化工进展 ›› 2025, Vol. 44 ›› Issue (1): 558-571.DOI: 10.16085/j.issn.1000-6613.2023-2282
气化细渣、铝灰的活化及其吸附性能
刘新维1( ), 高珊1,2(
), 高珊1,2( ), 王红涛1(
), 王红涛1( ), 王建成1,2
), 王建成1,2
- 1.太原理工大学环境科学与工程学院,山西 太原 030024
2.大气复合污染识别与控制山西省重点实验室,山西 太原 030024
-
收稿日期:2023-12-28修回日期:2024-05-17出版日期:2025-01-15发布日期:2025-02-13 -
通讯作者:高珊,王红涛 -
作者简介:刘新维(1999—),男,硕士研究生,研究方向为固体废物处理与资源化利用。E-mail:745581374@qq.com -
基金资助:山西省基础研究计划(20210302124132)
Activation of gasification fine slag and aluminum ash and their adsorption properties
LIU Xinwei1( ), GAO Shan1,2(
), GAO Shan1,2( ), WANG Hongtao1(
), WANG Hongtao1( ), WANG Jiancheng1,2
), WANG Jiancheng1,2
- 1.School of Environmental Science and Engineering, Taiyuan University of Science and Technology, Taiyuan 030024, Shanxi, China
2.Key Laboratory of Atmospheric Complex Pollution Identification and Control in Shanxi Province, Taiyuan 030024, Shanxi, China
-
Received:2023-12-28Revised:2024-05-17Online:2025-01-15Published:2025-02-13 -
Contact:GAO Shan, WANG Hongtao
摘要:
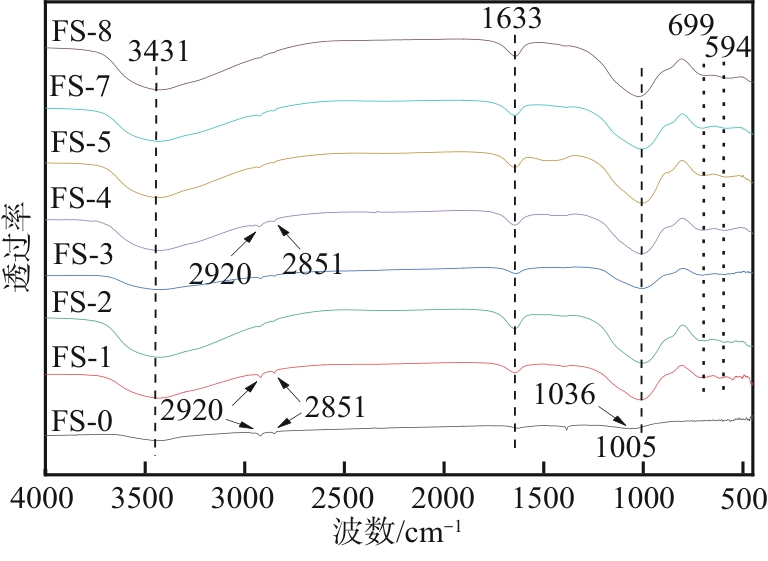
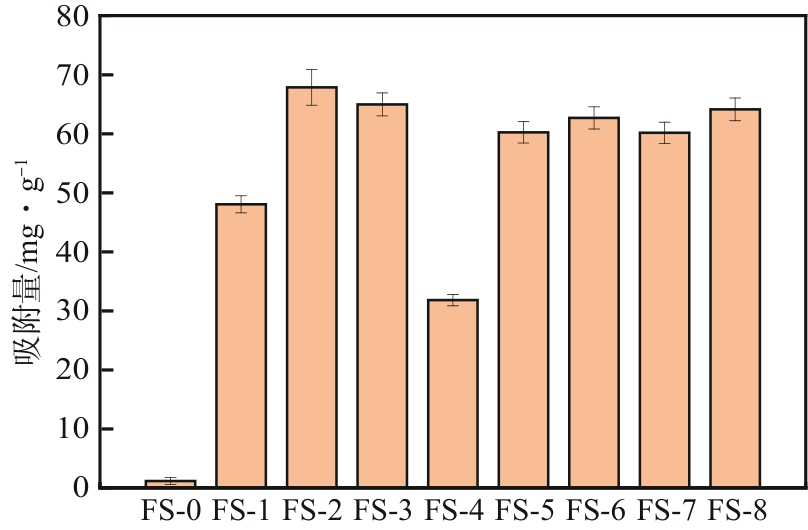
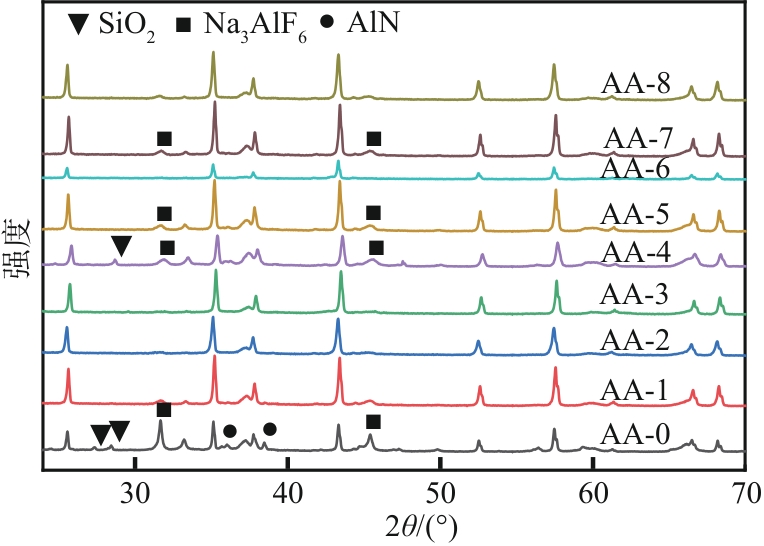
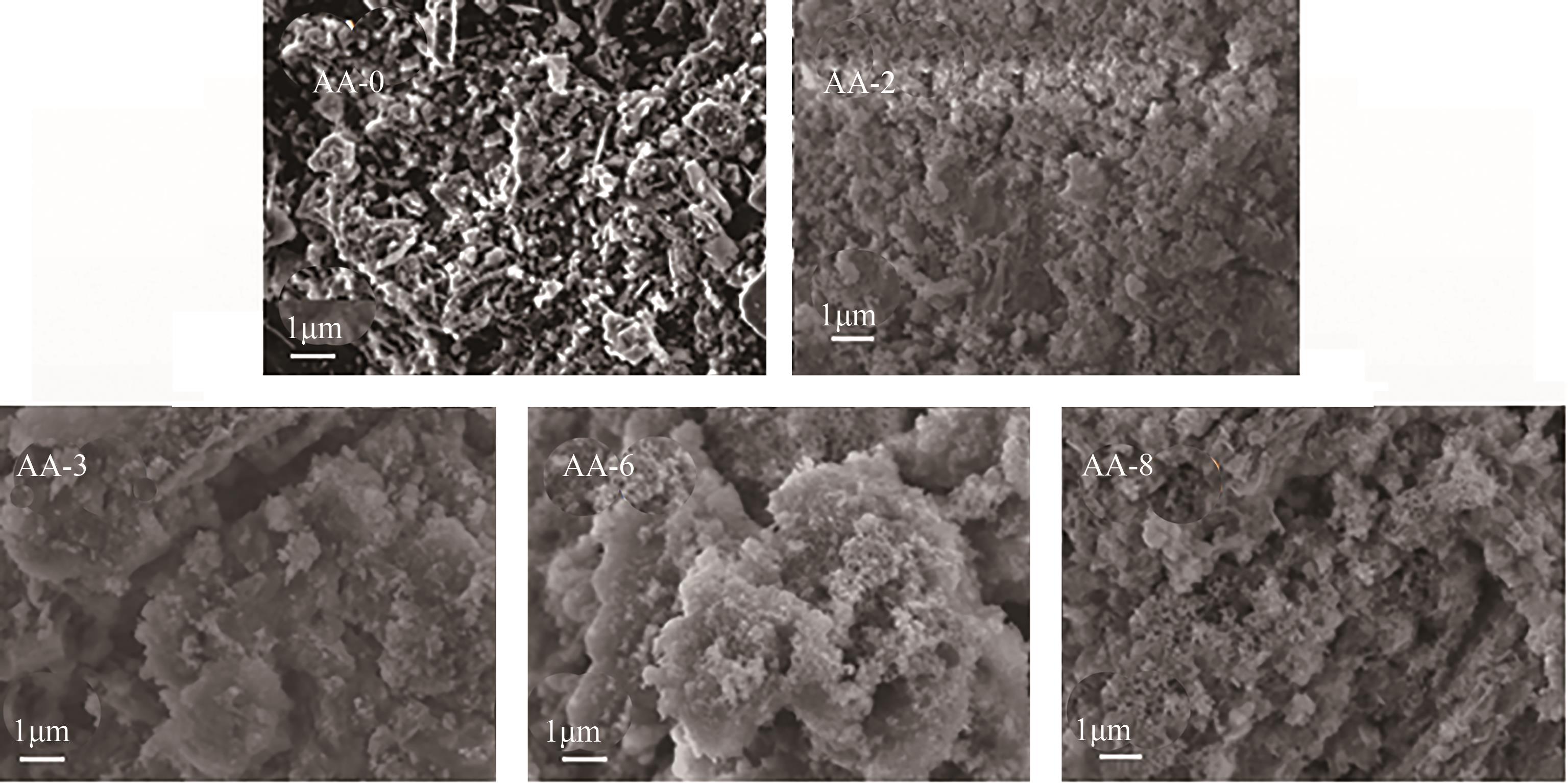
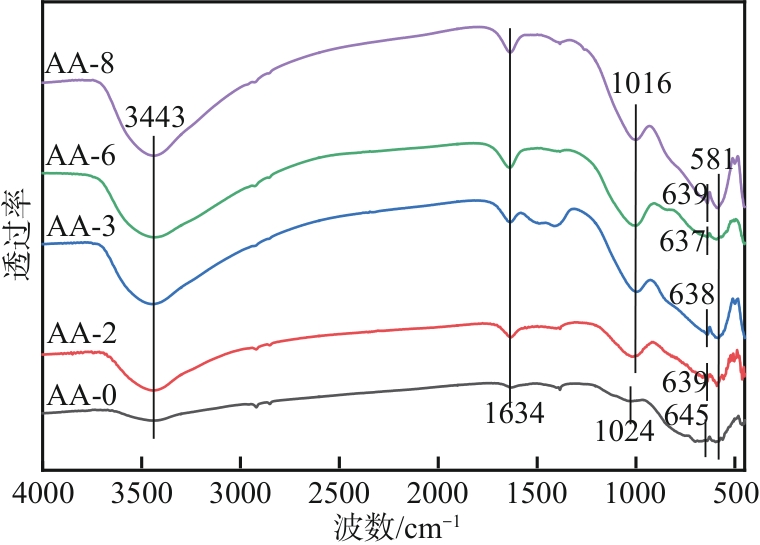
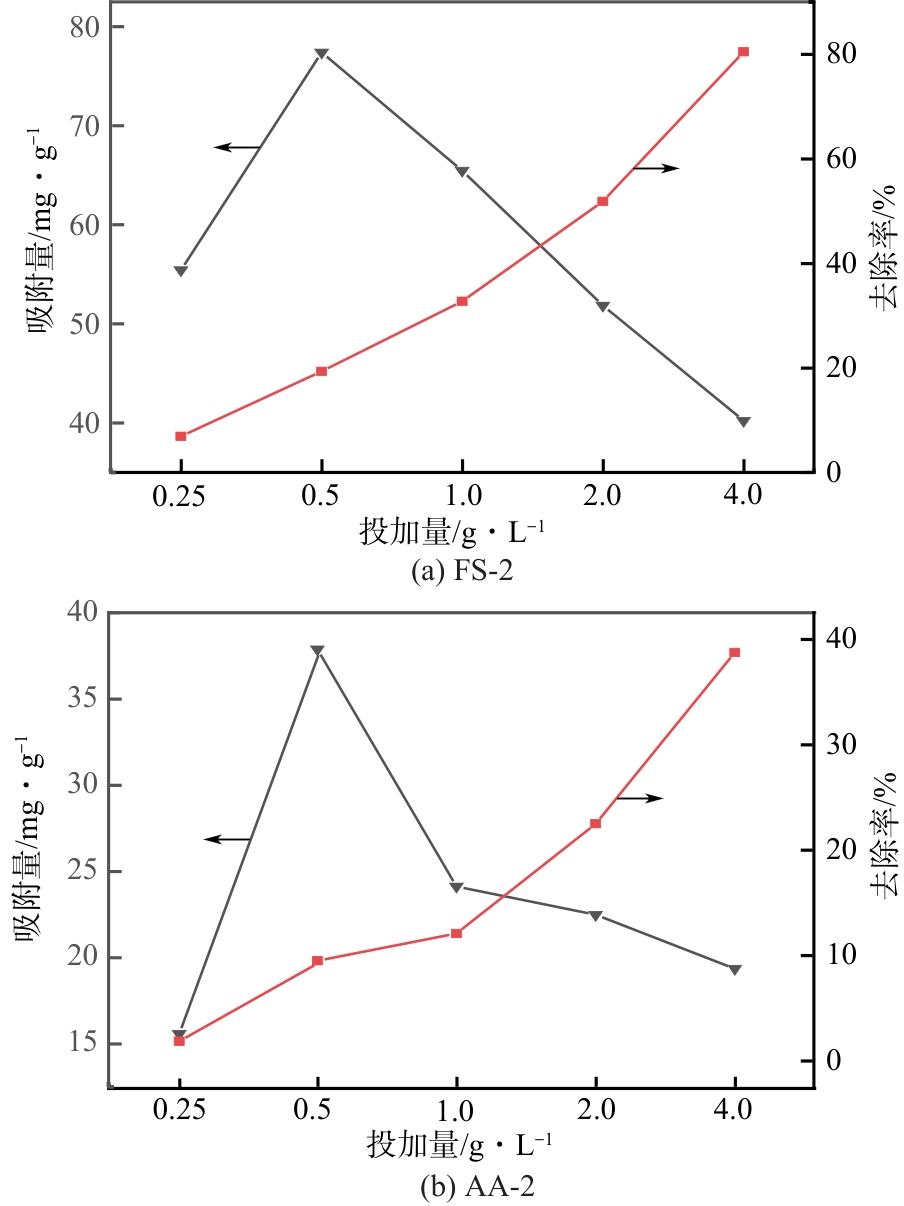
以工业废渣——煤气化细渣(FS)、铝灰(AA)为原料,一步碱熔法制备介孔吸附材料。研究了上述介孔材料对水中亚甲基蓝的吸附性能,结果表明,FS的最佳处理条件为:mFS∶mNaOH=5∶10、煅烧温度450℃、处理时间5h;AA的最佳处理条件为:mAA∶mNaOH=5∶8、煅烧温度550℃、处理时间3h。经碱熔处理后,FS材料对水中亚甲基蓝(MB)的吸附容量由1.16mg/g(FS-0)提升到67.85mg/g(FS-2),增长了近58倍;AA对水中亚甲基蓝的吸附容量由1.4mg/g(AA-0)提升到23.95mg/g(AA-2),增长了近17倍。X射线衍射、扫描电子显微镜、比表面积分析及傅里叶变换红外光谱等表征结果表明,两种材料在碱熔过程中形成的介孔孔道、带有氧缺陷位的 Si—O—基团以及大量的Al—OH和Si—OH键是其对亚甲基蓝吸附性能得到大大提升的原因。本研究表明FS和AA可以应用为一种廉价的亚甲基蓝吸附剂,为二者的资源化利用和印染废水的处理提供了一种潜在的方法。
中图分类号:
引用本文
刘新维, 高珊, 王红涛, 王建成. 气化细渣、铝灰的活化及其吸附性能[J]. 化工进展, 2025, 44(1): 558-571.
LIU Xinwei, GAO Shan, WANG Hongtao, WANG Jiancheng. Activation of gasification fine slag and aluminum ash and their adsorption properties[J]. Chemical Industry and Engineering Progress, 2025, 44(1): 558-571.
| 样品 | 改性条件 | ||
|---|---|---|---|
| 灰碱质量比 | 煅烧温度/℃ | 煅烧时间/h | |
| FS-0 | — | — | — |
| FS-1 | 5∶8 | 450 | 5 |
| FS-2 | 5∶10 | 450 | 5 |
| FS-3 | 5∶15 | 450 | 5 |
| FS-4 | 5∶10 | 350 | 5 |
| FS-5 | 5∶10 | 550 | 5 |
| FS-6 | 5∶10 | 650 | 5 |
| FS-7 | 5∶10 | 450 | 3 |
| FS-8 | 5∶10 | 450 | 7 |
| AA-0 | — | — | — |
| AA-1 | 5∶4 | 550 | 3 |
| AA-2 | 5∶8 | 550 | 3 |
| AA-3 | 5∶15 | 550 | 3 |
| AA-4 | 5∶8 | 350 | 3 |
| AA-5 | 5∶8 | 450 | 3 |
| AA-6 | 5∶8 | 650 | 3 |
| AA-7 | 5∶8 | 550 | 1 |
| AA-8 | 5∶8 | 550 | 5 |
表1 FS与AA的改性条件
| 样品 | 改性条件 | ||
|---|---|---|---|
| 灰碱质量比 | 煅烧温度/℃ | 煅烧时间/h | |
| FS-0 | — | — | — |
| FS-1 | 5∶8 | 450 | 5 |
| FS-2 | 5∶10 | 450 | 5 |
| FS-3 | 5∶15 | 450 | 5 |
| FS-4 | 5∶10 | 350 | 5 |
| FS-5 | 5∶10 | 550 | 5 |
| FS-6 | 5∶10 | 650 | 5 |
| FS-7 | 5∶10 | 450 | 3 |
| FS-8 | 5∶10 | 450 | 7 |
| AA-0 | — | — | — |
| AA-1 | 5∶4 | 550 | 3 |
| AA-2 | 5∶8 | 550 | 3 |
| AA-3 | 5∶15 | 550 | 3 |
| AA-4 | 5∶8 | 350 | 3 |
| AA-5 | 5∶8 | 450 | 3 |
| AA-6 | 5∶8 | 650 | 3 |
| AA-7 | 5∶8 | 550 | 1 |
| AA-8 | 5∶8 | 550 | 5 |
| 样品 | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | ClO2 | SO3 | Na2O |
|---|---|---|---|---|---|---|---|---|
| FS-0 | 37.30 | 26.04 | 14.90 | 11.72 | 0.59 | 0.53 | 5.47 | 0 |
| FS-2 | 34.79 | 23.08 | 21.84 | 17.00 | 0 | 0.48 | 0.08 | 0 |
| AA-0 | 4.22 | 74.95 | 0.70 | 1.48 | 5.83 | 9.14 | 1.09 | 0 |
| AA-2 | 13.23 | 61.89 | 3.81 | 3.43 | 10.14 | 0.61 | 0.06 | 0 |
表2 FS及AA处理前后的化学组成(质量分数,%)
| 样品 | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | ClO2 | SO3 | Na2O |
|---|---|---|---|---|---|---|---|---|
| FS-0 | 37.30 | 26.04 | 14.90 | 11.72 | 0.59 | 0.53 | 5.47 | 0 |
| FS-2 | 34.79 | 23.08 | 21.84 | 17.00 | 0 | 0.48 | 0.08 | 0 |
| AA-0 | 4.22 | 74.95 | 0.70 | 1.48 | 5.83 | 9.14 | 1.09 | 0 |
| AA-2 | 13.23 | 61.89 | 3.81 | 3.43 | 10.14 | 0.61 | 0.06 | 0 |
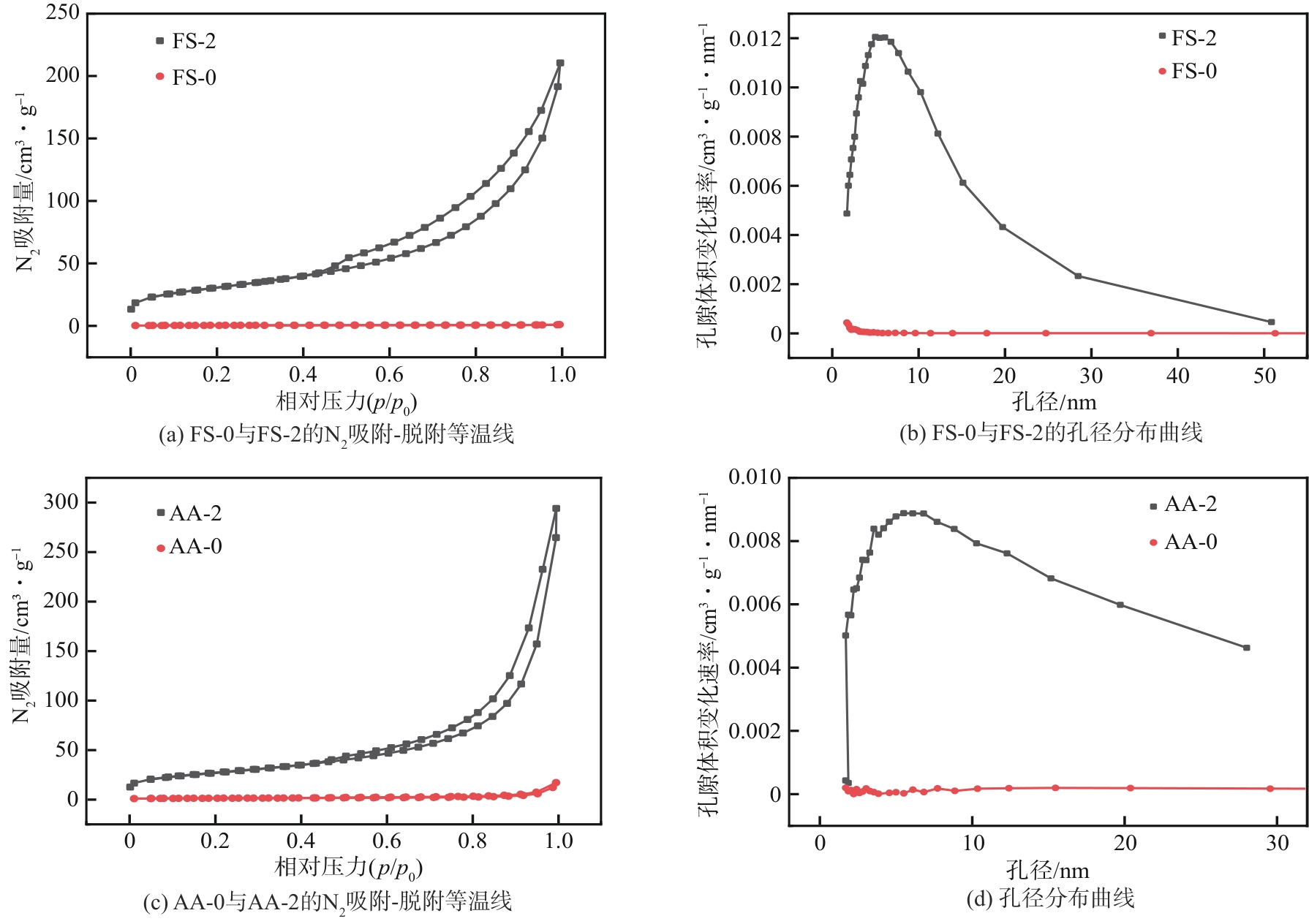
| 样品 | BET比表面积/m2·g-1 | 外比表面积/m2·g-1 | 微孔体积/cm3·g-1 | 介孔体积/cm3·g-1 | 孔径/nm |
|---|---|---|---|---|---|
| FS-0 | 1.87 | 1.10 | 0.0004 | 0.0009 | 5.572 |
| FS-2 | 109.29 | 102.78 | 0.0027 | 0.2250 | 8.231 |
| AA-0 | 4.55 | 2.77 | 0.0009 | 0.0080 | 10.384 |
| AA-2 | 96.32 | 89.40 | 0.0031 | 0.2394 | 13.430 |
表3 FS及AA处理前后的孔结构性质
| 样品 | BET比表面积/m2·g-1 | 外比表面积/m2·g-1 | 微孔体积/cm3·g-1 | 介孔体积/cm3·g-1 | 孔径/nm |
|---|---|---|---|---|---|
| FS-0 | 1.87 | 1.10 | 0.0004 | 0.0009 | 5.572 |
| FS-2 | 109.29 | 102.78 | 0.0027 | 0.2250 | 8.231 |
| AA-0 | 4.55 | 2.77 | 0.0009 | 0.0080 | 10.384 |
| AA-2 | 96.32 | 89.40 | 0.0031 | 0.2394 | 13.430 |
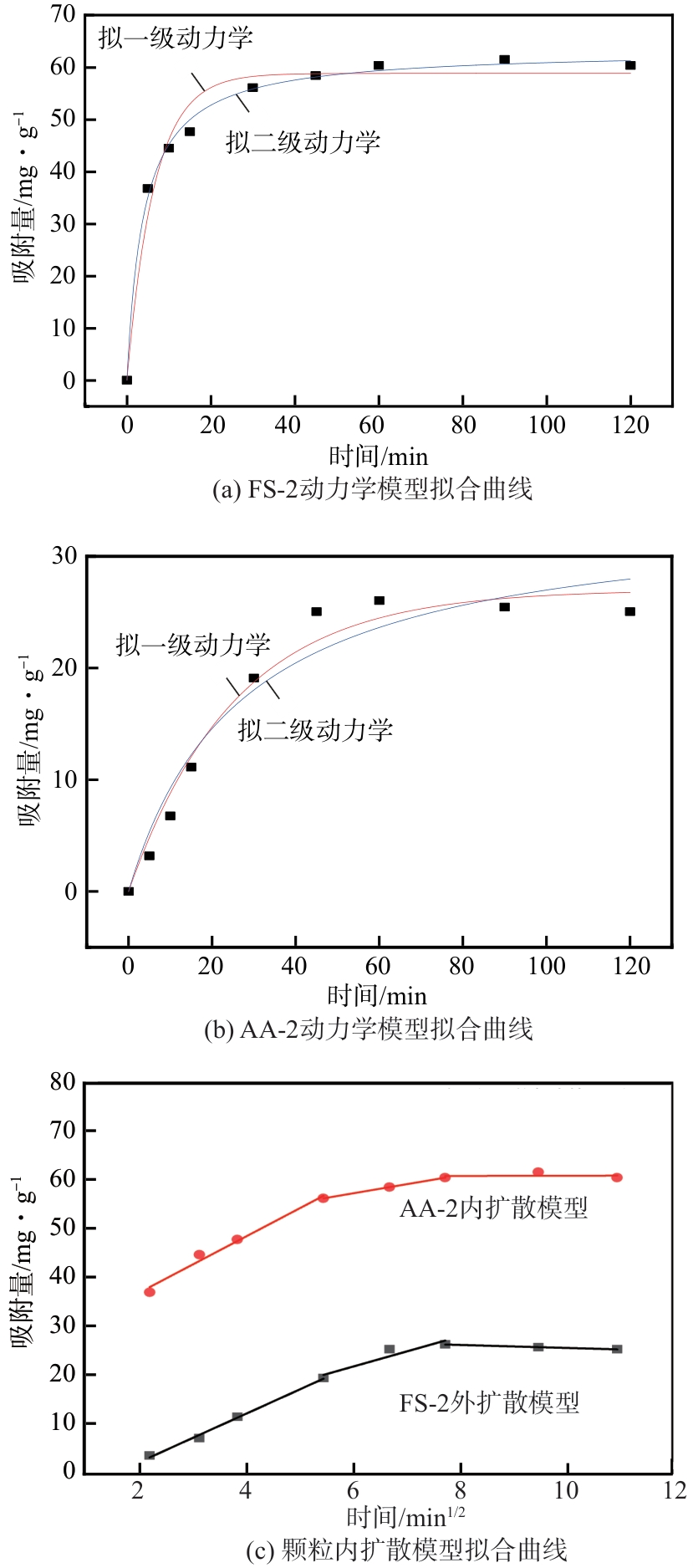
| 样品 | 拟一级动力学模型 | 拟二级动力学模型 | ||||
|---|---|---|---|---|---|---|
| qe/mg·g-1 | k1/min-1 | R2 | qe/mg·g-1 | k2/mg·g-1·min-1 | R2 | |
| FS-2 | 58.82 | 0.153 | 0.976 | 63.32 | 0.0039 | 0.996 |
| AA-2 | 27.01 | 0.039 | 0.977 | 34.27 | 0.0011 | 0.953 |
表4 FS-2与AA-2吸附MB的动力学拟合参数
| 样品 | 拟一级动力学模型 | 拟二级动力学模型 | ||||
|---|---|---|---|---|---|---|
| qe/mg·g-1 | k1/min-1 | R2 | qe/mg·g-1 | k2/mg·g-1·min-1 | R2 | |
| FS-2 | 58.82 | 0.153 | 0.976 | 63.32 | 0.0039 | 0.996 |
| AA-2 | 27.01 | 0.039 | 0.977 | 34.27 | 0.0011 | 0.953 |
| 样品 | MB在吸附剂表面上外扩散吸附阶段 | 吸附质在吸附剂孔隙中扩散阶段 | 吸附平衡阶段 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
kid,1 /mg·g-1·min-0.5 | Cid,1 /mg·g-1 | R2 | kid,2 /mg·g-1·min-0.5 | Cid,2 /mg·g-1 | R2 | kid,3 /mg·g-1·min-0.5 | Cid,3 /mg·g-1 | R2 | |||||||
| FS-2 | 5.798 | 24.868 | 0.983 | 1.878 | 45.807 | 0.999 | 0.0205 | 60.544 | 0.0024 | ||||||
| AA-2 | 4.999 | -8.391 | 0.996 | 3.113 | 2.711 | 0.887 | -0.309 | 28.421 | 0.996 | ||||||
表5 FS-2与AA-2吸附MB的颗粒内扩散模型拟合参数
| 样品 | MB在吸附剂表面上外扩散吸附阶段 | 吸附质在吸附剂孔隙中扩散阶段 | 吸附平衡阶段 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
kid,1 /mg·g-1·min-0.5 | Cid,1 /mg·g-1 | R2 | kid,2 /mg·g-1·min-0.5 | Cid,2 /mg·g-1 | R2 | kid,3 /mg·g-1·min-0.5 | Cid,3 /mg·g-1 | R2 | |||||||
| FS-2 | 5.798 | 24.868 | 0.983 | 1.878 | 45.807 | 0.999 | 0.0205 | 60.544 | 0.0024 | ||||||
| AA-2 | 4.999 | -8.391 | 0.996 | 3.113 | 2.711 | 0.887 | -0.309 | 28.421 | 0.996 | ||||||
| 样品 | T/K | Langmuir模型 | Freundlich模型 | ||||
|---|---|---|---|---|---|---|---|
| R2 | KL | qmax | R2 | KF | 1/n | ||
| FS-2 | 298.15 | 0.9929 | 0.0175 | 80.64 | 0.9214 | 11.16 | 0.3185 |
| 313.15 | 0.9902 | 0.0012 | 78.32 | 0.9320 | 6.09 | 0.404 | |
| 328.15 | 0.9906 | 0.0062 | 85.63 | 0.9440 | 2.66 | 0.532 | |
| AA-2 | 298.15 | 0.9932 | 0.0128 | 30.90 | 0.6120 | 2.19 | 0.439 |
| 313.15 | 0.9928 | 0.0091 | 30.27 | 0.6830 | 1.18 | 0.533 | |
| 328.15 | 0.9914 | 0.0044 | 35.10 | 0.7190 | 0.35 | 0.725 | |
表6 FS-2与AA-2吸附MB的等温线模型参数
| 样品 | T/K | Langmuir模型 | Freundlich模型 | ||||
|---|---|---|---|---|---|---|---|
| R2 | KL | qmax | R2 | KF | 1/n | ||
| FS-2 | 298.15 | 0.9929 | 0.0175 | 80.64 | 0.9214 | 11.16 | 0.3185 |
| 313.15 | 0.9902 | 0.0012 | 78.32 | 0.9320 | 6.09 | 0.404 | |
| 328.15 | 0.9906 | 0.0062 | 85.63 | 0.9440 | 2.66 | 0.532 | |
| AA-2 | 298.15 | 0.9932 | 0.0128 | 30.90 | 0.6120 | 2.19 | 0.439 |
| 313.15 | 0.9928 | 0.0091 | 30.27 | 0.6830 | 1.18 | 0.533 | |
| 328.15 | 0.9914 | 0.0044 | 35.10 | 0.7190 | 0.35 | 0.725 | |
| 样品 | T/K | ΔG/kJ·mol-1 | ΔH/kJ·mol-1 | ΔS/J·mol-1·K-1 |
|---|---|---|---|---|
| FS-2 | 298.15 | 4.38 | -5.92 | -34.54 |
| 313.15 | 4.93 | |||
| 328.15 | 5.40 | |||
| AA-2 | 298.15 | 6.59 | -5.04 | -38.98 |
| 313.15 | 7.17 | |||
| 328.15 | 7.75 |
表7 FS-2与AA-2吸附MB的热力学参数
| 样品 | T/K | ΔG/kJ·mol-1 | ΔH/kJ·mol-1 | ΔS/J·mol-1·K-1 |
|---|---|---|---|---|
| FS-2 | 298.15 | 4.38 | -5.92 | -34.54 |
| 313.15 | 4.93 | |||
| 328.15 | 5.40 | |||
| AA-2 | 298.15 | 6.59 | -5.04 | -38.98 |
| 313.15 | 7.17 | |||
| 328.15 | 7.75 |
| 1 | LAN Dawei, ZHU Huiwen, ZHANG Jianwen, et al. Adsorptive removal of organic dyes via porous materials for wastewater treatment in recent decades: A review on species, mechanisms and perspectives[J]. Chemosphere, 2022, 293: 133464. |
| 2 | Rania AL-TOHAMY, Sameh S ALI, LI Fanghua, et al. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety[J]. Ecotoxicology and Environmental Safety, 2022, 231: 113160. |
| 3 | 马超. 气化渣基氨氮吸附剂的制备及其性能研究[D]. 太原: 太原理工大学, 2021. |
| MA Chao. Preparation and properties of gasification slag-based NH4-N adsorbent[D]. Taiyuan: Taiyuan University of Technology, 2021. | |
| 4 | 范宁, 张逸群, 樊盼盼, 等. 煤气化渣特性分析及资源化利用研究进展[J]. 洁净煤技术, 2022, 28(8): 145-154. |
| FAN Ning, ZHANG Yiqun, FAN Panpan, et al. Research progress on characteristic analysis and resource utilization of coal gasification slag[J]. Clean Coal Technology, 2022, 28(8): 145-154. | |
| 5 | 沈汉林. 二次铝灰的理化特性和全组分资源化研究及应用[D]. 北京: 北京科技大学, 2022. |
| SHEN Hanlin. Characteristics of secondary aluminum dross and research and application of its total resource utilization[D]. Beijing: University of Science and Technology Beijing, 2022. | |
| 6 | 刘艳丽, 李强, 陈占飞, 等. 煤气化渣特性分析及综合利用研究进展[J]. 煤炭科学技术, 2022, 50(11): 251-257. |
| LIU Yanli, LI Qiang, CHEN Zhanfei, et al. Research progress characteristics analysis and comprehensive utilization of coal gasification slag[J]. Coal Science and Technology, 2022, 50(11): 251-257. | |
| 7 | YUAN Ning, TAN Kaiqi, ZHANG Xinling, et al. Synthesis and adsorption performance of ultra-low silica-to-alumina ratio and hierarchical porous ZSM-5 zeolites prepared from coal gasification fine slag[J]. Chemosphere, 2022, 303: 134839. |
| 8 | QIAO Qixia, ZHOU Huiming, GUO Feiqiang, et al. Facile and scalable synthesis of mesoporous composite materials from coal gasification fine slag for enhanced adsorption of malachite green[J]. Journal of Cleaner Production, 2022, 379: 134739. |
| 9 | WU Yuhua, XUE Kai, MA Qiaoling, et al. Removal of hazardous crystal violet dye by low-cost P-type zeolite/carbon composite obtained from in situ conversion of coal gasification fine slag[J]. Microporous and Mesoporous Materials, 2021, 312: 110742. |
| 10 | GAO Shan, LIU Yinghui, YUE Xiuping. Sustainable and low-cost SUZ-4 synthesis and its application for Cd2+ removal[J]. Journal of Cleaner Production, 2021, 312: 127825. |
| 11 | GAO Shan, LIU Yinghui. Potassium-assisted synthesis of SUZ-4 zeolite as an efficient adsorbent for Pb2+ removal from wastewater[J]. Separation and Purification Technology, 2022, 286: 120438. |
| 12 | BELVISO Claudia, CAVALCANTE Francesco, NICEFORO Giancarlo, et al. Sodalite, faujasite and A-type zeolite from 2∶1 dioctahedral and 2∶1∶1 trioctahedral clay minerals. A singular review of synthesis methods through laboratory trials at a low incubation temperature[J]. Powder Technology, 2017, 320: 483-497. |
| 13 | HUANG Xunrong, ZHAO Hanghang, HU Xiongfei, et al. Optimization of preparation technology for modified coal fly ash and its adsorption properties for Cd2+ [J]. Journal of Hazardous Materials, 2020, 392: 122461. |
| 14 | YU Miao, LI Jian, WANG Lijuan. KOH-activated carbon aerogels derived from sodium carboxymethyl cellulose for high-performance supercapacitors and dye adsorption[J]. Chemical Engineering Journal, 2017, 310: 300-306. |
| 15 | 赵佳, 李圣洁, 吉文欣, 等. 机械活化煤气化细渣资源化利用及残碳燃烧反应动力学探究[J]. 环境化学, 2022, 41(3): 1052-1059. |
| ZHAO Jia, LI Shengjie, JI Wenxin, et al. Mechanical activation and combustion kinetics of residual carbon in coal-gasification fine slag[J]. Environmental Chemistry, 2022, 41(3): 1052-1059. | |
| 16 | 程晓莹. 气化灰渣合成13X分子筛及其对重金属离子吸附性能的研究[D]. 淮南: 安徽理工大学, 2021. |
| CHENG Xiaoying. Preparation of 13X zeolite from coal gasification slag and its adsorption performance for heavy metal ions[D]. Huainan: Anhui University of Science & Technology, 2021. | |
| 17 | LUO Hongwei, HE Dongqin, ZHU Weiping, et al. Humic acid-induced formation of tobermorite upon hydrothermal treatment with municipal solid waste incineration bottom ash and its application for efficient removal of Cu(Ⅱ) ions[J]. Waste Management, 2019, 84: 83-90. |
| 18 | THOMMES Matthias, KANEKO Katsumi, NEIMARK Alexander V, et al. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report)[J]. Pure and Applied Chemistry, 2015, 87(9/10): 1051-1069. |
| 19 | ZHOU Jianmin, ZHENG Feng, LI Hui, et al. Optimization of post-treatment variables to produce hierarchical porous zeolites from coal gangue to enhance adsorption performance[J]. Chemical Engineering Journal, 2020, 381: 122698. |
| 20 | 高珊, 刘颖慧. SUZ-4分子筛的合成及其对亚甲基蓝的吸附[J]. 硅酸盐学报, 2021, 49(9): 2001-2008. |
| GAO Shan, LIU Yinghui. Synthesis of SUZ-4 zeolite and its adsorption for methylene blue[J]. Journal of the Chinese Ceramic Society, 2021, 49(9): 2001-2008. | |
| 21 | FAGHIHIAN Hossein, NOURMORADI Heshmatollah, SHOKOUHI Maryam. Performance of silica aerogels modified with amino functional groups in PB(Ⅱ) and CD(Ⅱ) removal from aqueous solutions[J]. PJCT, 2012, 14(1): 50-56. |
| 22 | 陈和生, 孙振亚, 邵景昌. 八种不同来源二氧化硅的红外光谱特征研究[J]. 硅酸盐通报, 2011, 30(4): 934-937. |
| CHEN Hesheng, SUN Zhenya, SHAO Jingchang. Investigation on FT-IR spectroscopy for eight different sources of SiO2 [J]. Bulletin of the Chinese Ceramic Society, 2011, 30(4): 934-937. | |
| 23 | 王志学, 王彩丽, 王斌, 等. Mg(OH)2@粉煤灰复合材料对重金属离子的去除研究[J]. 中国环境科学, 2022, 42(12): 5713-5724. |
| WANG Zhixue, WANG Caili, WANG Bin, et al. Study on removal of heavy metal ions by mg(OH)2 @ fly ash composite[J]. China Environmental Science, 2022, 42(12): 5713-5724. | |
| 24 | 王娜娜. 新型海绵状改性壳聚糖复合材料的制备及其对废水中Pb(Ⅱ)的净化应用研究[D]. 哈尔滨: 中国科学院大学(中国科学院东北地理与农业生态研究所), 2017. |
| WANG Nana. Preparation and application of novel spongy modified chitosan adsorbents for the removal of Pb(Ⅱ) from wastewaters[D]. Harbin: Northeast Institute of Geography and Agroecology (Chinese Academy of Sciences), 2017. | |
| 25 | 刘秀芸, 王刚, 雷雨昕, 等. 巯基改性玉米秸秆对水中Cu(Ⅱ)的吸附特性[J]. 中国环境科学, 2022, 42(3): 1220-1229. |
| LIU Xiuyun, WANG Gang, LEI Yuxin, et al. Adsorption performance and mechanism of mercaptoacetyl corn straw for Cu(Ⅱ) in aqueous solution[J]. China Environmental Science, 2022, 42(3): 1220-1229. | |
| 26 | DENG Hui, YANG Le, TAO Guanghui, et al. Preparation and characterization of activated carbon from cotton stalk by microwave assisted chemical activation—Application in methylene blue adsorption from aqueous solution[J]. Journal of Hazardous Materials, 2009, 166(2/3): 1514-1521. |
| 27 | SAHU Sumanta, KAR Padmaja, BISHOYI Nisarani, et al. Synthesis of polypyrrole-modified layered double hydroxides for efficient removal of Cr(Ⅵ)[J]. Journal of Chemical & Engineering Data, 2019, 64(10): 4357-4368. |
| 28 | MOMINA, MOHAMMAD Shahadat, SUZYLAWATI Isamil. Study of the adsorption/desorption of MB dye solution using bentonite adsorbent coating[J]. Journal of Water Process Engineering, 2020, 34: 101155. |
| [1] | 李琢宇, 余美琪, 陈孝彦, 胡若晖, 王庆宏, 陈春茂, 詹亚力. 炼油废催化剂吸附去除水中硝基苯的特性与机制[J]. 化工进展, 2025, 44(2): 1076-1087. |
| [2] | 周渝, 唐甜, 熊子悠, 韦奇. 基于两级微通道分离工艺的甲醇制烯烃废水深度处理[J]. 化工进展, 2025, 44(1): 100-108. |
| [3] | 杨润农, 白帆飞, 林梓荣, 孙永明, 尹祥. 分子筛吸附脱除有机硫的研究进展[J]. 化工进展, 2025, 44(1): 329-340. |
| [4] | 田晴, 刘青盟, 李方, 杨波, 张思远, 关自良. 细菌的耐盐调控及其在高盐生物脱氮除磷工艺中的应用[J]. 化工进展, 2025, 44(1): 465-476. |
| [5] | 倪鹏, 王先泓, 黄钰涵, 马晓彤, 马子轸, 谈琰, 张华伟, 刘亭. 活性炭类和磁性金属类吸附剂喷射脱汞技术应用对比及最新进展[J]. 化工进展, 2025, 44(1): 513-524. |
| [6] | 赵丽阳, 李倩, 何佩熹, 潘鸿辉, 刘艳, 刘细祥. 磷钼酸-Fe3O4球磨共改性污泥基生物炭对四环素的吸附特性[J]. 化工进展, 2025, 44(1): 583-595. |
| [7] | 张炜, 黄赳, 朱晓芳, 李鹏. 凹凸棒石基钴钨水滑石吸附铅的性能及机理[J]. 化工进展, 2025, 44(1): 596-606. |
| [8] | 周渝, 夏太阳, 韦奇, 唐甜, 田磊. 微通道耦合反渗透膜串联处理甲醇制烯烃废水工艺优化[J]. 化工进展, 2024, 43(S1): 43-51. |
| [9] | 朱昊, 刘汉飞, 高源, 黄益平, 费孝诚, 韩卫清. 盐分对电催化降解性能与机理的影响[J]. 化工进展, 2024, 43(S1): 571-580. |
| [10] | 石磊, 王倩, 赵晓胜, 刘宏臣, 车远军, 段玉, 李庆. 油页岩灰基分子筛的制备及对亚甲基蓝的吸附[J]. 化工进展, 2024, 43(S1): 650-661. |
| [11] | 刘丽, 冯博, 文洋, 古启雄. 硅基介孔材料的合成、功能化及对金属的吸附研究进展[J]. 化工进展, 2024, 43(9): 5063-5078. |
| [12] | 吴宇琦, 李江涛, 丁建智, 宋秀兰, 苏冰琴. 焙烧镁铝水滑石脱除厌氧消化沼气中CO2的效果及机制[J]. 化工进展, 2024, 43(9): 5250-5261. |
| [13] | 杨新衡, 纪志永, 郭志远, 刘萁, 张盼盼, 汪婧, 刘杰, 毕京涛, 赵颖颖, 袁俊生. 锂铝层状双金属氢氧化物的制备及其锂脱嵌过程[J]. 化工进展, 2024, 43(9): 5262-5274. |
| [14] | 安芳芳, 曹少磊, 连增帅, 舒大武, 张岩, 李万新, 韩博. 十二烷基甜菜碱对热活化过硫酸钠降解C.I.活性黑5的影响[J]. 化工进展, 2024, 43(9): 5302-5308. |
| [15] | 卞维柏, 张睿轩, 潘建明. 无机金属锂离子筛材料制备方法研究进展[J]. 化工进展, 2024, 43(8): 4173-4186. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||