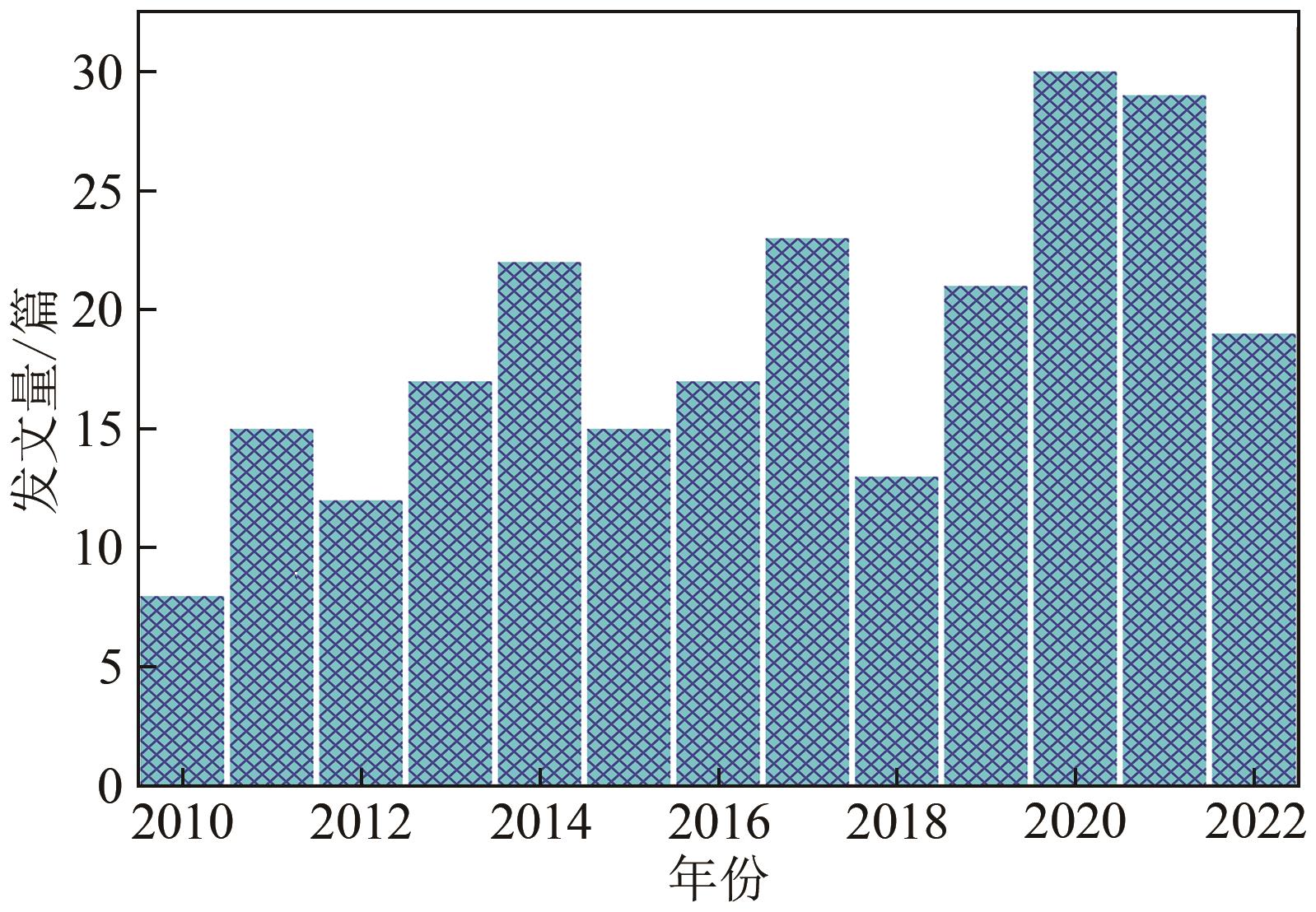
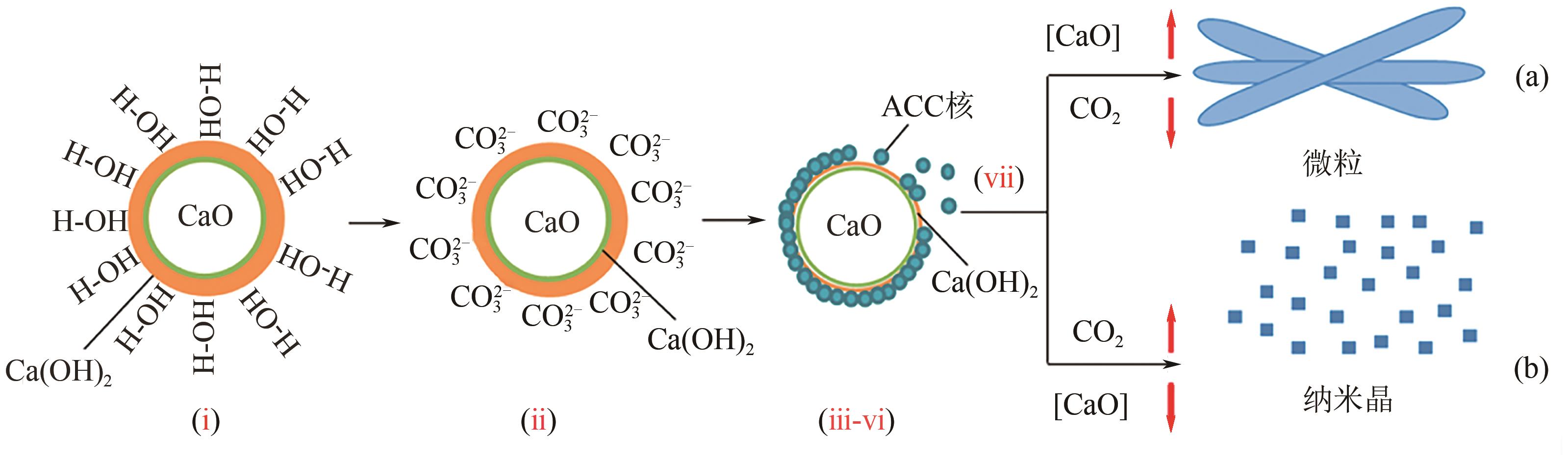
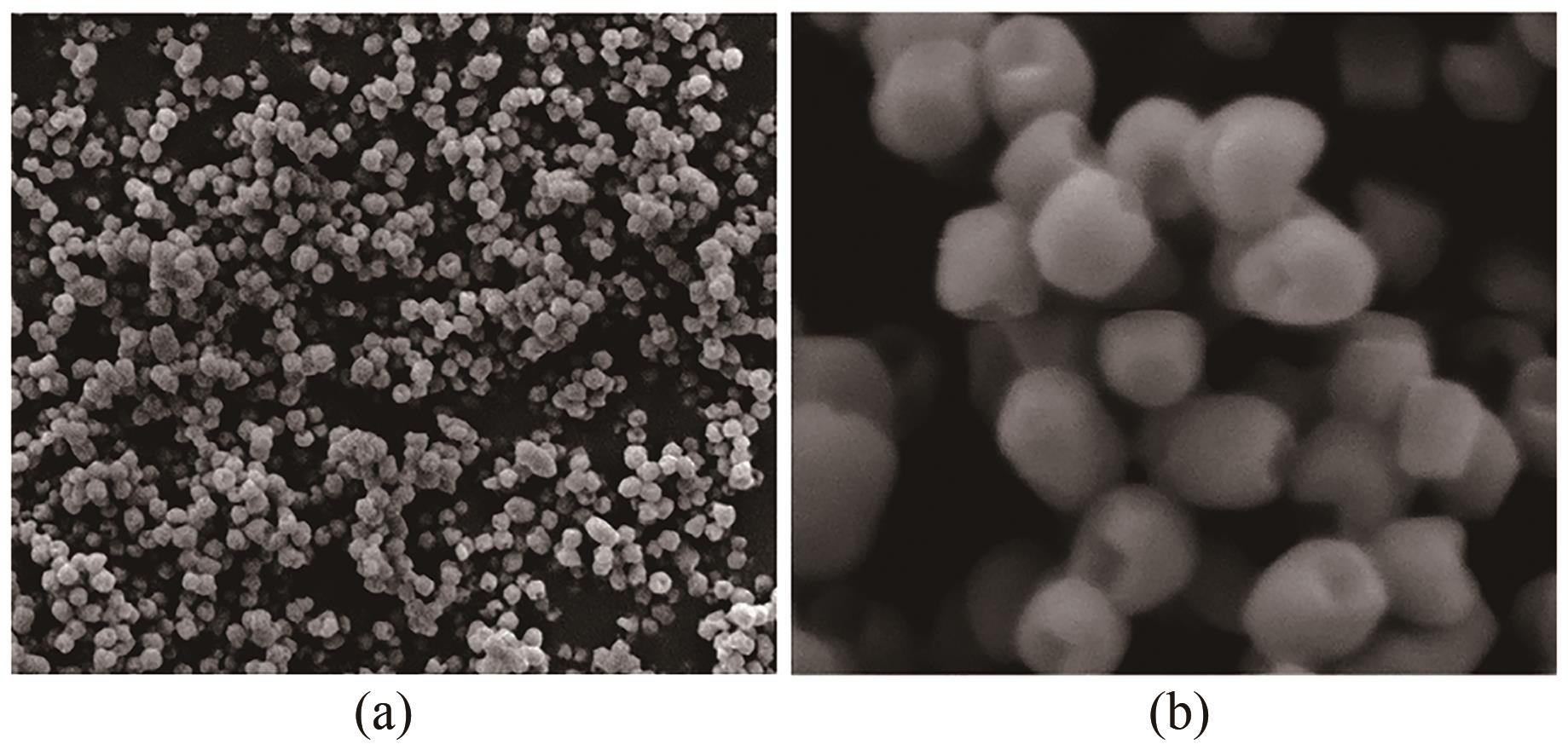
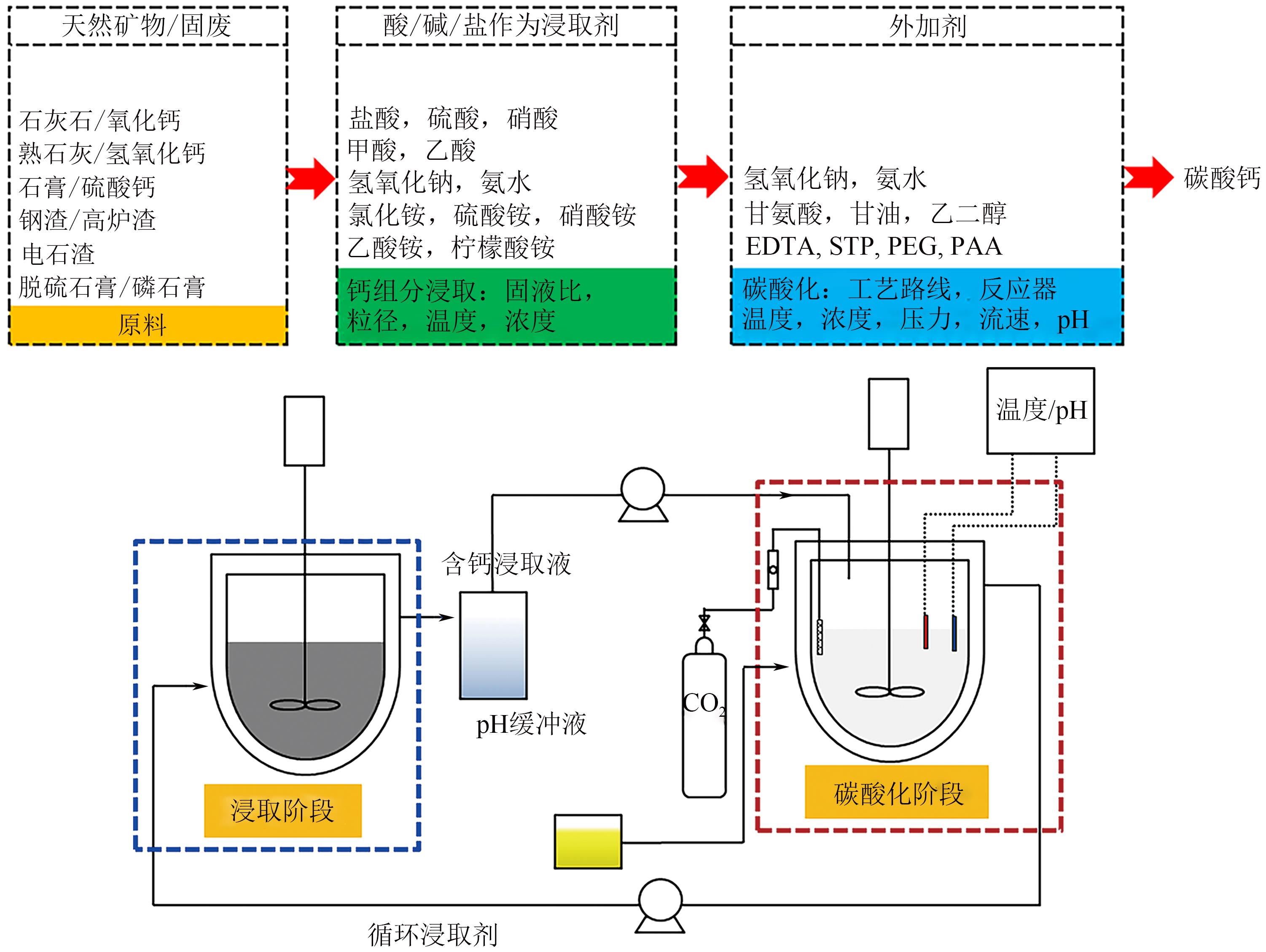
化工进展 ›› 2023, Vol. 42 ›› Issue (4): 2047-2057.DOI: 10.16085/j.issn.1000-6613.2022-1012
二氧化碳矿化高钙基固废制备微细碳酸钙研究进展
李文秀1( ), 杨宇航1, 黄艳2, 王涛1(
), 杨宇航1, 黄艳2, 王涛1( ), 王镭1, 方梦祥1
), 王镭1, 方梦祥1
- 1.浙江大学能源工程学院,浙江 杭州 310027
2.陕西国华锦界能源有限公司,陕西 榆林 719000
-
收稿日期:2022-05-31修回日期:2022-09-14出版日期:2023-04-25发布日期:2023-05-08 -
通讯作者:王涛 -
作者简介:李文秀(1988—),男,博士研究生,研究方向为二氧化碳矿化利用技术。E-mail:wenxiu.li@zju.edu.cn。 -
基金资助:浙江省属基本科研业务费专项资金(2021XZZX012);浙江省“尖兵”项目(2022C03040)
Preparation of ultrafine calcium carbonate by CO2 mineralization using high calcium-based solid waste
LI Wenxiu1( ), YANG Yuhang1, HUANG Yan2, WANG Tao1(
), YANG Yuhang1, HUANG Yan2, WANG Tao1( ), WANG Lei1, FANG Mengxiang1
), WANG Lei1, FANG Mengxiang1
- 1.College of Energy Engineering, Zhejiang University, Hangzhou 310027, Zhejiang, China
2.Shaanxi Guohua Jinjie Energy Co. , Ltd. , Yulin 719000, Shaanxi, China
-
Received:2022-05-31Revised:2022-09-14Online:2023-04-25Published:2023-05-08 -
Contact:WANG Tao
摘要:
微细碳酸钙作为一种重要的化工填充物,广泛地应用于造纸、油墨、橡胶、塑料等行业。随着当前碳减排行动的日趋深入,微细碳酸钙制备从原料到工艺路线已经越来越多元化。利用工业高钙原料,如钢渣、电石渣、废弃石膏等作为钙源耦合CO2制备微细碳酸钙的技术越来越被关注。本文综述了CO2矿化高钙固废制备微细碳酸钙的研究进展,分别对采用氧化钙、氢氧化钙、硫酸钙以及高钙基工业固废等材料制备微细碳酸钙进行了具体介绍。比较了在不同材料与CO2矿化反应制备碳酸钙的技术特性以及优化碳酸钙产品性能方面的最新成果,并对最具代表性的工业固体废弃物制备微细碳酸钙技术规模化应用及环境经济性评价进行了总结归纳。在初步技术经济分析的基础上,发现当前CO2矿化高钙固废制备微细碳酸钙的规模化应用主要受限于碳酸钙结晶速度快、运行成本高及能源强度大等方面。为更好地开展大规模工业化应用,建议如下:一是针对碳酸钙结晶过程,着眼于微观反应机制和高钙固废材料特性,开发有效适用于工业应用的控制方法;二是将工业固废资源化与合成精细化碳酸钙技术耦合,推进特定工艺开发和装置研发;三是基于控制方法和装备开发,对特定工艺路线展开全生命周期环境和经济性评估。
中图分类号:
引用本文
李文秀, 杨宇航, 黄艳, 王涛, 王镭, 方梦祥. 二氧化碳矿化高钙基固废制备微细碳酸钙研究进展[J]. 化工进展, 2023, 42(4): 2047-2057.
LI Wenxiu, YANG Yuhang, HUANG Yan, WANG Tao, WANG Lei, FANG Mengxiang. Preparation of ultrafine calcium carbonate by CO2 mineralization using high calcium-based solid waste[J]. Chemical Industry and Engineering Progress, 2023, 42(4): 2047-2057.
| 合成方法 | 条件参数 | 碳酸钙产品 | 参考文献 |
|---|---|---|---|
| 棕榈酸作模板剂 | 25℃,CO2 100mL/min | 立方形单颗粒,无表面活性剂100nm;1.5%(质量分数)棕榈酸20~4nm;2.5%(质量分数)棕榈酸500~800nm | [ |
| 超声波碳酸化 | 30℃,CO2 45L/h,Ca(OH)2 20mL/min | 菱面体方解石,224nm | [ |
| 微孔分散法 | 5℃/25℃,CO2 100mL/min,油酸作为表面活性剂 | 立方方解石单晶40~60nm,油酸作为表面活性剂时直径减小到30~40nm | [ |
| 超重力反应沉淀法 | 工业生产条件 | 方解石和球霰石碳酸钙,粒径80nm | [ |
| 连续喷雾法 | CO2 8%,60%饱和度的Ca(OH)2 | 纺锤状碳酸钙晶体,1~3µm | [ |
| 连续鼓泡搅拌法 | CO2 452.30mL/min,Ca(OH)2 2mol/L | 方解石晶体,0.1~0.5µm | [ |
表1 Ca(OH)2-H2O-CO2反应体系下合成微细碳酸钙
| 合成方法 | 条件参数 | 碳酸钙产品 | 参考文献 |
|---|---|---|---|
| 棕榈酸作模板剂 | 25℃,CO2 100mL/min | 立方形单颗粒,无表面活性剂100nm;1.5%(质量分数)棕榈酸20~4nm;2.5%(质量分数)棕榈酸500~800nm | [ |
| 超声波碳酸化 | 30℃,CO2 45L/h,Ca(OH)2 20mL/min | 菱面体方解石,224nm | [ |
| 微孔分散法 | 5℃/25℃,CO2 100mL/min,油酸作为表面活性剂 | 立方方解石单晶40~60nm,油酸作为表面活性剂时直径减小到30~40nm | [ |
| 超重力反应沉淀法 | 工业生产条件 | 方解石和球霰石碳酸钙,粒径80nm | [ |
| 连续喷雾法 | CO2 8%,60%饱和度的Ca(OH)2 | 纺锤状碳酸钙晶体,1~3µm | [ |
| 连续鼓泡搅拌法 | CO2 452.30mL/min,Ca(OH)2 2mol/L | 方解石晶体,0.1~0.5µm | [ |
| 原料 | CaO | MgO | SiO2 | Fe2O3 | Al2O3 | SO3 | 参考文献 |
|---|---|---|---|---|---|---|---|
| 钢渣 | 30~60 | 5~20 | 10~45 | 3~9 | 1~18 | <1 | [ |
| 高炉渣 | 30~50 | 5~15 | 25~45 | 10~30 | 0~10 | <1 | |
| 电石渣 | 80~90 | 0.1~1.5 | 1~20 | 0.2~1.5 | 1~3 | <1 | |
| 废弃石膏 | 30~45 | <1 | 0.1~6.5 | <1 | <1 | 40~45 |
表2 高钙基工业固体废物的化学组分分析(质量分数) (%)
| 原料 | CaO | MgO | SiO2 | Fe2O3 | Al2O3 | SO3 | 参考文献 |
|---|---|---|---|---|---|---|---|
| 钢渣 | 30~60 | 5~20 | 10~45 | 3~9 | 1~18 | <1 | [ |
| 高炉渣 | 30~50 | 5~15 | 25~45 | 10~30 | 0~10 | <1 | |
| 电石渣 | 80~90 | 0.1~1.5 | 1~20 | 0.2~1.5 | 1~3 | <1 | |
| 废弃石膏 | 30~45 | <1 | 0.1~6.5 | <1 | <1 | 40~45 |
| 1 | 黄浩, 王涛, 方梦祥. 二氧化碳矿化养护混凝土技术及新型材料研究进展[J]. 化工进展, 2019, 38(10): 4363-4373. |
| HUANG Hao, WANG Tao, FANG Mengxiang. Review on carbon dioxide mineral carbonation curing technology of concrete and novel material development[J]. Chemical Industry and Engineering Progress, 2019, 38(10): 4363-4373. | |
| 2 | Commission European. 2050 Long-term Strategy [EB/OL]. [2020-04-15]. |
| 3 | 张贤, 李阳, 马乔, 等. 我国碳捕集利用与封存技术发展研究[J]. 中国工程科学, 2021, 23(6): 70-80. |
| ZHANG Xian, LI Yang, MA Qiao, et al. Development of carbon capture, utilization and storage technology in China[J]. Strategic Study of CAE, 2021, 23(6): 70-80. | |
| 4 | XIE Heping, TANG Liang, WANG Yufei, et al. Feedstocks study on CO2 mineralization technology[J]. Environmental Earth Sciences, 2016, 75(7): 1-9. |
| 5 | WANG Bo, PAN Zihe, DU Zhiping, et al. Effect of impure components in flue gas desulfurization (FGD) gypsum on the generation of polymorph CaCO3 during carbonation reaction[J]. Journal of Hazardous Materials, 2019, 369: 236-243. |
| 6 | 王宇轩, 徐颖, 王东平, 等. 球霰石的性质及其应用进展[J]. 安徽理工大学学报(自然科学版), 2017, 37(2): 76-80. |
| WANG Yuxuan, XU Ying, WANG Dongping, et al. Properties and applications of vaterite[J]. Journal of Anhui University of Science and Technology (Natural Science), 2017, 37(2): 76-80. | |
| 7 | JIMOH Onimisi A, ARIFFIN Kamar Shah, HUSSIN Hashim Bin, et al. Synthesis of precipitated calcium carbonate: a review[J]. Carbonates and Evaporites, 2018, 33(2): 331-346. |
| 8 | CHEN Peng, LIU Yang, DI Mingwei. The effect of nano-filler on the damping properties of polyacrylic damping paint[J]. Advanced Materials Research, 2011, 183/184/185: 2154-2157. |
| 9 | TRUSHINA Daria B, BUKREEVA Tatiana V, KOVALCHUK Mikhail V, et al. CaCO3 vaterite microparticles for biomedical and personal care applications[J]. Materials Science and Engineering C, 2014, 45: 644-658. |
| 10 | WANG F, DREISINGER D B, JARVIS M, et al. The technology of CO2 sequestration by mineral carbonation: current status and future prospects[J]. Canadian Metallurgical Quarterly, 2018, 57(1): 46-58. |
| 11 | IBRAHIM Mohamed H, EL-NAAS Muftah H, ZEVENHOVEN Ron, et al. Enhanced CO2 capture through reaction with steel-making dust in high salinity water[J]. International Journal of Greenhouse Gas Control, 2019, 91: 102819. |
| 12 | BARHOUM Ahmed, VAN ASSCHE Guy, MAKHLOUF Abdel Salam Hamdy, et al. A green, simple chemical route for the synthesis of pure nanocalcite crystals[J]. Crystal Growth and Design, 2015, 15(2): 573-580. |
| 13 | ZHOU Jun, CAO Xun, YONG Xiaoyu, et al. Effects of various factors on biogas purification and nano-CaCO3 synthesis in a membrane reactor[J]. Industrial & Engineering Chemistry Research, 2014, 53(4): 1702-1706. |
| 14 | ERDOGAN Necmettin, EKEN Haci Ali. Precipitated calcium carbonate production, synthesis and properties[J]. Physicochemical Problems of Mineral Processing, 2016, 53: 57-68. |
| 15 | KEZUKA Yuki, KUMA Yoshiki, NAKAI Shinsuke, et al. Calcium carbonate chain-like nanoparticles: Synthesis, structural characterization, and dewaterability[J]. Powder Technology, 2018, 335: 195-203. |
| 16 | ULKERYILDIZ Eda, KILIC Sevgi, OZDEMIR Ekrem. Rice-like hollow nano-CaCO3 synthesis[J]. Journal of Crystal Growth, 2016, 450: 174-180. |
| 17 | ULKERYILDIZ Eda, KILIC Sevgi, OZDEMIR Ekrem. Nano-CaCO3 synthesis by jet flow[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2017, 512: 34-40. |
| 18 | ULKERYILDIZ Eda, KILIC Sevgi, OZDEMIR Ekrem. Nano-CaCO3 synthesis by jet flow[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2017, 512: 34-40. |
| 19 | JIANG Jiuxin, ZHANG Ying, YANG Xi, et al. Assemblage of nano-calcium carbonate particles on palmitic acid template[J]. Advanced Powder Technology, 2014, 25(2): 615-620. |
| 20 | SHIRSATH S R, SONAWANE S H, SAINI D R, et al. Continuous precipitation of calcium carbonate using sonochemical reactor[J]. Ultrasonics Sonochemistry, 2015, 24: 132-139. |
| 21 | JIANG Jiuxin, LIU Jie, LIU Chang, et al. Roles of oleic acid during micropore dispersing preparation of nano-calcium carbonate particles[J]. Applied Surface Science, 2011, 257(16): 7047-7053. |
| 22 | SHAN Dan, ZHU Mingjuan, HAN En, et al. Calcium carbonate nanoparticles: a host matrix for the construction of highly sensitive amperometric phenol biosensor[J]. Biosensors and Bioelectronics, 2007, 23(5): 648-654. |
| 23 | MA Liang, YANG Tingyu, WU Yu, et al. CO2 capture and preparation of spindle-like CaCO3 crystals for papermaking using calcium carbide residue waste via an atomizing approach[J]. Korean Journal of Chemical Engineering, 2019, 36(9): 1432-1440. |
| 24 | JIMOH Onimisi A, MAHMED Norsuria, OKOYE P U, et al. Utilization of milk of lime (MOL) originated from carbide lime waste and operating parameters optimization study for potential precipitated calcium carbonate (PCC) production[J]. Environmental Earth Sciences, 2016, 75(18): 1-7. |
| 25 | SHA Feng, ZHU Ning, BAI Yijia, et al. Controllable synthesis of various CaCO3 morphologies based on a CCUS idea[J]. ACS Sustainable Chemistry and Engineering, 2016, 4(6): 3032-3044. |
| 26 | KARTHIKA S, RADHAKRISHNAN T, KALAICHELVI P. A review of classical and nonclassical nucleation theories[J]. Crystal Growth & Design, 2016, 16: 6663-6681. |
| 27 | WANG Bo, PAN Zihe, CHENG Huaigang, et al. High-yield synthesis of vaterite microparticles in gypsum suspension system via ultrasonic probe vibration/magnetic stirring[J]. Journal of Crystal Growth, 2018, 492: 122-131. |
| 28 | GONG Yuan, WU Lin, LI Ji, et al. Modeling of multistep Ca2+ transfer in the carbonation system of CO2-NH4OH-CaSO4·2H2O-CaCO3 [J]. Journal of Crystal Growth, 2019, 522: 128-138. |
| 29 | ZHANG Xiaolei, CHEN Jiaxin, JIANG Jingjing, et al. The potential utilization of slag generated from iron- and steelmaking industries: A review[J]. Environmental Geochemistry and Health, 2020, 42(5): 1321-1334. |
| 30 | LIU Weizao, TENG Liumei, ROHANI Sohrab, et al. CO2 mineral carbonation using industrial solid wastes: A review of recent developments[J]. Chemical Engineering Journal, 2021, 416: 129093. |
| 31 | 赵红涛, 王树民, 刘志江, 等. 磷石膏矿化固定CO2制备高纯高白CaCO3 [J]. 材料导报, 2019, 33(18): 3031-3034, 3042. |
| ZHAO Hongtao, WANG Shumin, LIU Zhijiang, et al. Preparation of high-purity and high-white CaCO3 by phosphogypsum mineralization for CO2 capture[J]. Materials Reports, 2019, 33(18): 3031-3034, 3042. | |
| 32 | 赵立文, 朱干宇, 李少鹏, 等. 电石渣特性及综合利用研究进展[J]. 洁净煤技术, 2021, 27(3): 13-26. |
| ZHAO Liwen, ZHU Ganyu, LI Shaopeng, et al. Research progress on characteristics and comprehensive utilization of calcium carbide slag[J]. Clean Coal Technology, 2021, 27(3): 13-26. | |
| 33 | USGS. Mineral commodity summaries 2020. U.S. Geological Survey[EB/OL]. .[2020-04-15]. |
| 34 | TEIR Sebastian, KUUSIK Rein, FOGELHOLM Carl Johan, et al. Production of magnesium carbonates from serpentinite for long-term storage of CO2 [J]. International Journal of Mineral Processing, 2007, 85(1/2/3): 1-15. |
| 35 | ZHAO Qing, LI Jingyu, YOU Kaiwen, et al. Recovery of calcium and magnesium bearing phases from iron- and steelmaking slag for CO2 sequestration[J]. Process Safety and Environmental Protection, 2020, 135: 81-90. |
| 36 | BAO Weijun, LI Huiquan, YI Zhang. Selective leaching of steelmaking slag for indirect CO2 mineral sequestration[J]. Industrial and Engineering Chemistry Research, 2010, 49(5): 2055-2063. |
| 37 | OWAIS M, JÄRVINEN M, TASKINEN P, et al. Experimental study on the extraction of calcium, magnesium, vanadium and silicon from steelmaking slags for improved mineral carbonation of CO2 [J]. Journal of CO2 Utilization, 2019, 31: 1-7. |
| 38 | TONG Zhibo, MA Guojun, ZHOU Dan, et al. The indirect mineral carbonation of electric arc furnace slag under microwave irradiation[J]. Scientific Reports, 2019, 9(1): 7676. |
| 39 | PARK Ah-hyung alissa, FAN Liang-shih. CO2 mineral sequestration: Physically activated dissolution of serpentine and pH swing process[J]. Chemical Engineering Science, 2004, 59: 5241-5247. |
| 40 | WANG Xiaolong, Mercedes MAROTO-VALER M. Dissolution of serpentine using recyclable ammonium salts for CO2 mineral carbonation[J]. Fuel, 2011, 90(3): 1229-1237. |
| 41 | WANG Lin, LIU Weizao, HU Jingpeng, et al. Indirect mineral carbonation of titanium-bearing blast furnace slag coupled with recovery of TiO2 and Al2O3 [J]. Chinese Journal of Chemical Engineering, 2018, 26(3): 583-592. |
| 42 | CHIANG Yi wai, SANTOS Rafael M, ELSEN Jan, et al. Towards zero-waste mineral carbon sequestration via two-way valorization of ironmaking slag[J]. Chemical Engineering Journal, 2014, 249: 260-269. |
| 43 | WANG Yongjing, YE Baofang, HONG Zengchun, et al. Uniform calcite mircro/nanorods preparation from carbide slag using recyclable citrate extractant[J]. Journal of Cleaner Production, 2020, 253: 119930. |
| 44 | 吴琦文, 施利毅, 张仲燕. 利用电石渣制备纳米碳酸钙的研究[J]. 上海大学学报(自然科学版), 2002, 8(3): 247-250. |
| WU Qiwen, SHI Liyi, ZHANG Zhongyan. Preparation of nanometer calcium carbonate particles by calcium carbide residue[J]. Journal of Shanghai University (Natural Science Edition), 2002, 8(3): 247-250. | |
| 45 | 刘飞, 袁铭鸿, 曹建新. 利用电石渣制备碳酸钙晶须的初步研究[J]. 贵州大学学报(自然科学版), 2010, 27(2): 126-128, 132. |
| LIU Fei, YUAN Minghong, CAO Jianxin. Primary research of calcium carbonate whisker prepared from carbide slag[J]. Journal of Guizhou University (Natural Science Edition), 2010, 27(2): 126-128, 132. | |
| 46 | 张爱华, 朱敏, 关云山, 等. 氯化铵处理电石渣制备纳米碳酸钙的实验研究[J]. 科学技术与工程, 2013, 13(10): 2880-2883. |
| ZHANG Aihua, ZHU Min, GUAN Yunshan, et al. Experimental study on preparation of nanosized calcium carbonate from carbide slag treated by ammonium chloride[J]. Science Technology and Engineering, 2013, 13(10): 2880-2883. | |
| 47 | 张果龙, 刘跃进, 罗云峰, 等. 电石渣制备立方体晶型纳米碳酸钙研究[J]. 河北化工, 2008(6): 14-16, 49. |
| ZHANG Guolong, LIU Yuejin, LUO Yunfeng, et al. Study on preparation cubical mamometer calcium carbonate by calcium carbide residue[J]. Hebei Chemical Engineering and Industry, 2008(6): 14-16, 49. | |
| 48 | GUO Bo, ZHAO Tianxiang, SHA Feng, et al. Synthesis of vaterite CaCO3 micro-spheres by carbide slag and a novel CO2-storage material[J]. Journal of CO2 Utilization, 2017, 18: 23-29. |
| 49 | LI Wenxiu, HUANG Yan, WANG Tao, et al. Preparation of calcium carbonate nanoparticles from waste carbide slag based on CO2 mineralization[J]. Journal of Cleaner Production, 2022, 363: 132463. |
| 50 | 向兰, 张英才, 张清杰, 等. 一种工业副产石膏选择性固固分离制备高纯石膏的方法: CN109824078B[P]. 2019-03-05. |
| XIANG Lan, ZHANG Yingcai, ZHANG Qingjie, et al. A method for preparing high-purity gypsum by selective solid-solid separation of industrial by-product gypsum: CN109824078B[P]. 2019-03-05. | |
| 51 | PÉREZ-MORENO S M, GÁZQUEZ M J, BOLÍVAR J P. CO2 sequestration by indirect carbonation of artificial gypsum generated in the manufacture of titanium dioxide pigments[J]. Chemical Engineering Journal, 2015, 262: 737-746. |
| 52 | DE BEER M, DOUCET F J, MAREE J P, et al. Synthesis of high-purity precipitated calcium carbonate during the process of recovery of elemental sulphur from gypsum waste[J]. Waste Management, 2015, 46: 619-627. |
| 53 | DING Wenjin, CHEN Qiuju, SUN Hongjuan, et al. Modified mineral carbonation of phosphogypsum for CO2 sequestration[J]. Journal of CO2 Utilization, 2019, 34: 507-515. |
| 54 | HOSSEINI T, SELOMULYA C, HAQUE N, et al. Indirect carbonation of Victorian brown coal fly ash for CO2 sequestration: multiple-cycle leaching-carbonation and magnesium leaching kinetic modeling[J]. Energy & Fuels, 2014, 28: 6481-6493. |
| 55 | HE Lanlan, YU Dunxi, Weizhi LYU, et al. A novel method for CO2 sequestration via indirect carbonation of coal fly ash[J]. Industrial & Engineering Chemistry Research, 2013, 52(43): 15138-15145. |
| 56 | CUI Li, GUO Yanxia, WANG Xuming, et al. Dissolution kinetics of aluminum and iron from coal mining waste by hydrochloric acid[J]. Chinese Journal of Chemical Engineering, 2015, 23(3): 590-596. |
| 57 | WANG Jinyu, HUANG Xiaowei, WANG Liangshi, et al. Kinetics study on the leaching of rare earth and aluminum from FCC catalyst waste slag using hydrochloric acid[J]. Hydrometallurgy, 2017, 171: 312-319. |
| 58 | TRUSHINA Daria B, BUKREEVA Tatiana V, KOVALCHUK Mikhail V, et al. CaCO3 vaterite microparticles for biomedical and personal care applications[J]. Materials Science and Engineering, 2014, 45: 644-658. |
| 59 | ZHONG Yanjun, SHI Ting, CHEN Qiuge, et al. Leaching calcium from phosphogypsum desulfurization slag by using ammonium chloride solution: thermodynamics and kinetics study[J]. Chinese Journal of Chemical Engineering, 2020, 28(1): 208-215. |
| 60 | GEBAUER D. How can additives control the early stages of mineralisation[J]. Minerals, 2018, 8: 179. |
| 61 | SAID Arshe, LAUKKANEN Timo, Mika JÄRVINEN. Pilot-scale experimental work on carbon dioxide sequestration using steelmaking slag[J]. Applied Energy, 2016, 177: 602-611. |
| 62 | PARK Sanghyun, Yongtae AHN, LEE Sunjae, et al. Calcium carbonate synthesis from waste concrete for carbon dioxide capture: from laboratory to pilot scale[J]. Journal of Hazardous Materials, 2021, 403: 123862. |
| 63 | IIZUKA Atsushi, SASAKI Takeshi, HONMA Masato, et al. Pilot-scale operation of a concrete sludge recycling plant and simultaneous production of calcium carbonate[J]. Chemical Engineering Communications, 2017, 204(1): 79-85. |
| 64 | SANNA A, UIBU M, CARAMANNA G, et al. A review of mineral carbonation technologies to sequester CO2 [J]. Chemical Society Reviews, 2014, 43(23): 8049-8080. |
| 65 | TEIR Sebastian, KOTIRANTA Tuukka, PAKARINEN Jouko, et al. Case study for production of calcium carbonate from carbon dioxide in flue gases and steelmaking slag[J]. Journal of CO2 Utilization, 2016, 14: 37-46. |
| 66 | MATTILA Hannu Petteri, HUDD Hannes, ZEVENHOVEN Ron. Cradle-to-gate life cycle assessment of precipitated calcium carbonate production from steel converter slag[J]. Journal of Cleaner Production, 2014, 84: 611-618. |
| [1] | 舒斌, 陈建宏, 熊健, 吴其荣, 喻江涛, 杨平. 碳中和目标下推动绿色甲醇发展的必要性分析[J]. 化工进展, 2023, 42(9): 4471-4478. |
| [2] | 张丽宏, 金要茹, 程芳琴. 煤气化渣资源化利用[J]. 化工进展, 2023, 42(8): 4447-4457. |
| [3] | 丁文金, 刘卓齐, 卢海臣, 孙红娟, 彭同江. CH3COONa-NH4OH-H2O体系下磷石膏矿化CO2-联产高纯CaCO3[J]. 化工进展, 2023, 42(7): 3824-3833. |
| [4] | 孙晖, 孟祥海, 魏景海, 周红军, 徐春明. 绿电制氢生产氨的新场景与实践[J]. 化工进展, 2023, 42(2): 1098-1102. |
| [5] | 陶雨萱, 郭亮, 高聪, 宋伟, 陈修来. 代谢工程改造微生物固定二氧化碳研究进展[J]. 化工进展, 2023, 42(1): 40-52. |
| [6] | 高宁博, 胡雅迪, 全翠. 餐厨垃圾的热转化和生物处理研究进展[J]. 化工进展, 2022, 41(S1): 507-515. |
| [7] | 余正伟, 张晓霞, 雷杰, 李澳, 王光应, 丁祥, 龙红明. 废CeO x -MnO x 基SCR脱硝催化剂还原酸浸综合回收铈锰[J]. 化工进展, 2022, 41(9): 5122-5131. |
| [8] | 杨学萍. 碳中和背景下现代煤化工技术路径探索[J]. 化工进展, 2022, 41(7): 3402-3412. |
| [9] | 蔡思超, 周静, 杜金泽, 李方舟, 李源森, 何林, 李鑫钢, 王成扬. 煤化工酚基精馏釜残资源化利用过程初步分析[J]. 化工进展, 2022, 41(6): 3360-3371. |
| [10] | 甘凤丽, 江霞, 常玉龙, 靳紫恒, 汪华林, 师敬伟. 石化行业碳中和技术路径探索[J]. 化工进展, 2022, 41(3): 1364-1375. |
| [11] | 张春, 王学瑞, 刘华, 高雪超, 张玉亭, 顾学红. 面向工业过程碳减排的分子筛膜技术研究进展[J]. 化工进展, 2022, 41(3): 1376-1390. |
| [12] | 王建斌, 陈云, 王可华, 于学鹏, 陈聪, 刘建忠. 工业窑炉协同处置固废研究进展[J]. 化工进展, 2022, 41(3): 1494-1502. |
| [13] | 陈健, 姬存民, 卜令兵. 碳中和背景下工业副产气制氢技术研究与应用[J]. 化工进展, 2022, 41(3): 1479-1486. |
| [14] | 相宏伟, 杨勇, 李永旺. 碳中和目标下的煤化工变革与发展[J]. 化工进展, 2022, 41(3): 1399-1408. |
| [15] | 朱家华, 穆立文, 蒋管聪, 刘立, 熊晶晶, 陆小华. 生物质协同流程工业节能、降污、减碳路径思考[J]. 化工进展, 2022, 41(3): 1111-1114. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||