化工进展 ›› 2022, Vol. 41 ›› Issue (12): 6711-6722.DOI: 10.16085/j.issn.1000-6613.2022-0234
高温抗烧结吸附剂对SeO2捕集性能
杨晓域1( ), 于梦竹1(
), 于梦竹1( ), 黄亚继1(
), 黄亚继1( ), 李金壘1, 朱志成1, 李志远1, 王圣2, 李秋白2
), 李金壘1, 朱志成1, 李志远1, 王圣2, 李秋白2
- 1.东南大学能源热转换及其过程测控教育部重点实验室,江苏 南京 210096
2.国家能源集团科学技术研究院 有限公司,江苏 南京 210000
-
收稿日期:2022-02-15修回日期:2022-04-03出版日期:2022-12-20发布日期:2022-12-29 -
通讯作者:黄亚继 -
作者简介:杨晓域(1995—),男,硕士研究生,研究方向为气态污染物控制。E-mail:220194297@seu.edu.cn
于梦竹(1993—),女,博士研究生,研究方向为气态污染物控制。E-mail:yumz@seu.edu.cn。 -
基金资助:国家自然科学基金(51976036);国家重点研发计划(2018YFB0605102)
Improved capture capacity of SeO2 by sorbents with anti-sintering at high temperature
YANG Xiaoyu1( ), YU Mengzhu1(
), YU Mengzhu1( ), HUANG Yaji1(
), HUANG Yaji1( ), LI Jinlei1, ZHU Zhicheng1, LI Zhiyuan1, WANG Sheng2, LI Qiubai2
), LI Jinlei1, ZHU Zhicheng1, LI Zhiyuan1, WANG Sheng2, LI Qiubai2
- 1.Key Laboratory of Energy Thermal Conversion and Control of the Ministry of Education, Southeast University, Nanjing 210096, Jiangsu, China
2.China Energy Corporation Science and Technology Research Institute Co. , Ltd. , Nanjing 210000, Jiangsu, China
-
Received:2022-02-15Revised:2022-04-03Online:2022-12-20Published:2022-12-29 -
Contact:HUANG Yaji
摘要:
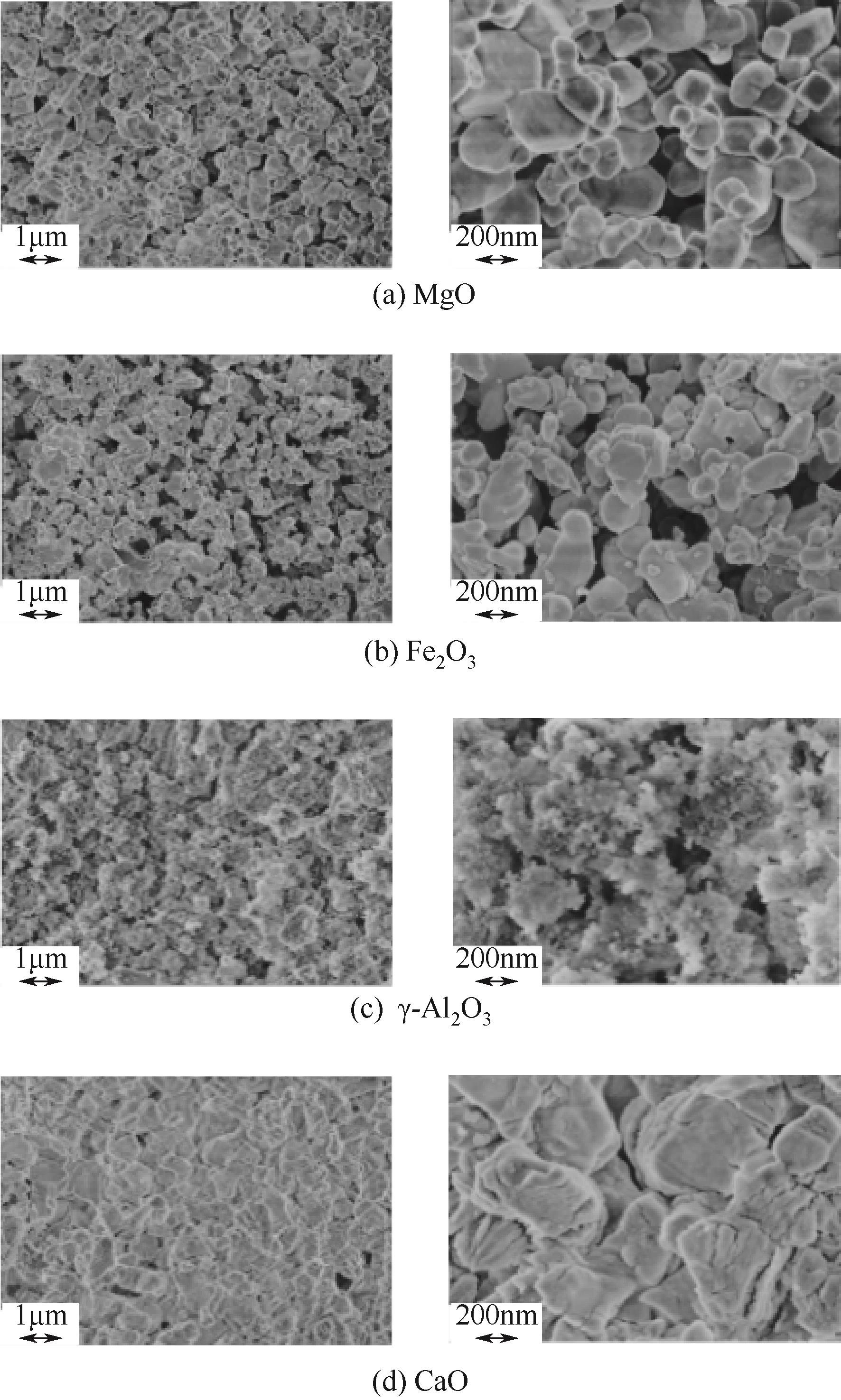
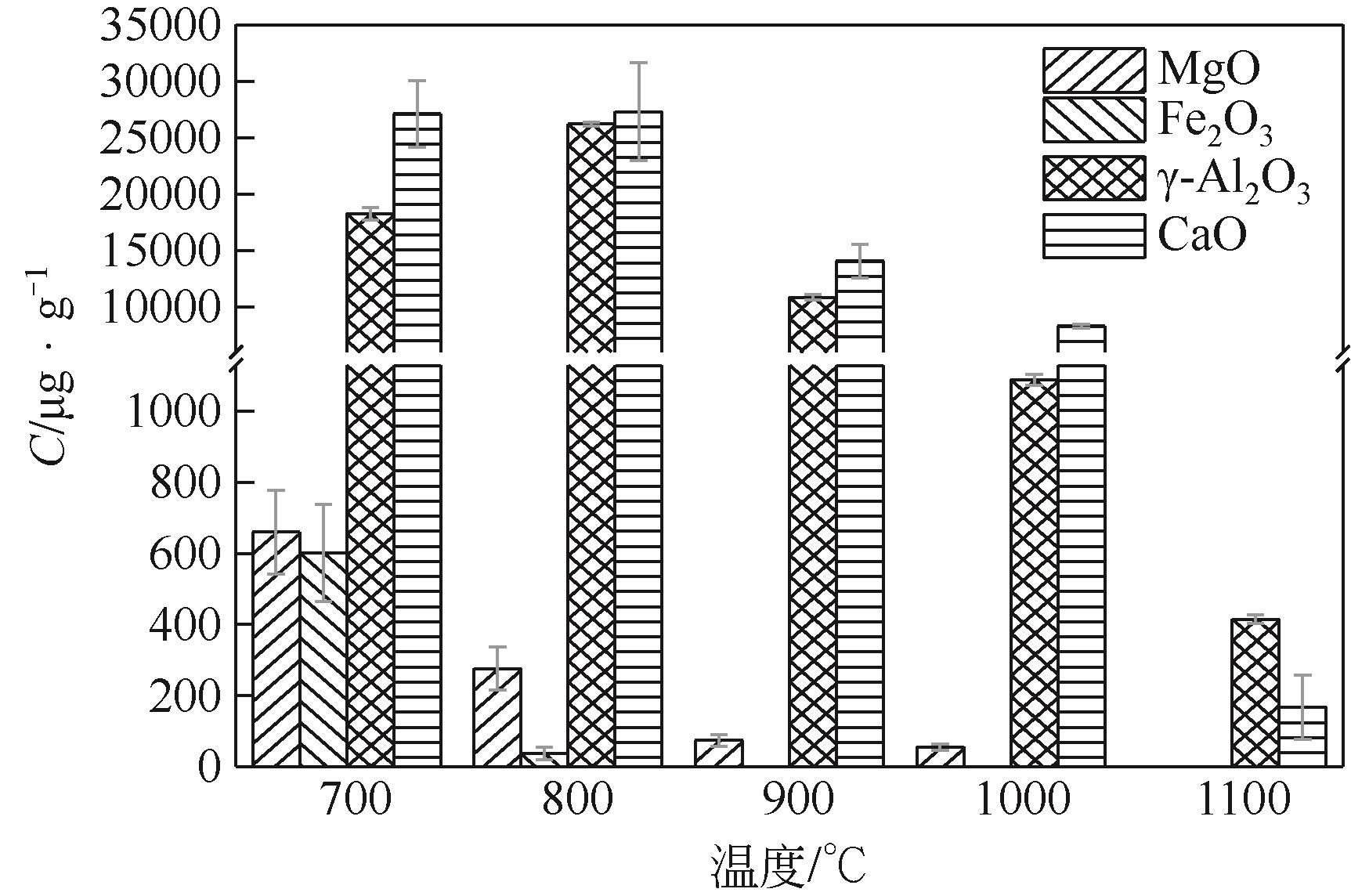
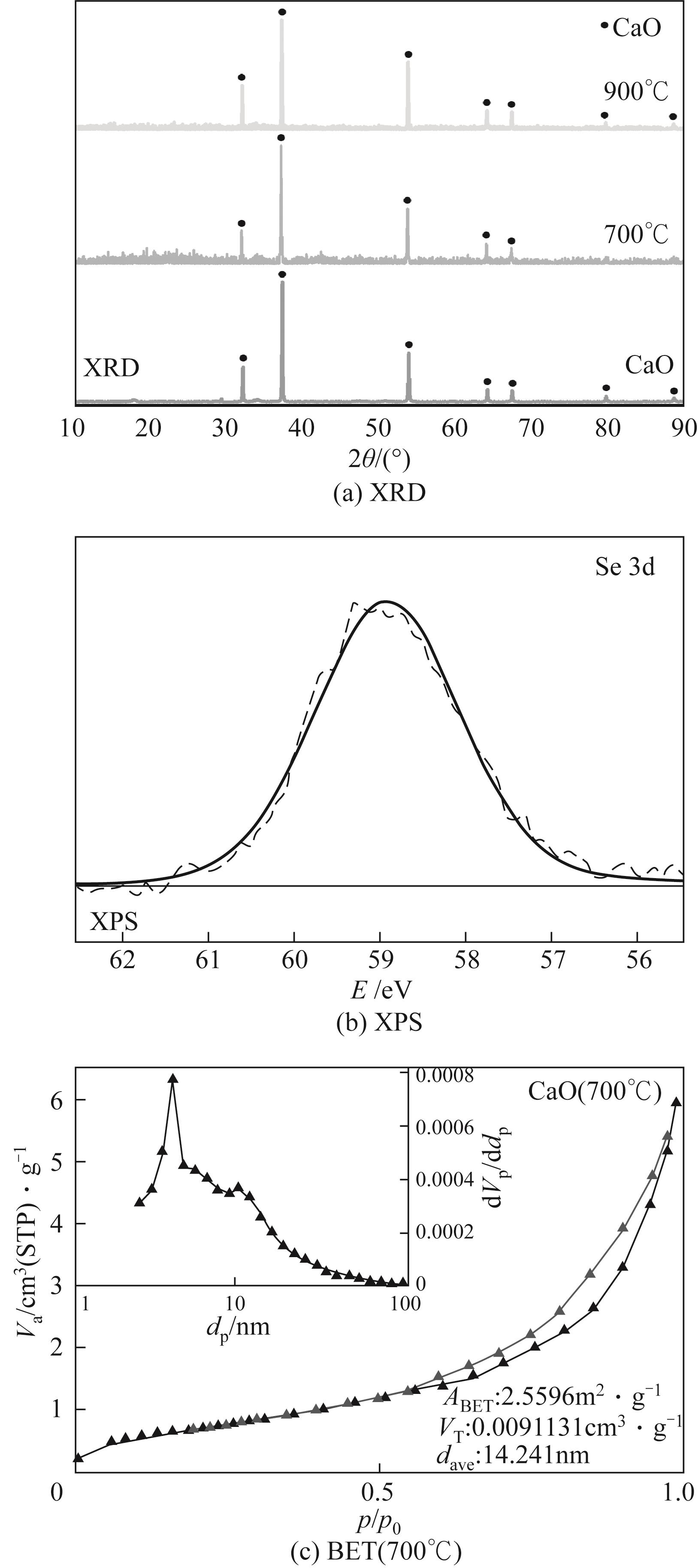
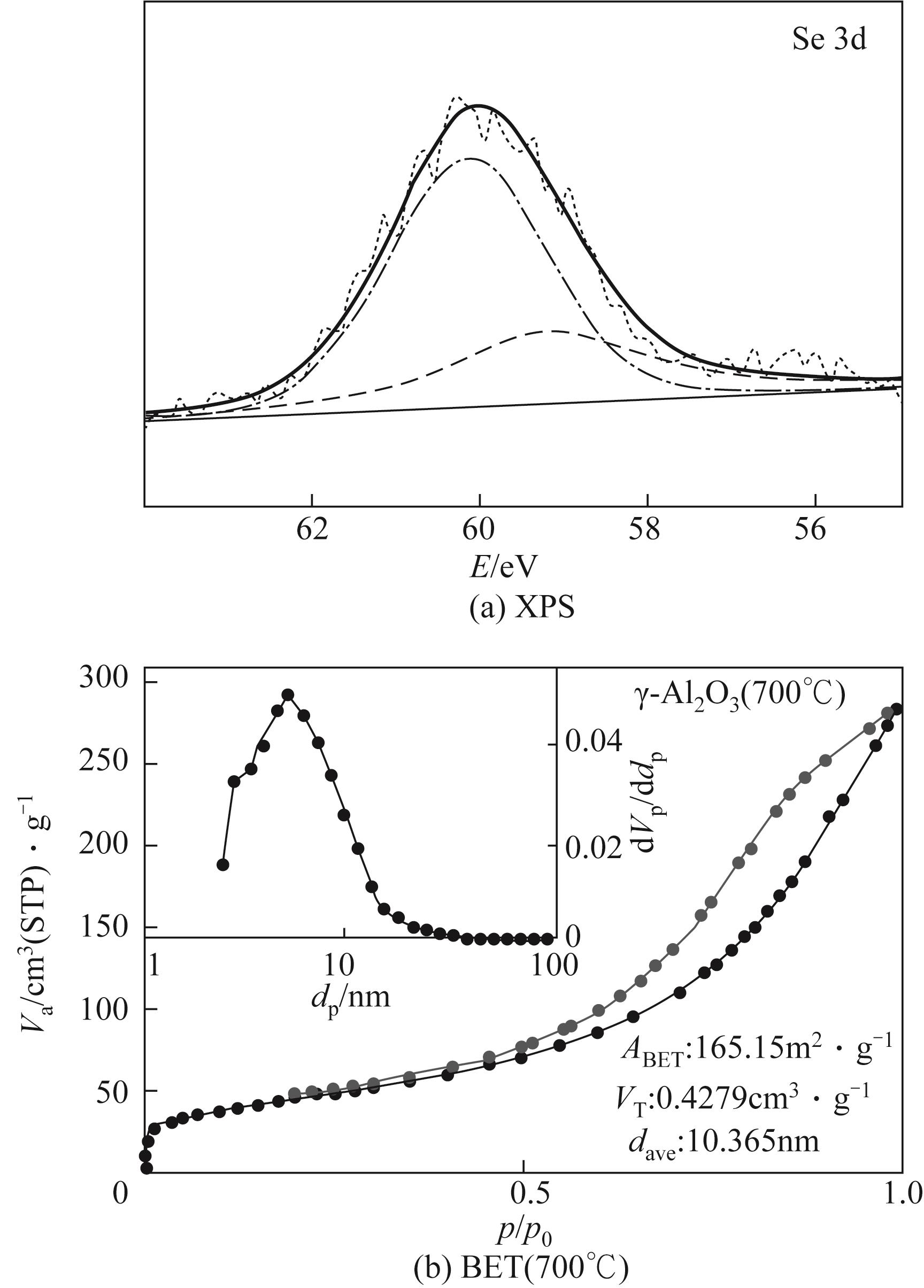
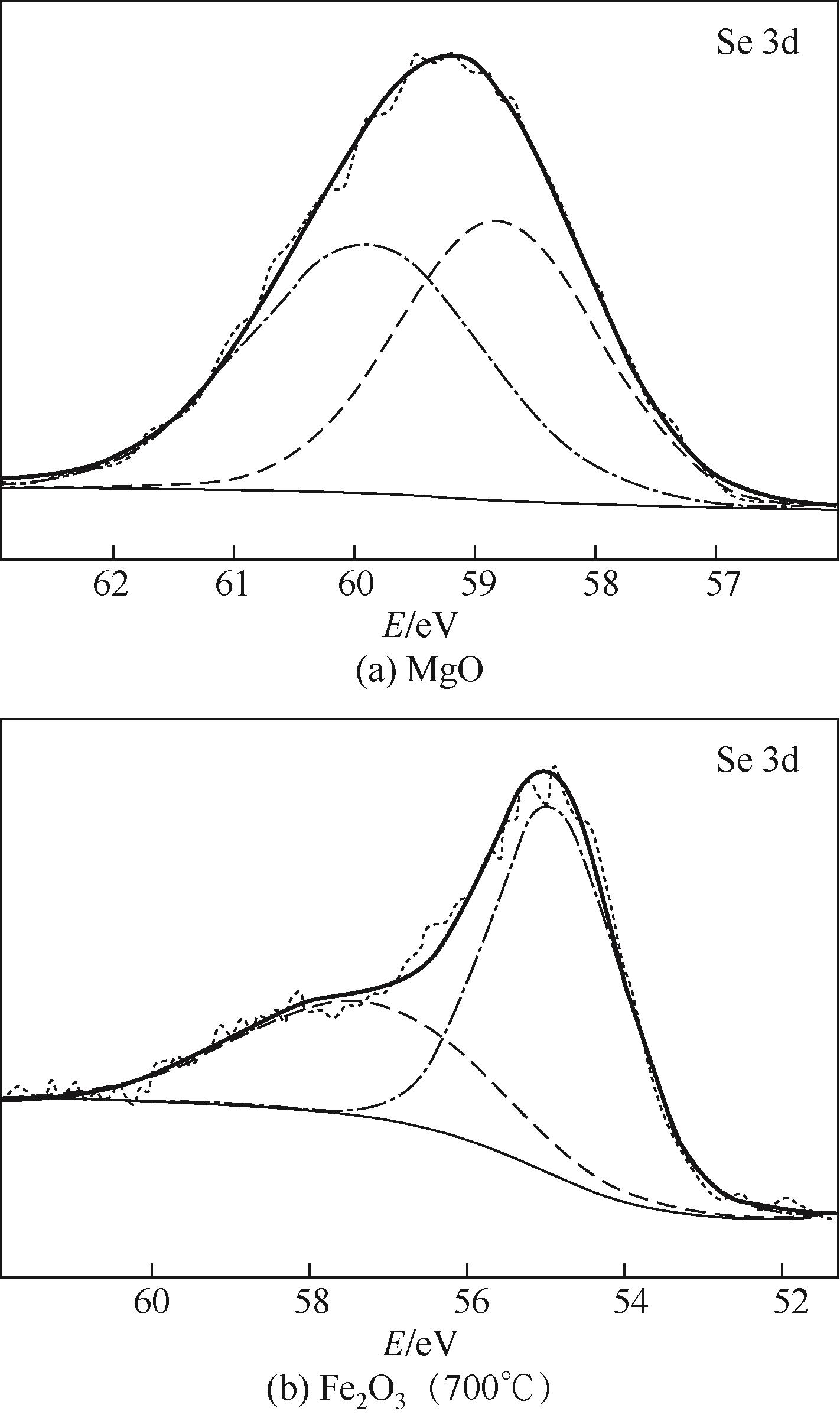
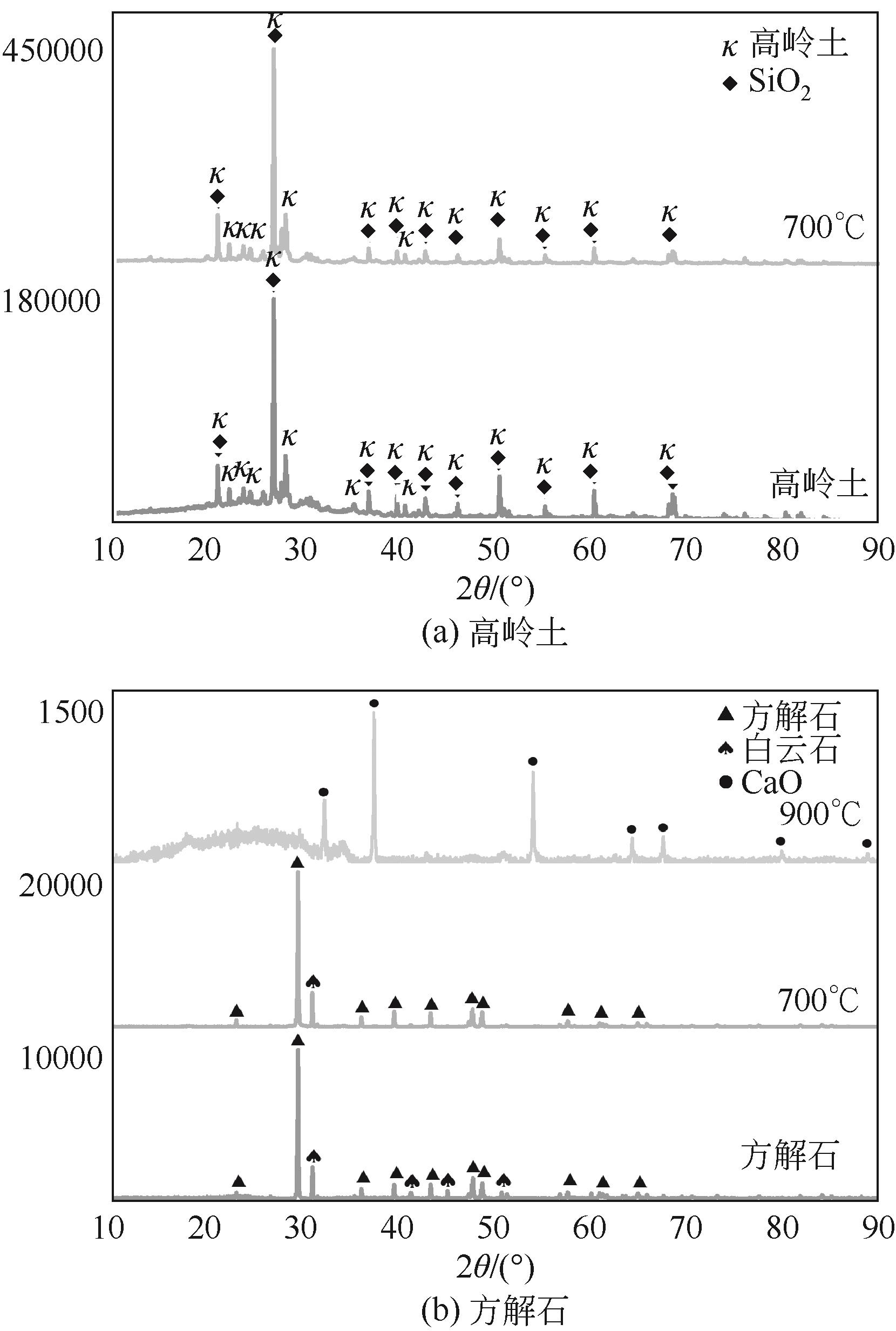
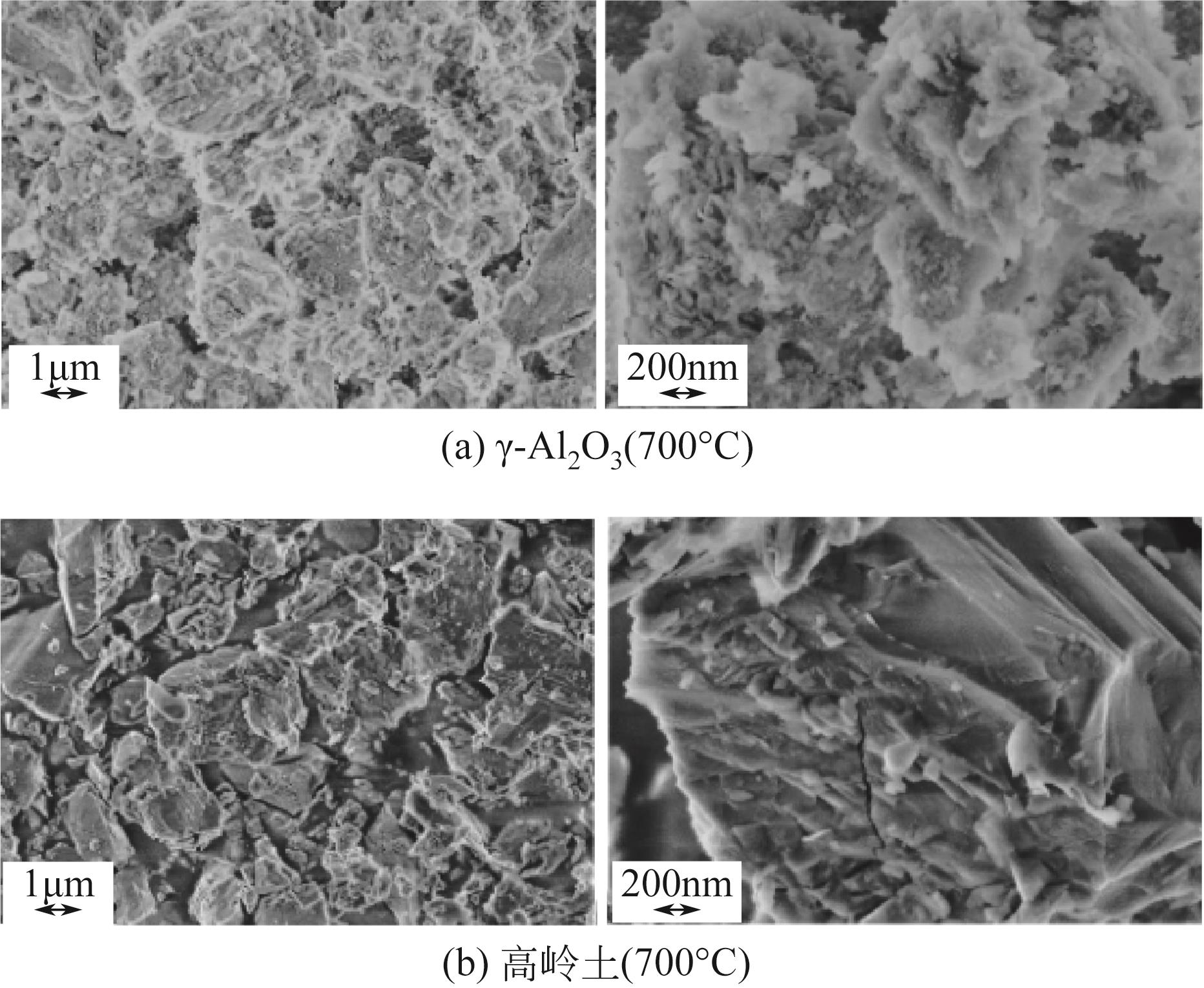
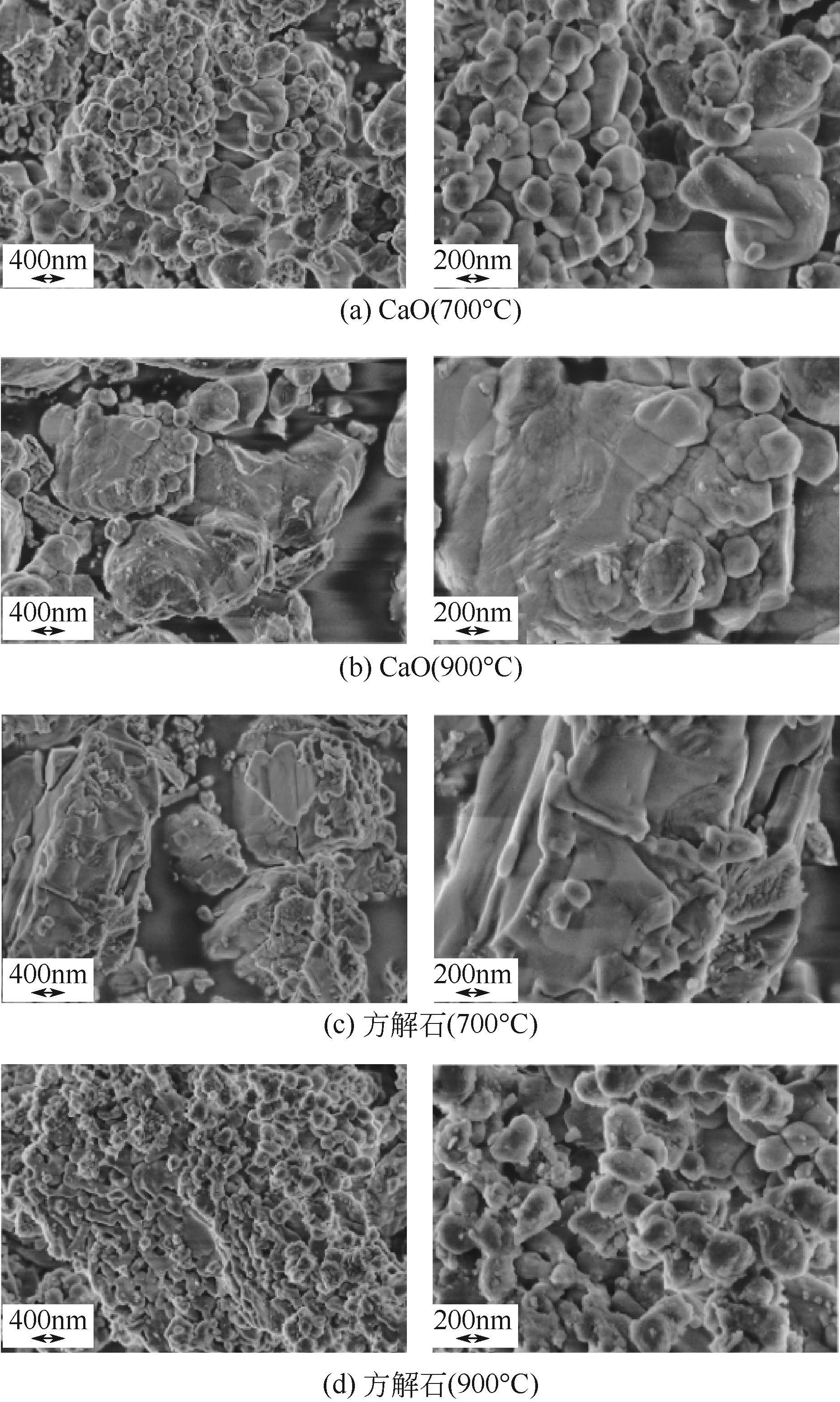
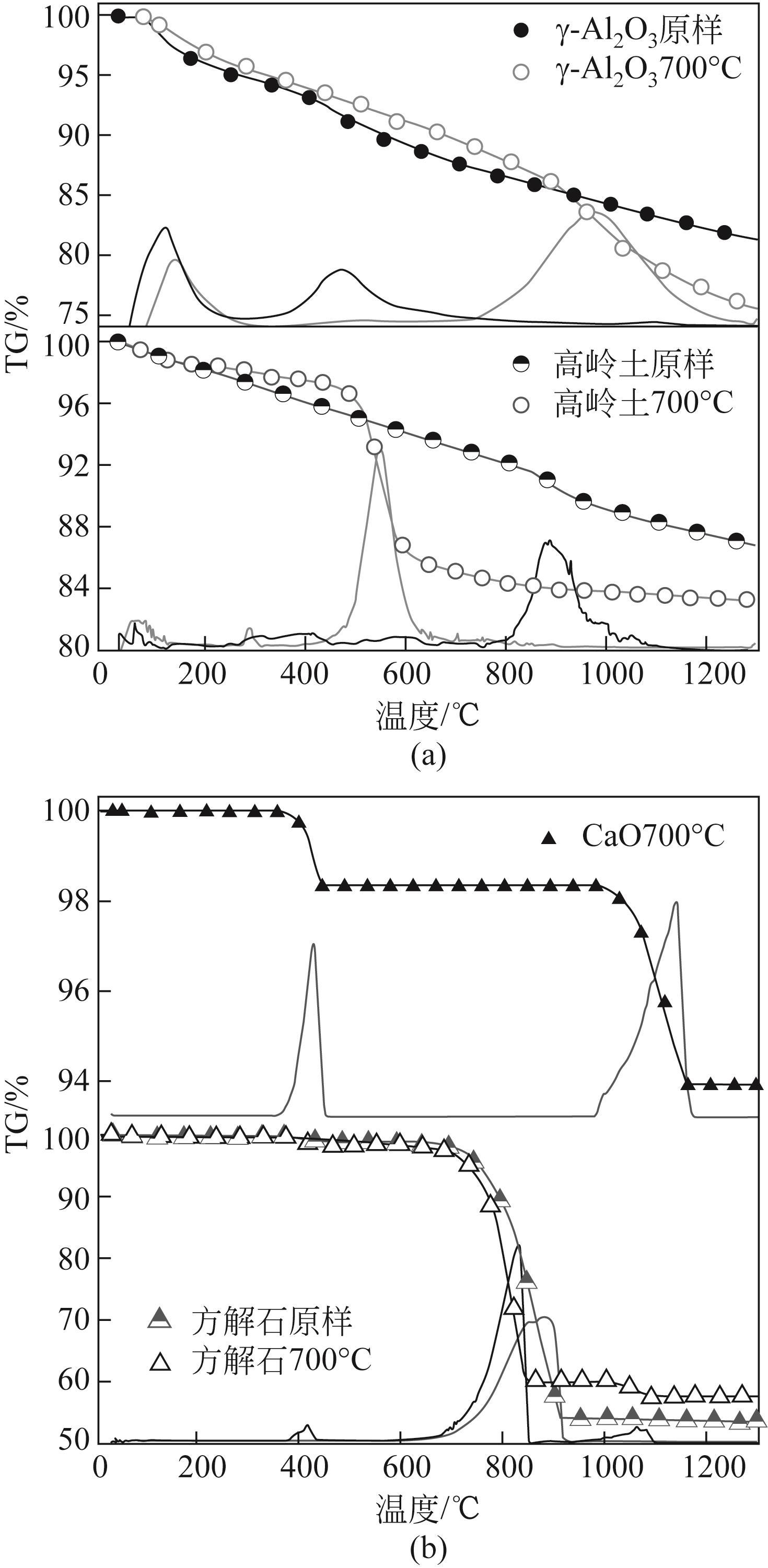
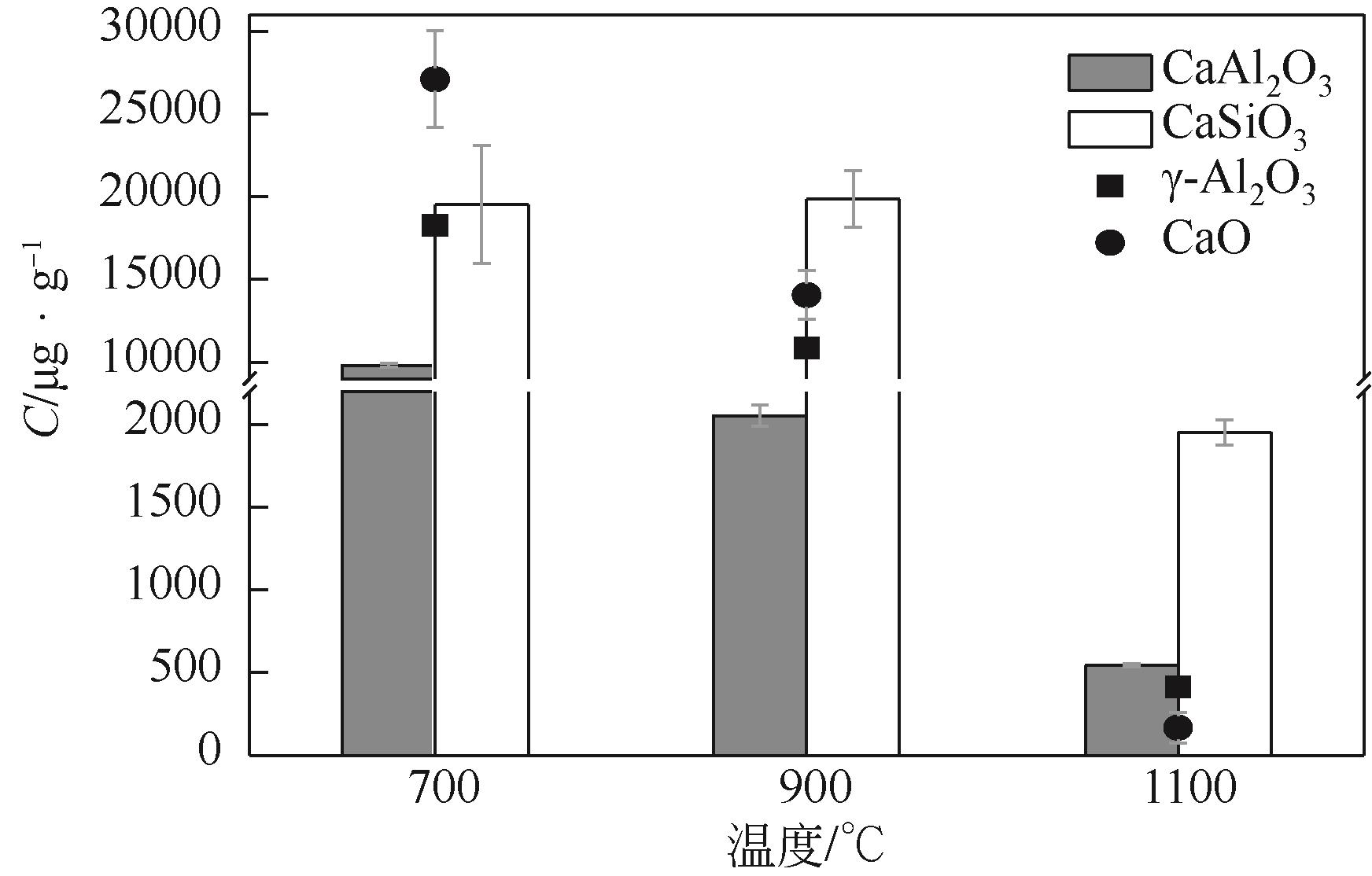
使用气固瞬时反应装置在700~1100℃下对不同氧化物(氧化镁、氧化铁、氧化铝、氧化钙)的硒捕集性能进行研究,确定吸附产物性质。基于此选取相应的典型矿物及双组分抗烧结吸附剂改善硒高温吸附能力。结果表明:900℃前氧化钙捕集效果最好,但1100℃时其吸附量迅速降低到167.59μg/g;γ-氧化铝在高温下捕集效果较好,1100℃时吸附量可达415.04μg/g,这与其优异的孔隙结构有关。钙基矿物方解石因其具有一定的抗烧结能力和发达的孔隙结构,表现出更好的高温捕集能力,1100℃时吸附量可提高到1064.97μg/g。双组分吸附剂高温捕集能力展现出不同程度的提高。钙-铝基吸附剂高温捕集性能提高相对较小;而钙-硅基吸附剂在高温下效果远高于单组分吸附剂,与氧化钙相比,1100℃时可提高1787.21μg/g。
中图分类号:
引用本文
杨晓域, 于梦竹, 黄亚继, 李金壘, 朱志成, 李志远, 王圣, 李秋白. 高温抗烧结吸附剂对SeO2捕集性能[J]. 化工进展, 2022, 41(12): 6711-6722.
YANG Xiaoyu, YU Mengzhu, HUANG Yaji, LI Jinlei, ZHU Zhicheng, LI Zhiyuan, WANG Sheng, LI Qiubai. Improved capture capacity of SeO2 by sorbents with anti-sintering at high temperature[J]. Chemical Industry and Engineering Progress, 2022, 41(12): 6711-6722.
| T/°C | ΔH/kcal | ΔS/cal·K-1 | ΔG/kcal | lgK | T/°C | ΔH/kcal | ΔS/cal·K-1 | ΔG/kcal | lgK |
|---|---|---|---|---|---|---|---|---|---|
| 500 | 84.156 | 108.146 | 0.543 | -0.153 | 900 | 94.349 | 118.762 | -44.976 | 8.379 |
| 600 | 86.677 | 111.212 | -10.428 | 2.610 | 1000 | 96.937 | 120.878 | -56.959 | 9.779 |
| 700 | 89.218 | 113.967 | -21.689 | 4.871 | 1100 | 99.539 | 122.846 | -69.147 | 11.006 |
| 800 | 91.776 | 116.469 | -33.213 | 6.764 | 1200 | 102.156 | 124.686 | -81.524 | 12.096 |
表1 HSC Chemistry 6.0热力学计算 [CaSeO3(ia)̿CaO+SeO2(g)]
| T/°C | ΔH/kcal | ΔS/cal·K-1 | ΔG/kcal | lgK | T/°C | ΔH/kcal | ΔS/cal·K-1 | ΔG/kcal | lgK |
|---|---|---|---|---|---|---|---|---|---|
| 500 | 84.156 | 108.146 | 0.543 | -0.153 | 900 | 94.349 | 118.762 | -44.976 | 8.379 |
| 600 | 86.677 | 111.212 | -10.428 | 2.610 | 1000 | 96.937 | 120.878 | -56.959 | 9.779 |
| 700 | 89.218 | 113.967 | -21.689 | 4.871 | 1100 | 99.539 | 122.846 | -69.147 | 11.006 |
| 800 | 91.776 | 116.469 | -33.213 | 6.764 | 1200 | 102.156 | 124.686 | -81.524 | 12.096 |
| T/°C | ΔH/kcal | ΔS/cal·K-1 | ΔG/kcal | lgK |
|---|---|---|---|---|
| 500 | 166.491 | 403.340 | -145.351 | 41.090 |
| 600 | 173.289 | 411.606 | -186.105 | 46.586 |
| 700 | 180.202 | 419.101 | -227.647 | 51.129 |
| 800 | 187.199 | 425.945 | -269.904 | 54.971 |
| 900 | 194.266 | 432.241 | -312.817 | 58.281 |
| 1000 | 201.390 | 438.069 | -356.336 | 61.174 |
| 1100 | 208.565 | 443.493 | -400.418 | 63.736 |
表2 热力学计算 [Al2(SeO3)3(ia)̿Al2O3+3SeO2(g)]
| T/°C | ΔH/kcal | ΔS/cal·K-1 | ΔG/kcal | lgK |
|---|---|---|---|---|
| 500 | 166.491 | 403.340 | -145.351 | 41.090 |
| 600 | 173.289 | 411.606 | -186.105 | 46.586 |
| 700 | 180.202 | 419.101 | -227.647 | 51.129 |
| 800 | 187.199 | 425.945 | -269.904 | 54.971 |
| 900 | 194.266 | 432.241 | -312.817 | 58.281 |
| 1000 | 201.390 | 438.069 | -356.336 | 61.174 |
| 1100 | 208.565 | 443.493 | -400.418 | 63.736 |
| T/°C | ΔH/kcal | ΔS/cal·K-1 | ΔG/kcal | lgK |
|---|---|---|---|---|
| 600 | 41.565 | 39.117 | 7.409 | -1.855 |
| 700 | 40.699 | 38.179 | 3.544 | -0.796 |
| 800 | 39.717 | 37.220 | -0.226 | 0.046 |
| 900 | 38.615 | 36.239 | -3.899 | 0.726 |
| 1000 | 37.389 | 35.237 | -7.473 | 1.283 |
| 1100 | 36.039 | 34.217 | -10.946 | 1.742 |
表3 热力学计算[MgSeO3̿MgO+SeO2(g)]
| T/°C | ΔH/kcal | ΔS/cal·K-1 | ΔG/kcal | lgK |
|---|---|---|---|---|
| 600 | 41.565 | 39.117 | 7.409 | -1.855 |
| 700 | 40.699 | 38.179 | 3.544 | -0.796 |
| 800 | 39.717 | 37.220 | -0.226 | 0.046 |
| 900 | 38.615 | 36.239 | -3.899 | 0.726 |
| 1000 | 37.389 | 35.237 | -7.473 | 1.283 |
| 1100 | 36.039 | 34.217 | -10.946 | 1.742 |
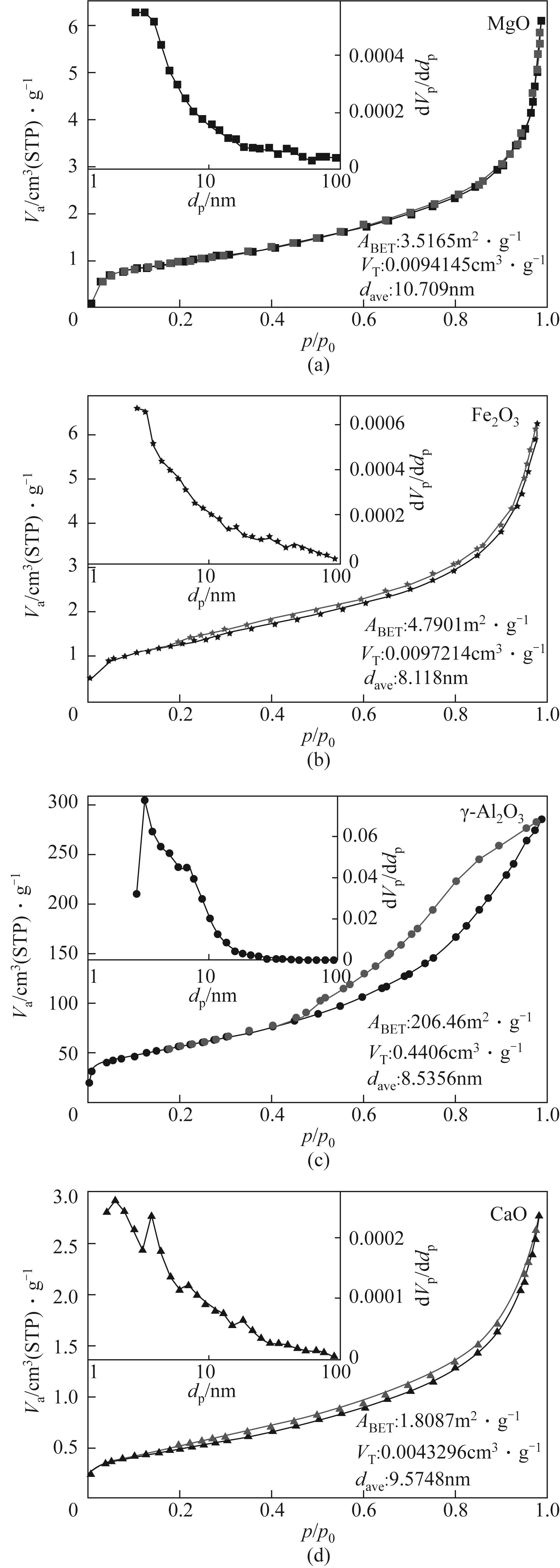
| 样品 | 比表面积/m2·g-1 | 孔容/cm3·g-1 | 孔径/nm |
|---|---|---|---|
| MgO(700℃) | 3.58 | 0.0096 | 10.75 |
| Fe2O3(700℃) | 2.92 | 0.0074 | 10.08 |
| 高岭土(700℃) | 2.61 | 0.0094 | 14.45 |
| 方解石(900℃) | 6.55 | 0.0203 | 12.39 |
| CaSiO3(700℃) | 3.44 | 0.0110 | 12.81 |
| CaSiO3(900℃) | 3.17 | 0.0056 | 7.08 |
表4 吸附产物比表面积、孔容与孔径
| 样品 | 比表面积/m2·g-1 | 孔容/cm3·g-1 | 孔径/nm |
|---|---|---|---|
| MgO(700℃) | 3.58 | 0.0096 | 10.75 |
| Fe2O3(700℃) | 2.92 | 0.0074 | 10.08 |
| 高岭土(700℃) | 2.61 | 0.0094 | 14.45 |
| 方解石(900℃) | 6.55 | 0.0203 | 12.39 |
| CaSiO3(700℃) | 3.44 | 0.0110 | 12.81 |
| CaSiO3(900℃) | 3.17 | 0.0056 | 7.08 |
| 1 | 中华人民共和国国家统计局. 中华人民共和国2008年国民经济和社会发展统计公报[J]. 中国统计, 2009(3): 4-10. |
| National Bureau of Statistics. Statistical bulletin of the People’s Republic of China on national economic and social development, 2020[J]. China Statistics, 2009(3): 4-10. | |
| 2 | 夏文青, 黄亚继, 王昕晔, 等. 非碳基吸附剂高温捕集氯化铅蒸气[J]. 化工进展, 2017, 36(9): 3508-3513. |
| XIA Wenqing, HUANG Yaji, WANG Xinye, et al. Experimental study on high temperature adsorption of lead chloride by non-carbon adsorbents[J]. Chemical Industry and Engineering Progress, 2017, 36(9): 3508-3513. | |
| 3 | FAN Yuyang, LIU Chao, KONG Xiangchen, et al. A new perspective on polyethylene-promoted lignin pyrolysis with mass transfer and radical explanation[J]. Green Energy & Environment, 2022, 7(6): 1318-1326. |
| 4 | ZHOU Chuncai, LIU Guijian, XU Zhongyu, et al. Retention mechanisms of ash compositions on toxic elements (Sb, Se and Pb) during fluidized bed combustion[J]. Fuel, 2018, 213: 98-105. |
| 5 | 程运, 王昕晔, 吕文婷, 等. 高岭土高温吸附重金属和碱金属的研究进展[J]. 化工进展, 2019, 38(8): 3852-3865. |
| CHENG Yun, WANG Xinye, Wenting LYU, et al. A review on heavy and alkali metals adsorption by Kaolin at high temperature[J]. Chemical Industry and Engineering Progress, 2019, 38(8): 3852-3865. | |
| 6 | WANG Lei, JU Yiwen, LIU Guijian, et al. Selenium in Chinese coals: distribution, occurrence, and health impact[J]. Environmental Earth Sciences, 2010, 60(8): 1641-1651. |
| 7 | TIAN H Z, ZHU C Y, GAO J J, et al. Quantitative assessment of atmospheric emissions of toxic heavy metals from anthropogenic sources in China: historical trend, spatial distribution, uncertainties, and control policies[J]. Atmospheric Chemistry and Physics, 2015, 15(17): 10127-10147. |
| 8 | FAN Yaming, ZHUO Yuqun, LI Liangliang. SeO2 adsorption on CaO surface: DFT and experimental study on the adsorption of multiple SeO2 molecules[J]. Applied Surface Science, 2017, 420: 465-471. |
| 9 | National emission standards for hazardous air pollutants from coal and oil-fired electric utility steam generating units and standards of performance for fossil-fuel-fired electric utility, industrial-commercial-institutional, and small industrial [S]. Federal Register, 2016. |
| 10 | 黄永达, 胡红云, 龚泓宇, 等. 燃煤电厂砷、硒、铅的排放与控制技术研究进展[J]. 燃料化学学报, 2020, 48(11): 1281-1297. |
| HUANG Yongda, HU Hongyun, GONG Hongyu, et al. Research progress on emission and control technologies of arsenic, selenium and lead in coal-fired power plants[J]. Journal of Fuel Chemistry and Technology, 2020, 48(11): 1281-1297. | |
| 11 | HU Jianjun, SUN Qiang, HE Huan. Thermal effects from the release of selenium from a coal combustion during high-temperature processing: a review[J]. Environmental Science and Pollution Research, 2018, 25(14): 13470-13478. |
| 12 | 张军营, 任德贻, 许德伟, 等. 煤中硒的研究现状[J]. 煤田地质与勘探, 1999, 27(2): 16-18. |
| ZHANG Junying, REN Deyi, XU Dewei, et al. Advances in the studies of selenium in coal[J]. Coal Geology & Exploration, 1999, 27(2): 16-18. | |
| 13 | JAMES D W, KRISHNAMOORTHY G, BENSON S A, et al. Modeling trace element partitioning during coal combustion[J]. Fuel Processing Technology, 2014, 126: 284-297. |
| 14 | SENIOR C L, TYREE C A, MEEKS N D, et al. Selenium partitioning and removal across a wet FGD scrubber at a coal-fired power plant[J]. Environmental Science & Technology, 2015, 49(24): 14376-14382. |
| 15 | LI Yuzhong, TONG Huiling, ZHUO Yuqun, et al. Simultaneous removal of SO2 and trace SeO2 from flue gas: effect of product layer on mass transfer[J]. Environmental Science & Technology, 2006, 40(13): 4306-4311. |
| 16 | ZOU Renjie, ZHANG Haoyu, LUO Guangqian, et al. Selenium migration behaviors in wet flue gas desulfurization slurry and an in situ treatment approach[J]. Chemical Engineering Journal, 2020, 385: 123891. |
| 17 | DIAZ-SOMOANO M, MARTINEZ-TARAZONA M R. Retention of arsenic and selenium compounds using limestone in a coal gasification flue gas[J]. Environmental Science & Technology, 2004, 38(3): 899-903. |
| 18 | GHOSH-DASTIDAR A, MAHULI S, AGNIHOTRI R, et al. Selenium capture using sorbent powders: mechanism of sorption by hydrated lime[J]. Environmental Science & Technology, 1996, 30(2): 447-452. |
| 19 | AGNIHOTRI R, CHAUK S, MAHULI S, et al. Selenium removal using Ca-based sorbents: reaction kinetics[J]. Environmental Science & Technology, 1998, 32(12): 1841-1846. |
| 20 | LOU Yu, FAN Yaming, PANG Chengkai, et al. The promotion by steam on CaO adsorbing SeO2 at medium temperature[J]. IOP Conference Series: Earth and Environmental Science, 2018, 159: 012010. |
| 21 | LI Yuzhong, TONG Huiling, ZHUO Yuqun, et al. Simultaneous removal of SO2 and trace SeO2 from flue gas: effect of SO2 on selenium capture and kinetics study[J]. Environmental Science & Technology, 2006, 40(24): 7919-7924. |
| 22 | SENIOR Constance, OTTEN Brydger Van, WENDT Jost O L, et al. Modeling the behavior of selenium in pulverized-coal combustion systems[J]. Combustion and Flame, 2010, 157(11): 2095-2105. |
| 23 | XU Shengrui, SHUAI Qin, HUANG Yunjie, et al. Se capture by a CaO-ZnO composite sorbent during the combustion of Se-rich stone coal[J]. Energy & Fuels, 2013, 27(11): 6880-6886. |
| 24 | FRANDSEN Flemming, Kim DAM-JOHANSEN, RASMUSSEN Peter. Trace elements from combustion and gasification of coal—An equilibrium approach[J]. Progress in Energy and Combustion Science, 1994, 20(2): 115-138. |
| 25 | WANG Jiawei, ZHANG Yongsheng, WANG Tao, et al. Effect of modified fly ash injection on As, Se, and Pb emissions in coal-fired power plant[J]. Chemical Engineering Journal, 2020, 380: 122561. |
| 26 | FURUZONO Takuya, NAKAJIMA Tsunenori, FUJISHIMA Hiroki, et al. Behavior of selenium in the flue gas of pulverized coal combustion system: influence of kind of coal and combustion conditions[J]. Fuel Processing Technology, 2017, 167: 388-394. |
| 27 | ZENG T, SAROFIM A F, SENIOR C L. Vaporization of arsenic, selenium and antimony during coal combustion[J]. Combustion and Flame, 2001, 126(3): 1714-1724. |
| 28 | 刘忠, 王硕, 白宝泉. 燃煤飞灰中矿物质对烟气中As、Se、Pb形态分布影响的热力学研究[J]. 燃料化学学报, 2020, 48(12): 1530-1536. |
| LIU Zhong, WANG Shuo, BAI Baoquan. Thermodynamic study on effect of minerals in fly ash on morphological distribution of As, Se and Pb in flue gas[J]. Journal of Fuel Chemistry and Technology, 2020, 48(12): 1530-1536. | |
| 29 | 于梦竹, 王海, 黄亚继, 等. 典型钙/镁基吸附剂对二氧化硒吸附特性研究[J]. 燃料化学学报, 2020, 48(11): 1335-1344. |
| YU Mengzhu, WANG Hai, HUANG Yaji, et al. Characteristics of selenium capture by typical Ca-/ Mg-based sorbents[J]. Journal of Fuel Chemistry and Technology, 2020, 48(11): 1335-1344. | |
| 30 | 王昕晔, 黄亚继, 仲兆平, 等. 炉内添加剂对垃圾焚烧过程中重金属捕集影响的试验研究[J]. 中国电机工程学报, 2012, 32(32): 15-21, 4. |
| WANG Xinye, HUANG Yaji, ZHONG Zhaoping, et al. Experimental study on the capture of heavy metals by in-furnace additives during MSW incineration[J]. Proceedings of the CSEE, 2012, 32(32): 15-21, 4. | |
| 31 | CAO Yue, SONG Bing, SONG Min, et al. Capture of arsenic in coal combustion flue gas at high temperature in the presence of CaSiO3 with good anti-sintering[J]. Fuel Processing Technology, 2020, 205: 106428. |
| 32 | CHENG Haoqiang, HUANG Yaji, ZHU Zhicheng, et al. Enhanced PbCl2 adsorption capacity of modified Kaolin in the furnace using a combined method of thermal pre-activation and acid impregnation[J]. Chemical Engineering Journal, 2021, 414: 128672. |
| 33 | 刘瑞卿, 王钧伟. 矿物质与矿物离子对煤中硒释放行为的影响[J]. 环境化学, 2013, 32(1): 100-105. |
| LIU Ruiqing, WANG Junwei. Influence of minerals and mineral ions on selenium release behaviors during coal pyrolysis[J]. Environmental Chemistry, 2013, 32(1): 100-105. | |
| 34 | YUAN Changle, ZHANG Cheng, YU Shenghui, et al. Experimental and density functional theory study of the adsorption characteristics of CaO for SeO2 in simulated flue gas and the effect of CO2 [J]. Energy & Fuels, 2020, 34(9): 10872-10881. |
| 35 | SHENASA Mohsen, SAINKAR Sudhakar, LICHTMAN David. XPS study of some selected selenium compounds[J]. Journal of Electron Spectroscopy and Related Phenomena, 1986, 40(4): 329-337. |
| 36 | SARODE P R, RAO K J, HEGDE M S, et al. Study of As2(Se, Te)3 glasses by X-ray absorption and photoelectron spectroscopy[J]. Journal of Physics C: Solid State Physics, 1979, 12(19): 4119-4128. |
| 37 | BAHL M K, WATSON R L, IRGOLIC K J. LMM Auger spectra of selenium and some of its compounds[J]. The Journal of Chemical Physics, 1980, 72(7): 4069-4077. |
| 38 | SATOH Kenji, TAKAHASHI Takashi, Hiroshi KATAYAMA-YOSHIDA, et al. Bound states in copper-based Cu-Ge and Cu-Se alloys studied by X-ray photoelectron spectroscopy[J]. Journal of the Physical Society of Japan, 1985, 54(3): 1214-1215. |
| 39 | CAHEN David, IRELAND P J, KAZMERSKI L L, et al. X-ray photoelectron and Auger electron spectroscopic analysis of surface treatments and electrochemical decomposition of CuInSe2 photoelectrodes[J]. Journal of Applied Physics, 1985, 57(10): 4761-4771. |
| 40 | FAN Yaming, ZHUO Yuqun, LOU Yu, et al. SeO2 adsorption on CaO surface: DFT study on the adsorption of a single SeO2 molecule[J]. Applied Surface Science, 2017, 413: 366-371. |
| 41 | ZHA Jianrui, HUANG Yaji, CLOUGH Peter T, et al. Green production of a novel sorbent from Kaolin for capturing gaseous PbCl2 in a furnace[J]. Journal of Hazardous Materials, 2021, 404(Pt B): 124045. |
| 42 | YU Mengzhu, HUANG Yaji, XIA Wenqing, et al. PbCl2 capture by Kaolin and metakaolin under different influencing factors of thermal treatment[J]. Energy & Fuels, 2020, 34(2): 2284-2292. |
| [1] | 郭志鹏, 卜宪标, 李华山, 龚宇烈, 王令宝. 基于热-流-化耦合作用的单井增强地热系统性能分析[J]. 化工进展, 2023, 42(2): 711-721. |
| [2] | 王庆宏, 姜晨旭, 王鑫, 余美琪, 朱帅, 李一鸣, 陈春茂. 天然矿物催化氧化水中难降解有机污染物研究进展[J]. 化工进展, 2023, 42(1): 417-434. |
| [3] | 王光绪, 金晶, 张云鹏, 刘薄鉴治, 梁诗雨, 翟中媛. 硅钙摩尔比对准东煤燃烧过程中矿物演变及灰熔融特性的影响[J]. 化工进展, 2022, 41(8): 4140-4146. |
| [4] | 何民宇, 刘维燥, 刘清才, 秦治峰. CO2矿物封存技术研究进展[J]. 化工进展, 2022, 41(4): 1825-1833. |
| [5] | 刘贺, 刘建忠, 陈建, 王建斌, 王明霞. 几种典型固废与神华煤掺烧的结渣特性[J]. 化工进展, 2022, 41(1): 443-452. |
| [6] | 王中辉, 苏胜, 尹子骏, 安晓雪, 赵志刚, 陈逸峰, 刘涛, 汪一, 胡松, 向军. CO2矿化及吸收-矿化一体化(IAM)方法研究进展[J]. 化工进展, 2021, 40(4): 2318-2327. |
| [7] | 于志浩, 金晶, 刘敦禹, 侯封校, 杭伊煊, 张瑞璞, 翟中媛. 鸟粪石添加剂对准东煤燃烧过程中钠、钙矿物转化和释放的影响[J]. 化工进展, 2021, 40(4): 2120-2129. |
| [8] | 郑烨, 李建波, 张锴, 关彦军, 杨凤玲, 程芳琴. 酸性氧化物和酸碱比对煤灰熔融行为的影响[J]. 化工进展, 2020, 39(9): 3617-3625. |
| [9] | 马杨杨, 仲兆平, 赖旭东. 矿物添加剂对煤燃烧过程中重金属的富集[J]. 化工进展, 2020, 39(6): 2479-2486. |
| [10] | 苗琪,张叶龙,贾旭,金翼,谈玲华,丁玉龙. 矿物基化学吸附储热技术的研究进展[J]. 化工进展, 2020, 39(4): 1308-1320. |
| [11] | 郑烨,李建波,关彦军,杨凤玲,张锴,程芳琴. 碱性氧化物对煤灰熔融特征行为的影响[J]. 化工进展, 2020, 39(2): 496-505. |
| [12] | 张志勇, 王慧敏, 陈媛媛, 张兵兵, 杨耀国. 新型硅铝矿物吸附剂用于废变压器油再生处理[J]. 化工进展, 2020, 39(10): 4297-4304. |
| [13] | 郑烨, 马志斌, 关彦军, 张锴, 程芳琴. 煤矸石灰添加对准东煤灰熔融特性影响[J]. 化工进展, 2019, 38(04): 1714-1720. |
| [14] | 王东旭, 陈裕辉, 王洋, 李文艳, 肖海平, 康志忠. MgO含量对高钠煤灰熔融特性的影响[J]. 化工进展, 2018, 37(04): 1392-1401. |
| [15] | 张胜寒, 孙晨皓, 陈玉强. 燃煤电厂脱硫废水中硒元素脱除技术研究进展[J]. 化工进展, 2017, 36(04): 1460-1469. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||