化工进展 ›› 2022, Vol. 41 ›› Issue (S1): 571-579.DOI: 10.16085/j.issn.1000-6613.2022-1049
可见光催化降解水中卤代有机污染物的研究进展
- 1.北京林业大学环境科学与工程学院,北京 100083
2.北京市水科学技术研究院,北京 100048
-
收稿日期:2022-06-06修回日期:2022-07-07出版日期:2022-10-20发布日期:2022-11-10 -
通讯作者:马伟芳 -
作者简介:刘怡璇(1999—),女,硕士研究生,研究方向为光催化。E-mail:504612986@qq.com。 -
基金资助:北京市科技计划(Z211100004321001)
Research progress on degradation of halogenated organic contaminants in water by visible light photocatalysis
LIU Yixuan1( ), LIN Yuechao2, MA Weifang1(
), LIN Yuechao2, MA Weifang1( )
)
- 1.College of Environmental Science and Engineering, Beijing Forestry University, Beijing 100083, China
2.Beijing Water Science and Technology Research Institute, Beijing 100048, China
-
Received:2022-06-06Revised:2022-07-07Online:2022-10-20Published:2022-11-10 -
Contact:MA Weifang
摘要:
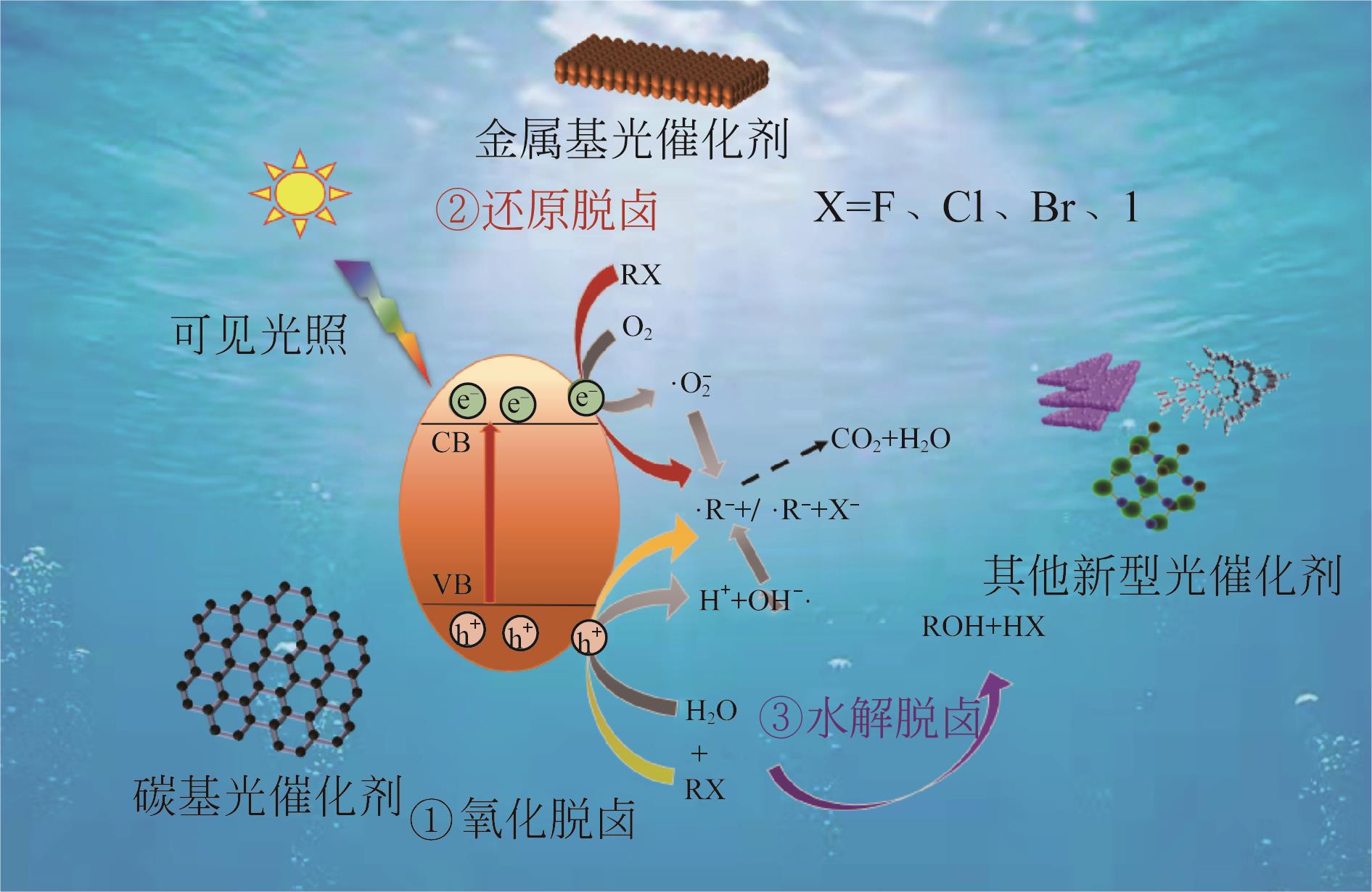
卤代有机污染物(HOCs)在水环境中检出频率高达45%,由于其具有毒性大、持久性强和易累积等特点,该类污染物引发的环境问题已引起越来越多的关注。可见光催化技术具有高效太阳能利用率、强选择性及反应条件温和、处理费用低等优点,对于降解水中HOCs具有独特的处理优势,因此近年来被广泛研究。本文梳理了可见光催化降解水中HOCs的核心脱卤机制,包括氧化脱卤、还原脱卤和水解脱卤,在脱卤机制的基础上汇总了三大类主流催化剂的脱卤贡献率,主要包括金属基光催化剂、碳基光催化剂及其他新型光催化剂三类光催化材料。基于可见光作用下降解水中HOCs的应用案例分析,探讨了光催化反应过程中的主要影响因素是溶液pH、催化剂用量及反应温度等。可见光催化降解去除效率高是本技术的核心优势,但由于催化剂的成本高和选择性差导致了其无法大规模应用,未来可见光催化材料设计应向成本低廉、精准匹配污染物从而实现高选择性的方向改进。
中图分类号:
引用本文
刘怡璇, 林跃朝, 马伟芳. 可见光催化降解水中卤代有机污染物的研究进展[J]. 化工进展, 2022, 41(S1): 571-579.
LIU Yixuan, LIN Yuechao, MA Weifang. Research progress on degradation of halogenated organic contaminants in water by visible light photocatalysis[J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 571-579.
| 溯源地 | HOCs名称 | 污染物检出浓度 /ng·L-1 | 参考 文献 |
|---|---|---|---|
| 太湖生态保护引领区 | 有机氯农药 | 22.03~166.24 | [ |
| 北江中上游地表水 | 多氯联苯 | 0.81~287.5 | [ |
| 洞庭湖及入湖河流 | 多氯联苯 | 0.077~10 | [ |
| 北京玉渊潭水体 | 多溴联苯醚 | 3.45~11.89 | [ |
| 贵州草海湖泊水体 | 全氟化合物 | 3.17~16.33 | [ |
| 千岛湖(新安江水库) | 全氟化合物 | 1.70~6.21 | [ |
| 沱江流域 | 全氟/多氟化合物 | 12.5~3789 | [ |
| 渤海湾天津滨海区 | 多溴联苯醚 | 43.8~94.3 | [ |
| 白洋淀水体 | 有机氯农药 | 0.05~24.01 | [ |
| 汕尾近岸水体 | 有机氯农药 | 0.26~6.77 | [ |
表1 国内部分地区地表水HOCs含量
| 溯源地 | HOCs名称 | 污染物检出浓度 /ng·L-1 | 参考 文献 |
|---|---|---|---|
| 太湖生态保护引领区 | 有机氯农药 | 22.03~166.24 | [ |
| 北江中上游地表水 | 多氯联苯 | 0.81~287.5 | [ |
| 洞庭湖及入湖河流 | 多氯联苯 | 0.077~10 | [ |
| 北京玉渊潭水体 | 多溴联苯醚 | 3.45~11.89 | [ |
| 贵州草海湖泊水体 | 全氟化合物 | 3.17~16.33 | [ |
| 千岛湖(新安江水库) | 全氟化合物 | 1.70~6.21 | [ |
| 沱江流域 | 全氟/多氟化合物 | 12.5~3789 | [ |
| 渤海湾天津滨海区 | 多溴联苯醚 | 43.8~94.3 | [ |
| 白洋淀水体 | 有机氯农药 | 0.05~24.01 | [ |
| 汕尾近岸水体 | 有机氯农药 | 0.26~6.77 | [ |
| 类别 | 名称 | 光源/波长 | HOCs种类 | HOCs浓度 /mol·L-1 | 反应 时间/h | 降解 效率/% | 参考文献 |
|---|---|---|---|---|---|---|---|
| 金属基光催化剂 | Pd/TiO2 | 300W氙灯/>400nm | 4-溴代二苯 | 2×10-5 | 0.5 | 100 | [ |
| (BiO)4CO3(OH)2/Bi2O2CO3 | 300W氙灯/>365nm | 4-氯苯酚 | 3.8×10-5 | 2 | 80 | [ | |
| Bi/Bi2WO6 | 300W氙灯/>420nm | 五氯酚钠 | 3.5×10-5 | 5 | 90 | [ | |
| Fe3O4/TiO2 | 125W汞灯/400~600nm | 2,4-二氯苯酚 | 3.1×10-4 | 0.8 | 92 | [ | |
| AgI/ TiO2 | 300W氙灯/>400nm | 十溴联苯醚 | 1×10-5 | 1 | 93 | [ | |
| TiO2/Cu2O | 300W氙灯/400~770nm | 四溴联苯醚 | 1×10-5 | 2 | 95 | [ | |
| Bi5O7I/ZnO | 500W氙灯/>420nm | 全氟辛酸 | 2.4×10-6 | 6 | 91 | [ | |
| 碳基光催化剂 | Ni-g-C3N4 | 300W氙灯/>420nm | 四溴联苯醚 | — | 24 | 94.5 | [ |
| Pd/HR-g- C3N4 | 300W氙灯/>400nm | 六溴环十二烷 | 1.6×10-6 | 1 | 92.6 | [ | |
| C/g-C3N4 | 1000W氙灯/>400nm | 三溴甲烷 | 4×10-6 | 1 | 51.8 | [ | |
| Pd /g-C3N4 | 300W氙灯/>400nm | 四溴二苯醚 | — | 2 | 100 | [ | |
| Fe3O4-g-C3N4 | 300W氙灯/>400nm | 多溴二苯醚 | 1×10-5 | 2.5 | 100 | [ | |
| PTH/SWNT | LED灯/420nm | 溴苯腈 | 6×10-5 | 16 | 99 | [ | |
| 其他新型光催化剂 | COFs/PTH | LED灯/420nm | 单溴联苯醚 | 3.3×10-6 | 24 | 85 | [ |
| COF-JLU22 | LED灯/520nm | 苯基溴 | 2.7×10-4 | 6 | 85 | [ | |
| PTBC-por COF | 150W氙灯/400~780nm | 2-溴苯乙酮 | 4×10-5 | 2 | 82 | [ | |
| 陶瓷BCN | LED灯/420nm | 4-溴联苯 | 3.3×10-5 | 30 | 95 | [ |
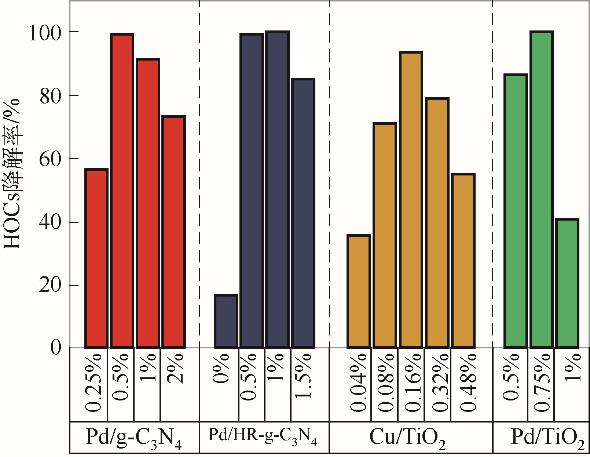
表2 可见光下不同催化剂对HOCs的催化性能
| 类别 | 名称 | 光源/波长 | HOCs种类 | HOCs浓度 /mol·L-1 | 反应 时间/h | 降解 效率/% | 参考文献 |
|---|---|---|---|---|---|---|---|
| 金属基光催化剂 | Pd/TiO2 | 300W氙灯/>400nm | 4-溴代二苯 | 2×10-5 | 0.5 | 100 | [ |
| (BiO)4CO3(OH)2/Bi2O2CO3 | 300W氙灯/>365nm | 4-氯苯酚 | 3.8×10-5 | 2 | 80 | [ | |
| Bi/Bi2WO6 | 300W氙灯/>420nm | 五氯酚钠 | 3.5×10-5 | 5 | 90 | [ | |
| Fe3O4/TiO2 | 125W汞灯/400~600nm | 2,4-二氯苯酚 | 3.1×10-4 | 0.8 | 92 | [ | |
| AgI/ TiO2 | 300W氙灯/>400nm | 十溴联苯醚 | 1×10-5 | 1 | 93 | [ | |
| TiO2/Cu2O | 300W氙灯/400~770nm | 四溴联苯醚 | 1×10-5 | 2 | 95 | [ | |
| Bi5O7I/ZnO | 500W氙灯/>420nm | 全氟辛酸 | 2.4×10-6 | 6 | 91 | [ | |
| 碳基光催化剂 | Ni-g-C3N4 | 300W氙灯/>420nm | 四溴联苯醚 | — | 24 | 94.5 | [ |
| Pd/HR-g- C3N4 | 300W氙灯/>400nm | 六溴环十二烷 | 1.6×10-6 | 1 | 92.6 | [ | |
| C/g-C3N4 | 1000W氙灯/>400nm | 三溴甲烷 | 4×10-6 | 1 | 51.8 | [ | |
| Pd /g-C3N4 | 300W氙灯/>400nm | 四溴二苯醚 | — | 2 | 100 | [ | |
| Fe3O4-g-C3N4 | 300W氙灯/>400nm | 多溴二苯醚 | 1×10-5 | 2.5 | 100 | [ | |
| PTH/SWNT | LED灯/420nm | 溴苯腈 | 6×10-5 | 16 | 99 | [ | |
| 其他新型光催化剂 | COFs/PTH | LED灯/420nm | 单溴联苯醚 | 3.3×10-6 | 24 | 85 | [ |
| COF-JLU22 | LED灯/520nm | 苯基溴 | 2.7×10-4 | 6 | 85 | [ | |
| PTBC-por COF | 150W氙灯/400~780nm | 2-溴苯乙酮 | 4×10-5 | 2 | 82 | [ | |
| 陶瓷BCN | LED灯/420nm | 4-溴联苯 | 3.3×10-5 | 30 | 95 | [ |
| 1 | RODAN B D, PENNINGTON D W, ECKLEY N, et al. Screening for persistent organic pollutants: techniques to provide a scientific basis for POPs criteria in international negotiations[J]. Environ. Sci. Technol., 1999, 33(20): 3482-3488. |
| 2 | LAINE D F, CHENG I F. The destruction of organic pollutants under mild reaction conditions: A review[J]. Microchemical Journal, 2007, 85(2): 183-193. |
| 3 | CABELLO-YEVES P J, PICAZO A, CAMACHO A, et al. Ecological and genomic features of two widespread freshwater picocyanobacteria[J]. Environmental Microbiology, 2018, 20(10): 3757-3771. |
| 4 | 陈燕, 任晓鸣, 邱阳, 等. 太湖生态保护引领区水体有机氯农药分布特征及生态风险评价[J]. 环境污染与防治, 2021, 43(7): 886-892. |
| CHEN Yan, REN Xiaoming, QIU Yang, et al. Distribution and risk assessment of organochlorine pesticide in waters of ecological protection leading: area in Taihu[J]. Environmental Pollution & Control, 2021, 43(7): 886-892. | |
| 5 | 昌盛, 白云松, 涂响, 等. 北江中上游地表水和沉积物中PAHs和PCBs污染特征和风险评估[J/OL]. 环境科学, 2022,doi:10.13227/j.hjkx.2022240 . |
| CHANG Sheng, BAI Yunsong, TU Xiang, et al. Pollution characteristics and risk assessment of PAHs and PCBs in surface water and sediments in middle and upper reaches of Beijiang River[J]. Environmental Science, 2022,doi:10.13227/j.hjkx.202202140 . | |
| 6 | 黄智峰, 郑丙辉, 尹大强, 等. 洞庭湖及入湖河流中209种多氯联苯同类物分布特征与风险评估[J]. 环境科学, 2022, 43(1): 363-368. |
| HUANG Zhifeng, ZHENG Binghui, YIN Daqiang, et al. Distribution characteristics and risk assessment of 209 polychlorinated biphenyls in Dongting Lake and the inflow rivers[J]. Environmental Science, 2022, 43(1): 363-368. | |
| 7 | 解琼玉. 北京城区典型地表水中有机卤素污染物的测定及其种态分布的研究[D]. 成都: 成都理工大学, 2014. |
| XIE Qiongyu.Study on the determianation and distribution of organohalogen in typical surface water of Beijing urban[D]. Chengdu: Chengdu University of Technology, 2014. | |
| 8 | 曾士宜, 杨鸿波, 彭洁, 等. 贵州草海湖泊表层水与沉积物中全氟化合物的污染特征及风险评估[J]. 环境化学, 2021, 40(4): 1193-1205. |
| ZENG Shiyi, YANG Hongbo, PENG Jie, et al. Pollution characteristics and risk assessment of perfluorinated compounds in surface water and sediments of Caohai Lake of Guizhou Province[J]. Environmental Chemistry, 2021, 40(4): 1193-1205. | |
| 9 | 张明, 唐访良, 程新良, 等. 千岛湖(新安江水库)表层水中全氟化合物的残留水平及分布特征[J]. 湖泊科学, 2020, 32(2): 337-345. |
| ZHANG Ming, TANG Fangliang, CHENG Xinliang, et al. Occurrence and distribution of perfluorinated compounds in surface water of Lake Qiandao( Xin'anjiang Reservoir)[J]. Journal of Lake Sciences, 2020, 32(2): 337-345. | |
| 10 | 宋娇娇, 汪艺梅, 孙静, 等. 沱江流域典型及新兴全氟/多氟化合物的污染特征及来源解析[J]. 环境科学, 2022, 43(9): 112-121. |
| SONG Jiaojiao, WANG Yimei, SUN Jing, et al. Pollution characteristics and source apportionment of typical and emerging per- and polyfluoroalkylated substances in Tuojiang River Basin [J].Environmental Science, 2022, 43(9): 112-121. | |
| 11 | 陈晓冉, 陈燕珍, 屠建波, 等. 渤海湾天津近岸典型海域PBDEs污染状况及分布规律研究[J].海洋环境科学, 2020, 39(3): 413-418. |
| CHEN Xiaoran, CHEN Yanzhen, TU Jianbo, et al. Pollution survey and distribution characteristics research of PBDEs in Tianjin typical coastal sea areas of Bohai Bay [J]. Marine Environmental Science, 2020, 39(3): 413-418. | |
| 12 | 段哲珊, 刘府延, 沈翔, 等. 白洋淀水体中有机氯农药的残留特征及其健康风险评估[J]. 安全与环境工程, 2021, 28(5): 161-175. |
| DUAN Zheshan, LIU Fuyan, SHEN Xiang, et al. Residual characteristics and health risk assessment of organochlorine pesticides in Baiyangdian water environment[J]. Safety and Environmental Engineering, 2021, 28(5): 161-175. | |
| 13 | 蔡一枝, 李发明, 刘凌峰, 等. 汕尾近岸水体和沉积物中有机氯农药的残留特征及生态风险评价[J]. 海洋环境科学, 2022, 41(3): 387-394. |
| CAI Yizhi, LI Faming, LIU Lingfeng, et al. Residue characteristics and ecological risk assessment of organochlorine pesticides in coastal waters and sediments of Shanwei[J]. Marine Environmental Science, 2022, 41(3): 387-394. | |
| 14 | MARK A K. Advances in greener separation processes-case study: Recovery of chlorinated aromatic compounds[J]. Green Chemistry, 2003, 5(3): 308-317. |
| 15 | KASTANEK F, MALETEROVA Y, KASTANEK P, et al. Complex treatment of wastewater and groundwater contaminated by halogenated organic compounds[J]. Desalination, 2007, 211(1/2/3): 261-271. |
| 16 | JIANG L B, XING Z, et al. Doping of graphitic carbon nitride for photocatalysis: a reveiw[J]. Applied Catalysis B:Environmental, 2017: 388-406. |
| 17 | SUN Q, LYU K, ZHANG Z, et al. Effect of contact interface between TiO2 and g-C3N4 on the photoreactivity of g-C3N4/TiO2 photocatalyst: (0 0 1) vs (1 0 1) facets of TiO2 [J]. Applied Catalysis B: Environmental, 2015, 164: 420-427. |
| 18 | JIANG L, YUAN X, ZENG G, et al. Metal-free efficient photocatalyst for stable visible-light photocatalytic degradation of refractory pollutant[J]. Applied Catalysis B: Environmental, 2018, 221: 715-25. |
| 19 | SHURBAJI S, HUONG P T, ALTAHTAMOUNI T M. Review on the visible light photocatalysis for the decomposition of ciprofloxacin, norfloxacin, tetracyclines, and sulfonamides antibiotics in wastewater[J]. Catalysts, 2021, 11(4): 437. |
| 20 | GUO W, ZOU J, GUO B, et al. Pd nanoclusters/TiO2(B) nanosheets with surface defects toward rapid photocatalytic dehalogenation of polyhalogenated biphenyls under visible light[J]. Applied Catalysis B: Environmental, 2020, 277: 119255. |
| 21 | XU Z, WANG F, ZHANG J, et al. In situ synthesis of p-n (BiO)4CO3(OH)2/Bi2O2CO3 internal polarized heterojunction for improved visible light photocatalytic performance[J]. Materials Research Express, 2020, 7(1): 015910. |
| 22 | DING X, ZHAO K, ZHANG L. Enhanced photocatalytic removal of sodium pentachlorophenate with self-doped Bi2WO6 under visible light by generating more superoxide ions[J]. Environ. Sci. Technol., 2014, 48(10): 5823-31. |
| 23 | DUBEY M, KUMAR R, SRIVASTAVA S K, et al. Visible light induced photodegradation of chlorinated organic pollutants using highly efficient magnetic Fe3O4/TiO2 nanocomposite[J]. Optik, 2021, 243: 167309. |
| 24 | SHAO Y Y, YE W D, SUN C Y, et al. Visible-light-induced degradation of polybrominated diphenyl ethers with AgI-TiO2 [J]. RSC Advances, 2017, 7(62): 39089-39095. |
| 25 | MING L, NAN W, ZHU L, et al. Photocatalytic reductive degradation of polybrominated diphenyl ethers on CuO/TiO2 nanocomposites: A mechanism based on the switching of photocatalytic reduction potential being controlled by the valence state of copper[J]. Applied Catalysis B: Environmental, 2016, 182: 414-423. |
| 26 | YANG Y, JI W, LI X, et al. Insights into the degradation mechanism of perfluorooctanoic acid under visible-light irradiation through fabricating flower-shaped Bi5O7I/ZnO n-n heterojunction microspheres[J]. Chemical Engineering Journal, 2021, 420: 129934. |
| 27 | WEI Y, GONG Y, ZHAO X, et al. Ligand directed debromination of tetrabromodiphenyl ether mediated by nickel under visible irradiation[J]. Environmental Science: Nano, 2019, 6(5): 1585-93. |
| 28 | WU M, LI Q, CHEN C, et al. Constructed palladium-anchored hollow-rod-like graphitic carbon nitride created rapid visible-light-driven debromination of hexabromocyclododecane[J]. Applied Catalysis B: Environmental, 2021, 297: 120409. |
| 29 | CHANG X, YAO X, DING N, et al. Photocatalytic degradation of trihalomethanes and haloacetonitriles on graphitic carbon nitride under visible light irradiation[J]. Sci Total Environ, 2019, 682: 200-207. |
| 30 | LEI M, WANG Z, ZHU L, et al. Complete debromination of 2,2′,4,4′-tetrabromodiphenyl ether by visible-light photocatalysis on g-C3N4 supported Pd[J]. Applied Catalysis B: Environmental, 2020, 261: 118236. |
| 31 | SHAO Y Y, YE W D, SUN C Y, et al. Enhanced photoreduction degradation of polybromodiphenyl ethers with Fe3O4-g-C3N4 under visible light irradiation[J]. RSC Advances, 2018, 8(20): 10914-10921. |
| 32 | GONZALEZ-MUNOZ D, MARTIN-SOMER A, STROBL K, et al. Enhancing visible-light photocatalysis via endohedral functionalization of single-walled carbon nanotubes with organic dyes[J]. ACS Applied Materials & Interfaces, 2021, 13(21): 24877-24886. |
| 33 | JIMÉNEZ-ALMARZA A, LÓPEZ-MAGANO A, CANO R, et al. Engineering covalent organic frameworks in the modulation of photocatalytic degradation of pollutants under visible light conditions[J]. Materials Today Chemistry, 2021, 22: 100548. |
| 34 | LI Z, ZHI Y, SHAO P, et al. Covalent organic framework as an efficient, metal-free, heterogeneous photocatalyst for organic transformations under visible light[J]. Applied Catalysis B: Environmental, 2019, 245: 334-342. |
| 35 | SHAN H, CAI D, ZHANG X, et al. Donor-acceptor type two-dimensional porphyrin-based covalent organic framework for visible-light-driven heterogeneous photocatalysis[J]. Chemical Engineering Journal, 2022, 432: 134288. |
| 36 | YUAN T, ZHENG M, ANTONIETTI M, et al. Ceramic boron carbonitrides for unlocking organic halides with visible light[J]. Chemical Science, 2021, 12(18): 6323-6332. |
| 37 | TYAGI H, CHAWLA H, BHANDARI H, et al. Recent-enhancements in visible-light photocatalytic degradation of organochlorines pesticides: a review[J]. Materials Today: Proceedings, 2022, 49: 3289-3305. |
| 38 | WANG Y, WEI Y, SONG W, et al. Photocatalytic hydrodehalogenation for the removal of halogenated aromatic contaminants[J]. ChemCatChem, 2019, 11(1): 258-268. |
| 39 | SAKAMOTO H, IMAI J, SHIRAISHI Y, et al. Photocatalytic dehalogenation of aromatic halides on Ta2O5-supported Pt–Pd bimetallic alloy nanoparticles activated by visible light[J]. ACS Catalysis, 2017, 7(8): 5194-5201. |
| 40 | LYU Y, CAO X, JIANG H, et al. Rapid photocatalytic debromination on TiO2 with in-situ formed copper co-catalyst: Enhanced adsorption and visible light activity[J]. Applied Catalysis B: Environmental, 2016, 194: 150-156. |
| 41 | ZHU F, LV Y, LI J, et al. Enhanced visible light photocatalytic performance with metal-doped Bi2WO6 for typical fluoroquinolones degradation: Efficiencies, pathways and mechanisms[J]. Chemosphere, 2020, 252: 126577. |
| 42 | 韩莉萍. 铋基材料的制备及其可见光催化应用[D]. 广州: 广东工业大学, 2021. |
| HAN Liping. Preparation of Bi-based materials for visible-light-driven photocatalytic applications[D]. Guangzhou: Guangdong University of Technology, 2021. | |
| 43 | LI H, SHANG H, LI Y, et al. Interfacial charging-decharging strategy for efficient and selective aerobic NO oxidation on oxygen vacancy[J]. Environmental Science & Technology, 2019, 53(12): 6964-6971. |
| 44 | WANG Y, ZHU Q, WEI Y, et al. Catalytic hydrodehalogenation over supported gold: Electron transfer versus hydride transfer[J]. Applied Catalysis B: Environmental, 2018, 231: 262-268. |
| 45 | CHEN C, SHI T, CHANG W, et al. Essential roles of proton transfer in photocatalytic redox reactions[J]. ChemCatChem, 2015, 7(5): 724-731. |
| 46 | WAN Z, MAO Q, CHEN Q. Proton-dependent photocatalytic dehalogenation activities caused by oxygen vacancies of In2O3 [J]. Chemical Engineering Journal, 2021, 403: 126389. |
| 47 | WANG Y, WEI Y, SONG W, et al. Photocatalytic hydrodehalogenation for the removal of halogenated aromatic contaminants[J]. ChemCatChem, 2019, 11(1): 258-268. |
| 48 | LONG X, CHEN W, LEI C, et al. Ultrafine Pd nanoparticles@g-C3N4 for highly efficient dehalogenation of chlorinated environmental pollutant: Structure, efficacy and mechanisms[J]. Science of The Total Environment, 2021, 775: 145178. |
| 49 | ONG W J, TAN L L, NG Y H, et al. Graphitic carbon nitride (g-C3N4)-based photocatalysts for artificial photosynthesis and environmental remediation: Are we a step closer to achieving sustainability?[J]. Chemical Reviews, 2016, 116(12): 7159-7329. |
| 50 | ZHAO Z, SUN Y, DONG F, et al. Template synthesis of carbon self-doped g-C3N4 with enhanced visible to near-infrared absorption and photocatalytic performance[J]. RSC Advances, 2015, 5(49): 39549-39556. |
| 51 | ZHENG Q, XU E, PARK E, et al. Looking at the overlooked hole oxidation: Photocatalytic transformation of organic contaminants on graphitic carbon nitride under visible light irradiation[J]. Applied Catalysis B: Environmental, 2019, 240: 262-269. |
| 52 | ONG C B, MOHAMMAD A W, NG L Y, et al. Solar photocatalytic and surface enhancement of ZnO/rGO nanocomposite: Degradation of perfluorooctanoic acid and dye[J]. Process Safety and Environmental Protection, 2017, 112: 298-307. |
| 53 | LUO Z, FANG Y, ZHOU M, et al. A borocarbonitride ceramic aerogel for photoredox catalysis[J]. Angewandte Chemie International Edition, 2019, 58(18): 6033-6037. |
| 54 | HAO J, WANG J, QIN S, et al. B/N co-doped carbon nanosphere frameworks as high-performance electrodes for supercapacitors [J]. Journal of Materials Chemistry A, 2018, 6(17): 8053-8058. |
| 55 | HAO Y, WANG S, SHAO Y, et al. High‐energy density Li‐ion capacitor with layered SnS2/reduced graphene oxide anode and BCN nanosheet cathode[J]. Advanced Energy Materials, 2020, 10(6): 1902836. |
| 56 | 张峰振, 吴超飞, 胡芸, 等. 卤代有机污染物的光化学降解[J].化学进展, 2014, 26(6): 1079-1098. |
| ZHANG Fengzhen, WU Chaofei, HU Yun, et al. Photochemical degradation of halogenated organic contaminants[J]. Progress in Chemistry, 2014, 26(6): 1079-1098. | |
| 57 | YANG W, YU W, RONGLIANG Q, et al. Reductive debromination and advanced oxidation of polybrominated diphenyl ethers (PBDEs) using zero-valent iron (ZVI) based materials[J]. Progress in Chemistry, 2018, 30(4): 420. |
| 58 | HE J, ZHANG Y, GUO Y, et al. Photocatalytic degradation of cephalexin by ZnO nanowires under simulated sunlight: Kinetics, influencing factors, and mechanisms[J]. Environment International, 2019, 132: 105105. |
| 59 | LI Y, ZHENG X, YANG J, et al. Enhanced photocatalytic degradation of 2, 4, 6-trichlorophenol and RhB with RhB-sensitized BiOClBr catalyst based on response surface methodology[J]. Journal of the Taiwan Institute of Chemical Engineers, 2021, 119: 213-223. |
| 60 | WANG Y, ZHU Q, WEI Y, et al. Catalytic hydrodehalogenation over supported gold: electron transfer versus hydride transfer[J]. Applied Catalysis B: Environmental, 2018, 231: 262-268. |
| 61 | CHOWDHURY P, MOREIRA J, GOMAA H, et al. Visible-solar-light-driven photocatalytic degradation of phenol with dye-sensitized TiO2: parametric and kinetic study[J]. Industrial & Engineering Chemistry Research, 2012, 51(12): 4523-4532. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [6] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [7] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [8] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| [9] | 张启, 赵红, 荣峻峰. 质子交换膜燃料电池中氧还原反应抗毒性电催化剂研究进展[J]. 化工进展, 2023, 42(9): 4677-4691. |
| [10] | 葛全倩, 徐迈, 梁铣, 王凤武. MOFs材料在光电催化领域应用的研究进展[J]. 化工进展, 2023, 42(9): 4692-4705. |
| [11] | 王伟涛, 鲍婷玉, 姜旭禄, 何珍红, 王宽, 杨阳, 刘昭铁. 醛酮树脂基非金属催化剂催化氧气氧化苯制备苯酚[J]. 化工进展, 2023, 42(9): 4706-4715. |
| [12] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [13] | 向阳, 黄寻, 魏子栋. 电催化有机合成反应的活性和选择性调控研究进展[J]. 化工进展, 2023, 42(8): 4005-4014. |
| [14] | 王耀刚, 韩子姗, 高嘉辰, 王新宇, 李思琪, 杨全红, 翁哲. 铜基催化剂电还原二氧化碳选择性的调控策略[J]. 化工进展, 2023, 42(8): 4043-4057. |
| [15] | 吕程远, 张函, 杨明旺, 杜健军, 樊江莉. 生物成像用二氧杂环丁烷余辉发光体系的研究进展[J]. 化工进展, 2023, 42(8): 4108-4122. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||