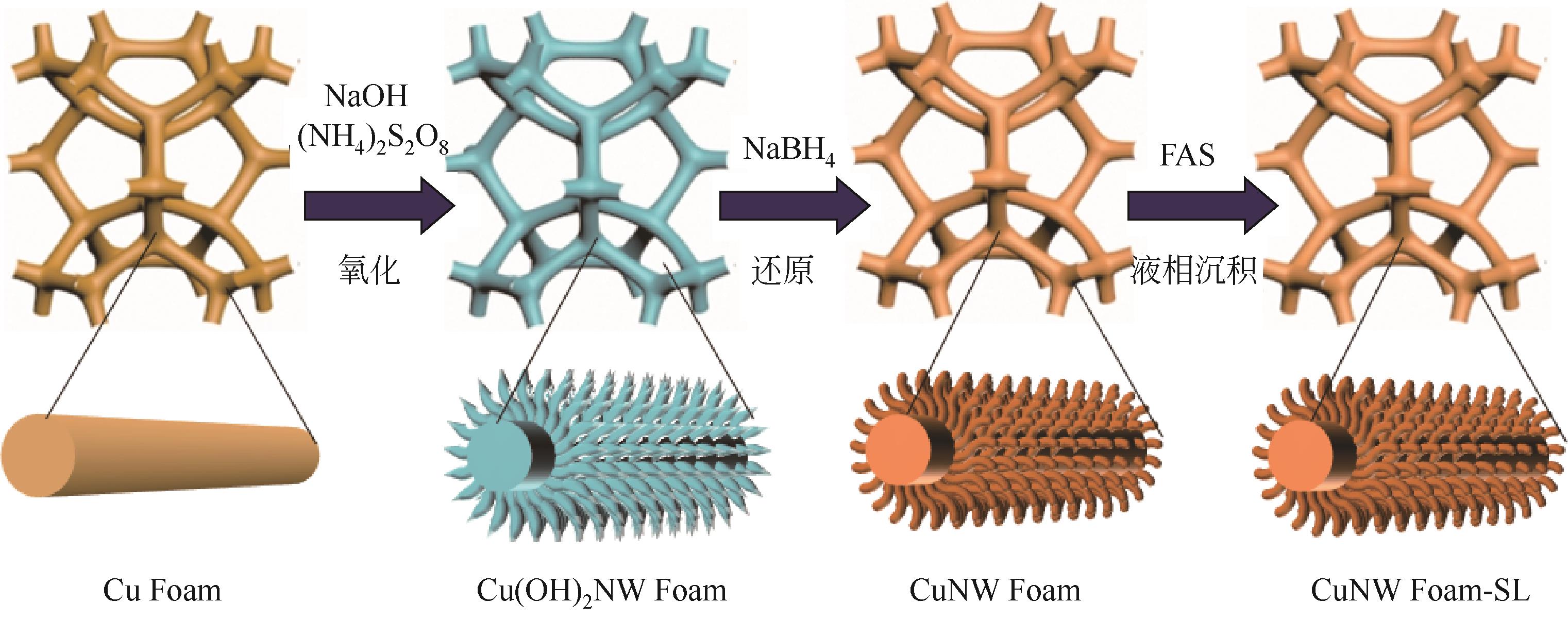
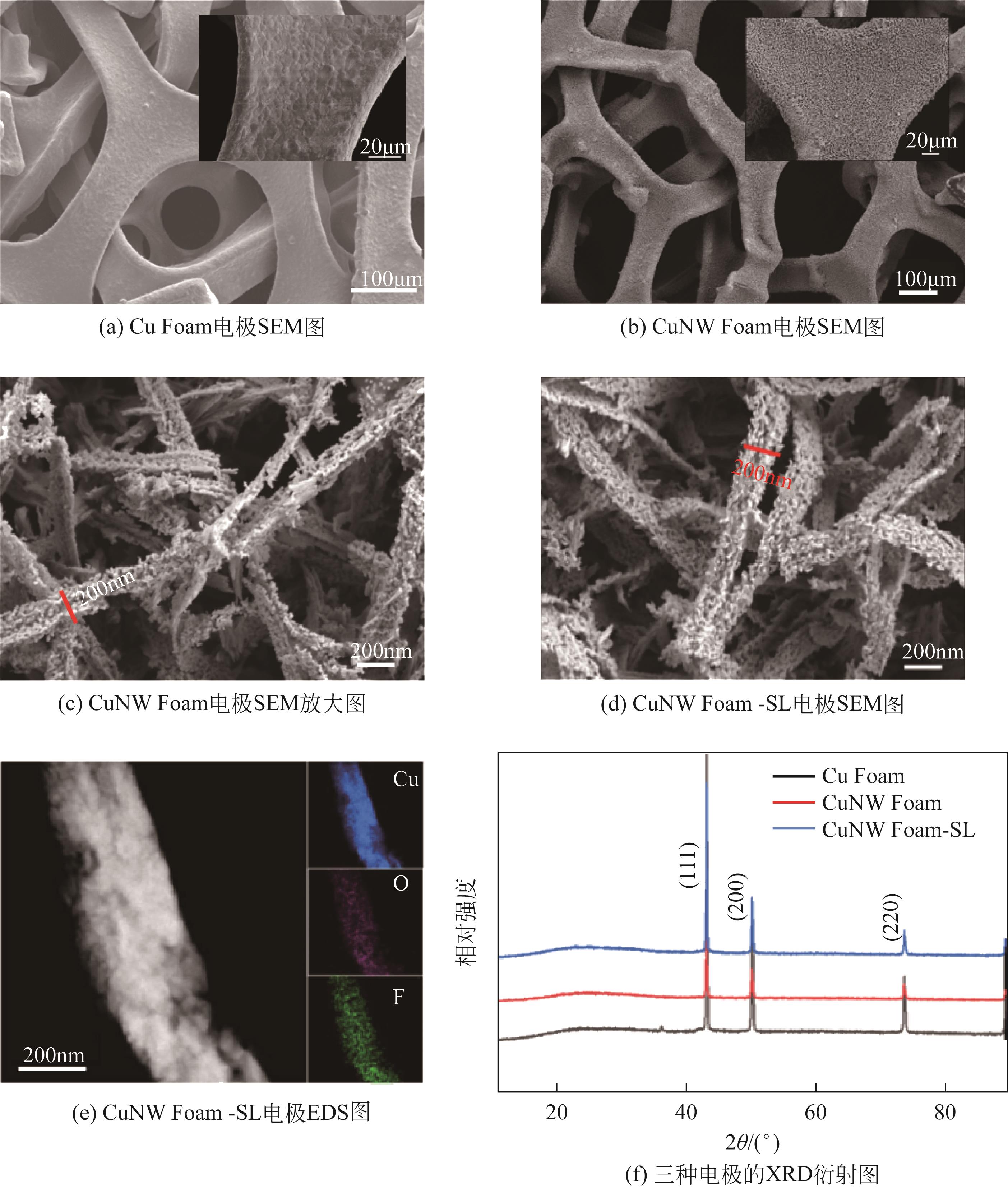
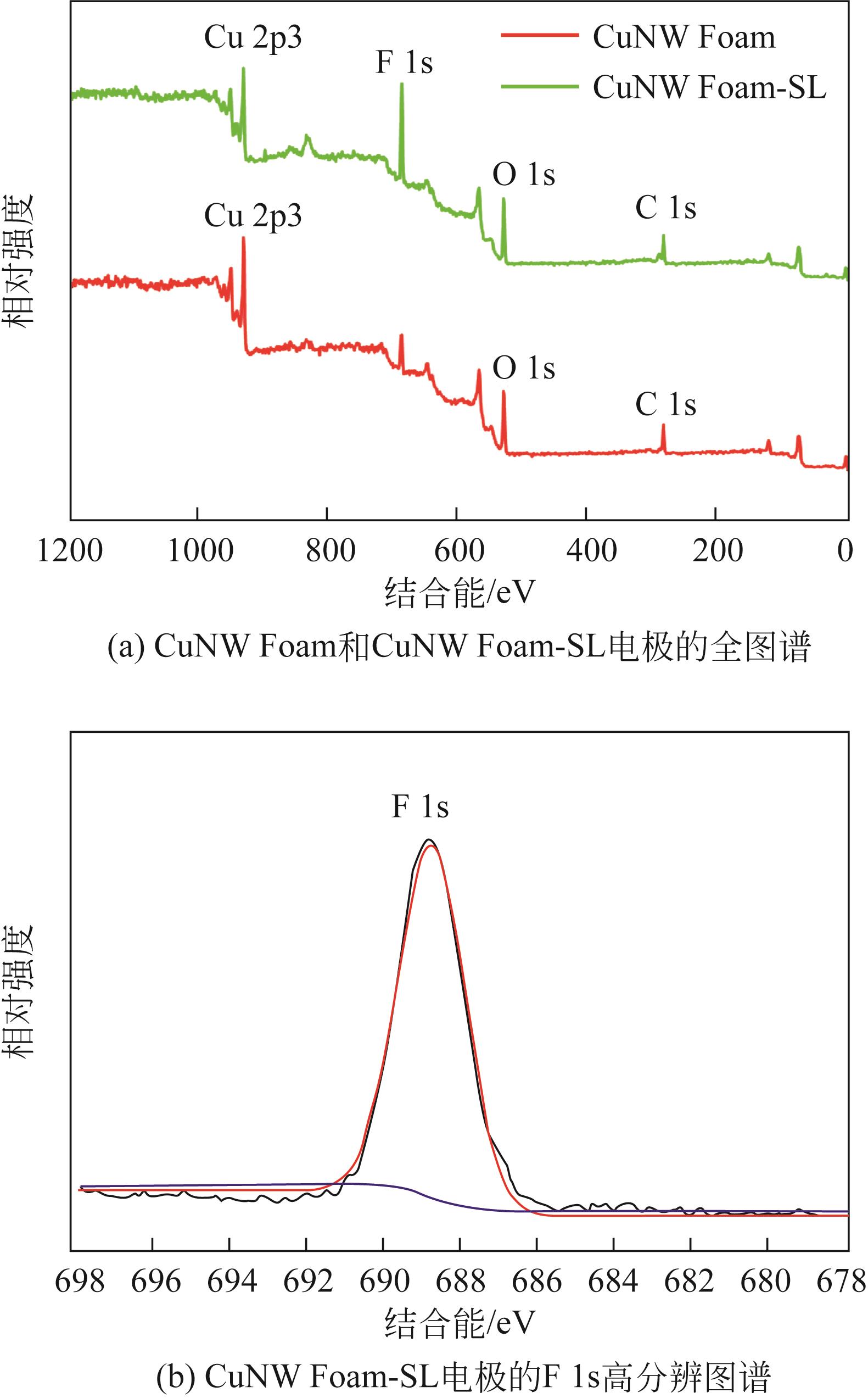
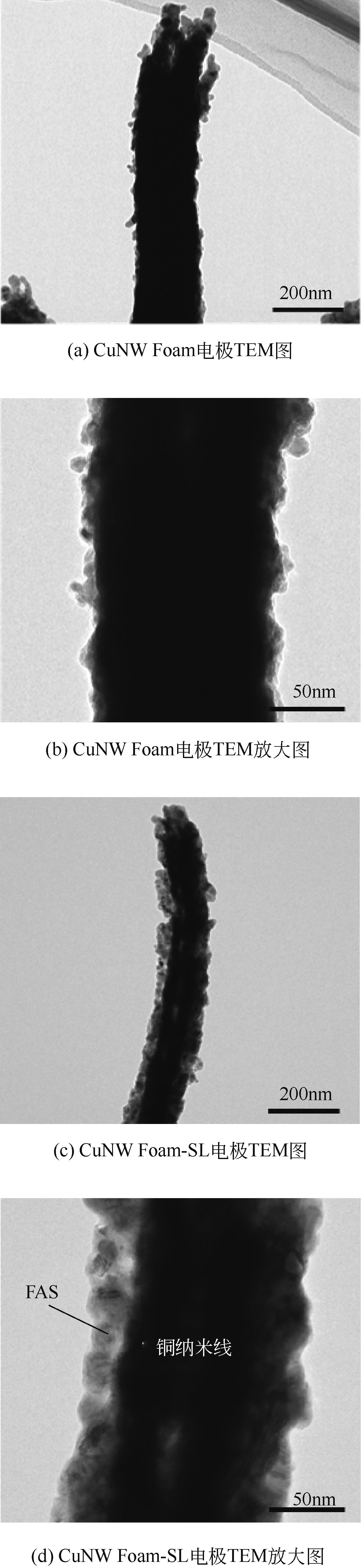
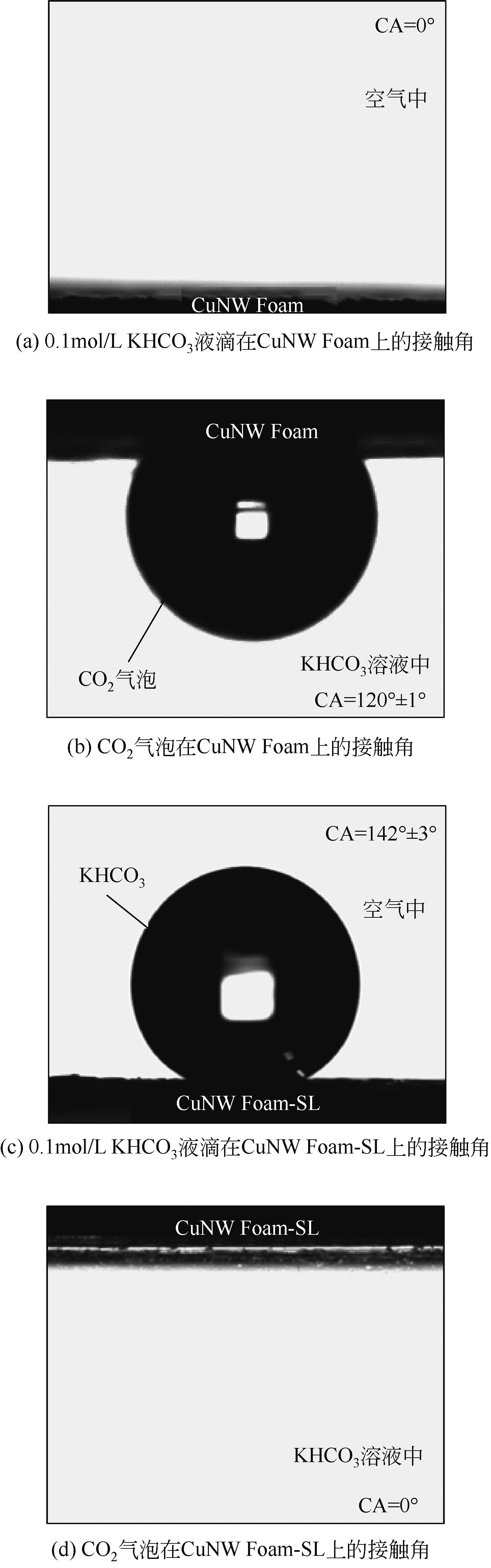
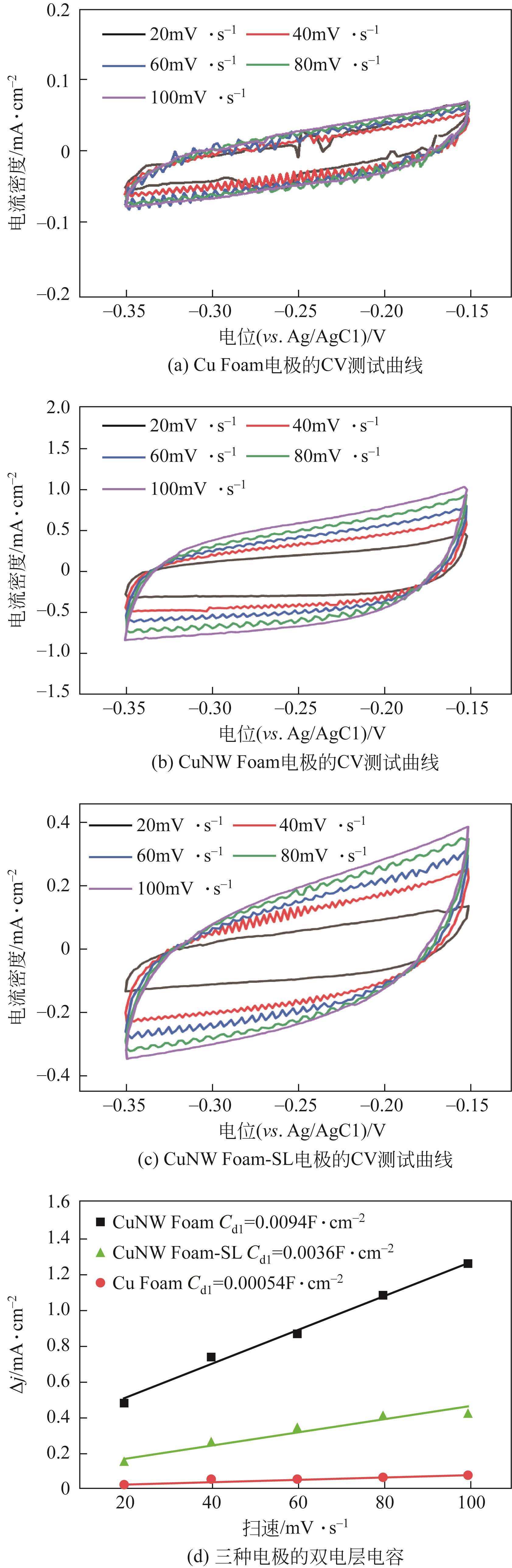
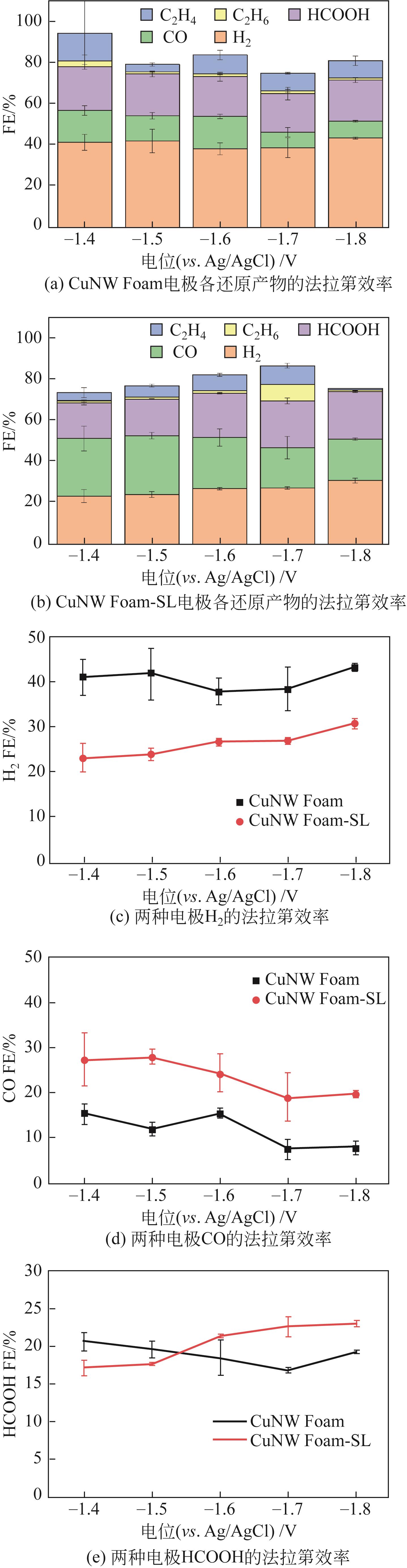
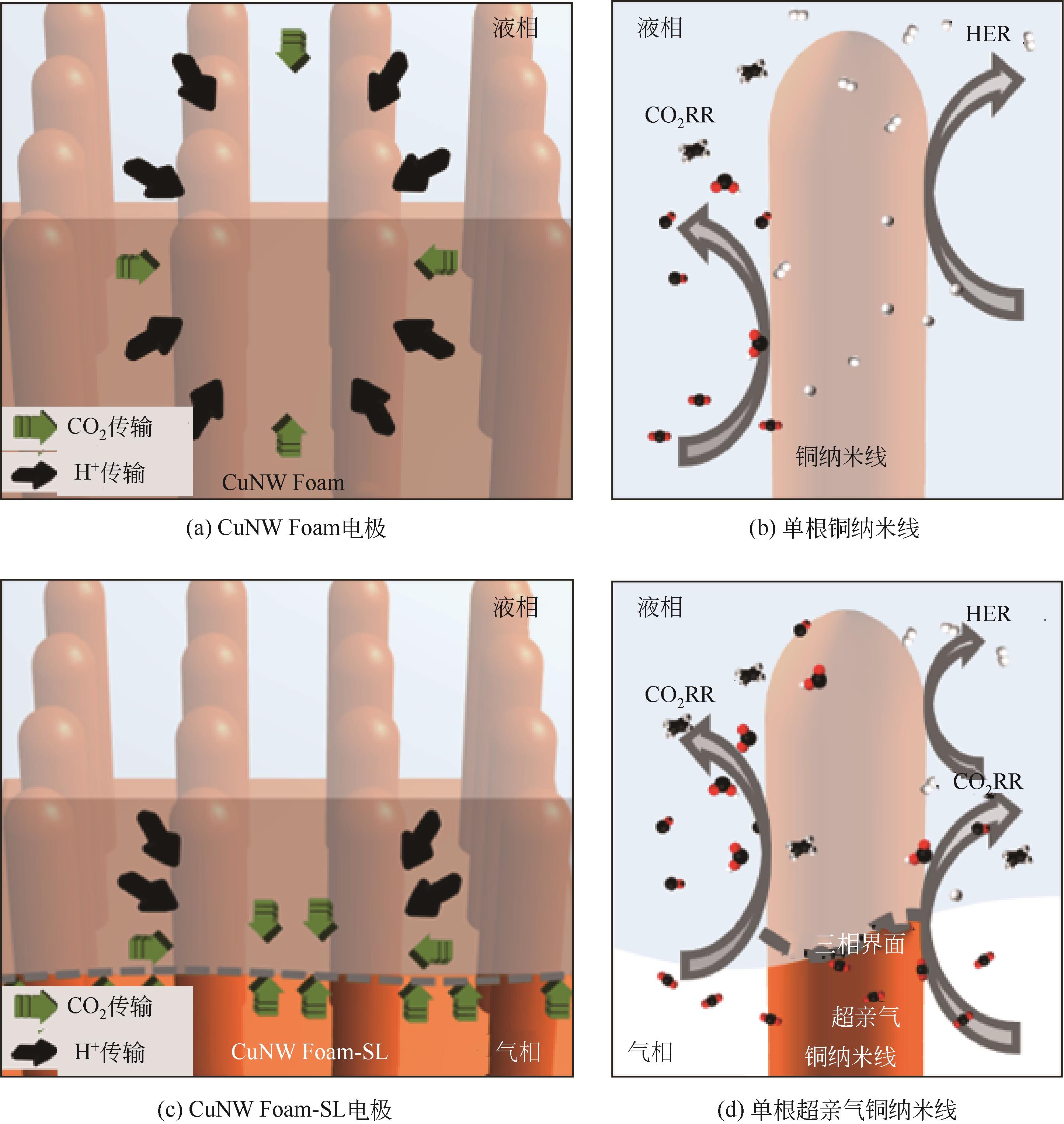
| 1 |
LEUNG Siufung, FU Huichun, ZHANG Maolin, et al. Blue energy fuels: Converting Ocean wave energy to carbon-based liquid fuels via CO2 reduction[J]. Energy & Environmental Science, 2020, 13(5): 1300-1308.
|
| 2 |
徐沛, 贾璇, 王勇, 等. 流场对MEC生物阴极CO2还原性能与产物的影响[J]. 化工进展, 2022, 41(7): 3816-3823.
|
|
XU Pei, JIA Xuan, WANG Yong, et al. Effect of flow field on the CO2 reduction performance and products of MEC biocathode[J]. Chemical Industry and Engineering Progress, 2022, 41(7): 3816-3823.
|
| 3 |
FU Qian, KURAMOCHI Yoshihiro, FUKUSHIMA Naoya, et al. Bioelectrochemical analyses of the development of a thermophilic biocathode catalyzing electromethanogenesis[J]. Environmental Science & Technology, 2015, 49(2): 1225-1232.
|
| 4 |
MICHL Josef. Towards an artificial leaf?[J]. Nature Chemistry, 2011, 3(4): 268-269.
|
| 5 |
蔡中杰, 田盼, 黄忠亮, 等. 基于生物模板制备二氧化碳加氢反应的Cu/ZnO催化剂[J]. 化工学报, 2021, 72(7): 3668-3679.
|
|
CAI Zhongjie, TIAN Pan, HUANG Zhongliang, et al. Preparation of Cu/ZnO nanocatalysts based on bio-templates for CO2 hydrogenation[J]. CIESC Journal, 2021, 72(7): 3668-3679.
|
| 6 |
KOLESNICHENKO N V, KREMLEVA E V, TELESHOV A T, et al. Carbon dioxide hydrogenation to formic acid in the presence of rhodium-oligoarylphosphonite catalyst systems[J]. Petroleum Chemistry, 2006, 46(1): 22-24.
|
| 7 |
LIU Yanming, CHEN Shuo, QUAN Xie, et al. Efficient electrochemical reduction of carbon dioxide to acetate on nitrogen-doped nanodiamond[J]. Journal of the American Chemical Society, 2015, 137(36): 11631-11636.
|
| 8 |
Sujat SEN, LIU Dan, PALMORE G Tayhas R. Electrochemical reduction of CO2 at copper nanofoams[J]. ACS Catalysis, 2014, 4(9): 3091-3095.
|
| 9 |
LEE Chang Hoon, KANAN Matthew W. Controlling H+ vs CO2 reduction selectivity on Pb electrodes[J]. ACS Catalysis, 2015, 5(1): 465-469.
|
| 10 |
LI Tengfei, LEES Eric W, GOLDMAN Maxwell, et al. Electrolytic conversion of bicarbonate into CO in a flow cell[J]. Joule, 2019, 3(6): 1487-1497.
|
| 11 |
YE Ke, CAO Ang, SHAO Jiaqi, et al. Synergy effects on Sn-Cu alloy catalyst for efficient CO2 electroreduction to formate with high mass activity[J]. Science Bulletin, 2020, 65(9): 711-719.
|
| 12 |
KIM Jun Hyuk, Hyunje WOO, CHOI Jihwan, et al. CO2 electroreduction on Au/TiC: Enhanced activity due to metal-support interaction[J]. ACS Catalysis, 2017, 7(3): 2101-2106.
|
| 13 |
李喆, 李泽洋, 杨宇森, 等. 电化学二氧化碳还原制甲酸催化剂的研究进展[J]. 化工进展, 2023, 42(1): 53-66.
|
|
LI Zhe, LI Zeyang, YANG Yusen, et al. Research progress on catalysts for electrocatalytic reduction of carbon dioxide to formic acid[J]. Chemical Industry and Engineering Progress, 2023, 42(1): 53-66.
|
| 14 |
张宇航, 叶丁丁, 朱恂, 等. 表面改性水热自生长SnO2碳布电极电化学还原CO2性能[J]. 科学通报, 2021, 66(26): 3488-3496.
|
|
ZHANG Yuhang, YE Dingding, ZHU Xun, et al. Performance of CO2 electrochemical reduction with surface modified self-growing SnO2 on carbon cloth electrode prepared by hydrothermal method[J]. Chinese Science Bulletin, 2021, 66(26): 3488-3496.
|
| 15 |
于丰收, 张鲁华. Cu基纳米材料电催化还原CO2的结构-性能关系[J]. 化工学报, 2021, 72(4): 1815-1824.
|
|
YU Fengshou, ZHANG Luhua. Structure-performance relationship of Cu-based nanocatalyst for electrochemical CO2 reduction[J]. CIESC Journal, 2021, 72(4): 1815-1824.
|
| 16 |
NIU Zhuangzhuang, GAO Feiyue, ZHANG Xiaolong, et al. Hierarchical copper with inherent hydrophobicity mitigates electrode flooding for high-rate CO2 electroreduction to multicarbon products[J]. Journal of the American Chemical Society, 2021, 143(21): 8011-8021.
|
| 17 |
LI Yifan, CUI Fan, ROSS Michael B, et al. Structure-sensitive CO2 electroreduction to hydrocarbons on ultrathin 5-fold twinned copper nanowires[J]. Nano Letters, 2017, 17(2): 1312-1317.
|
| 18 |
DAI Lei, QIN Qing, WANG Pei, et al. Ultrastable atomic copper nanosheets for selective electrochemical reduction of carbon dioxide[J]. Science Advances, 2017, 3(9): e1701069.
|
| 19 |
MA Sichao, SADAKIYO Masaaki, HEIMA Minako, et al. Electroreduction of carbon dioxide to hydrocarbons using bimetallic Cu-Pd catalysts with different mixing patterns[J]. Journal of the American Chemical Society, 2017, 139(1): 47-50.
|
| 20 |
SARFRAZ Saad, GARCIA-ESPARZA Angel T, JEDIDI Abdesslem, et al. Cu Sn bimetallic catalyst for selective aqueous electroreduction of CO2 to CO[J]. ACS Catalysis, 2016, 6(5): 2842-2851.
|
| 21 |
MA Ming, DJANASHVILI Kristina, SMITH Wilson A. Controllable hydrocarbon formation from the electrochemical reduction of CO2 over Cu nanowire arrays[J]. Angewandte Chemie International Edition, 2016, 55(23): 6680-6684.
|
| 22 |
KOTARO Ogura. Mediated reduction of CO and CO2 to methanol by surface-confined metal complexes in the presence of homogeneous catalysts[J]. Journal of Applied Electrochemistry, 1986, 16(5): 732-736.
|
| 23 |
CAI Zhao, ZHANG Yusheng, ZHAO Yuxin, et al. Selectivity regulation of CO2 electroreduction through contact interface engineering on superwetting Cu nanoarray electrodes[J]. Nano Research, 2019, 12(2): 345-349.
|
| 24 |
SHI Run, GUO Jiahao, ZHANG Xuerui, et al. Efficient wettability-controlled electroreduction of CO2 to CO at Au/C interfaces[J]. Nature Communications, 2020, 11(1): 3028.
|
| 25 |
LIN Ruoxu, ZHANG Shichao, REN Yanbiao, et al. Cu@Sn nanostructures based on light-weight current collectors for superior reversible lithium ion storage[J]. RSC Advances, 2016, 6(24): 20042-20050.
|
| 26 |
XU Wenwen, LU Zhiyi, SUN Xiaoming, et al. Superwetting electrodes for gas-involving electrocatalysis[J]. Accounts of Chemical Research, 2018, 51(7): 1590-1598.
|
 ), 叶丁丁1,2(
), 叶丁丁1,2( ), 朱恂1,2, 杨扬1,2, 陈蓉1,2, 廖强1,2
), 朱恂1,2, 杨扬1,2, 陈蓉1,2, 廖强1,2
 ), YE Dingding1,2(
), YE Dingding1,2( ), ZHU Xun1,2, YANG Yang1,2, CHEN Rong1,2, LIAO Qiang1,2
), ZHU Xun1,2, YANG Yang1,2, CHEN Rong1,2, LIAO Qiang1,2