化工进展 ›› 2023, Vol. 42 ›› Issue (5): 2724-2732.DOI: 10.16085/j.issn.1000-6613.2022-1230
蛋黄-壳介孔磁性炭微球的制备及其对红霉素的高效吸附
- 华东理工大学化工学院,上海 200237
-
收稿日期:2022-07-01修回日期:2022-09-05出版日期:2023-05-10发布日期:2023-06-02 -
通讯作者:陈葵 -
作者简介:刘念(1997—),男,硕士研究生,研究方向为材料化工。E-mail:ecustliunian@163.com。
Preparation of yolk-shell mesoporous magnetic carbon microspheres and its efficient adsorption of erythromycin
LIU Nian( ), CHEN Kui(
), CHEN Kui( ), WU Bin, JI Lijun, WU Yanyang, HAN Jinling
), WU Bin, JI Lijun, WU Yanyang, HAN Jinling
- College of Chemical Engineering, East China University of Science and Technology, Shanghai 200237, China
-
Received:2022-07-01Revised:2022-09-05Online:2023-05-10Published:2023-06-02 -
Contact:CHEN Kui
摘要:
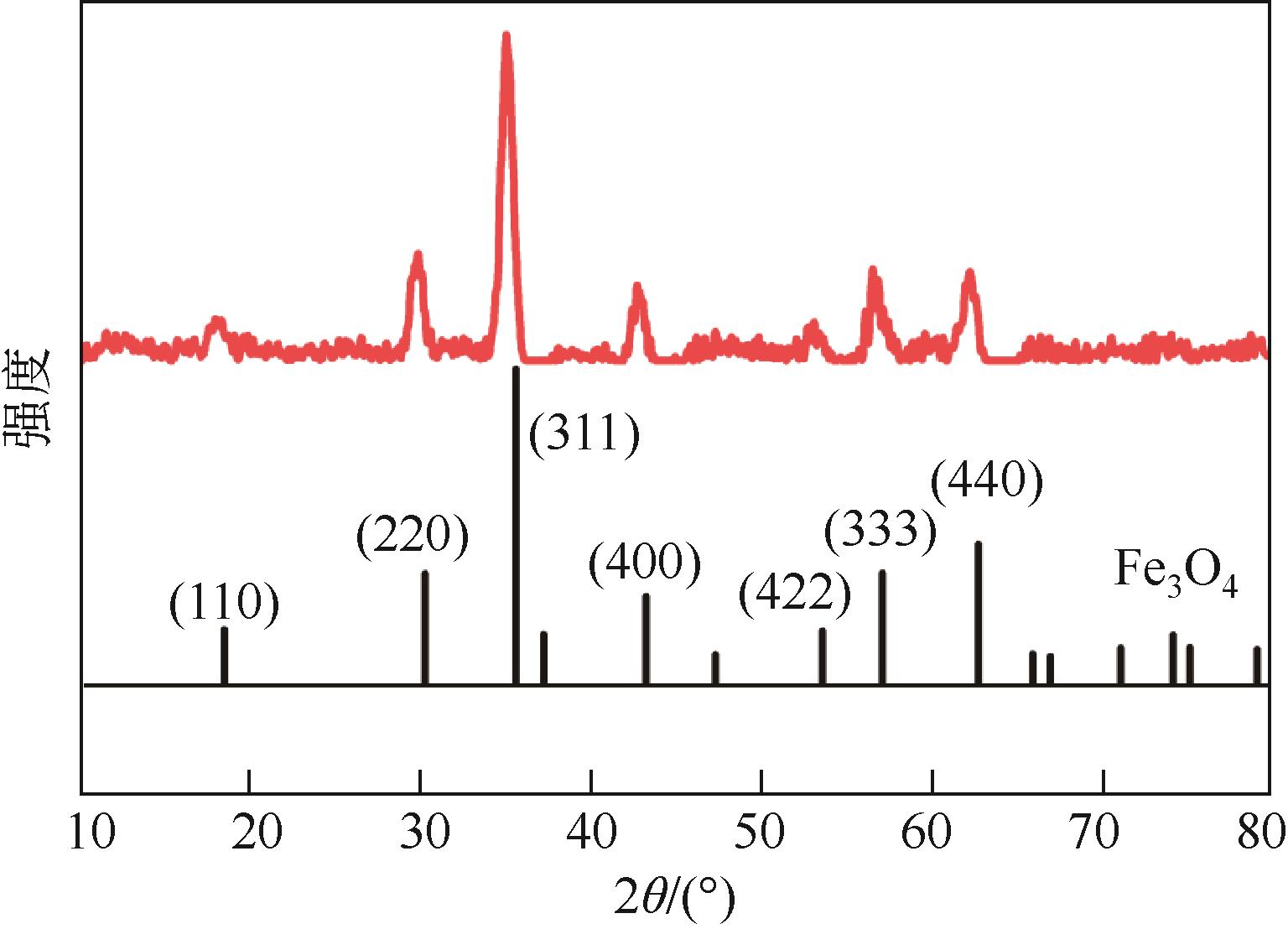
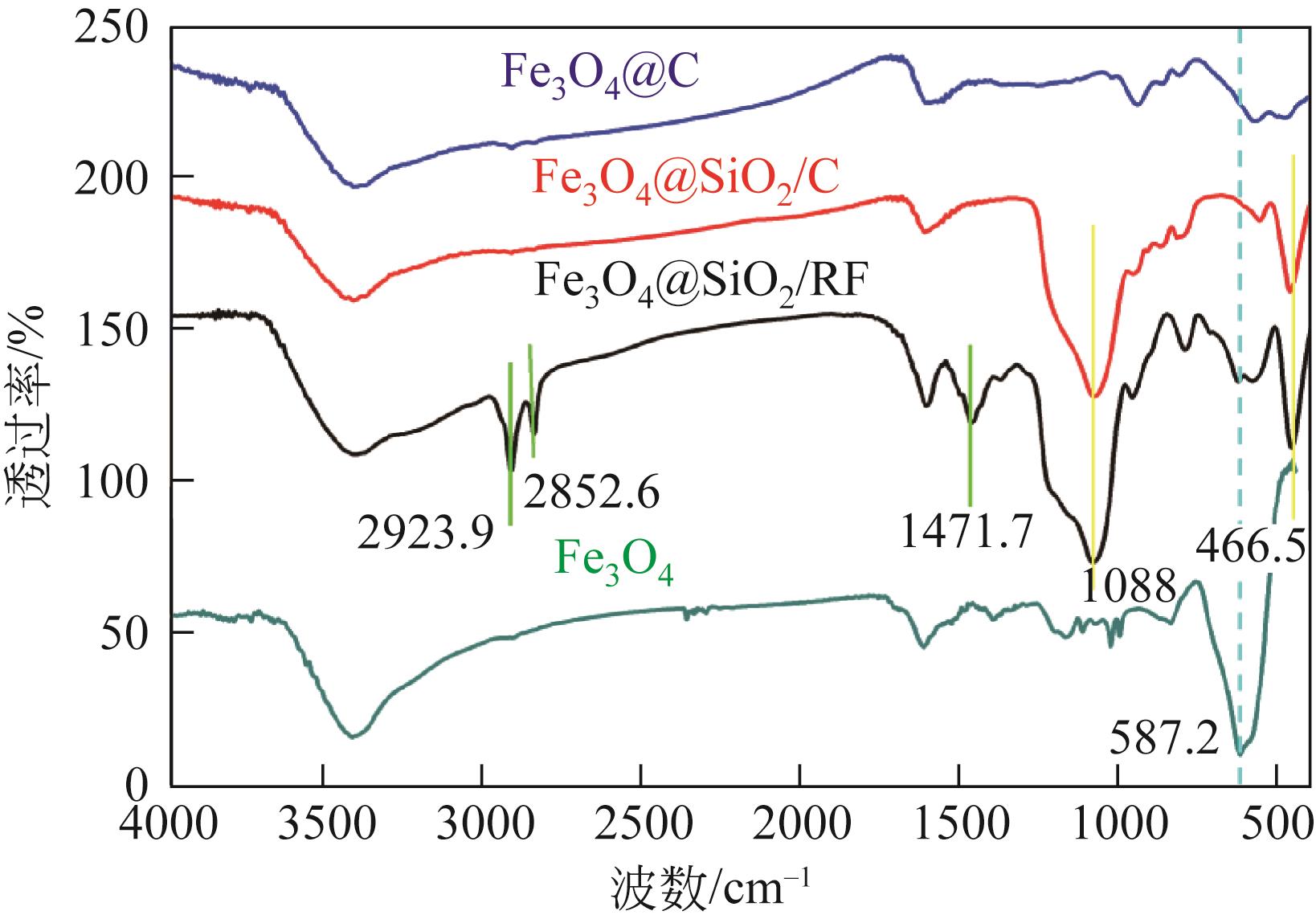
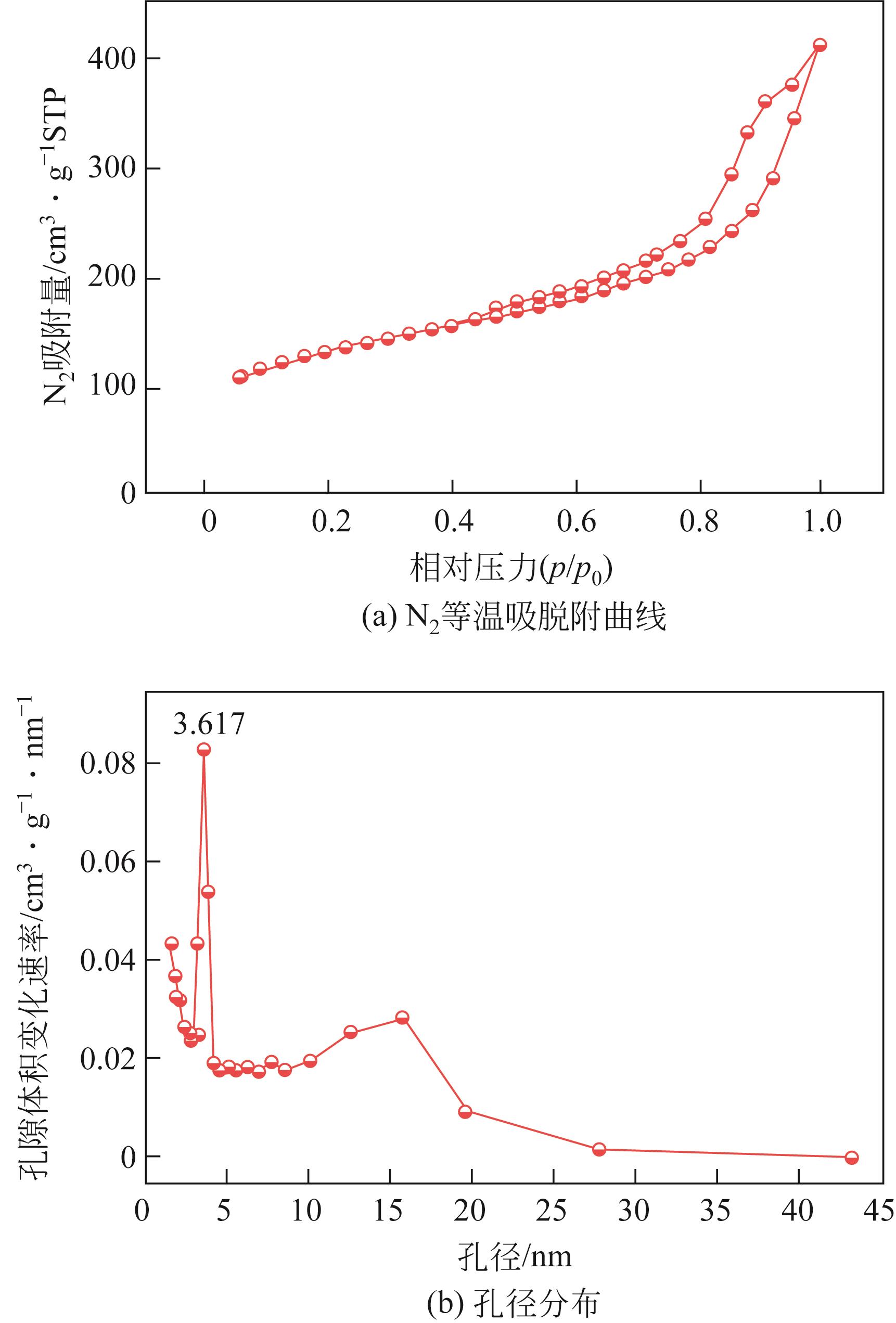
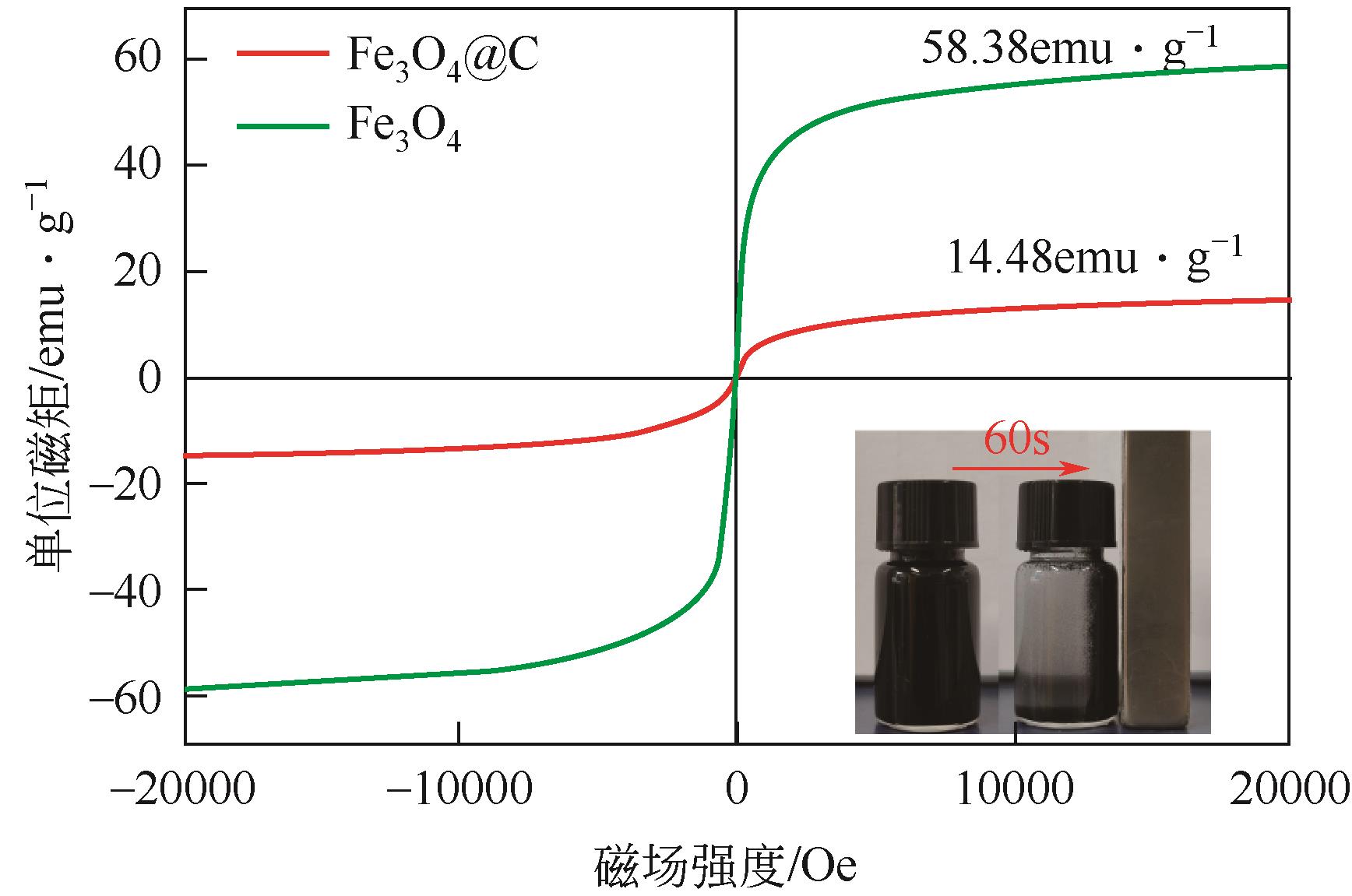
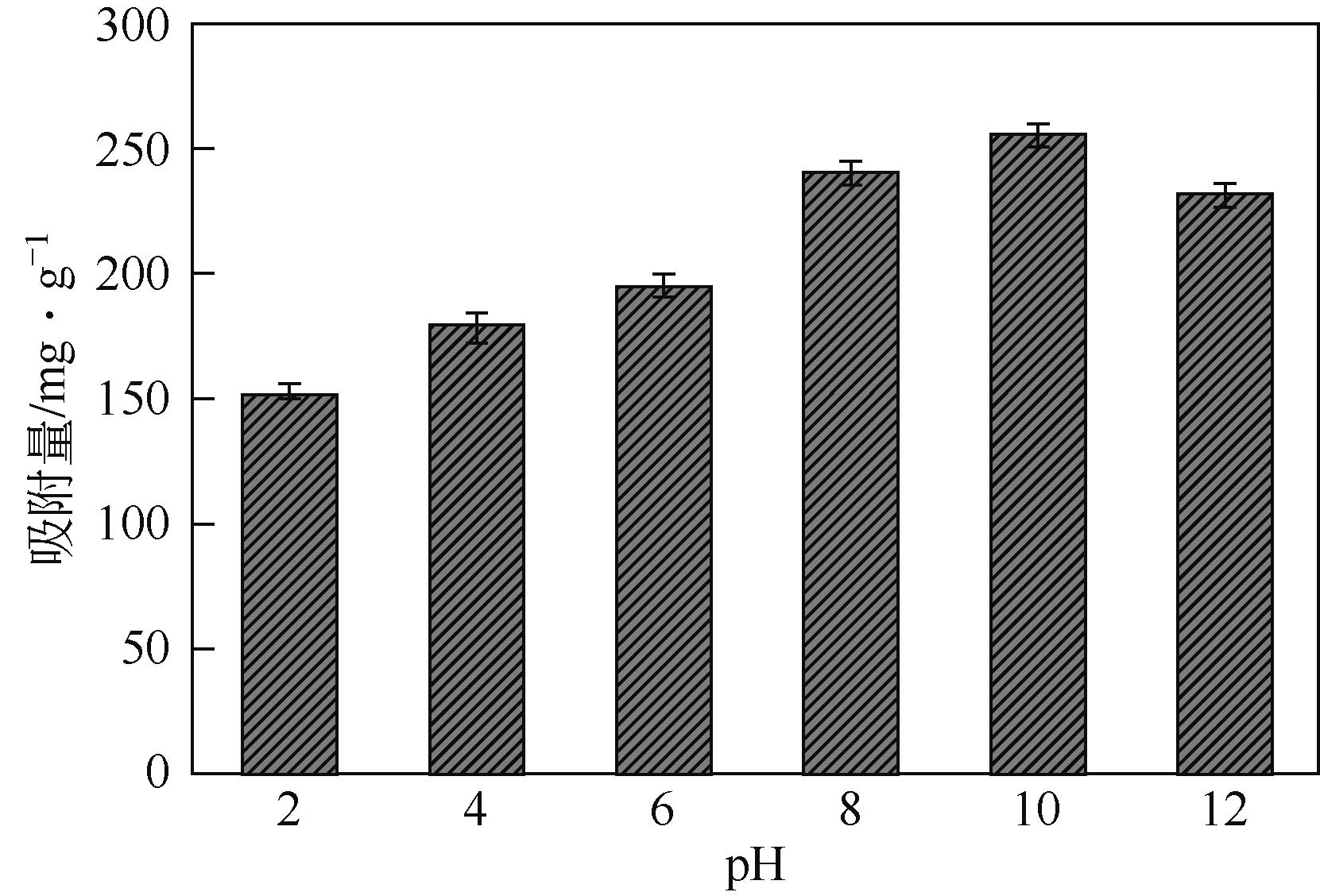
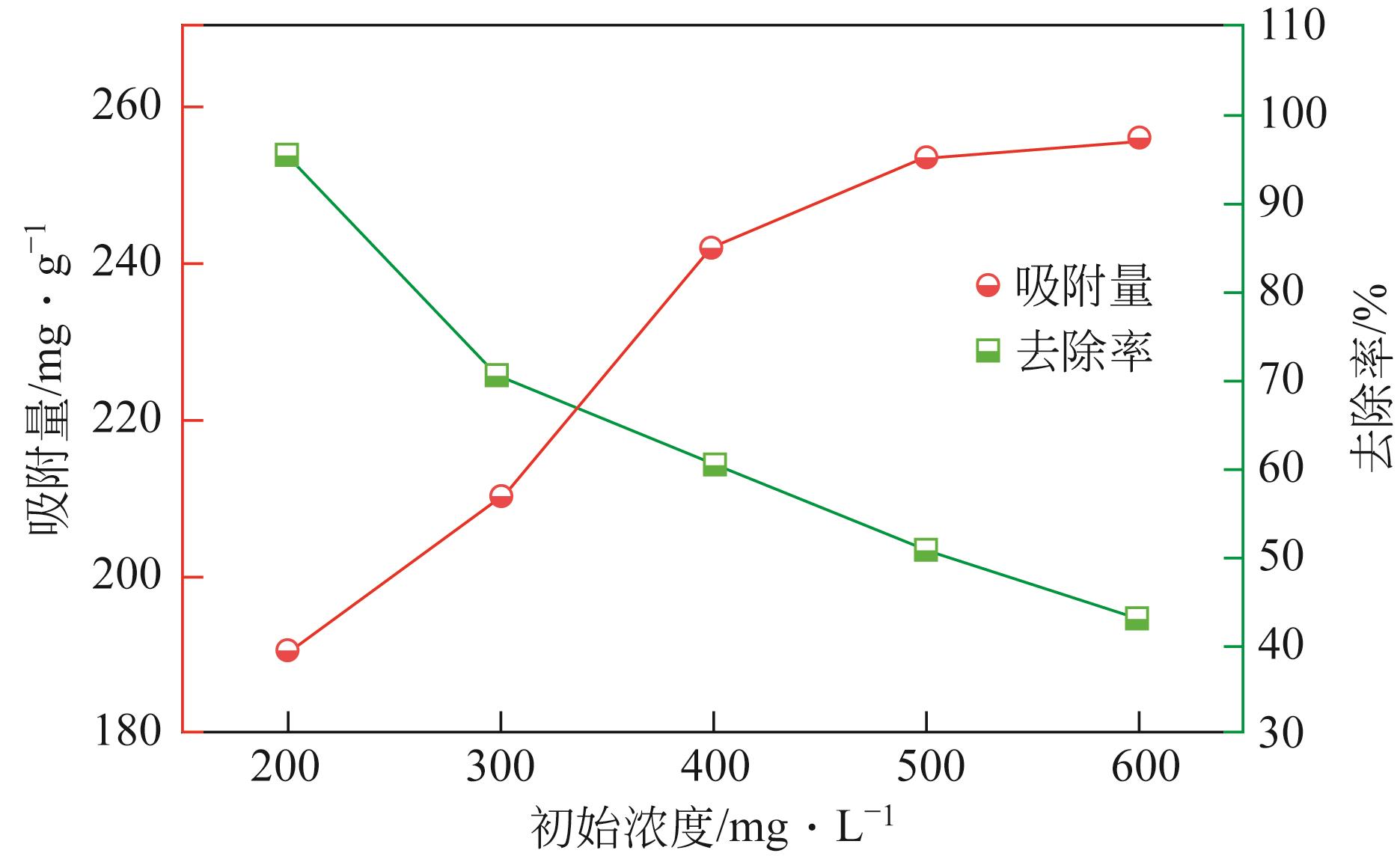
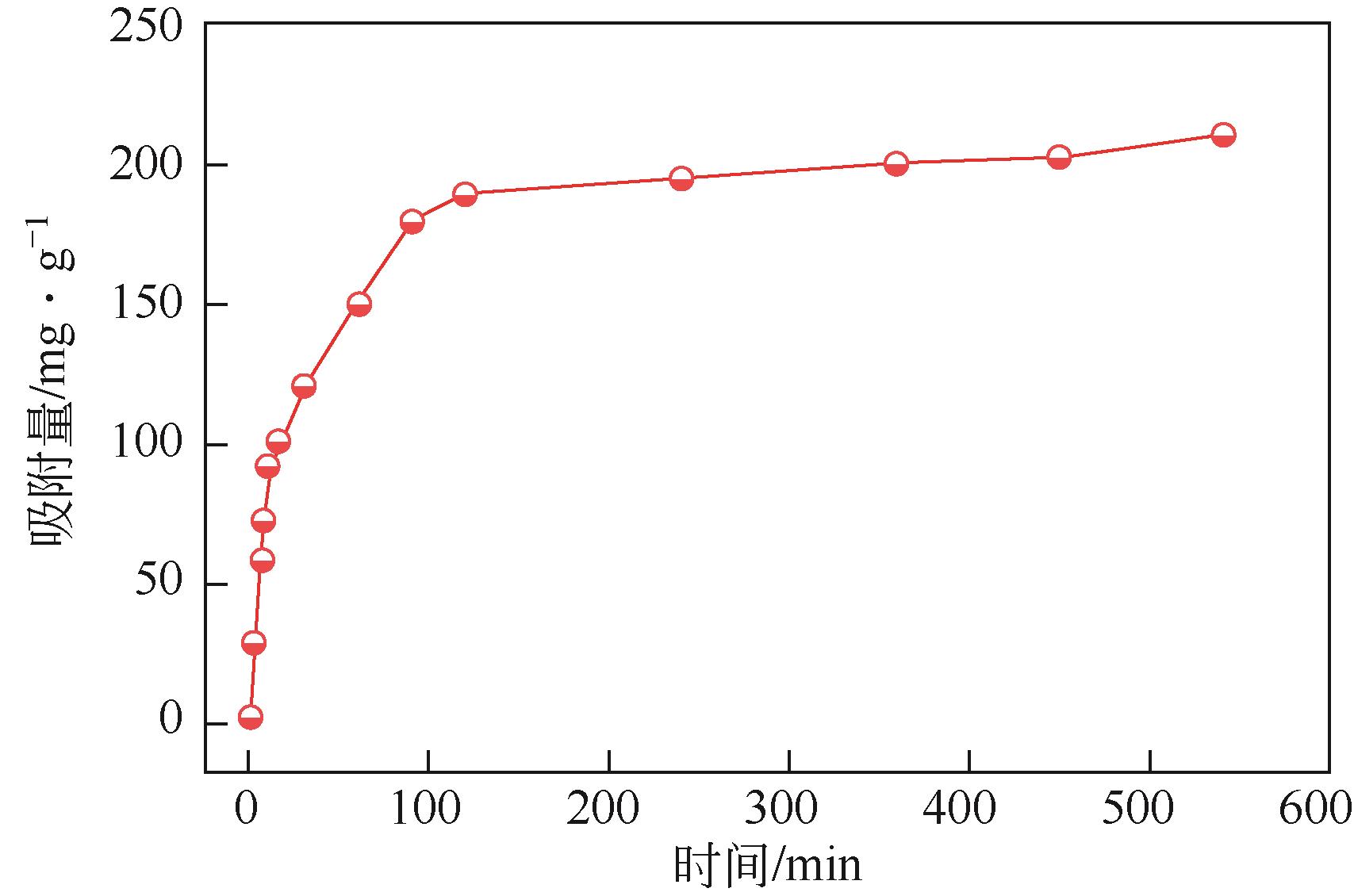
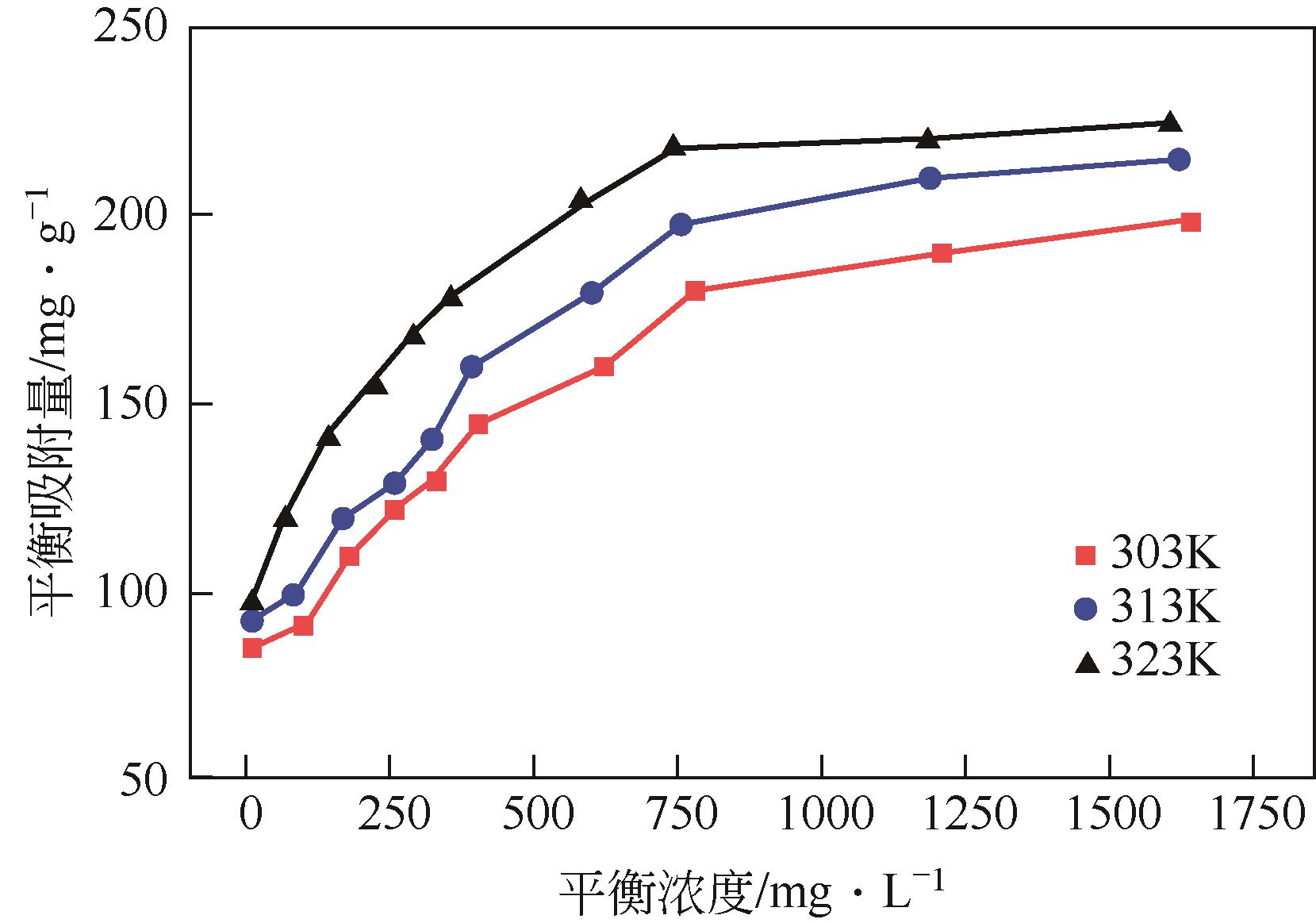
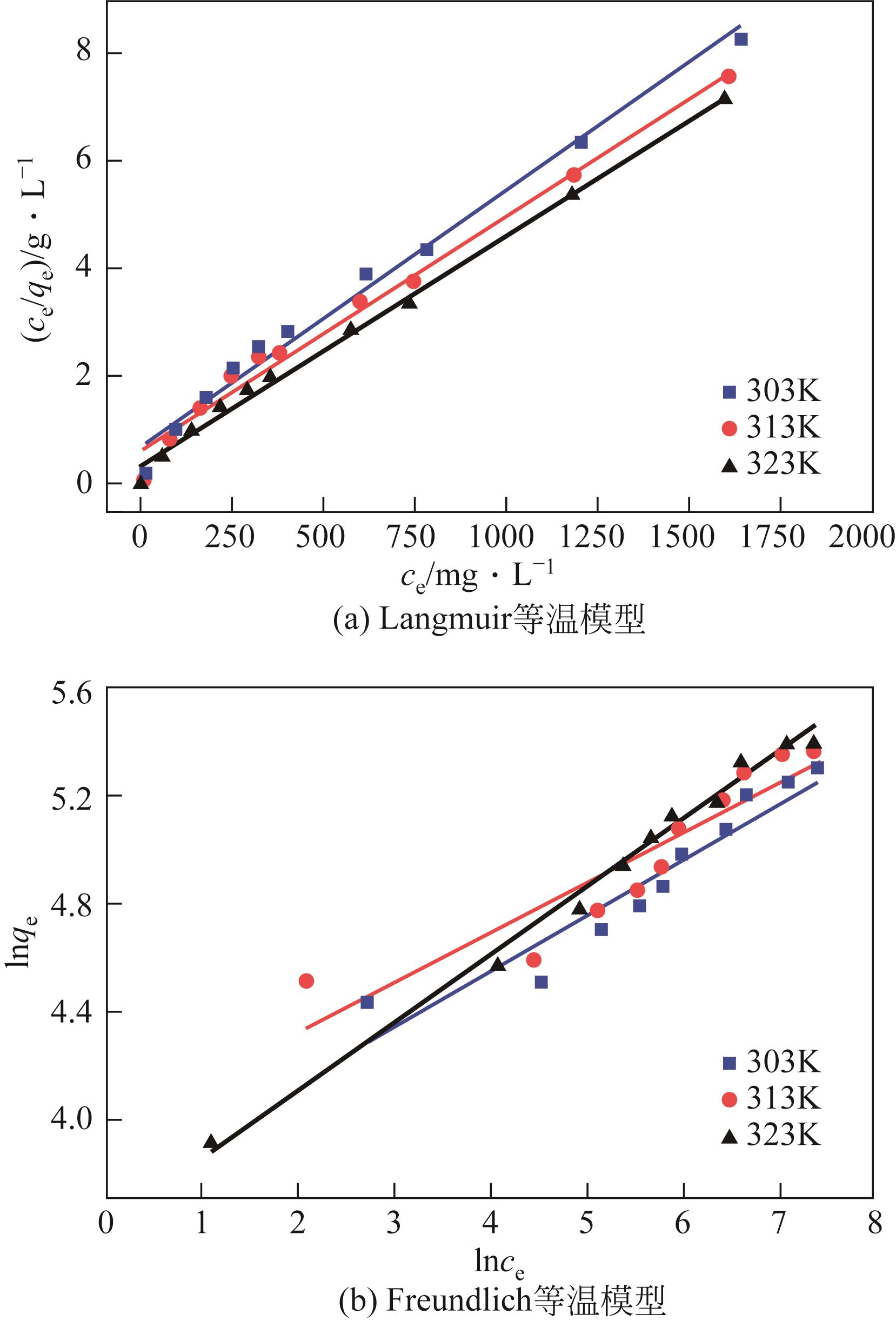
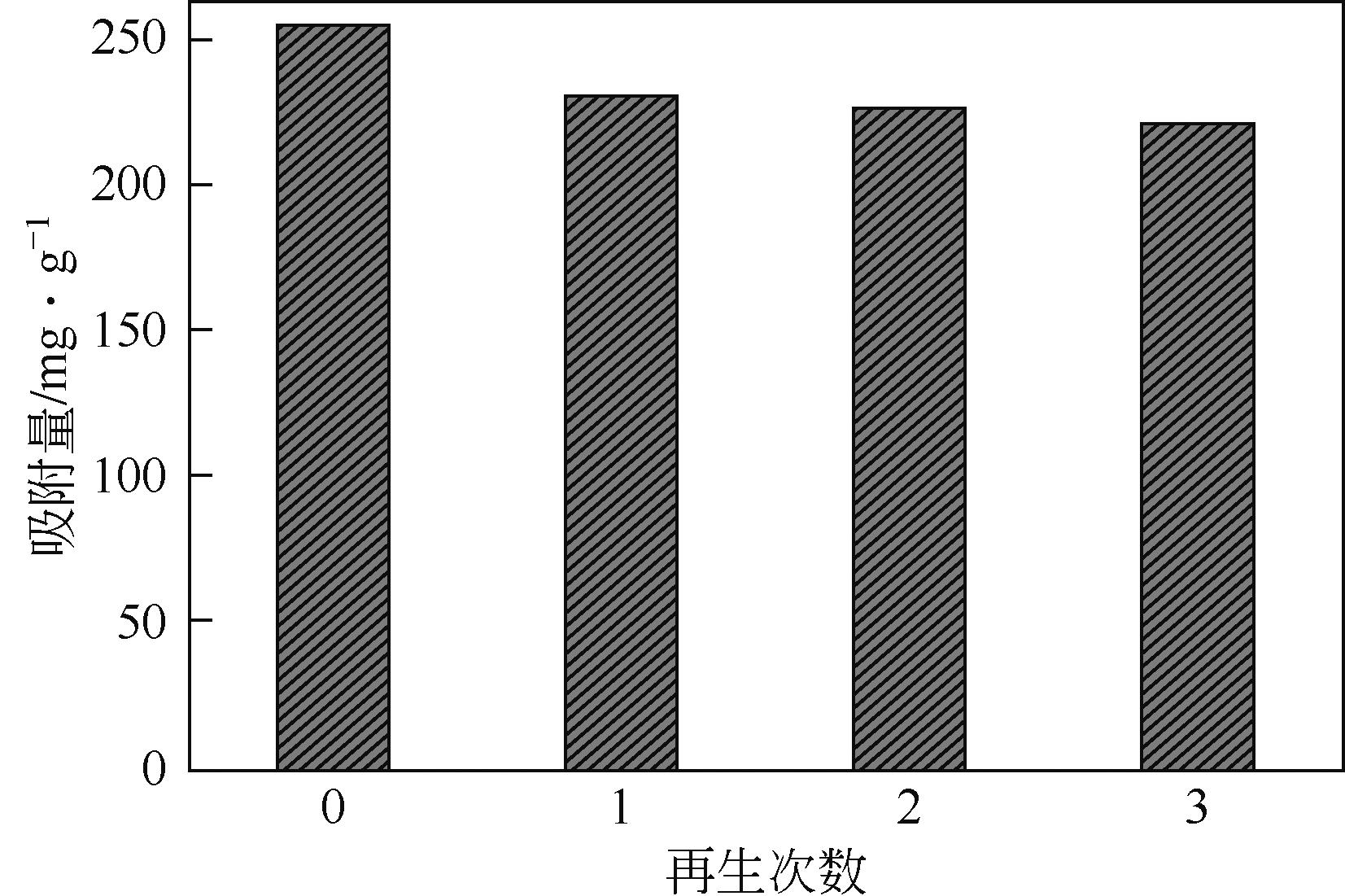
以溶剂热法制得的Fe3O4纳米颗粒为磁核,正硅酸四乙酯(TEOS)为造孔前体,间苯二酚-甲醛树脂(RF)为碳源,一步法制备蛋黄-壳介孔磁性炭微球(Fe3O4@C),并将其作为吸附剂用于去除水中的红霉素。采用TEM、XRD、FTIR、BET和VSM对Fe3O4@C进行表征。结果表明,Fe3O4@C核壳之间具有大空腔,比表面积为444m2/g,平均孔径为7.7nm,具有超顺磁性。通过静态吸附实验研究了Fe3O4@C对红霉素的吸附平衡和速率,并确定了优化的操作条件。结果表明,在吸附剂投加量为1.0g/L、初始红霉素浓度为300mg/L、pH为10的优化条件下,Fe3O4@C对红霉素的吸附量为210mg/g。Fe3O4@C对红霉素的吸附过程是自发的、吸热的和不可逆的,遵循准二级动力学和Langmuir等温线模型。经3次循环再生后,Fe3O4@C吸附量仍能维持在初始吸附量的86%以上。
中图分类号:
引用本文
刘念, 陈葵, 武斌, 纪利俊, 吴艳阳, 韩金玲. 蛋黄-壳介孔磁性炭微球的制备及其对红霉素的高效吸附[J]. 化工进展, 2023, 42(5): 2724-2732.
LIU Nian, CHEN Kui, WU Bin, JI Lijun, WU Yanyang, HAN Jinling. Preparation of yolk-shell mesoporous magnetic carbon microspheres and its efficient adsorption of erythromycin[J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2724-2732.
| 比表面积/m2·g-1 | 总孔容/cm3·g-1 | 介孔孔容占比/% | 平均孔径/nm |
|---|---|---|---|
| 444 | 0.637 | 74 | 7.7 |
表1 Fe3O4@C的孔结构数据
| 比表面积/m2·g-1 | 总孔容/cm3·g-1 | 介孔孔容占比/% | 平均孔径/nm |
|---|---|---|---|
| 444 | 0.637 | 74 | 7.7 |
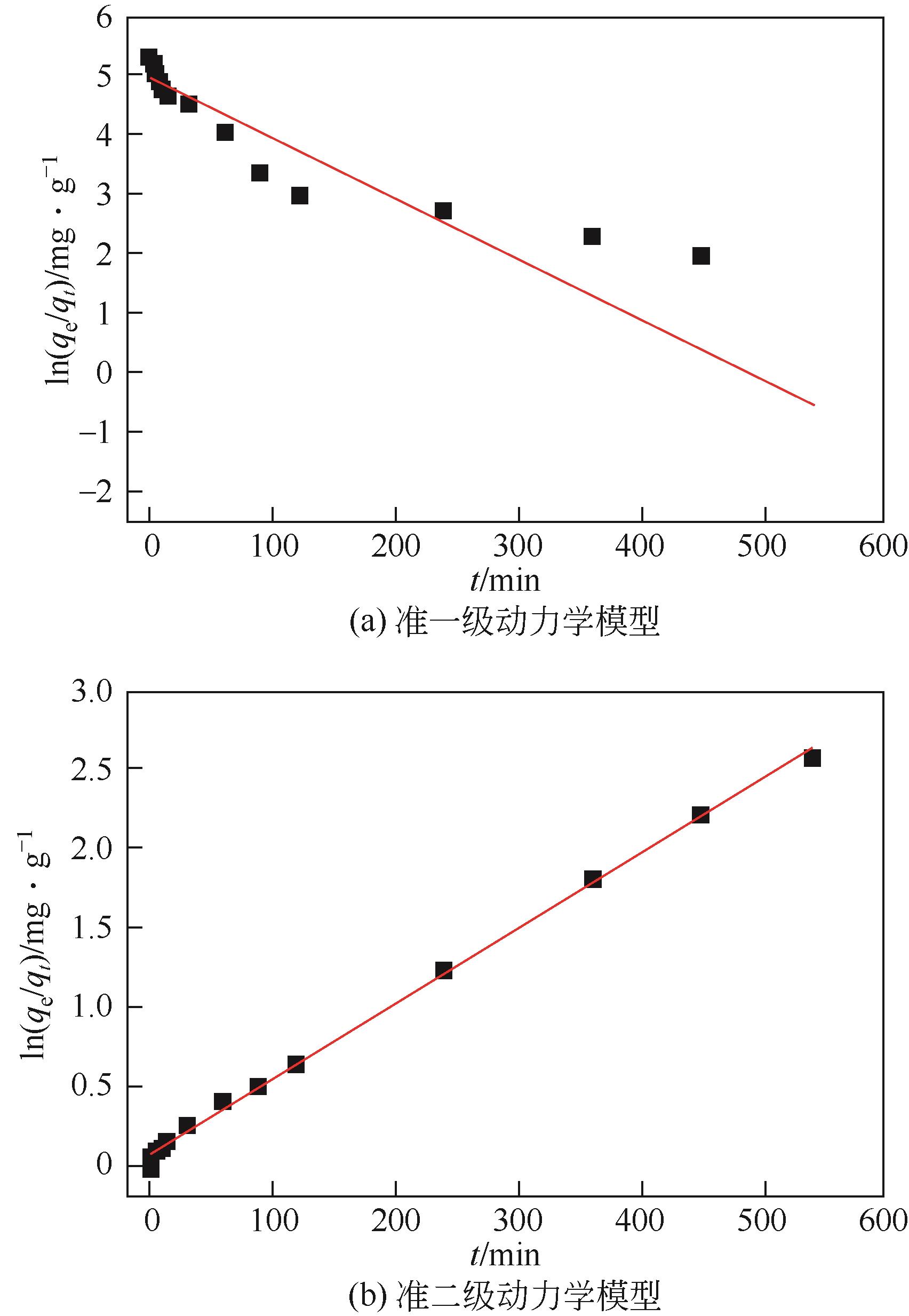
| 准一级动力学模型 | 准二级动力学模型 | 实验值qe /mg·g-1 | |||||
|---|---|---|---|---|---|---|---|
qe,cal /mg·g-1 | k1 /min-1 | R2 | qe,cal /mg·g-1 | k2 /mg·g-1·min-1 | R2 | ||
| 141.2 | 0.01 | 0.838 | 212.8 | 0.303×10-3 | 0.998 | 210 | |
表2 Fe3O4@C吸附红霉素的动力学常数
| 准一级动力学模型 | 准二级动力学模型 | 实验值qe /mg·g-1 | |||||
|---|---|---|---|---|---|---|---|
qe,cal /mg·g-1 | k1 /min-1 | R2 | qe,cal /mg·g-1 | k2 /mg·g-1·min-1 | R2 | ||
| 141.2 | 0.01 | 0.838 | 212.8 | 0.303×10-3 | 0.998 | 210 | |
| 温度/K | Langmuir等温模型 ce/qe=ce/qm+1/ (kLqm) | Freundlich等温模型 lnqe=lnkF+1/(nlnce) | ||||
|---|---|---|---|---|---|---|
| qm/mg·g-1 | kL/L·mg-1 | R2 | kF/L·g-1 | n | R2 | |
| 303 | 227.3 | 0.00782 | 0.992 | 41.72 | 4.854 | 0.895 |
| 313 | 263.2 | 0.00801 | 0.991 | 52.51 | 5.435 | 0.838 |
| 323 | 277.8 | 0.01293 | 0.995 | 37.08 | 4.027 | 0.990 |
表3 Fe3O4@C对红霉素的吸附等温线模型拟合数据
| 温度/K | Langmuir等温模型 ce/qe=ce/qm+1/ (kLqm) | Freundlich等温模型 lnqe=lnkF+1/(nlnce) | ||||
|---|---|---|---|---|---|---|
| qm/mg·g-1 | kL/L·mg-1 | R2 | kF/L·g-1 | n | R2 | |
| 303 | 227.3 | 0.00782 | 0.992 | 41.72 | 4.854 | 0.895 |
| 313 | 263.2 | 0.00801 | 0.991 | 52.51 | 5.435 | 0.838 |
| 323 | 277.8 | 0.01293 | 0.995 | 37.08 | 4.027 | 0.990 |
| 温度/K | lnKd | |||
|---|---|---|---|---|
| 303 | 2.62 | -6.60 | ||
| 313 | 3.02 | -7.87 | 12.12 | 0.0639 |
| 323 | 5.11 | -13.74 |
表4 红霉素对Fe3O4@C的热力学参数
| 温度/K | lnKd | |||
|---|---|---|---|---|
| 303 | 2.62 | -6.60 | ||
| 313 | 3.02 | -7.87 | 12.12 | 0.0639 |
| 323 | 5.11 | -13.74 |
| 吸附剂 | 吸附量/mg·g-1 | 参考文献 |
|---|---|---|
| SBA-15 | 172.4 | [ |
| 分子印迹聚合物 | 87.1 | [ |
| 磁性印迹聚合物 | 38.4 | [ |
| 磁性活性炭/壳聚糖 | 178.6 | [ |
| Fe3O4@C | 255.0 | 本文 |
表5 不同吸附剂对红霉素的吸附
| 吸附剂 | 吸附量/mg·g-1 | 参考文献 |
|---|---|---|
| SBA-15 | 172.4 | [ |
| 分子印迹聚合物 | 87.1 | [ |
| 磁性印迹聚合物 | 38.4 | [ |
| 磁性活性炭/壳聚糖 | 178.6 | [ |
| Fe3O4@C | 255.0 | 本文 |
| 1 | LENG Lijian, WEI Liang, XIONG Qinpan, et al. Use of microalgae based technology for the removal of antibiotics from wastewater: A review[J]. Chemosphere, 2020, 238: 124680. |
| 2 | LI Si, SHI Wanzi, LIU Wei, et al. A duodecennial national synthesis of antibiotics in China’s major rivers and seas (2005—2016)[J]. Science of the Total Environment, 2018, 615: 906-917. |
| 3 | LE PAGE Gareth, GUNNARSSON Lina, SNAPE Jason, et al. Integrating human and environmental health in antibiotic risk assessment: A critical analysis of protection goals, species sensitivity and antimicrobial resistance[J]. Environment International, 2017, 109: 155-169. |
| 4 | ZHOU Lijun, YING Guangguo, LIU Shan, et al. Occurrence and fate of eleven classes of antibiotics in two typical wastewater treatment plants in South China[J]. Science of the Total Environment, 2013, 452/453: 365-376. |
| 5 | Michaela TOKARČÍKOVÁ, Jana SEIDLEROVÁ, MOTYKA Oldřich, et al. Experimental verification of regenerable magnetically modified montmorillonite and its application for heavy metals removal from metallurgical waste leachates[J]. Journal of Water Process Engineering, 2021, 39: 101691. |
| 6 | MARKEB A A, LLIMÓS-TURET J, FERRER I, et al. The use of magnetic iron oxide based nanoparticles to improve microalgae harvesting in real wastewater[J]. Water Research, 2019, 159: 490-500. |
| 7 | LU Jianwei, FU Fenglian, DING Zecong, et al. Removal mechanism of selenite by Fe3O4-precipitated mesoporous magnetic carbon microspheres[J]. Journal of Hazardous Materials, 2017, 330: 93-104. |
| 8 | WANG Minghong, WANG Xiqing, YUE Qin, et al. Templated fabrication of core-shell magnetic mesoporous carbon microspheres in 3-dimensional ordered macroporous silicas[J]. Chemistry of Materials, 2014, 26(10): 3316-3321. |
| 9 | Zafar ALI, TIAN Lei, ZHANG Baoliang, et al. Synthesis of fibrous and non-fibrous mesoporous silica magnetic yolk-shell microspheres as recyclable supports for immobilization of Candida rugosa lipase[J]. Enzyme and Microbial Technology, 2017, 103: 42-52. |
| 10 | YIN Yuyong, ZHOU Shuxue, MIN Chen, et al. Preparation of rattle-type magnetic mesoporous carbon spheres and their highly efficient adsorption and separation[J]. Journal of Colloid and Interface Science, 2011, 361(2): 527-533. |
| 11 | GAO Jining, RAN Xinze, SHI Chunmeng, et al. One-step solvothermal synthesis of highly water-soluble, negatively charged superparamagnetic Fe3O4 colloidal nanocrystal clusters[J]. Nanoscale, 2013, 5(15): 7026-7033. |
| 12 | ZHAO Wenru, CHEN Hangrong, LI Yongsheng, et al. Uniform rattle-type hollow magnetic mesoporous spheres as drug delivery carriers and their sustained-release property[J]. Advanced Functional Materials, 2008, 18(18): 2780-2788. |
| 13 | LI Kexin, ZENG Zhenxing, XIONG Jingjing, et al. Fabrication of mesoporous Fe3O4@SiO2@CTAB-SiO2 magnetic microspheres with a core/shell structure and their efficient adsorption performance for the removal of trace PFOS from water[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2015, 465: 113-123. |
| 14 | MENG Qingnan, WANG Kai, TANG Yufei, et al. One-pot synthesis of Fe2O3 loaded SiO2 hollow particles as effective visible light photo-Fenton catalyst[J]. Journal of Alloys and Compounds, 2017, 722: 8-16. |
| 15 | HABILA M A, ALOTHMAN Z A, EI-TONI A M, et al. Mercaptobenzothiazole-functionalized magnetic carbon nanospheres of type Fe3O4@SiO2@C for the preconcentration of nickel, copper and lead prior to their determination by ICP-MS[J]. Microchimica Acta, 2016, 183(8): 2377-2384. |
| 16 | HORIKAWA T, DO D D, NICHOLSON D. Capillary condensation of adsorbates in porous materials[J]. Advances in Colloid and Interface Science, 2011, 169(1): 40-58. |
| 17 | KIM Y H, HEINZE T M, BEGER R, et al. A kinetic study on the degradation of erythromycin A in aqueous solution[J]. International Journal of Pharmaceutics, 2004, 271(1/2): 63-76. |
| 18 | LIMA E C, HOSSEINI-BANDEGHARAEI A, MORENO-PIRAJÁN J C, et al. A critical review of the estimation of the thermodynamic parameters on adsorption equilibria. Wrong use of equilibrium constant in the Van’t Hoof equation for calculation of thermodynamic parameters of adsorption[J]. Journal of Molecular Liquids, 2019, 273: 425-434. |
| 19 | PATHAN Soyeb, SOLANKI Priyanka, PATEL Anjali. Functionalized SBA-15 for controlled release of poorly soluble drug, erythromycin[J]. Microporous and Mesoporous Materials, 2018, 258: 114-121. |
| 20 | LI Tianhao, LI Xiufang, LIU Hui, et al. Preparation and characterization of molecularly imprinted polymers based on β-cyclodextrin-stabilized Pickering emulsion polymerization for selective recognition of erythromycin from river water and milk[J]. Journal of Separation Science, 2020, 43(18): 3683-3690. |
| 21 | Hongxiang OU, CHEN Qunhui, PAN Jianming, et al. Selective removal of erythromycin by magnetic imprinted polymers synthesized from chitosan-stabilized Pickering emulsion[J]. Journal of Hazardous Materials, 2015, 289: 28-37. |
| 22 | DANALıOĞLU S T, BAYAZIT Ş S, KERKEZ KUYUMCU Ö, et al. Efficient removal of antibiotics by a novel magnetic adsorbent: Magnetic activated carbon/chitosan (MACC) nanocomposite[J]. Journal of Molecular Liquids, 2017, 240: 589-596. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 胡喜, 王明珊, 李恩智, 黄思鸣, 陈俊臣, 郭秉淑, 于博, 马志远, 李星. 二硫化钨复合材料制备与储钠性能研究进展[J]. 化工进展, 2023, 42(S1): 344-355. |
| [3] | 崔守成, 徐洪波, 彭楠. 两种MOFs材料用于O2/He吸附分离的模拟分析[J]. 化工进展, 2023, 42(S1): 382-390. |
| [4] | 陈崇明, 陈秋, 宫云茜, 车凯, 郁金星, 孙楠楠. 分子筛基CO2吸附剂研究进展[J]. 化工进展, 2023, 42(S1): 411-419. |
| [5] | 李世霖, 胡景泽, 王毅霖, 王庆吉, 邵磊. 电渗析分离提取高值组分的研究进展[J]. 化工进展, 2023, 42(S1): 420-429. |
| [6] | 许春树, 姚庆达, 梁永贤, 周华龙. 共价有机框架材料功能化策略及其对Hg(Ⅱ)和Cr(Ⅵ)的吸附性能研究进展[J]. 化工进展, 2023, 42(S1): 461-478. |
| [7] | 顾永正, 张永生. HBr改性飞灰对Hg0的动态吸附及动力学模型[J]. 化工进展, 2023, 42(S1): 498-509. |
| [8] | 郭强, 赵文凯, 肖永厚. 增强流体扰动强化变压吸附甲硫醚/氮气分离的数值模拟[J]. 化工进展, 2023, 42(S1): 64-72. |
| [9] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [10] | 贺美晋. 分子管理在炼油领域分离技术中的应用和发展趋势[J]. 化工进展, 2023, 42(S1): 260-266. |
| [11] | 廖志新, 罗涛, 王红, 孔佳骏, 申海平, 管翠诗, 王翠红, 佘玉成. 溶剂脱沥青技术应用与进展[J]. 化工进展, 2023, 42(9): 4573-4586. |
| [12] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [13] | 林晓鹏, 肖友华, 管奕琛, 鲁晓东, 宗文杰, 傅深渊. 离子聚合物-金属复合材料(IPMC)柔性电极的研究进展[J]. 化工进展, 2023, 42(9): 4770-4782. |
| [14] | 杨莹, 侯豪杰, 黄瑞, 崔煜, 王兵, 刘健, 鲍卫仁, 常丽萍, 王建成, 韩丽娜. 利用煤焦油中酚类物质Stöber法制备碳纳米球用于CO2吸附[J]. 化工进展, 2023, 42(9): 5011-5018. |
| [15] | 张丽宏, 金要茹, 程芳琴. 煤气化渣资源化利用[J]. 化工进展, 2023, 42(8): 4447-4457. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||