化工进展 ›› 2023, Vol. 42 ›› Issue (3): 1606-1617.DOI: 10.16085/j.issn.1000-6613.2022-0994
氧化还原介体介导厌氧氨氧化生物脱氮的研究进展
赵星程( ), 贾方旭(
), 贾方旭( ), 蒋伟彧, 陈佳熠, 刘晨雨, 姚宏
), 蒋伟彧, 陈佳熠, 刘晨雨, 姚宏
- 北京交通大学环境学院,水中典型污染物控制与水质保障北京重点实验室,北京 100044
-
收稿日期:2022-05-27修回日期:2022-07-11出版日期:2023-03-15发布日期:2023-04-10 -
通讯作者:贾方旭 -
作者简介:赵星程(1992—),男,博士研究生,研究方向为厌氧氨氧化污水脱氮处理。E-mail:20115052@bjtu.edu.cn。 -
基金资助:中央高校基本科研业务费自由申报项目(2019JBM088)
Redox mediators-mediated anaerobic ammonium oxidation process for biological nitrogen removal: a review
ZHAO Xingcheng( ), JIA Fangxu(
), JIA Fangxu( ), JIANG Weiyu, CHEN Jiayi, LIU Chenyu, YAO Hong
), JIANG Weiyu, CHEN Jiayi, LIU Chenyu, YAO Hong
- Beijing Key Laboratory of Aqueous Typical Pollutants Control and Water Quality Safeguard, School of Environment, Beijing Jiaotong University, Beijing 100044, China
-
Received:2022-05-27Revised:2022-07-11Online:2023-03-15Published:2023-04-10 -
Contact:JIA Fangxu
摘要:
厌氧氨氧化(anaerobic ammonium oxidation,Anammox)工艺被认为是一种高效经济且环境友好的生物脱氮技术,是传统生物脱氮工艺的理想替代。然而,该工艺的核心微生物——厌氧氨氧化菌(AnAOB)生长速度缓慢,导致反应器启动时间长,限制了该工艺的实际应用与推广。因此,如何提高AnAOB活性,进一步缩短反应器启动时间成为亟待解决的问题。氧化还原介体(redox mediator,RMs)作为电子载体,通过加速电子传递过程,可增强AnAOB脱氮酶活性和代谢性能,从而提高整体脱氮效果。本文围绕RMs在强化Anammox性能中的作用进行讨论,介绍了RMs的特性、种类及其基本原理,从Anammox性能、胞外聚合物产量、脱氮酶活性及功能菌丰度等方面综述了RMs对Anammox过程的影响,并对其潜在的机理进行了分析和总结,以期为今后的实际应用提供理论依据和参考价值。
中图分类号:
引用本文
赵星程, 贾方旭, 蒋伟彧, 陈佳熠, 刘晨雨, 姚宏. 氧化还原介体介导厌氧氨氧化生物脱氮的研究进展[J]. 化工进展, 2023, 42(3): 1606-1617.
ZHAO Xingcheng, JIA Fangxu, JIANG Weiyu, CHEN Jiayi, LIU Chenyu, YAO Hong. Redox mediators-mediated anaerobic ammonium oxidation process for biological nitrogen removal: a review[J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1606-1617.
RMs 类型 | 反应器类型/ 有效体积 | 温度 /°C | pH | 运行 时间 | 添加量 /mg·L-1 | 进水浓度/mg·L-1 | 氮去除效果 | 参考 文献 | |
|---|---|---|---|---|---|---|---|---|---|
| NH | NO | ||||||||
| FA | 血清瓶/100mL | 37±0.2 | 7.8±0.1 | 120h | 308.24 | 50 | 50 | NRR①提高7.6% | [ |
| UASB②/7L | 32±1 | — | 70d | 50 | 150 | 150 | NRR提高12.7% | [ | |
| UASB/2.5L | 35±1 | 7.5±0.1 | 54d | 25.2~80.3 | 150~210 | 150~220 | NRR=1.7kg/(m3·d) | [ | |
| ABR③/1L | 15 | 7.8±0.2 | 77d | 15.4~30.8 | 75~115 | 100~132 | NRR提高39.2% | [ | |
| HA | SBR④/3.75L | 30 | 7.5~8.0 | 43d | 50~200 | 2300 | 2300 | 随HA浓度增加,NRE由58%降低至39.45% | [ |
| SNAP⑤/12L | 32~33 | 7.5~7.8 | 92d | 120~1637 | 200~700 | — | HA=576mg/L时,NRE=96% | [ | |
| SBBR⑥/7L | 33±2 | — | 54d | 50~200 | 600 | — | HA=50mg/L时,NRE提升了0.6% | [ | |
表1 可溶性腐殖质组分对Anammox性能的影响
RMs 类型 | 反应器类型/ 有效体积 | 温度 /°C | pH | 运行 时间 | 添加量 /mg·L-1 | 进水浓度/mg·L-1 | 氮去除效果 | 参考 文献 | |
|---|---|---|---|---|---|---|---|---|---|
| NH | NO | ||||||||
| FA | 血清瓶/100mL | 37±0.2 | 7.8±0.1 | 120h | 308.24 | 50 | 50 | NRR①提高7.6% | [ |
| UASB②/7L | 32±1 | — | 70d | 50 | 150 | 150 | NRR提高12.7% | [ | |
| UASB/2.5L | 35±1 | 7.5±0.1 | 54d | 25.2~80.3 | 150~210 | 150~220 | NRR=1.7kg/(m3·d) | [ | |
| ABR③/1L | 15 | 7.8±0.2 | 77d | 15.4~30.8 | 75~115 | 100~132 | NRR提高39.2% | [ | |
| HA | SBR④/3.75L | 30 | 7.5~8.0 | 43d | 50~200 | 2300 | 2300 | 随HA浓度增加,NRE由58%降低至39.45% | [ |
| SNAP⑤/12L | 32~33 | 7.5~7.8 | 92d | 120~1637 | 200~700 | — | HA=576mg/L时,NRE=96% | [ | |
| SBBR⑥/7L | 33±2 | — | 54d | 50~200 | 600 | — | HA=50mg/L时,NRE提升了0.6% | [ | |
生物炭制备 温度/℃ | 反应器类型/ 有效体积 | 温度 /℃ | pH | 运行 时间 | 最佳添加量 /g·L-1 | 进水浓度/mg·L-1 | 氮去除效果 | 参考 文献 | |
|---|---|---|---|---|---|---|---|---|---|
| NH | NO | ||||||||
| 300 | UASB/1L | 35±1 | — | 160d | 0.01 | 70~210 | 70~210 | 提高10.7% | [ |
| 500 | 提高7.1% | ||||||||
| 800 | 下降4.9% | ||||||||
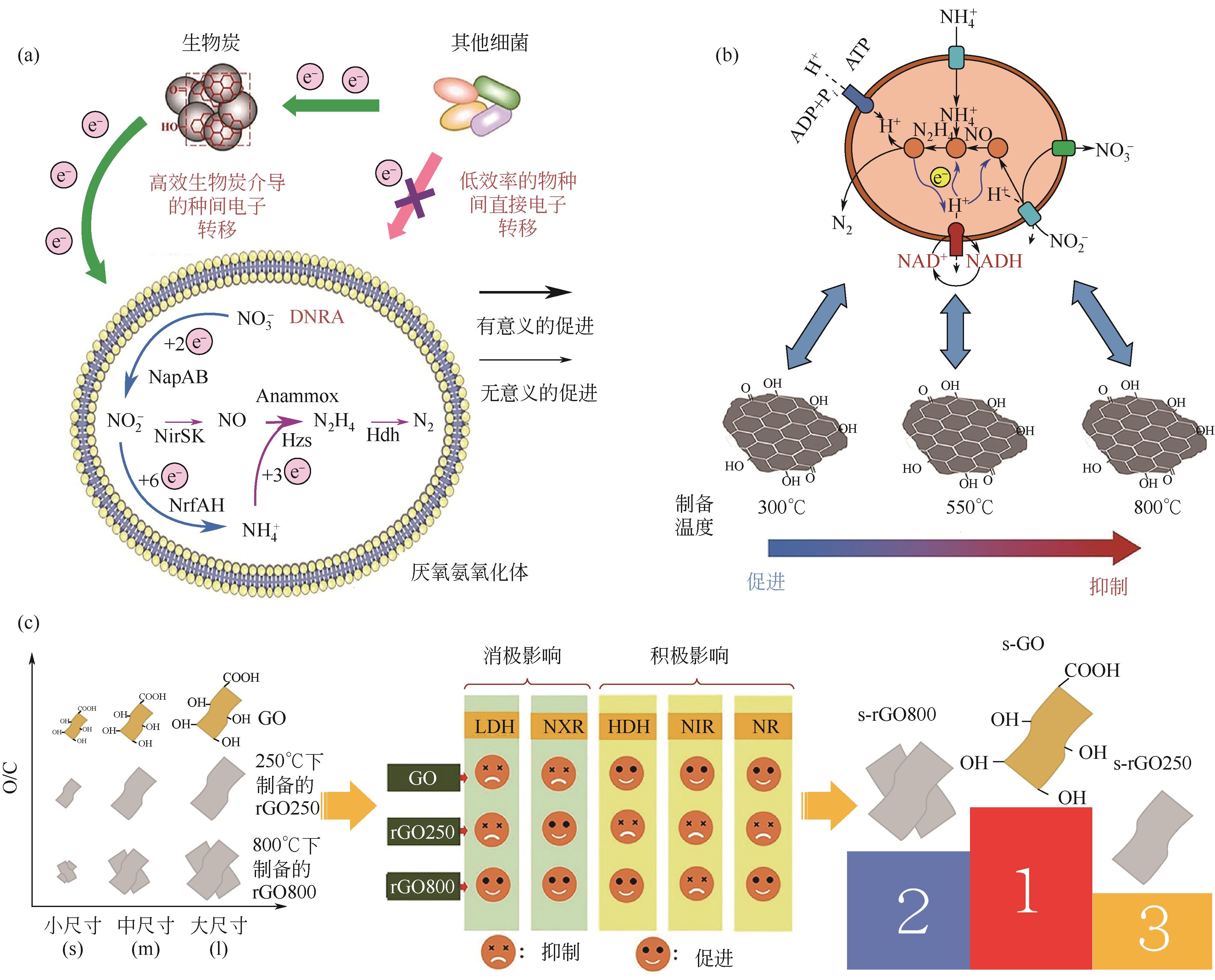
| 300 | 血清瓶/120mL | 32±1 | 约7.5 | 60h | 0.01 | 70 | 200 | 提高64.5% | [ |
| 500 | 提高63.6% | ||||||||
| 800 | 提高43.9% | ||||||||
| 300 | UASB/1L | 35±1 | 7.2~7.8 | 80d | 10 | 100 | 100 | 粒径10~30μm,NRE提高21.6% | [ |
| 粒径10~30μm,NRE提高21.1% | |||||||||
| 粒径10~30μm,NRE提高9.2% | |||||||||
| 300 | 血清瓶/100mL | 35 | — | 72h | 10 | 100 | 100 | 提高11.6% | [ |
| 400 | 血清瓶/100mL | 35±1 | 7.5±0.1 | 40d | 10 | TN=(220±10)~(300±15) | 提高11.6% | [ | |
| 400 | SBR/1L | 35±1 | — | 106d | 10 | 350±20 | 420±20 | C/N=0.5时,NRE下降3.1% | [ |
| C/N=0.7时,NRE提高0.4% | |||||||||
| 300 | 血清瓶/120mL | 35±1 | 7.5 | 56d | 10 | 70 | 100 | 粒径1~5μm,NRE提高17.5% | [ |
| 粒径500~1000μm,NRE提高34.6% | |||||||||
表2 生物炭对Anammox性能的影响
生物炭制备 温度/℃ | 反应器类型/ 有效体积 | 温度 /℃ | pH | 运行 时间 | 最佳添加量 /g·L-1 | 进水浓度/mg·L-1 | 氮去除效果 | 参考 文献 | |
|---|---|---|---|---|---|---|---|---|---|
| NH | NO | ||||||||
| 300 | UASB/1L | 35±1 | — | 160d | 0.01 | 70~210 | 70~210 | 提高10.7% | [ |
| 500 | 提高7.1% | ||||||||
| 800 | 下降4.9% | ||||||||
| 300 | 血清瓶/120mL | 32±1 | 约7.5 | 60h | 0.01 | 70 | 200 | 提高64.5% | [ |
| 500 | 提高63.6% | ||||||||
| 800 | 提高43.9% | ||||||||
| 300 | UASB/1L | 35±1 | 7.2~7.8 | 80d | 10 | 100 | 100 | 粒径10~30μm,NRE提高21.6% | [ |
| 粒径10~30μm,NRE提高21.1% | |||||||||
| 粒径10~30μm,NRE提高9.2% | |||||||||
| 300 | 血清瓶/100mL | 35 | — | 72h | 10 | 100 | 100 | 提高11.6% | [ |
| 400 | 血清瓶/100mL | 35±1 | 7.5±0.1 | 40d | 10 | TN=(220±10)~(300±15) | 提高11.6% | [ | |
| 400 | SBR/1L | 35±1 | — | 106d | 10 | 350±20 | 420±20 | C/N=0.5时,NRE下降3.1% | [ |
| C/N=0.7时,NRE提高0.4% | |||||||||
| 300 | 血清瓶/120mL | 35±1 | 7.5 | 56d | 10 | 70 | 100 | 粒径1~5μm,NRE提高17.5% | [ |
| 粒径500~1000μm,NRE提高34.6% | |||||||||
RMs 类型 | 反应器类型/ 有效体积 | 温度/℃ | pH | 运行 时间 | 添加量 /mg·L-1 | 进水浓度/mg·L-1 | 氮去除效果 | 参考 文献 | |
|---|---|---|---|---|---|---|---|---|---|
| NH | NO | ||||||||
| GN | 血清瓶/75mL | 35±2 | 7.5±0.1 | 45h | 10 | 100 | 100 | 46.0% | [ |
| GO | 血清瓶/100mL | 35 | — | 42h | 100 | 120 | 150 | — | [ |
| GO | 血清瓶/100mL | 35±1 | 7.5 | 4h | 100 | 50 | 50 | 17.2% | [ |
| GO | USFCWs①/1.5L | 37±1 | 7~8 | 61d | 1或10 | 50 | 66 | — | [ |
| rGO | SBR/0.3L | 35±1 | 7.0±0.2 | 220d | 100 | 50 | 65 | 27.4% | [ |
| rGO | SBR/0.5L | 35±1 | 7.0±0.2 | 200d | 100 | 120 | 150 | 13.7% | [ |
| rGO | 血清瓶/100mL | 13 | 7.3±0.2 | 2~8h | 15 | 25 | 30 | — | [ |
| rGO | SBR/5L | 10~30 | 7.6±0.3 | 316h | 15 | TN=124~269 | 温度大于15°C时,NRR可提升17% | [ | |
| rGO | SBR/1L | 15或23 | 7.5±0.4 | 26d | — | — | NRR与对照组相似 | [ | |
| GO/rGO | 血清瓶/100mL | 35 | 7.5±0.2 | 36h/18d | 25~150 | 100 | 132 | GO比RGO对Anammox具有更好的促进效果 | [ |
表3 石墨烯家族材料对Anammox性能的影响
RMs 类型 | 反应器类型/ 有效体积 | 温度/℃ | pH | 运行 时间 | 添加量 /mg·L-1 | 进水浓度/mg·L-1 | 氮去除效果 | 参考 文献 | |
|---|---|---|---|---|---|---|---|---|---|
| NH | NO | ||||||||
| GN | 血清瓶/75mL | 35±2 | 7.5±0.1 | 45h | 10 | 100 | 100 | 46.0% | [ |
| GO | 血清瓶/100mL | 35 | — | 42h | 100 | 120 | 150 | — | [ |
| GO | 血清瓶/100mL | 35±1 | 7.5 | 4h | 100 | 50 | 50 | 17.2% | [ |
| GO | USFCWs①/1.5L | 37±1 | 7~8 | 61d | 1或10 | 50 | 66 | — | [ |
| rGO | SBR/0.3L | 35±1 | 7.0±0.2 | 220d | 100 | 50 | 65 | 27.4% | [ |
| rGO | SBR/0.5L | 35±1 | 7.0±0.2 | 200d | 100 | 120 | 150 | 13.7% | [ |
| rGO | 血清瓶/100mL | 13 | 7.3±0.2 | 2~8h | 15 | 25 | 30 | — | [ |
| rGO | SBR/5L | 10~30 | 7.6±0.3 | 316h | 15 | TN=124~269 | 温度大于15°C时,NRR可提升17% | [ | |
| rGO | SBR/1L | 15或23 | 7.5±0.4 | 26d | — | — | NRR与对照组相似 | [ | |
| GO/rGO | 血清瓶/100mL | 35 | 7.5±0.2 | 36h/18d | 25~150 | 100 | 132 | GO比RGO对Anammox具有更好的促进效果 | [ |
| 1 | MULDER A, VAN DE GRAAF A A, ROBERTSON L A, et al. Anaerobic ammonium oxidation discovered in a denitrifying fluidized bed reactor[J]. FEMS Microbiology Ecology, 1995, 16(3): 177-183. |
| 2 | VAN DE GRAAF A A, MULDER A, DE BRUIJN P, et al. Anaerobic oxidation of ammonium is a biologically mediated process[J]. Applied and Environmental Microbiology, 1995, 61(4): 1246-1251. |
| 3 | Muhammad ALI, OKABE Satoshi. Anammox-based technologies for nitrogen removal: advances in process start-up and remaining issues[J]. Chemosphere, 2015, 141: 144-153. |
| 4 | JIA Fangxu, YANG Qing, HAN Jinhao, et al. Modeling optimization and evaluation of tightly bound extracellular polymeric substances extraction by sonication[J]. Applied Microbiology and Biotechnology, 2016, 100(19): 8485-8494. |
| 5 | JIA Fangxu, YANG Qing, LIU Xiuhong, et al. Stratification of extracellular polymeric substances (EPS) for aggregated Anammox microorganisms[J]. Environmental Science & Technology, 2017, 51(6): 3260-3268. |
| 6 | 贾方旭, 刘莹洁, 于晓华, 等. 尿素废水生物处理技术原理与工艺研究进展[J]. 中国环境科学, 2020, 40(12): 5270-5279. |
| JIA Fangxu, LIU Yingjie, YU Xiaohua, et al. Principle and application of urea wastewater biological treatment technology[J]. China Environmental Science, 2020, 40(12): 5270-5279. | |
| 7 | JIA Fangxu, PENG Yongzhen, LI Jianwei, et al. Metagenomic prediction analysis of microbial aggregation in Anammox-dominated community[J]. Water Environment Research, 2021, 93(11): 2549-2558. |
| 8 | 张星星, 张钰, 王超超, 等. 短程反硝化耦合厌氧氨氧化工艺及其应用前景研究进展[J]. 化工进展, 2020, 39(5): 1981-1991. |
| ZHANG Xingxing, ZHANG Yu, WANG Chaochao, et al. Research advances in application prospect of partial denitrification coupled with Anammox: a review[J]. Chemical Industry and Engineering Progress, 2020, 39(5): 1981-1991. | |
| 9 | VAN DE GRAAF A A, DE BRUIJN P, ROBERTSON L A, et al. Autotrophic growth of anaerobic ammonium-oxidizing micro-organisms in a fluidized bed reactor[J]. Microbiology, 1996, 142(8): 2187-2196. |
| 10 | KARTAL B, KUENEN J, VAN LOOSDRECHT M V. Sewage treatment with Anammox[J]. Science, 2010, 328(5979): 702-703. |
| 11 | 贾方旭, 彭永臻, 杨庆. 厌氧氨氧化菌与其他细菌之间的协同竞争关系[J]. 环境科学学报, 2014, 34(6): 1351-1361. |
| JIA Fangxu, PENG Yongzhen, YANG Qing. Competition and synergism between Anammox bacteria and other bacteria[J]. Acta Scientiae Circumstantiae, 2014, 34(6): 1351-1361. | |
| 12 | VAN DER STAR Wouter R L, ABMA Wiebe R, BLOMMERS Dennis, et al. Startup of reactors for anoxic ammonium oxidation: experiences from the first full-scale Anammox reactor in Rotterdam[J]. Water Research, 2007, 41(18): 4149-4163. |
| 13 | ZHANG Lei, NARITA Yuko, GAO Lin, et al. Maximum specific growth rate of Anammox bacteria revisited[J]. Water Research, 2017, 116: 296-303. |
| 14 | OSHIKI M, SATOH H, OKABE S. Ecology and physiology of anaerobic ammonium oxidizing bacteria[J]. Environmental Microbiology, 2016, 18(9): 2784-2796. |
| 15 | KARTAL Boran, KELTJENS Jan T. Anammox biochemistry: a tale of heme c proteins[J]. Trends in Biochemical Sciences, 2016, 41(12): 998-1011. |
| 16 | SINNINGHE DAMSTÉ Jaap S, STROUS Marc, RIJPSTRA W Irene C, et al. Linearly concatenated cyclobutane lipids form a dense bacterial membrane[J]. Nature, 2002, 4196908: 708-712. |
| 17 | YIN Xin, QIAO Sen, ZHOU Jiti, et al. Fast start-up of the Anammox process with addition of reduced graphene oxides[J]. Chemical Engineering Journal, 2016, 283: 160-166. |
| 18 | KUENEN J G. Anammox and beyond[J]. Environmental Microbiology, 2020, 22(2): 525-536. |
| 19 | WATANABE Kazuya, MANEFIELD Mike, LEE Matthew, et al. Electron shuttles in biotechnology[J]. Current Opinion in Biotechnology, 2009, 20(6): 633-641. |
| 20 | RABAEY Korneel, BOON Nico, Monica HÖFTE, et al. Microbial phenazine production enhances electron transfer in biofuel cells[J]. Environmental Science & Technology, 2005, 39(9): 3401-3408. |
| 21 | HUSAIN Maroof, HUSAIN Qayyum. Applications of redox mediators in the treatment of organic pollutants by using oxidoreductive enzymes: a review[J]. Critical Reviews in Environmental Science and Technology, 2007, 38(1): 1-42. |
| 22 | DAI Ruobin, CHEN Xiaoguang, MA Chengyu, et al. Insoluble/immobilized redox mediators for catalyzing anaerobic bio-reduction of contaminants[J]. Reviews in Environmental Science and Bio/Technology, 2016, 15(3): 379-409. |
| 23 | VAN DER ZEE Frank P, CERVANTES Francisco J. Impact and application of electron shuttles on the redox (bio)transformation of contaminants: a review[J]. Biotechnology Advances, 2009, 27(3): 256-277. |
| 24 | 陈甜甜, 王先宝, 张雨笛, 等. 氧化还原介体强化生物反硝化脱氮研究进展[J]. 环境化学, 2021, 40(10): 3199-3206. |
| CHEN Tiantian, WANG Xianbao, ZHANG Yudi, et al. Enhanced biological denitrification by redox mediators: a review[J]. Environmental Chemistry, 2021, 40(10): 3199-3206. | |
| 25 | WANG Jing, WANG Di, LIU Guangfei, et al. Enhanced nitrobenzene biotransformation by graphene-anaerobic sludge composite[J]. Journal of Chemical Technology & Biotechnology, 2014, 89(5): 750-755. |
| 26 | COLUNGA Alejandra, Rene RANGEL-MENDEZ J, CELIS Lourdes B, et al. Graphene oxide as electron shuttle for increased redox conversion of contaminants under methanogenic and sulfate-reducing conditions[J]. Bioresource Technology, 2015, 175: 309-314. |
| 27 | ZHANG Haikun, LU Hong, WANG Jing, et al. Accelerating effect of bio-reduced graphene oxide on decolorization of acid red 18 by shewanella algae [J]. Applied Biochemistry and Biotechnology, 2014, 174(2): 602-611. |
| 28 | CHEN Zehan, WANG Yue, WANG Jinxiu, et al. Enhanced activity and selectivity of electrocatalytic denitrification by highly dispersed CuPd bimetals on reduced graphene oxide[J]. Chemical Engineering Journal, 2021, 416: 129074. |
| 29 | LIAO Yinhao, LI Shengjie, ZHU Xianfang, et al. The promotion and inhibition effect of graphene oxide on the process of microbial denitrification at low temperature[J]. Bioresource Technology, 2021, 340: 125636. |
| 30 | XU Zhicheng, LI Yuran, GUO Junxiang, et al. An efficient and sulfur resistant K-modified activated carbon for SCR denitrification compared with acid- and Cu-modified activated carbon[J]. Chemical Engineering Journal, 2020, 395: 125047. |
| 31 | ROXON J J, RYAN A J, WRIGHT S E. Enzymatic reduction of tartrazine by proteus vulgaris from rats[J]. Food and Cosmetics Toxicology, 1967, 5(5): 645-656. |
| 32 | 苑宏英, 孙烨怡, 李原玲, 等. 不同碳源对低温投加氧化还原介体污水生物反硝化脱氮过程的影响[J]. 化工进展, 2018, 37(2): 783-788. |
| YUAN Hongying, SUN Yeyi, LI Yuanling, et al. Effects of different carbon sources on biological denitrification of wastewater at low temperature with adding redox mediator[J]. Chemical Industry and Engineering Progress, 2018, 37(2): 783-788. | |
| 33 | LIU Yuan, NIU Qigui, WANG Shaopo, et al. Upgrading of the symbiosis of nitrosomanas and Anammox bacteria in a novel single-stage partial nitritation-Anammox system: nitrogen removal potential and microbial characterization[J]. Bioresource Technology, 2017, 244: 463-472. |
| 34 | WANG Sike, YU Heng, SU Qingxian, et al. Exploring the role of heterotrophs in partial nitritation-Anammox process treating thermal hydrolysis process-anaerobic digestion reject water[J]. Bioresource Technology, 2021, 341: 125762. |
| 35 | GOTTSHALL Ekaterina Y, BRYSON Sam J, COGERT Kathryn I, et al. Sustained nitrogen loss in a symbiotic association of Comammox Nitrospira and Anammox bacteria[J]. Water Research, 2021, 202: 117426. |
| 36 | PROKOPENKO M G, HIRST M B, DE BRABANDERE L, et al. Nitrogen losses in anoxic marine sediments driven by Thioploca-Anammox bacterial consortia[J]. Nature, 2013, 500(7461): 194-198. |
| 37 | XIAO Rui, ZHU Wanlu, ZHENG Yuanzhu, et al. Active assimilators of soluble microbial products produced by wastewater Anammox bacteria and their roles revealed by DNA-SIP coupled to metagenomics[J]. Environment International, 2022, 164: 107265. |
| 38 | WANG Yujia, HU Xiaomin, JIANG Binhui, et al. Symbiotic relationship analysis of predominant bacteria in a lab-scale Anammox UASB bioreactor[J]. Environmental Science and Pollution Research, 2016, 23(8): 7615-7626. |
| 39 | XU Jisheng, ZHAO Bingzi, CHU Wenying, et al. Chemical nature of humic substances in two typical Chinese soils (upland vs. paddy soil): a comparative advanced solid state NMR study[J]. Science of the Total Environment, 2017, 576: 444-452. |
| 40 | LI Mu, SU Yinglong, CHEN Yinguang, et al. The effects of fulvic acid on microbial denitrification: promotion of NADH generation, electron transfer, and consumption[J]. Applied Microbiology and Biotechnology, 2016, 100(12): 5607-5618. |
| 41 | LIU Lingjie, JI Min, WANG Fen, et al. Insight into the short-term effect of fulvic acid on nitrogen removal performance and N-acylated-L-homoserine lactones (AHLs) release in the Anammox system[J]. Science of the Total Environment, 2020, 704: 135285. |
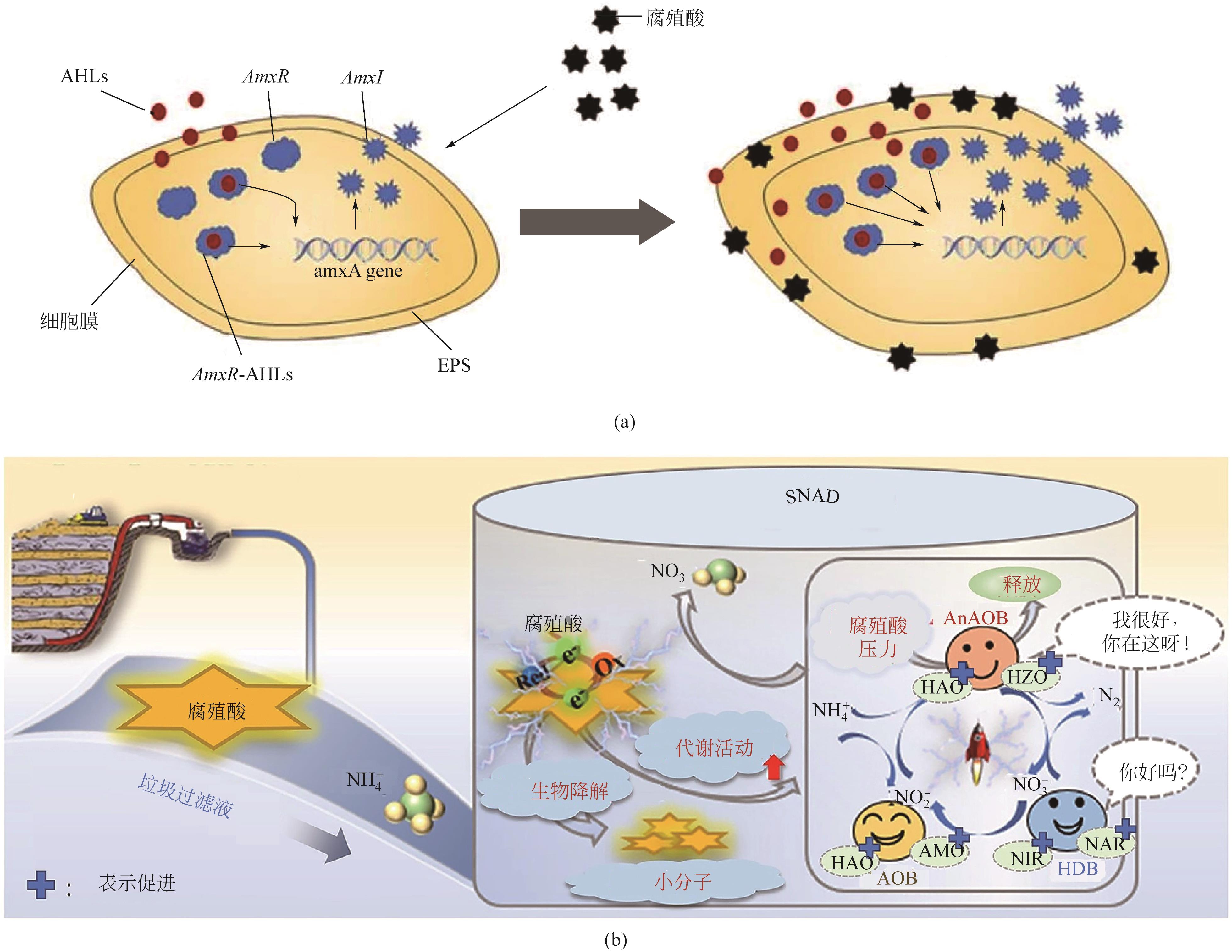
| 42 | 王家辉, 张苧文. 垃圾渗滤液中富里酸对厌氧氨氧化污泥的脱氮效能的影响[J]. 辽宁化工, 2021, 50(12): 1793-1798. |
| WANG Jiahui, ZHANG Ningwen. Effect of fulvic acid in landfill leachate on denitrification efficiency of Anammox sludge[J]. Liaoning Chemical Industry, 2021, 50(12): 1793-1798. | |
| 43 | LIU Lingjie, WANG Fen, XU Sihan, et al. Long-term effect of fulvic acid amendment on the Anammox biofilm system at 15℃: performance, microbial community and metagenomics analysis[J]. Bioresource Technology, 2022, 344(B): 126234. |
| 44 | CHEN Xiujuan, XU Yuan, FAN Mengjie, et al. The stimulatory effect of humic acid on the co-metabolic biodegradation of tetrabromobisphenol A in bioelectrochemical system[J]. Journal of Environmental Management, 2019, 235: 350-356. |
| 45 | KRAIEM Khadija, WAHAB Mohamed Ali, KALLALI Hamadi, et al. Effects of short- and long-term exposures of humic acid on the Anammox activity and microbial community[J]. Environmental Science and Pollution Research, 2019, 26(19): 19012-19024. |
| 46 | LU Changhao, YUAN Chunli, ZHU Tong, et al. Effect of humic acid on the single-stage nitrogen removal using Anammox and partial nitritation (SNAP) process: performance and bacterial communities[J]. Journal of Environmental Chemical Engineering, 2021, 9(6): 106680. |
| 47 | LIU Yinuo, HAN Yi, ZHANG Jianbing, et al. Deciphering effects of humic acid in landfill leachate on the simultaneous nitrification, Anammox and denitrification (SNAD) system from performance, electron transfer and microbial community[J]. Science of the Total Environment, 2022, 809: 151178. |
| 48 | ZHANG Li, WANG Yueping, SODA Satoshi, et al. Effect of fulvic acid on bioreactor performance and on microbial populations within the Anammox process[J]. Bioresource Technology, 2020, 318: 124094. |
| 49 | QIAO Sen, TIAN Tian, ZHOU Jiti. Effects of quinoid redox mediators on the activity of Anammox biomass[J]. Bioresource Technology, 2014, 152: 116-123. |
| 50 | DAPSON R W. The history, chemistry and modes of action of carmine and related dyes[J]. Biotechnic & Histochemistry: Official Publication of the Biological Stain Commission, 2007, 82(4/5): 173-187. |
| 51 | LIU Lingjie, JI Min, WANG Fen, et al. Response of nitrogen removal performance, functional genes abundances and N-acyl-homoserine lactones release to carminic acid of Anammox biomass[J]. Bioresource Technology, 2020, 299: 122567. |
| 52 | CHEN Guanhong, ZHANG Zhirong, ZHANG Zhiyuan, et al. Redox-active reactions in denitrification provided by biochars pyrolyzed at different temperatures[J]. Science of the Total Environment, 2018, 615: 1547-1556. |
| 53 | WANG Weigang, LIU Qinghua, XUE Hao, et al. The feasibility and mechanism of redox-active biochar for promoting Anammox performance[J]. Science of the Total Environment, 2022, 814: 152813. |
| 54 | WANG Weigang, WANG Tong, LIU Qinghua, et al. Biochar-mediated DNRA pathway of Anammox bacteria under varying COD/N ratios[J]. Water Research, 2022, 212: 118100. |
| 55 | XU Jiajia, LI Chao, ZHU Nanwen, et al. Particle size-dependent behavior of redox-active biochar to promote anaerobic ammonium oxidation (Anammox)[J]. Chemical Engineering Journal, 2021, 410: 127925. |
| 56 | XU Jiajia, WU Xiaohui, ZHU Nanwen, et al. Anammox process dosed with biochars for enhanced nitrogen removal: role of surface functional groups[J]. Science of the Total Environment, 2020, 748: 141367. |
| 57 | XU Jiajia, LI Chao, ZHU Nanwen, et al. Alleviating the nitrite stress on anaerobic ammonium oxidation by pyrolytic biochar[J]. Science of the Total Environment, 2021, 774: 145800. |
| 58 | LI Qian, JIA Ziwen, FU Jingwei, et al. Biochar enhances partial denitrification/Anammox by sustaining high rates of nitrate to nitrite reduction[J]. Bioresource Technology, 2022, 349: 126869. |
| 59 | XU Jiajia, LI Chao, SHEN Yanwen, et al. Anaerobic ammonium oxidation (Anammox) promoted by pyrogenic biochar: deciphering the interaction with extracellular polymeric substances (EPS)[J]. Science of the Total Environment, 2022, 802: 149884. |
| 60 | ZHANG Beichen, WANG Jingshu, HUANG Jinhui Jeanne, et al. Promotion of Anammox process by different graphene-based materials: roles of particle size and oxidation degree[J]. Science of the Total Environment, 2022, 831: 154816. |
| 61 | ELREEDY Ahmed, ISMAIL Sherif, Manal ALI, et al. Unraveling the capability of graphene nanosheets and γ-Fe2O3 nanoparticles to stimulate Anammox granular sludge[J]. Journal of Environmental Management, 2021, 277: 111495. |
| 62 | WANG Dong, WANG Guowen, ZHANG Guoquan, et al. Using graphene oxide to enhance the activity of Anammox bacteria for nitrogen removal[J]. Bioresource Technology, 2013, 131: 527-530. |
| 63 | LI Huai, CHI Zifang, YAN Baixing. Long-term impacts of graphene oxide and Ag nanoparticles on Anammox process: performance, microbial community and toxic mechanism[J]. Journal of Environmental Sciences, 2019, 79: 239-247. |
| 64 | YIN Xin, QIAO Sen, YU Cong, et al. Effects of reduced graphene oxide on the activities of Anammox biomass and key enzymes[J]. Chemical Engineering Journal, 2015, 276: 106-112. |
| 65 | TOMASZEWSKI Mariusz, CEMA Grzegorz, CIESIELSKI Slawomir, et al. Cold Anammox process and reduced graphene oxide -Varieties of effects during long-term interaction[J]. Water Research, 2019, 156: 71-81. |
| 66 | QIAO Sen, YIN Xin, ZHOU Jiti. Application of cathode modified by reduced graphene oxide/polypyrrole to enhance Anammox activity[J]. RSC Advances, 2016, 6(99): 97208-97215. |
| 67 | TOMASZEWSKI Mariusz, CEMA Grzegorz, Aleksandra ZIEMBIŃSKA-BUCZYŃSKA. Short-term effects of reduced graphene oxide on the Anammox biomass activity at low temperatures[J]. Science of the Total Environment, 2019, 646: 206-211. |
| 68 | Anna BANACH-WIŚNIEWSKA, TOMASZEWSKI Mariusz, HELLAL Mohamed S, et al. Effect of biomass immobilization and reduced graphene oxide on the microbial community changes and nitrogen removal at low temperatures[J]. Scientific Reports, 2021, 11(1): 840. |
| [1] | 史天茜, 石永辉, 武新颖, 张益豪, 秦哲, 赵春霞, 路达. Fe2+对厌氧氨氧化EGSB反应器运行性能的影响[J]. 化工进展, 2023, 42(9): 5003-5010. |
| [2] | 李白雪, 信欣, 朱羽蒙, 刘琴, 刘鑫. SASD-A体系构建及进水不同S/N对脱氮工艺的影响机制[J]. 化工进展, 2023, 42(6): 3261-3271. |
| [3] | 李华华, 李逸航, 金北辰, 李隆昕, 成少安. 厌氧氨氧化-生物电化学耦合废水处理系统的研究进展[J]. 化工进展, 2023, 42(5): 2678-2690. |
| [4] | 朱紫旋, 陈俊江, 张星星, 李祥, 刘文如, 吴鹏. 基于短程反硝化厌氧氨氧化新型污水生物脱氮工艺的研究进展[J]. 化工进展, 2023, 42(4): 2091-2100. |
| [5] | 张涵, 张肖静, 马冰冰, 佴灿, 刘烁烁, 马永鹏, 宋亚丽. 以城市废弃污泥为种泥启动厌氧氨氧化工艺的可行性[J]. 化工进展, 2023, 42(2): 1080-1088. |
| [6] | 祝佳欣, 朱雯喆, 徐俊, 谢靖, 王文标, 谢丽. 基于导电材料强化抗生素胁迫厌氧消化的研究进展[J]. 化工进展, 2023, 42(2): 1008-1019. |
| [7] | 池伟利, 杨宏. 厌氧氨氧化包埋填料处理稀土尾矿废水的中试脱氮和优化[J]. 化工进展, 2023, 42(1): 506-516. |
| [8] | 杨柳, 王名威, 张耀斌. 磁铁矿负载生物炭强化厌氧微生物处理2,4-二氯苯酚废水[J]. 化工进展, 2022, 41(9): 5065-5073. |
| [9] | 陈加波, 周鑫, 李旭. 以活性污泥为接种污泥厌氧氨氧化工艺的快速启动及脱氮效能[J]. 化工进展, 2022, 41(7): 3900-3907. |
| [10] | 王超超, 吴翼伶, 陈嘉巧, 蔡天宁, 刘文如, 李祥, 吴鹏. 新型厌氧水解酸化-短程反硝化厌氧氨氧化工艺同步处理生活污水和含硝酸盐模拟废水[J]. 化工进展, 2022, 41(7): 3890-3899. |
| [11] | 汪宇光, 张星星, 王超超, 夏云康, 王垚, 周澄, 吴翼伶, 吴鹏, 徐乐中. 反硝化除磷+短程反硝化厌氧氨氧化工艺的深度脱氮除磷效能[J]. 化工进展, 2022, 41(4): 2191-2201. |
| [12] | 刘锋, 张雪智, 王苏琴, 冯震, 葛丹丹, 杨洋. 硫代硫酸盐驱动自养反硝化耦合厌氧氨氧化强化总氮去除[J]. 化工进展, 2022, 41(2): 990-997. |
| [13] | 肖灿灿, 杨亚飞, 张耀斌. 针铁矿促进剩余污泥厌氧消化中的脱氮除碳[J]. 化工进展, 2022, 41(12): 6689-6697. |
| [14] | 庄海峰, 谢巧娜, 唐浩杰, 吴昊, 薛向东, 单胜道. 磁性材料强化厌氧工艺处理有机废水的研究进展[J]. 化工进展, 2021, 40(7): 3976-3983. |
| [15] | 雷欣, 闫荣, 慕玉洁, 章院灿, 付志敏. 铁元素对厌氧氨氧化菌脱氮效能的影响[J]. 化工进展, 2021, 40(5): 2730-2738. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||