化工进展 ›› 2022, Vol. 41 ›› Issue (12): 6698-6710.DOI: 10.16085/j.issn.1000-6613.2022-0246
低湿CO2/H2O混合气体吸附特性实验
- 北京建筑大学环境与能源工程学院,北京 100044
-
收稿日期:2022-02-16修回日期:2022-04-12出版日期:2022-12-20发布日期:2022-12-29 -
通讯作者:鲁军辉 -
作者简介:任可欣(1997—),女,硕士研究生,研究方向为电厂烟气碳捕集。E-mail:2108590020155@stu.bucea.edu.cn。 -
基金资助:国家重点研究计划(2016YFB0600105);北京学者计划(2015 022);北京市博士后工作经费项目(2021-ZZ-112);北京建筑大学市属高校基本科研业务专项资金项目(X21016)
Adsorption characteristics of CO2/H2O with low humidity
REN Kexin( ), LU Junhui(
), LU Junhui( ), WANG Suilin, TANG Jinjing
), WANG Suilin, TANG Jinjing
- School of Environment and Energy Engineering, Beijing University of Civil Engineering and Architecture, Beijing 100044, China
-
Received:2022-02-16Revised:2022-04-12Online:2022-12-20Published:2022-12-29 -
Contact:LU Junhui
摘要:
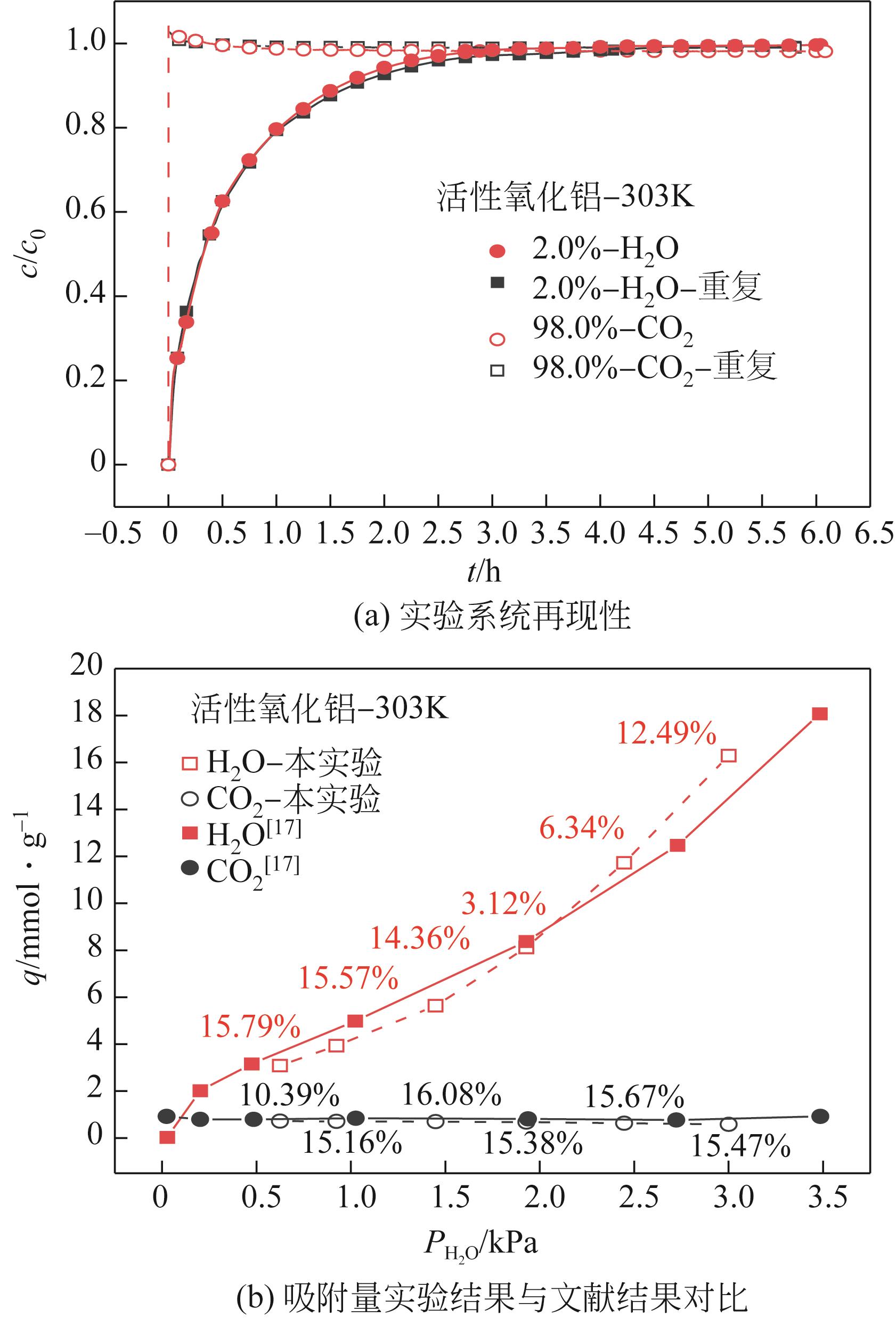
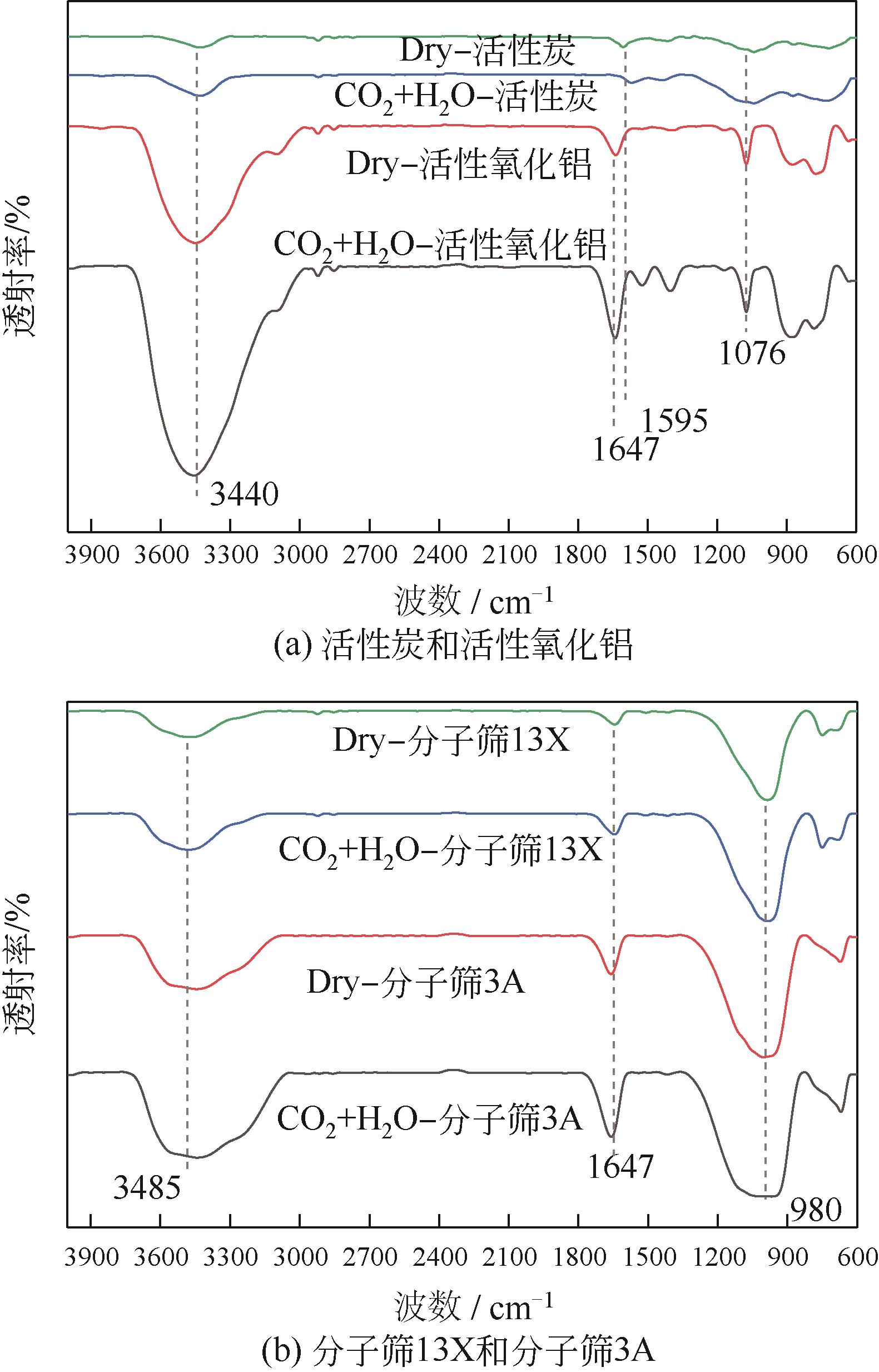
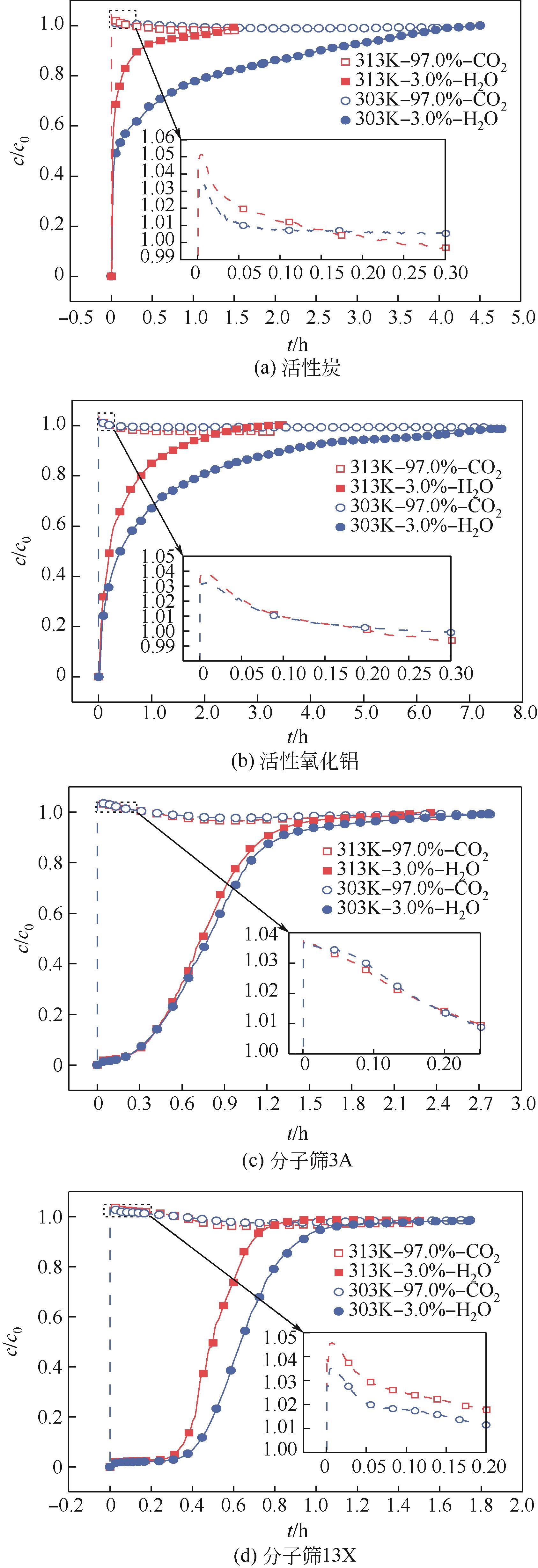
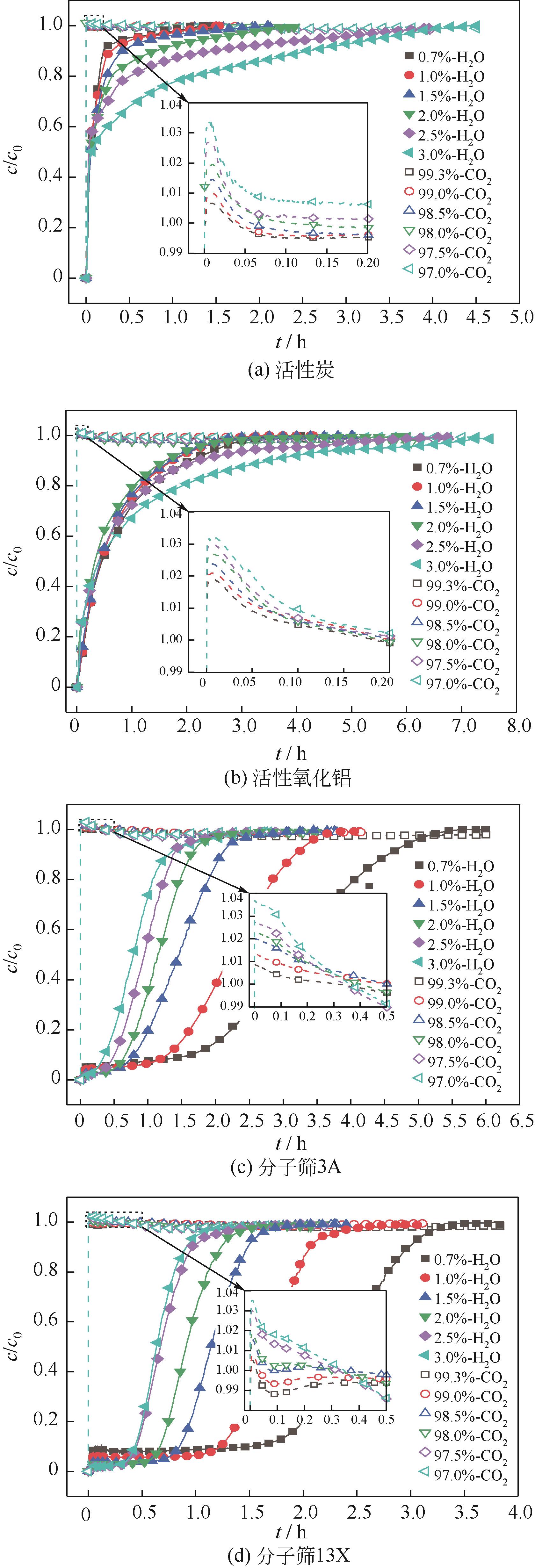
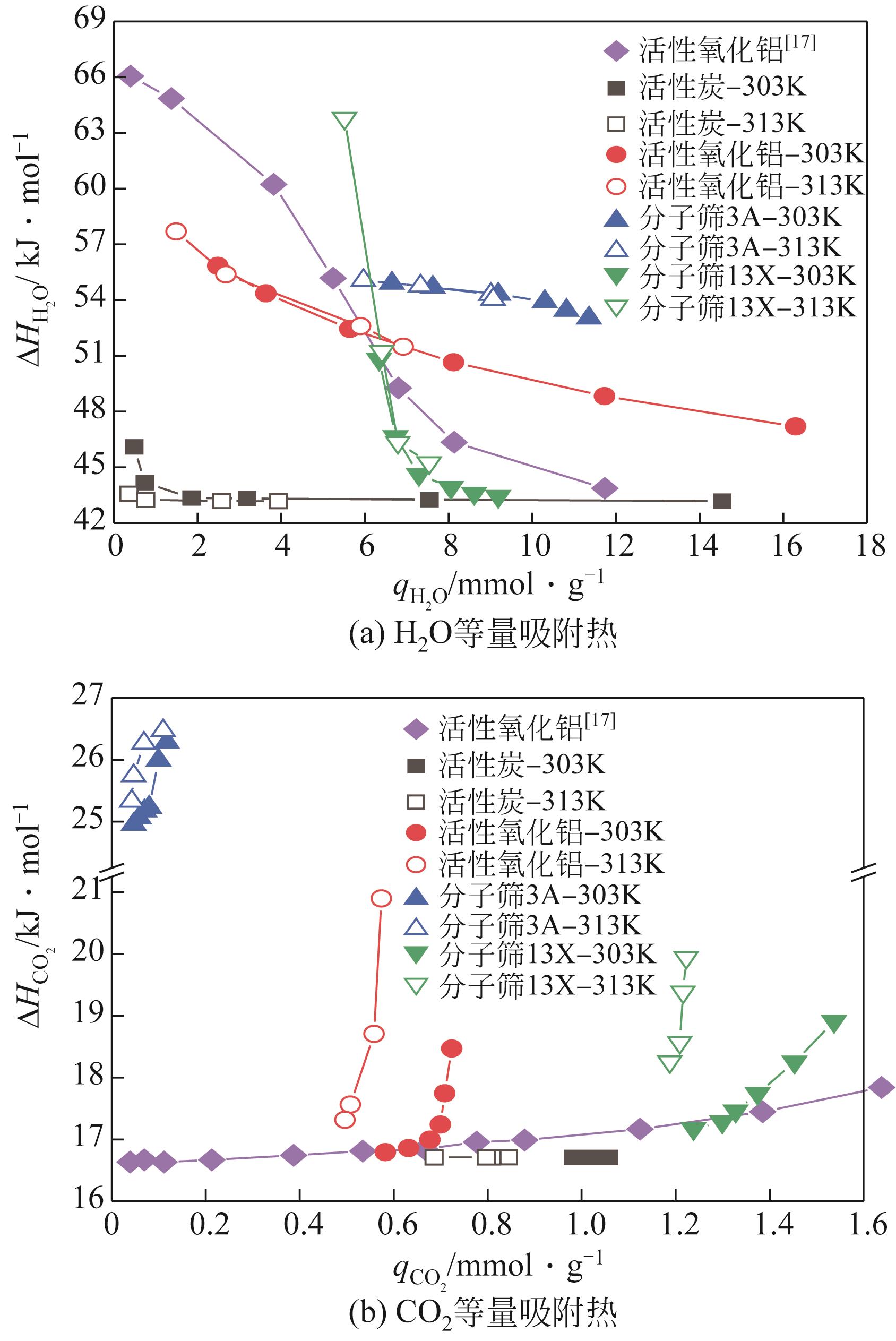
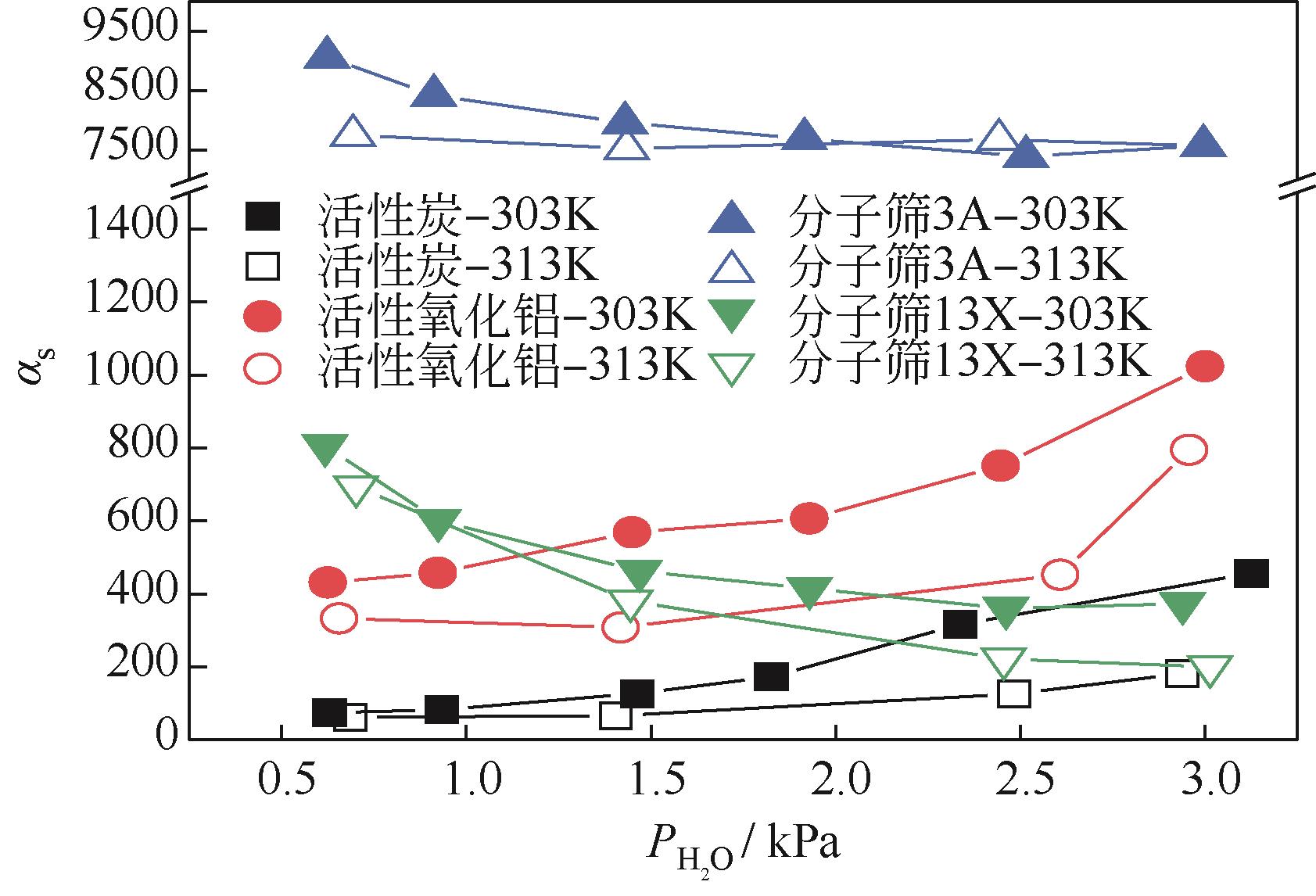
CO2捕集、封存及利用是实现“双碳”目标的重要途径,为将碳捕集后的低湿CO2/H2O进行CO2提纯和资源化利用,采用动态吸附实验研究了不同温度(303K、313K)、H2O含量(0.7%~3.0%)的CO2/H2O在活性炭、活性氧化铝、分子筛3A和13X四种吸附剂上的动态吸附穿透曲线、吸附床温度分布、吸附量,分析了CO2/H2O分离系数和吸附热。结果表明,在CO2/H2O动态吸附过程中,吸附床温度与各组分浓度随时间变化趋势相同。H2O饱和时间随进气温度升高而缩短;H2O含量增加,抑制CO2吸附;活性炭和氧化铝中H2O的饱和时间随H2O含量增加而增长,但分子筛3A和13X饱和时间缩短。H2O吸附量随H2O含量增加而增加,吸附热随吸附量增加而减小,CO2则相反。分子筛3A对CO2吸附量最小且CO2/H2O分离系数最大。H2O含量小于1%时,CO2吸附量最大的分子筛13X分离系数大于活性氧化铝,分子筛3A和13X适合分离低湿CO2/H2O。
中图分类号:
引用本文
任可欣, 鲁军辉, 王随林, 唐进京. 低湿CO2/H2O混合气体吸附特性实验[J]. 化工进展, 2022, 41(12): 6698-6710.
REN Kexin, LU Junhui, WANG Suilin, TANG Jinjing. Adsorption characteristics of CO2/H2O with low humidity[J]. Chemical Industry and Engineering Progress, 2022, 41(12): 6698-6710.
| 吸附剂 | 组分 | M/mol·kg-1 | K/kPa-1 | Rave/% | ||
|---|---|---|---|---|---|---|
| 303K | 313K | |||||
| 活性炭 | CO2 | 1.108 | 0.083 | 2.291 | 3.018 | |
| H2O | 25.709 | 0.333 | 0.051 | 7.561 | 7.638 | |
| CO2,H2O | 0.452 | 0.010 | — | — | ||
| 活性氧化铝 | CO2 | 1.110 | 0.025 | 2.216 | 1.447 | |
| H2O | 9.878 | 0.096 | 0.188 | 2.411 | 5.476 | |
| CO2,H2O | 0.578 | 0.125 | — | — | ||
| 分子筛3A | CO2 | 1.078 | 0.003 | 3.137 | 7.576 | |
| H2O | 12.747 | 0.014 | 0.021 | 1.192 | 3.809 | |
| CO2,H2O | 0.019 | 1.415 | — | — | ||
| 分子筛13X | CO2 | 1.008 | 0.010 | 3.089 | 0.881 | |
| H2O | 11.088 | 0.038 | 0.082 | 0.742 | 1.951 | |
| CO2,H2O | 2.688 | 0.026 | — | — | ||
表1 双组分吸附平衡R-LBET模型拟合参数
| 吸附剂 | 组分 | M/mol·kg-1 | K/kPa-1 | Rave/% | ||
|---|---|---|---|---|---|---|
| 303K | 313K | |||||
| 活性炭 | CO2 | 1.108 | 0.083 | 2.291 | 3.018 | |
| H2O | 25.709 | 0.333 | 0.051 | 7.561 | 7.638 | |
| CO2,H2O | 0.452 | 0.010 | — | — | ||
| 活性氧化铝 | CO2 | 1.110 | 0.025 | 2.216 | 1.447 | |
| H2O | 9.878 | 0.096 | 0.188 | 2.411 | 5.476 | |
| CO2,H2O | 0.578 | 0.125 | — | — | ||
| 分子筛3A | CO2 | 1.078 | 0.003 | 3.137 | 7.576 | |
| H2O | 12.747 | 0.014 | 0.021 | 1.192 | 3.809 | |
| CO2,H2O | 0.019 | 1.415 | — | — | ||
| 分子筛13X | CO2 | 1.008 | 0.010 | 3.089 | 0.881 | |
| H2O | 11.088 | 0.038 | 0.082 | 0.742 | 1.951 | |
| CO2,H2O | 2.688 | 0.026 | — | — | ||
| 1 | FU Wenfeng, WANG Lanjing, YANG Yongping. Optimal design for double reheat coal-fired power plants with post-combustion CO2 capture: a novel thermal system integration with a carbon capture turbine[J]. Energy, 2021, 221: 119838. |
| 2 | GUAN Yuru, SHAN Yuli, HUANG Qi, et al. Assessment to China’s recent emission pattern shifts[J]. Earth’s Future, 2021, 9(11). |
| 3 | GOUEDARD C, PICQ D, LAUNAY F, et al. Amine degradation in CO2 capture. I. A review[J]. International Journal of Greenhouse Gas Control, 2012, 10: 244-270. |
| 4 | WANG M, LAWAL A, STEPHENSON P, et al. Post-combustion CO2 capture with chemical absorption: a state-of-the-art review[J]. Chemical Engineering Research and Design, 2011, 89(9): 1609-1624. |
| 5 | GUO Liejin, JIN Hui. Boiling coal in water: hydrogen production and power generation system with zero net CO2 emission based on coal and supercritical water gasification[J]. International Journal of Hydrogen Energy, 2013, 38(29): 12953-12967. |
| 6 | CHEN Zhewen, ZHANG Xiaosong, HAN Wei, et al. A power generation system with integrated supercritical water gasification of coal and CO2 capture[J]. Energy, 2018, 142: 723-730. |
| 7 | PFAFF I, OEXMANN J, KATHER A. Optimised integration of post-combustion CO2 capture process in greenfield power plants[J]. Energy, 2010, 35(10): 4030-4041. |
| 8 | WEBLEY Paul A. Adsorption technology for CO2 separation and capture: a perspective[J]. Adsorption, 2014, 20(2/3): 225-231. |
| 9 | BEN-MANSOUR R, HABIB M A, BAMIDELE O E, et al. Carbon capture by physical adsorption: materials, experimental investigations and numerical modeling and simulations—A review[J]. Applied Energy, 2016, 161: 225-255. |
| 10 | LU Junhui, CAO Haishan, LI Junming. Energy and cost estimates for separating and capturing CO2 from CO2/H2O using condensation coupled with pressure/vacuum swing adsorption[J]. Energy, 2020, 202: 117604. |
| 11 | 赵振国. 吸附作用应用原理[M]. 北京: 化学工业出版社, 2005. |
| ZHAO Zhenguo. Application principle of adsorption[M]. Beijing: Chemical Industry Press, 2005. | |
| 12 | 近藤精一, 石川达雄, 安部郁夫. 吸附科学[M]. 李国希, 译. 北京: 化学工业出版社, 2006. |
| SEIICHI Kondo, TATSUO Ishikawa, IKUO Abe. Science of adsorption[M]. LI Guoxi, trans. Beijing: Chemical Industry Press, 2006. | |
| 13 | LIU X J, SHI Y F, KALBASSI M A, et al. A comprehensive description of water vapor equilibriums on alumina F-200: adsorption, desorption, and H2O/CO2 binary adsorption[J]. Separation and Purification Technology, 2014, 133: 276-281. |
| 14 | QASEM N A, BEN-MANSOUR R. Adsorption breakthrough and cycling stability of carbon dioxide separation from CO2/N2/H2O mixture under ambient conditions using 13X and Mg-MOF-74[J]. Applied Energy, 2018, 230: 1093-1107. |
| 15 | LI G, XIAO P, WEBLEY P A, et al. Competition of CO2/H2O in adsorption based CO2 capture[J]. Energy Procedia, 2009, 1(1): 1123-1130. |
| 16 | DANTAS T L, LUNA F M, SILVA I J, et al. Carbon dioxide-nitrogen separation through adsorption on activated carbon in a fixed bed[J]. Chemical Engineering Journal, 2011, 169(1/2/3): 11-19. |
| 17 | LI Gang, XIAO Penny, WEBLEY Paul. Binary adsorption equilibrium of carbon dioxide and water vapor on activated alumina[J]. Langmuir, 2009, 25(18): 10666-10675. |
| 18 | REGE S U, YANG R T. A novel FTIR method for studying mixed gas adsorption at low concentrations: H2O and CO2 on NaX zeolite and γ-alumina[J]. Chemical Engineering Science, 2001, 56(12): 3781-3796. |
| 19 | LI Gang, XIAO Penny, XU Dong, et al. Dual mode roll-up effect in multicomponent non-isothermal adsorption processes with multilayered bed packing[J]. Chemical Engineering Science, 2011, 66(9): 1825-1834. |
| 20 | 唐进京, 鲁军辉, 李俊明, 等. CO2/H2O吸附分离特性研究[J]. 中国电机工程学报, 2022, 42(6): 2216-2228. |
| TANG Jinjing, LU Junhui, LI Junming, et al. Study on adsorption characteristics of CO2/H2O[J]. Proceedings of the CSEE, 2022, 42(6): 2216-2228. | |
| 21 | 岑旗钢. 活性炭材料吸附分离烟气中二氧化碳研究[D]. 杭州: 浙江大学, 2017. |
| CEN Qigang. Research on CO2 adsorption from flue gas by activated carbons[D]. Hangzhou: Zhejiang University, 2017. | |
| 22 | 徐冬. 真空变压吸附分离燃烧后电厂废气中的高湿二氧化碳[D]. 沈阳: 东北大学, 2011. |
| XU Dong. Post-combustion CO2 capture from high humidity flue gas by vacuum swing adsorption[D]. Shenyang: Northeastern University, 2011. | |
| 23 | 叶贞成, 杨亚超, 罗娜. 真空变压吸附捕集烟道气中二氧化碳数值模拟研究[J]. 计算机与应用化学, 2016, 33(1): 21-26. |
| YE Zhencheng, YANG Yachao, LUO Na. Simulation of vacuum pressure swing adsorption process for CO2 capture from flue gas[J]. Computers and Applied Chemistry, 2016, 33(1): 21-26. | |
| 24 | 祝显强, 刘应书, 杨雄, 等. 中间气两步充压对快速真空变压吸附制氧的影响[J]. 化工学报, 2016, 67(10): 4264-4272. |
| ZHU Xianqiang, LIU Yingshu, YANG Xiong, et al. Effect of two-step pressurization with intermediate gas on rapid vacuum pressure swing adsorption process for oxygen generation[J]. CIESC Journal, 2016, 67(10): 4264-4272. | |
| 25 | 欧阳少波, 徐绍平, 张俊杰, 等. N2/CH4在吸附剂上的动态吸附特性[J]. 化工进展, 2014, 33(10): 2546-2551. |
| OUYANG Shaobo, XU Shaoping, ZHANG Junjie, et al. Study on N2/CH4 adsorption property in the adsorbents[J]. Chemical Industry and Engineering Progress, 2014, 33(10): 2546-2551. | |
| 26 | GARCÍA S, GIL M V, MARTÍN C F, et al. Breakthrough adsorption study of a commercial activated carbon for pre-combustion CO2 capture[J]. Chemical Engineering Journal, 2011, 171(2): 549-556. |
| 27 | XU Dong, GUO Hua, LIU Hanqiang, et al. CO2 and H2O capture from high humidity flue gas by single multi-layer vacuum swing adsorption unit[J]. Advanced Materials Research, 2012, 529: 402-407. |
| 28 | LI Gang, XIAO Penny, WEBLEY Paul, et al. Capture of CO2 from high humidity flue gas by vacuum swing adsorption with zeolite 13X[J]. Adsorption, 2008, 14(2/3): 415-422. |
| 29 | KOLLE J M, FAYAZ M, SAYARI A. Understanding the effect of water on CO2 adsorption[J]. Chemical Reviews, American Chemical Society, 2021, 121(13): 7280-7345. |
| 30 | 杨祖保. 吸附剂原理与应用[M]. 马丽萍, 宁平, 田森林, 译. 北京: 高等教育出版社, 2010. |
| YANG Ralph T. Adsorbents fundamentals and applications[M]. MA Liping, NING Ping, TIAN Senlin, trans. Beijing: Higher Education Press, 2010. | |
| 31 | 刘亚敏, 彭蕾, 苏凤英, 等. 多孔胺基化氧化石墨烯基材料对CO2的吸附性能研究[J]. 化工学报, 2019, 70(5): 2016-2024. |
| LIU Yamin, PENG Lei, SU Fengying, et al. Study of CO2 adsorption on amine functionalized graphene oxide porous materials[J]. CIESC Journal, 2019, 70(5):2016-2024. | |
| 32 | WURZBACHER J A, GEBALD C, STEINFELD A. Separation of CO2 from air by temperature-vacuum swing adsorption using diamine-functionalized silica gel[J]. Energy & Environmental Science, 2011, 4(9): 3584-3592. |
| 33 | 胡苏阳, 刘鑫博, 唐建峰, 等. 13X沸石分子筛对低浓度CO2动态吸附的实验研究[J]. 化工进展, 2022, 41(1): 153-160. |
| HU Suyang, LIU Xinbo, TANG Jianfeng, et al. Dynamic adsorption of low concentration CO2 over 13X zeolite[J]. Chemical Industry and Engineering Progress, 2022, 41(1): 153-160. | |
| 34 | 倪佳, 孙雪艳, 税子怡, 等. 湿法再生CO2空气捕集材料的能耗与性能优化[J]. 化工学报, 2021, 72(3): 1409-1418. |
| NI Jia, SUN Xueyan, SHUI Ziyi, et al. Energy consumption and performance optimization of moisture swing sorbents for direct air capture of CO2 [J]. CIESC Journal, 2021,72(3):1409-1418. | |
| 35 | DONG B X, ZHANG S Y, LIU W L, et al. Gas storage and separation in a water-stable [CuI5BTT3]4- anion framework comprising a giant multi-prismatic nanoscale cage[J]. Chemical Communications, 2015, 51(26): 5691-5694. |
| 36 | 费业泰. 误差理论与数据处理[M]. 北京: 机械工业出版社, 2010. |
| FEI Yetai. Error analysis and data processing[M]. Beijing: China Machine Press, 2010. | |
| 37 | SIRCAR S, MOHR R, RISTIC C, et al. Isosteric heat of adsorption: theory and experiment[J]. The Journal of Physical Chemistry B, 1999, 103(31): 6539-6546. |
| 38 | SIRCAR Shivaji. Excess properties and thermodynamics of multicomponent gas adsorption[J]. Journal of the Chemical Society, Faraday Transactions 1: Physical Chemistry in Condensed Phases, 1985, 81(7): 1527-1540. |
| 39 | SILVERSTEIN R M, WEBSTER F X, KIEMLE D J. 有机化合物的波谱解析[M]. 上海: 华东理工大学出版社, 2007. |
| SILVERSTEIN R M, WEBSTER F X, KIEMLE D J. Spectrometric identification of organic compounds[M]. Shanghai: East China University of Science and Technology Press, 2007. | |
| 40 | HERREROS Bruno, KLINOWSKI Jacek. Influence of the source of silicon and aluminium in the hydrothermal synthesis of sodalite[J]. Journal of the Chemical Society, Faraday Transactions, 1995, 91(7): 1147-1154. |
| 41 | MA Y K, RIGOLET S, MICHELIN L, et al. Facile and fast determination of Si/Al ratio of zeolites using FTIR spectroscopy technique[J]. Microporous and Mesoporous Materials, 2021, 311: 110683. |
| 42 | WANG Cheng, CAO Liyun, HUANG Jianfeng. Influences of acid and heat treatments on the structure and water vapor adsorption property of natural zeolite[J]. Surface and Interface Analysis, 2017, 49(12): 1249-1255. |
| 43 | YANG Kaizhong, YANG Guang, WU Jingyi. Insights into the enhancement of CO2 adsorption on faujasite with a low Si/Al ratio: understanding the formation sequence of adsorption complexes[J]. Chemical Engineering Journal, 2021, 404: 127056. |
| 44 | AUERBACH S M, CARRADO K A, DUTTA P K. Handbook of zeolite science and technology[M]. Beijing: CRC Press, 2003. |
| 45 | 邹勇, 吴肇亮, 陆绍信, 等. 微孔炭质吸附剂吸附贮存天然气的最佳孔径研究[J]. 石油与天然气化工, 1997(1): 15-16. |
| ZOU Yong, WU Zhaoliang, LU Shaoxin, et al. Research on optimum pore diameter of micropore carbon for adsorption of storage natural gas[J]. Chemical Engineering of Oil and Gas, 1997, 26(1): 15-16. | |
| 46 | PERI J B. A model for the surface of γ-alumina1 [J]. The Journal of Physical Chemistry, 1965, 69(1): 220-230. |
| 47 | KOTOH Kenji, ENOEDA Mikio, MATSUI Tetsuya, et al. A multilayer model for adsorption of water on activated alumina in relation to adsorption potential[J]. Journal of Chemical Engineering of Japan, 1993, 26(4): 355-360. |
| 48 | MCCAFFERTY E, WIGHTMAN J P. Determination of the concentration of surface hydroxyl groups on metal oxide films by a quantitative XPS method[J]. Surface and Interface Analysis, 1998, 26(8): 549-564. |
| 49 | BOLIS Vera, BUSCO Claudia, UGLIENGO Piero. Thermodynamic study of water adsorption in high-silica zeolites[J]. The Journal of Physical Chemistry B, 2006, 110(30): 14849-4859. |
| 50 | MAURIN G, LLEWELLYN P L, BELL R G. Adsorption mechanism of carbon dioxide in faujasites: grand canonical Monte Carlo simulations and microcalorimetry measurements[J]. The Journal of Physical Chemistry B, 2005, 109(33): 16084-16091. |
| [1] | 戴欢涛, 曹苓玉, 游新秀, 徐浩亮, 汪涛, 项玮, 张学杨. 木质素浸渍柚子皮生物炭吸附CO2特性[J]. 化工进展, 2023, 42(S1): 356-363. |
| [2] | 崔守成, 徐洪波, 彭楠. 两种MOFs材料用于O2/He吸附分离的模拟分析[J]. 化工进展, 2023, 42(S1): 382-390. |
| [3] | 陈崇明, 陈秋, 宫云茜, 车凯, 郁金星, 孙楠楠. 分子筛基CO2吸附剂研究进展[J]. 化工进展, 2023, 42(S1): 411-419. |
| [4] | 许春树, 姚庆达, 梁永贤, 周华龙. 共价有机框架材料功能化策略及其对Hg(Ⅱ)和Cr(Ⅵ)的吸附性能研究进展[J]. 化工进展, 2023, 42(S1): 461-478. |
| [5] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [6] | 顾永正, 张永生. HBr改性飞灰对Hg0的动态吸附及动力学模型[J]. 化工进展, 2023, 42(S1): 498-509. |
| [7] | 李化全, 王明华, 邱贵宝. 硫酸酸解钙钛矿相精矿的行为[J]. 化工进展, 2023, 42(S1): 536-541. |
| [8] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [9] | 郭强, 赵文凯, 肖永厚. 增强流体扰动强化变压吸附甲硫醚/氮气分离的数值模拟[J]. 化工进展, 2023, 42(S1): 64-72. |
| [10] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [11] | 王晋刚, 张剑波, 唐雪娇, 刘金鹏, 鞠美庭. 机动车尾气脱硝催化剂Cu-SSZ-13的改性研究进展[J]. 化工进展, 2023, 42(9): 4636-4648. |
| [12] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [13] | 朱传强, 茹晋波, 孙亭亭, 谢兴旺, 李长明, 高士秋. 固体高分子脱硝剂选择性非催化还原NO x 特性[J]. 化工进展, 2023, 42(9): 4939-4946. |
| [14] | 杨莹, 侯豪杰, 黄瑞, 崔煜, 王兵, 刘健, 鲍卫仁, 常丽萍, 王建成, 韩丽娜. 利用煤焦油中酚类物质Stöber法制备碳纳米球用于CO2吸附[J]. 化工进展, 2023, 42(9): 5011-5018. |
| [15] | 张丽宏, 金要茹, 程芳琴. 煤气化渣资源化利用[J]. 化工进展, 2023, 42(8): 4447-4457. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||