化工进展 ›› 2023, Vol. 42 ›› Issue (10): 5299-5309.DOI: 10.16085/j.issn.1000-6613.2022-2180
非金属元素掺杂石墨相氮化碳光催化材料的研究进展
宋亚丽1( ), 李紫燕1, 杨彩荣1, 黄龙2, 张宏忠1(
), 李紫燕1, 杨彩荣1, 黄龙2, 张宏忠1( )
)
- 1.郑州轻工业大学材料与化学工程学院,环境污染治理与生态修复河南省协同创新中心,河南 郑州 450001
2.郑州大学生态与环境学院,河南 郑州 450001
-
收稿日期:2022-11-23修回日期:2023-06-04出版日期:2023-10-15发布日期:2023-11-11 -
通讯作者:张宏忠 -
作者简介:宋亚丽(1988—),女,博士,讲师,主要研究方向为光催化技术在水体修复中的应用。E-mail:songyl@zzuli.edu.cn。 -
基金资助:国家自然科学基金(22006139);河南省创新型科技团队项目(CXTD2015023)
Research progress of non-metallic element doped graphitic carbon nitride photocatalytic materials
SONG Yali1( ), LI Ziyan1, YANG Cairong1, HUANG Long2, ZHANG Hongzhong1(
), LI Ziyan1, YANG Cairong1, HUANG Long2, ZHANG Hongzhong1( )
)
- 1.Henan Collaborative Innovation Center for Environmental Pollution Control and Ecological Restoration, College of Materials and Chemical Engineering, Zhengzhou University of Light Industry, Zhengzhou 450001, Henan, China
2.College of Ecology and Environment, Zhengzhou University, Zhengzhou 450001, Henan, China
-
Received:2022-11-23Revised:2023-06-04Online:2023-10-15Published:2023-11-11 -
Contact:ZHANG Hongzhong
摘要:
石墨相氮化碳(g-C3N4)是一种非金属光催化材料,其具有制备成本低、制备过程简单、绿色、无二次污染、带隙能可调控、热稳定性高等特点,使其成为人们在能源与环境领域研究和关注的焦点。然而,g-C3N4还存在比表面积小、禁带宽度较大、光生电子和空穴复合过快等缺点,限制了其发展。非金属元素掺杂可以对g-C3N4进行改性以有效解决以上问题,使其带隙减小,拓宽光谱响应范围,抑制光生电子-空穴对的复合,提高光吸收能力,来提高其光催化性能。本文对非金属元素掺杂g-C3N4的合成方法、应用等方面进行综述,从非金属单元素掺杂(单元素自掺杂和其他单元素掺杂)、非金属多元素共掺杂方面进行了总结。最后指出了在非金属元素掺杂g-C3N4方面,仍需要关注g-C3N4产量偏低、可见光利用效率不足、回收较难等问题,并强调了非金属元素掺杂g-C3N4在治理环境污染和应对能源危机方面的重要作用。
中图分类号:
引用本文
宋亚丽, 李紫燕, 杨彩荣, 黄龙, 张宏忠. 非金属元素掺杂石墨相氮化碳光催化材料的研究进展[J]. 化工进展, 2023, 42(10): 5299-5309.
SONG Yali, LI Ziyan, YANG Cairong, HUANG Long, ZHANG Hongzhong. Research progress of non-metallic element doped graphitic carbon nitride photocatalytic materials[J]. Chemical Industry and Engineering Progress, 2023, 42(10): 5299-5309.
| 序号 | 前体物质 | 掺杂元素 | 取代位点 | 带隙能量(掺杂后/未掺杂)/eV | 光催化活性评价指标 | 去除率或产氢率或产氨率(掺杂后/未掺杂) |
|---|---|---|---|---|---|---|
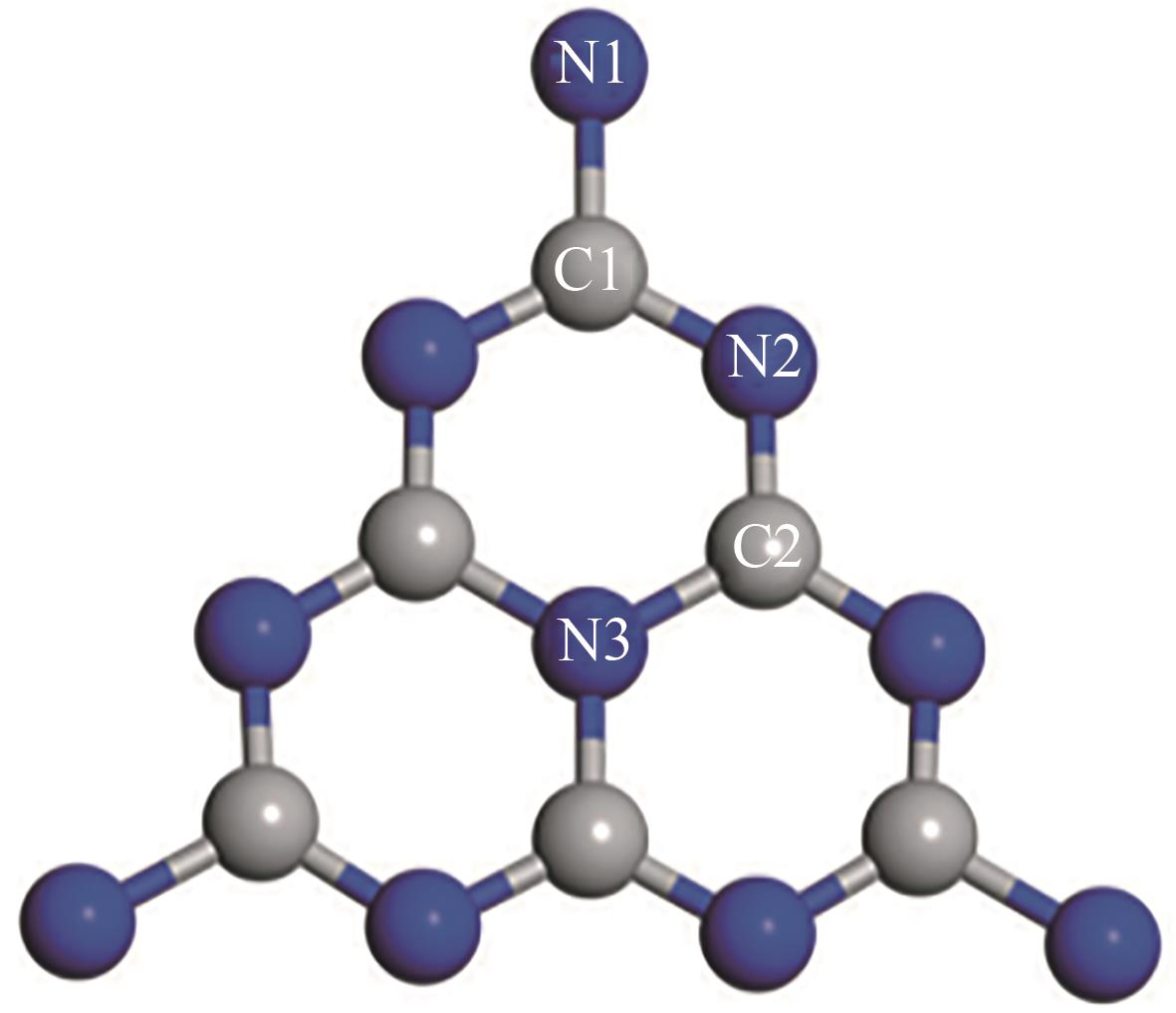
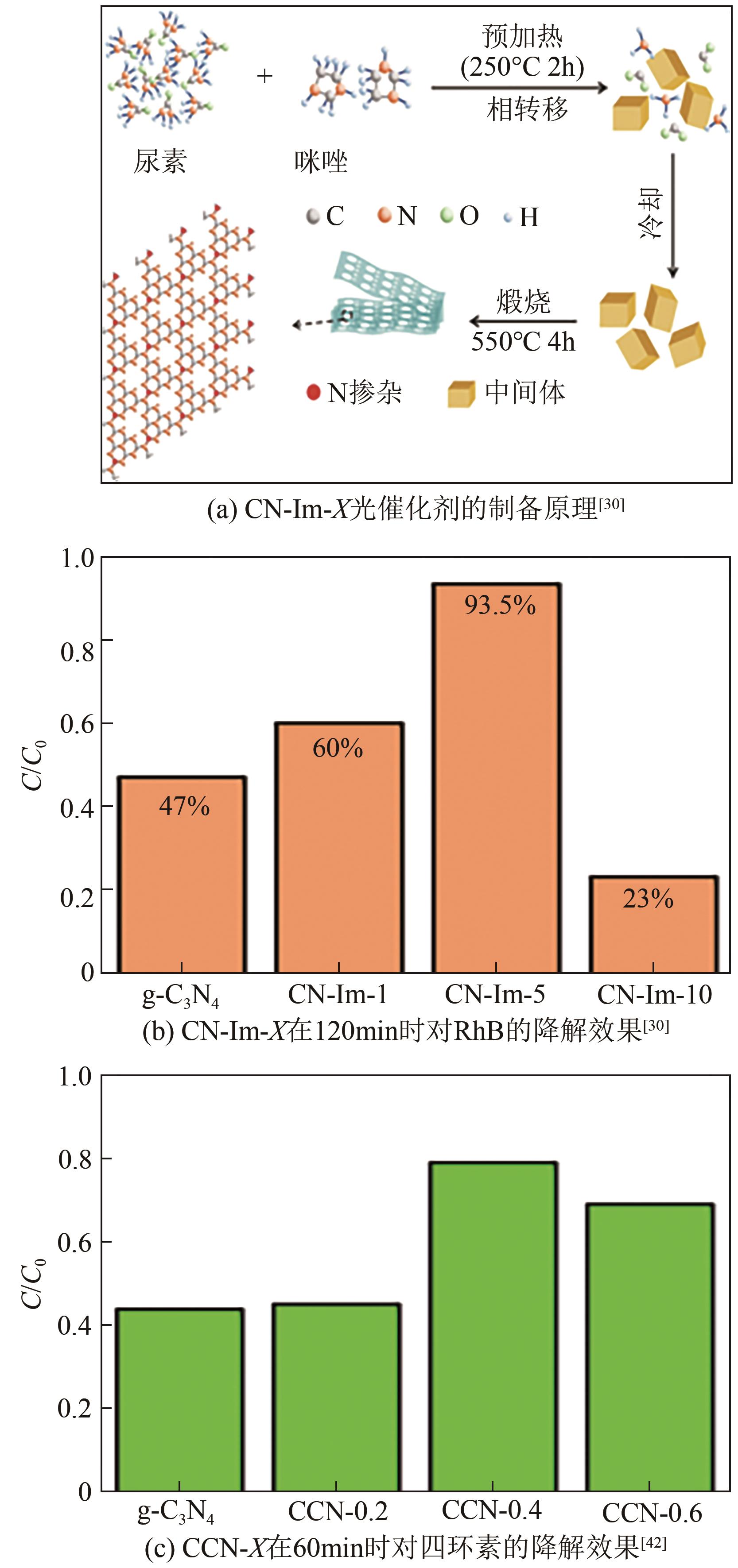
| 1 | 尿素+咪唑 | N | C1 | 2.26/2.72 | 罗丹明B去除率 | 93.5%/47%(提高2倍)[ |
| 2 | 双氰胺+二甲基甲酰胺 | C | N3 | 2.66/2.72 | 产氢率 | 8.88μmol·h-1/1.70μmol·h-1(提高5.2倍)[ |
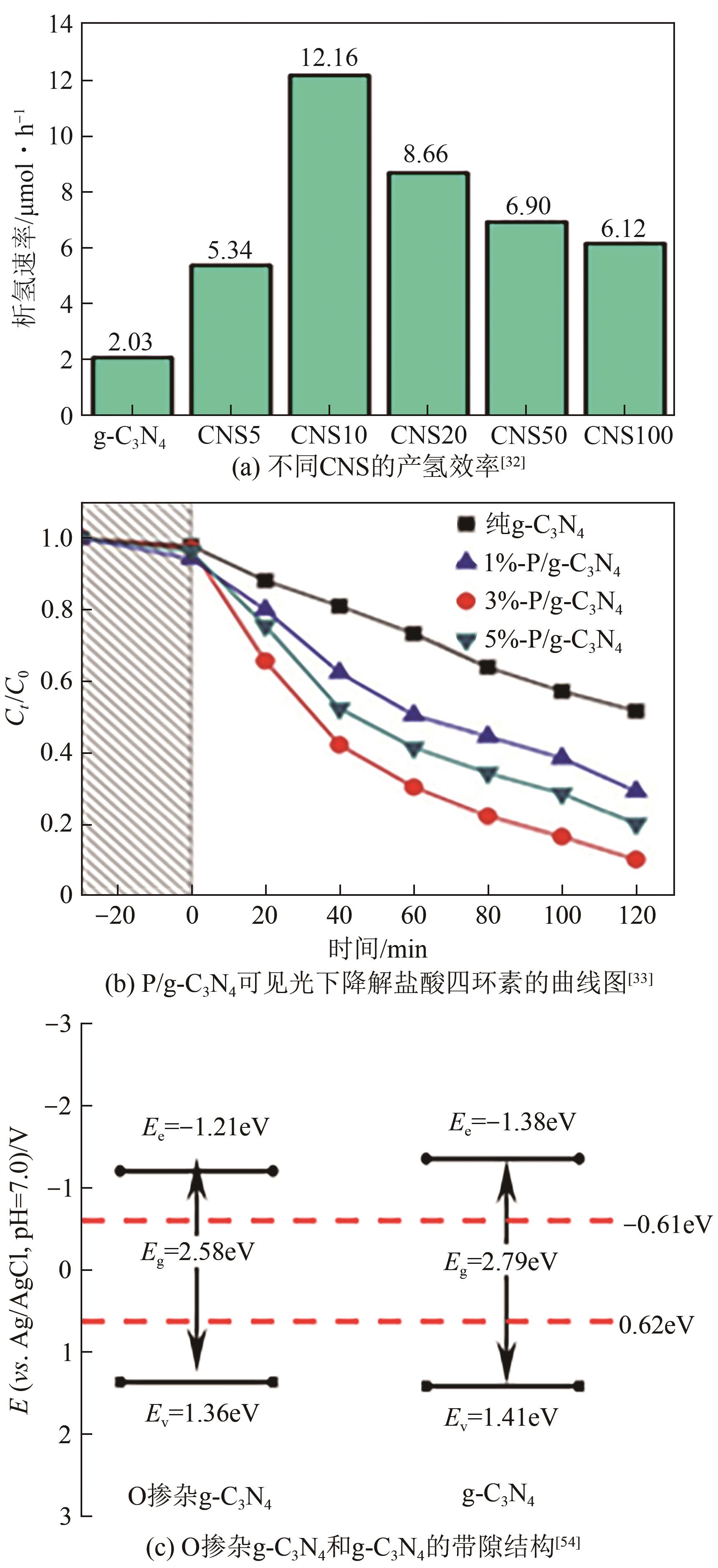
| 3 | 氰胺+硫脲 | S | N2 | — | 产氢率 | 12.16μmol·h-1/2.03μmol·h-1(提高6.0倍)[ |
| 4 | 尿素+1-丁基-3-甲基咪唑六氟磷酸盐 | P | — | 2.31/2.70 | 四环素去除率 | 85%/40%(提高2.1倍)[ |
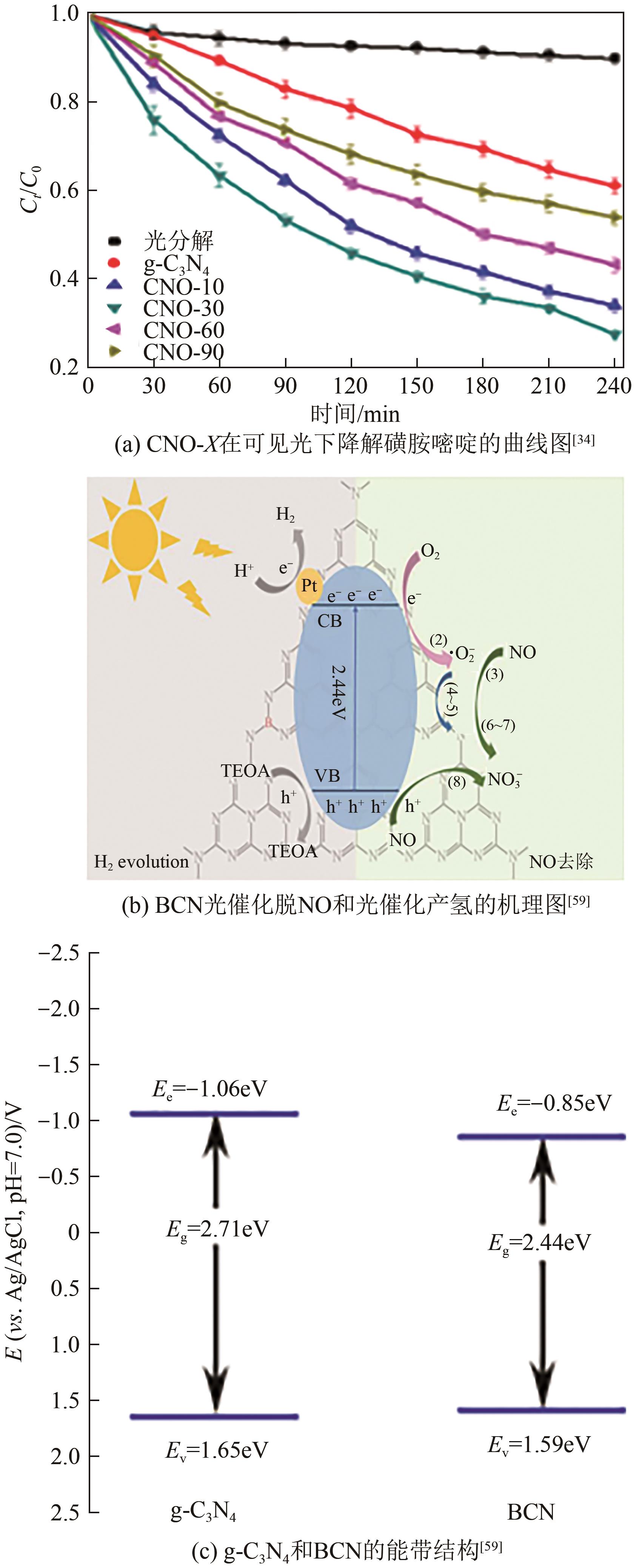
| 5 | 尿素 | O | N2 | 2.78/2.86 | 磺胺嘧啶去除率 | 72.64%/38.84%(提高1.8倍)[ |
| 6 | 尿素 | O | N1 | — | 罗丹明B去除率 | 99%/33%(提高3倍)[ |
| 7 | 硼酸+尿素 | B | — | 2.44/2.7 | 亚甲基蓝去除率 | 86%/11%(提高7.8倍)[ |
| 8 | 尿素+卤化铵 | F | N3 | 2.55/2.78 | 全氟辛酸去除率 | 74.3%/57.1%(提高1.3倍)[ |
| 9 | 三聚氯氰+双氰胺+乙腈 | Cl | N2 | 1.78/1.82 | 罗丹明B去除率 | 99.6%/16.8%(提高5.9倍)[ |
| 10 | 三聚氰胺+铵盐 | Br | 层间N | 2.75/2.78 | 土霉素去除率 | 75%/30%(提高2.5倍)[ |
| 11 | 双氰胺+碘酸钾 | I | N2 | 2.68/2.76 | 产氨率 | 200.8mg·L-1·g-1/71.9 mg·L-1·g-1(提高2.8倍)[ |
表1 非金属单元素掺杂g-C3N4的光催化特性
| 序号 | 前体物质 | 掺杂元素 | 取代位点 | 带隙能量(掺杂后/未掺杂)/eV | 光催化活性评价指标 | 去除率或产氢率或产氨率(掺杂后/未掺杂) |
|---|---|---|---|---|---|---|
| 1 | 尿素+咪唑 | N | C1 | 2.26/2.72 | 罗丹明B去除率 | 93.5%/47%(提高2倍)[ |
| 2 | 双氰胺+二甲基甲酰胺 | C | N3 | 2.66/2.72 | 产氢率 | 8.88μmol·h-1/1.70μmol·h-1(提高5.2倍)[ |
| 3 | 氰胺+硫脲 | S | N2 | — | 产氢率 | 12.16μmol·h-1/2.03μmol·h-1(提高6.0倍)[ |
| 4 | 尿素+1-丁基-3-甲基咪唑六氟磷酸盐 | P | — | 2.31/2.70 | 四环素去除率 | 85%/40%(提高2.1倍)[ |
| 5 | 尿素 | O | N2 | 2.78/2.86 | 磺胺嘧啶去除率 | 72.64%/38.84%(提高1.8倍)[ |
| 6 | 尿素 | O | N1 | — | 罗丹明B去除率 | 99%/33%(提高3倍)[ |
| 7 | 硼酸+尿素 | B | — | 2.44/2.7 | 亚甲基蓝去除率 | 86%/11%(提高7.8倍)[ |
| 8 | 尿素+卤化铵 | F | N3 | 2.55/2.78 | 全氟辛酸去除率 | 74.3%/57.1%(提高1.3倍)[ |
| 9 | 三聚氯氰+双氰胺+乙腈 | Cl | N2 | 1.78/1.82 | 罗丹明B去除率 | 99.6%/16.8%(提高5.9倍)[ |
| 10 | 三聚氰胺+铵盐 | Br | 层间N | 2.75/2.78 | 土霉素去除率 | 75%/30%(提高2.5倍)[ |
| 11 | 双氰胺+碘酸钾 | I | N2 | 2.68/2.76 | 产氨率 | 200.8mg·L-1·g-1/71.9 mg·L-1·g-1(提高2.8倍)[ |
| 序号 | 前驱体物质 | 掺杂元素 | 取代位置 | 催化活性评价 指标 | 去除率或产氢率 | 参考文献 | ||
|---|---|---|---|---|---|---|---|---|
| g-C3N4 | 单元素掺杂 | 多元素共掺杂 | ||||||
| 1 | 三聚氰胺+六氯三磷腈+硫 | P、S | P取代C1位点 S取代N2位点 | 甲基蓝去除率 | 58% | 69%(P掺杂) 55%(S掺杂) | 完全降解 | [ |
| 2 | 双氰胺+谷胱甘肽 | C、O | C取代N1位点 O取代N2位点 | 产氢率 | 0.23mmol·h-1·g-1 | — | 18.38mmol·h-1·g-1 | [ |
| 3 | 植酸+三聚氰胺 | P、C | — | 产氢率 | 153.9μmol·g-1·h-1 | — | 1493.3μmol·g-1·h-1 | [ |
| 4 | 三聚氰胺+乙醇 | C、O | C取代N3位点 O取代N2位点 | 产氢率 | 93μmol·g-1·h-1 | — | 395μmol·g-1·h-1 | [ |
| 5 | 双氰胺+尿素+ 四氟硼酸乙基吡啶 | B、F | — | 产氢率 | 36μmol·h-1 | — | 343.5μmol·h-1 | [ |
| 6 | 三聚氰胺+H2O2+三硫氰酸 | S、O | S取代N2位点 O取代N2位点 | 罗丹明B去除率 | 20 % | 48%(S掺杂) | 75% | [ |
| 7 | 三聚氰胺+磷酸铵+氯化铵 | P、Cl | P取代C1位点 Cl占据间隙位点 | 罗丹明B去除率 | 60% | 66.02%(P掺杂) 97.91%(Cl掺杂) | 99.62% | [ |
| 8 | 硫脲+磷酸氢二胺 | S、P | — | 罗丹明B去除率 | 15% | 23%(S掺杂) | 66% | [ |
| 9 | 双氰胺+硝酸+磷酸氢二铵 | P、O | — | 罗丹明B去除率 | 13% | 37%(O掺杂) | 95% | [ |
| 10 | 六氯环三磷腈+硫脲 | P、S | — | 四环素去除率 | 32.95% | 43.48%(P掺杂) 73.50%(S掺杂) | 85.85% | [ |
| 11 | 三聚氰胺+硫脲+ 磷酸氢二铵 | P、S和O | P取代C1位点 O取代N2位点 S占据间隙位点 | 产氢率 | 467.33μmol·g-1·h-1 | 574μmol·g-1·h-1(S掺杂) 1141μmol·g-1·h-1(P掺杂) | 2479μmol·g-1·h-1 | [ |
表2 非金属多元素掺杂g-C3N4的光催化特性
| 序号 | 前驱体物质 | 掺杂元素 | 取代位置 | 催化活性评价 指标 | 去除率或产氢率 | 参考文献 | ||
|---|---|---|---|---|---|---|---|---|
| g-C3N4 | 单元素掺杂 | 多元素共掺杂 | ||||||
| 1 | 三聚氰胺+六氯三磷腈+硫 | P、S | P取代C1位点 S取代N2位点 | 甲基蓝去除率 | 58% | 69%(P掺杂) 55%(S掺杂) | 完全降解 | [ |
| 2 | 双氰胺+谷胱甘肽 | C、O | C取代N1位点 O取代N2位点 | 产氢率 | 0.23mmol·h-1·g-1 | — | 18.38mmol·h-1·g-1 | [ |
| 3 | 植酸+三聚氰胺 | P、C | — | 产氢率 | 153.9μmol·g-1·h-1 | — | 1493.3μmol·g-1·h-1 | [ |
| 4 | 三聚氰胺+乙醇 | C、O | C取代N3位点 O取代N2位点 | 产氢率 | 93μmol·g-1·h-1 | — | 395μmol·g-1·h-1 | [ |
| 5 | 双氰胺+尿素+ 四氟硼酸乙基吡啶 | B、F | — | 产氢率 | 36μmol·h-1 | — | 343.5μmol·h-1 | [ |
| 6 | 三聚氰胺+H2O2+三硫氰酸 | S、O | S取代N2位点 O取代N2位点 | 罗丹明B去除率 | 20 % | 48%(S掺杂) | 75% | [ |
| 7 | 三聚氰胺+磷酸铵+氯化铵 | P、Cl | P取代C1位点 Cl占据间隙位点 | 罗丹明B去除率 | 60% | 66.02%(P掺杂) 97.91%(Cl掺杂) | 99.62% | [ |
| 8 | 硫脲+磷酸氢二胺 | S、P | — | 罗丹明B去除率 | 15% | 23%(S掺杂) | 66% | [ |
| 9 | 双氰胺+硝酸+磷酸氢二铵 | P、O | — | 罗丹明B去除率 | 13% | 37%(O掺杂) | 95% | [ |
| 10 | 六氯环三磷腈+硫脲 | P、S | — | 四环素去除率 | 32.95% | 43.48%(P掺杂) 73.50%(S掺杂) | 85.85% | [ |
| 11 | 三聚氰胺+硫脲+ 磷酸氢二铵 | P、S和O | P取代C1位点 O取代N2位点 S占据间隙位点 | 产氢率 | 467.33μmol·g-1·h-1 | 574μmol·g-1·h-1(S掺杂) 1141μmol·g-1·h-1(P掺杂) | 2479μmol·g-1·h-1 | [ |
| 1 | WEI Bo, WANG Wei, SUN Jianfei, et al. Insight into the effect of boron doping on electronic structure, photocatalytic and adsorption performance of g-C3N4 by first-principles study[J]. Applied Surface Science, 2020, 511: 145549. |
| 2 | ZHOU Yue, Wenhua LYU, ZHU Binglong, et al. Template-free one-step synthesis of g-C3N4 nanosheets with simultaneous porous network and S-doping for remarkable visible-light-driven hydrogen evolution[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(6): 5801-5807. |
| 3 | LIU Mingquan, JIAO Yingying, QIN Junchao, et al. Boron doped C3N4 nanodots/nonmetal element (S, P, F, Br) doped C3N4 nanosheets heterojunction with synergistic effect to boost the photocatalytic hydrogen production performance[J]. Applied Surface Science, 2021, 541: 148558. |
| 4 | WANG Xinchen, MAEDA Kazuhiko, THOMAS Arne, et al. A metal-free polymeric photocatalyst for hydrogen production from water under visible light[J]. Nature Materials, 2009, 8(1): 76-80. |
| 5 | 王衍坤, 方元行, 王心晨. 氮化碳光催化分解水的研究进展[J]. 福州大学学报(自然科学版), 2021, 49(5): 577-587. |
| WANG Yankun, FANG Yuanxing, WANG Xinchen. Progresses of carbon nitride polymers for water splitting[J]. Journal of Fuzhou University (Natural Science Edition), 2021, 49(5): 577-587. | |
| 6 | CHEN Jingling, HONG Zhenhua, CHEN Yilin, et al. One-step synthesis of sulfur-doped and nitrogen-deficient g-C3N4 photocatalyst for enhanced hydrogen evolution under visible light[J]. Materials Letters, 2015, 145: 129-132. |
| 7 | YANG Liuqing, HUANG Jianfeng, SHI Li, et al. A surface modification resultant thermally oxidized porous g-C3N4 with enhanced photocatalytic hydrogen production[J]. Applied Catalysis B: Environmental, 2017, 204: 335-345. |
| 8 | ARUMUGAM Malathi, TAHIR Muhammad, PRASERTHDAM Piyasan. Effect of nonmetals (B, O, P, and S) doped with porous g-C3N4 for improved electron transfer towards photocatalytic CO2 reduction with water into CH4 [J]. Chemosphere, 2022, 286: 131765. |
| 9 | 吴桧, 陈雪燕, 邹贵容, 等. 石墨相氮化碳在光催化降解领域的研究进展[J]. 辽宁化工, 2021, 50(9): 1318-1320. |
| WU Hui, SHEN Xueyan, ZOU Guirong, et al. Research progress of graphite carbon nitride in the photocatalytic degradation field[J]. Liaoning Chemical Industry, 2021, 50(9): 1318-1320. | |
| 10 | SHI Yuxing, LI Lingling, XU Zheng, et al. One-step simple green method to prepare carbon-doped graphitic carbon nitride nanosheets for boosting visible-light photocatalytic degradation of tetracycline[J]. Journal of Chemical Technology & Biotechnology, 2021, 96(11): 3122-3133. |
| 11 | PATTANAYAK D S, PAL D, MISHRA J, et al. Noble metal–free doped graphitic carbon nitride (g-C3N4) for efficient photodegradation of antibiotics: Progress, limitations, and future directions[J]. Environmental Science and Pollution Research, 2022, 30: 25546-25558. |
| 12 | WANG Xing, LIANG Yinghua, AN Weijia, et al. Removal of chromium (Ⅵ) by a self-regenerating and metal free g-C3N4/graphene hydrogel system via the synergy of adsorption and photo-catalysis under visible light[J]. Applied Catalysis B: Environmental, 2017, 219: 53-62. |
| 13 | XING Weinan, CHENG Ke, ZHANG Yichi, et al. Incorporation of nonmetal group dopants into g-C3N4 framework for highly improved photocatalytic H2 production[J]. Nanomaterials, 2021, 11(6): 1480. |
| 14 | MISHRA Bhagyashree P, BABU Pradeepta, PARIDA Kulamani. Phosphorous, boron and sulfur doped g-C3N4 nanosheet: Synthesis, characterization, and comparative study towards photocatalytic hydrogen generation[J]. Materials Today: Proceedings, 2021, 35: 258-262. |
| 15 | 谢磊, 刘帅, 孙有为, 等. 石墨相氮化碳光催化剂的研究进展[J]. 石油化工高等学校学报, 2021, 34(6): 27-34. |
| XIE Lei, LIU Shuai, SUN Youwei, et al. Research progress of graphite phase carbon nitride photocatalysts[J]. Journal of Petrochemical Universities, 2021, 34(6): 27-34. | |
| 16 | 李琛, 孙官超, 周烈兴, 等. g-C3N4结构调控及其复合体系的研究进展[J]. 水处理技术, 2022, 48(5): 18-23. |
| LI Chen, SUN Guanchao, ZHOU Liexing, et al. Research progress of structural regulation on g-C3N4 and corresponding composite system[J]. Technology of Water Treatment, 2022, 48(5): 18-23. | |
| 17 | 毛德贤, 王显恒, 王乾. 氮化碳复合材料光催化性能研究进展[J]. 内蒙古石油化工, 2020, 46(9): 18-19. |
| MAO Dexian, WANG Xianheng, WANG Qian. The research progress in photocatalytic performance for carbon nitride composites[J]. Inner Mongolia Petrochemical Industry, 2020, 46(9): 18-19. | |
| 18 | 梁海欧, 许瞳, 白杰, 等. 类石墨相氮化碳改性研究进展[J]. 化学通报, 2022, 85(1): 72-77, 51. |
| LIANG Haiou, XU Tong, BAI Jie, et al. Research advances in the modification of graphitic carbon nitride[J]. Chemistry, 2022, 85(1): 72-77, 51. | |
| 19 | 黎小芳, 沈群, 李覃, 等. 光催化材料石墨相氮化碳研究进展[J]. 中南民族大学学报(自然科学版), 2021, 40(5): 441-452. |
| LI Xiaofang, SHEN Qun, LI Qin, et al. Research progress of photocatalytic graphitic carbon nitride[J]. Journal of South-Central University for Nationalities (Natural Science Edition), 2021, 40(5): 441-452. | |
| 20 | LUO Yidan, WANG Jiaming, YU Shuohan, et al. Nonmetal element doped g-C3N4 with enhanced H2 evolution under visible light irradiation[J]. Journal of Materials Research, 2018, 33(9): 1268-1278. |
| 21 | MA Xinguo, Yanhui LYU, XU Jing, et al. A strategy of enhancing the photoactivity of g-C3N4 via doping of nonmetal elements: A first-principles study[J]. The Journal of Physical Chemistry C, 2012, 116(44): 23485-23493. |
| 22 | GUO Shien, DENG Zhaopeng, LI Mingxia, et al. Phosphorus-doped carbon nitride tubes with a layered micro-nanostructure for enhanced visible-light photocatalytic hydrogen evolution[J]. Angewandte Chemie International Edition, 2016, 55(5): 1830-1834. |
| 23 | HE Fang, WANG Zhenxing, LI Yuexiang, et al. The nonmetal modulation of composition and morphology of g-C3N4-based photocatalysts[J]. Applied Catalysis B: Environmental, 2020, 269: 118828. |
| 24 | JIANG Longbo, YUAN Xingzhong, PAN Yang, et al. Doping of graphitic carbon nitride for photocatalysis: A reveiw[J]. Applied Catalysis B: Environmental, 2017, 217: 388-406. |
| 25 | QUYEN Vu THI, KIM Hoon Jae, KIM JiTae, et al. Synthesizing S-doped graphitic carbon nitride for improvement photodegradation of tetracycline under solar light[J]. Solar Energy, 2021, 214: 288-293. |
| 26 | 彭小明, 罗文栋, 胡锋平, 等. 石墨类氮化碳改性方法的研究进展[J]. 水处理技术, 2019, 45(12): 1-6, 12. |
| PENG Xiaoming, LUO Wendong, HU Fengping, et al. Research progress of the modified method for graphitic carbon nitride[J]. Technology of Water Treatment, 2019, 45(12): 1-6, 12. | |
| 27 | 张家晶, 郑永杰, 金春雪, 等. g-C3N4基光催化剂改性的研究进展[J]. 现代化工, 2021, 41(3): 42-47. |
| ZHANG Jiajing, ZHENG Yongjie, JIN Chunxue, et al. Research progress on modification of g-C3N4-based photocatalyst[J]. Modern Chemical Industry, 2021, 41(3): 42-47. | |
| 28 | 王福禄, 王建华, 李子强. 氮化碳光催化材料的掺杂改性研究进展[J]. 精细石油化工, 2020, 37(3): 69-76. |
| WANG Fulu, WANG Jianhua, LI Ziqiang. Research progress on modification of carbon nitride photocatalyst by doping method[J]. Speciality Petrochemicals, 2020, 37(3): 69-76. | |
| 29 | HUANG Yan, NING Lichao, FENG Zibo, et al. Graphitic carbon nitride nanosheets with low ON1-doping content as efficient photocatalysts for organic pollutant degradation[J]. Environmental Science: Nano, 2021, 8(2): 460-469. |
| 30 | QI Huilan, LIU Yanan, LI Chengyun, et al. Precursor-reforming protocol to synthesis of porous N-doped g-C3N4 for highly improved photocatalytic water treatments[J]. Materials Letters, 2020, 264: 127329. |
| 31 | CAO Jingsheng, FAN Huiqing, WANG Chao, et al. Facile synthesis of carbon self-doped g-C3N4 for enhanced photocatalytic hydrogen evolution[J]. Ceramics International, 2020, 46(6): 7888-7895. |
| 32 | GE Lei, HAN Changcun, XIAO Xinlai, et al. Enhanced visible light photocatalytic hydrogen evolution of sulfur-doped polymeric g-C3N4 photocatalysts[J]. Materials Research Bulletin, 2013, 48(10): 3919-3925. |
| 33 | ZHAO Kun, KHAN Iltaf, QI Kezhen, et al. Ionic liquid assisted preparation of phosphorus-doped g-C3N4 photocatalyst for decomposition of emerging water pollutants[J]. Materials Chemistry and Physics, 2020, 253: 123322. |
| 34 | CAO Shihai, ZHANG Yu, DING Keqiang, et al. Efficient visible light driven degradation of antibiotic pollutants by oxygen-doped graphitic carbon nitride via the homogeneous supramolecular assembly of urea[J]. Environmental Research, 2022, 210: 112920. |
| 35 | YAN Qian, HUANG Guifang, LI Dongfeng, et al. Facile synthesis and superior photocatalytic and electrocatalytic performances of porous B-doped g-C3N4 nanosheets[J]. Journal of Materials Science & Technology, 2018, 34(12): 2515-2520. |
| 36 | CHEN Zesen, CHEN Weirui, LIAO Gaozu, et al. Flexible construct of N vacancies and hydrophobic sites on g-C3N4 by F doping and their contribution to PFOA degradation in photocatalytic ozonation[J]. Journal of Hazardous Materials, 2022, 428: 128222. |
| 37 | CAO Mengyu, WANG Ke, TUDELA Ignacio, et al. Improve photocatalytic performance of g-C3N4 through balancing the interstitial and substitutional chlorine doping[J]. Applied Surface Science, 2021, 536: 147784. |
| 38 | HONG Jeonghyun, HWANG Dae Kun, SELVARAJ Rengaraj, et al. Facile synthesis of Br-doped g-C3N4 nanosheets via one-step exfoliation using ammonium bromide for photodegradation of oxytetracycline antibiotics[J]. Journal of Industrial and Engineering Chemistry, 2019, 79: 473-481. |
| 39 | HU Xiuli, ZHANG Wenjun, YONG Yuwen, et al. One-step synthesis of iodine-doped g-C3N4 with enhanced photocatalytic nitrogen fixation performance[J]. Applied Surface Science, 2020, 510: 145413. |
| 40 | ZHOU Yajun, ZHANG Lingxia, HUANG Weimin, et al. N-doped graphitic carbon-incorporated g-C3N4 for remarkably enhanced photocatalytic H2 evolution under visible light[J]. Carbon, 2016, 99: 111-117. |
| 41 | JIANG Longbo, YUAN Xingzhong, ZENG Guangming, et al. Nitrogen self-doped g-C3N4 nanosheets with tunable band structures for enhanced photocatalytic tetracycline degradation[J]. Journal of Colloid and Interface Science, 2019, 536: 17-29. |
| 42 | LIU Wenshi, WANG Baogang. Biomimetic synthesis of C-doped g-C3N4 spinous hollow microspheres from sunflower pollen with enhanced visible-light photocatalytic performance[J]. Fullerenes, Nanotubes and Carbon Nanostructures, 2021, 29(12): 966-973. |
| 43 | CHEN Zhou, FAN Tingting, YU Xiang, et al. Gradual carbon doping of graphitic carbon nitride towards metal-free visible light photocatalytic hydrogen evolution[J]. Journal of Materials Chemistry A, 2018, 6(31): 15310-15319. |
| 44 | LIU Guangqing, XUE Mengwei, LIU Qinpu, et al. Facile synthesis of C-doped hollow spherical g-C3N4 from supramolecular self-assembly for enhanced photoredox water splitting[J]. International Journal of Hydrogen Energy, 2019, 44(47): 25671-25679. |
| 45 | WANG Wei, LIU Chengyin, XU Shanshan, et al. Intermediate-hydrothermal strategy of carbon doped g-C3N4 for improved photocatalytic degradation and disinfection capacity[J]. Inorganic Chemistry Communications, 2022, 139: 109335. |
| 46 | GUO Hui, SHU Zhu, CHEN Donghang, et al. One-step synthesis of S-doped g-C3N4 nanosheets for improved visible-light photocatalytic hydrogen evolution[J]. Chemical Physics, 2020, 533: 110714. |
| 47 | LIN Yanru, DIZON Gian Vincent Canlas, YAMADA Kanta, et al. Sulfur-doped g-C3N4 nanosheets for photocatalysis: Z-scheme water splitting and decreased biofouling[J]. Journal of Colloid and Interface Science, 2020, 567: 202-212. |
| 48 | LIU Gang, NIU Ping, SUN Chenghua, et al. Unique electronic structure induced high photoreactivity of sulfur-doped graphitic C3N4 [J]. Journal of the American Chemical Society, 2010, 132(33): 11642-11648. |
| 49 | WANG Ke, LI Qin, LIU Baoshun, et al. Sulfur-doped g-C3N4 with enhanced photocatalytic CO2-reduction performance[J]. Applied Catalysis B: Environmental, 2015, 176/177: 44-52. |
| 50 | WANG Yuelin, TIAN Yu, YAN Likai, et al. DFT study on sulfur-doped g-C3N4 nanosheets as a photocatalyst for CO2 reduction reaction[J]. The Journal of Physical Chemistry C, 2018, 122(14): 7712-7719. |
| 51 | LEBLANC Gabriel, CHEN Gongping, GIZZIE Evan A, et al. Enhanced photocurrents of photosystem I films on P-doped silicon[J]. Advanced Materials, 2012, 24(44): 5959-5962. |
| 52 | RAN Jingrun, MA Tianyi, GAO Guoping, et al. Porous P-doped graphitic carbon nitride nanosheets for synergistically enhanced visible-light photocatalytic H2 production[J]. Energy & Environmental Science, 2015, 8(12): 3708-3717. |
| 53 | ZHANG Wendong, ZHANG Jie, DONG Fan, et al. Facile synthesis of in situ phosphorus-doped g-C3N4 with enhanced visible light photocatalytic property for NO purification[J]. RSC Advances, 2016, 6(91): 88085-88089. |
| 54 | MEI Riguo, MA Lei, AN Liang, et al. Layered spongy-like O-doped g-C3N4: An efficient non-metal oxygen reduction catalyst for alkaline fuel cells[J]. Journal of the Electrochemical Society, 2017, 164(4): F354-F363. |
| 55 | SHE Xiaojie, LIU Liang, JI Haiyan, et al. Template-free synthesis of 2D porous ultrathin nonmetal-doped g-C3N4 nanosheets with highly efficient photocatalytic H2 evolution from water under visible light[J]. Applied Catalysis B: Environmental, 2016, 187: 144-153. |
| 56 | YANG Yajing, BIAN Zhaoyong. Oxygen doping through oxidation causes the main active substance in g-C3N4 photocatalysis to change from holes to singlet oxygen[J]. Science of the Total Environment, 2021, 753: 141908. |
| 57 | CHEN Pengfei, XING Pingxing, CHEN Zhiqiang, et al. Rapid and energy-efficient preparation of boron doped g-C3N4 with excellent performance in photocatalytic H2-evolution[J]. International Journal of Hydrogen Energy, 2018, 43(43): 19984-19989. |
| 58 | SAGARA Nobuhiro, KAMIMURA Sunao, TSUBOTA Toshiki, et al. Photoelectrochemical CO2 reduction by a p-type boron-doped g-C3N4 electrode under visible light[J]. Applied Catalysis B: Environmental, 2016, 192: 193-198. |
| 59 | XIA Xiang, XIE Cong, XU Baogang, et al. Role of B-doping in g-C3N4 nanosheets for enhanced photocatalytic NO removal and H2 generation[J]. Journal of Industrial and Engineering Chemistry, 2022, 105: 303-312. |
| 60 | WANG Dongbo, HUANG Xianqing, HUANG Ying, et al. Self-assembly synthesis of petal-like Cl-doped g-C3N4 nanosheets with tunable band structure for enhanced photocatalytic activity[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2021, 611: 125780. |
| 61 | TONG Jincheng, ZHANG Li, LI Fei, et al. Rapid and high-yield production of g-C3N4 nanosheets via chemical exfoliation for photocatalytic H2 evolution[J]. RSC Advances, 2015, 5(107): 88149-88153. |
| 62 | ZHU Bicheng, ZHANG Jinfeng, JIANG Chuanjia, et al. First principle investigation of halogen-doped monolayer g-C3N4 photocatalyst[J]. Applied Catalysis B: Environmental, 2017, 207: 27-34. |
| 63 | ZHAO Zaiwang, SUN Yanjuan, DONG Fan, et al. Template synthesis of carbon self-doped g-C3N4 with enhanced visible to near-infrared absorption and photocatalytic performance[J]. RSC Advances, 2015, 5(49): 39549-39556. |
| 64 | HU Chechia, HUNG Wei-Zeng, WANG Maosheng, et al. Phosphorus and sulfur codoped g-C3N4 as an efficient metal-free photocatalyst[J]. Carbon, 2018, 127: 374-383. |
| 65 | WU Jiaojiao, LI Nan, ZHANG Xiaohong, et al. Heteroatoms binary-doped hierarchical porous g-C3N4 nanobelts for remarkably enhanced visible-light-driven hydrogen evolution[J]. Applied Catalysis B: Environmental, 2018, 226: 61-70. |
| 66 | ZHAO Zhengliang, SHU Zhu, ZHOU Jun, et al. Facile one-pot synthesis of C, O-codoped nano-structured g-C3N4 with superior photocatalytic hydrogen evolution[J]. Materials Research Bulletin, 2022, 145: 111565. |
| 67 | WANG Hao, WANG Bo, BIAN Yaru, et al. Enhancing photocatalytic activity of graphitic carbon nitride by codoping with P and C for efficient hydrogen generation[J]. ACS Applied Materials & Interfaces, 2017, 9(26): 21730-21737. |
| 68 | LIU Qinqin, SHEN Jiyou, YU Xiaohui, et al. Unveiling the origin of boosted photocatalytic hydrogen evolution in simultaneously (S, P, O)-Codoped and exfoliated ultrathin g-C3N4 nanosheets[J]. Applied Catalysis B: Environmental, 2019, 248: 84-94. |
| 69 | WANG Hao, YANG Chuanfeng, LI Ming, et al. Enhanced photocatalytic hydrogen production of restructured B/F codoped g-C3N4 via post-thermal treatment[J]. Materials Letters, 2018, 212: 319-322. |
| 70 | YOU Ran, DOU Hailong, CHEN Lu, et al. Graphitic carbon nitride with S and O codoping for enhanced visible light photocatalytic performance[J]. RSC Advances, 2017, 7(26): 15842-15850. |
| 71 | YANG Yanqing, JIN Hufang, ZHANG Chi, et al. Nitrogen-deficient modified P-Cl co-doped graphitic carbon nitride with enhanced photocatalytic performance[J]. Journal of Alloys and Compounds, 2020, 821: 153439. |
| 72 | HU Shaozheng, MA Lin, XIE Ying, et al. Hydrothermal synthesis of oxygen functionalized S-P codoped g-C3N4 nanorods with outstanding visible light activity under anoxic conditions[J]. Dalton Transactions, 2015, 44(48): 20889-20897. |
| 73 | MA Huiqiang, LI Yang, LI Shuang, et al. Novel PO codoped g-C3N4 with large specific surface area: Hydrothermal synthesis assisted by dissolution-precipitation process and their visible light activity under anoxic conditions[J]. Applied Surface Science, 2015, 357: 131-138. |
| 74 | JIANG Longbo, YUAN Xingzhong, ZENG Guangming, et al. Phosphorus- and sulfur-codoped g-C3N4: Facile preparation, mechanism insight, and application as efficient photocatalyst for tetracycline and methyl orange degradation under visible light irradiation[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(7): 5831-5841. |
| [1] | 陈彰旭, 李燕辉, 任红云, 张丽丹, 郑炳云. g-C3N4/MIL-125(Ti)磁性复合材料制备及其去除罗丹明B[J]. 化工进展, 2022, 41(12): 6469-6476. |
| [2] | 周杰, 孙月, 包妍, 刘泽珏, 张沙沙, 朱蓓蓓, 王璐, 管国锋. 低维石墨相氮化碳合成方法研究进展[J]. 化工进展, 2022, 41(12): 6430-6442. |
| [3] | 王文霞, 刘小丰, 陈浠, 许艳虹, 蒙振邦, 郑俊霞, 安太成. 多孔g-C3N4基光催化材料的制备及应用研究进展[J]. 化工进展, 2022, 41(1): 300-309. |
| [4] | 张轩, 郑丽君. 光解水制氢单相催化剂研究进展[J]. 化工进展, 2021, 40(S1): 215-222. |
| [5] | 李酽, 宋双, 连晓雪. MoS2/ZnO纳米复合材料的光学和光催化性能[J]. 化工进展, 2021, 40(7): 3870-3877. |
| [6] | 段丽媛, 李国强, 张舒婷, 王宏宇, 赵永乐, 张永发. 二次等温热缩聚改性对g-C3N4光催化剂性能的影响[J]. 化工进展, 2021, 40(6): 3389-3400. |
| [7] | 孙金龙, 张宇, 刘福跃, 田浩然, 刘崎峰. 基于碳基催化剂活化过二硫酸盐降解有机污染物的研究进展[J]. 化工进展, 2021, 40(3): 1653-1666. |
| [8] | 李筱玲, 邓寒霜, 赵艳艳. Ag/g-C3N4光催剂的构建及降解7-氨基头孢烷酸机理[J]. 化工进展, 2020, 39(9): 3716-3722. |
| [9] | 周琱玉,李涛涛,王辉,乔珺威,梁伟. Au@TiO2纳米管阵列的制备及光催化性能[J]. 化工进展, 2019, 38(03): 1403-1410. |
| [10] | 杨冬, 周致远, 丁菲, 赵旭阳, 陈瑶, 姜忠义. 特殊形貌g-C3N4基光催化材料的研究进展[J]. 化工进展, 2019, 38(01): 495-504. |
| [11] | 张英杰, 朱子翼, 董鹏, 赵少博, 章艳佳, 杨成云, 杨城沣, 韦克毅, 李雪. 钠离子电池碳基负极材料的研究进展[J]. 化工进展, 2017, 36(11): 4106-4115. |
| [12] | 郭雅容, 陈志鸿, 刘琼, 张正国, 方晓明. 石墨相氮化碳光催化剂研究进展[J]. 化工进展, 2016, 35(07): 2063-2070. |
| [13] | 李家德, 方稳, 周晚琴, 余长林. 银基半导体光催化剂研究进展[J]. 化工进展, 2015, 34(1): 113-118. |
| [14] | 尹 莉,陈德良,李 涛,张 毅,张 锐. 贵金属/WO3复合纳米晶的气敏与光催化研究进展[J]. 化工进展, 2012, 31(01 ): 133-143. |
| [15] | 廖建军,李士普,曹献坤,曹 阳,林仕伟. 有序TiO2纳米管阵列光催化性能研究进展 [J]. 化工进展, 2011, 30(9): 2003-. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||