化工进展 ›› 2023, Vol. 42 ›› Issue (4): 1797-1810.DOI: 10.16085/j.issn.1000-6613.2022-1042
电催化氮还原合成氨MOF基催化剂研究进展
- 1.常州大学石油化工学院,江苏 常州 213164
2.江苏省绿色催化材料与技术重点实验室(常州大学),江苏 常州 213164
-
收稿日期:2022-06-06修回日期:2022-10-02出版日期:2023-04-25发布日期:2023-05-08 -
通讯作者:银凤翔 -
作者简介:郭朋举(1992—),男,博士研究生,研究方向为电催化。E-mail:ppjuguo086@163.com。 -
基金资助:国家自然科学基金(22078027)
Research progress in MOF-based catalysts for electrocatalytic nitrogen reduction to ammonia
GUO Pengju1,2( ), HE Xiaobo1,2, YIN Fengxiang1,2(
), HE Xiaobo1,2, YIN Fengxiang1,2( )
)
- 1.College of Petrochemical Engineering, Changzhou University, Changzhou 213164, Jiangsu, China
2.Jiangsu Key laboratory of Advanced Catalytic Materials and Technology, Changzhou University, Changzhou 213164, Jiangsu, China
-
Received:2022-06-06Revised:2022-10-02Online:2023-04-25Published:2023-05-08 -
Contact:YIN Fengxiang
摘要:
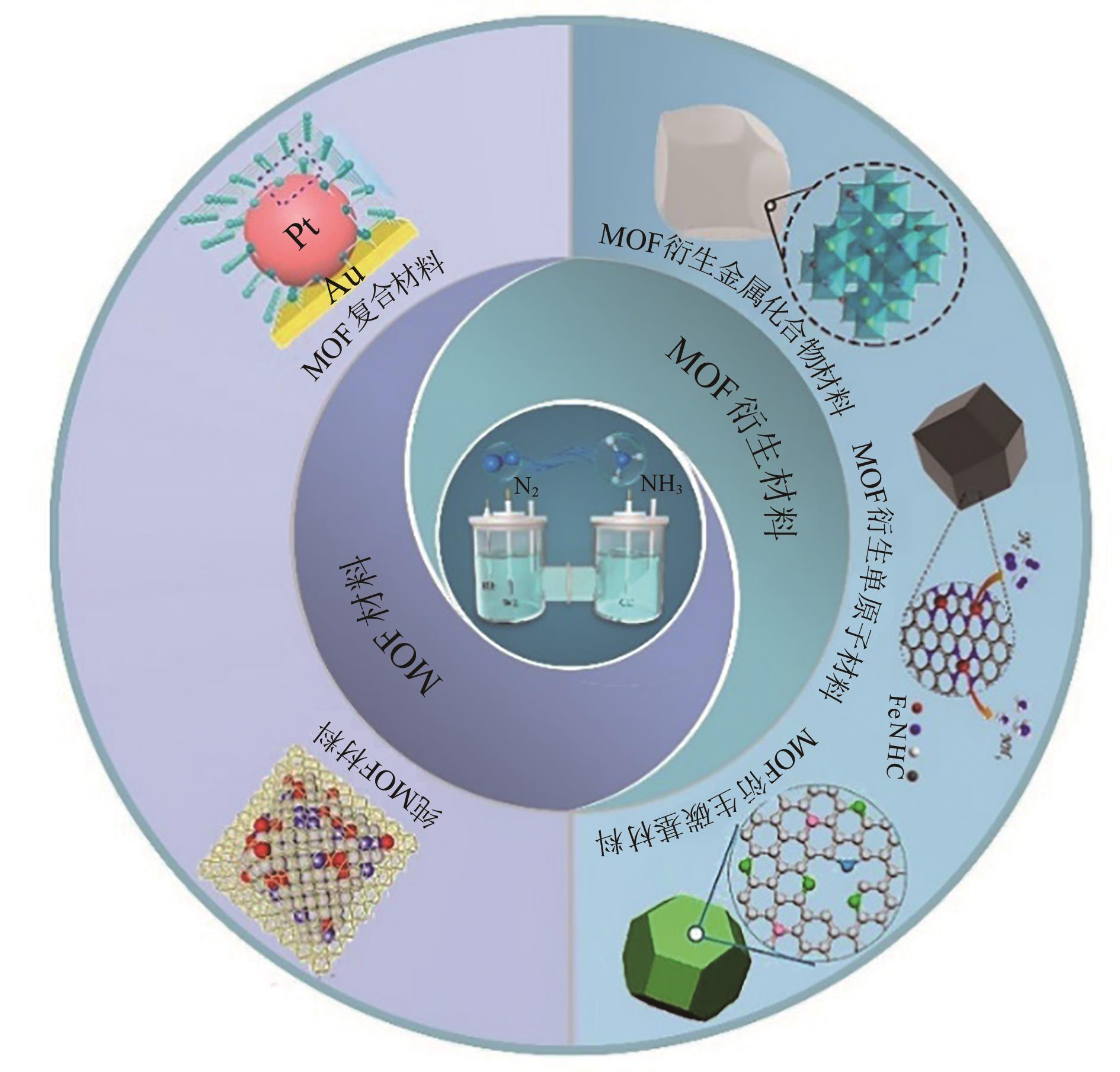
室温下电催化氮还原合成氨(nitrogen reduction reaction,NRR)因其较低的能耗而受到广泛关注。到目前为止,设计高效电催化剂以提高NRR性能是研究重点。其中,金属有机骨架(metal organic framework,MOF)及衍生材料因其独特的多孔结构和可控成分等优势促进了其在气体捕获、分离和催化中的应用。本文首先讨论NRR的反应机制,然后重点讨论MOF及衍生材料用作NRR电催化剂的研究进展,最后,对MOF基NRR电催化剂的设计策略、存在问题及NRR催化面临挑战进行了总结与展望。 此外,文中指出:① 自支撑MOF基电催化剂的合理设计对NRR性能有质的提升;② 机器学习、DFT计算及原位测试技术的有效结合对NRR机理、催化剂的高效设计及筛选等具有重要指导意义。因此这些也将成为NRR催化未来研究趋势。
中图分类号:
引用本文
郭朋举, 何小波, 银凤翔. 电催化氮还原合成氨MOF基催化剂研究进展[J]. 化工进展, 2023, 42(4): 1797-1810.
GUO Pengju, HE Xiaobo, YIN Fengxiang. Research progress in MOF-based catalysts for electrocatalytic nitrogen reduction to ammonia[J]. Chemical Industry and Engineering Progress, 2023, 42(4): 1797-1810.
| 催化剂 | 电解质 | 氨产率 | 法拉第效率/% | 参考文献 |
|---|---|---|---|---|
| NH2-MIL-88B-Fe(Fe基MOF) | 0.1mol/L Na2SO4 | 1.205×10-10mol·s-1·cm-2 | 12.45 | [ |
| Cu@Ce-MOF-2(铜网原位生长Ce-MOF) | 0.1mol/L KOH | 14.83μg·h-1·cm-2 | 10.81 | [ |
| Defective UiO-66-NH2(缺陷型UiO-66-NH2) | 0.1mol/L Na2SO4 | 2.071×10-10mol·s-1·cm-2 | 85.21 | [ |
| Fe-MOF(MIL-101-Fe) | 2mol/L KOH | 2.12×10-9mol·s-1·cm-2 | 1.43 | [ |
| HKUST-1(MOF:铜-BTC) | 0.1mol/L Na2SO4 | 46.63μg·h-1·mgcat-1 | 2.45 | [ |
| CuⅡ MOF(碳布自支撑CuⅡ-MOF催化剂) | 1mol/L Na2SO4 | 24.7mg·h-1·mgcat-1 | 11.9 | [ |
| Co3HHTP2(Co3(六羟基三苯)2纳米粒子) | 0.5mol/L LiClO4 | 22.14μg·h-1·mgcat-1 | 3.34 | [ |
| MIL-100-Al(Al基MOF) | 0.1mol/L KOH | 10.6μg·h-1·cm-2·mgcat-1 | 22.6 | [ |
| UiO-66-HAc(缺陷型UiO-66) | 0.1mol/L Na2SO4 | 31.81μg·h-1·mgcat-1 | 48.06 | [ |
| Co3Fe-MOF(二维双金属MOF纳米片) | 0.1mol/L KOH | 8.79μg·h-1·mgcat-1 | 25.64 | [ |
| NiFe-MOF(零维双金属MOF) | 0.1mol/L NaHCO3 | 9.3μg·h-1mgcat-1 | 11.5 | [ |
| MoS2@ZIF-71(ZIF-71封装MoS2纳米花) | 0.1mol/L Na2SO4 | 56.69μg·h-1·mgcat-1 | 30.91 | [ |
| CNT@CAU-17(碳纳米管插入CAU-17) | 0.05mol/L H2SO4 | 11.92μg·h-1·mgcat-1 | 31.27 | [ |
| MIL-101(Fe)/MoS3(纳米片杂化材料) | 0.1mol/L HCl | 25.7μg·h-1·mgcat-1 | 36.71 | [ |
| Ag-Au@ZIF(ZIF-71封装Ag-Au) | 0.2mol/L LiCF3SO3 | 0.623μg·h-1·cm-2 | 18 | [ |
| AuCu/ZIF-8(AuCu纳米合金负载ZIF-8) | 0.1mol/L HCl | 63.9μg·h-1·mgcat-1 | 14.2 | [ |
| HT Au@MOF(疏水性MOF自支撑Au) | 0.1mol/L Na2SO4 | 49.5μg·h-1·mgcat-1 | 60.9 | [ |
| Pt/Au@ZIF(ZIF-71封装Pt/Au) | 0.2mol/L LiCF3SO3 | 161μg·h-1·mgcat-1 | 44 | [ |
| Au@ZIF-8(纳米金嵌入ZIF-8) | 0.1mol/L Na2SO4 | 28.7μg·h-1·cm-2 | 44 | [ |
表1 近年来报道的MOF基材料作为NRR电催化剂及其性能
| 催化剂 | 电解质 | 氨产率 | 法拉第效率/% | 参考文献 |
|---|---|---|---|---|
| NH2-MIL-88B-Fe(Fe基MOF) | 0.1mol/L Na2SO4 | 1.205×10-10mol·s-1·cm-2 | 12.45 | [ |
| Cu@Ce-MOF-2(铜网原位生长Ce-MOF) | 0.1mol/L KOH | 14.83μg·h-1·cm-2 | 10.81 | [ |
| Defective UiO-66-NH2(缺陷型UiO-66-NH2) | 0.1mol/L Na2SO4 | 2.071×10-10mol·s-1·cm-2 | 85.21 | [ |
| Fe-MOF(MIL-101-Fe) | 2mol/L KOH | 2.12×10-9mol·s-1·cm-2 | 1.43 | [ |
| HKUST-1(MOF:铜-BTC) | 0.1mol/L Na2SO4 | 46.63μg·h-1·mgcat-1 | 2.45 | [ |
| CuⅡ MOF(碳布自支撑CuⅡ-MOF催化剂) | 1mol/L Na2SO4 | 24.7mg·h-1·mgcat-1 | 11.9 | [ |
| Co3HHTP2(Co3(六羟基三苯)2纳米粒子) | 0.5mol/L LiClO4 | 22.14μg·h-1·mgcat-1 | 3.34 | [ |
| MIL-100-Al(Al基MOF) | 0.1mol/L KOH | 10.6μg·h-1·cm-2·mgcat-1 | 22.6 | [ |
| UiO-66-HAc(缺陷型UiO-66) | 0.1mol/L Na2SO4 | 31.81μg·h-1·mgcat-1 | 48.06 | [ |
| Co3Fe-MOF(二维双金属MOF纳米片) | 0.1mol/L KOH | 8.79μg·h-1·mgcat-1 | 25.64 | [ |
| NiFe-MOF(零维双金属MOF) | 0.1mol/L NaHCO3 | 9.3μg·h-1mgcat-1 | 11.5 | [ |
| MoS2@ZIF-71(ZIF-71封装MoS2纳米花) | 0.1mol/L Na2SO4 | 56.69μg·h-1·mgcat-1 | 30.91 | [ |
| CNT@CAU-17(碳纳米管插入CAU-17) | 0.05mol/L H2SO4 | 11.92μg·h-1·mgcat-1 | 31.27 | [ |
| MIL-101(Fe)/MoS3(纳米片杂化材料) | 0.1mol/L HCl | 25.7μg·h-1·mgcat-1 | 36.71 | [ |
| Ag-Au@ZIF(ZIF-71封装Ag-Au) | 0.2mol/L LiCF3SO3 | 0.623μg·h-1·cm-2 | 18 | [ |
| AuCu/ZIF-8(AuCu纳米合金负载ZIF-8) | 0.1mol/L HCl | 63.9μg·h-1·mgcat-1 | 14.2 | [ |
| HT Au@MOF(疏水性MOF自支撑Au) | 0.1mol/L Na2SO4 | 49.5μg·h-1·mgcat-1 | 60.9 | [ |
| Pt/Au@ZIF(ZIF-71封装Pt/Au) | 0.2mol/L LiCF3SO3 | 161μg·h-1·mgcat-1 | 44 | [ |
| Au@ZIF-8(纳米金嵌入ZIF-8) | 0.1mol/L Na2SO4 | 28.7μg·h-1·cm-2 | 44 | [ |
| 催化剂 | 电解质 | 氨产率 | 法拉第效率/% | 参考文献 |
|---|---|---|---|---|
| Co@NC(Co纳米粒子负载氮掺杂碳基底) | 0.1mol/L Na2SO4 | 1.57×10-11mol·s-1·cm-2 | 21.79 | [ |
| Co/C-900(钴掺杂碳复合材料) | 0.1mol/L KOH | 4.66μmol·h-1·cm-2 | 11.53 | [ |
| Bi NPs@CRs(原位衍生铋纳米粒子) | 0.1mol/L HCl | 20.80μg·h-1·mgcat-1 | 11.50 | [ |
| Zn-Co3O4(Zn掺杂Co3O4纳米多面体) | 0.1mol/L HCl | 22.71μg·h-1·mgcat-1 | 11.9 | [ |
| Co3O4@NCs(氮掺杂碳/Co3O4核壳结构) | 0.05mol/L H2SO4 | 42.58μg·h-1·mgcat-1 | 8.5 | [ |
| Fe2O3@MoS2(双金属杂化材料) | 0.1mol/L Na2SO4 | 112.15μg·h-1·mgcat-1 | 8.62 | [ |
| Fe1.89 Mo4.11O7/FeS2@C(双金属杂化材料) | 0.1mol/L KOH | 105.3μg·h-1·mgcat-1 | 54.7 | [ |
| Mo-Co/NC(Mo-Co嵌入氮掺杂碳基底) | 0.1mol/L Na2SO4 | 89.8μmol·h-1·mgcat-1 | 13.5 | [ |
| CoRu@NC(双金属氮掺杂碳杂化材料) | 0.1mol/L KOH | 56.82μg·h-1·mgcat-1 | 2.02 | [ |
| CoP HNC(中空磷化钴纳米笼) | 0.1mol/L Na2SO4 | 10.78μg·h-1·mgcat-1 | 7.36 | [ |
| MoFe-Pc微球(Pc磷掺杂碳) | 0.1mol/L HCl | 34.23μg·h-1·mgcat-1 | 16.83 | [ |
| Ru SAc/N-C(Ru单原子催化剂) | 0.05mol/L H2SO4 | 120.9μg·h-1·mgcat-1 | 29.6 | [ |
| Ni-N x -C(Ni单原子催化剂) | 0.5mol/L LiClO4 | 115μg·h-1·cm-2 | 21.9 | [ |
| ISAS-Fe/NC(Fe单原子催化剂) | 0.1mol/L PBS | 62.9μg·h-1·mgcat-1 | 18.6 | [ |
| Co/N-doped C(钴/氮掺杂碳材料) | 0.1mol/L KOH | 0.4μmol·h-1·cm-2 | 10.1 | [ |
| Ru@ZrO2/NC(添加ZrO2的Ru单原子催化剂) | 0.1mol/L HCl | 3.665mg·h-1·mgRu-1 | 21 | [ |
| Fe1-N-C(Fe单原子嵌入氮掺杂碳基底) | 0.1mol/L HCl | 1.56×10-11mol·s-1·cm-2 | 4.51 | [ |
| NPC-750(氮掺杂多孔炭) | 0.05mol/L H2SO4 | 23.8μg·h-1·mgcat-1 | 1.42 | [ |
| C-ZIF-1100-1h(氮掺杂纳米多孔炭) | 0.1mol/L Na2SO4 | 3.4×10-6mol·cm-2·h-1 | 10.2 | [ |
| NP-C-MOF-5(氮、磷共掺杂多孔炭) | 0.1mol/L HCl | 1.08μg·h-1·mgcat-1 | 0.52 | [ |
| S/N-MPC(硫改性的氮掺杂多孔炭) | 0.05mol/L H2SO4 | 45.51μg·h-1·mgcat-1 | 25.16 | [ |
表2 近年来报道的MOF衍生物作为NRR电催化剂及其催化性能
| 催化剂 | 电解质 | 氨产率 | 法拉第效率/% | 参考文献 |
|---|---|---|---|---|
| Co@NC(Co纳米粒子负载氮掺杂碳基底) | 0.1mol/L Na2SO4 | 1.57×10-11mol·s-1·cm-2 | 21.79 | [ |
| Co/C-900(钴掺杂碳复合材料) | 0.1mol/L KOH | 4.66μmol·h-1·cm-2 | 11.53 | [ |
| Bi NPs@CRs(原位衍生铋纳米粒子) | 0.1mol/L HCl | 20.80μg·h-1·mgcat-1 | 11.50 | [ |
| Zn-Co3O4(Zn掺杂Co3O4纳米多面体) | 0.1mol/L HCl | 22.71μg·h-1·mgcat-1 | 11.9 | [ |
| Co3O4@NCs(氮掺杂碳/Co3O4核壳结构) | 0.05mol/L H2SO4 | 42.58μg·h-1·mgcat-1 | 8.5 | [ |
| Fe2O3@MoS2(双金属杂化材料) | 0.1mol/L Na2SO4 | 112.15μg·h-1·mgcat-1 | 8.62 | [ |
| Fe1.89 Mo4.11O7/FeS2@C(双金属杂化材料) | 0.1mol/L KOH | 105.3μg·h-1·mgcat-1 | 54.7 | [ |
| Mo-Co/NC(Mo-Co嵌入氮掺杂碳基底) | 0.1mol/L Na2SO4 | 89.8μmol·h-1·mgcat-1 | 13.5 | [ |
| CoRu@NC(双金属氮掺杂碳杂化材料) | 0.1mol/L KOH | 56.82μg·h-1·mgcat-1 | 2.02 | [ |
| CoP HNC(中空磷化钴纳米笼) | 0.1mol/L Na2SO4 | 10.78μg·h-1·mgcat-1 | 7.36 | [ |
| MoFe-Pc微球(Pc磷掺杂碳) | 0.1mol/L HCl | 34.23μg·h-1·mgcat-1 | 16.83 | [ |
| Ru SAc/N-C(Ru单原子催化剂) | 0.05mol/L H2SO4 | 120.9μg·h-1·mgcat-1 | 29.6 | [ |
| Ni-N x -C(Ni单原子催化剂) | 0.5mol/L LiClO4 | 115μg·h-1·cm-2 | 21.9 | [ |
| ISAS-Fe/NC(Fe单原子催化剂) | 0.1mol/L PBS | 62.9μg·h-1·mgcat-1 | 18.6 | [ |
| Co/N-doped C(钴/氮掺杂碳材料) | 0.1mol/L KOH | 0.4μmol·h-1·cm-2 | 10.1 | [ |
| Ru@ZrO2/NC(添加ZrO2的Ru单原子催化剂) | 0.1mol/L HCl | 3.665mg·h-1·mgRu-1 | 21 | [ |
| Fe1-N-C(Fe单原子嵌入氮掺杂碳基底) | 0.1mol/L HCl | 1.56×10-11mol·s-1·cm-2 | 4.51 | [ |
| NPC-750(氮掺杂多孔炭) | 0.05mol/L H2SO4 | 23.8μg·h-1·mgcat-1 | 1.42 | [ |
| C-ZIF-1100-1h(氮掺杂纳米多孔炭) | 0.1mol/L Na2SO4 | 3.4×10-6mol·cm-2·h-1 | 10.2 | [ |
| NP-C-MOF-5(氮、磷共掺杂多孔炭) | 0.1mol/L HCl | 1.08μg·h-1·mgcat-1 | 0.52 | [ |
| S/N-MPC(硫改性的氮掺杂多孔炭) | 0.05mol/L H2SO4 | 45.51μg·h-1·mgcat-1 | 25.16 | [ |
| 1 | YE Tiannan, PARK S W, LU Yangfan, et al. Vacancy-enabled N2 activation for ammonia synthesis on an Ni-loaded catalyst[J]. Nature, 2020, 583(7816): 391-395. |
| 2 | YAO Dazhi, TANG Cheng, LI Laiquan, et al. In situ fragmented bismuth nanoparticles for electrocatalytic nitrogen reduction[J]. Advanced Energy Materials, 2020, 10(33): 2001289. |
| 3 | KITANO M, INOUE Y, YAMAZAKI Y, et al. Ammonia synthesis using a stable electride as an electron donor and reversible hydrogen store[J]. Nature Chemistry, 2012, 4(11): 934-940. |
| 4 | SURYANTO B H R, DU H L, WANG Dabin, et al. Challenges and prospects in the catalysis of electroreduction of nitrogen to ammonia[J]. Nature Catalysis, 2019, 2(4): 290-296. |
| 5 | LIU Huimin, WEI Li, LIU Fei, et al. Homogeneous, heterogeneous, and biological catalysts for electrochemical N2 reduction toward NH3 under ambient conditions[J]. ACS Catalysis, 2019, 9(6): 5245-5267. |
| 6 | LIU Anmin, YANG Yanan, REN Xuefeng, et al. Current progress of electrocatalysts for ammonia synthesis through electrochemical nitrogen reduction under ambient conditions[J]. ChemSusChem, 2020, 13(15): 3766-3788. |
| 7 | LI Hongda, GU Shaonan, SUN Zijun, et al. The in-built bionic “MoFe cofactor” in Fe-doped two-dimensional MoTe2 nanosheets for boosting the photocatalytic nitrogen reduction performance[J]. Journal of Materials Chemistry A, 2020, 8(26): 13038-13048. |
| 8 | CHEN Gaofeng, REN Shiyu, ZHANG Lili, et al. Advances in electrocatalytic N2 reduction-Strategies to tackle the selectivity challenge[J]. Small Methods, 2018, 3(6): 1800337. |
| 9 | TANG Cheng, ZHENG Yao, JARONIEC M, et al. Electrocatalytic refinery for sustainable production of fuels and chemicals[J]. Angewandte Chemie International Edition, 2021, 60(36): 19572-19590. |
| 10 | XU Wence, FAN Guilan, CHEN Jialiang, et al. Nanoporous palladium hydride for electrocatalytic N2 reduction under ambient conditions[J]. Angewandte Chemie International Edition, 2020, 59(9): 3511-3516. |
| 11 | HU Xuemin, SUN Yuntong, GUO Shiying, et al. Identifying electrocatalytic activity and mechanism of Ce1/3NbO3 perovskite for nitrogen reduction to ammonia at ambient conditions[J]. Applied Catalysis B: Environmental, 2021, 280: 119419. |
| 12 | LIU Xi’en, DAI Liming. Carbon-based metal-free catalysts[J]. Nature Reviews Materials, 2016, 1(11): 16064. |
| 13 | MISTRY H, VARELA A S, KÜHL S, et al. Nanostructured electrocatalysts with tunable activity and selectivity[J]. Nature Reviews Materials, 2016, 1: 16009. |
| 14 | YANG Xiaofeng, WANG Aiqin, QIAO Botao, et al. Single-atom catalysts: A new frontier in heterogeneous catalysis[J]. Accounts of Chemical Research, 2013, 46(8): 1740-1748. |
| 15 | XUE Yuqi, ZHENG Shasha, XUE Huaiguo, et al. Metal-organic framework composites and their electrochemical applications[J]. Journal of Materials Chemistry A, 2019, 7(13): 7301-7327. |
| 16 | LIU Juan, HOU Shujin, LI Weijin, et al. Recent approaches to design electrocatalysts based on metal-organic frameworks and their derivatives[J]. Chemistry: an Asian Journal, 2019, 14(20): 3474-3501. |
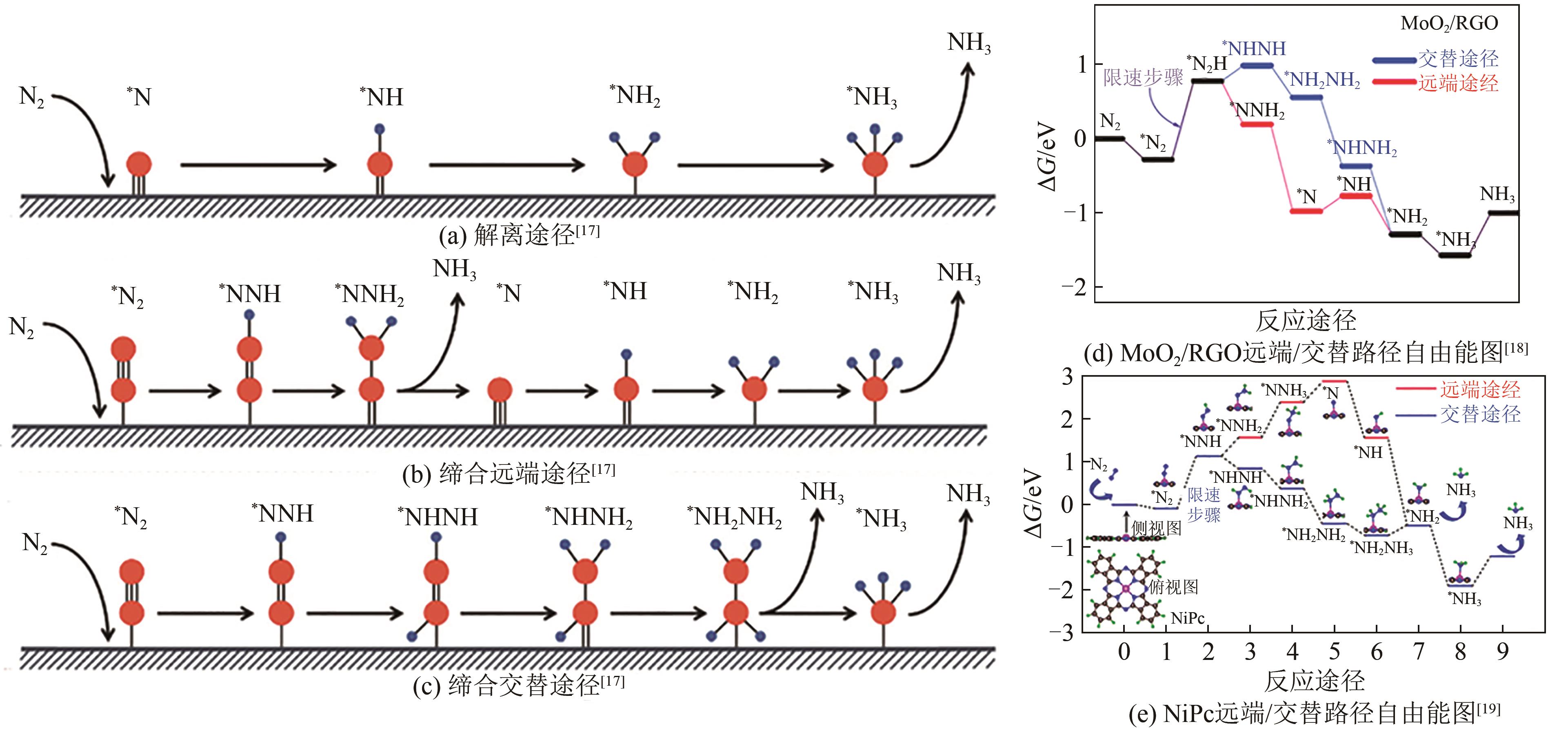
| 17 | CHEN Jiangyue, CHENG Hui, DING Liangxin, et al. Competing hydrogen evolution reaction: A challenge in electrocatalytic nitrogen fixation[J]. Materials Chemistry Frontiers, 2021, 5(16): 5954-5969. |
| 18 | WANG Jing, LIU Yaping, ZHANG Hu, et al. Ambient electrocatalytic nitrogen reduction on a MoO2/graphene hybrid: Experimental and DFT studies[J]. Catalysis Science & Technology, 2019, 9(16): 4248-4254. |
| 19 | MURMU S, PAUL S, KAPSE S, et al. Unveiling the genesis of the high catalytic activity in nickel phthalocyanine for electrochemical ammonia synthesis[J]. Journal of Materials Chemistry A, 2021, 9(25): 14477-14484. |
| 20 | JIAO Dongxu, LIU Yuejie, CAI Qinghai, et al. Coordination tunes the activity and selectivity of the nitrogen reduction reaction on single-atom iron catalysts: A computational study[J]. Journal of Materials Chemistry A, 2021, 9(2): 1240-1251. |
| 21 | LAN Jiao, LUO Min, HAN Jiuhui, et al. Nanoporous B13C2 towards highly efficient electrochemical nitrogen fixation[J]. Small, 2021, 17(39): 2102814. |
| 22 | YI Xuerui, HE Xiaobo, YIN Fengxiang, et al. NH2-MIL-88B-Fe for electrocatalytic N2 fixation to NH3 with high Faradaic efficiency under ambient conditions in neutral electrolyte[J]. Journal of Materials Science, 2020, 55(26): 12041-12052. |
| 23 | LIU Peixin, JING Peng, XU Xuan, et al. Structural reconstruction of Ce-MOF with active sites for efficient electrocatalytic N2 reduction[J]. ACS Applied Energy Materials, 2021, 4(11): 12128-12136. |
| 24 | HE Xiaobo, YIN Fengxiang, YI Xuerui, et al. Defective UiO-66-NH2 functionalized with stable superoxide radicals toward electrocatalytic nitrogen reduction with high faradaic efficiency[J]. ACS Applied Materials & Interfaces, 2022, 14(23): 26571-26586. |
| 25 | ZHAO Xinran, YIN Fengxiang, LIU Ning, et al. Highly efficient metal-organic-framework catalysts for electrochemical synthesis of ammonia from N2 (air) and water at low temperature and ambient pressure[J]. Journal of Materials Science, 2017, 52(17): 10175-10185. |
| 26 | CAO Yueming, LI Peipei, WU Tengteng, et al. Electrocatalysis of N2 to NH3 by HKUST-1 with high NH3 yield[J]. Chemistry-an Asian Journal, 2020, 15(8): 1272-1276. |
| 27 | ZHAO Lu, KUANG Xuan, CHEN Cheng, et al. Boosting electrocatalytic nitrogen fixation via energy-efficient anodic oxidation of sodium gluconate[J]. Chemical Communications (Cambridge, England), 2019, 55(68): 10170-10173. |
| 28 | YI Jundong, SI Duanhui, XIE Ruikuan, et al. Conductive two-dimensional phthalocyanine-based metal-organic framework nanosheets for efficient electroreduction of CO2 [J]. Angewandte Chemie International Edition, 2021, 60(31): 17108-17114. |
| 29 | XIONG Wei, CHENG Xin, WANG Ting, et al. Co3(hexahydroxytriphenylene)2: A conductive metal-organic framework for ambient electrocatalytic N2 reduction to NH3 [J]. Nano Research, 2020, 13(4): 1008-1012. |
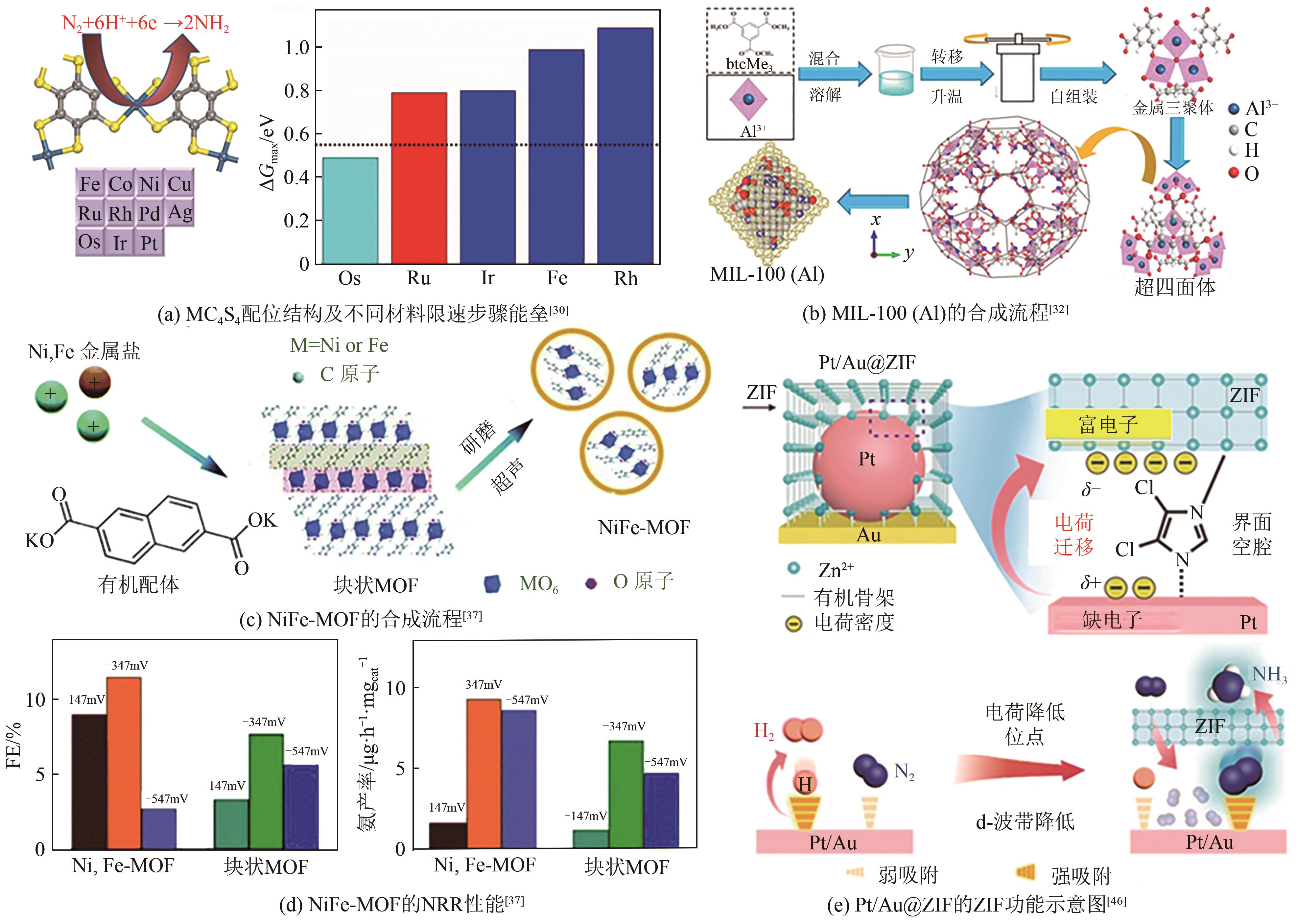
| 30 | LIU Xin, WANG Zhongxu, ZHAO Jia, et al. Two-dimensional π-conjugated osmium bis(dithiolene) complex (OsC4S4) as a promising electrocatalyst for ambient nitrogen reduction to ammonia[J]. Applied Surface Science, 2019, 487: 833-839. |
| 31 | LI Laiquan, TANG Cheng, JIN Huanyu, et al. Main-group elements boost electrochemical nitrogen fixation[J]. Chem, 2021, 7(12): 3232-3255. |
| 32 | FU Yang, LI Kangkang, BATMUNKH M, et al. Unsaturated p-metal-based metal-organic frameworks for selective nitrogen reduction under ambient conditions[J]. ACS Applied Materials & Interfaces, 2020, 12(40): 44830-44839. |
| 33 | HE Xioaobo, LIAO Yuanxiu, TAN Jiabin, et al. Defective UiO-66 toward boosted electrochemical nitrogen reduction to ammonia[J]. Electrochimica Acta, 2022, 409: 139988. |
| 34 | TIAN Di, SONG Na, ZHONG Mengxiao, et al. Bimetallic MOF nanosheets decorated on electrospun nanofibers for high-performance asymmetric supercapacitors[J]. ACS Applied Materials & Interfaces, 2020, 12(1): 1280-1291. |
| 35 | ZHOU Yingtang, ABAZARI R, CHEN Jing, et al. Bimetallic metal-organic frameworks and MOF-derived composites: Recent progress on electro- and photoelectrocatalytic applications[J]. Coordination Chemistry Reviews, 2022, 451: 214264. |
| 36 | LI Weixin, FANG Wei, WU Chen, et al. Bimetal-MOF nanosheets as efficient bifunctional electrocatalysts for oxygen evolution and nitrogen reduction reaction[J]. Journal of Materials Chemistry A, 2020, 8(7): 3658-3666. |
| 37 | DUAN Jingjing, SUN Yuntong, CHEN Sheng, et al. A zero-dimensional nickel, iron-metal-organic framework (MOF) for synergistic N2 electrofixation[J]. Journal of Materials Chemistry A, 2020, 8(36): 18810-18815. |
| 38 | WANG Zixi, HUANG Jianying, MAO Jiajun, et al. Metal-organic frameworks and their derivatives with graphene composites: Preparation and applications in electrocatalysis and photocatalysis[J]. Journal of Materials Chemistry A, 2020, 8(6): 2934-2961. |
| 39 | KEMPAHANUMAKKAGARI S, VELLINGIRI K, DEEP A, et al. Metal-organic framework composites as electrocatalysts for electrochemical sensing applications[J]. Coordination Chemistry Reviews, 2018, 357: 105-129. |
| 40 | DUAN Jihai, SHAO Dezan, HE Xin, et al. Model MoS2@ZIF-71 interface acts as a highly active and selective electrocatalyst for catalyzing ammonia synthesis[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2021, 619: 126529. |
| 41 | LV Yang, WANG Yiqi, YANG Miao, et al. Nitrogen reduction through confined electro-catalysis with carbon nanotube inserted metal-organic frameworks[J]. Journal of Materials Chemistry A, 2021, 9(3): 1480-1486. |
| 42 | XU Wanying, LI Cong, LI Feilong, et al. A hybrid catalyst for efficient electrochemical N2 fixation formed by decorating amorphous MoS3 nanosheets with MIL-101(Fe) nanodots[J]. Science China Chemistry, 2022, 65(5): 885-891. |
| 43 | LEE H K, KOH C S L, LEE Y H, et al. Favoring the unfavored: Selective electrochemical nitrogen fixation using a reticular chemistry approach[J]. Science Advances, 2018, 4(3): eaar3208. |
| 44 | LV Xianwei, WANG Lu, WANG Guichang, et al. ZIF-supported AuCu nanoalloy for ammonia electrosynthesis from nitrogen and thin air[J]. Journal of Materials Chemistry A, 2020, 8(18): 8868-8874. |
| 45 | HE Hongming, ZHU Qianqian, YAN Ying, et al. Metal-organic framework supported Au nanoparticles with organosilicone coating for high-efficiency electrocatalytic N2 reduction to NH3 [J]. Applied Catalysis B: Environmental, 2022, 302: 120840. |
| 46 | SIM H Y F, CHEN J R T, KOH C S L, et al. ZIF-iduced d-band modification in a bimetallic nanocatalyst: Achieving over 44 % efficiency in the ambient nitrogen reduction reaction[J]. Angewandte Chemie International Edition, 2020, 59(39): 16997-17003. |
| 47 | YANG Yijie, WANG Shuqi, WEN Haoming, et al. Nanoporous gold embedded ZIF composite for enhanced electrochemical nitrogen fixation[J]. Angewandte Chemie International Edition, 2019, 58(43): 15362-15366. |
| 48 | YIN Fengxiang, LIN Xin, HE Xiaobo, et al. High Faraday efficiency for electrochemical nitrogen reduction reaction on Co@N-doped carbon derived from a metal-organic framework under ambient conditions[J]. Materials Letters, 2019, 248: 109-113. |
| 49 | LIU Anmin, LIANG Xingyou, YANG Qiyue, et al. Metal-organic-framework-derived cobalt-doped carbon material for electrochemical ammonia synthesis under ambient conditions[J]. ChemElectroChem, 2020, 7(24): 4900-4905. |
| 50 | WANG Fengyi, ZHANG Longcheng, WANG Ting, et al. In situ derived Bi nanoparticles confined in carbon rods as an efficient electrocatalyst for ambient N2 reduction to NH3 [J]. Inorganic Chemistry, 2021, 60(10): 7584-7589. |
| 51 | WEN Lulu, LI Xinyang, ZHANG Rui, et al. Oxygen vacancy engineering of MOF-derived Zn-doped Co3O4 nanopolyhedrons for enhanced electrochemical nitrogen fixation[J]. ACS Applied Materials & Interfaces, 2021, 13(12): 14181-14188. |
| 52 | LUO Shijian, LI Xiaoman, ZHANG Baohai, et al. MOF-derived Co3O4@NC with core-shell structures for N2 electrochemical reduction under ambient conditions[J]. ACS Applied Materials & Interfaces, 2019, 11(30): 26891-26897. |
| 53 | MA Chaoqun, LIU Da, ZHANG Yanli, et al. MOF-derived Fe2O3@MoS2: An efficient electrocatalyst for ammonia synthesis under mild conditions[J]. Chemical Engineering Journal, 2022, 430: 132694. |
| 54 | WANG Xinming, FENG Zemin, XIAO Boxin, et al. Polyoxometalate-based metal-organic framework-derived bimetallic hybrid materials for upgraded electrochemical reduction of nitrogen[J]. Green Chemistry, 2020, 22(18): 6157-6169. |
| 55 | ZHANG Yizhen, HU Jue, ZHANG Chengxu, et al. Bimetallic Mo-Co nanoparticles anchored on nitrogen-doped carbon for enhanced electrochemical nitrogen fixation[J]. Journal of Materials Chemistry A, 2020, 8(18): 9091-9098. |
| 56 | CONG Linchuan, YAO Kaida, ZHANG Siqi, et al. Facile synthesis of bimetallic N-doped carbon hybrid material for electrochemical nitrogen reduction[J]. Journal of Energy Chemistry, 2021, 59: 715-720. |
| 57 | GUO Wenhan, LIANG Zibin, ZHAO Junliang, et al. Hierarchical cobalt phosphide hollow nanocages toward electrocatalytic ammonia synthesis under ambient pressure and room temperature[J]. Small Methods, 2018, 2(12): 1800204. |
| 58 | CHEN Silong, JANG H, WANG Jia, et al. Bimetallic metal-organic framework-derived MoFe-PC microspheres for electrocatalytic ammonia synthesis under ambient conditions[J]. Journal of Materials Chemistry A, 2020, 8(4): 2099-2104. |
| 59 | GENG Zhigang, LIU Yan, KONG Xiangdong, et al. Achieving a record-high yield rate of 120.9 for N2 electrochemical reduction over Ru single-atom catalysts[J]. Advanced Materials, 2018, 30(40): 1803498. |
| 60 | MUKHERJEE S, YANG Xiaoxuan, SHAN Weitao, et al. Atomically dispersed single Ni site catalysts for nitrogen reduction toward electrochemical ammonia synthesis using N2 and H2O[J]. Small Methods, 2020, 4(6): 1900821. |
| 61 | Fang LYU, ZHAO Shunzheng, GUO Ruijie, et al. Nitrogen-coordinated single Fe sites for efficient electrocatalytic N2 fixation in neutral media[J]. Nano Energy, 2019, 61: 420-427. |
| 62 | GAO Yunnan, HAN Zishan, HONG Song, et al. ZIF-67-derived cobalt/nitrogen-doped carbon composites for efficient electrocatalytic N2 reduction[J]. ACS Applied Energy Materials, 2019, 2(8): 6071-6077. |
| 63 | TAO Hengcong, CHOI C, DING Liangxin, et al. Nitrogen fixation by Ru single-atom electrocatalytic reduction[J]. Chem, 2019, 5(1): 204-214. |
| 64 | ZHANG Rui, JIAO Long, YANG Weijie, et al. Single-atom catalysts templated by metal-organic frameworks for electrochemical nitrogen reduction[J]. Journal of Materials Chemistry A, 2019, 7(46): 26371-26377. |
| 65 | LIU Yanming, SU Yan, QUAN Xie, et al. Facile ammonia synthesis from electrocatalytic N2 reduction under ambient conditions on N-doped porous carbon[J]. ACS Catalysis, 2018, 8(2): 1186-1191. |
| 66 | MUKHERJEE S, CULLEN D A, KARAKALOS S, et al. Metal-organic framework-derived nitrogen-doped highly disordered carbon for electrochemical ammonia synthesis using N2 and H2O in alkaline electrolytes[J]. Nano Energy, 2018, 48: 217-226. |
| 67 | SONG Pengfei, KANG Li, WANG Hao, et al. Nitrogen (N), phosphorus (P)-codoped porous carbon as a metal-free electrocatalyst for N2 reduction under ambient conditions[J]. ACS Applied Materials & Interfaces, 2019, 11(13): 12408-12414. |
| 68 | WANG Jin, HUANG Hao, WANG Peng, et al. N, S synergistic effect in hierarchical porous carbon for enhanced NRR performance[J]. Carbon, 2021, 179: 358-364. |
| 69 | LI Yang, ZHANG Qi, LI Can, et al. Atomically dispersed metal dimer species with selective catalytic activity for nitrogen electrochemical reduction[J]. Journal of Materials Chemistry A, 2019, 7(39): 22242-22247. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 胡喜, 王明珊, 李恩智, 黄思鸣, 陈俊臣, 郭秉淑, 于博, 马志远, 李星. 二硫化钨复合材料制备与储钠性能研究进展[J]. 化工进展, 2023, 42(S1): 344-355. |
| [6] | 张杰, 白忠波, 冯宝鑫, 彭肖林, 任伟伟, 张菁丽, 刘二勇. PEG及其复合添加剂对电解铜箔后处理的影响[J]. 化工进展, 2023, 42(S1): 374-381. |
| [7] | 崔守成, 徐洪波, 彭楠. 两种MOFs材料用于O2/He吸附分离的模拟分析[J]. 化工进展, 2023, 42(S1): 382-390. |
| [8] | 赵巍, 赵德银, 李世瀚, 刘洪达, 孙进, 郭艳秋. 三嗪型天然气管道缓蚀型减阻剂合成与应用[J]. 化工进展, 2023, 42(S1): 391-399. |
| [9] | 王正坤, 黎四芳. 双子表面活性剂癸炔二醇的绿色合成[J]. 化工进展, 2023, 42(S1): 400-410. |
| [10] | 高雨飞, 鲁金凤. 非均相催化臭氧氧化作用机理研究进展[J]. 化工进展, 2023, 42(S1): 430-438. |
| [11] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [12] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [13] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [14] | 王晋刚, 张剑波, 唐雪娇, 刘金鹏, 鞠美庭. 机动车尾气脱硝催化剂Cu-SSZ-13的改性研究进展[J]. 化工进展, 2023, 42(9): 4636-4648. |
| [15] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||