| 1 |
ZHOU J B, ZHUANG X G, ALASTUEY A, et al. Geochemistry and mineralogy of coal in the recently explored Zhundong large coal field in the Junggar basin, Xinjiang province, China[J]. International Journal of Coal Geology, 2010, 82: 51-67.
|
| 2 |
张守玉, 陈川, 施大钟, 等. 高钠煤燃烧利用现状[J]. 中国电机工程学报, 2013, 33(5): 1-12.
|
|
ZHANG Shouyu, CHEN Chuan, SHI Dazhong, et al. Situation of combustion utilization of high sodium coal[J]. Proceedings of the CSEE, 2013, 33(5): 1-12.
|
| 3 |
LI J B, ZHU M M, ZHANG Z Z, et al. The mineralogy, morphology and sintering characteristics of ash deposits on a probe at different temperatures during combustion of blends of Zhundong lignite and a bituminous coal in a drop tube furnace[J]. Fuel Processing Technology, 2016, 149: 176-186.
|
| 4 |
陈晓东, 孔令学, 白进, 等. 高温气化条件下Na2O对煤灰中矿物质演化行为的影响[J]. 燃料化学学报, 2016, 44(3): 263-272.
|
|
CHEN Xiaodong, KONG Lingxue, BAI Jin, et al. Effect of Na2O on mineral transformation of coal ash under high temperature gasification condition[J]. Journal of Fuel Chemistry and Technology, 2016, 44(3): 263-272.
|
| 5 |
VASSILEV S V, KITANO K, TAKEDAB S, et al. Influence of mineral and chemical composition of coal ashes on their fusibility[J]. Fuel Processing Technology, 1995(45): 27-51.
|
| 6 |
WANG Y, XIANG Y, WANG D X, et al. Effect of sodium oxides in ash composition on ash fusibility[J]. Energy & Fuels, 2016, 30: 1437-1444.
|
| 7 |
ZHAO Y L, ZHANG Y M, BAO S X, et al. Effect of stone coal chemical composition on sintering behavior during roasting[J]. Industrial & Engineering Chemistry Research, 2014, 53: 157-163.
|
| 8 |
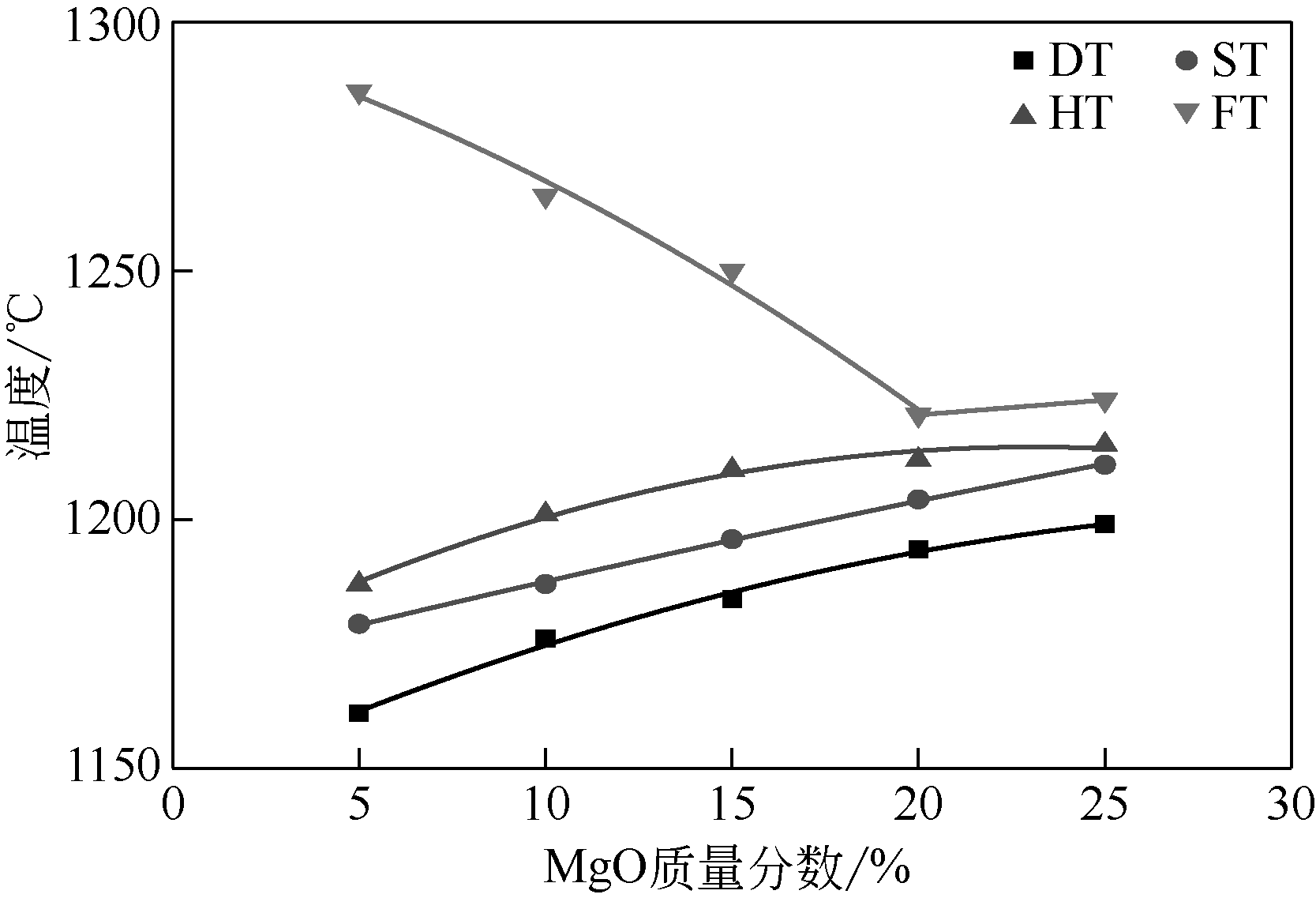
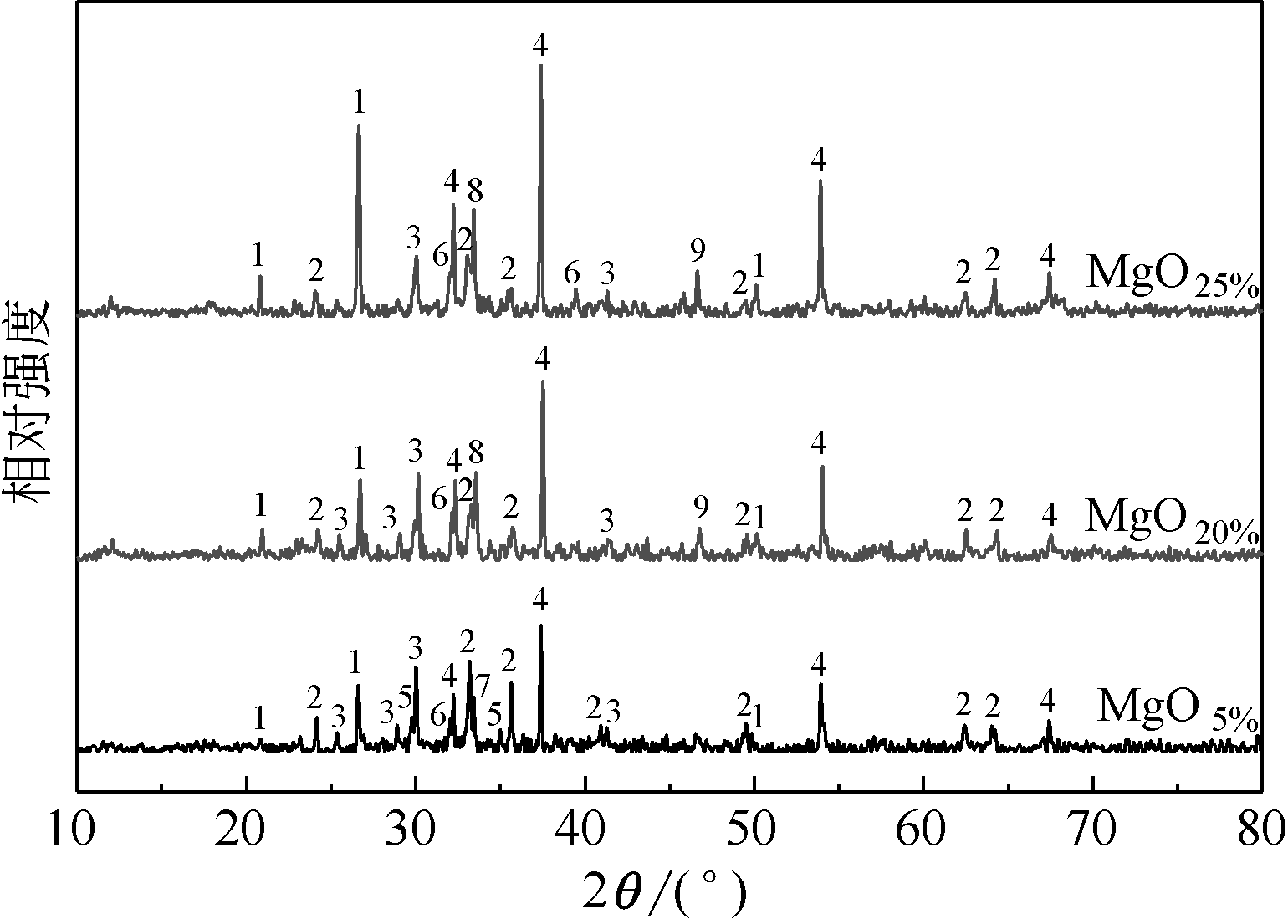
王东旭, 陈裕辉, 王洋, 等. MgO含量对高钠煤灰熔融特性的影响[J]. 化工进展, 2018, 37(4): 1392-1401.
|
|
WANG Dongxu, CHEN Yuhui, WANG Yang, et al. Effect of MgO content on the ash fusibility of high sodium coal[J]. Chemical Industry and Engineering Progress, 2018, 37(4): 1392-1401.
|
| 9 |
段锦, 李寒旭, 郝华东, 等. 钙镁复合助熔剂对长平煤灰熔融特性影响研究[J]. 硅酸盐通报, 2016, 35(12): 3936-3941.
|
|
DUAN Jin, LI Hanxu, HAO Huadong, et al. Effect of calcium and magnesium compound flux on Changping coal ash fusibility[J]. Bulletin of the Chinese Ceramic Society, 2016, 35(12): 3936-3941.
|
| 10 |
马志斌, 白宗庆, 白进, 等. 高温弱还原气氛下高硅铝比煤灰变化行为的研究[J]. 燃料化学学报, 2012, 40(3): 279-285.
|
|
MA Zhibin, BAI Zongqing, BAI Jin, et al. Evolution of coal ash with high Si/Al ratio under reducing atmosphere at high temperature[J]. Journal of Fuel Chemistry and Technology, 2012, 40(3): 279-285.
|
| 11 |
RAASK E. Mineral impurities in coal combustion: behavior, problems, and remedial measures[M]. Bristol: Hemisphere Pulishing, 1985: 160-166.
|
| 12 |
XU J, LIU X, ZHAO F, et al. Study on fusibility and flow behavior of high-calcium coal ash[J]. Journal of Chemical Engineering of Japan, 2014, 47(9): 711-716.
|
| 13 |
SONG W J, TANG L H, ZHU X D, et al. Prediction of Chinese coal ash fusion temperatures in Ar and H2 atmospheres[J]. Energy & Fuels, 2009, 23: 1990-1997.
|
| 14 |
PRONOBIS M. Evaluation of the influence of biomass co-combustion on boiler furnace slagging by means of fusibility correlations[J]. Biomass & Bioenergy, 2005, 28(4): 375-383.
|
| 15 |
郑烨, 马志斌, 关彦军, 等. 煤矸石灰添加对准东煤灰熔融特性影响[J]. 化工进展, 2019, 38(4): 1714-1720.
|
|
ZHENG Ye, MA Zhibin, GUAN Yanjun, et al. Effect of coal gangue ash addition on the fusibility of Zhundong coal ashes[J]. Chemical Industry and Engineering Progress, 2019, 38(4): 1714-1720.
|
| 16 |
王勤辉, 景妮洁, 骆仲泱, 等. 灰成分影响煤灰烧结温度的实验研究[J]. 煤炭学报, 2010, 35(6): 1015-1020.
|
|
WANG Qinhui, JING Nijie, LUO Zhongyang, et al. Experiments on the effect of chemical components of coal ash on the sintering temperature[J]. Journal of China Coal Society, 2010, 35(6): 1015-1020.
|
| 17 |
范建勇,周永刚,李培, 等. 准东煤灰熔融温度表征结渣特性的试验研究[J]. 煤炭学报, 2013, 38(s2): 478-482.
|
|
FAN Jianyong, ZHOU Yonggang, LI Pei, et al. Research on Zhundong coal’s ash melting temperature characterizing its slagging characteristics[J]. Journal of China Coal Society, 2013, 38(s2): 478-482.
|
| 18 |
付子文, 王长安, 车得福, 等. 成灰温度对准东煤灰理化特性影响的实验研究[J]. 工程热物理学报, 2014, 35(3): 609-613.
|
|
FU Ziwen, WANG Changan, CHE Defu, et al. Experimental study on the effect of ashing temperature on physicochemical properties of Zhundong coal ashes[J]. Journal of Engineering Thermophysics, 2014, 35(3): 609-613.
|
| 19 |
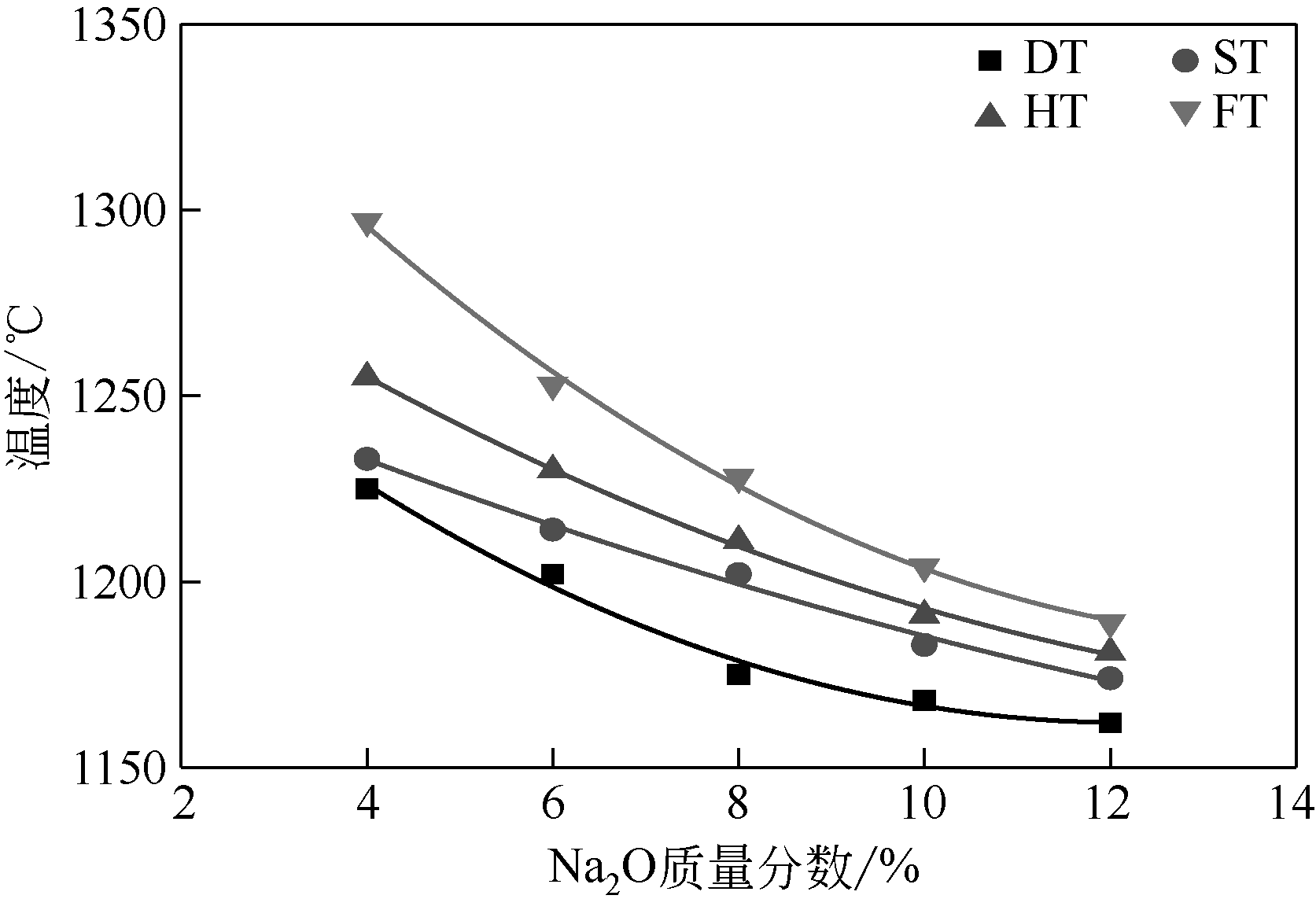
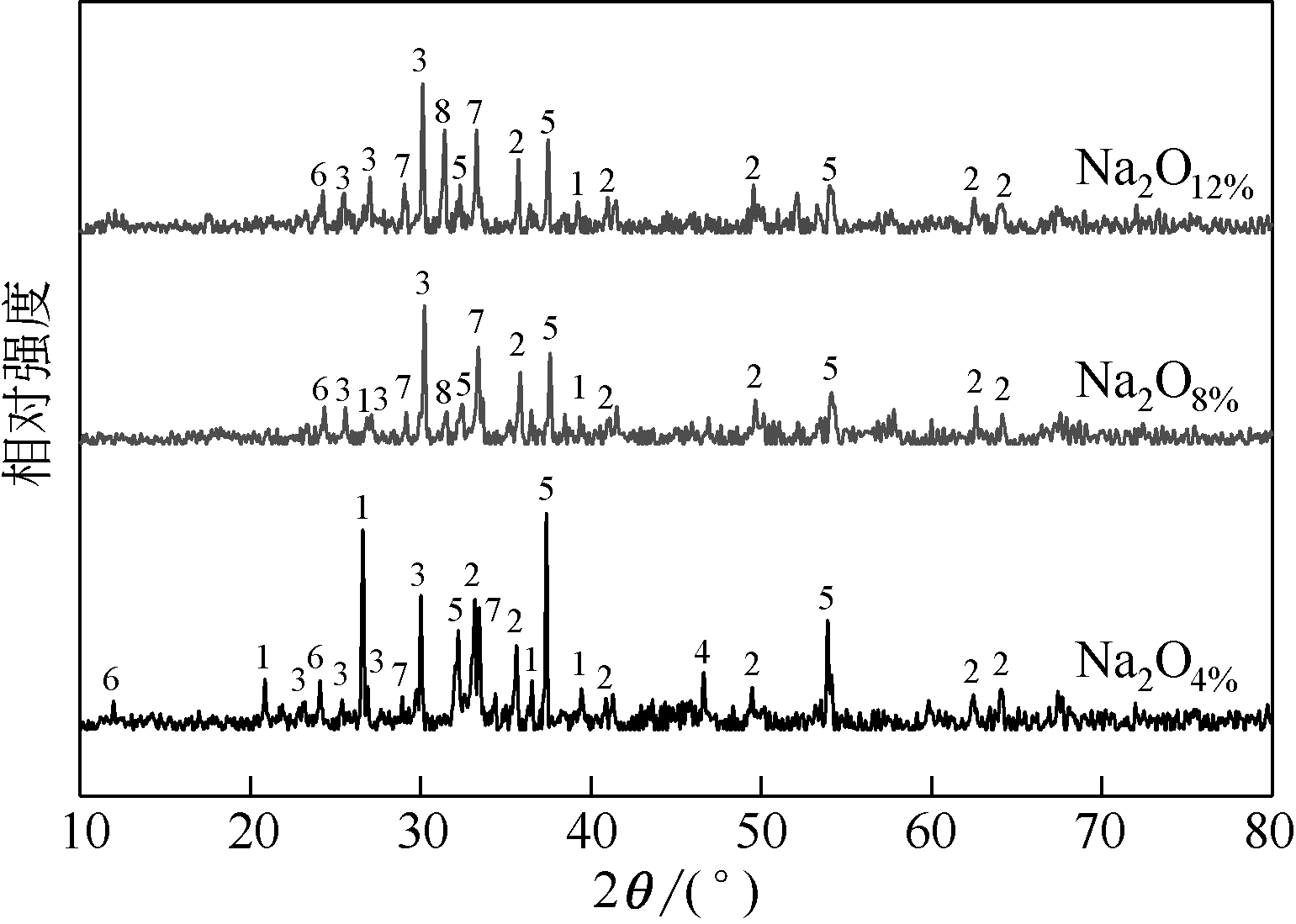
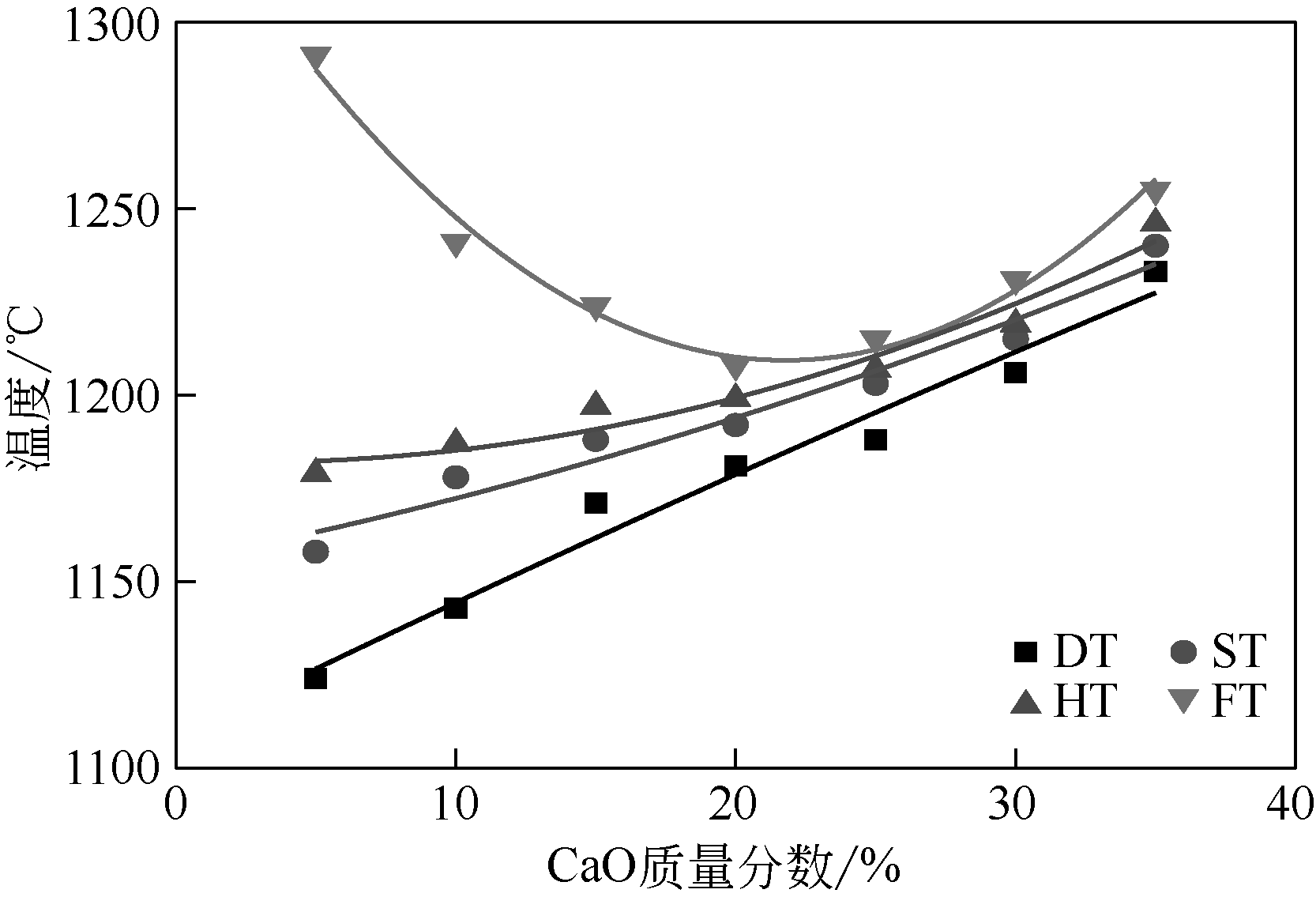
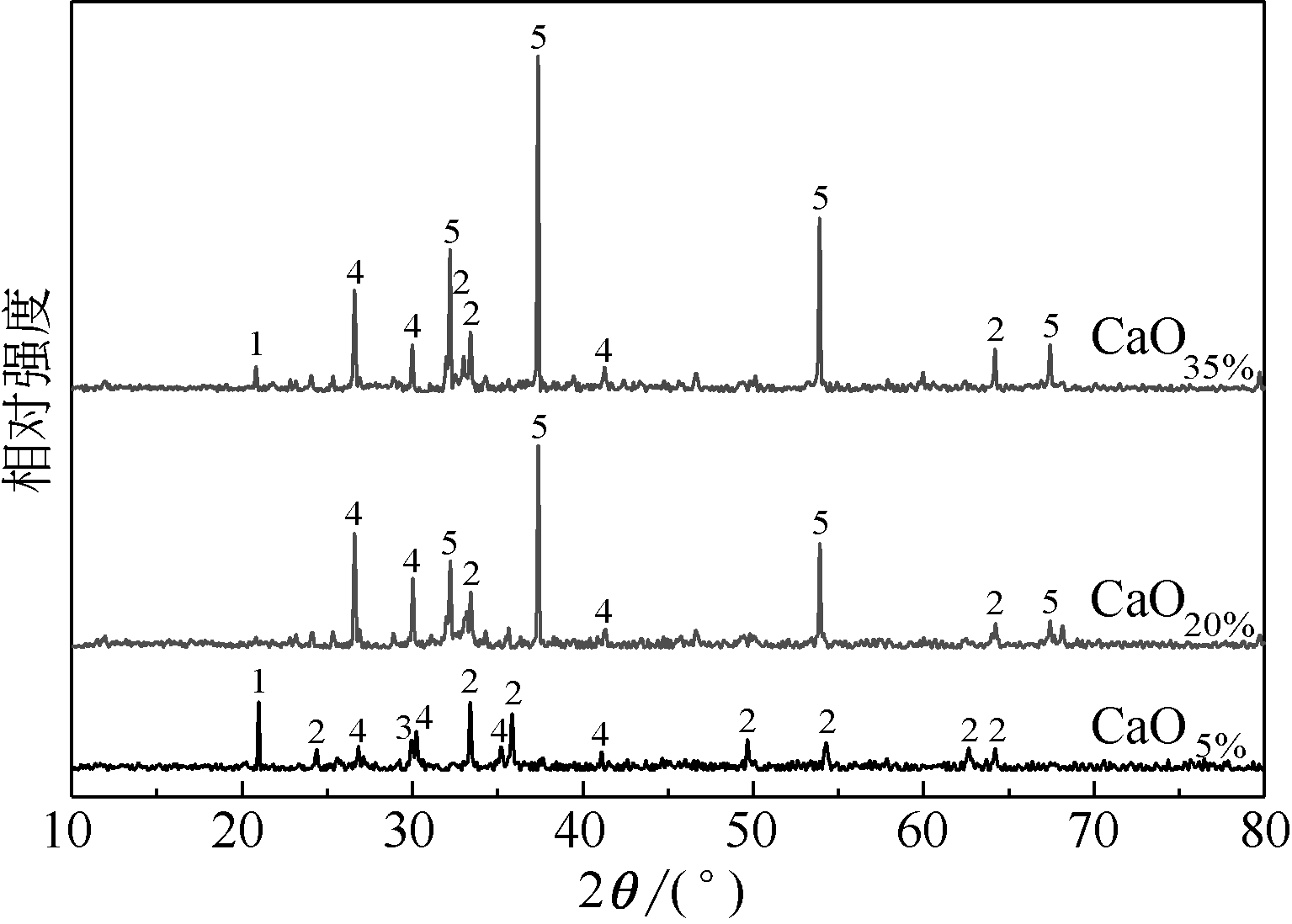
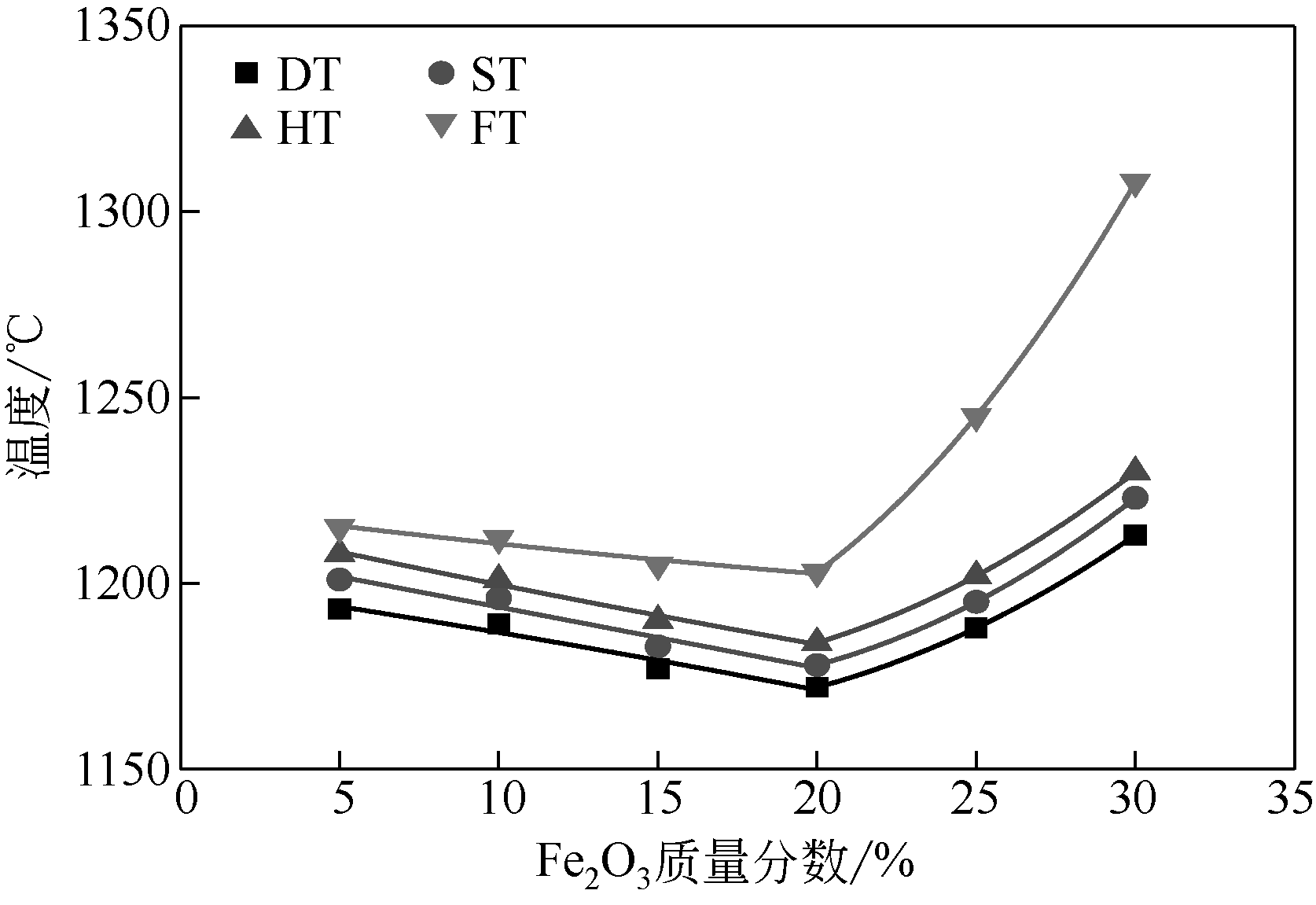
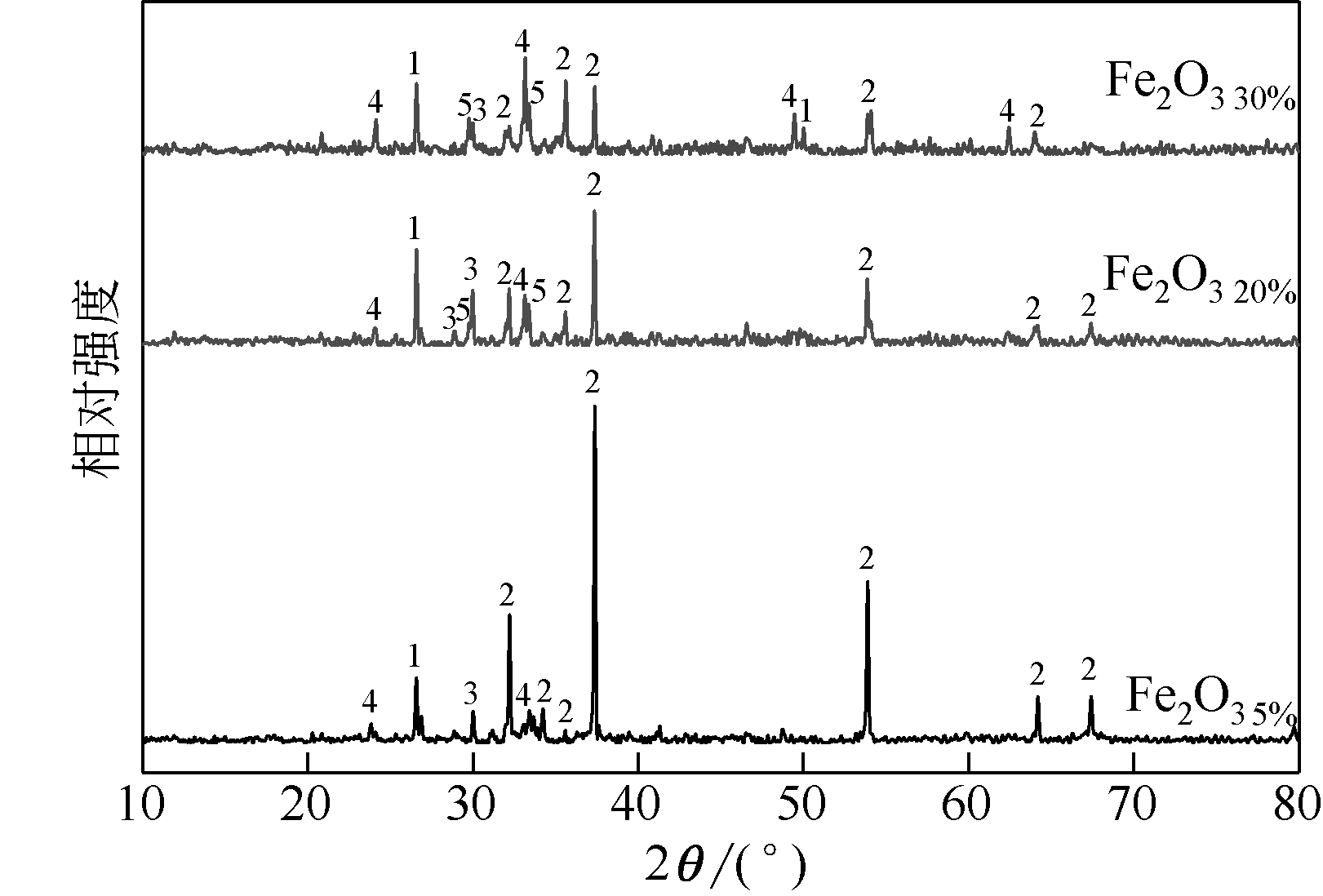
王东旭, 祁超, 王洋. 等. CaO含量对高钠煤灰熔融特性的影响[J]. 燃料化学学报, 2017, 45(9): 1025-1034.
|
|
WANG Dongxu, QI Chao, WANG Yang, et al. Effect of CaO content on the ash fusibility of high sodium coal[J]. Journal of Fuel Chemistry and Technology, 2017, 45(9): 1025-1034.
|
| 20 |
LI W D, LI M, LI W F, et al. Study on the ash fusion temperatures of coal and sewage sludge mixtures[J]. Fuel, 2010, 89: 1566-1572.
|
| 21 |
YAN T G, KONG L X, BAI J, et al. Thermo mechanical analysis of coal ash fusion behavior[J]. Chemical Engineering Science, 2016, 147: 74-82.
|
 ),李建波2,关彦军1,杨凤玲3,张锴1(
),李建波2,关彦军1,杨凤玲3,张锴1( ),程芳琴3
),程芳琴3
 ),Jianbo LI2,Yanjun GUAN1,Fengling YANG3,Kai ZHANG1(
),Jianbo LI2,Yanjun GUAN1,Fengling YANG3,Kai ZHANG1( ),Fangqin CHENG3
),Fangqin CHENG3