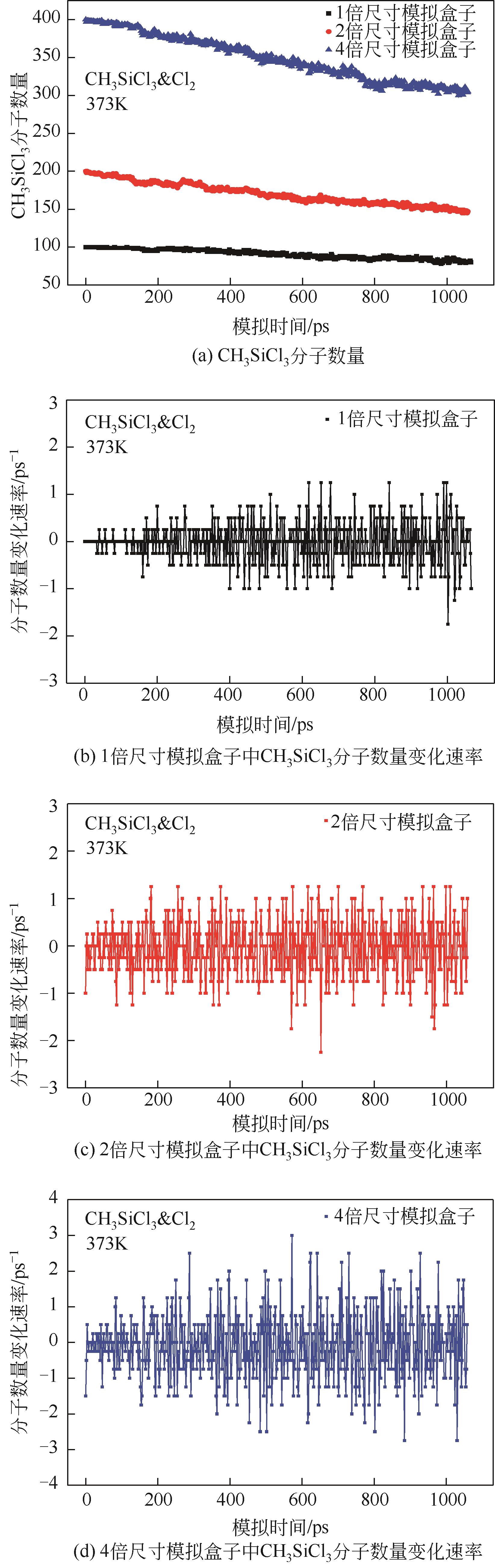
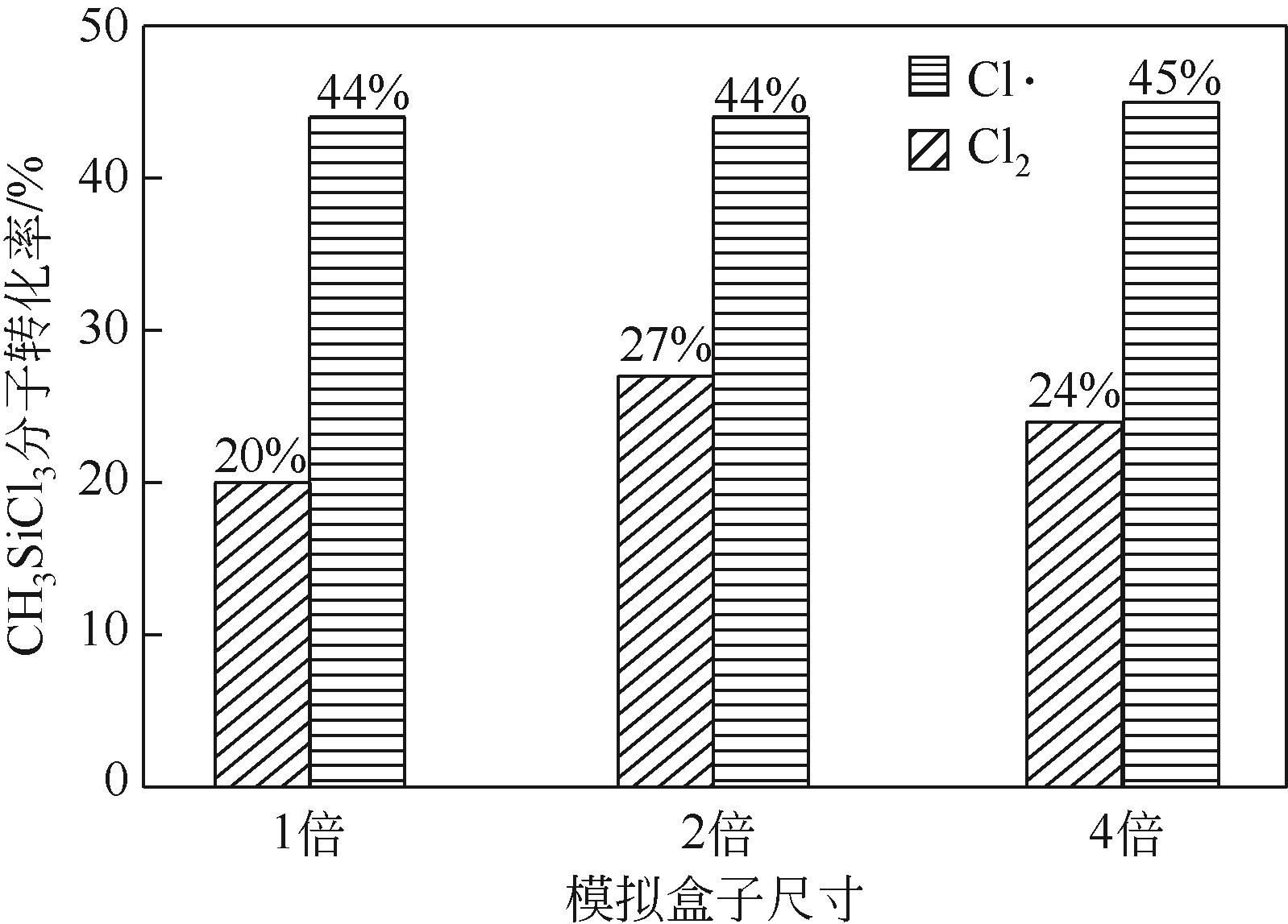
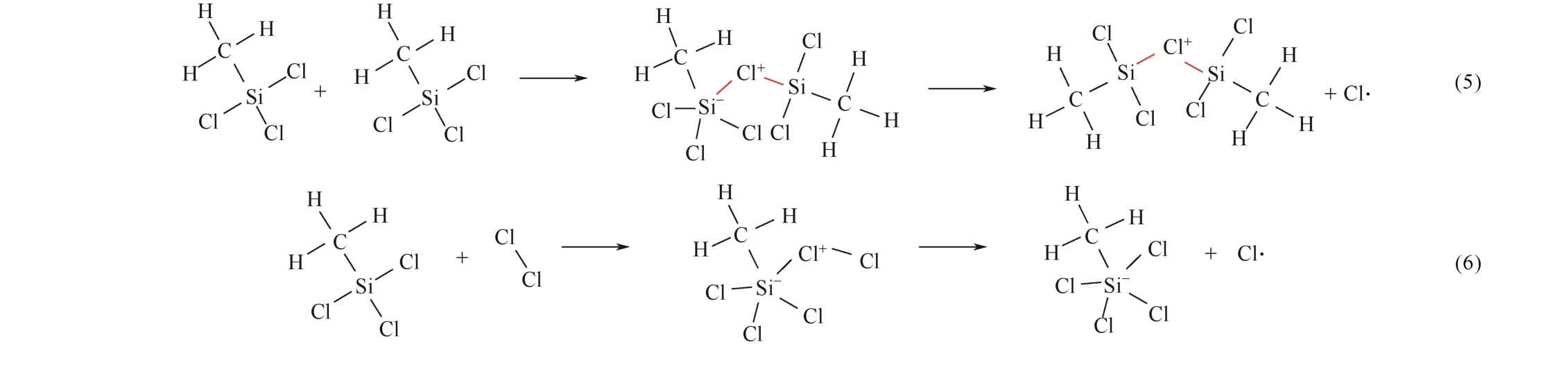
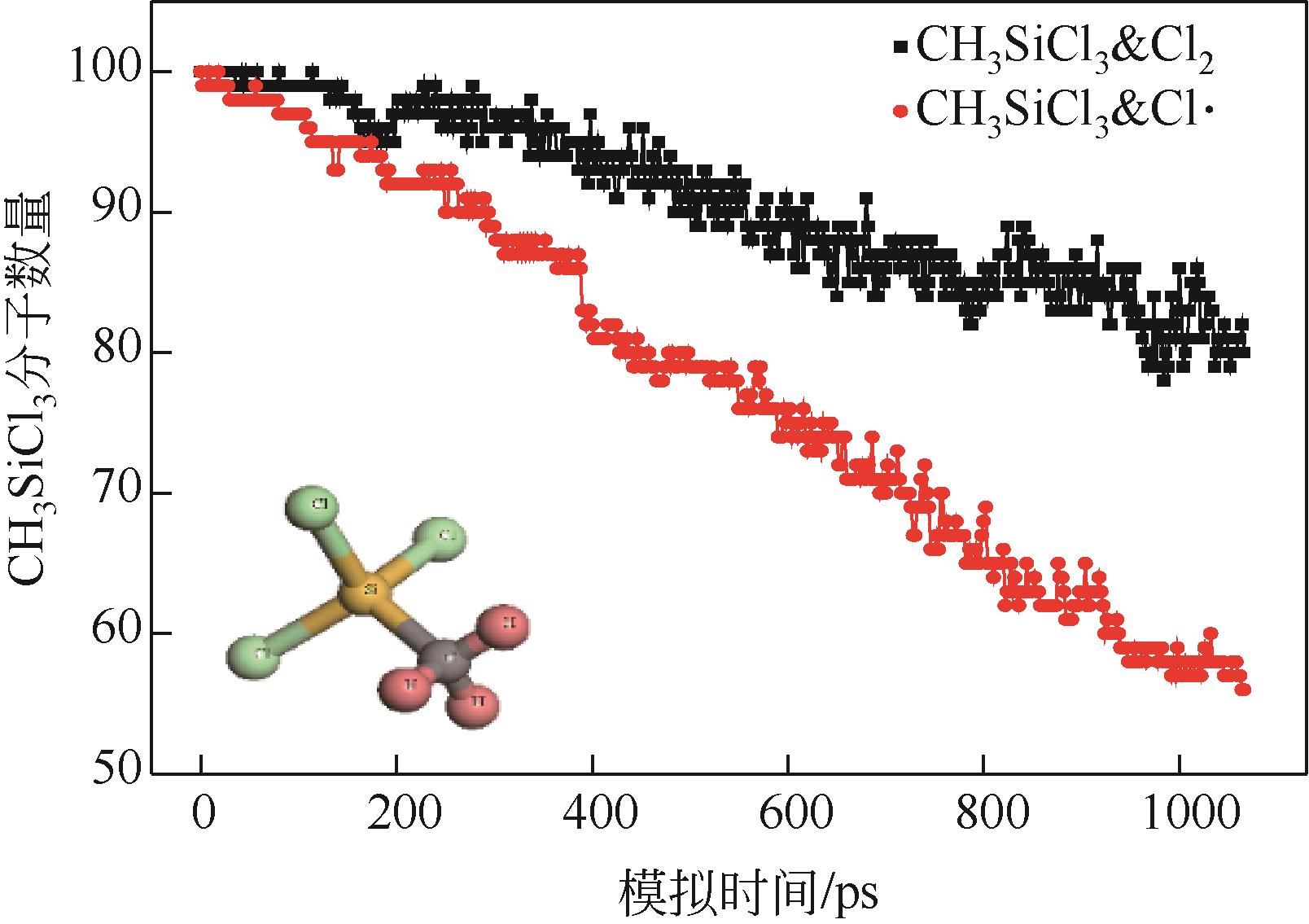
| 1 |
ZHANG L, SUN Y. Molecular simulation of adsorption and its implications to protein chromatography: a review[J]. Biochemical Engineering Journal, 2010, 48(3): 408-415.
|
| 2 |
GODDARD W, MERINOV B, VAN DUIN A, et al. Multi-paradigm multi-scale simulations for fuel cell catalysts and membranes[J]. Molecular Simulation, 2006, 32(3/4): 251-268.
|
| 3 |
贾建波. 神东煤镜质组结构模型的构建及其热解甲烷生成机理的分子模拟[D]. 太原: 太原理工大学, 2010.
|
|
JIA Jianbo. Construction of structural model and molecular simulation of methane formation mechanism during coal pyrolysis for Shendong vitrinite[D]. Taiyuan: Taiyuan University of Technology, 2010.
|
| 4 |
FRIESNER R A. Ab initio quantum chemistry: methodology and applications[J]. PNAS, 2005, 102(19): 6648-6653.
|
| 5 |
KLEIN M L, SHINODA W. Large-scale molecular dynamics simulations of self-assembling systems[J]. Science, 2008, 321(5890): 798-800.
|
| 6 |
MORTIER W J, GHOSH S K, SHANKAR S. Electronegativity-equalization method for the calculation of atomic charges in molecules[J]. Journal of the American Chemical Society, 1986, 108(15): 4315-4320.
|
| 7 |
STRACHAN A, VAN DUIN A C T, CHAKRABORTY D, et al. Shock waves in high-energy materials: the initial chemical events in nitramine RDX[J]. Physical Review Letters, 2003, 91(9): 098301.
|
| 8 |
WEN Y S, XUE X G, ZHOU X Q, et al. Twin induced sensitivity enhancement of HMX versus shock: a molecular reactive force field simulation[J]. The Journal of Physical Chemistry C, 2013, 117(46): 24368-24374.
|
| 9 |
CHENG X M, WANG Q D, LI J Q, et al. ReaxFF molecular dynamics simulations of oxidation of toluene at high temperatures[J]. The Journal of Physical Chemistry A, 2012, 116(40): 9811-9818.
|
| 10 |
ZHANG J L, WENG X X, HAN Y, et al. The effect of supercritical water on coal pyrolysis and hydrogen production: a combined ReaxFF and DFT study[J]. Fuel, 2013, 108: 682-690.
|
| 11 |
GODDARD W A, VAN DUIN A, CHENOWETH K, et al. Development of the ReaxFF reactive force field for mechanistic studies of catalytic selective oxidation processes on BiMoO x [J]. Topics in Catalysis, 2006, 38(1/2/3): 93-103.
|
| 12 |
LIU L C, JARAMILLO-BOTERO A, GODDARD W A, et al. Development of a ReaxFF reactive force field for ettringite and study of its mechanical failure modes from reactive dynamics simulations[J]. The Journal of Physical Chemistry A, 2012, 116(15): 3918-3925.
|
| 13 |
GAMALLO P, PRATS H, SAYÓS R. ReaxFF molecular dynamics simulations of CO collisions on an O-preadsorbed silica surface[J]. Journal of Molecular Modeling, 2014, 20(4): 1-11.
|
| 14 |
MANZANO H, MOEINI S, MARINELLI F, et al. Confined water dissociation in microporous defective silicates: mechanism, dipole distribution, and impact on substrate properties[J]. Journal of the American Chemical Society, 2012, 134(4): 2208-2215.
|
| 15 |
BERENDSEN H J C, POSTMA J P M, VAN GUNSTEREN W F, et al. Molecular dynamics with coupling to an external bath[J]. The Journal of Chemical Physics, 1984, 81(8): 3684-3690.
|
| 16 |
LIU J, LI X X, GUO L, et al. Reaction analysis and visualization of ReaxFF molecular dynamics simulations[J]. Journal of Molecular Graphics and Modelling, 2014, 53: 13-22.
|
| 17 |
JARAMILLO-BOTERO A, NASERIFAR S, GODDARD W A. General multiobjective force field optimization framework, with application to reactive force fields for silicon carbide[J]. Journal of Chemical Theory and Computation, 2014, 10(4): 1426-1439.
|
| 18 |
FENG M Y, JIANG X Z, MAO Q, et al. Initiation mechanisms of enhanced pyrolysis and oxidation of JP-10 (exo-tetrahydrodicyclopentadiene) on functionalized graphene sheets: insights from ReaxFF molecular dynamics simulations[J]. Fuel, 2019, 254: 115643.
|
| 19 |
CHENOWETH K, CHEUNG S, VAN DUIN A C T, et al. Simulations on the thermal decomposition of a poly(dimethylsiloxane) polymer using the ReaxFF reactive force field[J]. Journal of the American Chemical Society, 2005, 127(19): 7192-7202.
|
| 20 |
NIELSON K D, VAN DUIN A C T, OXGAARD J, et al. Development of the ReaxFF reactive force field for describing transition metal catalyzed reactions, with application to the initial stages of the catalytic formation of carbon nanotubes[J]. The Journal of Physical Chemistry A, 2005, 109(3): 493-499.
|
| 21 |
CHENOWETH K, VAN DUIN A C T, GODDARD W A. ReaxFF reactive force field for molecular dynamics simulations of hydrocarbon oxidation[J]. The Journal of Physical Chemistry A, 2008, 112(5): 1040-1053.
|
| 22 |
STRACHAN A, KOBER E M, VAN DUIN A C T, et al. Thermal decomposition of RDX from reactive molecular dynamics[J]. The Journal of Chemical Physics, 2005, 122(5): 054502.
|
| 23 |
YANG Z, SUN Y J, MA F, et al. Pyrolysis mechanisms of graphene oxide revealed by ReaxFF molecular dynamics simulation[J]. Applied Surface Science, 2020, 509: 145247.
|
| 24 |
万烨, 肖劲, 严大洲, 等. 光氯化反应脱除三氯氢硅中的甲基二氯硅烷[J]. 精细化工, 2020, 37(1): 201-206, 216.
|
|
WAN Ye, XIAO Jin, YAN Dazhou, et al. Removal of methyldichlorosilane from trichlorosilane via photochemical chlorination[J]. Fine Chemicals, 2020, 37(1): 201-206, 216.
|
 ), 严大洲1,2(
), 严大洲1,2( ), 杨涛1,2, 温国胜1,2, 韩治成1,2
), 杨涛1,2, 温国胜1,2, 韩治成1,2
 ), YAN Dazhou1,2(
), YAN Dazhou1,2( ), YANG Tao1,2, WEN Guosheng1,2, HAN Zhicheng1,2
), YANG Tao1,2, WEN Guosheng1,2, HAN Zhicheng1,2