化工进展 ›› 2022, Vol. 41 ›› Issue (S1): 210-220.DOI: 10.16085/j.issn.1000-6613.2022-0241
Si改性对NiMo/Al2O3催化剂加氢脱硫性能的影响
郭振雪1( ), 于海斌2, 张国辉2, 张景成2, 卢雁飞2, 何艳贞1, 孙彦民2, 韩恩山1(
), 于海斌2, 张国辉2, 张景成2, 卢雁飞2, 何艳贞1, 孙彦民2, 韩恩山1( )
)
- 1.河北工业大学化工学院,天津 300401
2.中海油天津化工研究设计院有限公司,天津 300131
-
收稿日期:2022-02-16修回日期:2022-05-05出版日期:2022-10-20发布日期:2022-11-10 -
通讯作者:韩恩山 -
作者简介:郭振雪(1997—),女,硕士研究生,研究方向为加氢催化剂开发。E-mail:gzxxxue@163.com。 -
基金资助:中国海洋石油集团有限公司“十三五”重大专项(CNOOC-KJ 135 ZDXM 32 TJY 001 TJY 2020)
Effect of silica modification on the performance of NiMo/Al2O3 catalyst in hydrodesulfurization
GUO Zhenxue1( ), YU Haibin2, ZHANG Guohui2, ZHANG Jingcheng2, LU Yanfei2, HE Yanzhen1, SUN Yanmin2, HAN Enshan1(
), YU Haibin2, ZHANG Guohui2, ZHANG Jingcheng2, LU Yanfei2, HE Yanzhen1, SUN Yanmin2, HAN Enshan1( )
)
- 1.School of Chemical Engineering and Technology, Hebei University of Technology, Tianjin 300401, China
2.CenerTech Tianjin Chemical Research and Design Institute Limited Company, Tianjin 300131, China
-
Received:2022-02-16Revised:2022-05-05Online:2022-10-20Published:2022-11-10 -
Contact:HAN Enshan
摘要:
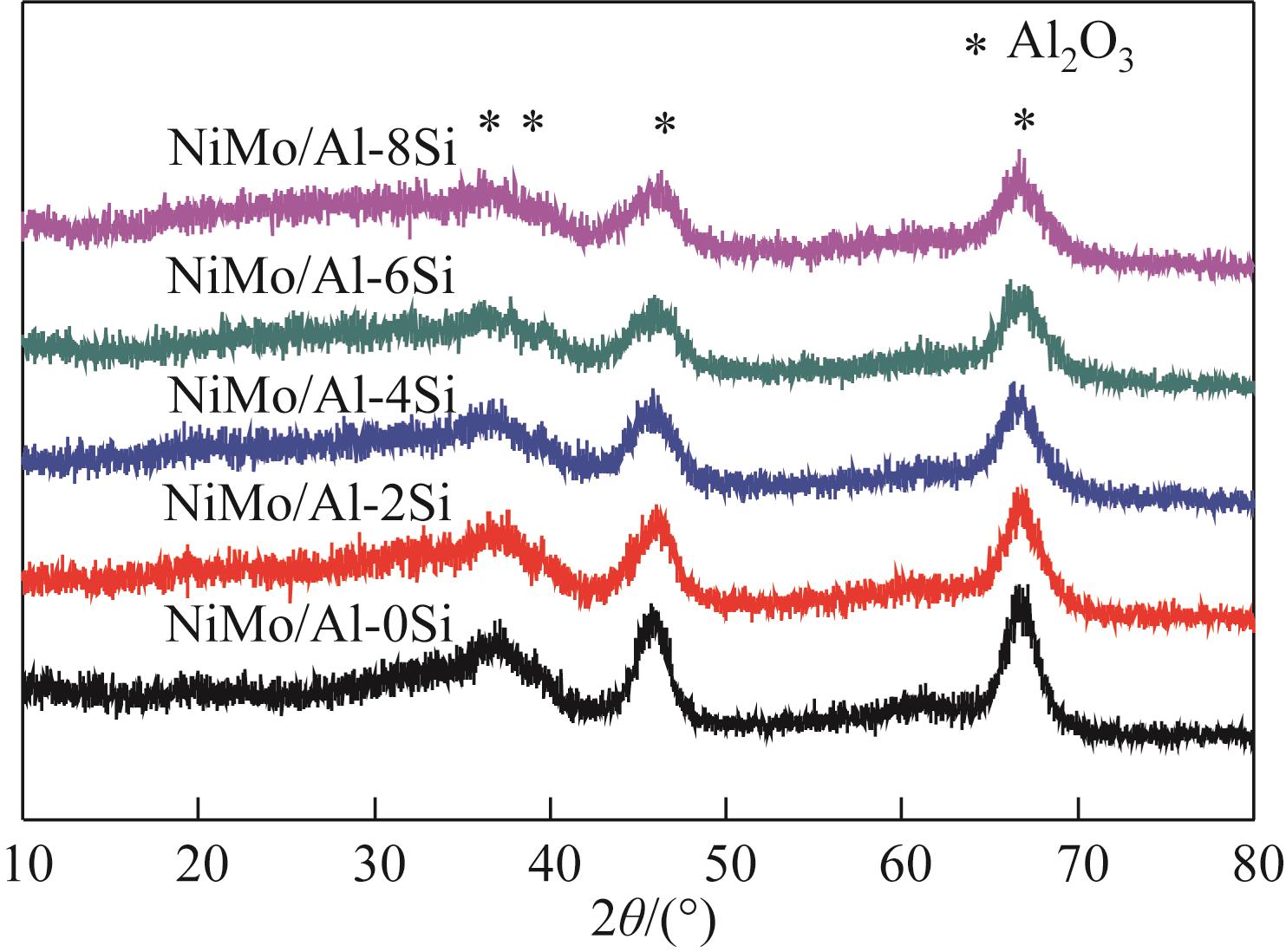
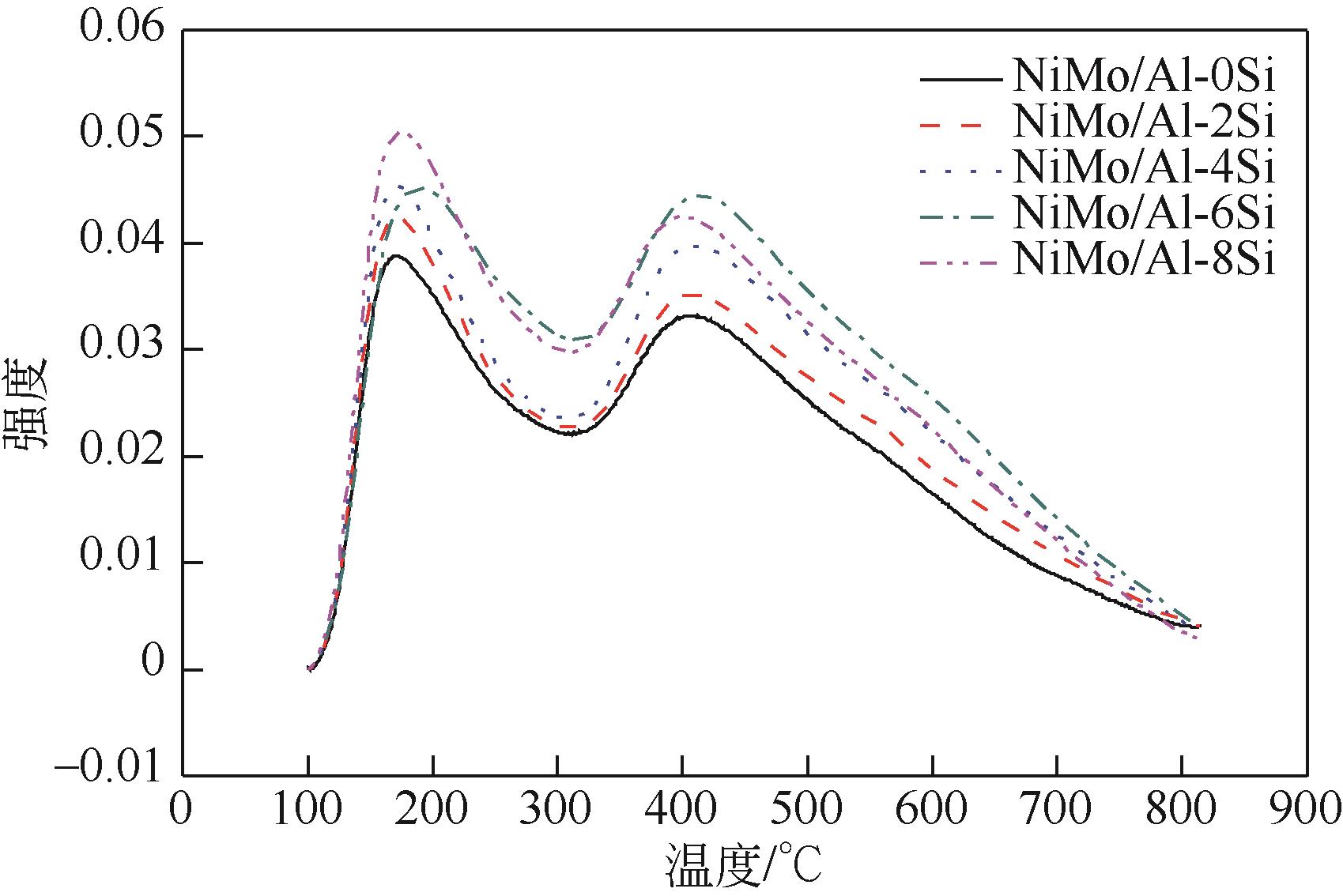
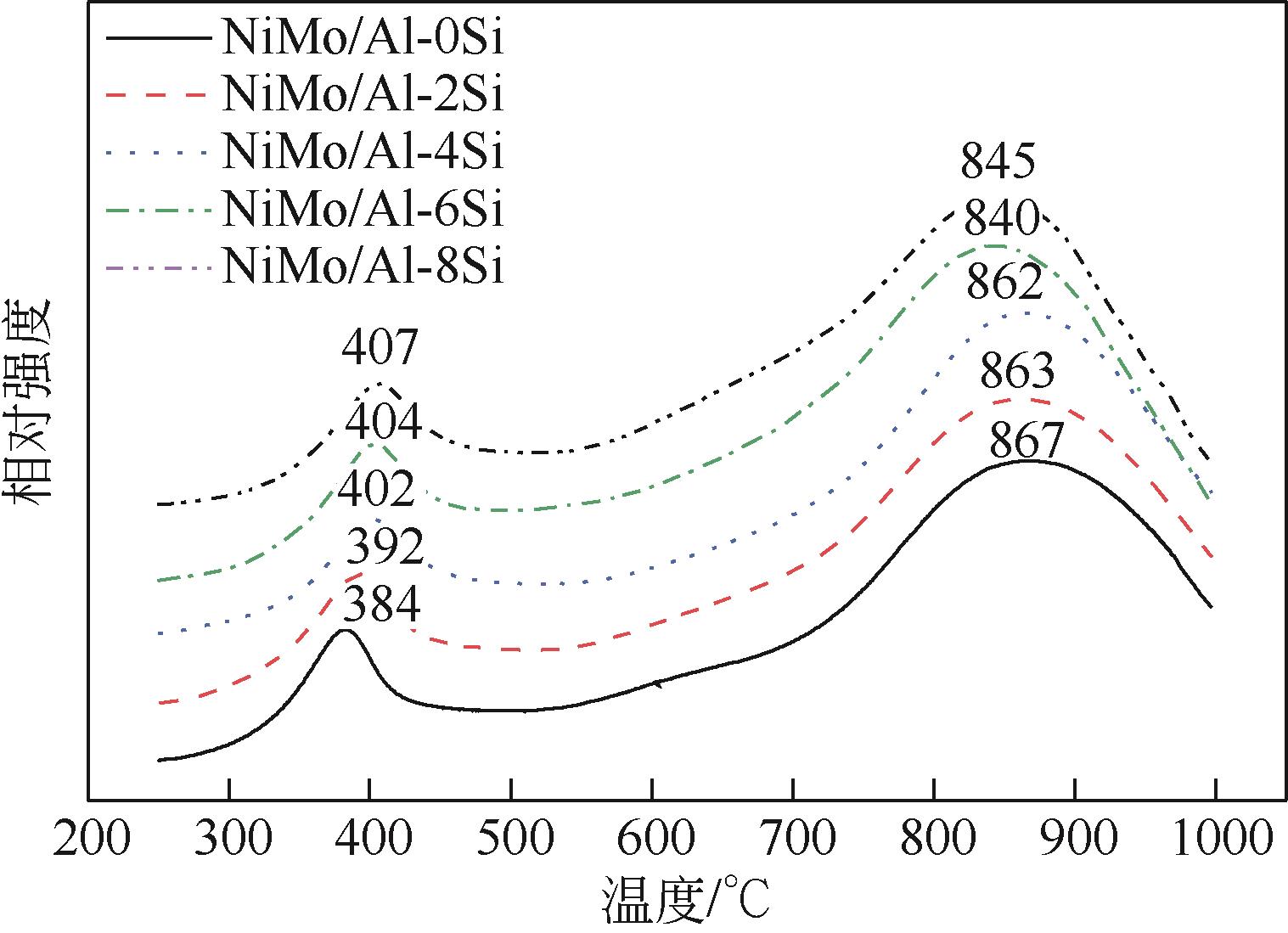
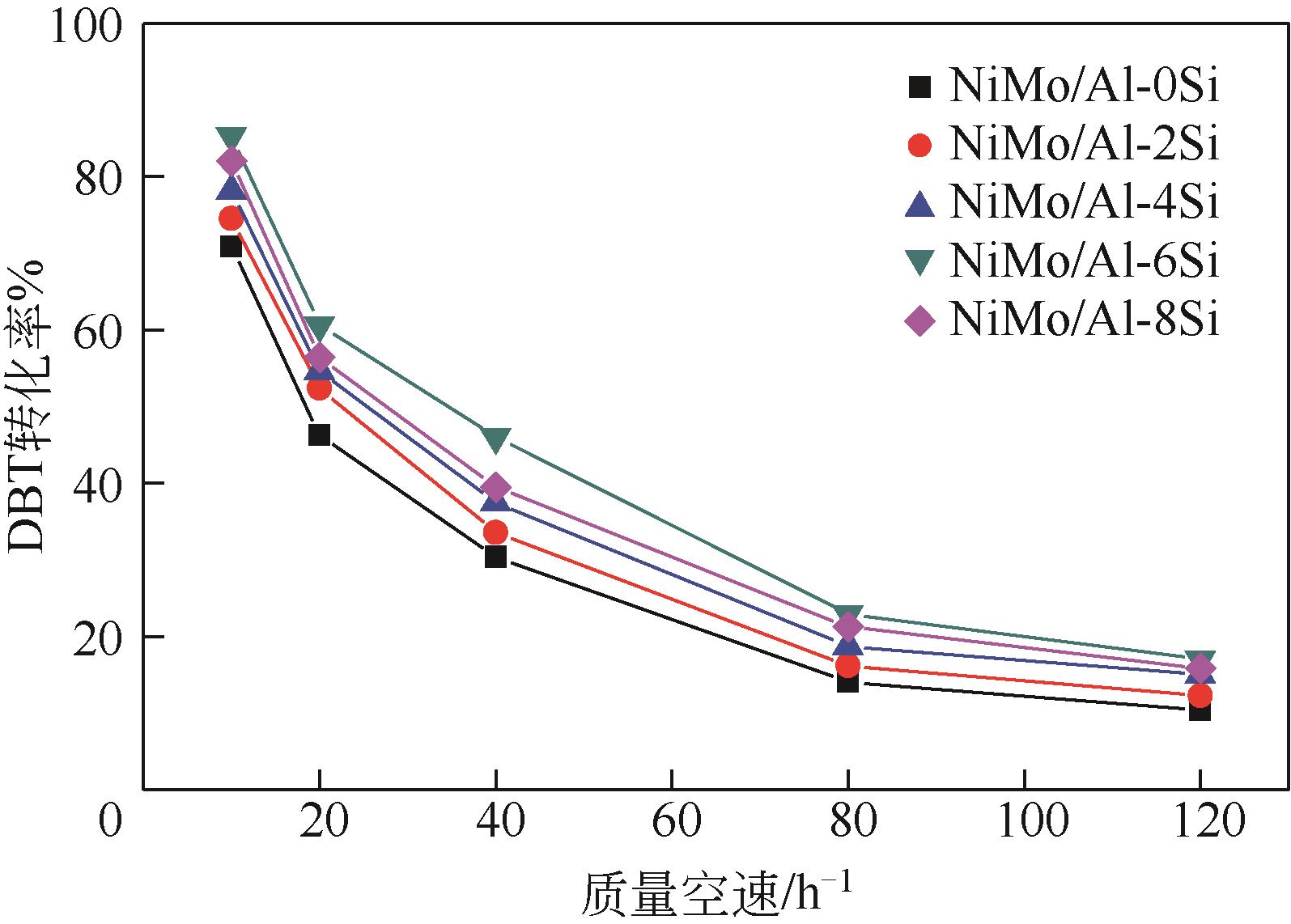
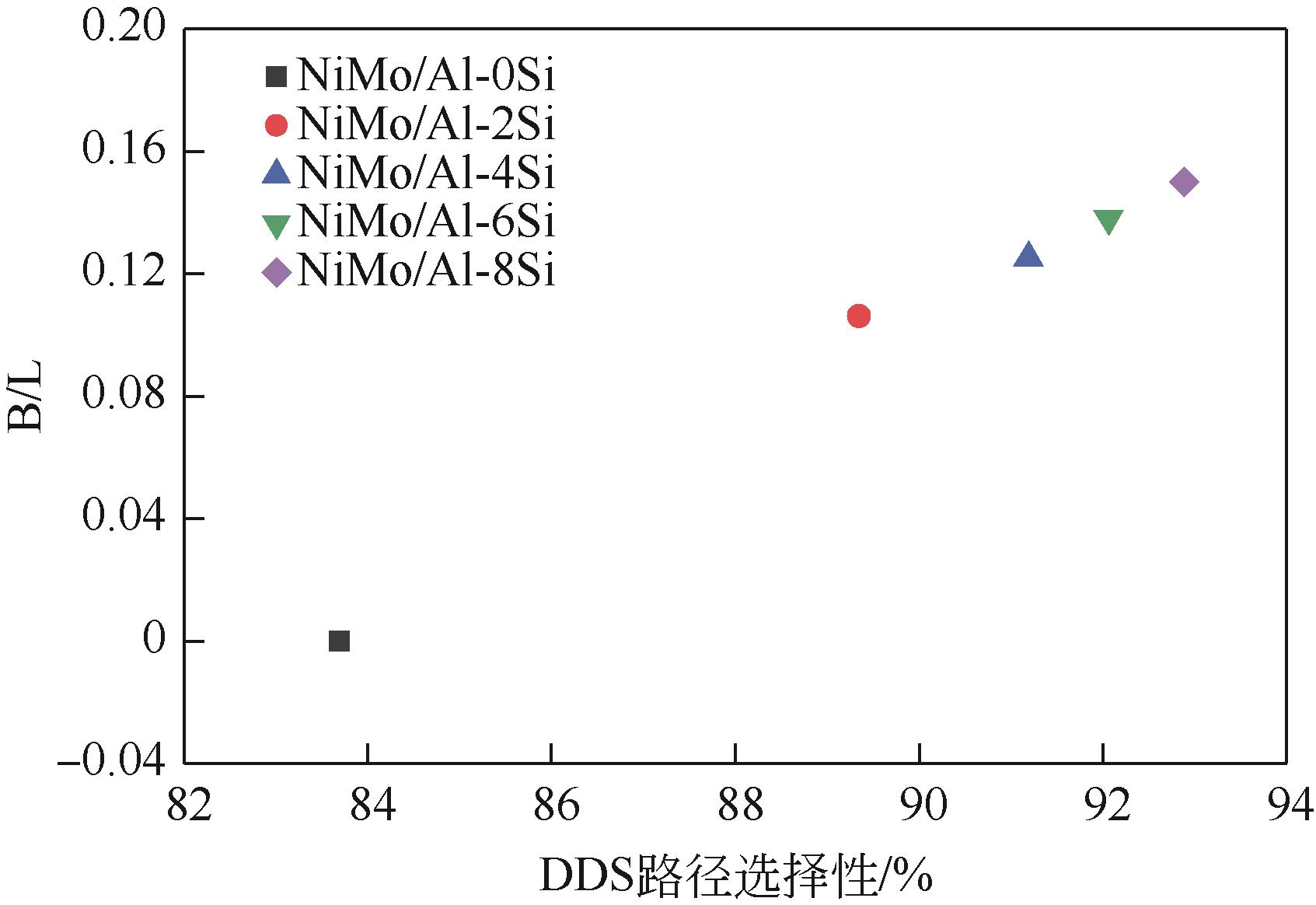
以中和法合成的不同SiO2含量的改性氧化铝为载体,本文制备系列Si改性的NiMo/Al2O3催化剂,采用X射线衍射(XRD)、N2物理吸附(BET)、程序升温脱附(NH3-TPD)、吡啶吸附红外光谱(Py-IR)、程序升温还原(H2-TPR)、高分辨透射电镜(HRTEM)和X射线光电子能谱(XPS)等分析手段进行详细表征。表征结果显示,引入Si减弱了活性金属与载体之间的相互作用,改善了催化剂的孔结构与表面酸性分布,提高了活性相分散度和金属硫化度,促使形成更多的II类NiMoS活性相。以二苯并噻吩(DBT)为模型化合物,在固定床加氢装置上考察了系列催化剂的加氢脱硫(HDS)性能,结果表明,引入Si可降低DBT的加氢反应活化能,提高反应速率常数,进而提高催化剂的加氢脱硫活性。对比DBT转化率在50%时的脱硫产物分布表明引入Si可影响催化剂的反应路径选择性,直接脱硫路径(DDS)选择性从83.69%增加至92.89%,证实了催化剂的表征规律。
中图分类号:
引用本文
郭振雪, 于海斌, 张国辉, 张景成, 卢雁飞, 何艳贞, 孙彦民, 韩恩山. Si改性对NiMo/Al2O3催化剂加氢脱硫性能的影响[J]. 化工进展, 2022, 41(S1): 210-220.
GUO Zhenxue, YU Haibin, ZHANG Guohui, ZHANG Jingcheng, LU Yanfei, HE Yanzhen, SUN Yanmin, HAN Enshan. Effect of silica modification on the performance of NiMo/Al2O3 catalyst in hydrodesulfurization[J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 210-220.
| 催化剂 | 比表面积 /m2·g-1 | 孔体积 /cm3·g-1 | 孔径比例/% | ||
|---|---|---|---|---|---|
| <4nm | 4~10nm | >10nm | |||
| NiMo/Al-0Si | 225 | 0.51 | 8.6 | 73.8 | 17.6 |
| NiMo/Al-2Si | 228 | 0.53 | 6.4 | 75.9 | 17.7 |
| NiMo/Al-4Si | 241 | 0.54 | 5.5 | 76.5 | 18.0 |
| NiMo/Al-6Si | 255 | 0.58 | 4.4 | 77.3 | 18.3 |
| NiMo/Al-8Si | 256 | 0.59 | 2.8 | 78.4 | 18.8 |
表1 NiMo/Al-xSi催化剂的织构性质
| 催化剂 | 比表面积 /m2·g-1 | 孔体积 /cm3·g-1 | 孔径比例/% | ||
|---|---|---|---|---|---|
| <4nm | 4~10nm | >10nm | |||
| NiMo/Al-0Si | 225 | 0.51 | 8.6 | 73.8 | 17.6 |
| NiMo/Al-2Si | 228 | 0.53 | 6.4 | 75.9 | 17.7 |
| NiMo/Al-4Si | 241 | 0.54 | 5.5 | 76.5 | 18.0 |
| NiMo/Al-6Si | 255 | 0.58 | 4.4 | 77.3 | 18.3 |
| NiMo/Al-8Si | 256 | 0.59 | 2.8 | 78.4 | 18.8 |
| 催化剂 | B酸量 /mmol·g-1 | L酸量 /mmol·g-1 | 总酸量 /mmol·g-1 | B/L |
|---|---|---|---|---|
| NiMoS/Al-0Si | — | 0.102 | 0.102 | — |
| NiMoS/Al-2Si | 0.012 | 0.113 | 0.125 | 0.106 |
| NiMoS/Al-4Si | 0.015 | 0.120 | 0.135 | 0.125 |
| NiMoS/Al-6Si | 0.018 | 0.130 | 0.148 | 0.138 |
| NiMoS/Al-8Si | 0.020 | 0.133 | 0.153 | 0.150 |
表2 NiMoS/Al-xSi催化剂的B酸与L酸分布
| 催化剂 | B酸量 /mmol·g-1 | L酸量 /mmol·g-1 | 总酸量 /mmol·g-1 | B/L |
|---|---|---|---|---|
| NiMoS/Al-0Si | — | 0.102 | 0.102 | — |
| NiMoS/Al-2Si | 0.012 | 0.113 | 0.125 | 0.106 |
| NiMoS/Al-4Si | 0.015 | 0.120 | 0.135 | 0.125 |
| NiMoS/Al-6Si | 0.018 | 0.130 | 0.148 | 0.138 |
| NiMoS/Al-8Si | 0.020 | 0.133 | 0.153 | 0.150 |
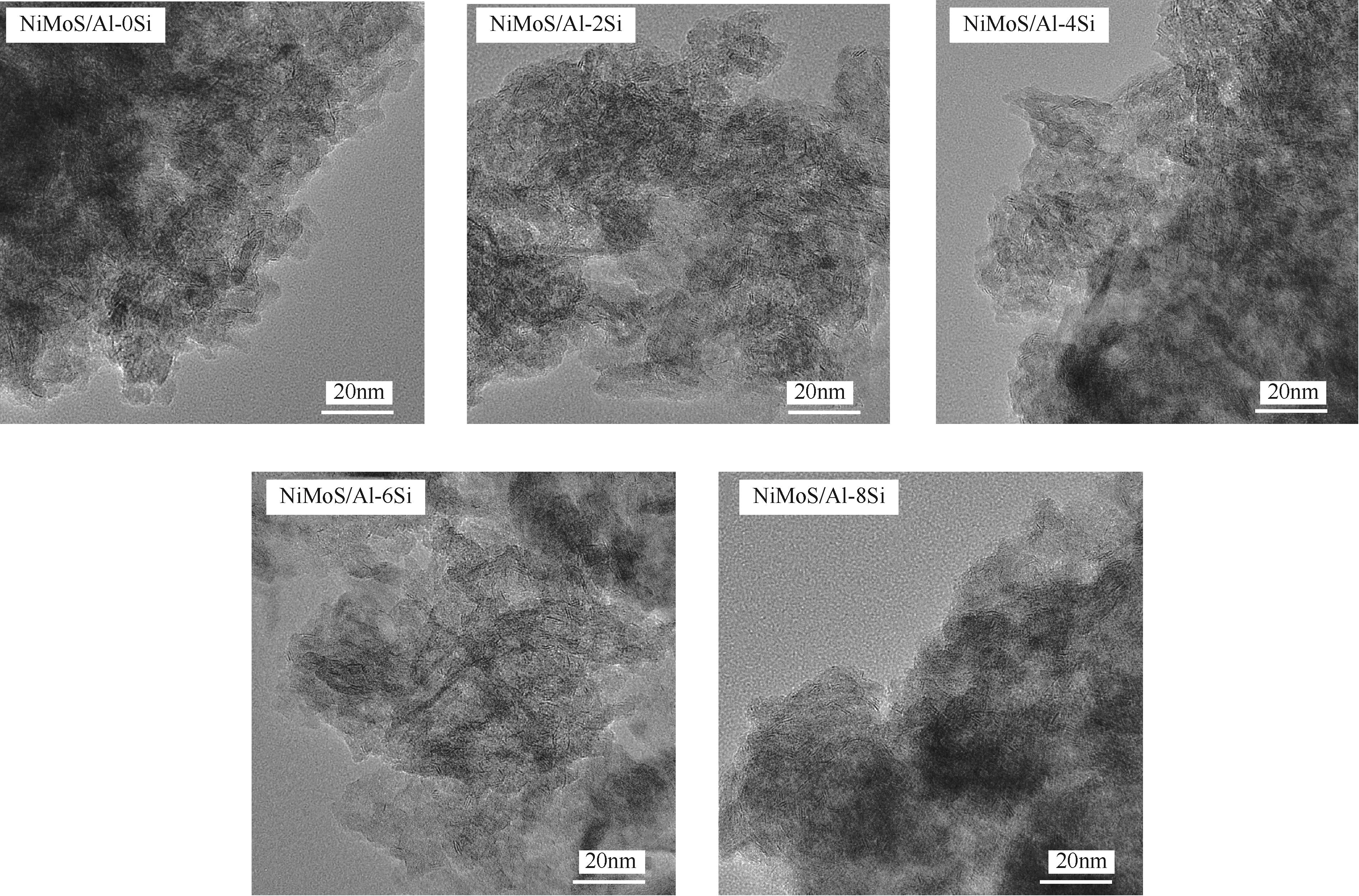
| 催化剂 | 平均长度/nm | 平均堆垛层数 | fMo |
|---|---|---|---|
| NiMoS/Al-0Si | 3.91 | 2.81 | 0.30 |
| NiMoS/Al-2Si | 3.84 | 3.15 | 0.33 |
| NiMoS/Al-4Si | 3.73 | 3.18 | 0.34 |
| NiMoS/Al-6Si | 3.51 | 3.23 | 0.38 |
| NiMoS/Al-8Si | 3.67 | 3.33 | 0.35 |
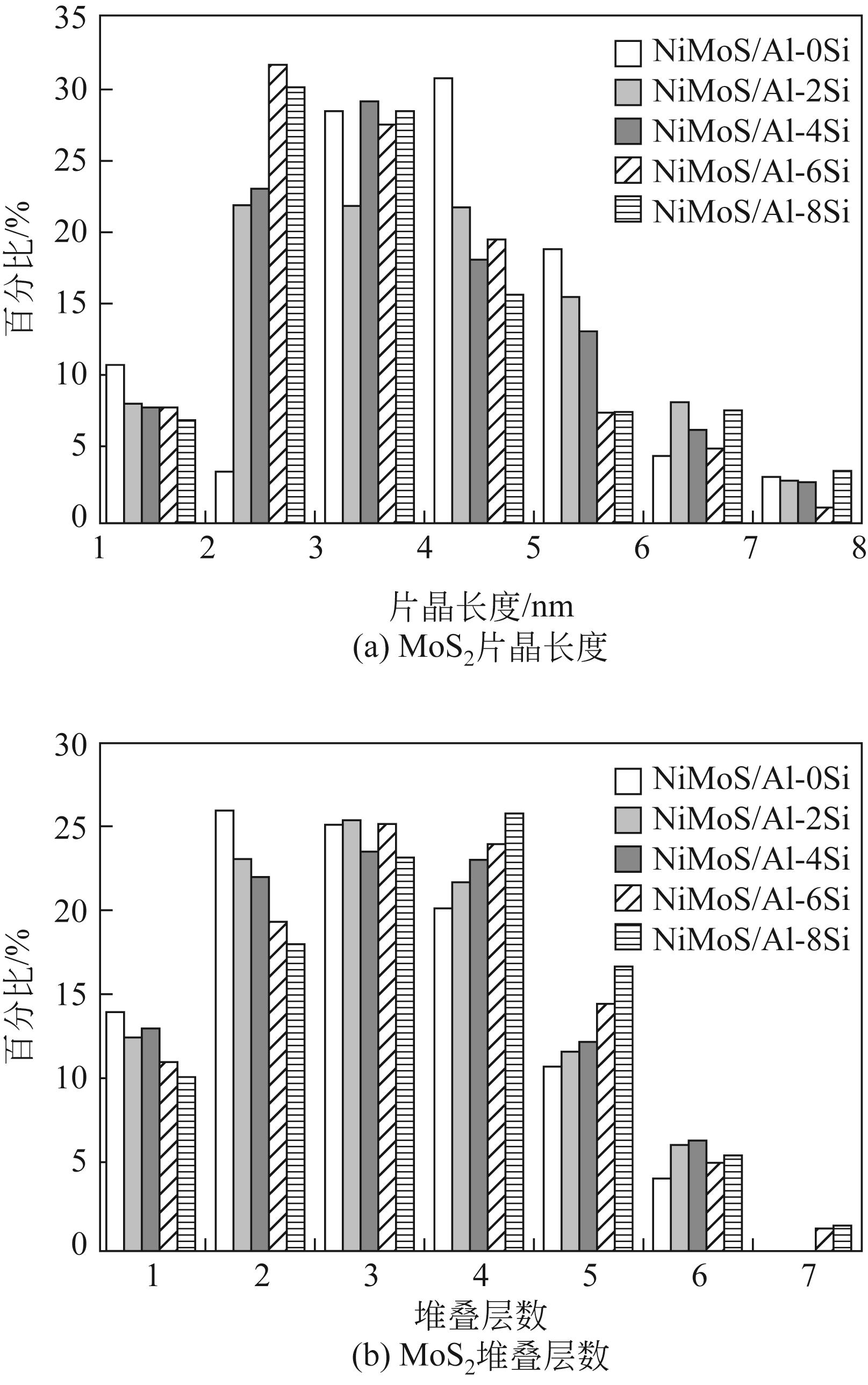
表3 催化剂中MoS2片晶的平均片晶长度、堆叠层数及fMo
| 催化剂 | 平均长度/nm | 平均堆垛层数 | fMo |
|---|---|---|---|
| NiMoS/Al-0Si | 3.91 | 2.81 | 0.30 |
| NiMoS/Al-2Si | 3.84 | 3.15 | 0.33 |
| NiMoS/Al-4Si | 3.73 | 3.18 | 0.34 |
| NiMoS/Al-6Si | 3.51 | 3.23 | 0.38 |
| NiMoS/Al-8Si | 3.67 | 3.33 | 0.35 |
| 催化剂 | Mo4+/% | Mo5+/% | Mo6+/% | NiS x /% | NiMoS/% | NiO/% |
|---|---|---|---|---|---|---|
| NiMoS/Al-0Si | 47.13 | 15.44 | 37.43 | 21.70 | 38.30 | 40.00 |
| NiMoS/Al-2Si | 51.02 | 15.28 | 33.70 | 16.94 | 39.31 | 43.75 |
| NiMoS/Al-4Si | 54.41 | 8.77 | 36.82 | 19.72 | 47.02 | 33.26 |
| NiMoS/Al-6Si | 55.43 | 13.57 | 31.00 | 16.94 | 48.97 | 34.09 |
| NiMoS/Al-8Si | 53.46 | 10.98 | 35.56 | 24.87 | 45.80 | 29.33 |
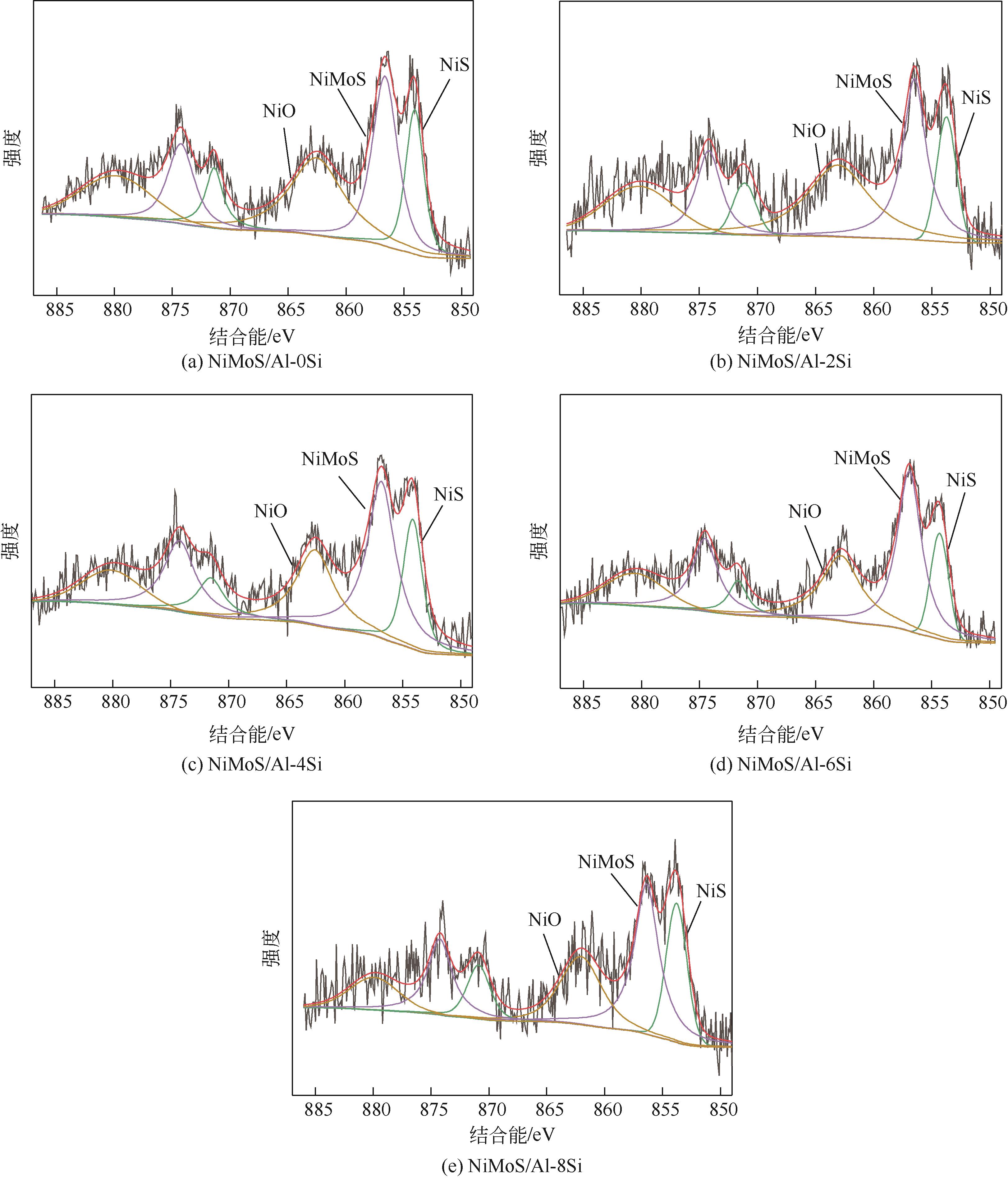
表4 NiMoS/Al-xSi催化剂中Mo和Ni原子XPS分析
| 催化剂 | Mo4+/% | Mo5+/% | Mo6+/% | NiS x /% | NiMoS/% | NiO/% |
|---|---|---|---|---|---|---|
| NiMoS/Al-0Si | 47.13 | 15.44 | 37.43 | 21.70 | 38.30 | 40.00 |
| NiMoS/Al-2Si | 51.02 | 15.28 | 33.70 | 16.94 | 39.31 | 43.75 |
| NiMoS/Al-4Si | 54.41 | 8.77 | 36.82 | 19.72 | 47.02 | 33.26 |
| NiMoS/Al-6Si | 55.43 | 13.57 | 31.00 | 16.94 | 48.97 | 34.09 |
| NiMoS/Al-8Si | 53.46 | 10.98 | 35.56 | 24.87 | 45.80 | 29.33 |
| 催化剂 | Ea /kJ·mol-1 | EaDDS /kJ·mol-1 | EaHYD /kJ·mol-1 | KHDS /10-4mol·g-1·h-1 |
|---|---|---|---|---|
| NiMo/Al-0Si | 105.5 | 96.4 | 106.9 | 3.37 |
| NiMo/Al-2Si | 98.5 | 92.5 | 102.2 | 3.80 |
| NiMo/Al-4Si | 93.0 | 90.7 | 97.8 | 4.21 |
| NiMo/Al-6Si | 91.4 | 88.8 | 93.7 | 5.25 |
| NiMo/Al-8Si | 92.4 | 86.7 | 98.3 | 4.67 |
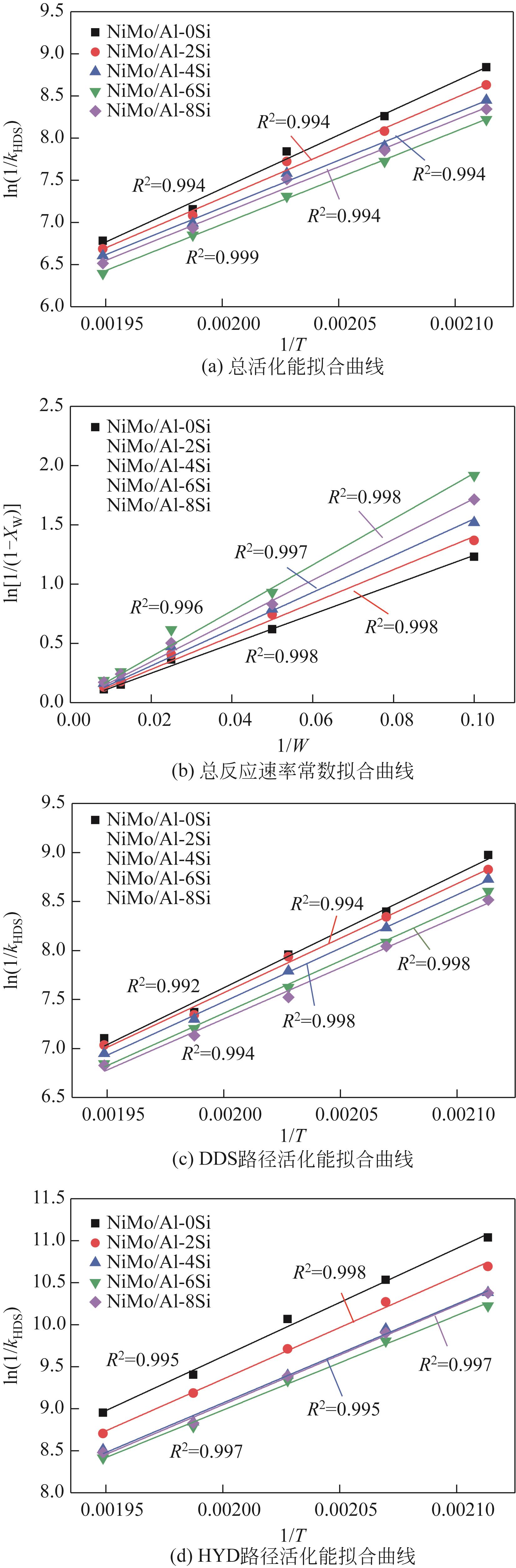
表5 催化剂活化能及反应速率常数计算汇总表
| 催化剂 | Ea /kJ·mol-1 | EaDDS /kJ·mol-1 | EaHYD /kJ·mol-1 | KHDS /10-4mol·g-1·h-1 |
|---|---|---|---|---|
| NiMo/Al-0Si | 105.5 | 96.4 | 106.9 | 3.37 |
| NiMo/Al-2Si | 98.5 | 92.5 | 102.2 | 3.80 |
| NiMo/Al-4Si | 93.0 | 90.7 | 97.8 | 4.21 |
| NiMo/Al-6Si | 91.4 | 88.8 | 93.7 | 5.25 |
| NiMo/Al-8Si | 92.4 | 86.7 | 98.3 | 4.67 |
| 催化剂 | DDS(BP①)/% | HYD/% | 转化率(DBT)/% | |||
|---|---|---|---|---|---|---|
| THDBT② | HHDBT③ | CHB④ | DCH⑤ | |||
| NiMo/Al-0Si | 83.69 | 0.33 | 13.13 | 0.52 | 2.33 | 49.59 |
| NiMo/Al-2Si | 89.35 | 0.14 | 7.94 | 0.42 | 2.15 | 49.63 |
| NiMo/Al-4Si | 91.20 | 0.13 | 6.46 | 0.27 | 1.94 | 50.25 |
| NiMo/Al-6Si | 92.07 | 0.20 | 5.74 | 0.09 | 1.90 | 50.00 |
| NiMo/Al-8Si | 92.89 | 0.13 | 4.75 | 0.28 | 1.95 | 49.70 |
表6 不同催化剂的加氢脱硫产物对比表
| 催化剂 | DDS(BP①)/% | HYD/% | 转化率(DBT)/% | |||
|---|---|---|---|---|---|---|
| THDBT② | HHDBT③ | CHB④ | DCH⑤ | |||
| NiMo/Al-0Si | 83.69 | 0.33 | 13.13 | 0.52 | 2.33 | 49.59 |
| NiMo/Al-2Si | 89.35 | 0.14 | 7.94 | 0.42 | 2.15 | 49.63 |
| NiMo/Al-4Si | 91.20 | 0.13 | 6.46 | 0.27 | 1.94 | 50.25 |
| NiMo/Al-6Si | 92.07 | 0.20 | 5.74 | 0.09 | 1.90 | 50.00 |
| NiMo/Al-8Si | 92.89 | 0.13 | 4.75 | 0.28 | 1.95 | 49.70 |
| 1 | WANG H Y, LIU S D, SMITH K J. Understanding selectivity changes during hydrodesulfurization of dibenzothiophene on Mo2C/carbon catalysts[J]. Journal of Catalysis, 2019, 369: 427-439. |
| 2 | ZHOU W W, WEI Q, ZHOU Y S, et al. Hydrodesulfurization of 4,6-dimethyldibenzothiophene over NiMo sulfide catalysts supported on meso-microporous Y zeolite with different mesopore sizes[J]. Applied Catalysis B: Environmental, 2018, 238: 212-224. |
| 3 | ZHOU W W, ZHOU A N, ZHANG Y T, et al. Hydrodesulfurization of 4,6-dimethyldibenzothiophene over NiMo supported on Ga-modified Y zeolites catalysts[J]. Journal of Catalysis, 2019, 374: 345-359. |
| 4 | WANG X L, MEI J L, ZHAO Z, et al. Restrictive diffusion in the hydrodesulfurization over Ni-MoS2/Al2O3 with different crystal forms[J]. Industrial & Engineering Chemistry Research, 2017, 56(36): 10018-10027. |
| 5 | GRØNBORG S S, ŠARIĆ M, MOSES P G, et al. Atomic scale analysis of sterical effects in the adsorption of 4,6-dimethyldibenzothiophene on a CoMoS hydrotreating catalyst[J]. Journal of Catalysis, 2016, 344: 121-128. |
| 6 | JAF Z N, ALTARAWNEH M, MIRAN H A, et al. Hydrodesulfurization of thiophene over γ-Mo2N catalyst[J]. Molecular Catalysis, 2018, 459: 21-30. |
| 7 | TOPSØE H, CLAUSEN B S, CANDIA R, et al. In situ Mössbauer emission spectroscopy studies of unsupported and supported sulfided CoMo hydrodesulfurization catalysts: evidence for and nature of a CoMoS phase[J]. Journal of Catalysis, 1981, 68(2): 433-452. |
| 8 | YU Q, ZHANG J C, NAN J, et al. Synthesis and hydrodesulfurization performance of NiMo sulfide catalysts supported on an Al-Si mixed oxide[C]//Preceedings of the 2016 International Conference on Civil, Architecture and Environmental Engineering. Taibei, China, 2017: 404-407. |
| 9 | RAYO P, TORRES-MANCERA P, CENTENO G, et al. Effect of silicon incorporation method in the supports of NiMo catalysts for hydrotreating reactions[J]. Fuel, 2019, 239: 1293-1303. |
| 10 | ROMERO-GALARZA A, RAMíREZ J, GUTIÉRREZ-ALEJANDRE A, et al. Relevant changes in the properties of Co(Ni)Mo/Al2O3 HDS catalysts modified by small amounts of SiO2 [J]. Journal of Materials Research, 2018, 33(21): 3570-3579. |
| 11 | ABOUTALEB W A, NAGGER A M A EL, SAYED M A, et al. Influence of CeO2 loading on the catalytic performance of CoNiMoS/CeO2-Al2O3 toward vacuum gas oil hydrotreatment[J]. Materials Chemistry and Physics, 2022, 276: 125165. |
| 12 | WANG S, ZHANG J C, LIU D, et al. Deep insights into enhanced direct-desulfurization selectivity of thiourea-modified CoMoP/ γ-Al2O3: an investigation of catalyst microstructures[J]. Fuel, 2020, 267: 116993. |
| 13 | 张国辉, 张玉婷, 张景成, 等. 制备方法对Ni-Mo/Al2O3加氢催化剂性能的影响[J]. 化工进展, 2018, 37(11): 4315-4321. |
| ZHANG Guohui, ZHANG Yuting, ZHANG Jingcheng, et al. Influence of preparation method on hydrotreating activity of Ni-Mo/Al2O3 [J]. Chemical Industry and Engineering Progress, 2018, 37(11): 4315-4321. | |
| 14 | 汪佩华, 秦志峰, 吴琼笑, 等. 磷添加方式对NiMo/Al2O3催化剂加氢脱硫性能的影响[J]. 化工进展, 2021, 40(2): 890-900. |
| WANG Peihua, QIN Zhifeng, WU Qiongxiao, et al. Effect of phosphorus adding manners on the performance of NiMo/Al2O3 catalyst in hydrodesulfurization[J]. Chemical Industry and Engineering Progress, 2021, 40(2): 890-900. | |
| 15 | ŠARIĆ M, ROSSMEISL J, MOSES P G. Modeling the adsorption of sulfur containing molecules and their hydrodesulfurization intermediates on the Co-promoted MoS2 catalyst by DFT[J]. Journal of Catalysis, 2018, 358: 131-140. |
| 16 | CAO Z K, ZHANG X, GUO R, et al. Synergistic effect of acidity and active phases for NiMo catalysts on dibenzothiophene hydrodesulfurization performance[J]. Chemical Engineering Journal, 2020, 400: 125886. |
| 17 | LIU B, LIU L, WANG Z, et al. Effect of hydrogen spillover in selective hydrodesulfurization of FCC gasoline over the CoMo catalyst[J]. Catalysis Today, 2017, 282: 214-221. |
| 18 | HENKER M, WENDLANDT K P, VALYON J, et al. Structure of MoO3/Al2O3-SiO2 catalysts[J]. Applied Catalysis, 1991, 69(1): 205-220. |
| 19 | ARNOLDY P, JONGE J D, MOULIJN J. Temperature-programed reduction of molybdenum (Ⅵ) oxide and molybdenum (Ⅳ) oxide[J]. The Journal of Physical Chemistry, 1985, 89(21): 4517-4526. |
| 20 | 岳凡, 李蒙, 杨祝红, 等. 介孔TiO2晶须-γ-Al2O3复合载体催化剂的制备及对二苯并噻吩的加氢脱硫性能[J]. 石油学报(石油加工), 2020, 36(4): 667-676. |
| YUE Fan, LI Meng, YANG Zhuhong, et al. Preparation of mesoporous TiO2 whisker-γ-Al2O3 composite supported catalyst and its catalytic performance in dibenzothiophene hydrodesulfurization[J]. Acta Petrolei Sinica (Petroleum Processing Section), 2020, 36(4): 667-676. | |
| 21 | RAJAGOPAL S, MARINI H, MARZARI J, et al. Silica-alumina-supported acidic molybdenum catalysts-TPR and XRD characterization[J]. Journal of Catalysis, 1994, 147(2): 417-428. |
| 22 | XU J, HUANG T, FAN Y. Highly efficient NiMo/SiO2-Al2O3 hydrodesulfurization catalyst prepared from gemini surfactant-dispersed Mo precursor[J]. Applied Catalysis B: Environmental, 2017, 203: 839-850. |
| 23 | HAO L, XIONG G, LIU L P, et al. Preparation of highly dispersed desulfurization catalysts and their catalytic performance in hydrodesulfurization of dibenzothiophene[J]. Chinese Journal of Catalysis, 2016, 37(3): 412-419. |
| 24 | GLOTOV A P, VUTOLKINA A V, VINOGRADOV N A, et al. Enhanced HDS and HYD activity of sulfide Co-PMo catalyst supported on alumina and structured mesoporous silica composite[J]. Catalysis Today, 2021, 377: 82-91. |
| 25 | HARRIS S, CHINANELLI R R. Catalysis by transition metal sulfides: the relation between calculated electronic trends and HDS activity[J]. Journal of Catalysis, 1984, 86(2): 400-412. |
| 26 | SÁNCHEZ-MINERO F, RAMÍIREZ J, NCHEZ-MINERO F, et al. Analysis of the HDS of 4,6-DMDBT in the presence of naphthalene and carbazole over NiMo/Al2O3-SiO2(x) catalysts[J]. Catalysis Today, 2008, 133: 267-276. |
| 27 | PRINS R, EGOROVA M, RÖTHLISBERGER A, et al. Mechanisms of hydrodesulfurization and hydrodenitrogenation[J]. Catalysis Today, 2006, 111(1-2): 84-93. |
| 28 | 赵瑞玉, 曹东炜, 曾令有, 等. 助剂Ni与载体的相互作用及其对NiMo/γ-Al2O3催化剂加氢脱硫性能的影响[J]. 燃料化学学报, 2016, 44(5): 564-569. |
| ZHAO Ruiyu, CAO Dongwei, ZENG Lingyou, et al. Interaction between Ni promoter and Al2O3 support and its effect on the performance of NiMo/γ-Al2O3 catalyst in hydrodesulphurization[J]. Journal of Fuel Chemistry and Technology, 2016, 44(5): 564-569. | |
| 29 | LÓPEZ-BENÍTEZ A, BERHAULT G, GUEVARA-LARA.A. Addition of manganese to alumina and its influence on the formation of supported NiMo catalysts for dibenzothiophene hydrodesulfurization application[J]. Journal of Catalysis, 2016, 344: 59-76. |
| 30 | LEBEAU B, BONNE M, COMPAROT J D, et al. HDS of 4,6-dimethyldibenzothiophene over CoMoS supported mesoporous SiO2-TiO2 materials[J]. Catalysis Today, 2020: 675-683. |
| 31 | 李翔, 王安杰, 柳广厦, 等. 芳香杂环含硫化合物C—S键断裂方式[J]. 石油学报(石油加工), 2017, 33(6): 1039-1052. |
| LI Xiang, WANG Anjie, LIU Guangxia, et al. Mechanisms of the cleavage of C—S Bonds in polyaromatic sulfur-containing compounds[J]. Acta Petrolei Sinica (Petroleum Processing Section), 2017, 33(6): 1039-1052. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [6] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [7] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [8] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| [9] | 张启, 赵红, 荣峻峰. 质子交换膜燃料电池中氧还原反应抗毒性电催化剂研究进展[J]. 化工进展, 2023, 42(9): 4677-4691. |
| [10] | 王伟涛, 鲍婷玉, 姜旭禄, 何珍红, 王宽, 杨阳, 刘昭铁. 醛酮树脂基非金属催化剂催化氧气氧化苯制备苯酚[J]. 化工进展, 2023, 42(9): 4706-4715. |
| [11] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [12] | 王尚彬, 欧红香, 薛洪来, 曹海珍, 王钧奇, 毕海普. 黄原胶和纳米二氧化硅对无氟泡沫性能的影响[J]. 化工进展, 2023, 42(9): 4856-4862. |
| [13] | 吴海波, 王希仑, 方岩雄, 纪红兵. 3D打印催化材料开发与应用进展[J]. 化工进展, 2023, 42(8): 3956-3964. |
| [14] | 向阳, 黄寻, 魏子栋. 电催化有机合成反应的活性和选择性调控研究进展[J]. 化工进展, 2023, 42(8): 4005-4014. |
| [15] | 王耀刚, 韩子姗, 高嘉辰, 王新宇, 李思琪, 杨全红, 翁哲. 铜基催化剂电还原二氧化碳选择性的调控策略[J]. 化工进展, 2023, 42(8): 4043-4057. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||