethanol synthesis via C-N and C-C coupling[J]. Journal of Materials Chemistry A, 2022, 10(44): 23843-23853. |
| 21 | WAN Hao, WANG Xingli, TAN Lei, et al. Electrochemical synthesis of urea: Co-reduction of nitric oxide and carbon monoxide[J]. ACS Catalysis, 2023, 13(3):1926-1933. |
| 22 | WEI Xiaoxiao, LIU Yingying, ZHU Xiaorong, et al. Dynamic reconstitution between copper single atoms and clusters for electrocatalytic urea synthesis[J]. Advanced Materials, 2023, 35(18): 2300020. |
| 23 | ZHENG Meng, MA Haiqing, LI Zhiming, et al. Theoretical insights on C-N coupling mechanism and guidance for screening the catalysts of electrocatalytic urea synthesis by descriptors[J]. Applied Catalysis B: Environmental, 2024, 342: 123366. |
| 24 | YANG Guolin, HSIEH Chi-Tien, Yeu-Shiuan HO, et al. Gaseous CO2 coupling with N-containing intermediates for key C—N bond formation during urea production from coelectrolysis over Cu[J]. ACS Catalysis, 2022, 12(18):11494-11504. |
| 25 | ZHAO Qinglan, ZHANG Yan, CAO Dapeng, et al. The prospects of urea manufacturing via electrochemical co-reduction of CO2 and nitrates[J]. Current Opinion in Electrochemistry, 2024, 45: 101479. |
| 26 | Chade LYU, LEE Carmen, ZHONG Lixiang, et al. A defect engineered electrocatalyst that promotes high-efficiency urea synthesis under ambient conditions[J]. ACS Nano, 2022, 16(5): 8213-8222. |
| 27 | SHIBATA Masami, YOSHIDA Kohji, FURUYA Nagakazu. Electrochemical synthesis of urea on reduction of carbon dioxide with nitrate and nitrite ions using Cu-loaded gas-diffusion electrode[J]. Journal of Electroanalytical Chemistry, 1995, 387(1/2): 143-145. |
| 28 | SHIBATA Masami, YOSHIDA Kohji, FURUYA Nagakazu. Electrochemical synthesis of urea at gas-diffusion electrodes: Part Ⅱ. Simultaneous reduction of carbon dioxide and nitrite ions at Cu, Ag and Au catalysts[J]. Journal of Electroanalytical Chemistry, 1998, 442(1/2): 67-72. |
| 29 | LI Hsien-Chin, Yeu-Shiuan HO, YANG Guolin, et al. Linking CO to urea production from CO2 and NO3 -/NO2 - co-electrolysis on transition metals[J]. The Journal of Physical Chemistry C, 2024, 128(3): 1058-1067. |
| 30 | KUNIMATSU K, ARAMATA A, NAKAJIMA N, et al. Infrared spectra of carbon monoxide adsorbed on a smooth gold electrode: Part Ⅱ. Emirs and polarization-modulated irras stuidy of the adsorbed CO layer in acidic and alkaline solutions[J]. Journal of Electroanalytical Chemistry and Interfacial Electrochemistry, 1986, 207(1/2): 293-307. |
| 31 | TAO Zixu, ROONEY Conor L, LIANG Yongye, et al. Accessing organonitrogen compounds via C-N coupling in electrocatalytic CO2 reduction[J]. Journal of the American Chemical Society, 2021, 143(47): 19630-19642. |
| 32 | ZHAO Yilong, DING Yunxuan, LI Wenlong, et al. Efficient urea electrosynthesis from carbon dioxide and nitrate via alternating Cu-W bimetallic C-N coupling sites[J]. Nature Communications, 2023, 14(1): 4491. |
| 33 | WEI Xiaoxiao, WEN Xiaojian, LIU Yingying, et al. Oxygen vacancy-mediated selective C-N coupling toward electrocatalytic urea synthesis[J]. Journal of the American Chemical Society, 2022, 144(26): 11530-11535. |
| 34 | WANG Hua, JIANG Yong, LI Sijun, et al. Realizing efficient C-N coupling via electrochemical co-reduction of CO2 and NO3 - on AuPd nanoalloy to form urea: Key C-N coupling intermediates[J]. Applied Catalysis B: Environmental, 2022, 318: 121819. |
| 35 | QIN Jiangzhou, LIU Nengsheng, CHEN Liuzhou, et al. Selective electrochemical urea synthesis from nitrate and CO2 using in situ Ru anchoring onto a three-dimensional copper electrode[J]. ACS Sustainable Chemistry & Engineering, 2022, 10(48): 15869-15875. |
| 36 | FENG Yonggang, YANG Hao, ZHANG Ying, et al. Te-doped Pd nanocrystal for electrochemical urea production by efficiently coupling carbon dioxide reduction with nitrite reduction[J]. Nano Letters, 2020, 20(11): 8282-8289. |
| 37 | WANG Yan, XIA Shuai, ZHANG Jianfang, et al. Spatial management of CO diffusion on tandem electrode promotes NH2 intermediate formation for efficient urea electrosynthesis[J]. ACS Energy Letters, 2023, 8(8): 3373-3380. |
| 38 | JOUNY Matthew, Jingjing LYU, CHENG Tao, et al. Formation of carbon-nitrogen bonds in carbon monoxide electrolysis[J]. Nature Chemistry, 2019, 11(9): 846-851. |
| 39 | JIANG Jiadi, WU Guanzheng, SUN Mengmiao, et al. Cu-Mo dual sites in Cu-doped MoSe2 for enhanced electrosynthesis of urea[J]. ACS Nano, 2024, 18(21): 13745-13754. |
| 40 | LIU Xiaowen, KUMAR Priyank Vijaya, CHEN Qing, et al. Carbon nanotubes with fluorine-rich surface as metal-free electrocatalyst for effective synthesis of urea from nitrate and CO2 [J]. Applied Catalysis B: Environmental, 2022, 316: 121618. |
| 41 | CHEN Chen, LI Shuang, ZHU Xiaorong, et al. Balancing sub-reaction activity to boost electrocatalytic urea synthesis using a metal-free electrocatalyst[J]. Carbon Energy, 2023, 5(10): e345. |
| 42 | LIU Junxian, SMITH Sean C, GU Yuantong, et al. C-N coupling enabled by N-N bond breaking for electrochemical urea production[J]. Advanced Functional Materials, 2023, 33(47): 2305894. |
| 43 | ZHOU Hangyan, XIONG Bingyan, CHEN Lisong, et al. Modulation strategies of Cu-based electrocatalysts for efficient nitrogen reduction[J]. Journal of Materials Chemistry A, 2020, 8(39): 20286-20293. |
| 44 | JIANG Minghang, ZHU Mengfei, WANG Mengjun, et al. Review on electrocatalytic coreduction of carbon dioxide and nitrogenous species for urea synthesis[J]. ACS Nano, 2023, 17(4): 3209-3224. |
| 45 | XU Zifan, YANG Zhengwu, LU Huan, et al. Atomic defects engineering boosts urea synthesis toward carbon dioxide and nitrate coelectroreduction[J]. Nano Letters, 2024, 24(37): 11730-11737. |
| 46 | WAN Yuchi, ZHENG Muyun, YAN Wei, et al. Fundamentals and rational design of heterogeneous C-N coupling electrocatalysts for urea synthesis at ambient conditions[J]. Advanced Energy Materials, 2024, 14(28): 2303588. |
| 47 | YE Wei, ZHANG Ye, CHEN Liang, et al. A strongly coupled metal/hydroxide heterostructure cascades carbon dioxide and nitrate reduction reactions toward efficient urea electrosynthesis[J]. Angewandte Chemie International Edition, 2024, 63(48): e202410105. |
| 48 | GENG Jing, JI Sihan, JIN Meng, et al. Ambient electrosynthesis of urea with nitrate and carbon dioxide over iron-based dual-sites[J]. Angewandte Chemie International Edition, 2023, 62(6): e202210958. |
| 49 | HE Jingfu, JOHNSON Noah J J, HUANG Aoxue, et al. Electrocatalytic alloys for CO2 reduction[J]. ChemSusChem, 2018, 11(1): 48-57. |
| 50 | CHEN Kejun, CAO Maoqi, NI Ganghai, et al. Nickel polyphthalocyanine with electronic localization at the nickel site for enhanced CO2 reduction reaction[J]. Applied Catalysis B: Environmental, 2022, 306: 121093. |
| 51 | WANG Jing, WANG Yian, CAI Chao, et al. Cu-doped iron oxide for the efficient electrocatalytic nitrate reduction reaction[J]. Nano Letters, 2023, 23(5): 1897-1903. |
| 52 | HOU Tong, DING Junyang, ZHANG Hao, et al. FeNi3 nanoparticles for electrocatalytic synthesis of urea from carbon dioxide and nitrate[J]. Materials Chemistry Frontiers, 2023, 7(20): 4952-4960. |
| 53 | ZHANG Shengbo, GENG Jing, ZHAO Zhong, et al. High-efficiency electrosynthesis of urea over bacterial cellulose regulated Pd-Cu bimetallic catalyst[J]. EES Catalysis, 2023, 1(1): 45-53. |
| 54 | LIU Juan, CAI Yanming, SONG Rongbin, et al. Recent progress on single-atom catalysts for CO2 electroreduction[J]. Materials Today, 2021, 48: 95-114. |
| 55 | WANG Liming, CHEN Wenlong, ZHANG Doudou, et al. Surface strategies for catalytic CO2 reduction: From two-dimensional materials to nanoclusters to single atoms[J]. Chemical Society Reviews, 2019, 48(21): 5310-5349. |
| 56 | LEVERETT Josh, Thanh TRAN-PHU, YUWONO Jodie A, et al. Tuning the coordination structure of Cu-N-C single atom catalysts for simultaneous electrochemical reduction of CO2 and NO3 – to urea[J]. Advanced Energy Materials, 2022, 12(32): 2201500. |
| 57 | LI Yang, ZHENG Shisheng, LIU Hao, et al. Sequential co-reduction of nitrate and carbon dioxide enables selective urea electrosynthesis[J]. Nature Communications, 2024, 15(1): 176. |
| 58 | HU Yifan, LI Zesheng, LI Bolin, et al. Recent progress of diatomic catalysts: General design fundamentals and diversified catalytic applications[J]. Small, 2022, 18(46): 2203589. |
| 59 | LI Runze, WANG Dingsheng. Superiority of dual-atom catalysts in electrocatalysis: One step further than single-atom catalysts[J]. Advanced Energy Materials, 2022, 12(9): 2103564. |
| 60 | LIU Chongchong, TONG Haili, WANG Peifang, et al. The asymmetric orbital hybridization in single-atom-dimers for urea synthesis by optimizing the C-N coupling reaction pathway[J]. Applied Catalysis B: Environmental, 2023, 336: 122917. |
| 61 | CHEN Kai, MA Danyang, ZHANG Ying, et al. Urea electrosynthesis from nitrate and CO2 on diatomic alloys[J]. Advanced Materials, 2024, 36(30): 2402160. |
| 62 | AZEEM Babar, KUSHAARI KuZilati, MAN Zakaria B, et al. Review on materials & methods to produce controlled release coated urea fertilizer[J]. Journal of Controlled Release, 2014, 181: 11-21. |
| 63 | KAYAN Didem Balun, Fatih KÖLELI. Simultaneous electrocatalytic reduction of dinitrogen and carbon dioxide on conducting polymer electrodes[J]. Applied Catalysis B: Environmental, 2016, 181: 88-93. |
| 64 | MAO Yini, JIANG Yong, GOU Qiao, et al. Indium-activated bismuth-based catalysts for efficient electrocatalytic synthesis of urea[J]. Applied Catalysis B: Environmental, 2024, 340: 123189. |
| 65 | CONG Meiyu, LIU Qi, WANG Dongping, et al. Electrocatalytic urea synthesis from CO2 and nitrate co-reduction on natural vitamin B12 coupled carbon nanotubes[J]. Applied Catalysis B: Environmental and Energy, 2024, 351: 123941. |
| 66 | QIU Weibin, QIN Shimei, LI Yibao, et al. Overcoming electrostatic interaction via pulsed electroreduction for boosting the electrocatalytic urea synthesis[J]. Angewandte Chemie International Edition, 2024, 63(24): e202402684. |
| 67 | MAO Yini, REN Fei, GOU Qiao, et al. Enhanced performance of oxygen vacancy-rich In-TiO2 materials for electrocatalytic urea synthesis via a relay catalysis strategy[J]. Chemical Engineering Journal, 2024, 485: 150052. |
| 68 | ZHANG Shengbo, JIN Meng, XU Hui, et al. An oxygen-coordinated cobalt single-atom electrocatalyst boosting urea and urea peroxide production[J]. Energy & Environmental Science, 2024, 17(5): 1950-1960. |
| 69 | LIU Xiaofang, FENG Jie, CHENG Xuefeng, et al. High C-selectivity for urea synthesis through O-philic adsorption to form *OCO intermediate on Ti-MOF based electrocatalysts[J]. Advanced Functional Materials, 2024, 34(34): 2400892. |
| 70 | ZHAN Peng, ZHUANG Jinjie, YANG Shuai, et al. Efficient electrosynthesis of urea over single-atom alloy with electronic metal support interaction[J]. Angewandte Chemie International Edition, 2024, 63(33): e202409019. |
| 71 | FAN Xiaoya, LIU Chaozhen, HE Xun, et al. Efficient electrochemical co-reduction of carbon dioxide and nitrate to urea with high faradaic efficiency on cobalt-based dual-sites[J]. Advanced Materials, 2024, 36(25): 2401221. |
| 72 | GAO Yuhang, WANG Jingnan, SUN Menglong, et al. Tandem catalysts enabling efficient C-N coupling toward the electrosynthesis of urea[J]. Angewandte Chemie International Edition, 2024, 63(23): e202402215. |
| 73 | LI Ning, GAO Hui, LIU Zhixin, et al. Metalphthalocyanine frameworks grown on TiO2 nanotubes for synergistically and efficiently electrocatalyzing urea production from CO2 and nitrate[J]. Science China Chemistry, 2023, 66(5): 1417-1424. |
| 74 | SHIN Seokmin, SULTAN Siraj, CHEN Zongxian, et al. Copper with an atomic-scale spacing for efficient electrocatalytic co-reduction of carbon dioxide and nitrate to urea[J]. Energy & Environmental Science, 2023, 16(5): 2003-2013. |
| 75 | MENG Nannan, MA Xiaomin, WANG Changhong, et al. Oxide-derived core-shell Cu@Zn nanowires for urea electrosynthesis from carbon dioxide and nitrate in water[J]. ACS Nano, 2022, 16(6): 9095-9104. |
| 76 | ZHOU Weiliang, FENG Chao, LI Xuan, et al. Boosting electrochemical urea synthesis via constructing ordered Pd-Zn active pair[J]. Nano-Micro Letters, 2024, 16(1): 247. |
| 77 | LIU Yingying, TU Xiaojin, WEI Xiaoxiao, et al. C-bound or O-bound surface: Which one boosts electrocatalytic urea synthesis?[J]. Angewandte Chemie International Edition, 2023, 62(19): e202300387. |
| 78 | WANG Yan, XIA Shuai, CAI Rui, et al. Dynamic reconstruction of two-dimensional defective Bi nanosheets for efficient electrocatalytic urea synthesis[J]. Angewandte Chemie International Edition, 2024, 63(16): e202318589. |
| [1] | 丁阿静, 周巧巧, 顾学红. 膜反应器中杨木催化气化制清洁合成气[J]. 化工进展, 2025, 44(5): 2716-2723. |
| [2] | 何志勇. 分步脱羟/脱碳催化剂实现高效裂解甲醇制氢[J]. 化工进展, 2025, 44(5): 2724-2732. |
| [3] | 宋坤莉, 肖雷, 马丹丹, 肖朋, 杨莎莎, 石建稳. 超低温氨气选择性脱硝催化剂的研究进展[J]. 化工进展, 2025, 44(4): 2028-2035. |
| [4] | 王淑媛, 尹玲玲, 高志华, 黄伟. 插层Cu比例对CuZnAl-LDHs催化剂结构及催化性能的影响[J]. 化工进展, 2025, 44(4): 2036-2044. |
| [5] | 张舒茜, 陈佩婷, 蒲建波, 王宇作, 阮殿波, 乔志军. 进风量对硅/碳负极材料二次颗粒尺寸及电化学性能的影响[J]. 化工进展, 2025, 44(4): 2196-2201. |
| [6] | 张绎如, 韩东梅, 马伟芳. 铁基复合卤氧化铋磁性材料强化可见光催化处理难降解有机废水研究进展[J]. 化工进展, 2025, 44(4): 2258-2273. |
| [7] | 刘江涛, 彭冲, 张永春. Zn调控Fe基催化剂催化CO2加氢制低碳烯烃[J]. 化工进展, 2025, 44(3): 1396-1405. |
| [8] | 陶金泉, 贾亦静, 白天瑜, 姚荣鹏, 黄文斌, 崔岩, 周亚松, 魏强. Silicalite-1分子筛的低成本合成及其MTP催化性能[J]. 化工进展, 2025, 44(3): 1550-1558. |
| [9] | 陈宇航, 李巧艳, 梁美生, 宋天远, 汪玥, 李思萌, 周宇璇. Sn掺杂Cu/CeZrO2/γ-Al2O3对三效催化(TWC)反应的作用:提高低温活性和抗硫性[J]. 化工进展, 2025, 44(3): 1368-1377. |
| [10] | 张馨儿, 裴刘军, 周雨蝶, 靳凯丽, 王际平. 基于TiO2的光催化剂利用太阳能裂解水制氢研究进展[J]. 化工进展, 2025, 44(3): 1298-1308. |
| [11] | 刘俊杰, 吴建民, 孙启文, 王建成, 孙燕. 茂金属催化线性α-烯烃聚合获取高分子量产物研究进展[J]. 化工进展, 2025, 44(3): 1309-1322. |
| [12] | 朱国瑜, 葛棋, 付名利. 甲醇重整制氢催化剂耐久性评价和寿命预测方法[J]. 化工进展, 2025, 44(3): 1338-1346. |
| [13] | 谢鑫瑶, 万芬, 伏炫羽, 范雨婷, 陈令修, 李鹏. Cu-Ag纳米团簇CO2电催化还原性能和机理[J]. 化工进展, 2025, 44(3): 1387-1395. |
| [14] | 左骥, 罗莉, 谢永锴, 陈文尧, 钱刚, 周兴贵, 段学志. 甲醇无氧脱氢制甲醛Cu催化剂的粒径效应[J]. 化工进展, 2025, 44(3): 1347-1354. |
| [15] | 毕文涛, 王学林, 曲炜, 王从新, 田志坚. Mg改性对低铂载量Pt/ZSM-22烷烃加氢异构性能的影响[J]. 化工进展, 2025, 44(3): 1355-1367. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||