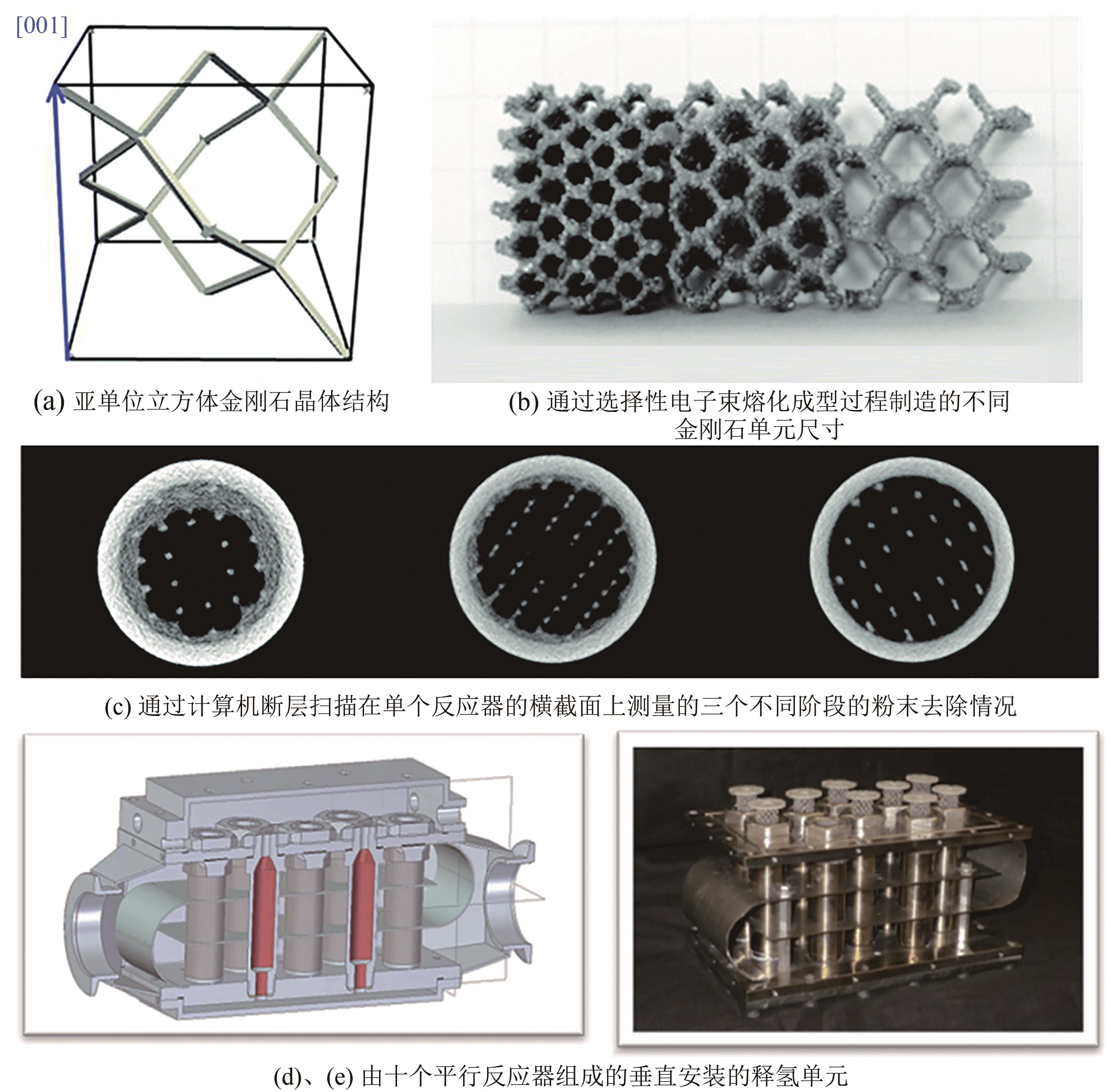
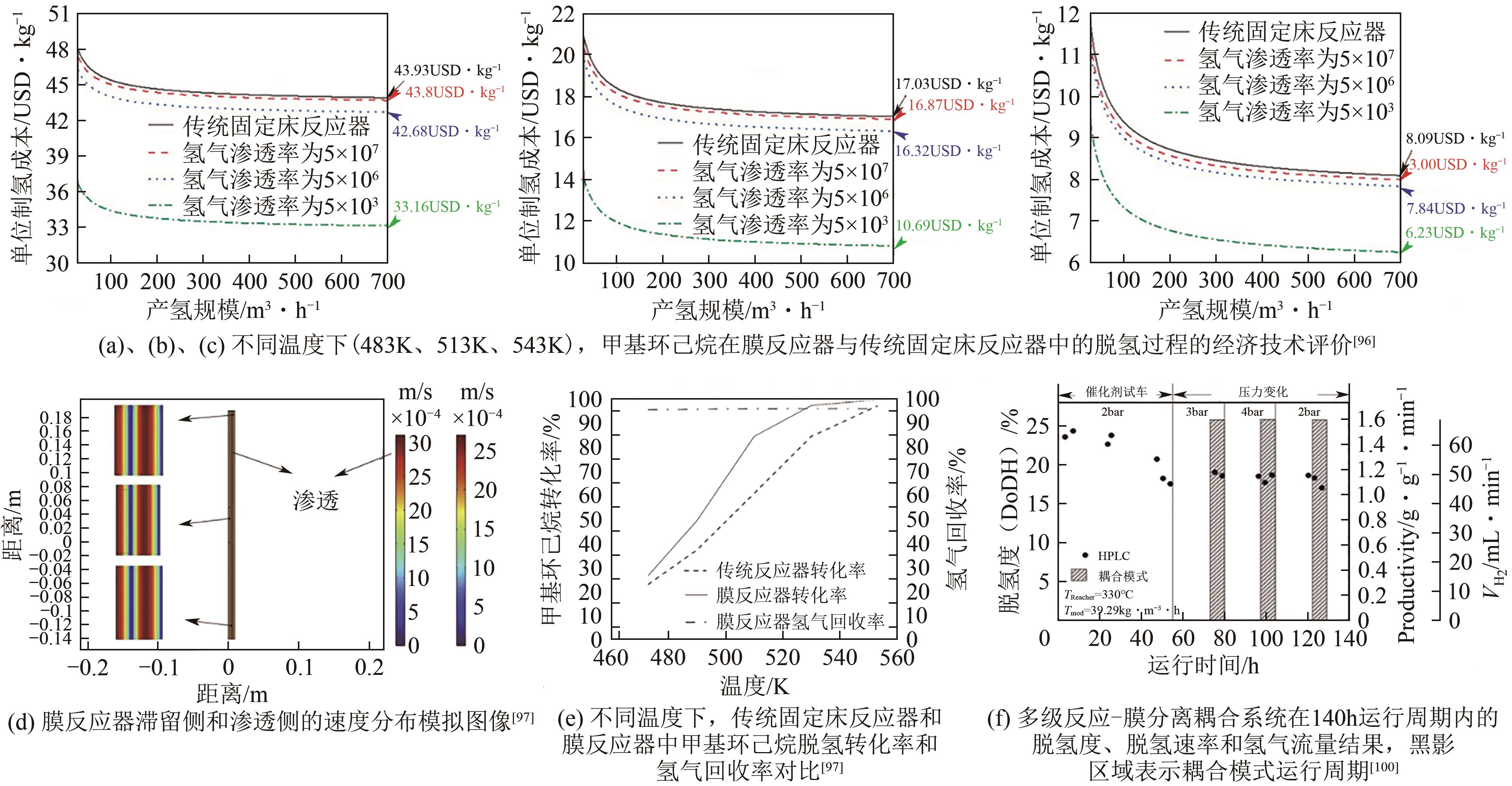
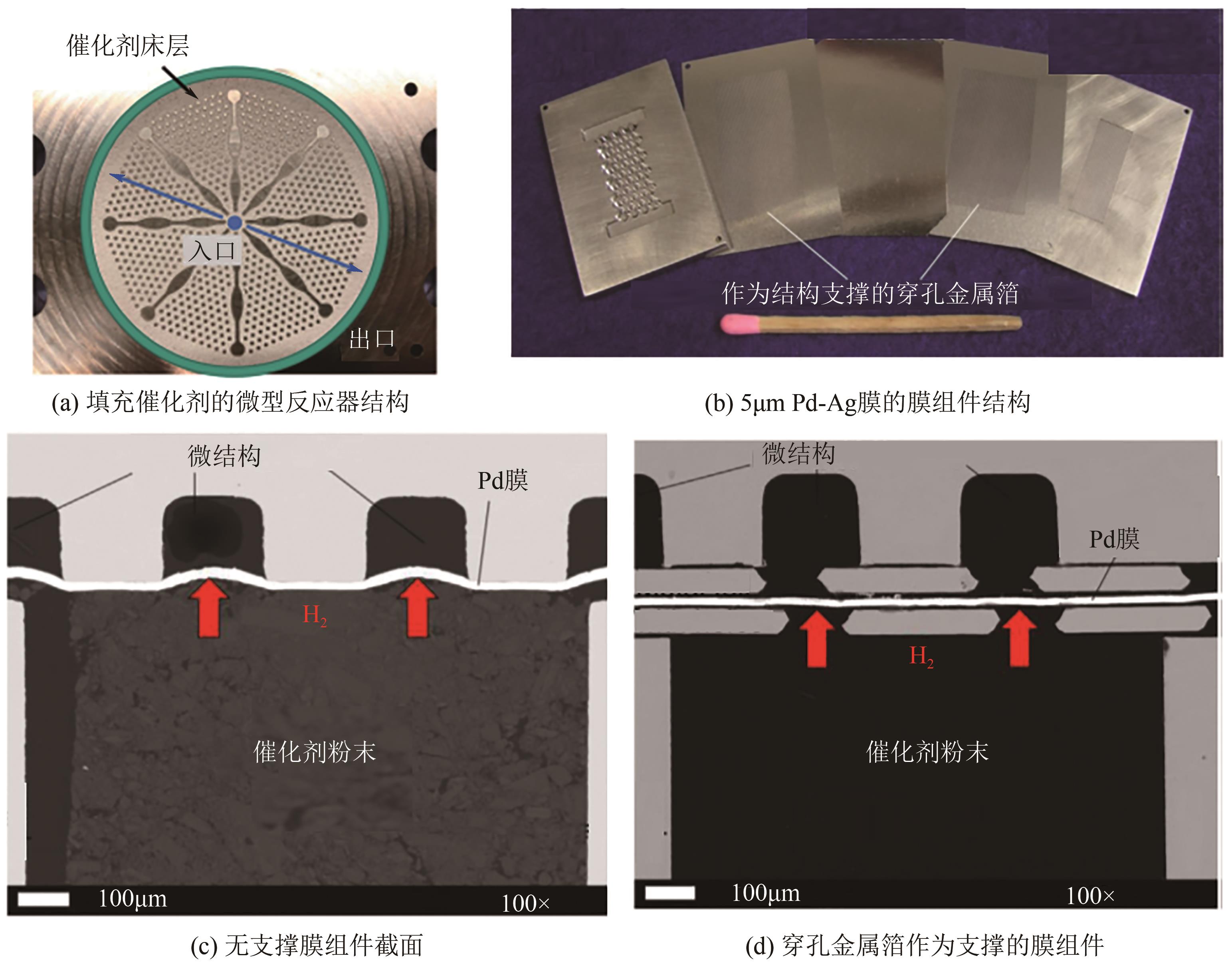
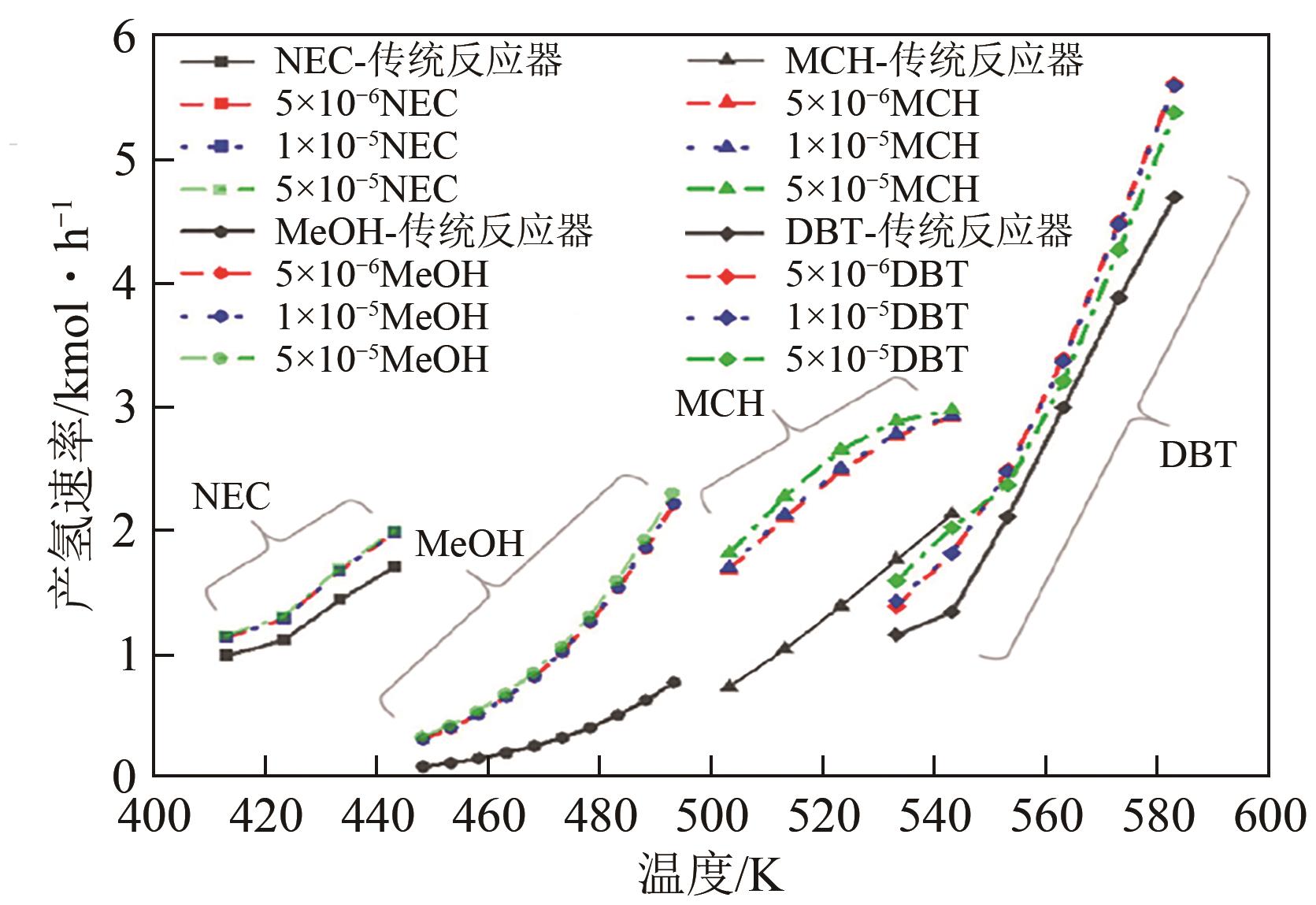
化工进展 ›› 2024, Vol. 43 ›› Issue (1): 164-185.DOI: 10.16085/j.issn.1000-6613.2023-1265
• 专栏:化工过程强化 • 上一篇
有机液体储氢技术催化脱氢过程强化研究进展
盖宏伟1( ), 张辰君2, 屈晶莹3, 孙怀禄3, 脱永笑1(
), 张辰君2, 屈晶莹3, 孙怀禄3, 脱永笑1( ), 王斌4, 金旭2, 张茜2, 冯翔3(
), 王斌4, 金旭2, 张茜2, 冯翔3( ), CHEN De1,5
), CHEN De1,5
- 1.中国石油大学(华东)新能源学院,山东 青岛 266580
2.中国石油天然气股份有限公司勘探开发研究院,北京 100083
3.中国石油大学(华东)化学化工学院,山东 青岛 266580
4.西安交通大学化学工程与技术学院,陕西 西安 710049
5.挪威科技大学化学工程系,挪威 特隆赫姆N -7491
-
收稿日期:2023-07-23修回日期:2023-11-07出版日期:2024-01-20发布日期:2024-02-05 -
通讯作者:脱永笑,冯翔 -
作者简介:盖宏伟(2000—),男,硕士研究生,研究方向为有机储氢介质催化脱氢过程强化。E-mail:2634617620@qq.com。 -
基金资助:国家自然科学基金(22208374);山东省自然科学基金(ZR2020QB173);中国石油科技创新基金(2022DQ02-0607)
Research progress on catalytic dehydrogenation process intensification for liquid organic hydride carrier hydrogen storage
GAI Hongwei1( ), ZHANG Chenjun2, QU Jingying3, SUN Huailu3, TUO Yongxiao1(
), ZHANG Chenjun2, QU Jingying3, SUN Huailu3, TUO Yongxiao1( ), WANG Bin4, JIN Xu2, ZHANG Xi2, FENG Xiang3(
), WANG Bin4, JIN Xu2, ZHANG Xi2, FENG Xiang3( ), CHEN De1,5
), CHEN De1,5
- 1.College of New Energy, China University of Petroleum (East China), Qingdao 266580, Shangdong, China
2.Research Institute of Petroleum Exploration & Development, China National Petroleum Corporation, Beijing 100083, China
3.College of Chemistry and Chemical Engineering, China University of Petroleum (East China), Qingdao 266580, Shangdong, China
4.College of Chemical Engineering and Technology, Xi'an Jiaotong University, Xi'an 710049, Shaanxi
5.Department of Chemical Engineering, Norwegian University of Science and Technology, Trondheim N -7491, Norway
-
Received:2023-07-23Revised:2023-11-07Online:2024-01-20Published:2024-02-05 -
Contact:TUO Yongxiao, FENG Xiang
摘要:
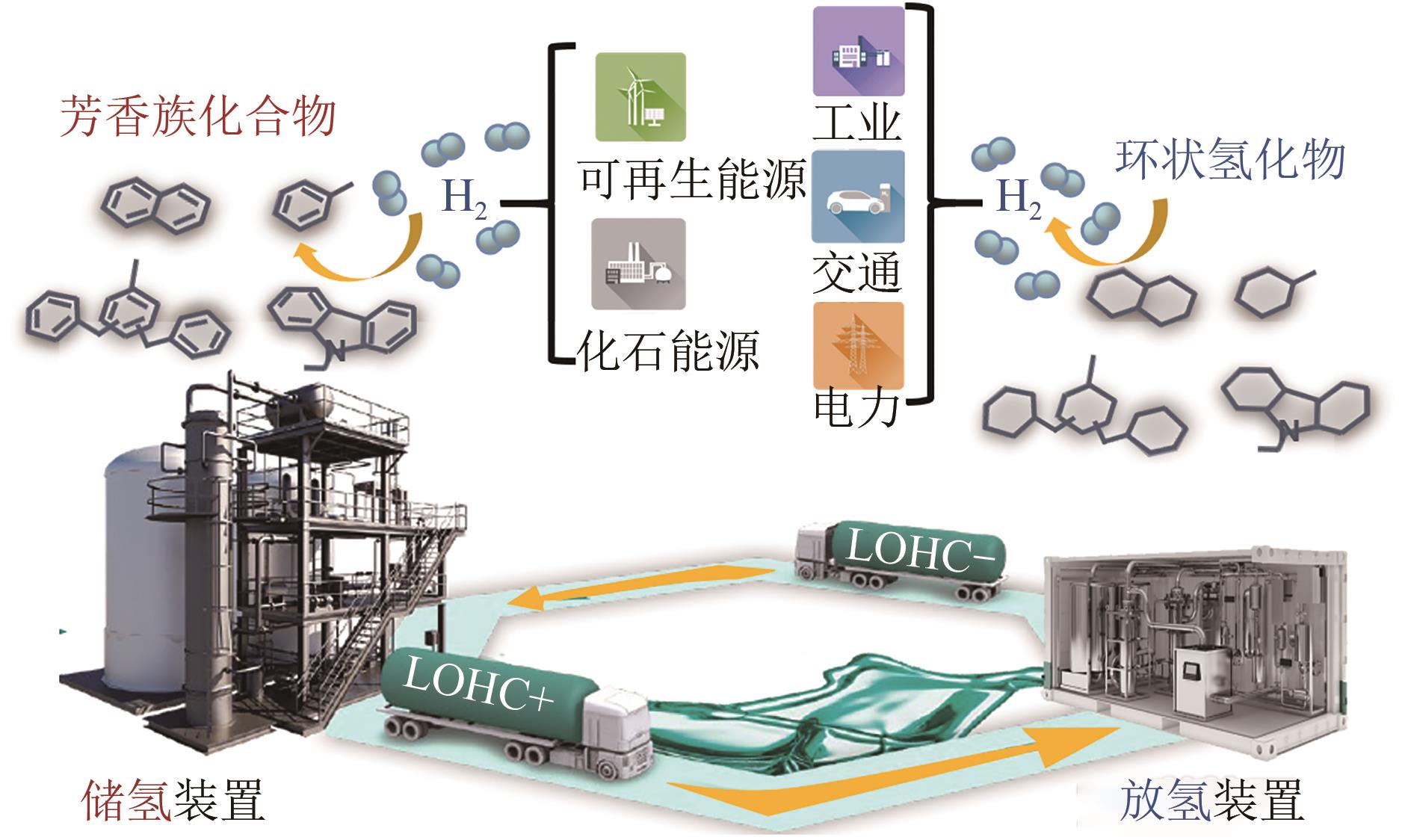
氢能是实现化石能源清洁高效利用和支撑可再生能源大规模发展的理想互联媒介,然而氢的储运是制约氢能规模化应用的关键技术瓶颈。有机氢化物(LOHC)储氢技术具有成本低、储氢密度大、安全稳定等优势,可匹配现有化石能源输运架构,有望在大规模、长距离和分布式的氢储运场景中发挥重要作用。但是,在LOHC储氢循环中,相对于发展较为成熟的加氢技术,LOHC脱氢过程效率低、稳定性差,是制约该技术发展的关键。基于此,本文综述了LOHC储氢技术催化脱氢过程强化的研究进展和发展趋势,概述了LOHC储氢基本概念和催化脱氢反应基本原理,从催化过程强化、产物分离强化、能量效率强化等方面总结了脱氢过程强化策略,通过对比不同技术手段的特点,分析了LOHC储氢技术催化脱氢过程目前亟需解决的难题,即开发高效的脱氢催化剂、提高催化脱氢过程的传热传质效率以及降低脱氢过程能耗,这对LOHC储氢技术的实际应用具有重要的参考和借鉴意义。
中图分类号:
引用本文
盖宏伟, 张辰君, 屈晶莹, 孙怀禄, 脱永笑, 王斌, 金旭, 张茜, 冯翔, CHEN De. 有机液体储氢技术催化脱氢过程强化研究进展[J]. 化工进展, 2024, 43(1): 164-185.
GAI Hongwei, ZHANG Chenjun, QU Jingying, SUN Huailu, TUO Yongxiao, WANG Bin, JIN Xu, ZHANG Xi, FENG Xiang, CHEN De. Research progress on catalytic dehydrogenation process intensification for liquid organic hydride carrier hydrogen storage[J]. Chemical Industry and Engineering Progress, 2024, 43(1): 164-185.
| LOHC | 全氢化物熔点/℃ | 全氢化物沸点/℃ | 质量储氢密度(%) /体积储氢密度(kg/m3) | 全氢化物 脱氢温度/℃ | 反应方程式 |
|---|---|---|---|---|---|
| 苯 | 6.5 | 80.7 | 7.2/55.9 | 300~320 |  |
| 甲苯 | -126.6 | 101 | 6.2/47.4 | 300~350 | 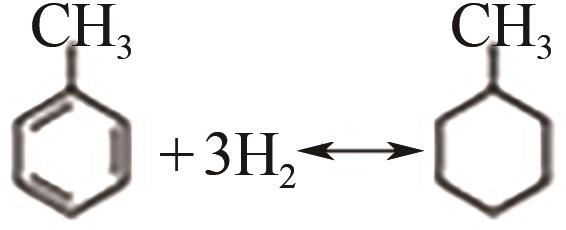 |
| 萘 | -31 | 187 | 7.3/65.4 | 320~340 |  |
| 联苯 | 3 | 227 | 7.27/— | 310~330 |  |
| 二苯基甲烷 | -18.6 | 153 | 6.66/— | 340~360 | 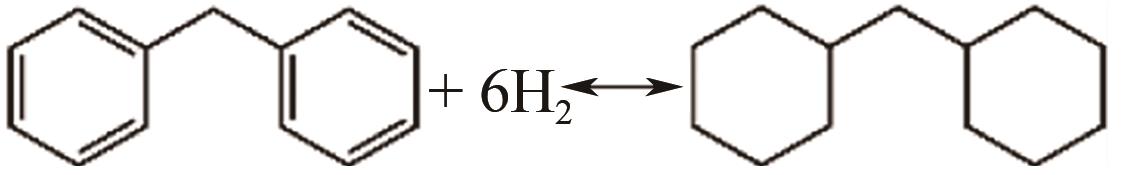 |
| 咔唑 | 65 | 124 | 6.7/— | 150~170 |  |
| N-乙基咔唑 | -84.5 | — | 5.8/— | 170~200 |  |
| 二苄基甲苯 | -39 | 390 | 6.2/5.7 | 260~310 |  |
表1 常见的LOHC体系
| LOHC | 全氢化物熔点/℃ | 全氢化物沸点/℃ | 质量储氢密度(%) /体积储氢密度(kg/m3) | 全氢化物 脱氢温度/℃ | 反应方程式 |
|---|---|---|---|---|---|
| 苯 | 6.5 | 80.7 | 7.2/55.9 | 300~320 |  |
| 甲苯 | -126.6 | 101 | 6.2/47.4 | 300~350 | 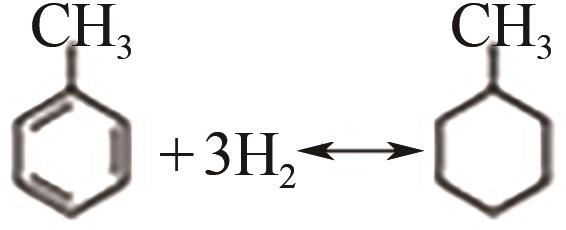 |
| 萘 | -31 | 187 | 7.3/65.4 | 320~340 |  |
| 联苯 | 3 | 227 | 7.27/— | 310~330 |  |
| 二苯基甲烷 | -18.6 | 153 | 6.66/— | 340~360 |  |
| 咔唑 | 65 | 124 | 6.7/— | 150~170 |  |
| N-乙基咔唑 | -84.5 | — | 5.8/— | 170~200 |  |
| 二苄基甲苯 | -39 | 390 | 6.2/5.7 | 260~310 |  |
| 催化剂载体 | 传质因子JD | 气相传质系数kg/mm·s-1 | 外扩散判据ηe·Da | 有效扩散系数De/mm2·g-1 | 内扩散判据φ2·ηi |
|---|---|---|---|---|---|
| Ni-C2H6 | 3.50 | 7.07 | 9.07×10-5 | 8.98 | 2.63×10-7 |
| Fe-CO | 3.74 | 7.32 | 1.79×10-4 | 8.46 | 7.18×10-7 |
| AC球-A | 2.03 | 4.06 | 3.65×10-3 | 1.37 | 0.02 |
| AC球-B | 2.04 | 4.04 | 5.94×10-2 | 1.37 | 0.31 |
表2 四种催化剂载体传质计算结果
| 催化剂载体 | 传质因子JD | 气相传质系数kg/mm·s-1 | 外扩散判据ηe·Da | 有效扩散系数De/mm2·g-1 | 内扩散判据φ2·ηi |
|---|---|---|---|---|---|
| Ni-C2H6 | 3.50 | 7.07 | 9.07×10-5 | 8.98 | 2.63×10-7 |
| Fe-CO | 3.74 | 7.32 | 1.79×10-4 | 8.46 | 7.18×10-7 |
| AC球-A | 2.03 | 4.06 | 3.65×10-3 | 1.37 | 0.02 |
| AC球-B | 2.04 | 4.04 | 5.94×10-2 | 1.37 | 0.31 |
| 强化策略 | 传热、传质性能 | 脱氢效率分析 | 评价 |
|---|---|---|---|
| 催化过程强化 | |||
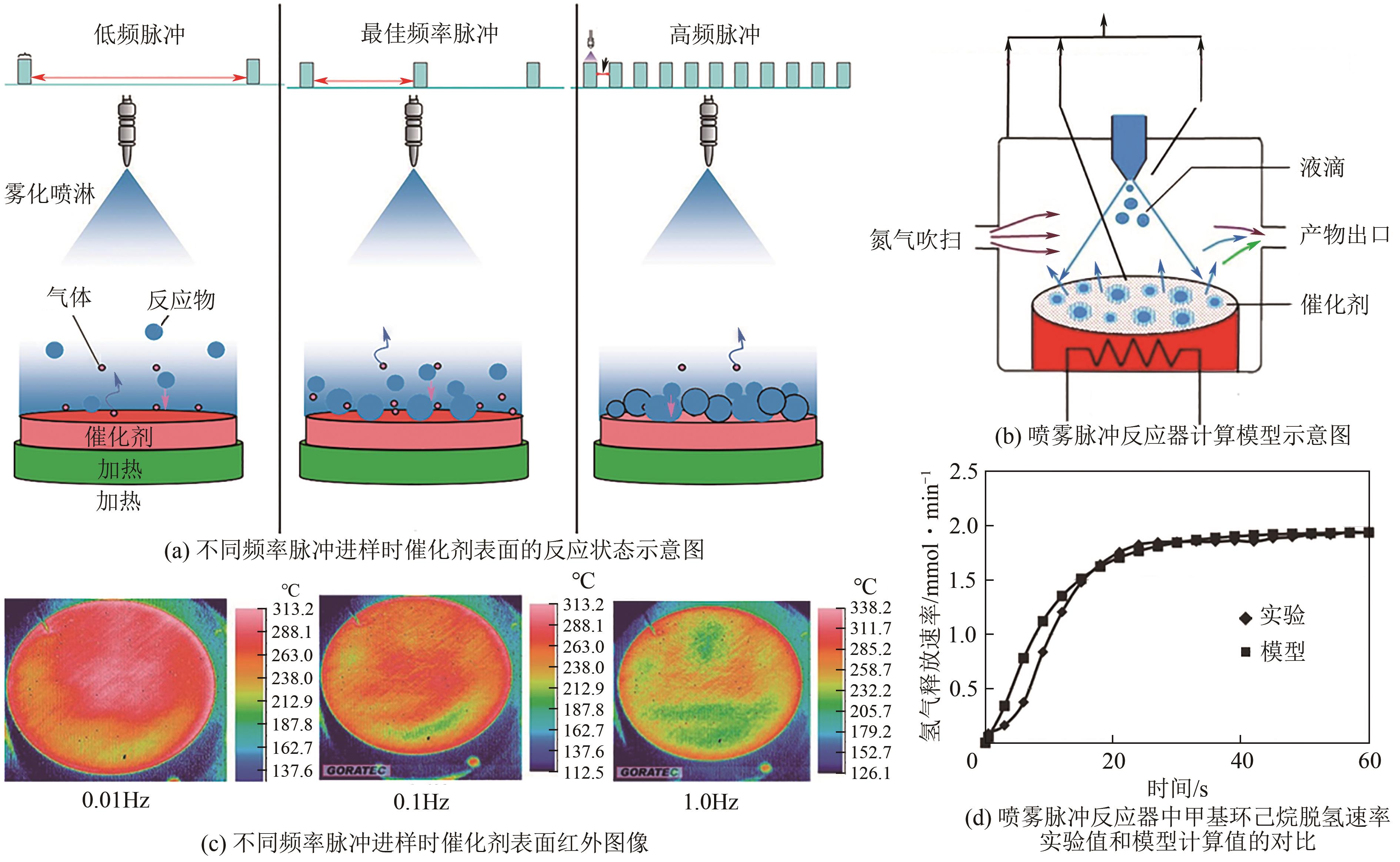
| 液膜态/湿干多相态 | 催化剂表面经历湿干两相,催化剂表面温度较高,传递介质使用过热液体代替气体,传热和传质效率均提高 | 湿干多相态下,环己烷在375℃时脱氢反应速率可达3.8mol/gPt/min[ | 反应物与催化剂用量比例、喷雾条件等要求严格,难以应用于大规模制氢场景 |
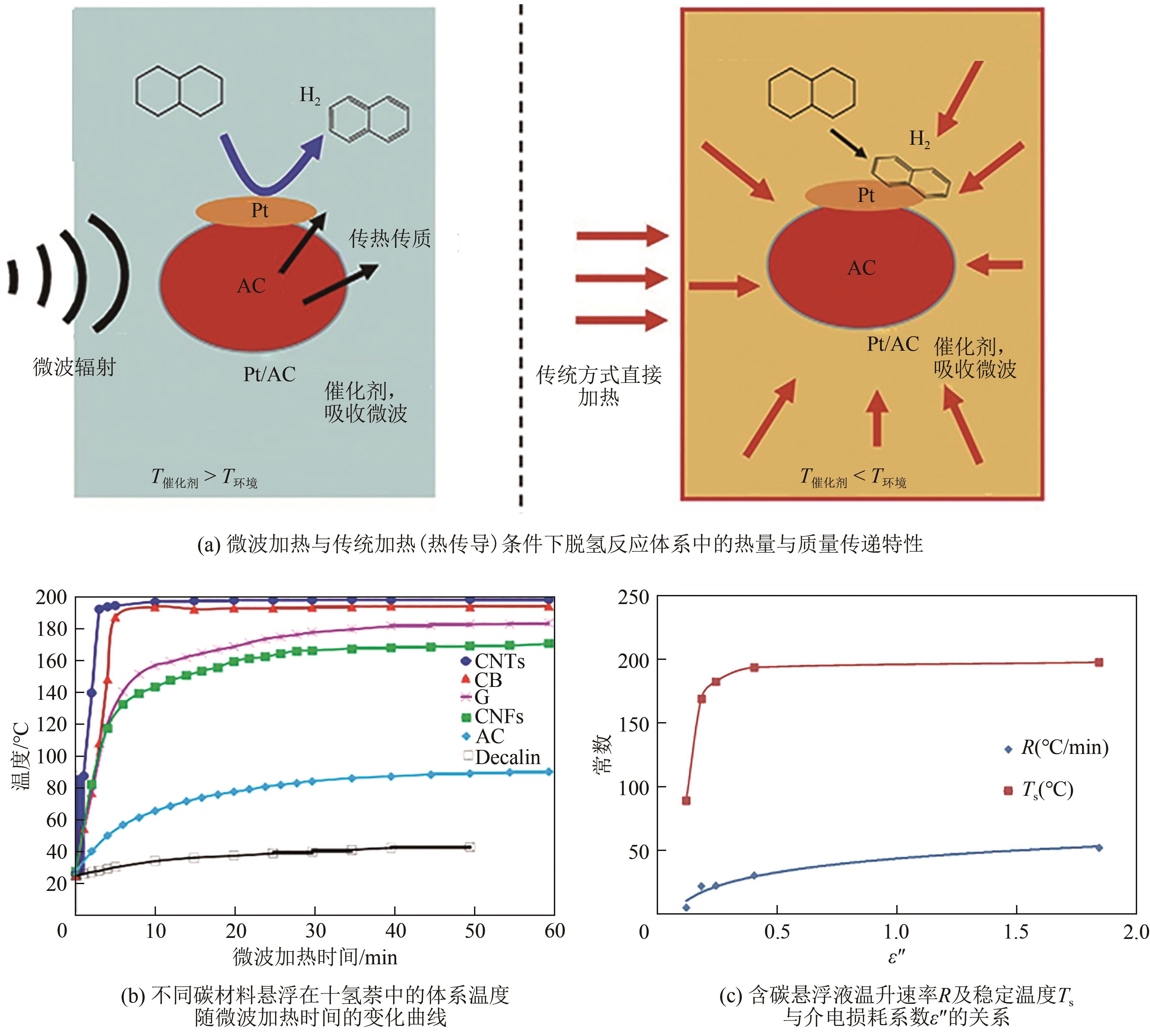
| 微波耦合 | 催化剂由内向外发热,传热效率提高 | 微波加热至196℃,十氢萘[ | 加热更加均匀,传热效率提高,能耗降低且催化剂表面不易结焦 |
| 电场强化 | 质子扩散速率加强 | 在甲基环己烷脱氢时,施加电场降低了反应活化能25kJ/mol;448K时,甲基环己烷转化率可达约37%[ | 电场强化加速了质子碰撞,降低了反应活化能,可以显著降低脱氢温度 |
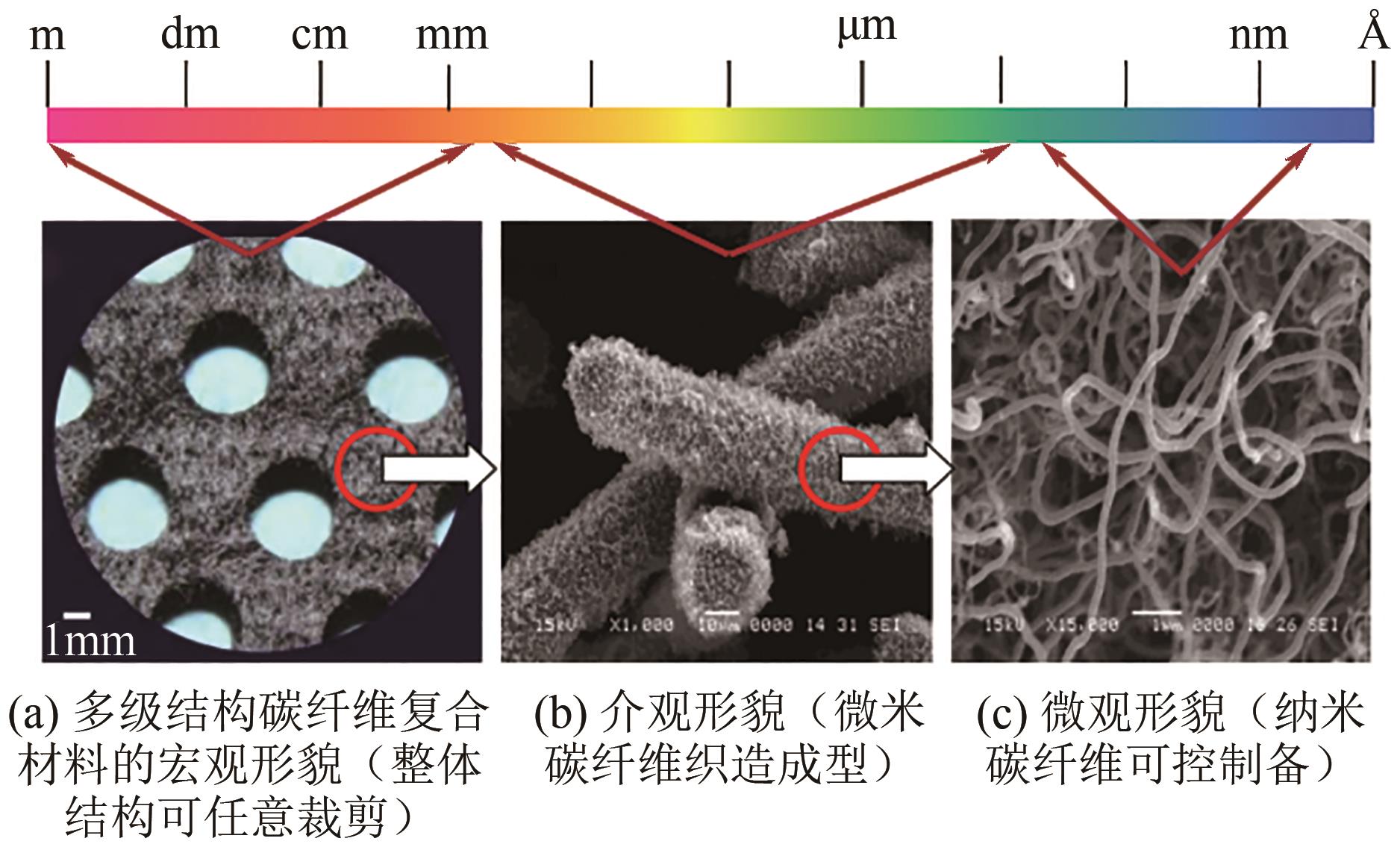
| 规整结构催化剂及反应器 | 规整化的三维空间结构的催化剂 载体具有特殊的流体流动、传热传 质性能 | 在243℃下,2h内,十氢萘脱氢速率可达2.16mol/(gPt·min),高于同种类型的粉末状催化剂脱氢速率(约2~3倍)[ | 作为实验室向工业应用过渡的枢纽,是科学研究的重要方向 |
| 产物分离强化 | |||
| 膜分离 | 在反应过程中,将产物及时分离,避免产物带走过多热量,促使化学反应平衡向生成产物的方向移动,打破了热力学限制,传热以及传质速率均提高 | 使用膜分离反应器进行环己烷脱氢,转化率可达70%以上,渗透流H2的纯度可达99.9%[ | 膜分离反应器提高LOHC脱氢转化率以及H2纯度 |
| 反应精馏 | — | 反应精馏相比于一般液相脱氢,其脱氢度提高约30%[ | — |
| 能量效率强化 | |||
| 与燃料电池耦合 | — | 与燃料电池联合运行,氢气发电效率可达45%[ | 将LOHC脱氢过程与燃料电池联合运行,将有效提高系统能量利用率,进一步节约能耗 |
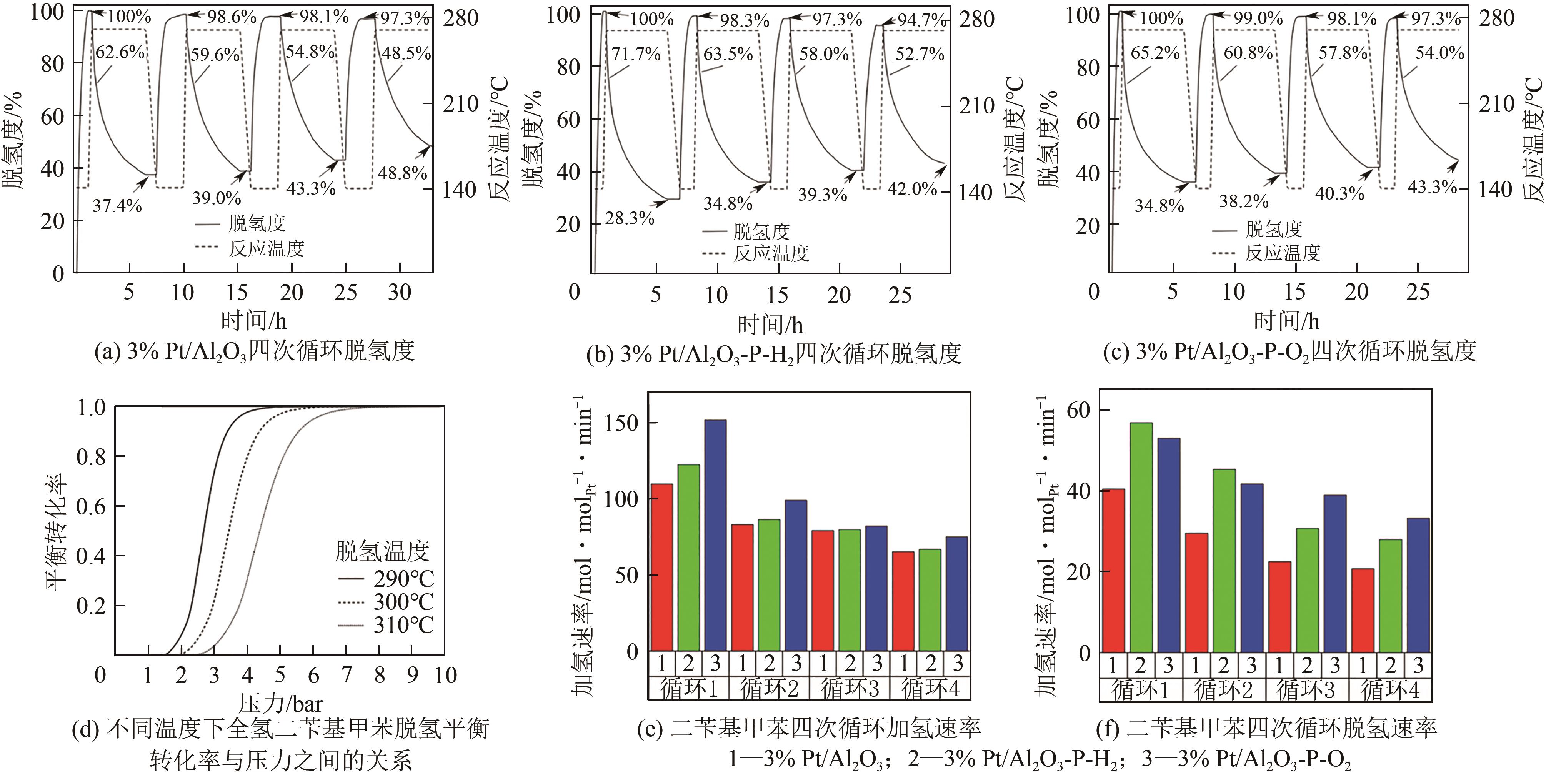
| 加/脱氢一体化 | — | 使用双功能催化剂,二苄基甲苯的储氢效率可达84%[ | 加氢放热和脱氢吸热的高效耦合,提高能量利用率;反应装置体积缩小,成本以及能耗降低 |
表3 不同强化策略对脱氢效率的影响
| 强化策略 | 传热、传质性能 | 脱氢效率分析 | 评价 |
|---|---|---|---|
| 催化过程强化 | |||
| 液膜态/湿干多相态 | 催化剂表面经历湿干两相,催化剂表面温度较高,传递介质使用过热液体代替气体,传热和传质效率均提高 | 湿干多相态下,环己烷在375℃时脱氢反应速率可达3.8mol/gPt/min[ | 反应物与催化剂用量比例、喷雾条件等要求严格,难以应用于大规模制氢场景 |
| 微波耦合 | 催化剂由内向外发热,传热效率提高 | 微波加热至196℃,十氢萘[ | 加热更加均匀,传热效率提高,能耗降低且催化剂表面不易结焦 |
| 电场强化 | 质子扩散速率加强 | 在甲基环己烷脱氢时,施加电场降低了反应活化能25kJ/mol;448K时,甲基环己烷转化率可达约37%[ | 电场强化加速了质子碰撞,降低了反应活化能,可以显著降低脱氢温度 |
| 规整结构催化剂及反应器 | 规整化的三维空间结构的催化剂 载体具有特殊的流体流动、传热传 质性能 | 在243℃下,2h内,十氢萘脱氢速率可达2.16mol/(gPt·min),高于同种类型的粉末状催化剂脱氢速率(约2~3倍)[ | 作为实验室向工业应用过渡的枢纽,是科学研究的重要方向 |
| 产物分离强化 | |||
| 膜分离 | 在反应过程中,将产物及时分离,避免产物带走过多热量,促使化学反应平衡向生成产物的方向移动,打破了热力学限制,传热以及传质速率均提高 | 使用膜分离反应器进行环己烷脱氢,转化率可达70%以上,渗透流H2的纯度可达99.9%[ | 膜分离反应器提高LOHC脱氢转化率以及H2纯度 |
| 反应精馏 | — | 反应精馏相比于一般液相脱氢,其脱氢度提高约30%[ | — |
| 能量效率强化 | |||
| 与燃料电池耦合 | — | 与燃料电池联合运行,氢气发电效率可达45%[ | 将LOHC脱氢过程与燃料电池联合运行,将有效提高系统能量利用率,进一步节约能耗 |
| 加/脱氢一体化 | — | 使用双功能催化剂,二苄基甲苯的储氢效率可达84%[ | 加氢放热和脱氢吸热的高效耦合,提高能量利用率;反应装置体积缩小,成本以及能耗降低 |
| 1 | 袁胜楠, 张龙龙, 赵宁, 等. 液态有机物储氢技术发展历程与问题分析[J]. 太阳能, 2022(9): 5-14. |
| YUAN Shengnan, ZHANG Longlong, ZHAO Ning, et al. Development course and problem analysis of hydrogen storage technology for liquid organic matter[J]. Solar Energy, 2022(9): 5-14. | |
| 2 | 蔡卫权, 陈进富. 有机液态氢化物可逆储放氢技术进展[J]. 现代化工, 2001, 21(11): 21-23, 25. |
| CAI Weiquan, CHEN Jinfu. Progresses on liquid organic hydrides in hydrogen storage and transportation[J]. Modern Chemical Industry, 2001, 21(11): 21-23, 25. | |
| 3 | JIANG Zhao, PAN Qi, XU Jie, et al. Current situation and prospect of hydrogen storage technology with new organic liquid[J]. International Journal of Hydrogen Energy, 2014, 39(30): 17442-17451. |
| 4 | 曹军文, 覃祥富, 耿嘎, 等. 氢气储运技术的发展现状与展望[J]. 石油学报(石油加工), 2021, 37(6): 1461-1478. |
| CAO Junwen, QIN Xiangfu, GENG Ga, et al. Current status and prospects of hydrogen storage and transportation technology[J]. Acta Petrolei Sinica (Petroleum Processing Section), 2021, 37(6): 1461-1478. | |
| 5 | NIERMANN Matthias, BECKENDORFF Alexander, KALTSCHMITT Martin, et al. Liquid organic hydrogen carrier (LOHC)—Assessment based on chemical and economic properties[J]. International Journal of Hydrogen Energy, 2019, 44(13): 6631-6654. |
| 6 | HIRSCHER M, YARTYS V A, BARICCO M, et al. Materials for hydrogen-based energy storage-past, recent progress and future outlook[J]. Journal of Alloys and Compounds, 2020, 827: 153548. |
| 7 | LANG C G, JIA Y, YAO X. Recent advances in liquid-phase chemical hydrogen storage[J]. Energy Storage Materials, 2020, 26: 290-312. |
| 8 | HE Teng, PEI Qijun, CHEN Ping. Liquid organic hydrogen carriers[J]. Journal of Energy Chemistry, 2015, 24(5): 587-594. |
| 9 | KUSTOV L M, TARASOV A L, SUNG J, et al. Hydrogen storage materials[J]. Mendeleev Communications, 2014, 24(1): 1-8. |
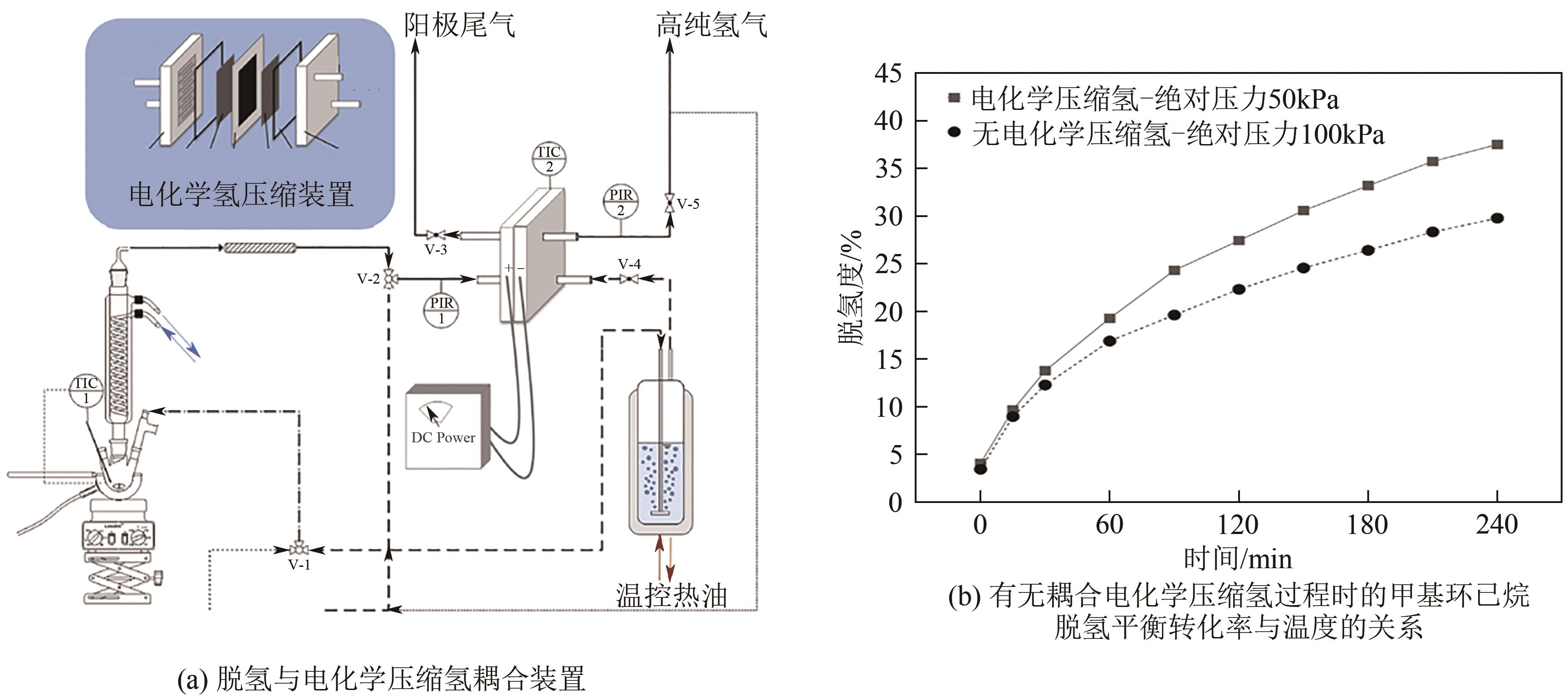
| 10 | BOURANE A, ELANANY M, PHAM T V, et al. An overview of organic liquid phase hydrogen carriers[J]. International Journal of Hydrogen Energy, 2016, 41(48): 23075-23091. |
| 11 | Boris BRIGLJEVIĆ, BYUN Manhee, Hankwon LIM. Design, economic evaluation, and market uncertainty analysis of LOHC-based, CO2 free, hydrogen delivery systems[J]. Applied Energy, 2020, 274: 115314. |
| 12 | KWAK Yeonsu, KIRK Jaewon, MOON Seongeun, et al. Hydrogen production from homocyclic liquid organic hydrogen carriers (LOHCs): Benchmarking studies and energy-economic analyses[J]. Energy Conversion and Management, 2021, 239: 114124. |
| 13 | MODISHA P M, OUMA C N M, GARIDZIRAI R, et al. The prospect of hydrogen storage using liquid organic hydrogen carriers[J]. Energy & Fuels, 2019, 33(4): 2778-2796. |
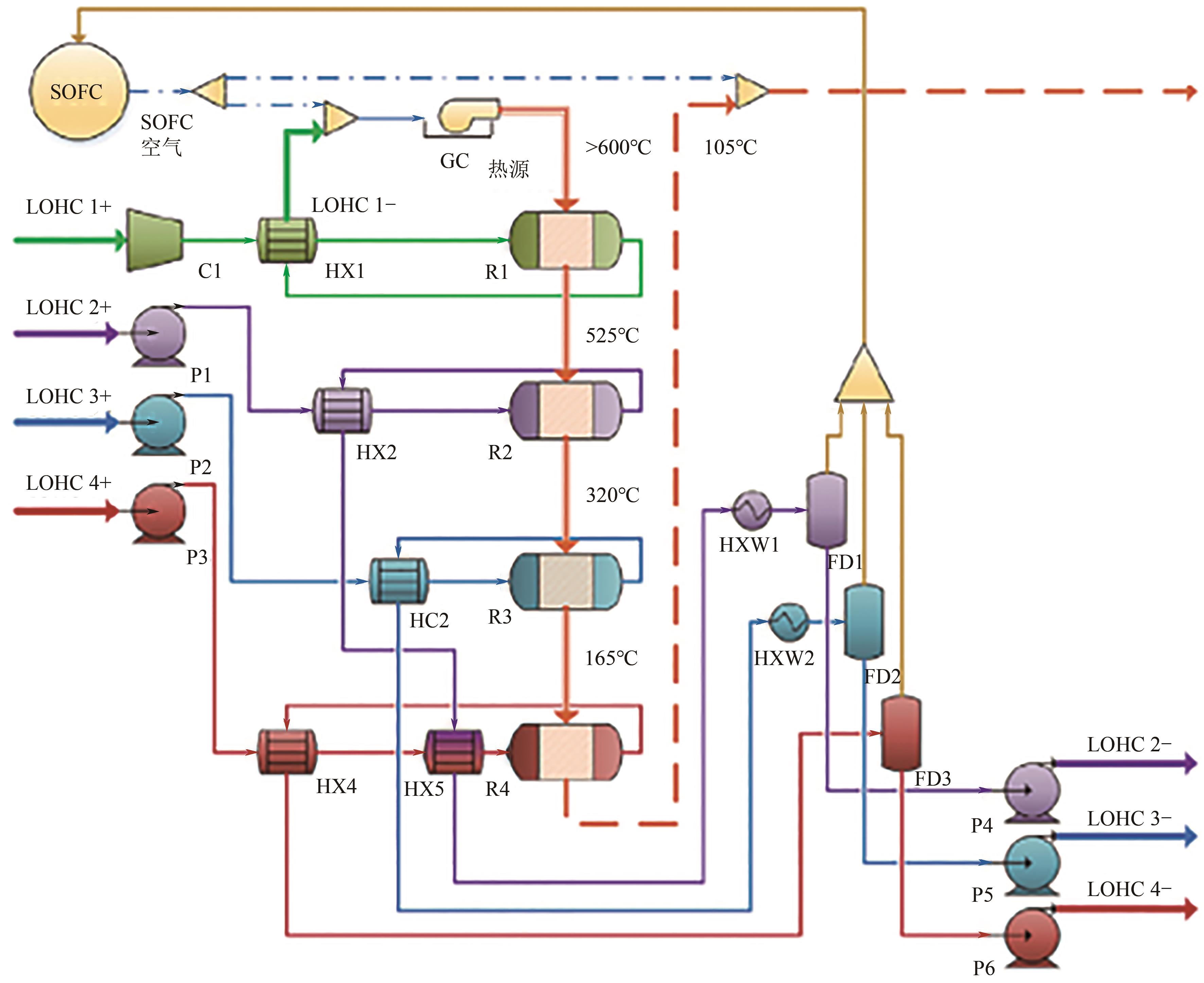
| 14 | GIANOTTI Elia, Mélanie TAILLADES-JACQUIN, Jacques ROZIÈRE, et al. High-purity hydrogen generation via dehydrogenation of organic carriers: A review on the catalytic process[J]. ACS Catalysis, 2018, 8(5): 4660-4680. |
| 15 | MENG Junchi, ZHOU Feng, MA Huixia, et al. A review of catalysts for methylcyclohexane dehydrogenation[J]. Topics in Catalysis, 2021, 64(7/8): 509-520. |
| 16 | 刘安鼐, 任靖, 赵保槐, 等. 液相有机氢载体的催化研究与应用[J]. 北京化工大学学报(自然科学版), 2021, 48(4): 1-18. |
| LIU Annai, REN Jing, ZHAO Baohuai, et al. Catalysis and applications of liquid organic hydrogen carriers[J]. Journal of Beijing University of Chemical Technology (Natural Science Edition), 2021, 48(4): 1-18. | |
| 17 | QI Suitao, HUANG Jun, CHEN Hao, et al. Development of dehydrogenation catalyst for reversible hydrogen storage in organic hydrides[J]. Acta Chimica Sinica, 2012, 70(24): 2467. |
| 18 | RAO Purna, YOON Minyoung. Potential liquid-organic hydrogen carrier (LOHC) systems: A review on recent progress[J]. Energies, 2020, 13(22): 6040. |
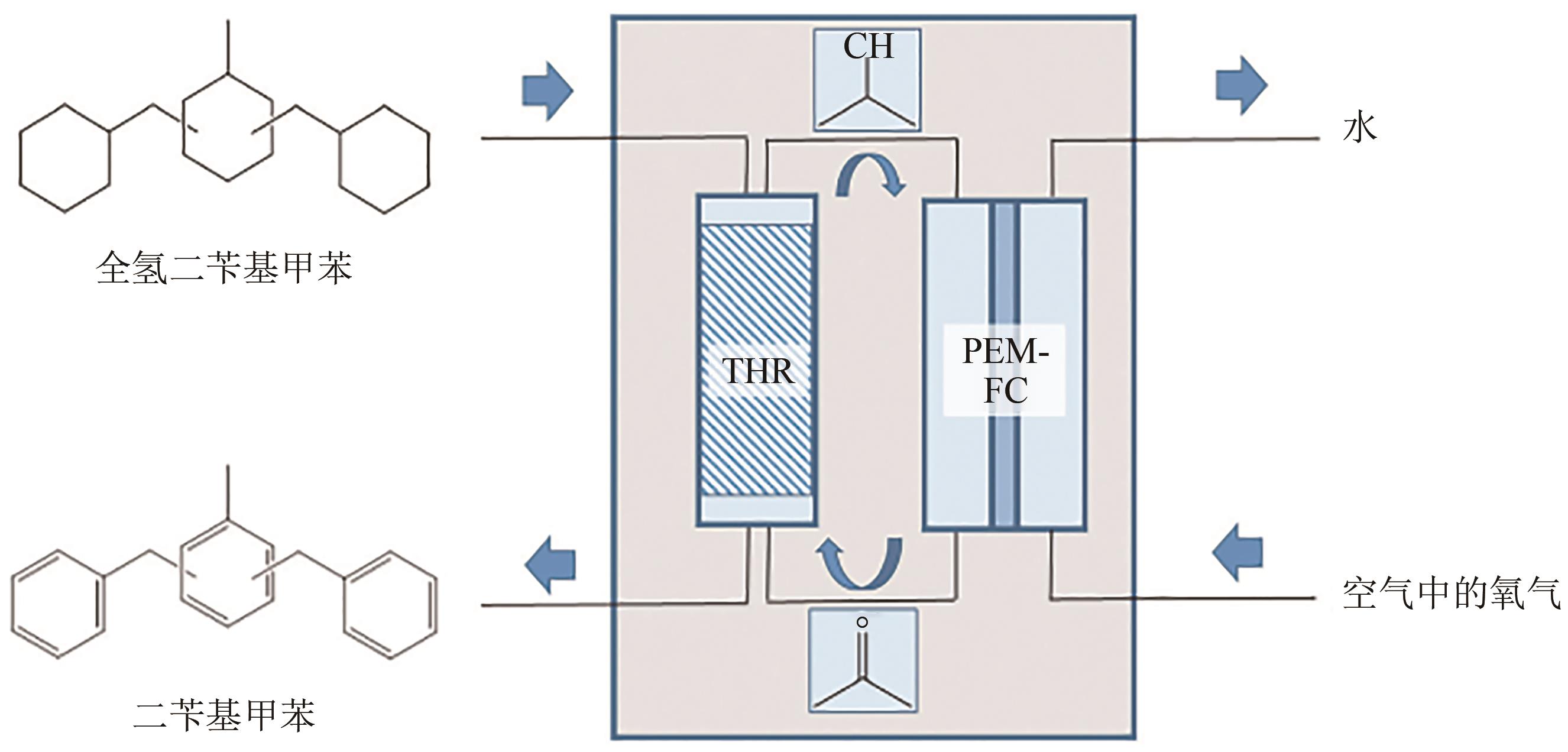
| 19 | GEMECHU Desalegn Nigatu, MOHAMMED Ahmed Mustefa, REDI Mesfin, et al. First principles-based approaches for catalytic activity on the dehydrogenation of liquid organic hydrogen carriers: A review[J]. International Journal of Hydrogen Energy, 2023, 48(85): 33186-33206. |
| 20 | 王子宗, 刘罡, 王振维. 乙烯丙烯生产过程强化技术进展及思考[J]. 化工进展, 2023, 42(4): 1669-1676. |
| WANG Zizong, LIU Gang, WANG Zhenwei. Progress and reflection on process intensification technology for ethylene/propylene production[J]. Chemical Industry and Engineering Progress, 2023, 42(4): 1669-1676. | |
| 21 | CHU Chenyang, WU Kai, LUO Bingbing, et al. Hydrogen storage by liquid organic hydrogen carriers: Catalyst, renewable carrier, and technology—A review[J]. Carbon Resources Conversion, 2023, 6(4): 334-351. |
| 22 | Guido Peter PEZ, SCOTT Aaron Raymond, COOPER Alan Charles, et al. Hydrogen storage by reversible hydrogenation of pi-conjugated substrates: CA20042524846[P]. 2005-01-06. |
| 23 | WANG Bin, CHEN You-Tao, CHANG Tie-Yan, et al. Facet-dependent catalytic activities of Pd/rGO: Exploring dehydrogenation mechanism of dodecahydro-N-ethylcarbazole[J]. Applied Catalysis B: Environmental, 2020, 266: 118658. |
| 24 | WANG Bin, CHANG Tieyan, JIANG Zhao, et al. Component controlled synthesis of bimetallic PdCu nanoparticles supported on reduced graphene oxide for dehydrogenation of dodecahydro-N-ethylcarbazole[J]. Applied Catalysis B: Environmental, 2019, 251: 261-272. |
| 25 | WANG Bin, CHANG Tieyan, JIANG Zhao, et al. Catalytic dehydrogenation study of dodecahydro-N-ethylcarbazole by noble metal supported on reduced graphene oxide[J]. International Journal of Hydrogen Energy, 2018, 43(15): 7317-7325. |
| 26 | WANG Bin, CHANG Tieyan, GONG Xiang, et al. One-pot synthesis of Au/Pd core/shell nanoparticles supported on reduced graphene oxide with enhanced dehydrogenation performance for dodecahydro-N-ethylcarbazole[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(1): 1760-1768. |
| 27 | WANG Bin, YAN Ting, CHANG Tieyan, et al. Palladium supported on reduced graphene oxide as a high-performance catalyst for the dehydrogenation of dodecahydro-N-ethylcarbazole[J]. Carbon, 2017, 122: 9-18. |
| 28 | DONG Yuan, ZHAO Haoming, LIU Zhenjie, et al. Understanding the mechanism of the competitive adsorption in 8-methylquinoline hydrogenation over a Ru catalyst[J]. RSC Advances, 2020, 10(19): 11039-11045. |
| 29 | NICOLE Brückner, KATHARINA Obesser, Bösmann ANDREAS, et al. Evaluation of industrially applied heat-transfer fluids as liquid organic hydrogen carrier systems[J]. ChemSusChem, 2014, 7(1): 229-235. |
| 30 | MI Chengjing, HUANG Yanping, CHEN Fengtao, et al. Density functional theory study on dehydrogenation of methylcyclohexane on Ni-Pt(111)[J]. International Journal of Hydrogen Energy, 2021, 46(1): 875-885. |
| 31 | 周一鸣, 齐随涛, 周宇亮, 等. 多环芳烃类液体有机氢载体储放氢技术研究进展[J]. 化工进展, 2023, 42(2): 1000-1007. |
| ZHOU Yiming, QI Suitao, ZHOU Yuliang, et al. Research progress in the hydrogenation and dehydrogenation technology of polycyclic aromatic hydrocarbon liquid organic hydrogen carriers[J]. Chemical Industry and Engineering Progress, 2023, 42(2): 1000-1007. | |
| 32 | ALHUMAIDAN Faisal, CRESSWELL David, GARFORTH Arthur. Hydrogen storage in liquid organic hydride: Producing hydrogen catalytically from methylcyclohexane[J]. Energy & Fuels, 2011, 25(10): 4217-4234. |
| 33 | KIM Kyeounghak, Jinho OH, KIM Tae Wan, et al. Different catalytic behaviors of Pd and Pt metals in decalin dehydrogenation to naphthalene[J]. Catalysis Science & Technology, 2017, 7(17): 3728-3735. |
| 34 | WANG Ruibing, BING Qiming, LIU Jingyao. Insights into the mechanism of formic acid dehydrogenation on Pd-Co@Pd core-shell catalysts: A theoretical study[J]. Applied Surface Science, 2020, 505: 144532. |
| 35 | ZHAO Wei, CHIZALLET Céline, SAUTET Philippe, et al. Dehydrogenation mechanisms of methyl-cyclohexane on γ-Al2O3 supported Pt13: Impact of cluster ductility[J]. Journal of Catalysis, 2019, 370: 118-129. |
| 36 | CHEN Fengtao, HUANG Yanping, MI Chengjing, et al. Density functional theory study on catalytic dehydrogenation of methylcyclohexane on Pt(111)[J]. International Journal of Hydrogen Energy, 2020, 45(11): 6727-6737. |
| 37 | WANG Weitao, LIU Huizhen, WU Tianbin, et al. Ru catalyst supported on bentonite for partial hydrogenation of benzene to cyclohexene[J]. Journal of Molecular Catalysis A: Chemical, 2012, 355: 174-179. |
| 38 | SOTOODEH F, HUBER B J M, SMITH K J. The effect of the N atom on the dehydrogenation of heterocycles used for hydrogen storage[J]. Applied Catalysis A: General, 2012, 419/420: 67-72. |
| 39 | ERIC C, ODILE E, CRABTREE R H. Computational structure-activity relationships in H2 storage: How placement of N atoms affects release temperatures in organic liquid storage materials[J]. Chemical Communications, 2007(22): 2231-2233. |
| 40 | MARTYNENKO E A, PIMERZIN Al A, SAVINOV A A, et al. Hydrogen release from decalin by catalytic dehydrogenation over supported platinum catalysts[J]. Topics in Catalysis, 2020, 63(1): 178-186. |
| 41 | Yongxiao TUO, MENG Ying, CHEN Chen, et al. Partial positively charged Pt in Pt/MgAl2O4 for enhanced dehydrogenation activity[J]. Applied Catalysis B: Environmental, 2021, 288: 119996. |
| 42 | Yongxiao TUO, MENG Ying, LU Qing, et al. Taming Pt 5d state occupancy via Pt—O—Mn electronic linkage for enhanced dehydrogenation activity[J]. AIChE Journal, 2023, 69(9): e18149. |
| 43 | Yongxiao TUO, YANG Liu, MA Xuefei, et al. Carbon nanotubes-supported Pt catalysts for decalin dehydrogenation to release hydrogen: A comparison between nitrogen-and oxygen-surface modification[J]. International Journal of Hydrogen Energy, 2021, 46(1): 930-942. |
| 44 | Yongxiao TUO, LIU Xiaojun, SHI Liujie, et al. Searching for efficient defect types in carbon nanofibers to promote supported Pt catalytic activity for dehydrogenation reaction[J]. Catalysis Today, 2020, 347: 87-95. |
| 45 | Yongxiao TUO, YANG Liu, CHENG Hongye, et al. Density functional theory study of decalin dehydrogenation for hydrogen release on Pt(111) and Pt(211)[J]. International Journal of Hydrogen Energy, 2018, 43(42): 19575-19588. |
| 46 | NAVEEN Kanagaraj, Tahereh MAHVELATI-SHAMSABADI, SHARMA Pragyan, et al. MOF-derived Co/Zn single-atom catalysts for reversible hydrogenation and dehydrogenation of quinoline hydrogen carrier[J]. Applied Catalysis B: Environmental, 2023, 328: 122482. |
| 47 | ALCONADA K, BARRIO V L. Evaluation of bimetallic Pt-Co and Pt-Ni catalysts in LOHC dehydrogenation[J]. International Journal of Hydrogen Energy, 2023 |
| 48 | WANG Min, CHEN Huimin, WANG Min, et al. Tuning C1/C2 selectivity of CO2 electrochemical reduction over in situ evolved CuO/SnO2 heterostructure[J]. Angewandte Chemie International Edition, 2023, 62(40): e202306456. |
| 49 | LIN Yan, CHEN Xiaomeng, Yongxiao TUO, et al. In-situ doping-induced lattice strain of NiCoP/S nanocrystals for robust wide pH hydrogen evolution electrocatalysis and supercapacitor[J]. Journal of Energy Chemistry, 2022, 70: 27-35. |
| 50 | MAO Jianing, MEI Bingbao, LI Ji, et al. Unraveling the dynamic structural evolution of phthalocyanine catalysts during CO2 electroreduction[J]. Chinese Journal of Structural Chemistry, 2022, 41(10): 7. |
| 51 | ZHU Gangli, YANG Bolun, WANG Shuyan. Nanocrystallites-forming hierarchical porous Ni/Al2O3-TiO2 catalyst for dehydrogenation of organic chemical hydrides[J]. International Journal of Hydrogen Energy, 2011, 36(21): 13603-13613. |
| 52 | YOLCULAR Sevim, Özden OLGUN. Ni/Al2O3 catalysts and their activity in dehydrogenation of methylcyclohexane for hydrogen production[J]. Catalysis Today, 2008, 138(3/4): 198-202. |
| 53 | GULYAEVA Y K, ALEKSEEVA (BYKOVA) M V, ERMAKOV D Y, et al. High-loaded nickel based sol-gel catalysts for methylcyclohexane dehydrogenation[J]. Catalysts, 2020, 10(10): 1198. |
| 54 | YAN Jing, WANG Weiyan, MIAO Lei, et al. Dehydrogenation of methylcyclohexane over PtSn supported on MgAl mixed metal oxides derived from layered double hydroxides[J]. International Journal of Hydrogen Energy, 2018, 43(19): 9343-9352. |
| 55 | Cuncai LYU, LOU Pingping, CHANG Jiarong, et al. Nickel single atoms/cerium oxide hybrid for hydrogen production via solar-heating catalytic dehydrogenation of methyl cyclohexane[J]. Journal of Power Sources, 2023, 559: 232674. |
| 56 | ZHOU Liu, SUN Lin, XU Lixin, et al. Recent developments of effective catalysts for hydrogen storage technology using N-ethylcarbazole[J]. Catalysts, 2020, 10(6): 648. |
| 57 | DONG Chunyang, GAO Zirui, LI Yinlong, et al. Fully exposed palladium cluster catalysts enable hydrogen production from nitrogen heterocycles[J]. Nature Catalysis, 2022, 5(6): 485-493. |
| 58 | DENG Yuchen, GUO Yu, JIA Zhimin, et al. Few-atom Pt ensembles enable efficient catalytic cyclohexane dehydrogenation for hydrogen production[J]. Journal of the American Chemical Society, 2022, 144(8): 3535-3542. |
| 59 | 李佳豪, 杨锦, 潘伦, 等. 含氮有机液体储放氢催化体系研究进展[J/OL]. 化工进展, 2023, 42(12): 6325-6344. |
| LI Jiahao, YANG Jin, PAN Lun, et al. Research progress in catalytic system for hydrogen storage and release from nitrogen-containing liquid organic carriers[J/OL]. Chemical Industry and Engineering Progress, 2023, 42(12): 6325-6344. | |
| 60 | Yongxiao TUO, SHI Liujie, CHENG Hongye, et al. Insight into the support effect on the particle size effect of Pt/C catalysts in dehydrogenation[J]. Journal of Catalysis, 2018, 360: 175-186. |
| 61 | JANG Munjeong, CHOI Subin, KIM Yoondo, et al. Effect of CeO2 redox properties on the catalytic activity of Pt-CeO x over irreducible SiO2 support for methylcyclohexane (MCH) dehydrogenation[J]. Applied Surface Science, 2023, 627: 157134. |
| 62 | CHEN Luning, VERMA Pragya, HOU Kaipeng, et al. Reversible dehydrogenation and rehydrogenation of cyclohexane and methylcyclohexane by single-site platinum catalyst[J]. Nature Communications, 2022, 13: 1092. |
| 63 | ITO Hiroya, OSHIMA Kazumasa, YAMAMOTO Tsuyoshi, et al. Improved catalytic stability of Pt/TiO2 catalysts for methylcyclohexane dehydrogenation via selenium addition[J]. International Journal of Hydrogen Energy, 2022, 47(91): 38635-38643. |
| 64 | KUZMIN A O. Confined multiphase swirled flows in chemical engineering[J]. Reviews in Chemical Engineering, 2021.38(1): 31-68. |
| 65 | BINIWALE R B, ICHIKAWA M. Thermal imaging of catalyst surface during catalytic dehydrogenation of cyclohexane under spray-pulsed conditions[J]. Chemical Engineering Science, 2007, 62(24): 7370-7377. |
| 66 | HODOSHIMA Shinya, SHONO Atsushi, SAITO Yasukazu. Chemical recuperation of low-quality waste heats by catalytic dehydrogenation of organic chemical hydrides and its exergy analysis[J]. Energy & Fuels, 2008, 22(4): 2559-2569. |
| 67 | HODOSHIMA Shinya, SAITO Yasukazu. Characteristics of decalin dehydrogenation catalysis in the superheated liquid-film state for mobile storage of hydrogen[J]. Journal of Chemical Engineering of Japan, 2004, 37(3): 391-398. |
| 68 | HODOSHIMA Shinya, TAKAIWA Shigeki, SHONO Atsushi, et al. Hydrogen storage by decalin/naphthalene pair and hydrogen supply to fuel cells by use of superheated liquid-film-type catalysis[J]. Applied Catalysis A: General, 2005, 283(1/2): 235-242. |
| 69 | SHONO Atsushi, HASHIMOTO Takanori, HODOSHIMA Shinya, et al. Continuous catalytic dehydrogenation of decalin under mild conditions[J]. Journal of Chemical Engineering of Japan, 2006, 39(2): 211-215. |
| 70 | HODOSHIMA Shinya, NAGATA Hiroaki, SAITO Yasukazu. Efficient hydrogen supply from tetralin with superheated liquid-film-type catalysis for operating fuel cells[J]. Applied Catalysis A: General, 2005, 292: 90-96. |
| 71 | SAITO Yasukazu, ARAMAKI Kiyoshi, HODOSHIMA Shinya, et al. Efficient hydrogen generation from organic chemical hydrides by using catalytic reactor on the basis of superheated liquid-film concept[J]. Chemical Engineering Science, 2008, 63(20): 4935-4941. |
| 72 | LÁZARO M P, GARCÍA-BORDEJÉ E, SEBASTIÁN D, et al. In situ hydrogen generation from cycloalkanes using a Pt/CNF catalyst[J]. Catalysis Today, 2008, 138(3/4): 203-209. |
| 73 | SEBASTIÁN D, BORDEJÉ E G, CALVILLO L, et al. Hydrogen storage by decalin dehydrogenation/naphthalene hydrogenation pair over platinum catalysts supported on activated carbon[J]. International Journal of Hydrogen Energy, 2008, 33(4): 1329-1334. |
| 74 | LEE Gihoon, JEONG Yeojin, KIM Bong-Geun, et al. Hydrogen production by catalytic decalin dehydrogenation over carbon-supported platinum catalyst: Effect of catalyst preparation method[J]. Catalysis Communications, 2015, 67: 40-44. |
| 75 | ZHOU Qi, LI Ping, WANG Xilong, et al. Preparation of CNF-supported Pt catalysts for hydrogen evolution from decalin[J]. Materials Chemistry and Physics, 2011, 126(1/2): 41-45. |
| 76 | LI Ping, HUANG Yili, CHEN De, et al. CNFs-supported Pt catalyst for hydrogen evolution from decalin[J]. Catalysis Communications, 2009, 10(6): 815-818. |
| 77 | KARIYA Nobuko, FUKUOKA Atsushi, ICHIKAWA Masaru. Efficient evolution of hydrogen from liquid cycloalkanes over Pt-containing catalysts supported on active carbons under “wet-dry multiphase conditions”[J]. Applied Catalysis A: General, 2002, 233(1/2): 91-102. |
| 78 | BINIWALE R B, KARIYA N, YAMASHIRO H, et al. Heat transfer and thermographic analysis of catalyst surface during multiphase phenomena under spray-pulsed conditions for dehydrogenation of cyclohexane over Pt catalysts[J]. The Journal of Physical Chemistry B, 2006, 110(7): 3189-3196. |
| 79 | KARIYA Nobuko, FUKUOKA Atsushi, UTAGAWA Tadashi, et al. Efficient hydrogen production using cyclohexane and decalin by pulse-spray mode reactor with Pt catalysts[J]. Applied Catalysis A: General, 2003, 247(2): 247-259. |
| 80 | BINIWALE R B, HIKARU Y, MASARU I. In-situ infrared thermographic analysis during dehydrogenationof cyclohexane over carbon-supported Pt catalysts using spray-pulsed reactor[J]. Catalysis Letters, 2005, 102(1): 23-31. |
| 81 | BINIWALE R B, NOBUKO K, MASARU I. Dehydrogenation of cyclohexane over Ni based catalysts supported on activated carbon using spray-pulsed reactor and enhancement in activity by addition of a small amount of Pt[J]. Catalysis Letters, 2005, 105(1): 83-87. |
| 82 | SILUVAI A P, SOHONY R A, BINIWALE R B. An insight into spray pulsed reactor through mathematical modeling of catalytic dehydrogenation of cyclohexane[J]. International Journal of Hydrogen Energy, 2014, 39(13): 6944-6952. |
| 83 | SUN Jianchen, SHANG Hui, MIAO Chao, et al. Microwave enhanced hydrogen production from liquid organic hydrogen carriers: A review[J]. Chemical Engineering and Processing - Process Intensification, 2023, 190: 109432. |
| 84 | LI Xing, Yongxiao TUO, LI Ping, et al. Effects of carbon support on microwave-assisted catalytic dehydrogenation of decalin[J]. Carbon, 2014, 67: 775-783. |
| 85 | SUTTISAWAT Yindee, SAKAI Hideki, Masahiko ABE, et al. Microwave effect in the dehydrogenation of tetralin and decalin with a fixed-bed reactor[J]. International Journal of Hydrogen Energy, 2012, 37(4): 3242-3250. |
| 86 | SHI Liujie, LIU Xiaojun, Yongxiao TUO, et al. Graphene-CNT composite as catalyst support for microwave-assisted hydrogen releasing from liquid organic hydride[J]. International Journal of Hydrogen Energy, 2017, 42(27): 17403-17413. |
| 87 | SEKINE Yasushi, HARAGUCHI Masayuki, TOMIOKA Masahiko, et al. Low-temperature hydrogen production by highly efficient catalytic system assisted by an electric field[J]. The Journal of Physical Chemistry A, 2010, 114(11): 3824-3833. |
| 88 | KENT Takise, AYAKA Sato, KOTA Murakami, et al. Irreversible catalytic methylcyclohexane dehydrogenation by surface protonics at low temperature[J]. RSC Advances, 2019, 9(11): 5918-5924. |
| 89 | 龙军, 邵潜, 贺振富, 等. 规整结构催化剂及反应器研究进展[J]. 化工进展, 2004, 23(9): 925-932. |
| LONG Jun, SHAO Qian, HE Zhenfu, et al. Monolithic catalysts and monolithic reactors[J]. Chemical Industry and Engineering Progress, 2004, 23(9): 925-932. | |
| 90 | David SEBASTIÁN, ALEGRE Cinthia, CALVILLO Laura, et al. Carbon supports for the catalytic dehydrogenation of liquid organic hydrides as hydrogen storage and delivery system[J]. International Journal of Hydrogen Energy, 2014, 39(8): 4109-4115. |
| 91 | LI Xing, Yongxiao TUO, JIANG Hao, et al. Engineering Pt/carbon-nanofibers/carbon-paper composite towards highly efficient catalyst for hydrogen evolution from liquid organic hydride[J]. International Journal of Hydrogen Energy, 2015, 40: 12217-12226. |
| 92 | Yongxiao TUO, JIANG Hao, LI Xing, et al. Kinetic behavior of Pt catalyst supported on structured carbon nanofiber bed during hydrogen releasing from decalin[J]. International Journal of Hydrogen Energy, 2016, 41(25): 10755-10765. |
| 93 | MOULIJN J A, KAPTEIJN F. Monolithic reactors in catalysis: Excellent control[J]. Current Opinion in Chemical Engineering, 2013, 2(3): 346-353. |
| 94 | PETERS W, EYPASCH M, FRANK T, et al. Efficient hydrogen release from perhydro-N-ethylcarbazole using catalyst-coated metallic structures produced by selective electron beam melting[J]. Energy & Environmental Science, 2015, 8(2): 641-649. |
| 95 | ALI J K, NEWSON E J, RIPPIN D W T. Deactivation and regeneration of Pd-Ag membranes for dehydrogenation reactions[J]. Journal of Membrane Science, 1994, 89(1/2): 171-184. |
| 96 | LI Gang, YADA Kazuya, KANEZASHI Masakoto, et al. Methylcyclohexane dehydrogenation in catalytic membrane reactors for efficient hydrogen production[J]. Industrial & Engineering Chemistry Research, 2013, 52(37): 13325-13332. |
| 97 | GHASEMZADEH Kamran, GHAHREMANI Milad, YOUSEFI AMIRI Taher, et al. Hydrogen production by silica membrane reactor during dehydrogenation of methylcyclohexane: CFD analysis[J]. International Journal of Hydrogen Energy, 2021, 46(37): 19768-19777. |
| 98 | JEONG Byeong-Heon, SOTOWA Ken-Ichiro, KUSAKABE Katsuki. Catalytic dehydrogenation of cyclohexane in an FAU-type zeolite membrane reactor[J]. Journal of Membrane Science, 2003, 224(1/2): 151-158. |
| 99 | SUN Yiming, KHANG Soon Jai. Catalytic membrane for simultaneous chemical reaction and separation applied to a dehydrogenation reaction[J]. Industrial & Engineering Chemistry Research, 1988, 27(7): 1136-1142. |
| 100 | ALEXANDER Wunsch, TATJANA Berg, PETER Pfeifer. Hydrogen production from the LOHC perhydro-dibenzyl-toluene and purification using a 5µm PdAg-membrane in a coupled microstructured system[J]. Materials, 2020, 13(2): 277. |
| 101 | KREUDER H, BOELTKEN T, CHOLEWA M, et al. Heat storage by the dehydrogenation of methylcyclohexane—Experimental studies for the design of a microstructured membrane reactor[J]. International Journal of Hydrogen Energy, 2016, 41(28): 12082-12092. |
| 102 | WAGNER Christin, CHOLEWA Martin, ULMER Ulrich, et al. Konzept zur chemischen Wärmespeicherung mit flüssigen organischen hydriden[J]. Chemie Ingenieur Technik, 2017, 89(3): 341-345. |
| 103 | BYUN Manhee, KIM Heehyang, CHOE Changgwon, et al. Conceptual feasibility studies for cost-efficient and bi-functional methylcyclohexane dehydrogenation in a membrane reactor for H2 storage and production[J]. Energy Conversion and Management, 2021, 227: 113576. |
| 104 | BYUN Manhee, CHOE Changgwon, CHEON Seunghyun, et al. Statistical and stochastic feasibility studies of potential liquid organic hydrogen carriers in a membrane reactor for simultaneous hydrogen storage and production: Technical, economic, and environmental aspects[J]. Renewable Energy, 2022, 195: 1393-1411. |
| 105 | 陶贤湖, 杨伯伦, 华贲. 反应精馏过程中的场协同分析[J]. 高校化学工程学报, 2003, 17(4): 389-394. |
| TAO Xianhu, YANG Bolun, HUA Ben. Analysis for fields synergy in the reactive distillation process[J]. Journal of Chemical Engineering of Chinese Universities, 2003, 17(4): 389-394. | |
| 106 | GEIßELBRECHT M, MRUSEK S, MÜLLER K, et al. Highly efficient, low-temperature hydrogen release from perhydro-benzyltoluene using reactive distillation[J]. Energy & Environmental Science, 2020, 13(9): 3119-3128. |
| 107 | KIERMAIER Stephan, LEHMANN Daniel, Andreas BÖSMANN, et al. Dehydrogenation of perhydro-N-ethylcarbazole under reduced total pressure[J]. International Journal of Hydrogen Energy, 2021, 46(29): 15660-15670. |
| 108 | MÜLLER K, STARK K, EMEL’YANENKO V N, et al. Liquid organic hydrogen carriers:Thermophysical and thermochemical studies of benzyl-and dibenzyl-toluene derivatives[J]. Industrial & Engineering Chemistry Research, 2015, 54(32): 7967-7976. |
| 109 | MRUSEK Stephan, PREUSTER Patrick, Karsten MÜLLER, et al. Pressurized hydrogen from charged liquid organic hydrogen carrier systems by electrochemical hydrogen compression[J]. International Journal of Hydrogen Energy, 2021, 46(29): 15624-15634. |
| 110 | Karsten MÜLLER, SKELEDZIC Tanja, WASSERSCHEID Peter. Strategies for low-temperature liquid organic hydrogen carrier dehydrogenation[J]. Energy & Fuels, 2021, 35(13): 10929-10936. |
| 111 | WANG Chaoyang. Fundamental models for fuel cell engineering[J]. Chemical Reviews, 2004, 104(10): 4727-4766. |
| 112 | Karsten MÜLLER, THIELE Simon, WASSERSCHEID Peter. Evaluations of concepts for the integration of fuel cells in liquid organic hydrogen carrier systems[J]. Energy & Fuels, 2019, 33(10): 10324-10330. |
| 113 | PREUSTER Patrick, FANG Qingping, PETERS Roland, et al. Solid oxide fuel cell operating on liquid organic hydrogen carrier-based hydrogen-making full use of heat integration potentials[J]. International Journal of Hydrogen Energy, 2018, 43(3): 1758-1768. |
| 114 | PETERS Roland, DEJA Robert, FANG Qingping, et al. A solid oxide fuel cell operating on liquid organic hydrogen carrier-based hydrogen—A kinetic model of the hydrogen release unit and system performance[J]. International Journal of Hydrogen Energy, 2019, 44(26): 13794-13806. |
| 115 | Boris BRIGLJEVIĆ, LEE Boreum, DICKSON Rofice, et al. Concept for temperature-cascade hydrogen release from organic liquid carriers coupled with SOFC power generation[J]. Cell Reports Physical Science, 2020, 1(3): 100032. |
| 116 | SIEVI Gabriel, GEBURTIG Denise, SKELEDZIC Tanja, et al. Towards an efficient liquid organic hydrogen carrier fuel cell concept[J]. Energy & Environmental Science, 2019, 12(7): 2305-2314. |
| 117 | JORSCHICK H, PREUSTER P, DÜRR S, et al. Hydrogen storage using a hot pressure swing reactor[J]. Energy & Environmental Science, 2017, 10(7): 1652-1659. |
| 118 | MARKIEWICZ M, ZHANG Y Q, BÖSMANN A, et al. Environmental and health impact assessment of liquid organic hydrogen carrier (LOHC) systems-challenges and preliminary results[J]. Energy & Environmental Science, 2015, 8(3): 1035-1045. |
| 119 | SHI Libin, QI Suitao, QU Jifeng, et al. Integration of hydrogenation and dehydrogenation based on dibenzyltoluene as liquid organic hydrogen energy carrier[J]. International Journal of Hydrogen Energy, 2019, 44(11): 5345-5354. |
| 120 | SHI Libin, ZHOU Yiming, QI Suitao, et al. Pt catalysts supported on H2 and O2 plasma-treated Al2O3 for hydrogenation and dehydrogenation of the liquid organic hydrogen carrier pair dibenzyltoluene and perhydrodibenzyltoluene[J]. ACS Catalysis, 2020, 10(18): 10661-10671. |
| 121 | XUE Wenjie, LIU Hongxia, ZHAO Binbin, et al. Single Rh1Co catalyst enabling reversible hydrogenation and dehydrogenation of N-ethylcarbazole for hydrogen storage[J]. Applied Catalysis B: Environmental, 2023, 327: 122453. |
| [1] | 王达锐, 孙洪敏, 王一棪, 唐智谋, 李芮, 范雪研, 杨为民. 分子筛催化反应过程高效化的技术进展[J]. 化工进展, 2024, 43(1): 1-18. |
| [2] | 苏梦军, 刘剑, 辛靖, 陈禹霏, 张海洪, 韩龙年, 朱元宝, 李洪宝. 气液混合强化在固定床加氢过程中的应用进展[J]. 化工进展, 2024, 43(1): 100-110. |
| [3] | 田时泓, 郭磊, 李娜, 宇文超, 许磊, 郭胜惠, 巨少华. 微波加热强化闪蒸工艺的科学基础及发展趋势[J]. 化工进展, 2024, 43(1): 135-144. |
| [4] | 罗芬, 杨晓琪, 段方麟, 李小江, 吴亮, 徐铜文. 双极膜研究进展及应用展望[J]. 化工进展, 2024, 43(1): 145-163. |
| [5] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [6] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [7] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [8] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [9] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [10] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [11] | 邵博识, 谭宏博. 锯齿波纹板对挥发性有机物低温脱除过程强化模拟分析[J]. 化工进展, 2023, 42(S1): 84-93. |
| [12] | 肖辉, 张显均, 兰治科, 王苏豪, 王盛. 液态金属绕流管束流动传热进展[J]. 化工进展, 2023, 42(S1): 10-20. |
| [13] | 盛维武, 程永攀, 陈强, 李小婷, 魏嘉, 李琳鸽, 陈险峰. 微气泡和微液滴双强化脱硫反应器操作分析[J]. 化工进展, 2023, 42(S1): 142-147. |
| [14] | 赵晨, 苗天泽, 张朝阳, 洪芳军, 汪大海. 负压状态窄缝通道乙二醇水溶液传热特性[J]. 化工进展, 2023, 42(S1): 148-157. |
| [15] | 黄益平, 李婷, 郑龙云, 戚傲, 陈政霖, 史天昊, 张新宇, 郭凯, 胡猛, 倪泽雨, 刘辉, 夏苗, 主凯, 刘春江. 三级环流反应器中气液流动与传质规律[J]. 化工进展, 2023, 42(S1): 175-188. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||