化工进展 ›› 2023, Vol. 42 ›› Issue (7): 3802-3815.DOI: 10.16085/j.issn.1000-6613.2022-1671
纳米流体用于二氧化碳吸收分离研究进展
娄宝辉1,2,3( ), 吴贤豪2,3, 张驰1,2,3, 陈臻2,3, 冯向东2,3(
), 吴贤豪2,3, 张驰1,2,3, 陈臻2,3, 冯向东2,3( )
)
- 1.浙江大学材料科学与工程学院,浙江 杭州,310027
2.浙能技术研究院有限公司,浙江 杭州 311121
3.浙江省火力发电高效节能与污染物控制技术研究重点实验室,浙江 杭州 311121
-
收稿日期:2022-09-08修回日期:2022-11-21出版日期:2023-07-15发布日期:2023-08-14 -
通讯作者:冯向东 -
作者简介:娄宝辉(1992—),男,博士研究生,研究方向为能源高效清洁转化与低碳发展。E-mail:loubaohui@qq.com。 -
基金资助:中国博士后科学基金(2022M712738)
Advances in nanofluid for CO2 absorption and separation
LOU Baohui1,2,3( ), WU Xianhao2,3, ZHANG Chi1,2,3, CHEN Zhen2,3, FENG Xiangdong2,3(
), WU Xianhao2,3, ZHANG Chi1,2,3, CHEN Zhen2,3, FENG Xiangdong2,3( )
)
- 1.School of Materials Science and Engineering, Zhejiang University, Hangzhou 310027, Zhejiang, China
2.Zhejiang Energy R&D Institute, Hangzhou 311121, Zhejiang, China
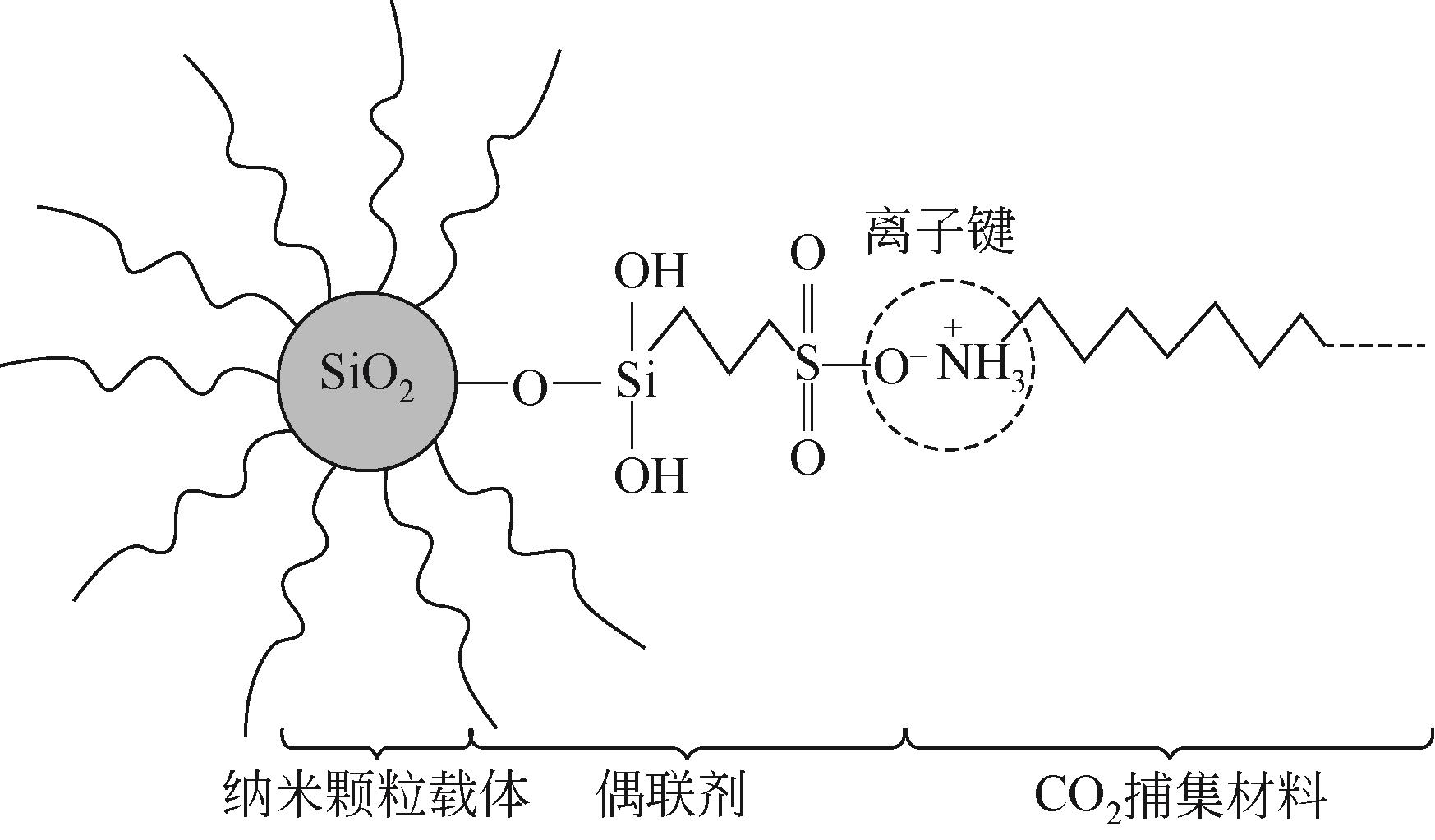
3.Key Research Laboratory of High Efficiency Energy Saving and Pollutant Control Technology in Thermal Power Generation of Zhejiang Province, Hangzhou 311121, Zhejiang, China
-
Received:2022-09-08Revised:2022-11-21Online:2023-07-15Published:2023-08-14 -
Contact:FENG Xiangdong
摘要:
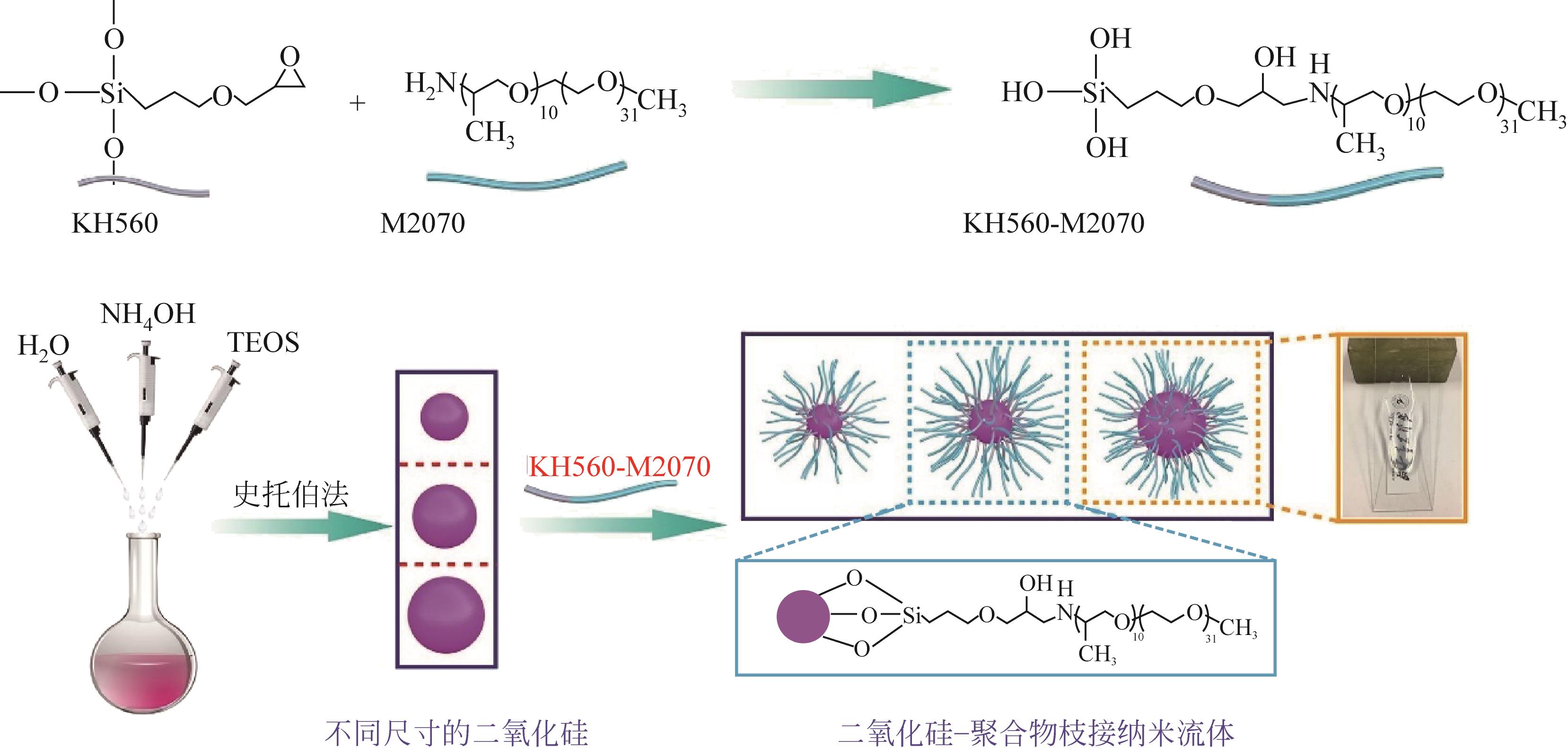
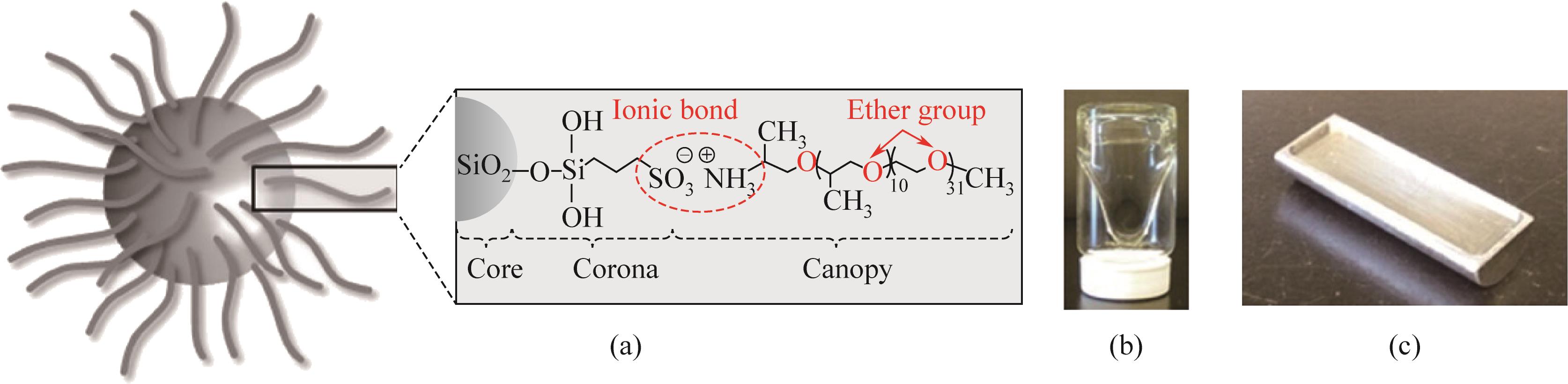
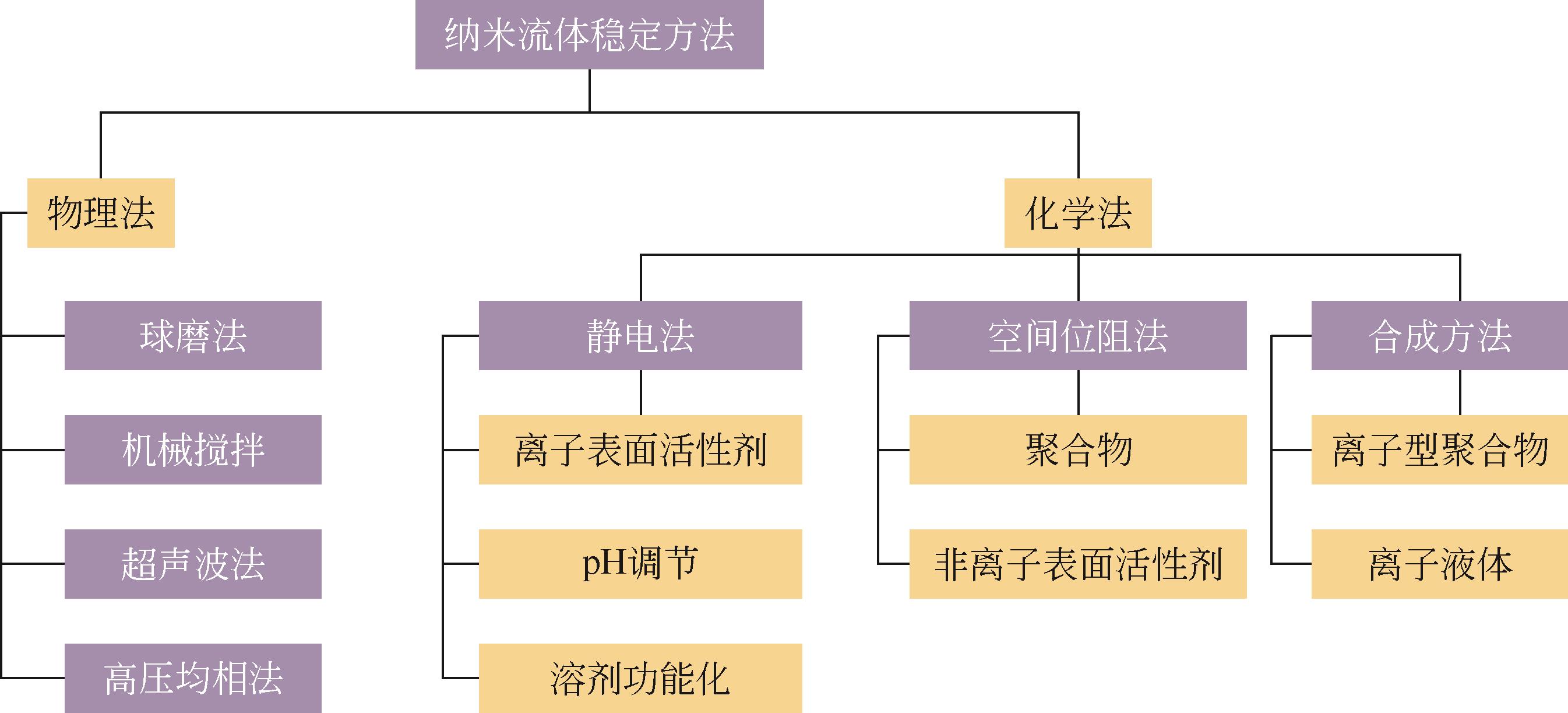
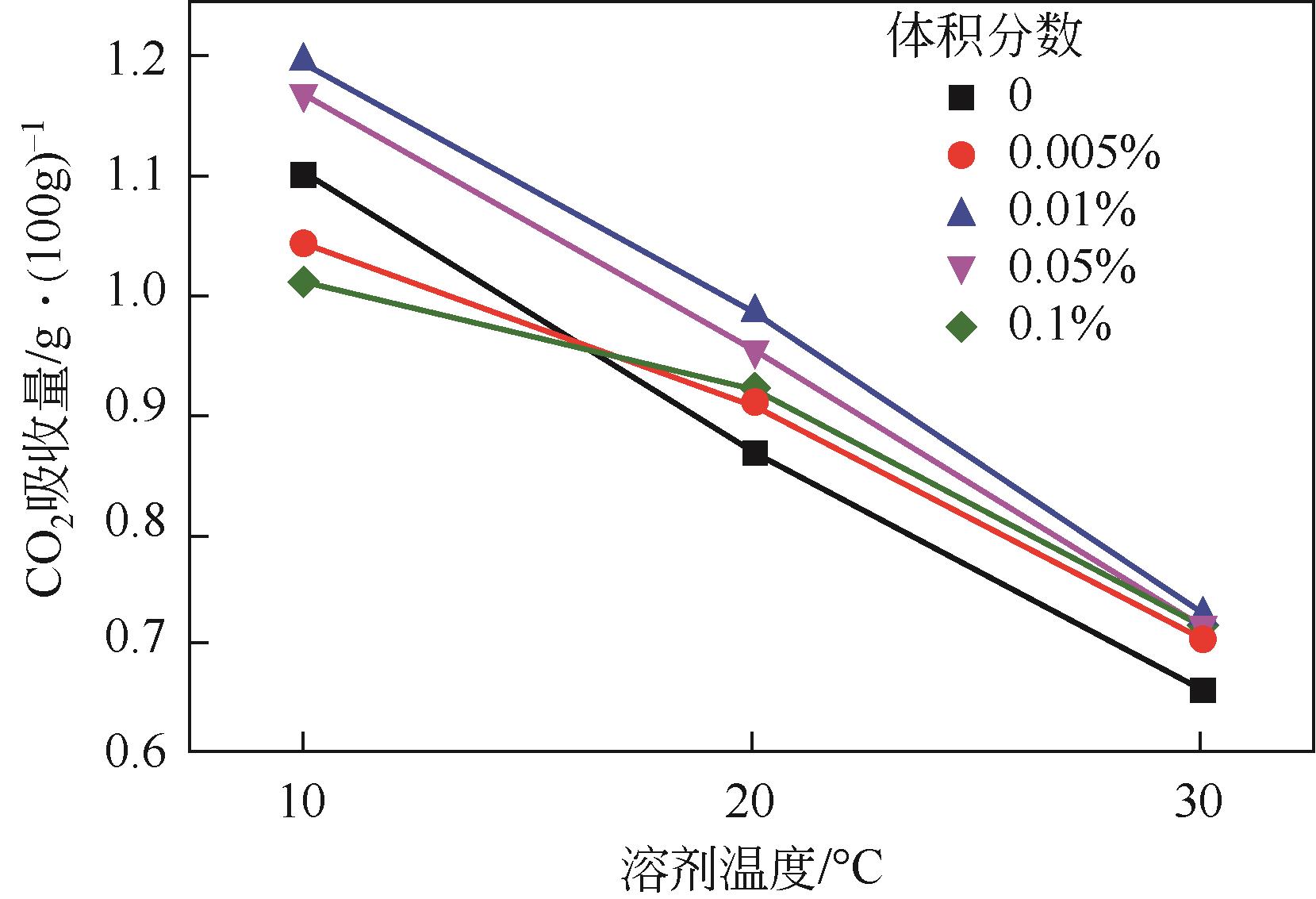
二氧化碳是全球气候变暖的主要诱因,随着我国“碳达峰、碳中和”战略的提出,二氧化碳的捕集、储存、利用技术快速发展。纳米流体是以由纳米颗粒以预先指定的比例分散在无机或有机液相中产生的具有稳定均匀性的胶体分散系统,兼有纳米材料和液体的特性。纳米颗粒由于对传热和传质过程有着明显的强化作用,因此对二氧化碳的化学吸收具有较大的潜在工业应用价值。本文基于纳米流体的概念,从基液选择、稳定性以及传质增强机制机理阐述了纳米流体在二氧化碳吸收领域的应用;进一步综述了目前纳米流体用于二氧化碳吸收分离的研究进展,分析了基液组成、二氧化碳分压、物化特性等对二氧化碳吸收性能的影响及机理研究;最后展望了纳米流体在二氧化碳吸收分离领域的未来发展趋势。
中图分类号:
引用本文
娄宝辉, 吴贤豪, 张驰, 陈臻, 冯向东. 纳米流体用于二氧化碳吸收分离研究进展[J]. 化工进展, 2023, 42(7): 3802-3815.
LOU Baohui, WU Xianhao, ZHANG Chi, CHEN Zhen, FENG Xiangdong. Advances in nanofluid for CO2 absorption and separation[J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3802-3815.
纳米 颗粒 | 平均尺寸 /nm | 形貌 | 比表面积 /m2·g-1 | 密度 /kg·m-3 | 热导率 /W·m-1·K-1 |
|---|---|---|---|---|---|
| Fe3O4 | 4~5 | 球形 | 40~60 | 5200 | 17.65 |
| SiO2 | 10~15 | 球形 | 180~270 | 2200 | — |
| TiO2 | <50 | 球形 | 50±15 | 5500~6000 | — |
| ZnO | 10~30 | 纳米棒 | 20~60 | 5606 | 29 |
| NiO2 | 50 | — | — | 6670 | — |
| Al2O3 | <40 | 球形 | — | 4700 | 36~40 |
| MgO | — | 立方体 | — | 2900 | 48.4 |
表1 常见纳米颗粒的热物理性质
纳米 颗粒 | 平均尺寸 /nm | 形貌 | 比表面积 /m2·g-1 | 密度 /kg·m-3 | 热导率 /W·m-1·K-1 |
|---|---|---|---|---|---|
| Fe3O4 | 4~5 | 球形 | 40~60 | 5200 | 17.65 |
| SiO2 | 10~15 | 球形 | 180~270 | 2200 | — |
| TiO2 | <50 | 球形 | 50±15 | 5500~6000 | — |
| ZnO | 10~30 | 纳米棒 | 20~60 | 5606 | 29 |
| NiO2 | 50 | — | — | 6670 | — |
| Al2O3 | <40 | 球形 | — | 4700 | 36~40 |
| MgO | — | 立方体 | — | 2900 | 48.4 |
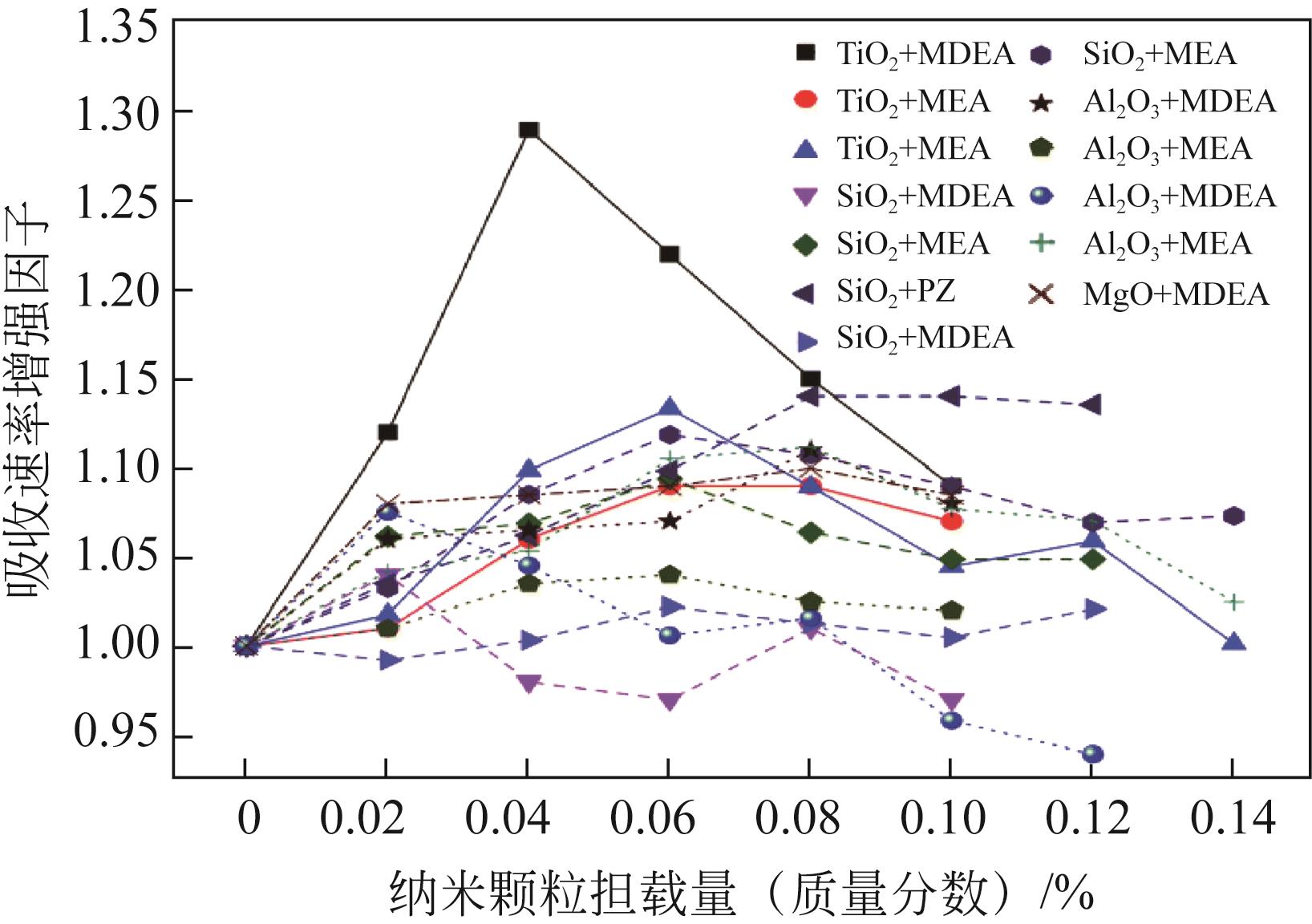
| 研究人员 | 反应器 | 纳米颗粒类型 | 溶剂 | 增强/% | 负载 |
|---|---|---|---|---|---|
| Jiang等[ | 鼓泡反应器 | TiO2 | MEA | 0.7 | 0.06%(质量分数) |
| Al2O3 | 0.02 | 0.06%(质量分数) | |||
| Lu等[ | 搅拌式反应器 | CNT | 水 | 100 | 1.6kg/m3 |
| Al2O3 | 5 | 1.6kg/m3 | |||
| Pineda等[ | 设有托盘的吸收塔 | TiO2 | 甲醇 | 5 | 0.05%(体积分数) |
| SiO2 | 6 | 0.05%(体积分数) | |||
| A12O3 | 10 | 0.05%(体积分数) | |||
| Zhang等[ | 搅拌式反应器 | TiO2 | 碳酸丙烯酯 | 60 | 1.0kg/m3 |
| Golkhar等[ | 中空纤维膜气液反应器 | SiO2 | 水 | 20 | 0.5%(质量分数) |
| CNT | 40 | 0.5%(质量分数) | |||
| Haghtalab等[ | 准静态等温高压搅拌反应器 | SiO2 | 水 | 7 | 0.1%(质量分数) |
| ZnO | 水 | 14 | 0.1%(质量分数) | ||
| Nabipour等[ | 准静态高压反应器 | Fe3O4 | Sulfinol-M | 14.7 | 0.02%(质量分数) |
| Kim等[ | 鼓泡吸收器 | SiO2 | 水 | 24 | 0.21%(质量分数) |
| Pang等[ | 吸收柱 | Ag | 水/NH3混合物 | 55 | 0.02%(质量分数) |
| Lee和Kang[ | 鼓泡反应器 | Al2O3 | NaCl溶液 | 12.5 | 0.01%(体积分数) |
| Zhu等[ | 微型搅拌反应器 | MCM41(中孔SiO2) | 水 | 60 | 0.4%(质量分数) |
| Lee等[ | 鼓泡反应器 | Al2O3 | 甲醇 | 5.6 | 0.01%(体积分数) |
| Jung等[ | 鼓泡反应器 | Al2O3 | 甲醇 | 8 | 0.01%(体积分数) |
表2 不同纳米流体吸收特性
| 研究人员 | 反应器 | 纳米颗粒类型 | 溶剂 | 增强/% | 负载 |
|---|---|---|---|---|---|
| Jiang等[ | 鼓泡反应器 | TiO2 | MEA | 0.7 | 0.06%(质量分数) |
| Al2O3 | 0.02 | 0.06%(质量分数) | |||
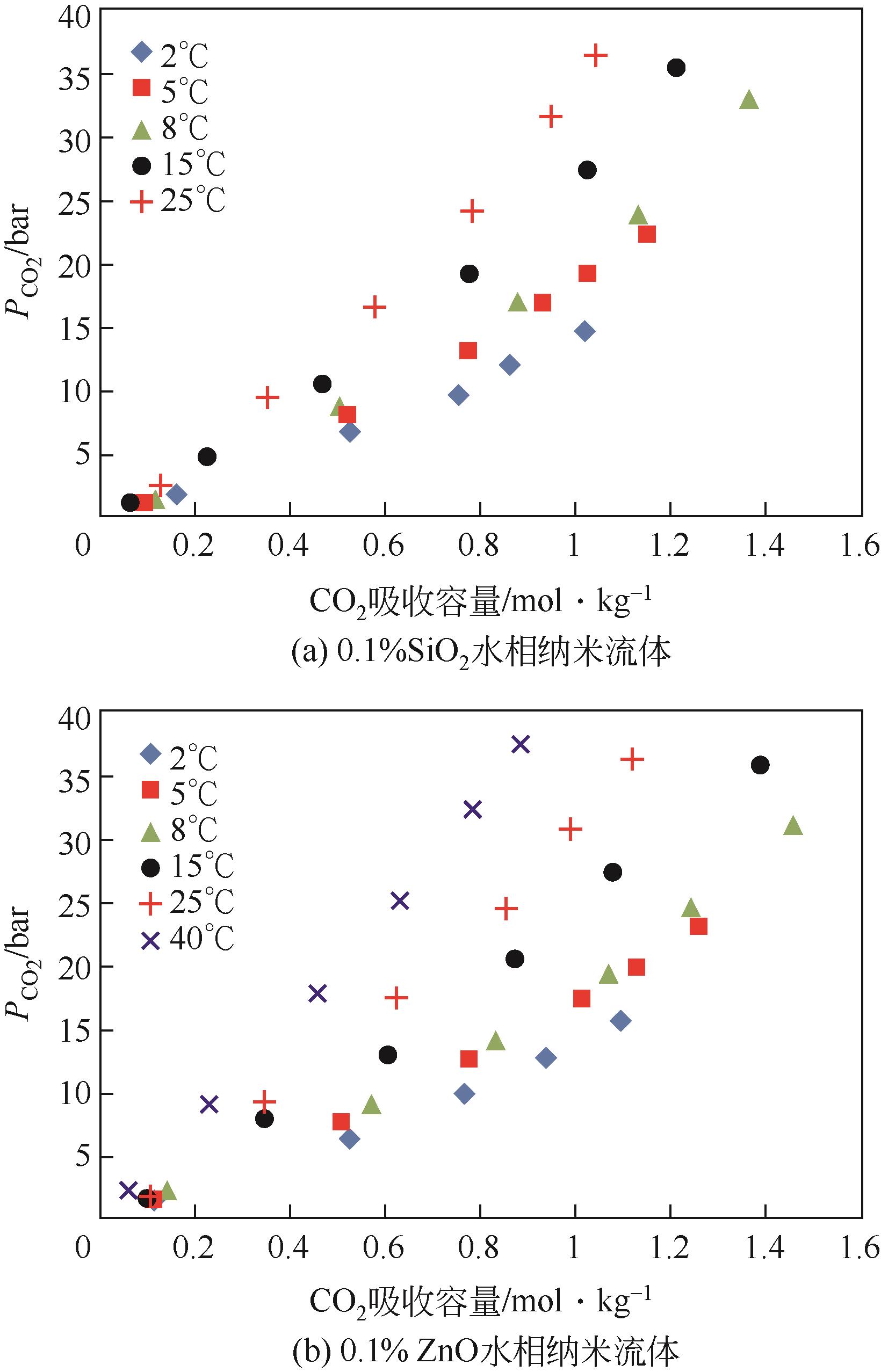
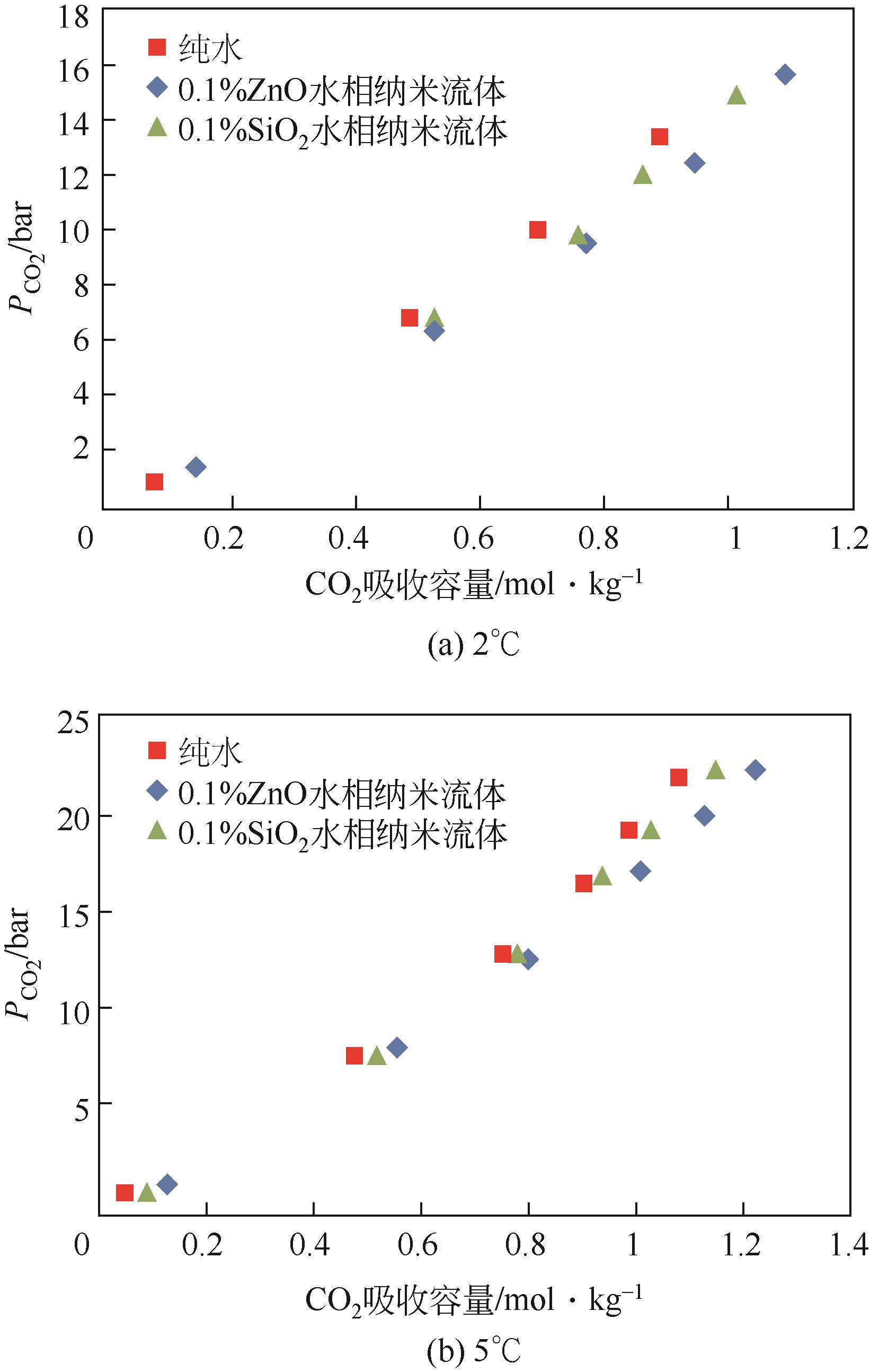
| Lu等[ | 搅拌式反应器 | CNT | 水 | 100 | 1.6kg/m3 |
| Al2O3 | 5 | 1.6kg/m3 | |||
| Pineda等[ | 设有托盘的吸收塔 | TiO2 | 甲醇 | 5 | 0.05%(体积分数) |
| SiO2 | 6 | 0.05%(体积分数) | |||
| A12O3 | 10 | 0.05%(体积分数) | |||
| Zhang等[ | 搅拌式反应器 | TiO2 | 碳酸丙烯酯 | 60 | 1.0kg/m3 |
| Golkhar等[ | 中空纤维膜气液反应器 | SiO2 | 水 | 20 | 0.5%(质量分数) |
| CNT | 40 | 0.5%(质量分数) | |||
| Haghtalab等[ | 准静态等温高压搅拌反应器 | SiO2 | 水 | 7 | 0.1%(质量分数) |
| ZnO | 水 | 14 | 0.1%(质量分数) | ||
| Nabipour等[ | 准静态高压反应器 | Fe3O4 | Sulfinol-M | 14.7 | 0.02%(质量分数) |
| Kim等[ | 鼓泡吸收器 | SiO2 | 水 | 24 | 0.21%(质量分数) |
| Pang等[ | 吸收柱 | Ag | 水/NH3混合物 | 55 | 0.02%(质量分数) |
| Lee和Kang[ | 鼓泡反应器 | Al2O3 | NaCl溶液 | 12.5 | 0.01%(体积分数) |
| Zhu等[ | 微型搅拌反应器 | MCM41(中孔SiO2) | 水 | 60 | 0.4%(质量分数) |
| Lee等[ | 鼓泡反应器 | Al2O3 | 甲醇 | 5.6 | 0.01%(体积分数) |
| Jung等[ | 鼓泡反应器 | Al2O3 | 甲醇 | 8 | 0.01%(体积分数) |
| 5 | NARUKULLA R, CHATURVEDI K R, OJHA U, et al. Carbon dioxide capturing evaluation of polyacryloyl hydrazide solutions via rheological analysis for carbon utilization applications[J]. Energy, 2022, 241: 122929. |
| 6 | SIDDIG A, WANG Wenju, ATIF A. A brief review for chemical looping combustion as a promising CO2 capture technology: Fundamentals and progress[J]. Science of the Total Environment, 2021, 764: 142892. |
| 7 | MADEJSKI P, CHMIEL K, SUBRAMANIAN N, et al. Methods and techniques for CO2 capture: Review of potential solutions and applications in modern energy technologies[J]. Energies, 2022, 15(3): 887. |
| 8 | JANG S PIL, CHOI S U S. Effects of various parameters on nanofluid thermal conductivity[J]. Journal of Heat Transfer, 2007, 129(5): 617-623. |
| 9 | EASTMAN J A, CHOI U S, LI S, et al. Enhanced thermal conductivity through the development of nanofluids[J]. MRS Online Proceedings Library, 1996, 457(1): 3-11. |
| 10 | KRISHNAMURTHY S, BHATTACHARYA P, PHELAN P E, et al. Enhanced mass transport in nanofluids[J]. Nano Letters, 2006, 6(3): 419-423. |
| 11 | TAVAKOLI A, RAHIMI K, SAGHANDALI F, et al. Nanofluid preparation, stability and performance for CO2 absorption and desorption enhancement: A review[J]. Journal of Environmental Management, 2022, 313: 114955. |
| 12 | LU Suming, XING Min, SUN Yan, et al. Experimental and theoretical studies of CO2 absorption enhancement by nano-Al2O3 and carbon nanotube particles[J]. Chinese Journal of Chemical Engineering, 2013, 21(9): 983-990. |
| 13 | HAJATZADEH P A, AGHAKHANI S, AFRAND M, et al. An updated review on application of nanofluids in heat exchangers for saving energy[J]. Energy Conversion and Management, 2019, 198: 111886. |
| 14 | ZARE P, KESHAVARZ P, MOWLA D. Membrane absorption coupling process for CO2 capture: Application of water-based ZnO, TiO2, and multi-walled carbon nanotube nanofluids[J]. Energy & Fuels, 2019, 33(2): 1392-1403. |
| 15 | DARVANJOOGHI M H K, ESFAHANY M N, ESMAEILI-FARAJ S H. Investigation of the effects of nanoparticle size on CO2 absorption by silica-water nanofluid[J]. Separation and Purification Technology, 2018, 195: 208-215. |
| 16 | KARIMI DARVANJOOGHI M H, PAHLEVANINEZHAD M, ABDOLLAHI A, et al. Investigation of the effect of magnetic field on mass transfer parameters of CO2 absorption using Fe3O4-water nanofluid[J]. AIChE Journal, 2017, 63(6): 2176-2186. |
| 17 | SALIMI J, HAGHSHENASFARD M, ETEMAD S G. CO2 absorption in nanofluids in a randomly packed column equipped with magnetic field[J]. Heat and Mass Transfer, 2015, 51(5): 621-629. |
| 18 | AGHEL B, SAHRAIE S, HEIDARYAN E, et al. Experimental study of carbon dioxide absorption by mixed aqueous solutions of methyl diethanolamine (MDEA) and piperazine (PZ) in a microreactor[J]. Process Safety and Environmental Protection, 2019, 131: 152-159. |
| 19 | LIN K-Y A, PARK A-H A. Effects of bonding types and functional groups on CO2 capture using novel multiphase systems of liquid-like nanoparticle organic hybrid materials[J]. Environmental Science & Technology, 2011, 45(15): 6633-6639. |
| 20 | BOURLINOS A B, CHOWDHURY S RAY, HERRERA R, et al. Functionalized nanostructures with liquid-like behavior: Expanding the gallery of available nanostructures[J]. Advanced Functional Materials, 2005, 15(8): 1285-1290. |
| 21 | RODRIGUEZ R, HERRERA R, BOURLINOS A B, et al. The synthesis and properties of nanoscale ionic materials[J]. Applied Organometallic Chemistry, 2010, 24(8): 581-589. |
| 22 | ZHANG Na, ZHANG Xiaoyang, PAN Zhen, et al. A brief review of enhanced co2 absorption by nanoparticles[J]. International Journal of Energy for a Clean Environment, 2018, 19(3/4): 201-215. |
| 23 | AKOH H, TSUKASAKI Y, YATSUYA S, et al. Magnetic properties of ferromagnetic ultrafine particles prepared by vacuum evaporation on running oil substrate[J]. Journal of Crystal Growth, 1978, 45: 495-500. |
| 24 | MA B, SHIN D, BANERJEE D. One-step synthesis of molten salt nanofluid for thermal energy storage application—A comprehensive analysis on thermophysical property, corrosion behavior, and economic benefit[J]. Journal of Energy Storage, 2021, 35: 102278. |
| 25 | ELSALAMONY R A, MORSI R E, ALSABAGH A M. Influence of gamma radiation on the improvement of stability, conductivity and photoactivity of titania nanofluid[J]. Journal of Nanofluids, 2015, 4(4): 442-448. |
| 26 | SARKAR J, GHOSH P, ADIL A. A review on hybrid nanofluids: Recent research, development and applications[J]. Renewable and Sustainable Energy Reviews, 2015, 43: 164-177. |
| 27 | ZHANG Zhien, CAI Jianchao, CHEN Feng, et al. Progress in enhancement of CO2 absorption by nanofluids: A mini review of mechanisms and current status[J]. Renewable Energy, 2018, 118: 527-535. |
| 28 | SOLANGI K H, KAZI S N, LUHUR M R, et al. A comprehensive review of thermo-physical properties and convective heat transfer to nanofluids[J]. Energy, 2015, 89: 1065-1086. |
| 29 | MEHDIPOUR M, KESHAVARZ P, RAHIMPOUR M R. Rotating liquid sheet contactor: A new gas-liquid contactor system in CO2 absorption by nanofluids[J]. Chemical Engineering and Processing: Process Intensification, 2021, 165: 108447. |
| 30 | MA Binjian, BANERJEE D. A review of nanofluid synthesis[M]//Balasubramanian G, In Advances in Nanomaterials. Springer Cham, 2018: 135-176. |
| 31 | BOURLINOS A B, HERRERA R, CHALKIAS N, et al. Surface-functionalized nanoparticles with liquid-like behavior[J]. Advanced Materials, 2005, 17(2): 234-237. |
| 32 | RODRIGUEZ R, HERRERA R, ARCHER L A, et al. Nanoscale ionic materials[J]. Advanced Materials, 2008, 20(22): 4353-4358. |
| 33 | PETIT C, BHATNAGAR S, PARK A-H A. Effect of water on the physical properties and carbon dioxide capture capacities of liquid-like nanoparticle organic hybrid materials and their corresponding polymers[J]. Journal of Colloid and Interface Science, 2013, 407: 102-108. |
| 34 | LIN K-Y A, PARK Y, PETIT C, et al. Thermal stability, swelling behavior and CO2 absorption properties of Nanoscale Ionic Materials (NIMs)[J]. RSC Advances, 2014, 4(110): 65195-65204. |
| 35 | PARK Y, PETIT C, HAN P, et al. Effect of canopy structures and their steric interactions on CO2 sorption behavior of liquid-like nanoparticle organic hybrid materials[J]. RSC Advances, 2014, 4(17): 8723-8726. |
| 36 | LI Y, XIE H . Preparation of nanofluid by selecting nanoparticle from alumina, dioxide titanium, oxide zinc, nickel, and copper as additive, adding surfactant to base liquid, strongly stirring, and vibrating with ultrasonic wave to obtain nanofluid, CN 101735775-A [P/OL]. |
| 37 | BIRMINGHAM J G, ROOT D W. Apparatus for generating electrical energy, has nanoparticle clusters having nanoparticle work function between anode work function and cathode work function, US2015229013-A1 |
| 38 | CHA J H, KYOUNG W M, SONG K H, et al. Method for evaluating dispersion degree of nanoparticle dispersed in nanofluid utilized in vehicle, involves reflecting result determining coherence between nanoparticles for computing dispersion degree of nanofluid, KR1704280-B1 [P/OL]. |
| 39 | CHOI C, OH J M, YOO H S. Oil-based nanofluid preparing method, involves replacing liquid solvent with predetermined oil, and obtaining nanofluid by dispersing certain volume percentage of metal, non-metal or ceramic-based nano powder in oil, KR2008038625-A KR851649-B1 [P/OL]. |
| 40 | LI Y, LI X, LI R, et al. Preparation of MXene nanofluid for solar collector, involves mixing MXene with dispersant to obtain mixture, and dispersing mixture into base liquid using ultrasonic instrument, CN111928500-A [P/OL]. |
| 1 | Plc BP. Statistical review of world energy[R]. 2022. |
| 2 | KOSAKA F, YAMAGUCHI T, ANDO Y, et al. Thermal management of CO2 methanation with axial staging of active metal concentration in Ni-YSZ tubular catalysts[J]. International Journal of Hydrogen Energy, 2021, 46(5): 4116-4125. |
| 3 | KWAWU C R, ANIAGYEI A. A review on the computational studies of the reaction mechanisms of CO2 conversion on pure and bimetals of late 3D metals[J]. Journal of Molecular Modeling, 2021, 27(7): 200. |
| 4 | LEE C T, TSAI C C, WU P J, et al. Screening of CO2 utilization routes from process simulation: Design, optimization, environmental and techno-economic analysis[J]. Journal of CO2 Utilization, 2021, 53: 101722. |
| 41 | FRANCE D M, HEIFETZ A, ROUTBORT J, et al. Nanofluid, useful in a heat transfer application, comprises a base heat transfer fluid and many ceramic nanoparticles dispersed throughout the base heat transfer fluid at a particle concentration and size to form stable nanofluid: US2011001081-A1, US9340720-B2 [P]. |
| 42 | SAID Z, HACHICHA A A, ABEROUMAND S, et al. Recent advances on nanofluids for low to medium temperature solar collectors: Energy, exergy, economic analysis and environmental impact[J]. Progress in Energy and Combustion Science, 2021, 84: 100898. |
| 43 | HAN J, LIN Y, WANG Q, et al. Preparation of zinc oxide nanofluid comprises dissolving soluble zinc salt in alcohol-water mixed solvent, adding dispersant, heating to boil, adding alkali, boiling, and cooling to room temperature: CN102502781[P]. 2012-06-20. |
| 44 | CHAKRABORTY S, PANIGRAHI P K. Stability of nanofluid: A review[J]. Applied Thermal Engineering, 2020, 174: 115259. |
| 45 | WALKER D A, KOWALCZYK B, DE LA CRUZ M O, et al. Electrostatics at the nanoscale[J]. Nanoscale, 2011, 3(4): 1316-1344. |
| 46 | SOFIAH A G N, SAMYKANO M, SHAHABUDDIN S, et al. A comparative experimental study on the physical behavior of mono and hybrid RBD palm olein based nanofluids using CuO nanoparticles and PANI nanofibers[J]. International Communications in Heat and Mass Transfer, 2021, 120: 105006. |
| 47 | KONG Linghui, SUN Jianlin, BAO Yueyue. Preparation, characterization and tribological mechanism of nanofluids[J]. RSC Advances, 2017, 7(21): 12599-12609. |
| 48 | ETTEFAGHI E, GHOBADIAN B, RASHIDI A, et al. Preparation and investigation of the heat transfer properties of a novel nanofluid based on graphene quantum dots[J]. Energy Conversion and Management, 2017, 153: 215-223. |
| 49 | YANG Xuefei, LIU Zhenhua. Pool boiling heat transfer of functionalized nanofluid under sub-atmospheric pressures[J]. International Journal of Thermal Sciences, 2011, 50(12): 2402-2412. |
| 50 | THAKUR P, SONAWANE S S, SONAWANE S H, et al. Nanofluids-based delivery system, encapsulation of nanoparticles for stability to make stable nanofluids[M]//Encapsulation of Active Molecules and Their Delivery System. Amsterdam: Elsevier, 2020: 141-152. |
| 51 | SOURAV B. DLS and zeta potential—What they are and what they are not?[J]. Journal of Controlled Release: Official Journal of the Controlled Release Society, 2016, 235: 337-351. |
| 52 | JUNG J-Y, YOO J Y. Thermal conductivity enhancement of nanofluids in conjunction with electrical double layer (EDL)[J]. International Journal of Heat and Mass Transfer, 2009, 52(1/2): 525-528. |
| 53 | KAMALGHARIBI M, HORMOZI F, ZAMZAMIAN Seyed Amir Hossein, et al. Experimental studies on the stability of CuO nanoparticles dispersed in different base fluids: Influence of stirring, sonication and surface active agents[J]. Heat and Mass Transfer, 2016, 52(1): 55-62. |
| 54 | ZHU Haitao, ZHANG Canying, TANG Yaming, et al. Preparation and thermal conductivity of suspensions of graphite nanoparticles[J]. Carbon, 2007, 45(1): 226-228. |
| 55 | CHOUDHARY R, KHURANA D, KUMAR A, et al. Stability analysis of Al2O3/water nanofluids[J]. Journal of Experimental Nanoscience, 2017,12(1): 140-151. |
| 56 | MOHAMMAD M, EMAD S, SARA T L, et al. Investigation of thermal conductivity and rheological properties of nanofluids containing graphene nanoplatelets[J]. Nanoscale Research Letters, 2014, 9(1): 15. |
| 57 | KHALED E, OLABI A G, TABBI W, et al. Environmental impacts of nanofluids: A review[J]. Science of the Total Environment, 2021, 763: 144202. |
| 58 | RUTHIYA K C, VAN DER SCHAAF J, KUSTER B F M, et al. Mechanisms of physical and reaction enhancement of mass transfer in a gas inducing stirred slurry reactor[J]. Chemical Engineering Journal, 2003, 96(1/2/3): 55-69. |
| 59 | KRISHNAMURTHY S, BHATTACHARYA P, PHELAN P E, et al. Enhanced mass transport in nanofluids[J]. Nano Letters, 2006, 6(3): 419-423. |
| 60 | JIANG Jiazong, ZHAO Bo, ZHUO Yuqun, et al. Experimental study of CO2 absorption in aqueous MEA and MDEA solutions enhanced by nanoparticles[J]. International Journal of Greenhouse Gas Control, 2014, 29: 135-141. |
| 61 | ZARENEZHAD B, MONTAZERI V. Nanofluid-assisted gas to hydrate (GTH) energy conversion for promoting CO2 recovery and sequestration processes in the petroleum industry[J]. Petroleum Science and Technology, 2016, 34(1): 37-43. |
| 62 | RASHIDI H, MAMIVAND S. Experimental and numerical mass transfer study of carbon dioxide absorption using Al2O3/water nanofluid in wetted wall column[J]. Energy, 2022, 238: 121670. |
| 63 | SELVI P, BASKAR R. CO2 absorption in nanofluid with magnetic field[J]. Chemical Industry and Chemical Engineering Quarterly, 2020, 26(4): 321-328. |
| 64 | JIANG Jiazong, ZHANG Song, FU Xuelong, et al. Review of gas-liquid mass transfer enhancement by nanoparticles from macro to microscopic[J]. Heat and Mass Transfer, 2019, 55(8): 2061-2072. |
| 65 | 叶航, 刘琦, 彭勃, 等. 纳米颗粒强化胺法吸收CO2研究进展[J]. 热力发电, 2021, 50(1): 74-81. |
| YE Hang, LIU Qi, PENG Bo, et al. Review on CO2 absorption enhancement by nanoparticles in amine solutions[J]. Thermal Power Generation, 2021, 50(1): 74-81. | |
| 66 | RAHMATMAND B, KESHAVARZ P, AYATOLLAHI S. Study of absorption enhancement of CO2 by SiO2, Al2O3, CNT, and Fe3O4 nanoparticles in water and amine solutions[J]. Journal of Chemical & Engineering Data, 2016, 61(4): 1378-1387. |
| 67 | KOMATI S, SURESH A K. CO2 absorption into amine solutions: A novel strategy for intensification based on the addition of ferrofluids[J]. Journal of Chemical Technology & Biotechnology, 2008, 83(8): 1094-1100. |
| 68 | KIM E S, JUNG J Y, KANG Y T. The effect of surface area on pool boiling heat transfer coefficient and CHF of Al2O3/water nanofluids[J]. Journal of Mechanical Science and Technology, 2013, 27(10): 3177-3182. |
| 69 | GANAPATHY H, SHOOSHTARI A, DESSIATOUN S, et al. Experimental investigation of enhanced absorption of carbon dioxide in diethanolamine in a microreactor[C]//Proceedings of ASME 2013 11th International Conference on Nanochannels, Microchannels, and Minichannels, June 16-19, 2013, Sapporo, Japan, 2013. |
| 70 | GARCIA M, KNUUTILA H K, ARONU U E, et al. Influence of substitution of water by organic solvents in amine solutions on absorption of CO2 [J]. International Journal of Greenhouse Gas Control, 2018, 78: 286-305. |
| 71 | PINEDA I T, CHOI C K, KANG Y T. CO2 gas absorption by CH3OH based nanofluids in an annular contactor at low rotational speeds[J]. International Journal of Greenhouse Gas Control, 2014, 23: 105-112. |
| 72 | ZHANG Yu, ZHAO Bo, JIANG Jiazong, et al. The use of TiO2 nanoparticles to enhance CO2 absorption[J]. International Journal of Greenhouse Gas Control, 2016, 50: 49-56. |
| 73 | GOLKHAR A, KESHAVARZ P, MOWLA D. Investigation of CO2 removal by silica and CNT nanofluids in microporous hollow fiber membrane contactors[J]. Journal of Membrane Science, 2013, 433: 17-24. |
| 74 | HAGHTALAB A, MOHAMMADI M, FAKHROUEIAN Z. Absorption and solubility measurement of CO2 in water-based ZnO and SiO2 nanofluids[J]. Fluid Phase Equilibria, 2015, 392: 33-42. |
| 75 | NABIPOUR M, KESHAVARZ P, RAEISSI S. Experimental investigation on CO2 absorption in Sulfinol-M based Fe3 O4 and MWCNT nanofluids[J]. International Journal of Refrigeration, 2017, 73: 1-10. |
| 76 | KIM W G, KANG H U, JUNG K M, et al. Synthesis of silica nanofluid and application to CO2 absorption[J]. Separation Science and Technology, 2008, 43(11/12): 3036-3055. |
| 77 | PANG Changwei, WU Weidong, SHENG Wei, et al. Mass transfer enhancement by binary nanofluids (NH3/H2O+Ag nanoparticles) for bubble absorption process[J]. International Journal of Refrigeration, 2012, 35(8): 2240-2247. |
| 78 | LEE J W, KANG Y T. CO2 absorption enhancement by Al2O3 nanoparticles in NaCl aqueous solution[J]. Energy, 2013, 53: 206-211. |
| 79 | IRANI V, TAUASOLI A, MALEKI A, et al. Polyethyleneimine-functionalized HKUST-1/MDEA nanofluid to enhance the absorption of CO2 in gas sweetening process[J]. International Journal of Hydrogen Energy, 2018, 43(11): 5610-5619. |
| 80 | MANIKANDAN S P, AKILA S, DEEPAPRIYA N. Mass transfer performance of Al2O3 nanofluids for CO2 absorption in a wetted wall column[J]. International Research Journal of Engineering and Technology, 2019, 6(6): 1329-1331. |
| 81 | HWANG B J, PARK S W, PARK D W, et al. Absorption of carbon dioxide into aqueous colloidal silica solution with different sizes of silica particles containing monoethanolamine[J]. Korean Journal of Chemical Engineering, 2009, 26(3): 775-782. |
| 82 | PARK S W, CHOI B S, KIM S S, et al. Absorption of carbon dioxide into aqueous colloidal silica solution with diisopropanolamine[J]. Journal of Industrial and Engineering Chemistry, 2008, 14(2): 166-174. |
| 83 | PARK S W, CHOI B S, KIM S S, et al. Chemical absorption of carbon dioxide into aqueous colloidal silica solution containing monoethanolamine[J]. Journal of Industrial and Engineering Chemistry, 2007, 13: 133-142. |
| 84 | PARK S W, CHOI B S, LEE J W. Effect of elasticity of aqueous colloidal silica solution on chemical absorption of carbon dioxide with 2-amino-2-methyl-1-propanol[J]. Korea-Australia Rheology Journal, 2006, 18(3): 133-141. |
| 85 | LEE J W, PINEDA I T, LEE J H, et al. Combined CO2 absorption/regeneration performance enhancement by using nanoabsorbents[J]. Applied Energy, 2016, 178: 164-176. |
| 86 | SAID S, GOVINDARAJ V, HERRI J M, et al. A study on the influence of nanofluids on gas hydrate formation kinetics and their potential: Application to the CO2 capture process[J]. Journal of Natural Gas Science and Engineering, 2016, 32: 95-108. |
| 87 | NAGY E, FECZKO T, KOROKNAI B. Enhancement of oxygen mass transfer rate in the presence of nanosized particles[J]. Chemical Engineering Science, 2007, 62(24): 7391-7398. |
| 88 | ZHU Haiyang, SHANKS Brent H, HEINDEL Theodore J. Enhancing CO-water mass transfer by functionalized MCM41 nanoparticles[J]. Industrial & Engineering Chemistry Research, 2008, 47(20): 7881-7887. |
| 89 | KIM J H, JUNG C W, KANG Y T. Mass transfer enhancement during CO2 absorption process in methanol/Al2O3 nanofluids[J]. International Journal of Heat and Mass Transfer, 2014, 76: 484-491. |
| 90 | KIM J K, PARK C W, KANG Y T. The effect of micro-scale surface treatment on heat and mass transfer performance for a falling film H2O/LiBr absorber[J]. International Journal of Refrigeration, 2003, 26(5): 575-585. |
| 91 | LEE J W, JUNG J Y, LEE S G, et al. CO2 bubble absorption enhancement in methanol-based nanofluids[J]. International Journal of Refrigeration, 2011, 34(8): 1727-1733. |
| 92 | JUNG Jung-Yewl, LEE Jae Won, KANG Yong Tab. CO2 absorption characteristics of nanoparticle suspensions in methanol[J]. Journal of Mechanical Science and Technology, 2012, 26: 2285-2290. |
| 93 | DALMOLIN I, SKOVROINSKI E, BIASI A, et al. Solubility of carbon dioxide in binary and ternary mixtures with ethanol and water[J]. Fluid Phase Equilibria, 2006, 245(2): 193-200. |
| 94 | SIQUEIRA CAMPOS C E P, H G D’A VILLARDI, PESSOA F L P, et al. Solubility of carbon dioxide in water and hexadecane: Experimental measurement and thermodynamic modeling[J]. Journal of Chemical & Engineering Data, 2009, 54(10): 2881-2886. |
| 95 | VALTZ A, CHAPOY A, COQUELET C, et al. Vapour-liquid equilibria in the carbon dioxide-water system, measurement and modelling from 278.2 to 318.2K[J]. Fluid Phase Equilibria, 2004, 226: 333-344. |
| 96 | AGHEHROCHABOKI R, CHABOKI Y A, MALEKNIA S A, et al. Polyethyleneimine functionalized graphene oxide/methyldiethanolamine nanofluid: Preparation, characterization, and investigation of CO2 absorption[J]. Journal of Environmental Chemical Engineering, 2019, 7(5): 103285. |
| 97 | CRAIG V S J. Bubble coalescence and specific-ion effects[J]. Current Opinion in Colloid & Interface Science, 2004, 9(1/2): 178-184. |
| 98 | KARS R L, BEST R J, DRINKENBURG A A H. The sorption of propane in slurries of active carbon in water[J]. The Chemical Engineering Journal, 1979, 17(3): 201-210. |
| 99 | VINKE H, HAMERSMA P J, FORTUIN J M H. Enhancement of the gas-absorption rate in agitated slurry reactors by gas-adsorbing particles adhering to gas bubbles[J]. Chemical Engineering Science, 1993, 48(12): 2197-2210. |
| 100 | KLUYTMANS J H J, VAN WACHEM B G M, KUSTER B F M, et al. Mass transfer in sparged and stirred reactors: Influence of carbon particles and electrolyte[J]. Chemical Engineering Science, 2003, 58(20): 4719-4728. |
| 101 | HOLSTVOOGD R D, VANSWAAIJ W P M. The influence of adsorption capacity on enhanced gas absorption in activated carbon slurries[J]. Chemical Engineering Science, 1990, 45(1): 151-162. |
| 102 | KOO J, KLEINSTREUER C. Impact analysis of nanoparticle motion mechanisms on the thermal conductivity of nanofluids[J]. International Communications in Heat and Mass Transfer, 2005, 32(9): 1111-1118. |
| [1] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [2] | 郑谦, 官修帅, 靳山彪, 张长明, 张小超. 铈锆固溶体Ce0.25Zr0.75O2光热协同催化CO2与甲醇合成DMC[J]. 化工进展, 2023, 42(S1): 319-327. |
| [3] | 戴欢涛, 曹苓玉, 游新秀, 徐浩亮, 汪涛, 项玮, 张学杨. 木质素浸渍柚子皮生物炭吸附CO2特性[J]. 化工进展, 2023, 42(S1): 356-363. |
| [4] | 孙玉玉, 蔡鑫磊, 汤吉海, 黄晶晶, 黄益平, 刘杰. 反应精馏合成甲基丙烯酸甲酯工艺优化及节能[J]. 化工进展, 2023, 42(S1): 56-63. |
| [5] | 杨寒月, 孔令真, 陈家庆, 孙欢, 宋家恺, 王思诚, 孔标. 微气泡型下向流管式气液接触器脱碳性能[J]. 化工进展, 2023, 42(S1): 197-204. |
| [6] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [7] | 张岱凌, 丁玉梅, 左夏华, 黎昊为, 杨卫民, 阎华, 安瑛. 废弃墨粉纳米流体的光热特性[J]. 化工进展, 2023, 42(9): 4791-4798. |
| [8] | 王耀刚, 韩子姗, 高嘉辰, 王新宇, 李思琪, 杨全红, 翁哲. 铜基催化剂电还原二氧化碳选择性的调控策略[J]. 化工进展, 2023, 42(8): 4043-4057. |
| [9] | 刘毅, 房强, 钟达忠, 赵强, 李晋平. Ag/Cu耦合催化剂的Cu晶面调控用于电催化二氧化碳还原[J]. 化工进展, 2023, 42(8): 4136-4142. |
| [10] | 黄玉飞, 李子怡, 黄杨强, 金波, 罗潇, 梁志武. 光催化CO2和CH4重整催化剂研究进展[J]. 化工进展, 2023, 42(8): 4247-4263. |
| [11] | 白亚迪, 邓帅, 赵睿恺, 赵力, 杨英霞. 变温吸附碳捕集机组标准化测试方案探讨及性能实验[J]. 化工进展, 2023, 42(7): 3834-3846. |
| [12] | 顾诗亚, 董亚超, 刘琳琳, 张磊, 庄钰, 都健. 考虑中间节点的碳捕集管路系统设计与优化[J]. 化工进展, 2023, 42(6): 2799-2808. |
| [13] | 吕超, 张习文, 金理健, 杨林军. 新型两相吸收剂-离子液体系统高效捕获CO2[J]. 化工进展, 2023, 42(6): 3226-3232. |
| [14] | 王科菊, 赵成, 胡晓玫, 云军阁, 魏凝涵, 姜雪迎, 邹昀, 陈志航. 金属氧化物低温催化氧化VOCs的研究进展[J]. 化工进展, 2023, 42(5): 2402-2412. |
| [15] | 马源, 肖晴月, 岳君容, 崔彦斌, 刘姣, 许光文. CeO2-Al2O3复合载体负载Ni基催化剂催化CO x 共甲烷化性能[J]. 化工进展, 2023, 42(5): 2421-2428. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||