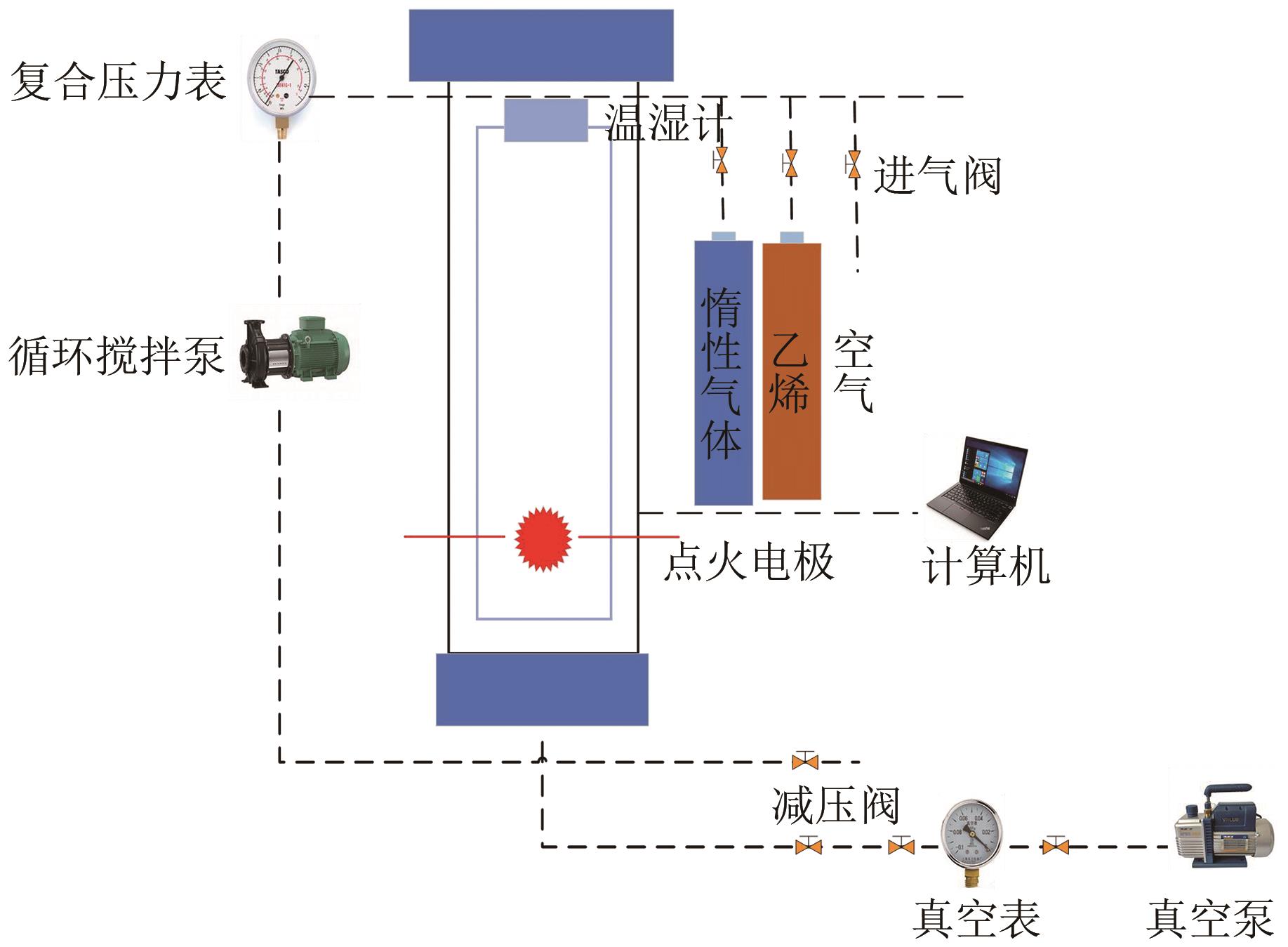
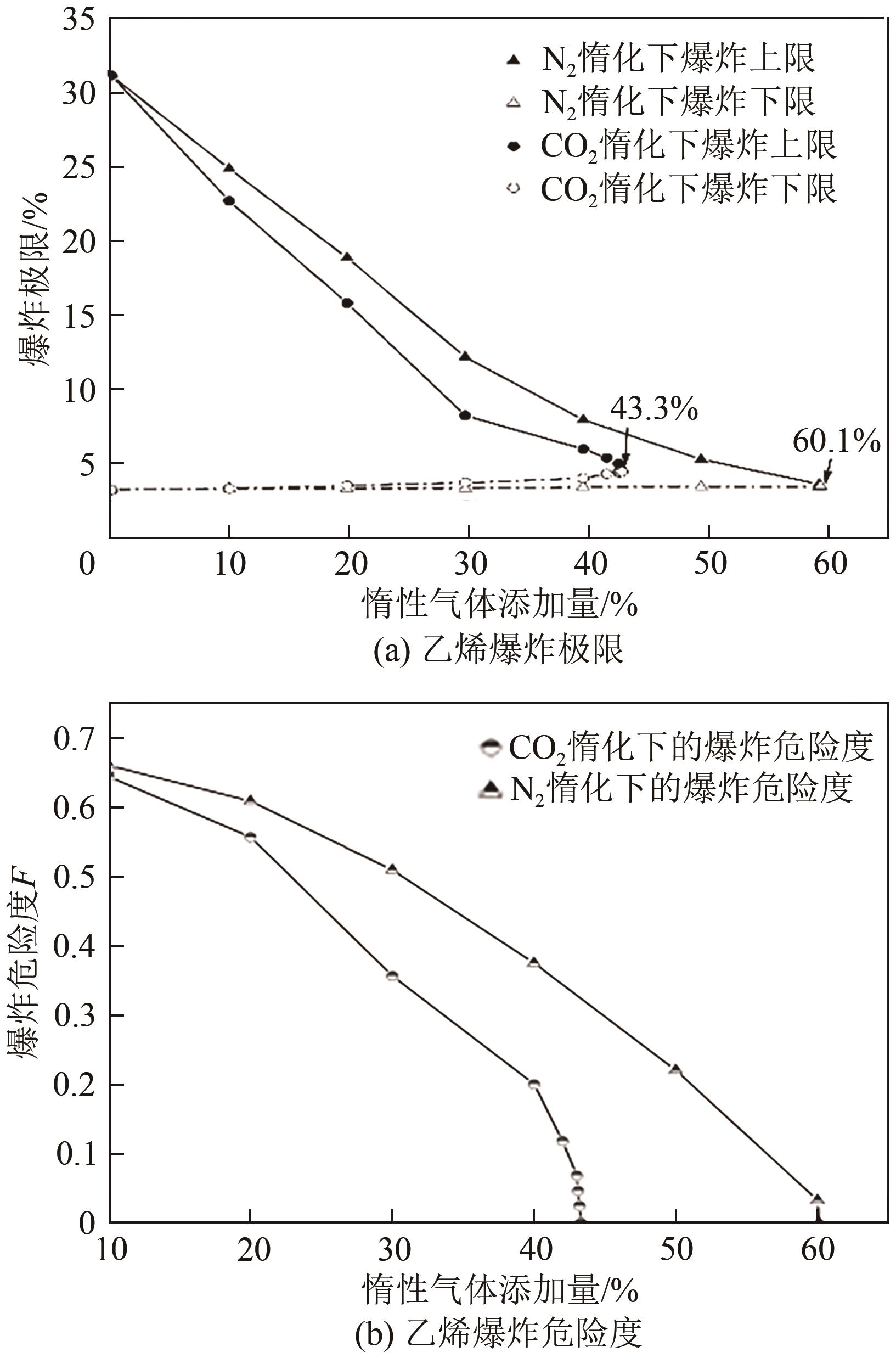
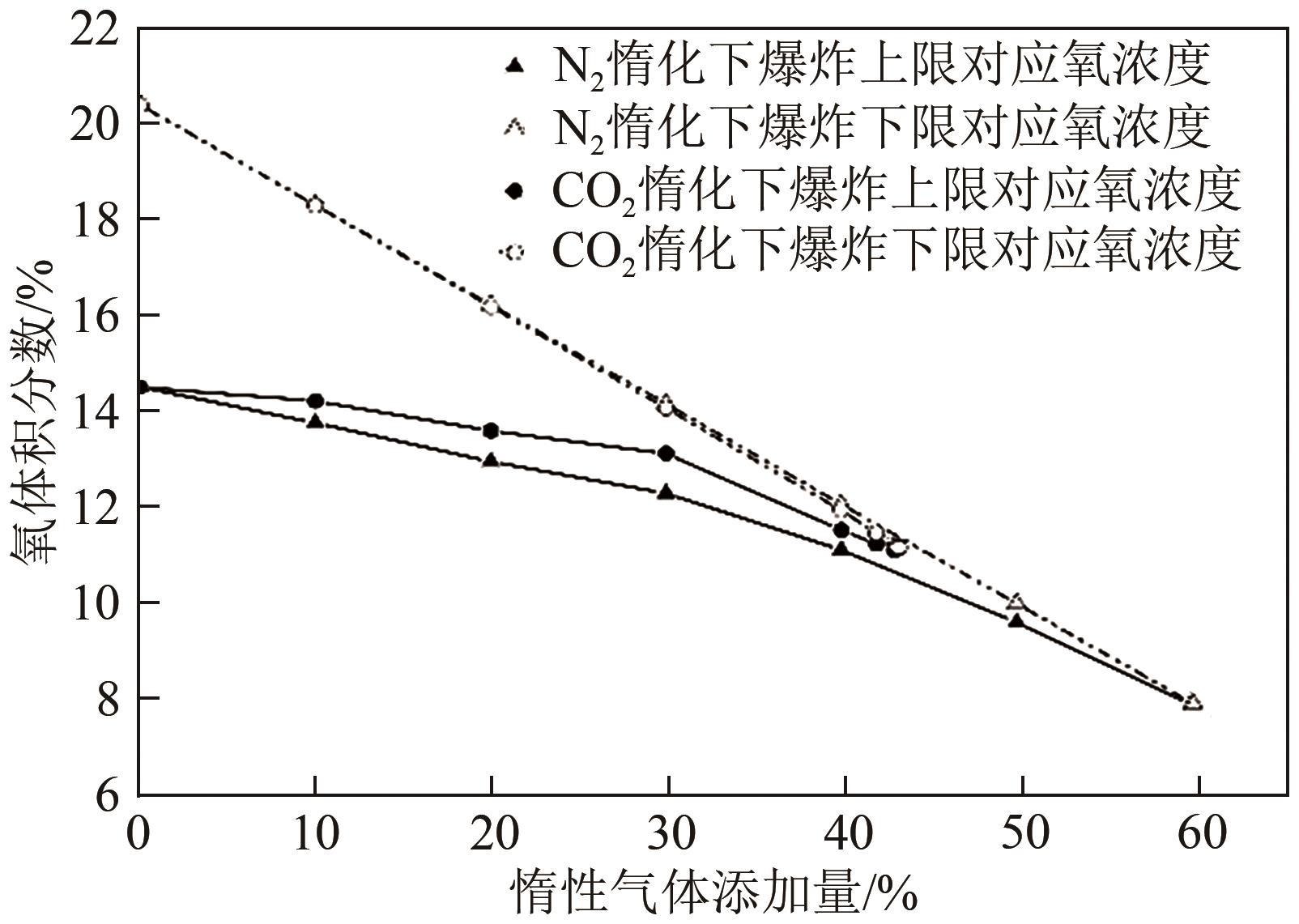
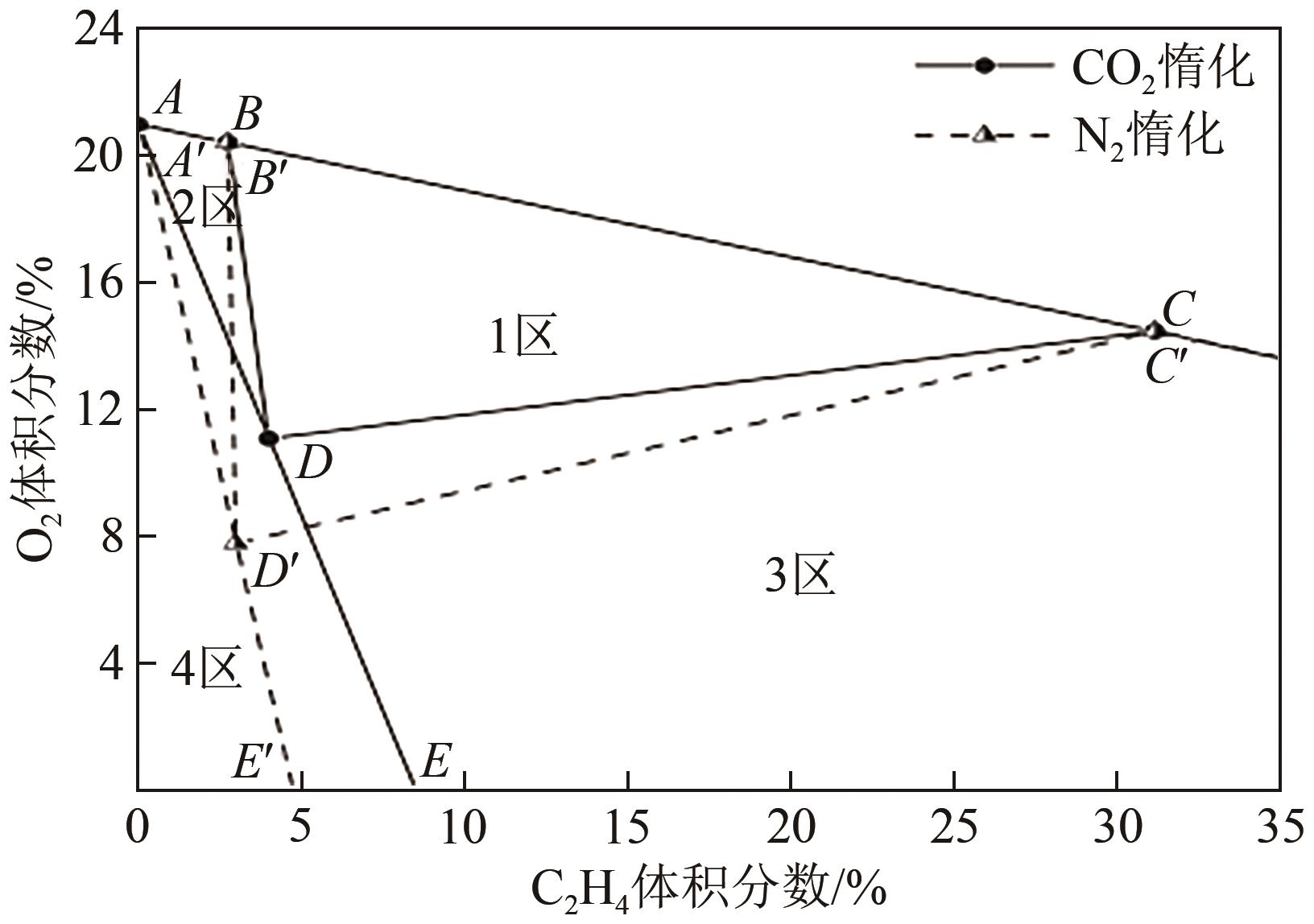
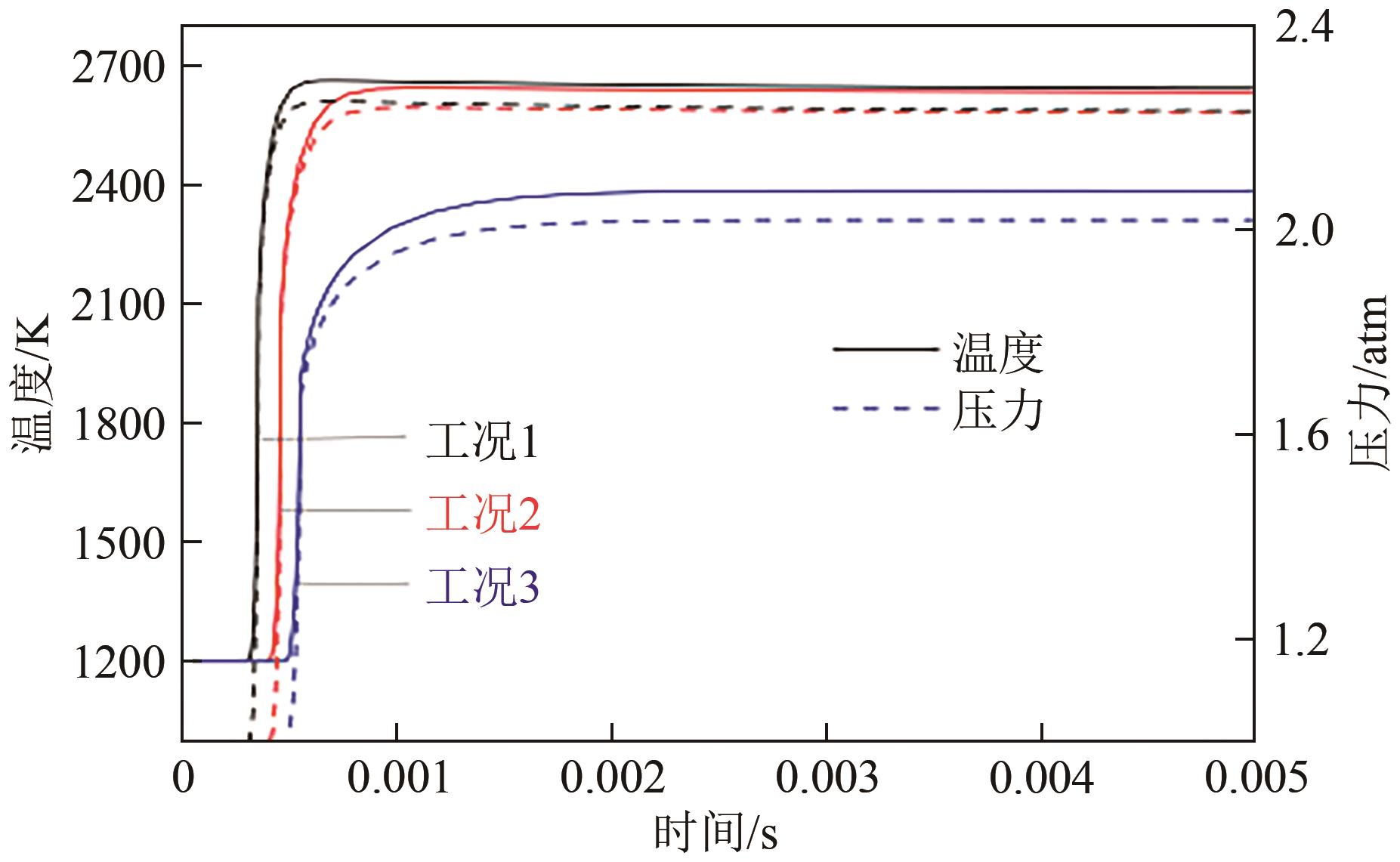
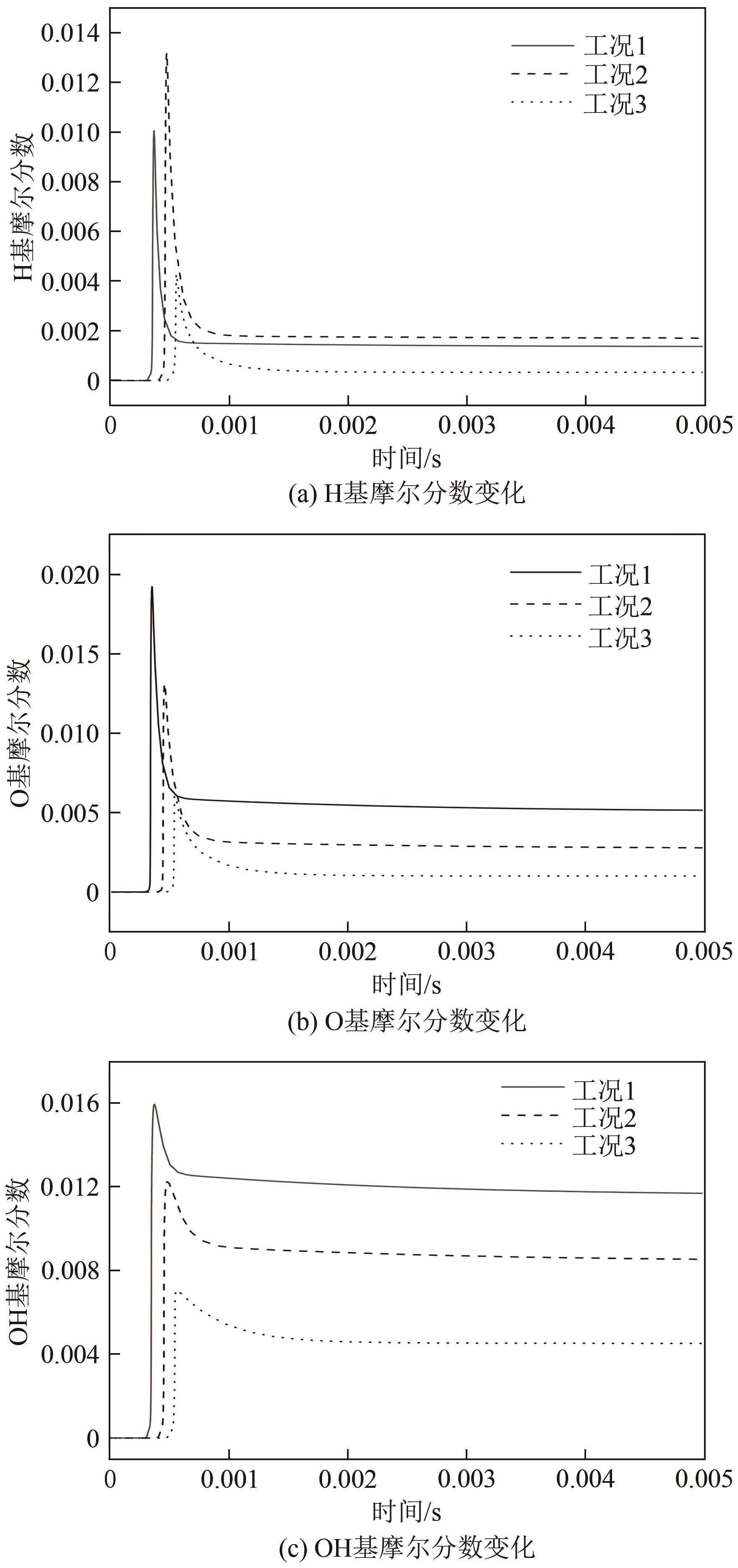
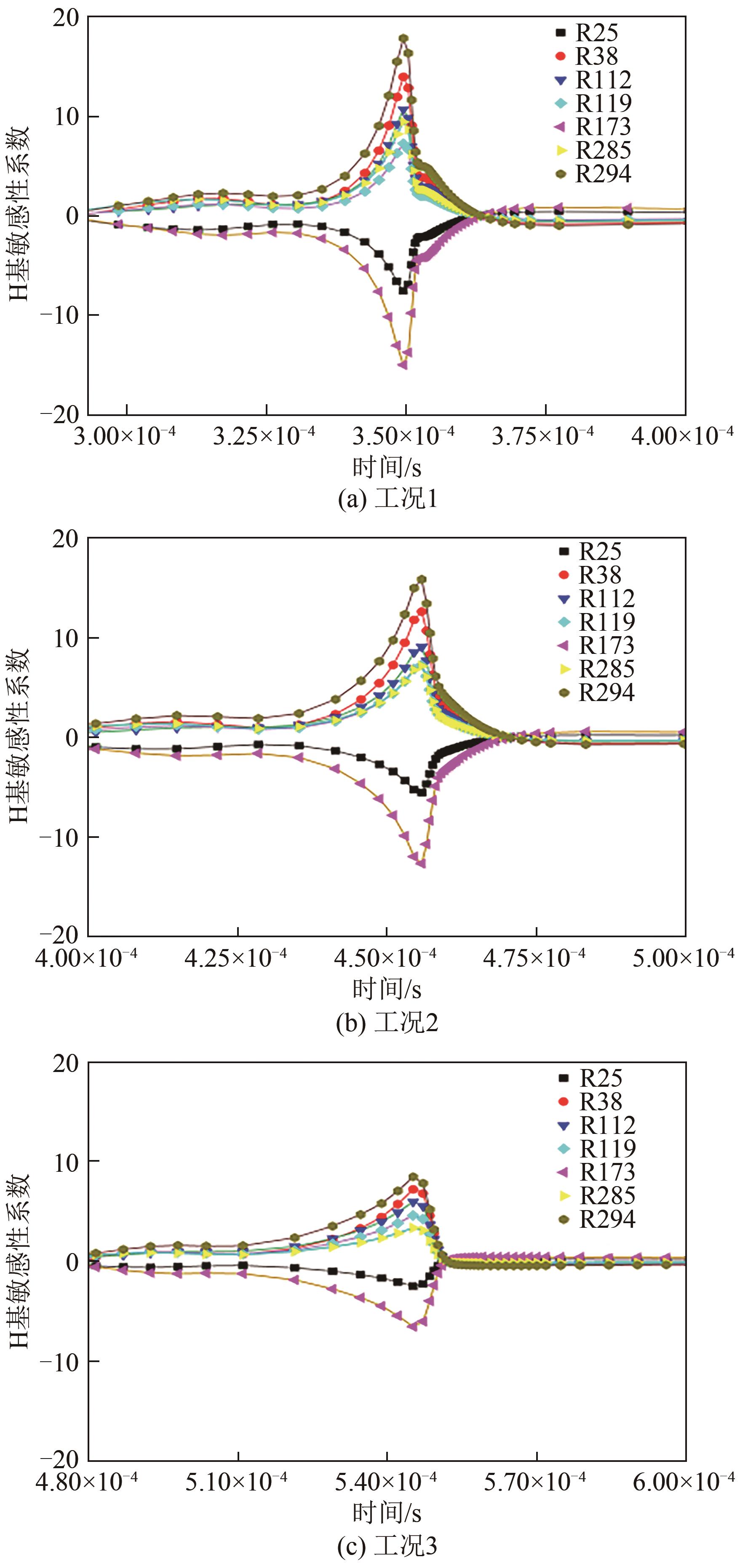
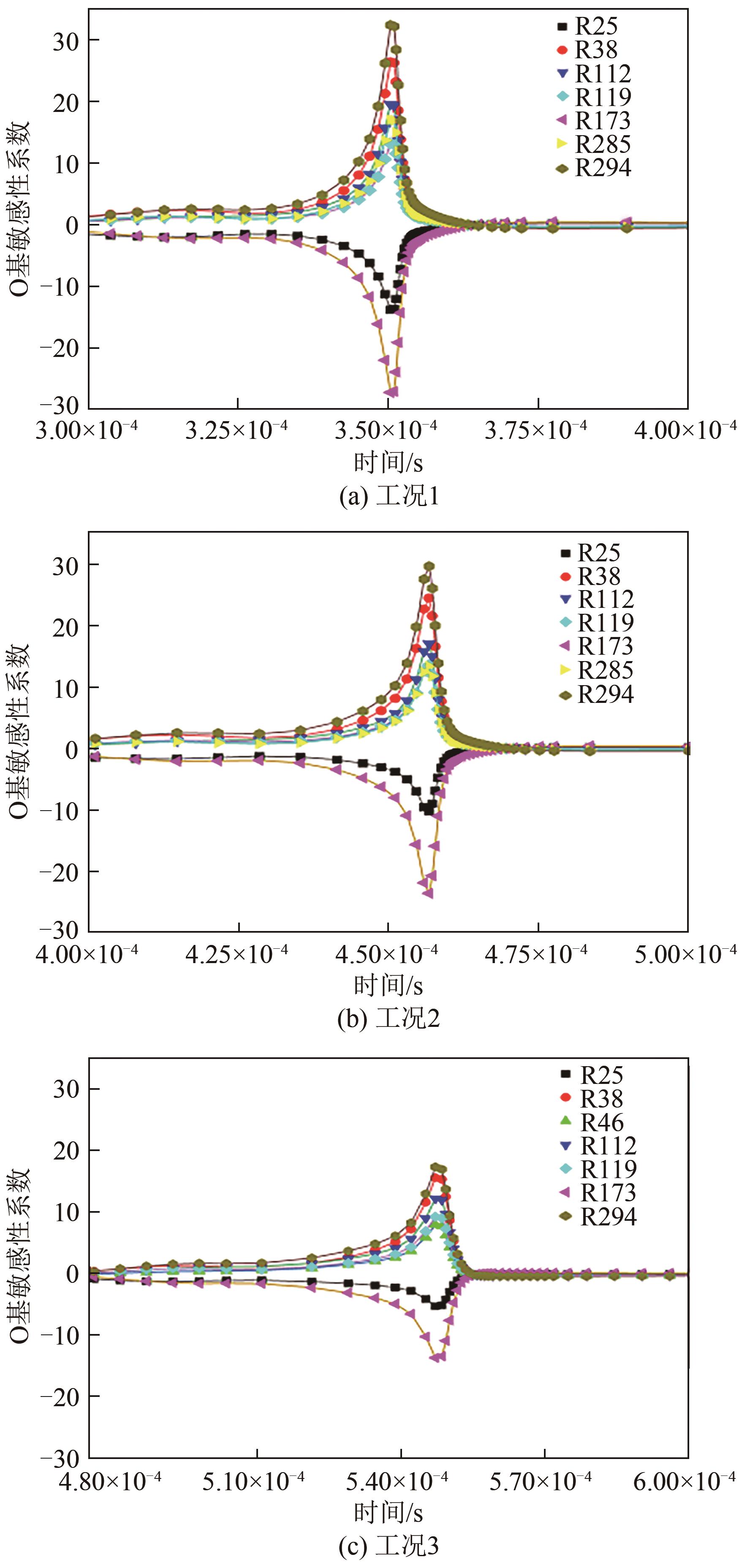
| 1 |
罗振敏, 解超, 王九柱, 等. N2和CO2对液化石油气(LPG)惰化抑爆效能对比分析[J]. 化工进展, 2019, 38(6): 2574-2580.
|
|
LUO Zhenmin, XIE Chao, WANG Jiuzhu, et al. Comparative analysis of the inert effects of N2 and CO2 on LPG explosion[J]. Chemical Industry and Engineering Progress, 2019, 38(6): 2574-2580.
|
| 2 |
罗振敏, 杨勇, 程方明, 等. N2和CO2惰化丙烯爆炸极限参数实验研究[J]. 化工学报, 2020, 71(4): 1922-1928.
|
|
LUO Zhenmin, YANG Yong, CHENG Fangming, et al. Experimental study on explosion limits parameters of propylene with dilution of nitrogen and carbon dioxide[J]. CIESC Journal, 2020, 71(4): 1922-1928.
|
| 3 |
周宁, 李海涛, 任常兴, 等. 氮气、二氧化碳对液化石油气的惰化抑爆研究[J]. 消防科学与技术, 2016, 35(6): 733-737.
|
|
ZHOU Ning, LI Haitao, REN Changxing, et al. The liquefied petroleum gas inert gas explosion suppression about of nitrogen and carbon dioxide[J]. Fire Science and Technology, 2016, 35(6): 733-737.
|
| 4 |
周宁, 李海涛, 任常兴, 等. 多元混合气体爆炸特性及惰化防爆研究[J]. 安全与环境学报, 2018, 18(1): 165-171.
|
|
ZHOU Ning, LI Haitao, REN Changxing, et al. On the bursting and explosion characteristic features and inert inhibition of the mixed gas[J]. Journal of Safety and Environment, 2018, 18(1): 165-171.
|
| 5 |
周宁, 王宇飞, 赵会军, 等. 惰化系统极限氧浓度变化规律及监测预警研究[J]. 中国安全科学学报, 2018, 28(1): 75-80.
|
|
ZHOU Ning, WANG Yufei, ZHAO Huijun, et al. Study on change regulation of limit oxygen concentration and monitoring early warning system for inerting system[J]. China Safety Science Journal, 2018, 28(1): 75-80.
|
| 6 |
TREVIÑO C, MÉNDEZ F. Reduced kinetic mechanism for methane ignition[J]. Symposium (International) on Combustion, 1992, 24(1): 121-127.
|
| 7 |
MEDVEDEV V G, TELEGIN V G, TELEGIN G G. Statistical analysis of kinetics of an adiabatic thermal explosion[J]. Combustion, Explosion, and Shock Waves, 2009, 45(3): 274-277.
|
| 8 |
NIE Baisheng, YANG Longlong, GE Boqing, et al. Chemical kinetic characteristics of methane/air mixture explosion and its affecting factors[J]. Journal of Loss Prevention in the Process Industries, 2017, 49:675-682.
|
| 9 |
罗振敏, 张群, 王华, 等. 基于FLACS的受限空间瓦斯爆炸数值模拟[J]. 煤炭学报, 2013, 38(8): 1381-1387.
|
|
LUO Zhenmin, ZHANG Qun, WANG Hua, et al. Numerical simulation of gas explosion in confined space with FLACS[J]. Journal of China Coal Society, 2013, 38(8): 1381-1387.
|
| 10 |
罗振敏, 邓军, 郭晓波. 基于Gaussian的瓦斯爆炸微观反应机理[J]. 辽宁工程技术大学学报(自然科学版), 2008, 27(3): 325-328.
|
|
LUO Zhenmin, DENG Jun, GUO Xiaobo. Microcosmic mechanism of gas explosion based on Gaussian[J]. Journal of Liaoning Technical University (Natural Science), 2008, 27(3): 325-328.
|
| 11 |
梁运涛, 曾文. 定容燃烧弹中瓦斯爆炸的反应动力学模拟[J]. 燃烧科学与技术, 2010, 16(4): 375-381.
|
|
LIANG Yuntao, ZENG Wen. Kinetic simulation of gas explosion in constant volume bomb[J]. Journal of Combustion Science and Technology, 2010, 16(4): 375-381.
|
| 12 |
梁运涛, 王连聪, 罗海珠, 等. 定容燃烧反应器中瓦斯爆炸反应动力学计算模型[J]. 煤炭学报, 2015, 40(8): 1853-1858.
|
|
LIANG Yuntao, Wang Liancong, Luo Haizhu, et al. Computational model of reaction kinetic for gas explosion in constant volume combustion reactor[J]. Journal of China Coal Society, 2015, 40(8): 1853-1858.
|
| 13 |
贾宝山, 李艳红, 曾文, 等. 定容体系中氮气影响瓦斯爆炸反应的动力学模拟[J]. 过程工程学报, 2011, 11(5): 812-817.
|
|
JIA Baoshan, LI Yanhong, ZENG Wen, et al. Kinetic simulation for the effect of N2 content on gas explosion in an constant volume system[J]. The Chinese Journal of Process Engineering, 2011, 11(5): 812-817.
|
| 14 |
贾宝山, 温海燕, 梁运涛, 等. 煤矿巷道内N2及CO2抑制瓦斯爆炸的机理特性[J]. 煤炭学报, 2013, 38(3): 361-366.
|
|
JIA Baoshan, WEN Haiyan, Liang Yuntao, et al. Mechanism characteristics of CO2 and N2 inhibiting methane explosions in coal mine roadways[J]. Journal of China Coal Society, 2013, 38(3): 361-366.
|
| 15 |
贾宝山, 胡如霞, 李春苗, 等. 受限空间内C2H6对瓦斯爆炸的促进机理研究[J]. 中国安全生产科学技术, 2015, 11(10): 17-22.
|
|
JIA Baoshan, HU Ruxia, LI Chunmiao, et al. Study on promoting mechanism of C2H6 on gas explosion in confined space[J]. Journal of Safety Science and Technology, 2015, 11(10): 17-22.
|
| 16 |
贾宝山, 李艳红, 曾文, 等. 受限空间瓦斯爆炸链式反应机理的敏感性分析[J]. 环境工程, 2011, 29(S1): 318-323.
|
|
JIA Baoshan, LI Yanhong, ZENG Wen, et al. Sensitive analysis of chain reaction mechanism of gas explosion[J]. Environmental Engineering, 2011, 29(S1): 318-323.
|
| 17 |
贾宝山, 温海燕, 梁运涛, 等. 受限空间瓦斯爆炸与氢气促进机理研究[J]. 中国安全科学学报, 2012, 22(2): 81-87.
|
|
JIA Baoshan, WEN Haiyan, LIANG Yuntao, et al. Study on the methane explosion in an enclosed space and hydrogen promoting mechanism[J]. China Safety Science Journal, 2012, 22(2): 81-87.
|
| 18 |
贾宝山, 王小云, 张师一, 等. 受限空间中CO与水蒸汽阻尼瓦斯爆炸的反应动力学模拟研究[J]. 火灾科学, 2013, 22(3): 131-139.
|
|
JIA Baoshan, WANG Xiaoyun, ZHANG Shiyi, et al. Kinetic simulation of CO and water vapor damping the gas explosion in an enclosed space[J]. Fire Safety Science, 2013, 22(3): 131-139.
|
| 19 |
李孝斌, 李会荣, 何昆, 等. 甲烷爆炸感应期内CN/CH/CHO/CH2O/NCN特征光谱分析[J]. 煤炭学报, 2014, 39(10): 2042-2046.
|
|
LI Xiaobin, LI Huirong, HE Kun, et al. Analysis of characteristic spectrum of CN/CH/CHO/CH2O/NCN in induction period of methane explosion[J]. Journal of China Coal Society, 2014, 39(10): 2042-2046.
|
| 20 |
杨春丽, 刘艳, 胡玢, 等. 氮气和水蒸气对瓦斯爆炸基元反应的影响及抑爆机理分析[J]. 高压物理学报, 2017, 31(3): 301-308.
|
|
YANG Chunli, LIU Yan, HU Bin, et al. Effect of nitrogen and water vapor on methane-air mixture explosion elementary reaction and suppression mechanism[J]. Chinese Journal of High Pressure Physics, 2017, 31(3): 301-308.
|
| 21 |
YU Xiaozhe, YAN Xingqing, JI Wentao, et al. Effect of super-ambient conditions on the upper explosion limit of ethane/oxygen and ethylene/oxygen mixtures[J]. Journal of Loss Prevention in the Process Industries, 2019, 59: 100-105.
|
| 22 |
罗灿. 高温高压下乙烷、乙烯在纯氧中爆炸极限研究[D]. 大连: 大连理工大学, 2018.
|
|
LUO Can. Research on the explosion limit of ethane-oxygen and ethylene-oxygen mixtures at elevated temperatures and pressures[D]. Dalian: Dalian University of Technology, 2018.
|
| 23 |
喻健良, 纪文涛, 孙会利, 等. 乙烯/聚乙烯两相体系爆炸特性[J]. 化工学报, 2017, 68(12): 4841-4847.
|
|
YU Jianliang, JI Wentao, SUN Huili, et al. Explosibility of hybrid mixtures of ethylene and polyethylene dust[J]. CIESC Journal, 2017, 68(12): 4841-4847.
|
| 24 |
杨理, 饶国宁, 解立峰, 等. 扩散时间对乙烯-空气燃爆特性的影响[J]. 高压物理学报, 2015, 29(5): 369-376.
|
|
YANG Li, RAO Guoning, XIE Lifeng, et al. Effect of diffusion time on deflagration and detonation parameters of ethene-air[J]. Chinese Journal of High Pressure Physics, 2015, 29(5): 369-376.
|
| 25 |
乐慧慧, 张浩, 朱中南, 等. 乙烯-氧-醋酸-氮气-二氧化碳系统爆炸极限的测定研究[J]. 化学反应工程与工艺, 1995, 11(1): 80-85.
|
|
LE Huihui, ZHANG Hao, ZHU Zhongnan, et al. A study on the explosion limits for the system of ethylene-oxygen-acetic acid nitrogen-carbon dioxide[J].Chemical Reaction Engineering and Technology, 1995, 11(1): 80-85.
|
| 26 |
HOLTAPPELS K, BRINKMANN C, DIETLEN S, et al. Measurement and prediction of the inert gas influence on explosion limits for ethylene/nitrogen/air and ethylene/carbon-dioxide/air mixtures at elevated pressures[J]. Chemical Engineering & Technology, 2001, 24(12): 1263-1267.
|
| 27 |
张欣, 任常兴, 张琰, 等. 三种测试装置与判定标准对比研究可燃气体爆炸[J]. 消防科学与技术, 2018, 37(7): 863-866.
|
|
ZHANG Xin, REN Changxing, ZHANG Yan, et al. Research on flammable gas by three test devices and three criterions[J]. Fire Science and Technology, 2018, 37(7): 863-866.
|
| 28 |
LUTZ A E, KEE R J, MILLER J A. SENKIN: A Fortran program for predicting homogeneous gas phase chemical kinetics with sensitivity analysis[R]. Sandia National Laboratories Report, 1987.
|
| 29 |
RODAT S, ABANADES S, COULIÉ J, et al. Kinetic modelling of methane decomposition in a tubular solar reactor[J]. Chemical Engineering Journal, 2009, 146(1): 120-127.
|
| 30 |
CARRASCO N, ALCARAZ C, DUTUIT O, et al. Sensitivity of a Titan ionospheric model to the ion-molecule reaction parameters[J]. Planetary and Space Science, 2008, 56(12): 1644-1657.
|
| 31 |
RASMUSSEN C L, RASMUSSEN A E, GLARBORG P. Sensitizing effects of NO x on CH4 oxidation at high pressure[J]. Combustion and Flame, 2008, 154(3): 529-545.
|
| 32 |
GREGORY P, SMITH D G. Gri-Mech at 3.0[EB/OL]. , 2007.
|
| 33 |
韩美. 新的爆炸危险度F值[J]. 低温与特气, 1995, 13(2): 57-60.
|
|
HAN Mei. A new explosive hazard degree F [J]. Low Temperature and Specialty Gases, 1995, 13(2): 57-60.
|
 ), LIU Lu1, SU Bin1, SONG Fangzhi1
), LIU Lu1, SU Bin1, SONG Fangzhi1