化工进展 ›› 2025, Vol. 44 ›› Issue (5): 2724-2732.DOI: 10.16085/j.issn.1000-6613.2024-1844
• 可再生能源利用 • 上一篇
分步脱羟/脱碳催化剂实现高效裂解甲醇制氢
- 中石化南京化工研究院有限公司,江苏 南京 210048
-
收稿日期:2024-11-12修回日期:2025-04-10出版日期:2025-05-25发布日期:2025-05-20 -
作者简介:何志勇(1975—),男,博士,研究方向为精细化工和工业催化。E-mail:hezy.nhgs@sinopec.com。 -
基金资助:中国石油化工集团有限公司资助项目(123048)
Catalyst evolved by stepwise dehydroxylation/decarbonization method achieves efficient methanol decomposition to produce hydrogen
- SINOPEC Nanjing Research Institute of Chemical Industry Co. , Ltd. , Nanjing 210048, Jiangsu, China
-
Received:2024-11-12Revised:2025-04-10Online:2025-05-25Published:2025-05-20
摘要:
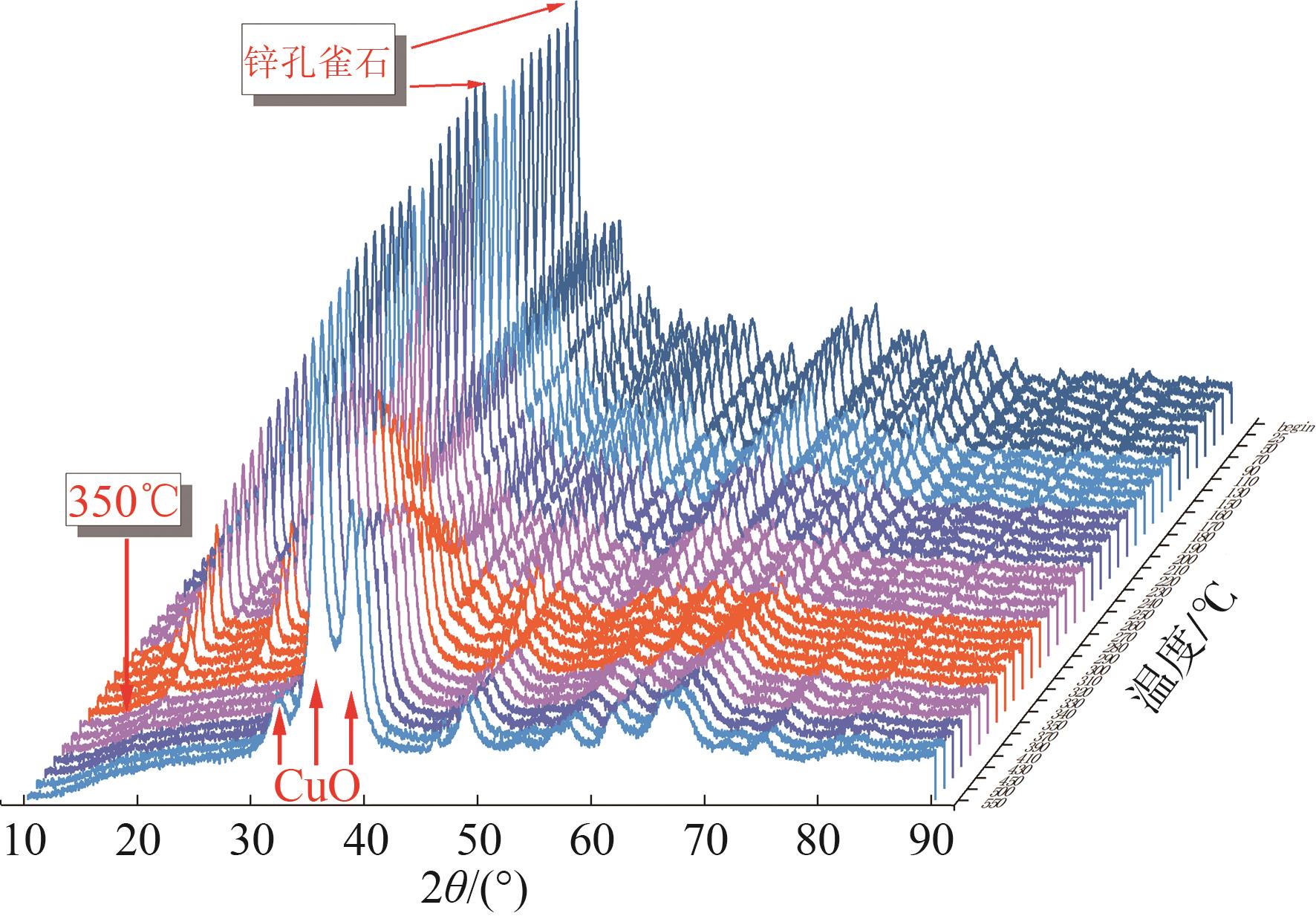
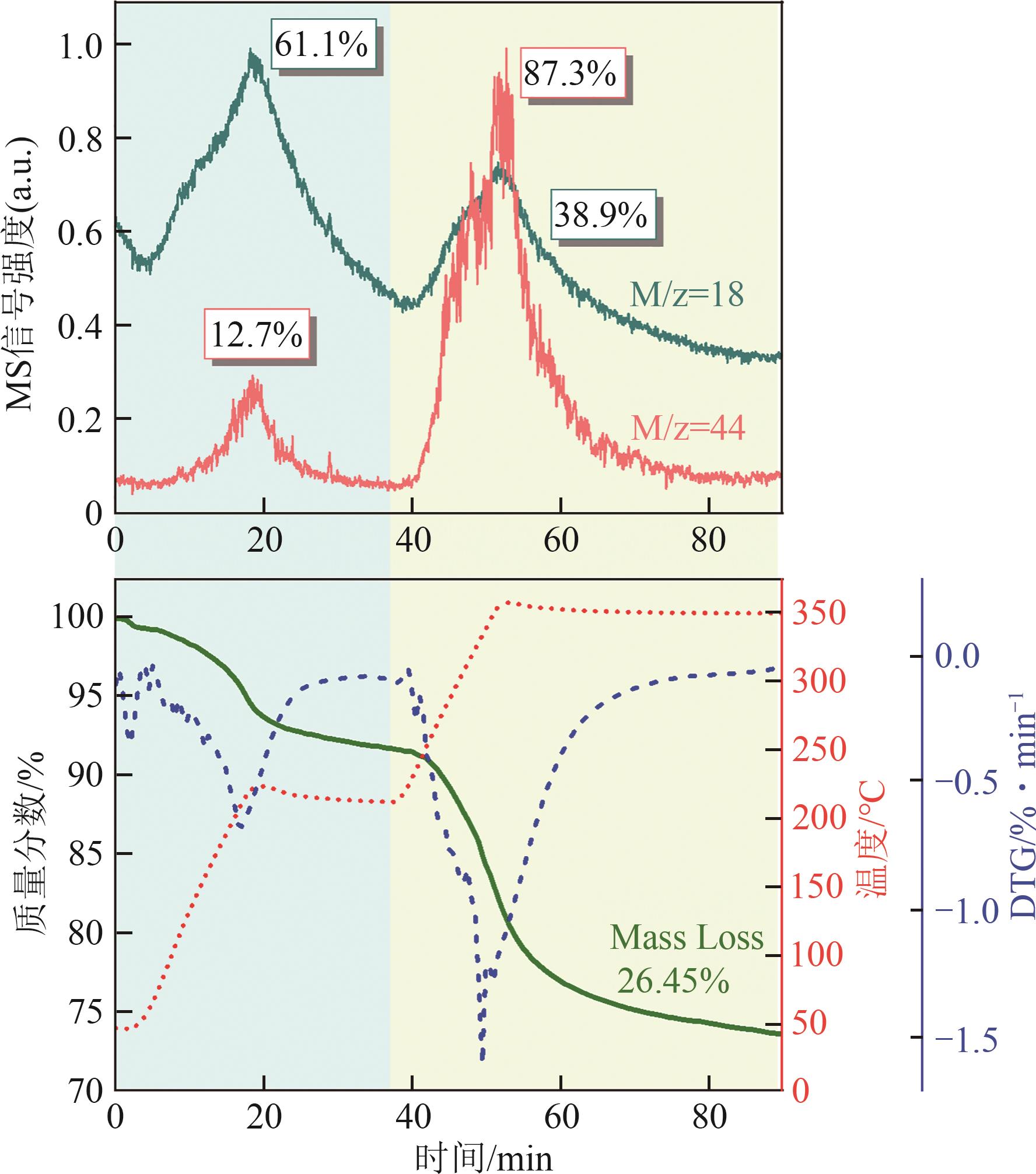
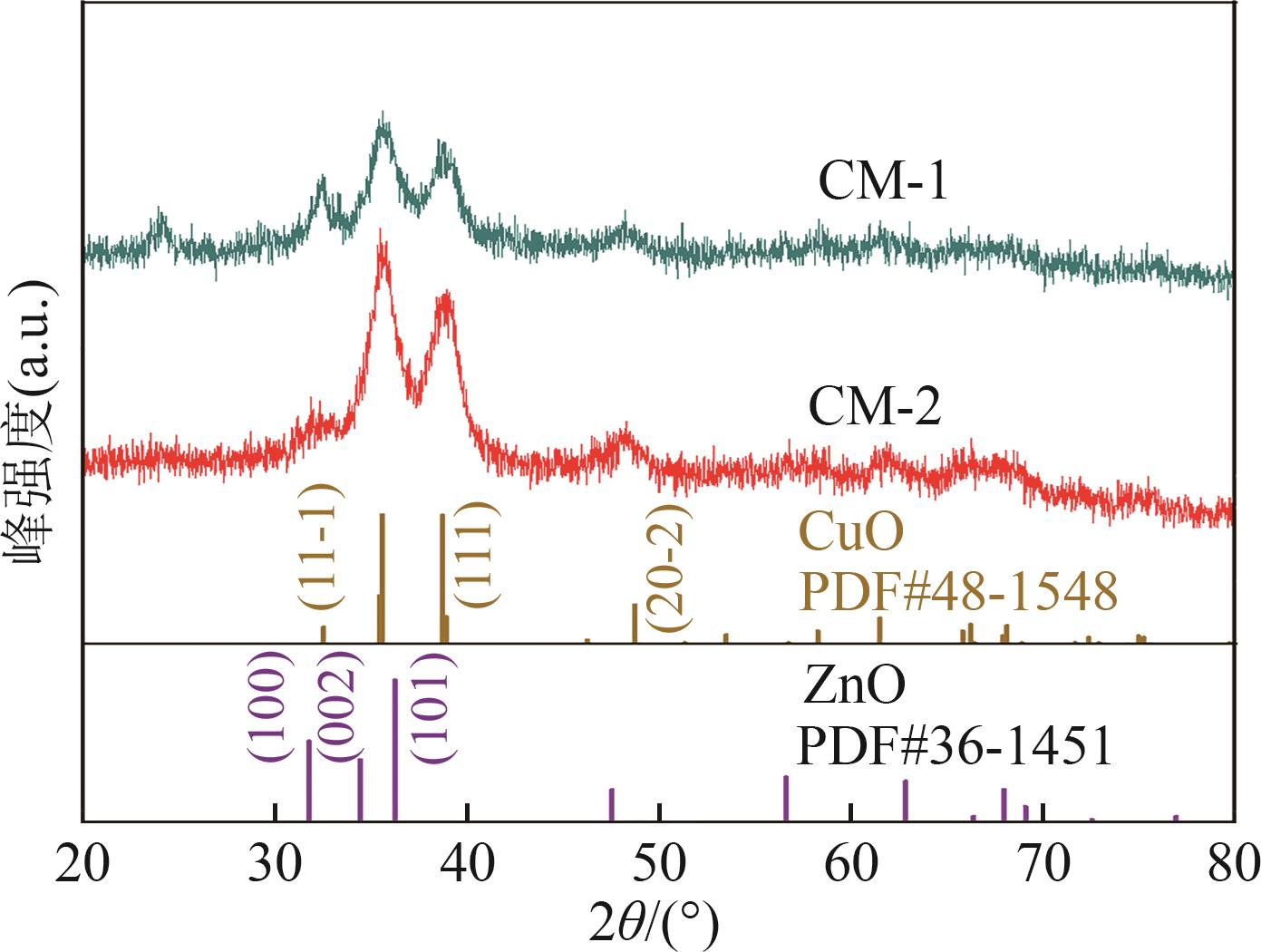
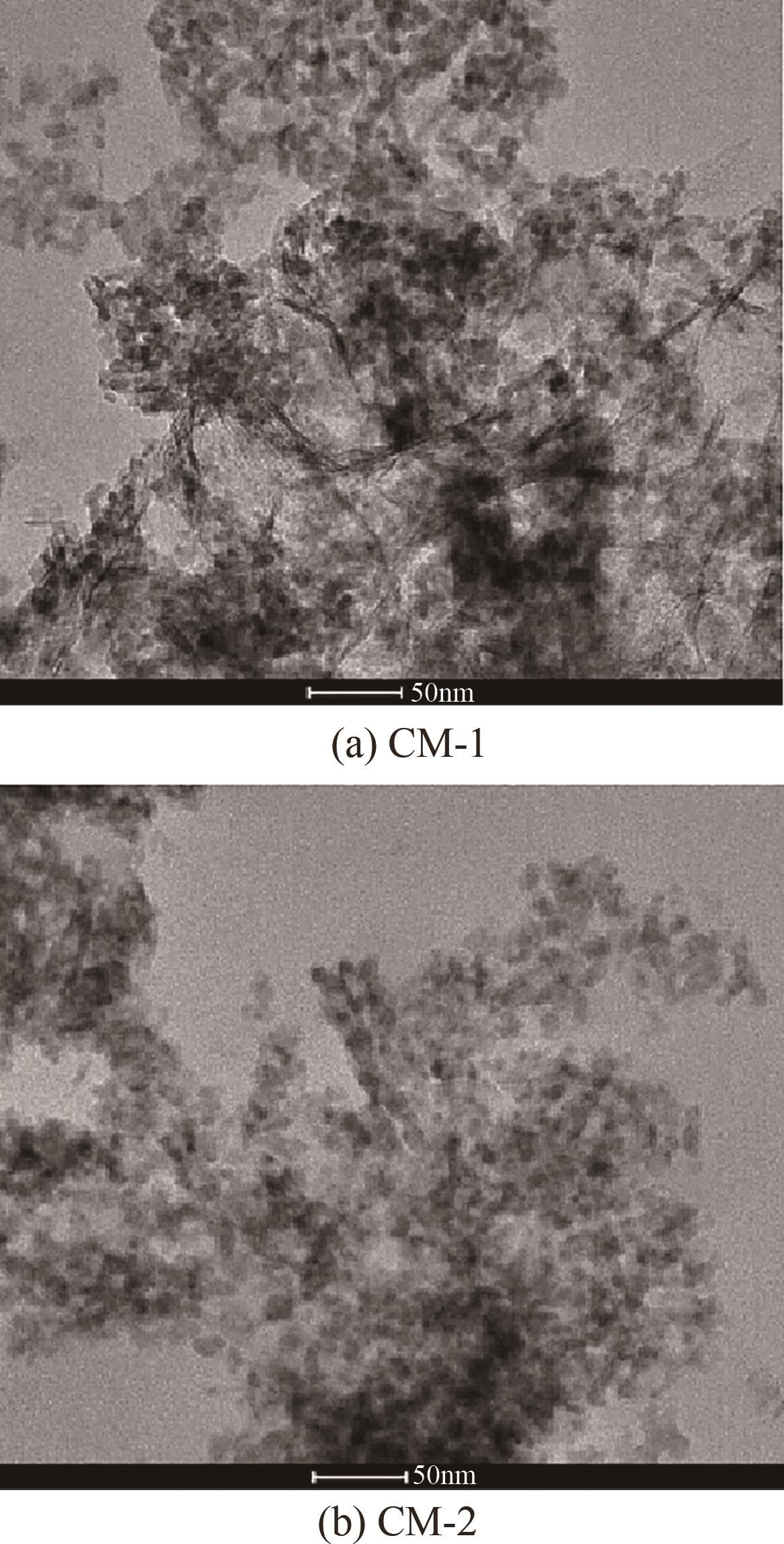
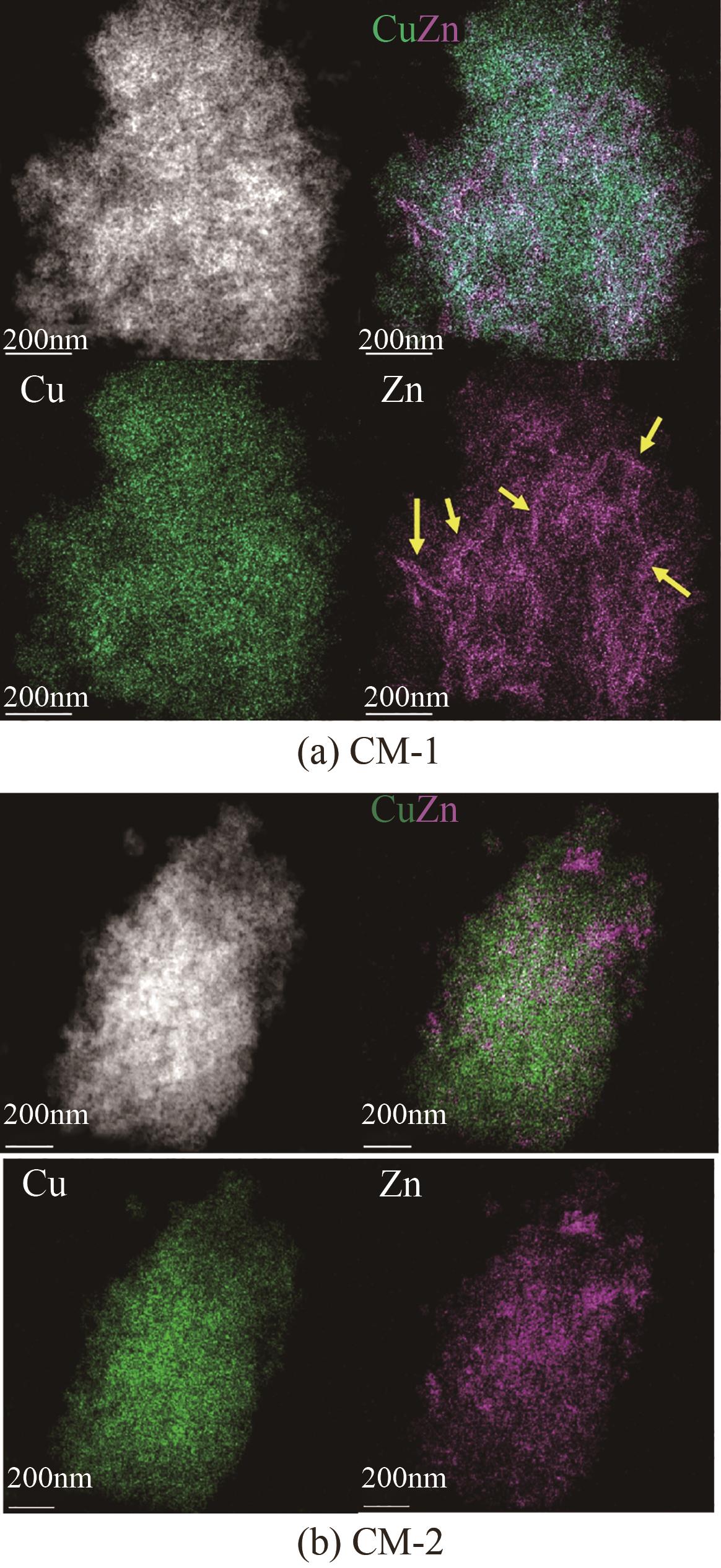
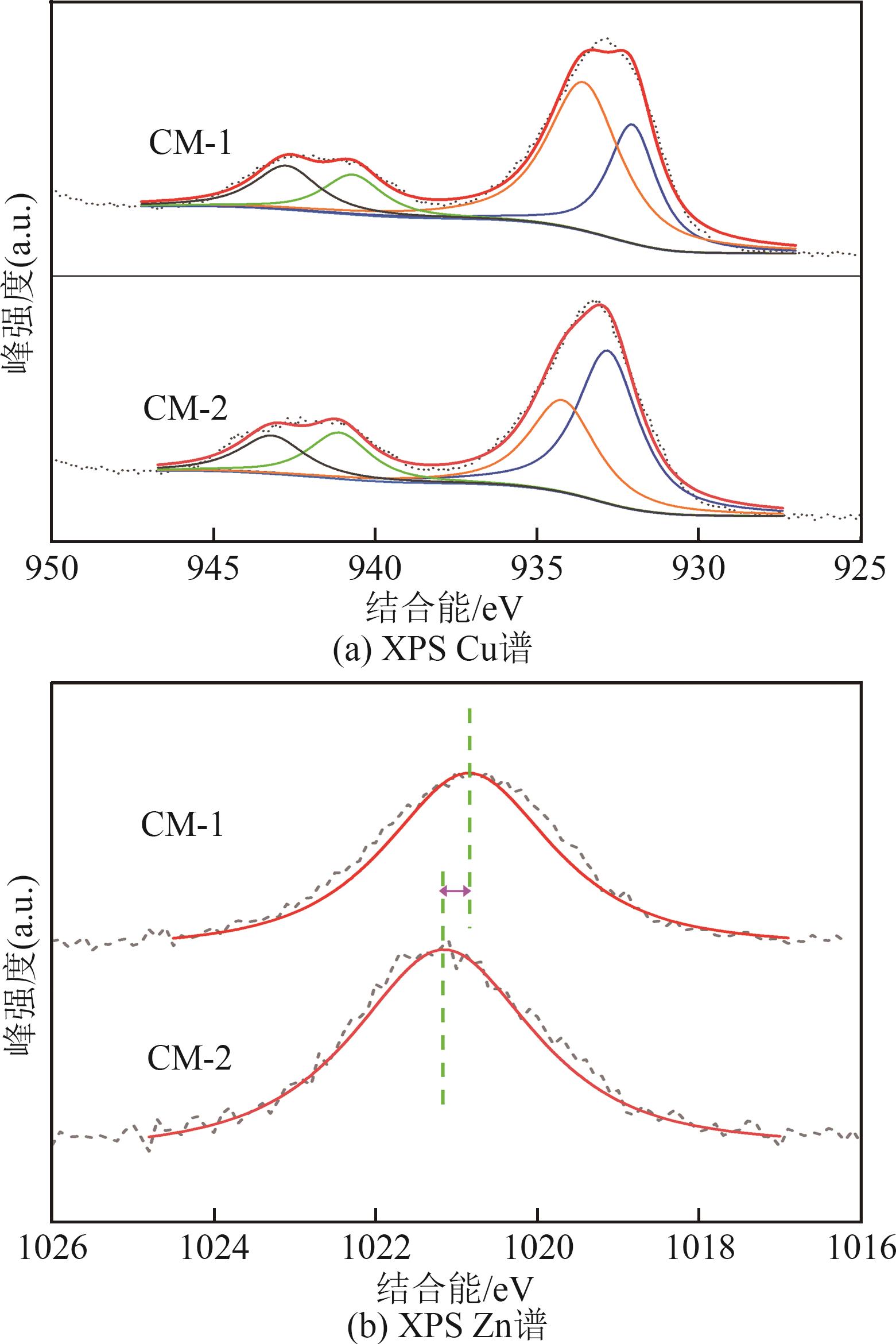
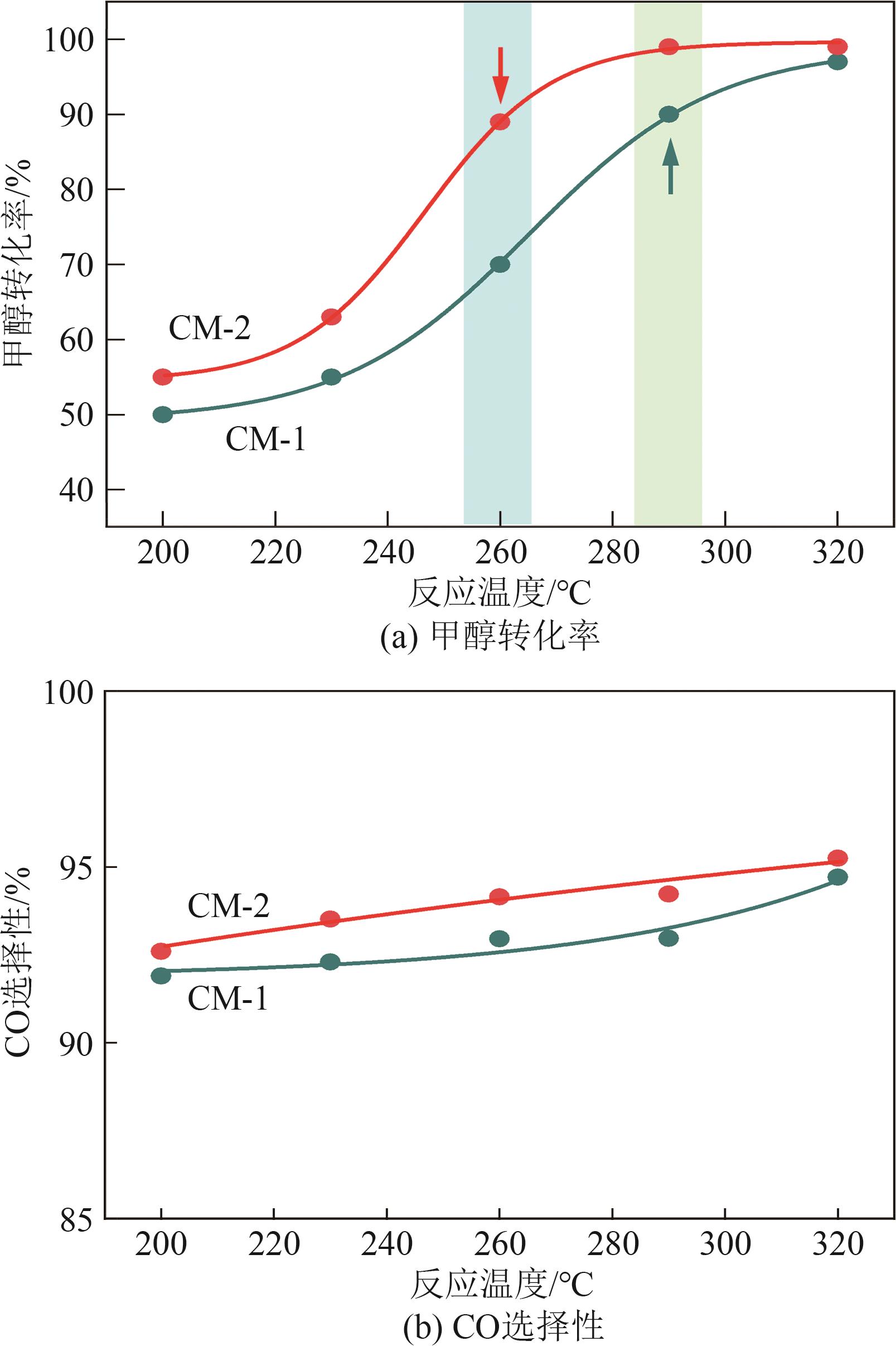
甲醇是一种重要的氢能载体,利用甲醇裂解制氢是解决氢能储存、运输难题的有效解决方案。铜基催化剂是目前工业化应用最为广泛的甲醇裂解制氢催化剂,其常规制备方法为中和沉淀得到前体、再焙烧成相应金属氧化物。然而,热焙烧过程往往会导致铜活性组分团聚、晶粒度增大,影响活性位点的可接触程度,从而制约甲醇裂解制氢的反应活性。本工作剖析了铜基催化剂前体锌孔雀石物相组成,创新性地将前体焙烧过程拆分为脱羟和脱碳两部分,避免过于剧烈的热解过程导致活性颗粒过热烧结。相较于传统一步焙烧催化剂,经脱羟/脱碳分步焙烧制得的催化剂,CuO晶粒度从9.0nm减至6.3nm,最可几孔径由36.5nm缩至8.1nm,孔道结构规整度更高。因此,分步焙烧催化剂实现了比一步焙烧催化剂更低的甲醇裂解起活温度,同时甲醇裂解转化率和选择性也显著提升。
中图分类号:
引用本文
何志勇. 分步脱羟/脱碳催化剂实现高效裂解甲醇制氢[J]. 化工进展, 2025, 44(5): 2724-2732.
HE Zhiyong. Catalyst evolved by stepwise dehydroxylation/decarbonization method achieves efficient methanol decomposition to produce hydrogen[J]. Chemical Industry and Engineering Progress, 2025, 44(5): 2724-2732.
| 温度/℃ | CuO粒径①/nm |
|---|---|
| 300 | 6.8 |
| 350 | 8.8 |
| 400 | 9.3 |
| 450 | 9.8 |
| 500 | 10.1 |
| 550 | 10.6 |
表1 典型温度下的CuO晶粒度
| 温度/℃ | CuO粒径①/nm |
|---|---|
| 300 | 6.8 |
| 350 | 8.8 |
| 400 | 9.3 |
| 450 | 9.8 |
| 500 | 10.1 |
| 550 | 10.6 |
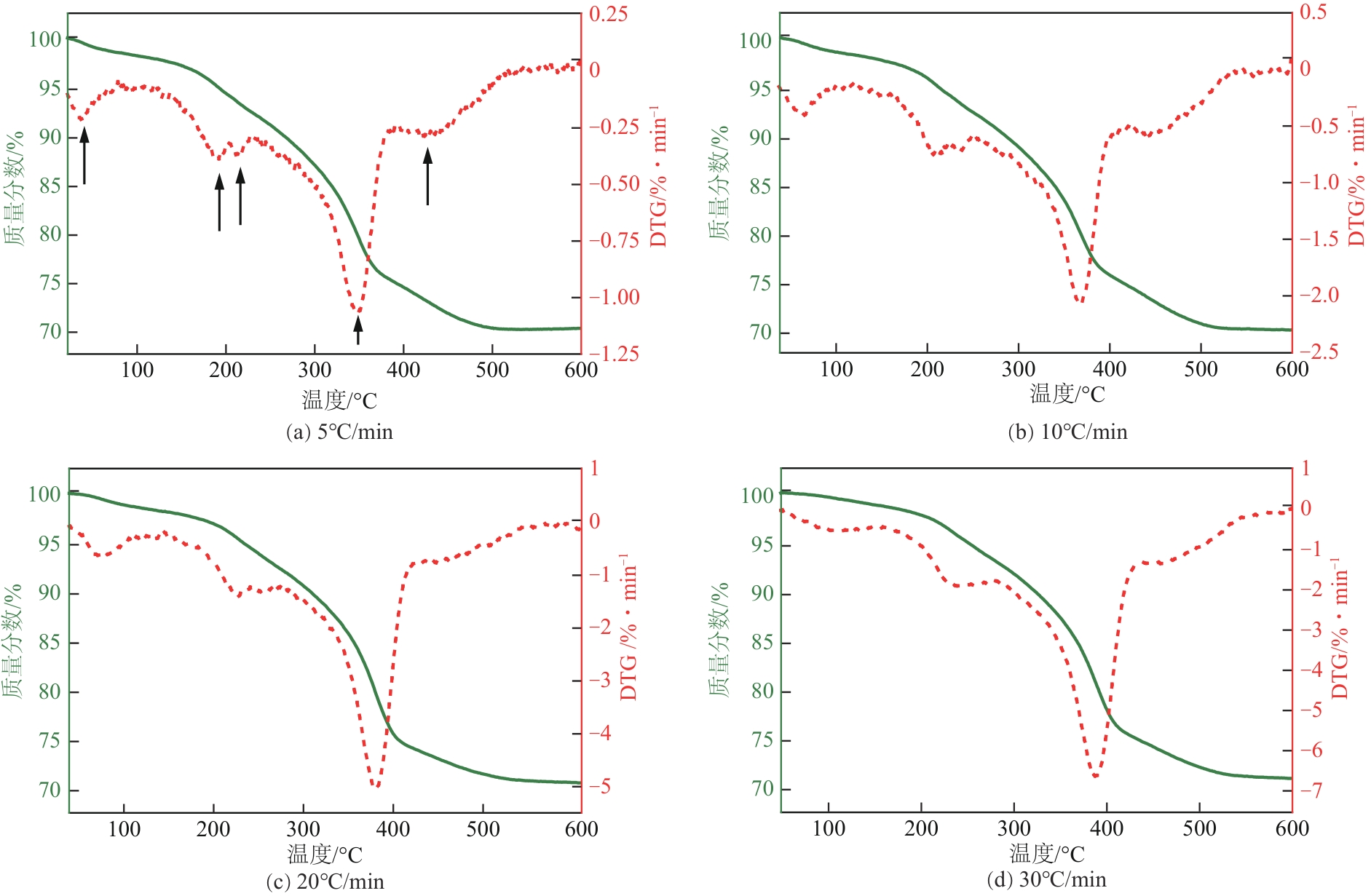
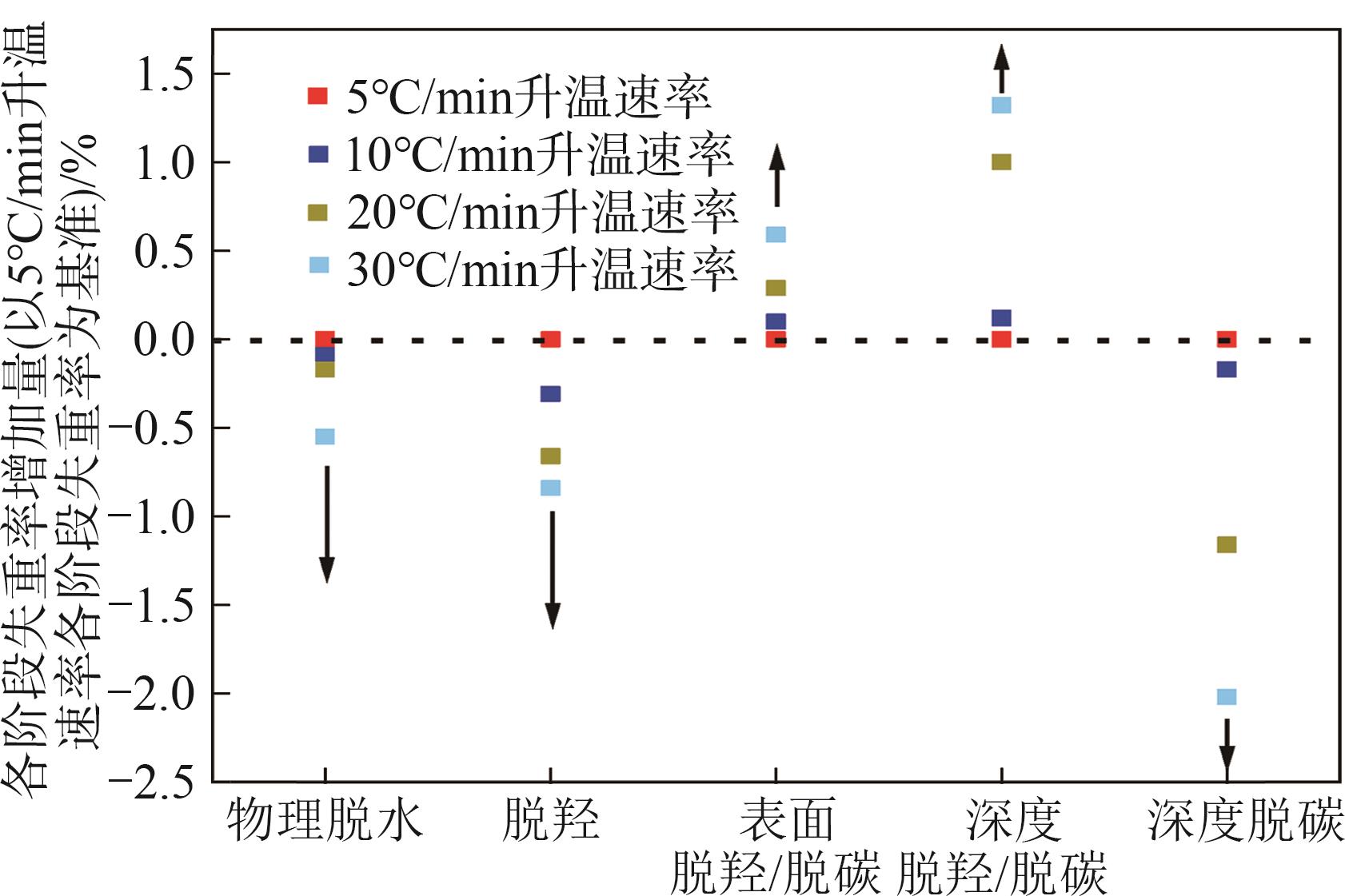
| 升温速率 /℃·min-1 | 失重峰1 (物理吸附水) | 失重峰2 (脱羟) | 失重峰3 (表面脱羟/脱碳) | 失重峰4 (深度脱羟/脱碳) | 失重峰5 (深度脱碳) | 总失重 /% | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 温度/℃ | 失重/% | 温度/℃ | 失重/% | 温度/℃ | 失重/% | 温度/℃ | 失重/% | 温度/℃ | 失重/% | |||||||||
| 5 | 47 | 1.96 | 198 | 4.23 | 218 | 1.82 | 351 | 17.37 | 425 | 4.89 | 29.91 | |||||||
| 10 | 58 | 1.87 | 206 | 3.92 | 231 | 1.92 | 365 | 17.49 | 437 | 4.72 | 30.01 | |||||||
| 20 | 70 | 1.79 | 218 | 3.57 | 244 | 2.11 | 372 | 18.37 | 454 | 3.73 | 29.41 | |||||||
| 30 | 93 | 1.41 | 222 | 3.39 | 246 | 2.41 | 381 | 18.69 | 453 | 2.87 | 29.01 | |||||||
表2 各失重阶段的中心温度和失重率
| 升温速率 /℃·min-1 | 失重峰1 (物理吸附水) | 失重峰2 (脱羟) | 失重峰3 (表面脱羟/脱碳) | 失重峰4 (深度脱羟/脱碳) | 失重峰5 (深度脱碳) | 总失重 /% | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 温度/℃ | 失重/% | 温度/℃ | 失重/% | 温度/℃ | 失重/% | 温度/℃ | 失重/% | 温度/℃ | 失重/% | |||||||||
| 5 | 47 | 1.96 | 198 | 4.23 | 218 | 1.82 | 351 | 17.37 | 425 | 4.89 | 29.91 | |||||||
| 10 | 58 | 1.87 | 206 | 3.92 | 231 | 1.92 | 365 | 17.49 | 437 | 4.72 | 30.01 | |||||||
| 20 | 70 | 1.79 | 218 | 3.57 | 244 | 2.11 | 372 | 18.37 | 454 | 3.73 | 29.41 | |||||||
| 30 | 93 | 1.41 | 222 | 3.39 | 246 | 2.41 | 381 | 18.69 | 453 | 2.87 | 29.01 | |||||||
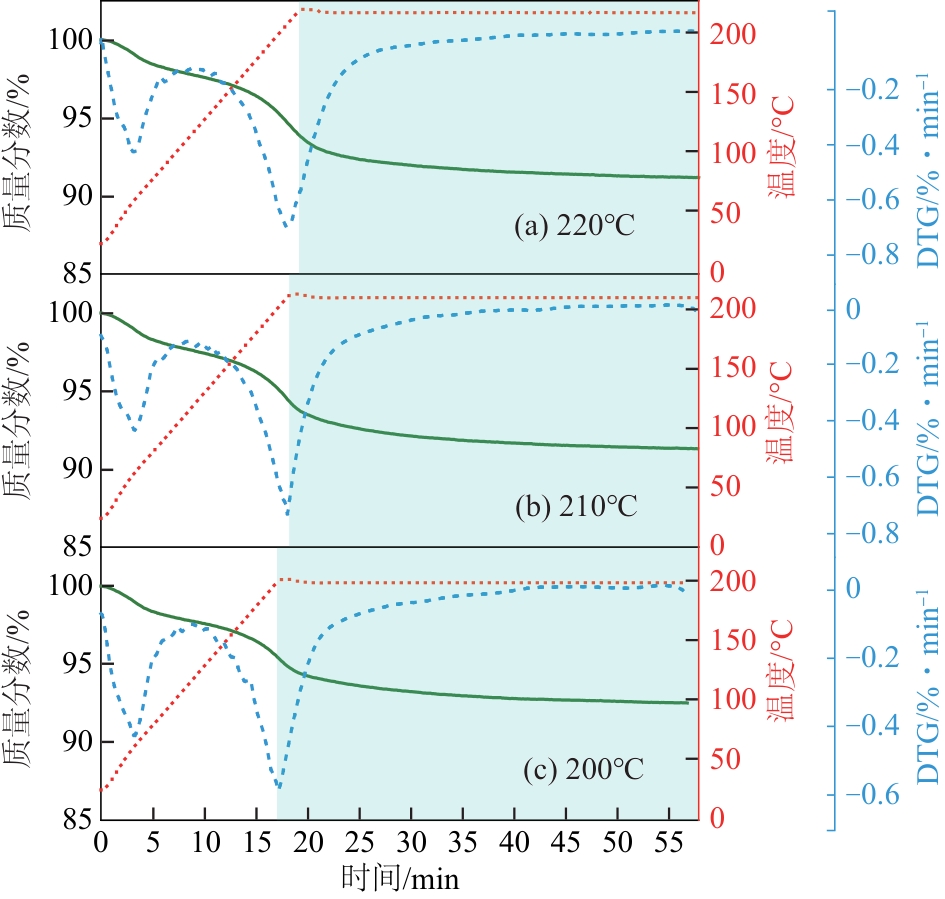
| 恒温 温度/℃ | 失重峰1 | 失重峰2 | 总失重 /% | ||
|---|---|---|---|---|---|
| 温度/℃ | 失重/% | 温度/℃ | 失重/% | ||
| 220 | 62 | 2.28 | 210 | 6.19 | 8.47 |
| 210 | 63 | 2.33 | 210 | 5.99 | 8.32 |
| 200 | 62 | 2.24 | 201 | 5.00 | 7.24 |
表3 第一段不同恒温温度下失重中心温度及失重率
| 恒温 温度/℃ | 失重峰1 | 失重峰2 | 总失重 /% | ||
|---|---|---|---|---|---|
| 温度/℃ | 失重/% | 温度/℃ | 失重/% | ||
| 220 | 62 | 2.28 | 210 | 6.19 | 8.47 |
| 210 | 63 | 2.33 | 210 | 5.99 | 8.32 |
| 200 | 62 | 2.24 | 201 | 5.00 | 7.24 |
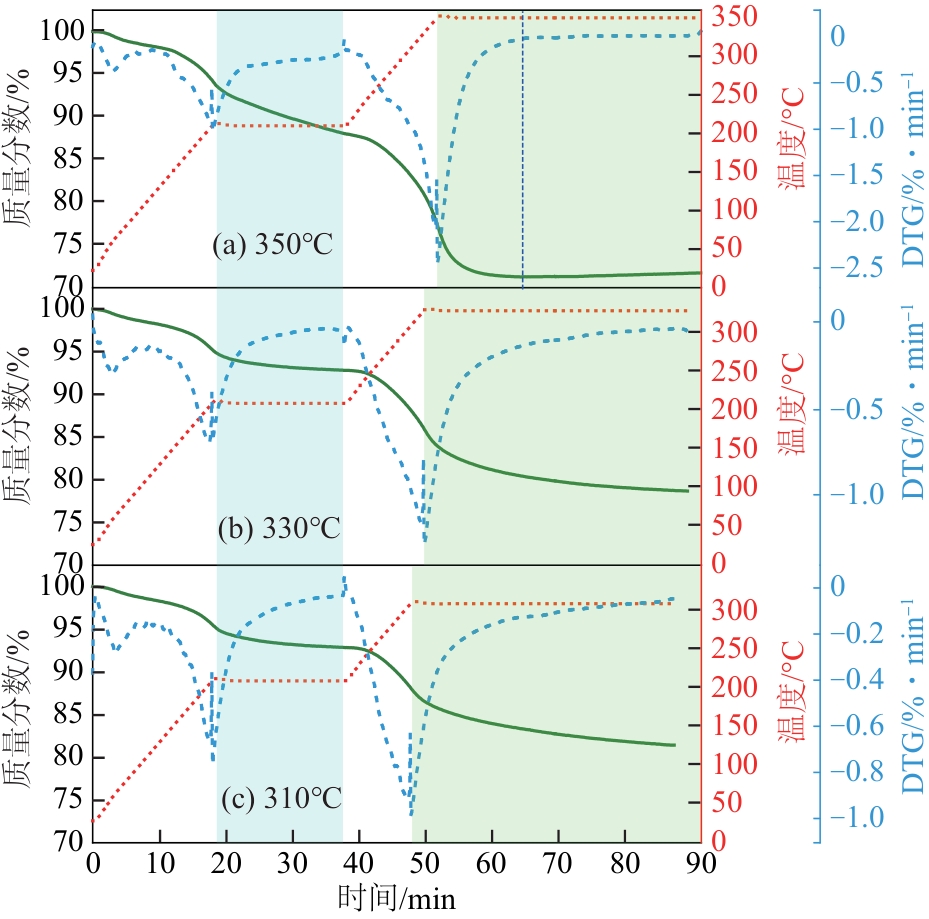
恒温温度 /℃ | 失重峰1 | 失重峰2 | 失重峰3 | 失重峰2+失重峰3总失重 /% | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 温度/℃ | 失重/% | 温度/℃ | 失重/% | 温度/℃ | 失重/% | |||||||
| 350 | 60.5 | 1.60 | 210 | 10.35 | 350 | 16.78 | 27.13 | |||||
| 330 | 58.6 | 1.63 | 210 | 5.53 | 330 | 14.12 | 19.65 | |||||
| 310 | 64.5 | 1.49 | 210 | 5.64 | 310 | 11.42 | 17.06 | |||||
表4 第二段不同恒温温度下失重中心温度及失重率
恒温温度 /℃ | 失重峰1 | 失重峰2 | 失重峰3 | 失重峰2+失重峰3总失重 /% | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 温度/℃ | 失重/% | 温度/℃ | 失重/% | 温度/℃ | 失重/% | |||||||
| 350 | 60.5 | 1.60 | 210 | 10.35 | 350 | 16.78 | 27.13 | |||||
| 330 | 58.6 | 1.63 | 210 | 5.53 | 330 | 14.12 | 19.65 | |||||
| 310 | 64.5 | 1.49 | 210 | 5.64 | 310 | 11.42 | 17.06 | |||||
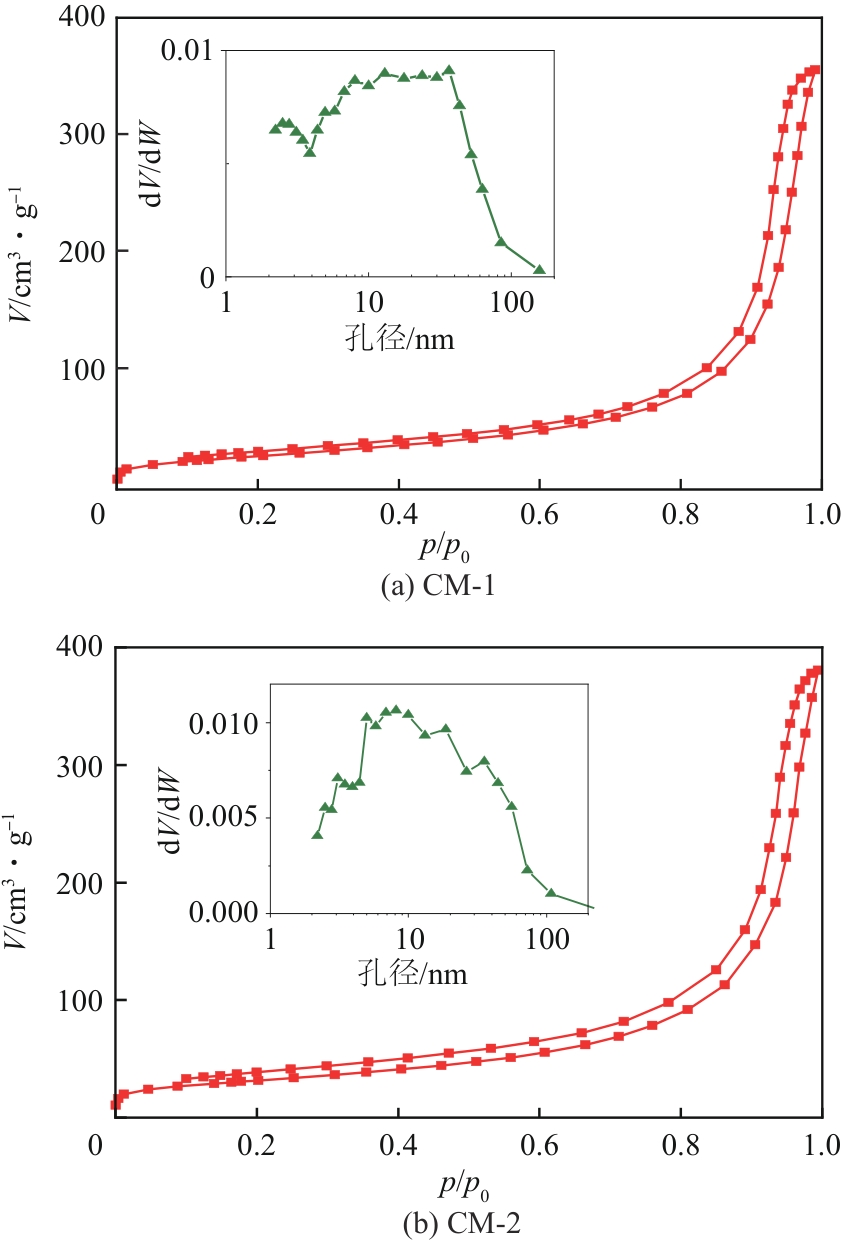
| 催化剂 | BET比表面积/m2·g-1 | 孔容/cm3·g-1 | 平均孔径/nm | 最可几孔径/nm |
|---|---|---|---|---|
| CM-1 | 102 | 0.56 | 20.6 | 36.5 |
| CM-2 | 113 | 0.60 | 20.7 | 8.1 |
表6 不同催化剂织构特征
| 催化剂 | BET比表面积/m2·g-1 | 孔容/cm3·g-1 | 平均孔径/nm | 最可几孔径/nm |
|---|---|---|---|---|
| CM-1 | 102 | 0.56 | 20.6 | 36.5 |
| CM-2 | 113 | 0.60 | 20.7 | 8.1 |
| 1 | BYUN Manhee, KIM Heehyang, LEE Hyunjun, et al. Conceptual design for methanol steam reforming in serial packed-bed reactors and membrane filters: Economic and environmental perspectives[J]. Energy, 2022, 241: 122516. |
| 2 | ANANDARAJAH Gabrial, MCDOWALL Will, EKINS Paul. Decarbonising road transport with hydrogen and electricity: Long term global technology learning scenarios[J]. International Journal of Hydrogen Energy, 2013, 38(8): 3419-3432. |
| 3 | NUNES Paula, OLIVEIRA Fabricio, HAMACHER Silvio, et al. Design of a hydrogen supply chain with uncertainty[J]. International Journal of Hydrogen Energy, 2015, 40(46): 16408-16418. |
| 4 | SIDDIQUI Osamah, DINCER Ibrahim. A well to pump life cycle environmental impact assessment of some hydrogen production routes[J]. International Journal of Hydrogen Energy, 2019, 44(12): 5773-5786. |
| 5 | ZHAO Jiaqi, SHI Run, LI Zhenhua, et al. How to make use of methanol in green catalytic hydrogen production[J]. Nano Select, 2020, 1(1): 12-29. |
| 6 | BALCOMBE Paul, SPEIRS Jamie, JOHNSON Erin, et al. The carbon credentials of hydrogen gas networks and supply chains[J]. Renewable and Sustainable Energy Reviews, 2018, 91: 1077-1088. |
| 7 | GARCIA Gabriel, ARRIOLA Emmanuel, CHEN Wei-Hsin, et al. A comprehensive review of hydrogen production from methanol thermochemical conversion for sustainability[J]. Energy, 2021, 217: 119384. |
| 8 | XIAO Ze, MENG Qingwei, YUAN Qingchun, et al. High-performance metal-base bifunctional catalysts (Ni x Mg y -MMO) for aqueous phase reforming of methanol to hydrogen[J]. Fuel, 2023, 350: 128808. |
| 9 | YANG Huanhuan, CHEN Yanyan, CUI Xiaojing, et al. A highly stable copper-based catalyst for clarifying the catalytic roles of Cu0 and Cu+ species in methanol dehydrogenation[J]. Angewandte Chemie International Edition, 2018, 57(7): 1836-1840. |
| 10 | MAURI Silvia, Gianluca D’OLIMPIO, GHICA Corneliu, et al. Hydrogen production mechanism in low-temperature methanol decomposition catalyzed by Ni3Sn4 intermetallic compound: A combined operando and density functional theory investigation[J]. The Journal of Physical Chemistry Letters, 2023, 14(5): 1334-1342. |
| 11 | BAGHERZADEH Seyed Behnam, HAGHIGHI Mohammad. Plasma-enhanced comparative hydrothermal and coprecipitation preparation of CuO/ZnO/Al2O3 nanocatalyst used in hydrogen production via methanol steam reforming[J]. Energy Conversion and Management, 2017, 142: 452-465. |
| 12 | XU Xinhai, SHUAI Kaipeng, XU Ben. Review on copper and palladium based catalysts for methanol steam reforming to produce hydrogen[J]. Catalysts, 2017, 7(6): 183. |
| 13 | HUANG Gang, LIAW Biing-Jye, JHANG Cheng-Jyun, et al. Steam reforming of methanol over CuO/ZnO/CeO2/ZrO2/Al2O3 catalysts[J]. Applied Catalysis A: General, 2009, 358(1): 7-12. |
| 14 | LI Guangjun, GU Chuantao, ZHU Wanbin, et al. Hydrogen production from methanol decomposition using Cu-Al spinel catalysts[J]. Journal of Cleaner Production, 2018, 183: 415-423. |
| 15 | LIU X Y, TOYIR J, DE LA PISCINA P R, et al. Hydrogen production from methanol steam reforming over Al2O3 and ZrO2-modified CuOZnOGa2O3 catalysts[J]. International Journal of Hydrogen Energy, 2017, 42(19): 13704-13711. |
| LIU Xianyun, TOYIR Jamil, RAMÍREZ DE LA PISCINA Pilar, et al. Hydrogen production from methanol steam reforming over Al2O3- and ZrO2-modified CuOZnOGa2O3 catalysts[J]. International Journal of Hydrogen Energy, 2017, 42(19): 13704-13711. | |
| 16 | MATTER Paul H, BRADEN Drew J, OZKAN Umit S. Steam reforming of methanol to H2 over nonreduced Zr-containing CuO/ZnO catalysts[J]. Journal of Catalysis, 2004, 223(2): 340-351. |
| 17 | TWIGG Martyn V, SPENCER Michael S. Deactivation of supported copper metal catalysts for hydrogenation reactions[J]. Applied Catalysis A: General, 2001, 212(1/2): 161-174. |
| 18 | MARTÍN Antonio J, MITCHELL Sharon, MONDELLI Cecilia, et al. Unifying views on catalyst deactivation[J]. Nature Catalysis, 2022, 5(10): 854-866. |
| 19 | Sandra SÁ, SILVA Hugo, Lúcia BRANDÃO, et al. Catalysts for methanol steam reforming—A review[J]. Applied Catalysis B: Environmental, 2010, 99(1/2): 43-57. |
| 20 | YANG Jun, ZHENG Hongyan, ZHU Yulei, et al. Effects of calcination temperature on performance of Cu-Zn-Al catalyst for synthesizing γ-butyrolactone and 2-methylfuran through the coupling of dehydrogenation and hydrogenation[J]. Catalysis Communications, 2004, 5(9): 505-510. |
| 21 | BEHRENS Malte, GIRGSDIES Frank. Structural effects of Cu/Zn substitution in the malachite-rosasite system[J]. Zeitschrift Für Anorganische und Allgemeine Chemie, 2010, 636(6): 919-927. |
| 22 | KLOKISHNER Sophia, BEHRENS Malte, Oleg REU, et al. Cation ordering in natural and synthetic (Cu1– x Zn x )2CO3(OH)2 and (Cu1– x Zn x )5(CO3)2(OH)6 [J]. The Journal of Physical Chemistry A, 2011, 115(35): 9954-9968. |
| 23 | WELLS A F. Malachite: re-examination of crystal structure[J]. Acta Crystallographica, 1951, 4(3): 200-204. |
| 24 | ZHANG Fan, XU Xiaoying, QIU Zhengpu, et al. Improved methanol synthesis performance of Cu/ZnO/Al2O3 catalyst by controlling its precursor structure[J]. Green Energy & Environment, 2022, 7(4): 772-781. |
| 25 | Stefanie KÜHL, TARASOV Andrey, ZANDER Stefan, et al. Cu-based catalyst resulting from a Cu, Zn, Al hydrotalcite-like compound: A microstructural, thermoanalytical, and In Situ XAS study[J]. Chemistry:A European Journal, 2014, 20(13): 3782-3792. |
| 26 | SCHUMANN Julia, LUNKENBEIN Thomas, TARASOV Andrey, et al. Synthesis and characterisation of a highly active Cu/ZnO: Al catalyst[J]. ChemCatChem, 2014, 6(10): 2889-2897. |
| 27 | BEMS Bettina, SCHUR Michael, DASSENOY Alina, et al. Relations between synthesis and microstructural properties of copper/zinc hydroxycarbonates[J]. Chemistry:A European Journal, 2003, 9(9): 2039-2052. |
| 28 | SING Kenneth. The use of nitrogen adsorption for the characterisation of porous materials[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2001, 187: 3-9. |
| 29 | WANG Zixiang, ZOU Ying, FANG Zhibin, et al. Partitioning of pore space in hydrogen-bonded organic frameworks for enhanced CO2 photoreduction[J]. Science China Materials, 2024, 67(6): 1846-1850. |
| 30 | ZHANG Haoqing, ZHANG Limin, et al. Controllable synthesis of hollow mesoporous ZSM-5 with improved catalytic performance for tetralin hydrocracking to light aromatics[J]. Industrial & Engineering Chemistry Research, 2025, 64(8): 4330-4341. |
| 31 | IVANOVA T M, MASLAKOV K I, SIDOROV A A, et al. XPS detection of unusual Cu( Ⅱ) to Cu( Ⅰ ) transition on the surface of complexes with redox-active ligands[J]. Journal of Electron Spectroscopy and Related Phenomena, 2020, 238: 146878. |
| 32 | DIVINS Núria J, KORDUS David, TIMOSHENKO Janis, et al. operando high-pressure investigation of size-controlled CuZn catalysts for the methanol synthesis reaction[J]. Nature Communications, 2021, 12(1): 1435. |
| [1] | 刘威, 侯雪兰, 杨贵东. 氢-氨绿色循环研究进展与展望[J]. 化工进展, 2025, 44(5): 2625-2641. |
| [2] | 丁阿静, 周巧巧, 顾学红. 膜反应器中杨木催化气化制清洁合成气[J]. 化工进展, 2025, 44(5): 2716-2723. |
| [3] | 张绎如, 韩东梅, 马伟芳. 铁基复合卤氧化铋磁性材料强化可见光催化处理难降解有机废水研究进展[J]. 化工进展, 2025, 44(4): 2258-2273. |
| [4] | 高建刚, 姜亚鹏, 包宝青, 王书琦, 崔书明. 绿氢转化制绿色甲醇与绿氨[J]. 化工进展, 2025, 44(4): 1987-1997. |
| [5] | 宋坤莉, 肖雷, 马丹丹, 肖朋, 杨莎莎, 石建稳. 超低温氨气选择性脱硝催化剂的研究进展[J]. 化工进展, 2025, 44(4): 2028-2035. |
| [6] | 王淑媛, 尹玲玲, 高志华, 黄伟. 插层Cu比例对CuZnAl-LDHs催化剂结构及催化性能的影响[J]. 化工进展, 2025, 44(4): 2036-2044. |
| [7] | 黄娇, 朱亚明, 岳佳兴, 王莹, 程俊霞, 赵雪飞. 球形活性炭的制备、改性及应用研究进展[J]. 化工进展, 2025, 44(4): 2081-2101. |
| [8] | 陈宇航, 李巧艳, 梁美生, 宋天远, 汪玥, 李思萌, 周宇璇. Sn掺杂Cu/CeZrO2/γ-Al2O3对三效催化(TWC)反应的作用:提高低温活性和抗硫性[J]. 化工进展, 2025, 44(3): 1368-1377. |
| [9] | 张馨儿, 裴刘军, 周雨蝶, 靳凯丽, 王际平. 基于TiO2的光催化剂利用太阳能裂解水制氢研究进展[J]. 化工进展, 2025, 44(3): 1298-1308. |
| [10] | 刘俊杰, 吴建民, 孙启文, 王建成, 孙燕. 茂金属催化线性α-烯烃聚合获取高分子量产物研究进展[J]. 化工进展, 2025, 44(3): 1309-1322. |
| [11] | 朱国瑜, 葛棋, 付名利. 甲醇重整制氢催化剂耐久性评价和寿命预测方法[J]. 化工进展, 2025, 44(3): 1338-1346. |
| [12] | 谢鑫瑶, 万芬, 伏炫羽, 范雨婷, 陈令修, 李鹏. Cu-Ag纳米团簇CO2电催化还原性能和机理[J]. 化工进展, 2025, 44(3): 1387-1395. |
| [13] | 左骥, 罗莉, 谢永锴, 陈文尧, 钱刚, 周兴贵, 段学志. 甲醇无氧脱氢制甲醛Cu催化剂的粒径效应[J]. 化工进展, 2025, 44(3): 1347-1354. |
| [14] | 毕文涛, 王学林, 曲炜, 王从新, 田志坚. Mg改性对低铂载量Pt/ZSM-22烷烃加氢异构性能的影响[J]. 化工进展, 2025, 44(3): 1355-1367. |
| [15] | 张茂润, 孙伟如, 马天麟, 辛志玲. Mo改性MnCe/SiC低温SCR脱硝催化剂抗SO2中毒性能[J]. 化工进展, 2025, 44(3): 1378-1386. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||