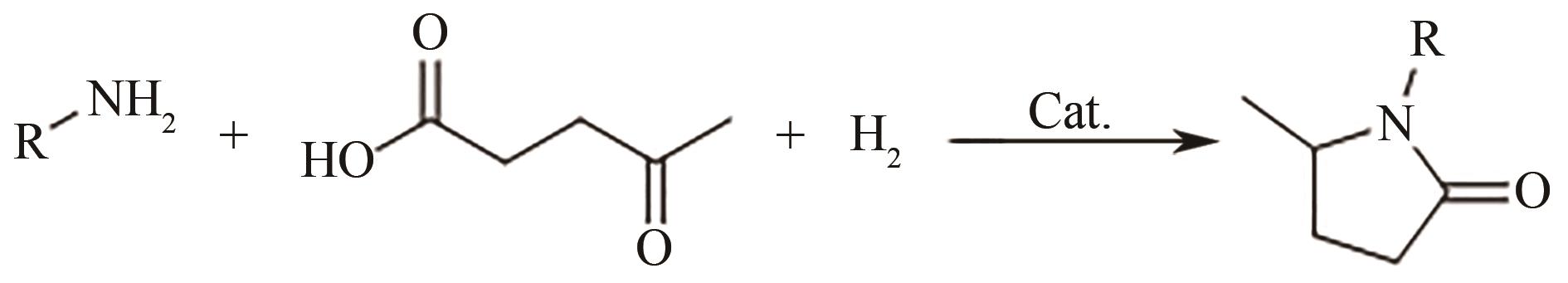化工进展 ›› 2024, Vol. 43 ›› Issue (1): 186-197.DOI: 10.16085/j.issn.1000-6613.2023-1479
• 专栏:化工过程强化 • 上一篇
微反应器中连续还原胺化反应的研究进展
- 清华大学化学工程系,化学工程联合国家重点实验室,北京 100084
-
收稿日期:2023-08-23修回日期:2023-11-21出版日期:2024-01-20发布日期:2024-02-05 -
通讯作者:张吉松 -
作者简介:张家昊(1999—),男,博士研究生,研究方向为微填充床反应器内还原胺化。E-mail: zhang-jh21@mails.tsinghua.edu.cn。 -
基金资助:国家自然科学基金(22022809)
Research advancement of continuous reductive amination in microreactors
ZHANG Jiahao( ), LI Yingying, XU Yanlin, YIN Jiabin, ZHANG Jisong(
), LI Yingying, XU Yanlin, YIN Jiabin, ZHANG Jisong( )
)
- State Key Laboratory of Chemical Engineering, Department of Chemical Engineering, Tsinghua University, Beijing 100084, China
-
Received:2023-08-23Revised:2023-11-21Online:2024-01-20Published:2024-02-05 -
Contact:ZHANG Jisong
摘要:
还原胺化反应是一种把醛(酮)转化为胺类物质的有效方法。还原胺化反应路径复杂,影响因素众多,合适的反应条件能够提升反应效率和选择性。本文总结了还原胺化反应常见的催化体系及催化剂、溶剂、温度、底物性质以及氨/水/酸的加入对反应的影响。基于这些影响因素,进一步介绍了连续微反应器技术在还原胺化过程中的应用,总结了以伯胺/仲胺/叔胺为目标产物的连续还原胺化过程、以硝基化合物为原料的连续还原胺化过程、酶催化及无催化剂的连续还原胺化过程。微反应器中的温度控制、传质强化和停留时间分布能进一步实现反应强化和选择性提升。基于微反应器的连续还原胺化技术及该技术与新型催化材料的结合有望在胺类物质的生产领域扮演越来越重要的角色。
中图分类号:
引用本文
张家昊, 李盈盈, 徐彦琳, 尹佳滨, 张吉松. 微反应器中连续还原胺化反应的研究进展[J]. 化工进展, 2024, 43(1): 186-197.
ZHANG Jiahao, LI Yingying, XU Yanlin, YIN Jiabin, ZHANG Jisong. Research advancement of continuous reductive amination in microreactors[J]. Chemical Industry and Engineering Progress, 2024, 43(1): 186-197.
| 催化剂 | 模板反应 | 反应条件 | 收率/% | 参考文献 |
|---|---|---|---|---|
| Ru/Cox | 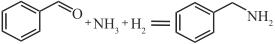 | 90℃,4MPa | 98 | [ |
| Ru1/NC |  | 100℃,2MPa | 97 | [ |
| Ru/TiP | 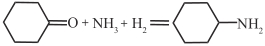 | 30℃,1.4MPa | 97 | [ |
| [Rh(cod)Cl]2 |  | 135℃,6.5MPa | 86 | [ |
| Rh/Al2O3 |  | 80℃,2MPa | 92 | [ |
| Ni/Al2O3 | 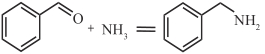 | 80℃,1MPa | 99 | [ |
| Ni y AlO x | 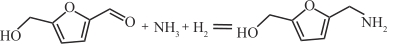 | 100℃,0.1MPa | 99 | [ |
| Ni/SiO2 |  | 70℃,1MPa | 99 | [ |
| Fe/SiC |  | 140℃,6.5MPa | 99 | [ |
| Co/SiC |  | 50℃,1MPa | 99 | [ |
| Pd/C | 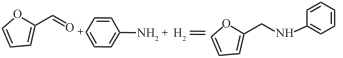 | 80℃,3.5MPa | 100 | [ |
| Pd/NiO |  | 25℃,0.1MPa | 98 | [ |
| Pt/Al2O3 |  | 25℃,0.5MPa | 99 | [ |
| Au/CeO2/TiO2 | 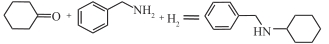 | 100℃,3MPa | 79 | [ |
| Ru(DMP)2Cl2 |  | 60℃,1.2MPa | 98 | [ |
| Co/NC |  | 110℃,1MPa | 98 | [ |
| [Rh(COD)Cl]2 | 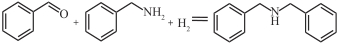 | 100℃,1.4MPa | 86 | [ |
| Cu/AlO x |  | 80℃,1MPa | 99 | [ |
| Cu/SiO2TiO3 | 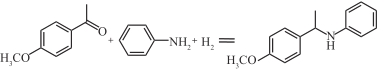 | 100℃,0.1MPa | 97 | [ |
| Pt nanowire |  | 80℃,0.1MPa | 93.3 | [ |
| PtMo nanowire |  | 100℃,0.1MPa | 96.1 | [ |
| Pd/Fe2O3 | 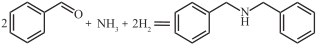 | 0℃,0.1MPa | 96.6 | [ |
表1 常见还原胺化反应中用于高选择性胺类物质合成的案例
| 催化剂 | 模板反应 | 反应条件 | 收率/% | 参考文献 |
|---|---|---|---|---|
| Ru/Cox | 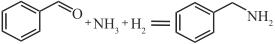 | 90℃,4MPa | 98 | [ |
| Ru1/NC |  | 100℃,2MPa | 97 | [ |
| Ru/TiP | 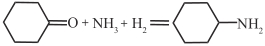 | 30℃,1.4MPa | 97 | [ |
| [Rh(cod)Cl]2 |  | 135℃,6.5MPa | 86 | [ |
| Rh/Al2O3 |  | 80℃,2MPa | 92 | [ |
| Ni/Al2O3 | 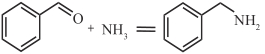 | 80℃,1MPa | 99 | [ |
| Ni y AlO x | 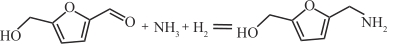 | 100℃,0.1MPa | 99 | [ |
| Ni/SiO2 |  | 70℃,1MPa | 99 | [ |
| Fe/SiC |  | 140℃,6.5MPa | 99 | [ |
| Co/SiC |  | 50℃,1MPa | 99 | [ |
| Pd/C | 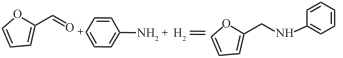 | 80℃,3.5MPa | 100 | [ |
| Pd/NiO |  | 25℃,0.1MPa | 98 | [ |
| Pt/Al2O3 |  | 25℃,0.5MPa | 99 | [ |
| Au/CeO2/TiO2 | 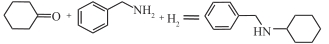 | 100℃,3MPa | 79 | [ |
| Ru(DMP)2Cl2 |  | 60℃,1.2MPa | 98 | [ |
| Co/NC |  | 110℃,1MPa | 98 | [ |
| [Rh(COD)Cl]2 | 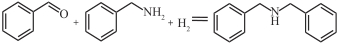 | 100℃,1.4MPa | 86 | [ |
| Cu/AlO x |  | 80℃,1MPa | 99 | [ |
| Cu/SiO2TiO3 | 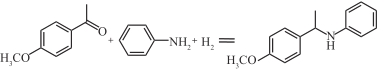 | 100℃,0.1MPa | 97 | [ |
| Pt nanowire |  | 80℃,0.1MPa | 93.3 | [ |
| PtMo nanowire |  | 100℃,0.1MPa | 96.1 | [ |
| Pd/Fe2O3 | 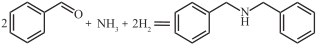 | 0℃,0.1MPa | 96.6 | [ |
| 1 | SMITH M. March’s advanced organic chemistry: Reactions, mechanisms, and structure[M]. 7th Edition. Hoboken, New Jersey: Wiley, 2013 |
| 2 | LINDLEY James. Tetrahedron report number 163[J]. Tetrahedron, 1984, 40(9): 1433-1456. |
| 3 | BELFIELD A J, BROWN G R, FOUBISTER A J. Recent synthetic advances in the nucleophilic amination of benzenes[J]. Tetrahedron, 1999, 55(38): 11399-11428. |
| 4 | AFANASYEV O I, KUCHUK E, USANOV D L, et al. Reductive amination in the synthesis of pharmaceuticals[J]. Chemical Reviews, 2019, 119(23): 11857-11911. |
| 5 | TOKMIC K, JACKSON B J, SALAZAR A, et al. Cobalt-catalyzed and lewis acid-assisted nitrile hydrogenation to primary amines: A combined effort[J]. Journal of the American Chemical Society, 2017, 139(38): 13554-13561. |
| 6 | BAGAL D B, BHANAGE B M. Recent advances in transition metal-catalyzed hydrogenation of nitriles[J]. Advanced Synthesis and Catalysis, 2015, 357(5): 883-900. |
| 7 | TOMKINS Patrick, Ewa GEBAUER-HENKE, LEITNER Walter, et al. Concurrent hydrogenation of aromatic and nitro groups over carbon-supported ruthenium catalysts[J]. ACS Catalysis, 2015, 5(1): 203-209. |
| 8 | SARMAH P P, DUTTA D K. Chemoselective reduction of a nitro group through transfer hydrogenation catalysed by Ru0-nanoparticles stabilized on modified montmorillonite clay[J]. Green Chemistry, 2012, 14(4): 1086-1093. |
| 9 | LANG Fengrui, ZEWGE Daniel, HOUPIS Ioannis N, et al. Amination of aryl halides using copper catalysis[J]. Tetrahedron Letters, 2001, 42(19): 3251-3254. |
| 10 | AUBIN Y, FISCHMEISTER C, THOMAS C M, et al. Direct amination of aryl halides with ammonia[J]. Chemical Society Reviews, 2010, 39(11): 4130-4145. |
| 11 | GOMEZ S, J A PETERS, MASCHMEYER T. The reductive amination of aldehydes and ketones and the hydrogenation of nitriles: Mechanistic aspects and selectivity control[J]. Advanced Synthesis & Catalysis, 2002, 344(10): 1037-1057. |
| 12 | YUAN Ziliang, LIU Bing, ZHOU Peng, et al. Preparation of nitrogen-doped carbon supported cobalt catalysts and its application in the reductive amination[J]. Journal of Catalysis, 2019, 370: 347-356. |
| 13 | MURUGESAN K, SENTHAMARAI T, CHANDRASHEKHAR V G, et al. Catalytic reductive aminations using molecular hydrogen for synthesis of different kinds of amines[J]. Chemical Society Reviews, 2020, 49(17): 6273-6328. |
| 14 | IRRGANG Torsten, KEMPE Rhett. Transition-metal-catalyzed reductive amination employing hydrogen[J]. Chemical Reviews, 2020, 120(17): 9583-9674. |
| 15 | HAHN G, KUNNAS P, DE JONGE N, et al. General synthesis of primary amines via reductive amination employing a reusable nickel catalyst[J]. Nature Catalysis, 2019, 2(1): 71-77. |
| 16 | QI Haifeng, YANG Ji, LIU Fei, et al. Highly selective and robust single-atom catalyst Ru1/NC for reductive amination of aldehydes/ketones[J]. Nature Communications, 2021, 12: 3295. |
| 17 | HEINEN A W, PETERS J A, VAN BEKKUM H. The reductive amination of benzaldehyde over Pd/C catalysts: Mechanism and effect of carbon modifications on the selectivity[J]. European Journal of Organic Chemistry, 2000, 2000(13): 2501-2506. |
| 18 | LU Shuanglong, XU Pengyao, CAO Xueqin, et al. A highly active worm-like PtMo nanowire for the selective synthesis of dibenzylamines[J]. RSC Advances, 2018, 8(16): 8755-8760. |
| 19 | FROST C G, MUTTON L. Heterogeneous catalytic synthesis using microreactor technology[J]. Green Chemistry, 2010, 12(10): 1687-1703. |
| 20 | 骆广生, 王凯, 王玉军, 等. 微化工系统的原理和应用[J]. 化工进展, 2011, 30(8): 1637-1642. |
| LUO Guangsheng, WANG Kai, WANG Yujun, et al. Principles and applications of micro-structured chemical system[J]. Chemical Industry and Engineering Progress, 2011, 30(8): 1637-1642. | |
| 21 | MASON B P, PRICE K E, STEINBACHER J L, et al. Greener approaches to organic synthesis using microreactor technology[J]. Chemical Reviews, 2007, 107(6): 2300-2318. |
| 22 | CHAMBERS R D, SPINK R C H. Microreactors for elemental fluorine[J]. Chemical Communications, 1999(10): 883-884. |
| 23 | NAGY K D, SHEN Bo, JAMISON T F, et al. Mixing and dispersion in small-scale flow systems[J]. Organic Process Research & Development, 2012, 16(5): 976-981. |
| 24 | 章承浩, 罗京, 张吉松. 微反应器内基于氮氧自由基催化剂连续氧气/空气氧化反应的研究进展[J]. 化工学报, 2023, 74(2): 511-524. |
| ZHANG Chenghao, LUO Jing, ZHANG Jisong. Advances in continuous aerobic oxidation based on nitroxyl radical catalyst in microreactors[J]. CIESC Journal, 2023, 74(2): 511-524. | |
| 25 | 屠佳成, 桑乐, 艾宁, 等. 连续微反应加氢技术在有机合成中的研究进展[J]. 化工学报, 2019, 70(10): 3859-3868. |
| TU Jiacheng, SANG Le, AI Ning, et al. Research progress of continuous hydrogenation in organic synthesis[J]. CIESC Journal, 2019, 70(10): 3859-3868. | |
| 26 | GENET C, NGUYEN X, BAYATSARMADI B, et al. Reductive aminations using a 3D printed supported metal(0) catalyst system[J]. Journal of Flow Chemistry, 2018, 8(2): 81-88. |
| 27 | GOMEZ S, PETERS J A, VAN DER WAAL J C, et al. Preparation of benzylamine by highly selective reductive amination of benzaldehyde over Ru on an acidic activated carbon support as the catalyst[J]. Catalysis Letters, 2002, 84(1): 1-5. |
| 28 | DONG Chenglong, WU Yushan, WANG Hongtao, et al. Facile and efficient synthesis of primary amines via reductive amination over a Ni/Al2O3 catalyst[J]. ACS Sustainable Chemistry & Engineering, 2021, 9(21): 7318-7327. |
| 29 | GOMEZ S, PETERS J A, VAN DER WAAL J C, et al. The rationalization of catalyst behaviour in the reductive amination of benzaldehyde with ammonia using a simple computer model[J]. Applied Catalysis A: General, 2004, 261(1): 119-125. |
| 30 | LUO Dan, HE Yurong, YU Xin, et al. Intrinsic mechanism of active metal dependent primary amine selectivity in the reductive amination of carbonyl compounds[J]. Journal of Catalysis, 2021, 395: 293-301. |
| 31 | GOULD N S, LANDFIELD H, DINKELACKER B, et al. Selectivity control in catalytic reductive amination of furfural to furfurylamine on supported catalysts[J]. ChemCatChem, 2020, 12(7): 2106-2115. |
| 32 | XIE Chao, SONG Jinliang, HUA Manli, et al. Ambient-temperature synthesis of primary amines via reductive amination of carbonyl compounds[J]. ACS Catalysis, 2020, 10(14): 7763-7772. |
| 33 | GROSS Thoralf, SEAYAD Abdul Majeed, AHMAD Moballigh, et al. Synthesis of primary amines: First homogeneously catalyzed reductive amination with ammonia[J]. Organic Letters, 2002, 4(12): 2055-2058. |
| 34 | CHATTERJEE Maya, ISHIZAKA Takayuki, KAWANAMI Hajime. Reductive amination of furfural to furfurylamine using aqueous ammonia solution and molecular hydrogen: An environmentally friendly approach[J]. Green Chemistry, 2016, 18(2): 487-496. |
| 35 | EMERSON W S. The preparation of amines by reductive alkylation[M]. New Yourk: Wiley, 2011: 174-255. |
| 36 | KRUPKA Jiri, Libor DLUHOŠ, Lech MRÓZEK. Evaluation of benzylamine production via reductive amination of benzaldehyde in a slurry reactor[J]. Chemical Engineering & Technology, 2017, 40(5): 870-877. |
| 37 | YUAN Hangkong, LI Jerry-Peng, SU Fangzheng, et al. Reductive amination of furanic aldehydes in aqueous solution over versatile Ni y AlO x catalysts[J]. ACS Omega, 2019, 4(2): 2510-2516. |
| 38 | ZHANG Jiahao, YIN Jiabin, DUAN Xiaonan, et al. Continuous reductive amination to synthesize primary amines with high selectivity in flow[J]. Journal of Catalysis, 2023, 420: 89-98. |
| 39 | Christoph BÄUMLER, BAUER Christof, KEMPE Rhett. The synthesis of primary amines through reductive amination employing an iron catalyst[J]. ChemSusChem, 2020, 13(12): 3110-3114. |
| 40 | ELFINGER Matthias, Timon SCHÖNAUER, THOMÄ Sabrina L J, et al. Co-catalyzed synthesis of primary amines via reductive amination employing hydrogen under very mild conditions[J]. ChemSusChem, 2021, 14(11): 2360-2366. |
| 41 | GARCÍA-ORTIZ A, VIDAL J D, CLIMENT M J, et al. Chemicals from biomass: Selective synthesis of N-substituted furfuryl amines by the one-pot direct reductive amination of furanic aldehydes[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(6): 6243-6250. |
| 42 | YANG Huimin, CUI Xinjiang, DENG Youquan, et al. Reductive amination of aldehydes and amines with an efficient Pd/NiO catalyst[J]. Synthetic Communications, 2014, 44(9): 1314-1322. |
| 43 | NUZHDIN A L, SIMONOV P A, BUKHTIYAROVA G A, et al. Reductive amination of 5-acetoxymethylfurfural over Pt/Al2O3 catalyst in a flow reactor[J]. Molecular Catalysis, 2021, 499: 111297. |
| 44 | LAROCHE Benjamin, ISHITANI Haruro, KOBAYASHI Shū. Direct reductive amination of carbonyl compounds with H2 using heterogeneous catalysts in continuous flow as an alternative to N-alkylation with alkyl halides[J]. Advanced Synthesis & Catalysis, 2018, 360(24): 4699-4704. |
| 45 | GAO Liang, LAI Liangchuan, YE Baijun, et al. Continuous-flow synthesis of N, N'-bis(2, 2, 6, 6-tetramethyl-4-piperidinyl)-1, 6-hexanediamine (DTMPA) in a micro fixed-bed reactor[J]. Journal of Flow Chemistry, 2022, 12(4): 419-427. |
| 46 | KOLOBOVA E, MÄKI-ARVELA P, PESTRYAKOV A, et al. Reductive amination of ketones with benzylamine over gold supported on different oxides[J]. Catalysis Letters, 2019, 149(12): 3432-3446. |
| 47 | XU Zhanwei, YAN Peifang, XU Wenjuan, et al. Direct reductive amination of 5-hydroxymethylfurfural with primary/secondary amines via Ru-complex catalyzed hydrogenation[J]. RSC Advances, 2014, 4(103): 59083-59087. |
| 48 | MAO Fei, SUI Dejun, QI Zhengliang, et al. Heterogeneous cobalt catalysts for reductive amination with H2: General synthesis of secondary and tertiary amines[J]. RSC Advances, 2016, 6(96): 94068-94073. |
| 49 | ROBICHAUD André, NAIT AJJOU Abdelaziz. First example of direct reductive amination of aldehydes with primary and secondary amines catalyzed by water-soluble transition metal catalysts[J]. Tetrahedron Letters, 2006, 47(22): 3633-3636. |
| 50 | NUZHDIN A L, BUKHTIYAROVA M V, BUKHTIYAROV V I. Two-step one-pot reductive amination of furanic aldehydes using CuAlO x catalyst in a flow reactor[J]. Molecules, 2020, 25(20): 4771. |
| 51 | SANTORO Federica, PSARO Rinaldo, RAVASIO Nicoletta, et al. Reductive amination of ketones or amination of alcohols over heterogeneous Cu catalysts: Matching the catalyst support with the N-alkylating agent[J]. ChemCatChem, 2012, 4(9): 1249-1254. |
| 52 | QI Fenqiang, HU Lei, LU Shuanglong, et al. Selective synthesis of secondary amines by Pt nanowire catalyzed reductive amination of aldehydes and ketones with ammonia[J]. Chemical Communications, 2012, 48(77): 9631-9633. |
| 53 | YUAN Ziliang, ZHOU Peng, LIU Xixi, et al. Mild and selective synthesis of secondary amines direct from the coupling of two aldehydes with ammonia[J]. Industrial & Engineering Chemistry Research, 2017, 56(50): 14766-14770. |
| 54 | SONG Song, WANG Yunzhu, YAN Ning. A remarkable solvent effect on reductive amination of ketones[J]. Molecular Catalysis, 2018, 454: 87-93. |
| 55 | VIDAL J D, CLIMENT M J, CONCEPCION P, et al. Chemicals from biomass: Chemoselective reductive amination of ethyl levulinate with amines[J]. ACS Catalysis, 2015, 5(10): 5812-5821. |
| 56 | WU Hongguo, YU Zhaozhuo, LI Yan, et al. Hot water-promoted catalyst-free reductive cycloamination of (bio-) keto acids with HCOONH4 toward cyclic amides[J]. The Journal of Supercritical Fluids, 2020, 157: 104698. |
| 57 | MA Tengfei, ZHANG Hongyu, YIN Guohui, et al. Catalyst-free reductive amination of levulinic acid to N-substituted pyrrolidinones with formic acid in continuous-flow microreactor[J]. Journal of Flow Chemistry, 2018, 8(1): 35-43. |
| 58 | CHRISTIE Francesca, Antonio ZANOTTI-GEROSA, GRAINGER Damian. Hydrogenation and reductive amination of aldehydes using triphos ruthenium catalysts[J]. ChemCatChem, 2018, 10(5): 1012-1018. |
| 59 | HUANG Haizhou, LIU Xiaoyan, ZHOU Le, et al. Direct asymmetric reductive amination for the synthesis of chiral β-arylamines[J]. Angewandte Chemie, 2016, 128(17): 5395-5398. |
| 60 | CHEN Yuzhen, ZHOU Yuxiao, WANG Hengwei, et al. Multifunctional PdAg@MIL-101 for one-pot cascade reactions: Combination of host-guest cooperation and bimetallic synergy in catalysis[J]. ACS Catalysis, 2015, 5(4): 2062-2069. |
| 61 | SAINI Ms Kanika, KUMAR Sahil, LI Hu, et al. Advances in the catalytic reductive amination of furfural to furfural amine: The momentous role of active metal sites[J]. ChemSusChem, 2022, 15(7): e202200107. |
| 62 | DENG Dian, KITA Yusuke, KAMATA Keigo, et al. Low-temperature reductive amination of carbonyl compounds over Ru deposited on Nb2O5·nH2O[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(5): 4692-4698. |
| 63 | NUZHDIN A L, BUKHTIYAROVA M V, BUKHTIYAROVA G A. Cu-Al mixed oxide derived from layered double hydroxide as an efficient catalyst for continuous-flow reductive amination of aromatic aldehydes[J]. Journal of Chemical Technology & Biotechnology, 2020, 95(12): 3292-3299. |
| 64 | CAO Pengwei, MA Tengfei, ZHANG Hongyu, et al. Conversion of levulinic acid to N-substituted pyrrolidinones over a nonnoble bimetallic catalyst Cu15Pr3/Al2O3 [J]. Catalysis Communications, 2018, 116: 85-90. |
| 65 | BOMANN M D, GUCH I C, DIMARE M. A mild, pyridine-borane-based reductive amination protocol[J]. The Journal of Organic Chemistry, 1995, 60(18): 5995-5996. |
| 66 | LE Ngoc-Thuc, BYUN Areum, HAN Yohan, et al. Preparation of 2,5-bis(aminomethyl)furan by direct reductive amination of 2,5-diformylfuran over nickel-raney catalysts[J]. Green and Sustainable Chemistry, 2015, 5(3): 115-127. |
| 67 | ENTHALER Stephan. Synthesis of secondary amines by iron-catalyzed reductive amination[J]. ChemCatChem, 2010, 2(11): 1411-1415. |
| 68 | BLANDEN A R, MUKHERJEE K, DILEK O, et al. 4-Aminophenylalanine as a biocompatible nucleophilic catalyst for hydrazone ligations at low temperature and neutral pH[J]. Bioconjugate Chemistry, 2011, 22(10): 1954-1961. |
| 69 | 朱建新. 醛/酮与有机胺的缩合反应研究[D]. 南京: 南京大学, 2018. |
| ZHU Jianxin. Studies on the condensation reactions of aldehydes/ketones with amines[D]. Nanjing: Nanjing University, 2018. | |
| 70 | LEI Qian, WEI Yawen, TALWAR Dinesh, et al. Fast reductive amination by transfer hydrogenation “on water”[J]. Chemistry – A European Journal, 2013, 19(12): 4021-4029. |
| 71 | LAWRENCE S A. Amines: Synthesis, properties and applications[M]. Cambridge, UK: Cambridge University Press, 2004. |
| 72 | SALVATORE R N, YOON C H, JUNG K W. Synthesis of secondary amines[J]. Tetrahedron, 2001, 57(37): 7785-7811. |
| 73 | POLIDORO Daniele, Daily RODRIGUEZ-PADRON, PEROSA Alvise, et al. Chitin-derived nanocatalysts for reductive amination reactions[J]. Materials, 2023, 16(2): 575. |
| 74 | CHIEFFI Gianpaolo, BRAUN Max, ESPOSITO Davide. Continuous reductive amination of biomass-derived molecules over carbonized filter paper-supported FeNi alloy[J]. ChemSusChem, 2015, 8(21): 3590-3594. |
| 75 | WANG Y, NUZHDIN A L, SHAMANAEV I V, et al. Flow synthesis of N-alkyl-5-methyl-2-pyrrolidones over Ni2P/SiO2 catalyst[J]. Molecular Catalysis, 2021, 515: 111884. |
| 76 | WANG Y, NUZHDIN A L, SHAMANAEV I V, et al. Effect of phosphorus precursor, reduction temperature, and support on the catalytic properties of nickel phosphide catalysts in continuous-flow reductive amination of ethyl levulinate[J]. International Journal of Molecular Sciences, 2022, 23(3): 1106. |
| 77 | GUNANATHAN Chidambaram, MILSTEIN David. Selective synthesis of primary amines directly from alcohols and ammonia[J]. Angewandte Chemie, 2008, 120(45): 8789-8792. |
| 78 | FALUS Péter, BOROS Zoltn, Gbor HORNYÁNSZKY, et al. Reductive amination of ketones: Novel one-step transfer hydrogenations in batch and continuous-flow mode[J]. Tetrahedron Letters, 2011, 52(12): 1310-1312. |
| 79 | LI Xinyue, NISHIMURA Shun. Synthesis of 5-hydroxymethyl-2-furfurylamine via reductive amination of 5-hydroxymethyl-2-furaldehyde with supported Ni-Co bimetallic catalysts[J]. Catalysis Letters, 2022: 1-8. |
| 80 | ZHANG Jiahao, YIN Jiabin, DUAN Xiaonan, et al. Continuous reductive amination of carbonyl compounds with ammonia to synthesize secondary amines with high selectivity[J]. Journal of Catalysis, 2023, 427: 115123. |
| 81 | ARTIUKHA E A, NUZHDIN A L, BUKHTIYAROVA Galina A, et al. Flow synthesis of secondary amines over Ag/Al2O3 catalyst by one-pot reductive amination of aldehydes with nitroarenes[J]. RSC Advances, 2017, 7(72): 45856-45861. |
| 82 | ARTIUKHA E A, NUZHDIN A L, BUKHTIYAROVA G A, et al. One-pot reductive amination of aldehydes with nitroarenes over an Au/Al2O3 catalyst in a continuous flow reactor[J]. Catalysis Science & Technology, 2015, 5(10): 4741-4745. |
| 83 | NUZHDIN A L, ARTIUKHA E A, BUKHTIYAROVA G A, et al. Synthesis of unsaturated secondary amines by direct reductive amination of aliphatic aldehydes with nitroarenes over Au/Al2O3 catalyst in continuous flow mode[J]. RSC Advances, 2016, 6(91): 88366-88372. |
| 84 | NUZHDIN A L, ARTIUKHA E A, BUKHTIYAROVA G A, et al. Synthesis of secondary amines by reductive amination of aldehydes with nitroarenes over supported copper catalysts in a flow reactor[J]. Catalysis Communications, 2017, 102: 108-113. |
| 85 | MANGAS-SANCHEZ J, SHARMA M, COSGROVE S C, et al. Asymmetric synthesis of primary amines catalyzed by thermotolerant fungal reductive aminases[J]. Chemical Science, 2020, 11(19): 5052-5057. |
| 86 | CROCI F, VILÍM J, ADAMOPOULOU T, et al. Continuous flow biocatalytic reductive amination by co‐entrapping dehydrogenases with agarose gel in a 3D‐printed mould reactor[J]. ChemBioChem, 2022, 23(22): e202200549. |
| 87 | KIM Hong Won, BYUN Sangmoon, KIM Seong Min, et al. Simple reversible fixation of a magnetic catalyst in a continuous flow system: Ultrafast reduction of nitroarenes and subsequent reductive amination using ammonia borane[J]. Catalysis Science & Technology, 2020, 10(4): 944-949. |
| 88 | GILMORE K, VUKELIĆ S, MCQUADE D T, et al. Continuous reductions and reductive aminations using solid NaBH4 [J]. Organic Process Research & Development, 2014, 18(12): 1771-1776. |
| [1] | 王达锐, 孙洪敏, 王一棪, 唐智谋, 李芮, 范雪研, 杨为民. 分子筛催化反应过程高效化的技术进展[J]. 化工进展, 2024, 43(1): 1-18. |
| [2] | 苏梦军, 刘剑, 辛靖, 陈禹霏, 张海洪, 韩龙年, 朱元宝, 李洪宝. 气液混合强化在固定床加氢过程中的应用进展[J]. 化工进展, 2024, 43(1): 100-110. |
| [3] | 罗芬, 杨晓琪, 段方麟, 李小江, 吴亮, 徐铜文. 双极膜研究进展及应用展望[J]. 化工进展, 2024, 43(1): 145-163. |
| [4] | 盖宏伟, 张辰君, 屈晶莹, 孙怀禄, 脱永笑, 王斌, 金旭, 张茜, 冯翔, CHEN De. 有机液体储氢技术催化脱氢过程强化研究进展[J]. 化工进展, 2024, 43(1): 164-185. |
| [5] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [6] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [7] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [8] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [9] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [10] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [11] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [12] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| [13] | 张启, 赵红, 荣峻峰. 质子交换膜燃料电池中氧还原反应抗毒性电催化剂研究进展[J]. 化工进展, 2023, 42(9): 4677-4691. |
| [14] | 王伟涛, 鲍婷玉, 姜旭禄, 何珍红, 王宽, 杨阳, 刘昭铁. 醛酮树脂基非金属催化剂催化氧气氧化苯制备苯酚[J]. 化工进展, 2023, 42(9): 4706-4715. |
| [15] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||