化工进展 ›› 2025, Vol. 44 ›› Issue (9): 5377-5390.DOI: 10.16085/j.issn.1000-6613.2024-2118
• 资源与环境化工 • 上一篇
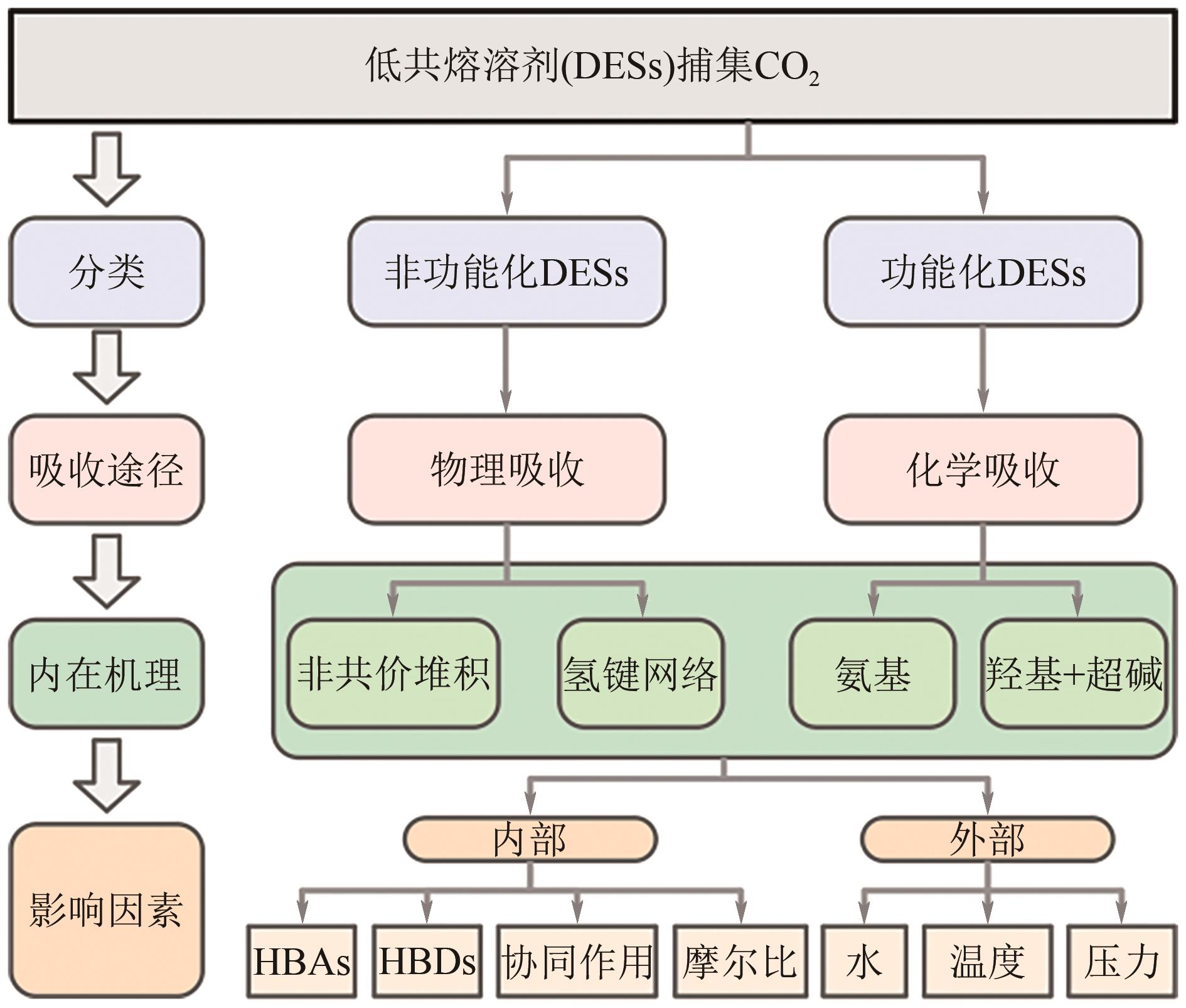
低共熔溶剂在二氧化碳捕集中的发展与应用
陈思铭1,2,3( ), 刘景超4, 钟志轩5, 张新柱3, 祝天浩6, 彭毅勍6, 游赛6, 王一凯6, 袁嘉骏6, 张永春7(
), 刘景超4, 钟志轩5, 张新柱3, 祝天浩6, 彭毅勍6, 游赛6, 王一凯6, 袁嘉骏6, 张永春7( )
)
- 1.中国矿业大学碳中和研究院,江苏 徐州 221116
2.中国矿业大学江苏省煤基温室气体减排与资源化利用重点实验室,江苏 徐州 221116
3.中国矿业大学化工学院,江苏 徐州 221116
4.青岛大学环境科学与工程学院,山东 青岛 2660754
5.中国矿业大学资源与地球科学学院,江苏 徐州 221116
6.中国矿业大学孙越崎学院,江苏 徐州 221116
7.大连理工大学化工学院,辽宁 大连 116024
-
收稿日期:2024-12-30修回日期:2025-05-09出版日期:2025-09-25发布日期:2025-09-30 -
通讯作者:陈思铭,张永春 -
作者简介:陈思铭(1988—),女,博士,副研究员,硕士生导师,研究方向为CO2捕集与转化。E-mail:chensiming@cumt.edu.cn。 -
基金资助:国家自然科学基金青年基金(52006112);徐州市重点研发计划社会发展项目(KC23293);中国博士后科学基金(2023M743768)
Development and application of deep eutectic solvents in carbon dioxide capture
CHEN Siming1,2,3( ), LIU Jingchao4, ZHONG Zhixuan5, ZHANG Xinzhu3, ZHU Tianhao6, PENG Yiqing6, YOU Sai6, WANG Yikai6, YUAN Jiajun6, ZHANG Yongchun7(
), LIU Jingchao4, ZHONG Zhixuan5, ZHANG Xinzhu3, ZHU Tianhao6, PENG Yiqing6, YOU Sai6, WANG Yikai6, YUAN Jiajun6, ZHANG Yongchun7( )
)
- 1.Institute of Carbon Neutralization, China University of Mining and Technology, Xuzhou 221116, Jiangsu, China
2.Jiangsu Key Laboratory of Coal-based Greenhouse Gas Control and Utilization, China University of Mining and Technology, Xuzhou 221116, Jiangsu, China
3.School of Chemical Engineering, China University of Mining and Technology, Xuzhou 221116, Jiangsu, China
4.School of Environmental Science and Engineering, Qingdao University, Qingdao 266075, Shandong, China
5.School of Resources and Geosciences, China University of Mining and Technology, Xuzhou 221116, Jiangsu, China
6.SUNYUEQI Honors College, China University of Mining and Technology, Xuzhou 221116, Jiangsu, China
7.School of Chemical Engineering, Dalian University of Technology, Dalian 116024, Liaoning, China
-
Received:2024-12-30Revised:2025-05-09Online:2025-09-25Published:2025-09-30 -
Contact:CHEN Siming, ZHANG Yongchun
摘要:
CO2捕集、利用和封存(CCUS)技术的开发是遏制全球CO2浓度增高的关键措施。其中以化学吸收为代表的CO2捕集技术尤为关键,然而传统化学吸收方法存在着低寿命、高能耗与腐蚀性等问题。低共熔溶剂(DESs)以其优异的物理化学性质及环境友好性,已成为开发新型CO2化学吸收剂的热点。本文总结了DESs在CO2捕集领域的研究现状,并深入探讨了其定义、物理化学特性及应用分类。随后进一步分析了影响DESs捕集CO2性能的因素,包括氢键受体(HBAs)和氢键供体(HBDs)的选择、摩尔比、水分含量、温度和压力等。最后,综合现有DESs种类及性能,提出了高效捕集CO2的DESs分子设计策略,旨在为未来DESs的开发和技术优化提供科学依据,以推进绿色溶剂在捕集CO2和促进可持续发展中的应用。
中图分类号:
引用本文
陈思铭, 刘景超, 钟志轩, 张新柱, 祝天浩, 彭毅勍, 游赛, 王一凯, 袁嘉骏, 张永春. 低共熔溶剂在二氧化碳捕集中的发展与应用[J]. 化工进展, 2025, 44(9): 5377-5390.
CHEN Siming, LIU Jingchao, ZHONG Zhixuan, ZHANG Xinzhu, ZHU Tianhao, PENG Yiqing, YOU Sai, WANG Yikai, YUAN Jiajun, ZHANG Yongchun. Development and application of deep eutectic solvents in carbon dioxide capture[J]. Chemical Industry and Engineering Progress, 2025, 44(9): 5377-5390.
| 类型 | 通式 | 组成 |
|---|---|---|
| Ⅰ | Cat+X-zMCl x | M=Zn, Sn, Fe, Al, Ga, In等 |
| Ⅱ | Cat+X-zMCl x ·yH2O | M=Cr, Co, Cu, Ni, Fe等 |
| Ⅲ | Cat+X-zR*Z | Z=CONH2, COOH, OH |
| Ⅳ | MCl x +R*Z | M=Al, Zn等; Z=CONH2, OH |
表1 DESs的分类
| 类型 | 通式 | 组成 |
|---|---|---|
| Ⅰ | Cat+X-zMCl x | M=Zn, Sn, Fe, Al, Ga, In等 |
| Ⅱ | Cat+X-zMCl x ·yH2O | M=Cr, Co, Cu, Ni, Fe等 |
| Ⅲ | Cat+X-zR*Z | Z=CONH2, COOH, OH |
| Ⅳ | MCl x +R*Z | M=Al, Zn等; Z=CONH2, OH |
| HBA | HBD | HBA∶HBD | T/K | P/kPa | CO2溶解度/mol·kg-1 | 文献 |
|---|---|---|---|---|---|---|
| BHDE | AC | 1∶2 | 298.15 | 210~2026 | 0.064~0.84 | [ |
| BHDE | LA | 1∶2 | 298.15 | 283~2086 | 0.016~0.50 | [ |
| BTEA | AC | 1∶2 | 298.15 | 325~2054 | 0.13~0.97 | [ |
| BTMA | AC | 1∶2 | 298.15 | 219~2037 | 0.078~1.45 | [ |
| BTMA | Gly | 1∶2 | 298.15 | 394~2026 | 0.037~0.26 | [ |
| [BTPP]Br | EG | 1∶12 | 298.15 | 1000 | 0.6 | [ |
| [BTPP]Cl | Gly | 1∶12 | 298.15 | 1000 | 0.47 | [ |
| ChCl | MEA | 1∶7 | 298.15 | 182~2035 | 0.78~3.58 | [ |
| Gua | MEA | 1∶2 | 298.15 | 226~2025 | 0.31~1.66 | [ |
| MTPP | PG | 1∶4 | 298.15 | 220~2026 | 0.022~0.55 | [ |
| MTPP | AC | 1∶4 | 298.15 | 173~2014 | 0.073~3.02 | [ |
| MTPP | EG | 1∶3 | 298.15 | 192~2018 | 0.045~0.35 | [ |
| MTPP | Gly | 1∶4 | 298.15 | 161~2026 | 0.009~0.29 | [ |
| MTPP | LV | 1∶3 | 298.15 | 301~2068 | 0.024~0.69 | [ |
| MTPP | MEA | 1∶6 | 298.15 | 1000 | 1.63 | [ |
| MTPP | MEA | 1∶7 | 298.15 | 1000 | 1.46 | [ |
| MTPP | MEA | 1∶8 | 298.15 | 1000 | 1.44 | [ |
| TBAC | DecA | 1∶2 | 298.15~323.15 | 90~1990 | 0.042~1.52 | [ |
| [N8881]Br | DecA | 1∶2 | 298.15~323.15 | 90~1990 | 0.041~1.31 | [ |
| [N8881]Cl | DecA | 1∶2 | 298.15~308.15 | 90~1990 | 0.045~1.35 | [ |
| [N8888]Br | DecA | 1∶2 | 298.15~323.15 | 90~1990 | 0.039~1.33 | [ |
| [N8888]Cl | DecA | 1∶1.5 | 298.15~323.15 | 90~1990 | 0.041~1.41 | [ |
| [N8888]Cl | DecA | 1∶2 | 298.15~323.15 | 90~1990 | 0.042~1.41 | [ |
| TBAB | AC | 1∶2 | 298.15 | 388~2011 | 0.14~1.13 | [ |
| TBAB | DEA | 1∶6 | 298.15 | 1000 | 0.85 | [ |
| TBAB | MEA | 1∶6 | 298.15 | 1000 | 1.34 | [ |
| TBAB | MEA | 1∶6 | 298.15 | 351~2021 | 0.44~2.78 | [ |
| TBAB | MEA | 1∶7 | 298.15 | 381~2040 | 0.53~3.01 | [ |
| TBAB | TEA | 1∶3 | 298.15 | 1000 | 0.47 | [ |
| TBAC | AC | 1∶2 | 298.15 | 348~2002 | 0.18~1.41 | [ |
| TEAC | AC | 1∶2 | 298.15 | 281~2018 | 0.14~1.18 | [ |
| TEAC | AC | 1∶3 | 298.15 | 397~2016 | 0.13~1.23 | [ |
| TEAC | OCT | 1∶3 | 298.15 | 353~2018 | 0.16~1.39 | [ |
| TEMA | AC | 1∶2 | 298.15 | 198~1837 | 0.081~1.18 | [ |
| TEMA | EG | 1∶2 | 298.15 | 138~1345 | 0.062~0.63 | [ |
| TEMA | Gly | 1∶2 | 298.15 | 150~1648 | 0.017~0.43 | [ |
| TEMA | LA | 1∶2 | 298.15 | 143~1863 | 0.047~0.53 | [ |
| TEMA | LV | 1∶2 | 298.15 | 136~1617 | 0.057~0.61 | [ |
| TMAC | AC | 1∶4 | 298.15 | 294~2096 | 0.12~1.56 | [ |
| TPAC | AC | 1∶6 | 298.15 | 350~2030 | 0.25~1.72 | [ |
| TPAC | MEA | 1∶4 | 298.15 | 481~2009 | 0.34~1.43 | [ |
| TPAC | MEA | 1∶7 | 298.15 | 357~2019 | 1.71~3.53 | [ |
表2 非功能化DESs的CO2捕集性能
| HBA | HBD | HBA∶HBD | T/K | P/kPa | CO2溶解度/mol·kg-1 | 文献 |
|---|---|---|---|---|---|---|
| BHDE | AC | 1∶2 | 298.15 | 210~2026 | 0.064~0.84 | [ |
| BHDE | LA | 1∶2 | 298.15 | 283~2086 | 0.016~0.50 | [ |
| BTEA | AC | 1∶2 | 298.15 | 325~2054 | 0.13~0.97 | [ |
| BTMA | AC | 1∶2 | 298.15 | 219~2037 | 0.078~1.45 | [ |
| BTMA | Gly | 1∶2 | 298.15 | 394~2026 | 0.037~0.26 | [ |
| [BTPP]Br | EG | 1∶12 | 298.15 | 1000 | 0.6 | [ |
| [BTPP]Cl | Gly | 1∶12 | 298.15 | 1000 | 0.47 | [ |
| ChCl | MEA | 1∶7 | 298.15 | 182~2035 | 0.78~3.58 | [ |
| Gua | MEA | 1∶2 | 298.15 | 226~2025 | 0.31~1.66 | [ |
| MTPP | PG | 1∶4 | 298.15 | 220~2026 | 0.022~0.55 | [ |
| MTPP | AC | 1∶4 | 298.15 | 173~2014 | 0.073~3.02 | [ |
| MTPP | EG | 1∶3 | 298.15 | 192~2018 | 0.045~0.35 | [ |
| MTPP | Gly | 1∶4 | 298.15 | 161~2026 | 0.009~0.29 | [ |
| MTPP | LV | 1∶3 | 298.15 | 301~2068 | 0.024~0.69 | [ |
| MTPP | MEA | 1∶6 | 298.15 | 1000 | 1.63 | [ |
| MTPP | MEA | 1∶7 | 298.15 | 1000 | 1.46 | [ |
| MTPP | MEA | 1∶8 | 298.15 | 1000 | 1.44 | [ |
| TBAC | DecA | 1∶2 | 298.15~323.15 | 90~1990 | 0.042~1.52 | [ |
| [N8881]Br | DecA | 1∶2 | 298.15~323.15 | 90~1990 | 0.041~1.31 | [ |
| [N8881]Cl | DecA | 1∶2 | 298.15~308.15 | 90~1990 | 0.045~1.35 | [ |
| [N8888]Br | DecA | 1∶2 | 298.15~323.15 | 90~1990 | 0.039~1.33 | [ |
| [N8888]Cl | DecA | 1∶1.5 | 298.15~323.15 | 90~1990 | 0.041~1.41 | [ |
| [N8888]Cl | DecA | 1∶2 | 298.15~323.15 | 90~1990 | 0.042~1.41 | [ |
| TBAB | AC | 1∶2 | 298.15 | 388~2011 | 0.14~1.13 | [ |
| TBAB | DEA | 1∶6 | 298.15 | 1000 | 0.85 | [ |
| TBAB | MEA | 1∶6 | 298.15 | 1000 | 1.34 | [ |
| TBAB | MEA | 1∶6 | 298.15 | 351~2021 | 0.44~2.78 | [ |
| TBAB | MEA | 1∶7 | 298.15 | 381~2040 | 0.53~3.01 | [ |
| TBAB | TEA | 1∶3 | 298.15 | 1000 | 0.47 | [ |
| TBAC | AC | 1∶2 | 298.15 | 348~2002 | 0.18~1.41 | [ |
| TEAC | AC | 1∶2 | 298.15 | 281~2018 | 0.14~1.18 | [ |
| TEAC | AC | 1∶3 | 298.15 | 397~2016 | 0.13~1.23 | [ |
| TEAC | OCT | 1∶3 | 298.15 | 353~2018 | 0.16~1.39 | [ |
| TEMA | AC | 1∶2 | 298.15 | 198~1837 | 0.081~1.18 | [ |
| TEMA | EG | 1∶2 | 298.15 | 138~1345 | 0.062~0.63 | [ |
| TEMA | Gly | 1∶2 | 298.15 | 150~1648 | 0.017~0.43 | [ |
| TEMA | LA | 1∶2 | 298.15 | 143~1863 | 0.047~0.53 | [ |
| TEMA | LV | 1∶2 | 298.15 | 136~1617 | 0.057~0.61 | [ |
| TMAC | AC | 1∶4 | 298.15 | 294~2096 | 0.12~1.56 | [ |
| TPAC | AC | 1∶6 | 298.15 | 350~2030 | 0.25~1.72 | [ |
| TPAC | MEA | 1∶4 | 298.15 | 481~2009 | 0.34~1.43 | [ |
| TPAC | MEA | 1∶7 | 298.15 | 357~2019 | 1.71~3.53 | [ |
| HBA | HBD | HBA∶HBD | T/K | P/kPa | CO2溶解度(摩尔分数) | 文献 |
|---|---|---|---|---|---|---|
| ChCl | EG | 1∶2 | 303.15 | 10000 | 0.046 | [ |
| ChCl | DEG | 1∶3 | 303.15 | 8200 | 0.028 | [ |
| ChCl | DEG | 1∶4 | 303.15 | 8300 | 0.028 | [ |
| TBAB | EG | 1∶2 | 303.15 | 7800 | 0.031 | [ |
| TBAB | EG | 1∶3 | 303.15 | 8800 | 0.039 | [ |
| TBAB | EG | 1∶4 | 303.15 | 8300 | 0.032 | [ |
| TBAB | DEG | 1∶2 | 303.15 | 7200 | 0.034 | [ |
| TBAB | DEG | 1∶3 | 303.15 | 6900 | 0.040 | [ |
| TBAB | DEG | 1∶4 | 303.15 | 9000 | 0.084 | [ |
| ChCl | MDEA | 1∶6 | 303.15 | 7100 | 0.160 | [ |
| ChCl | MDEA | 1∶7 | 303.15 | 5900 | 0.120 | [ |
| ChCl | DEA | 1∶6 | 303.15 | 8000 | 0.078 | [ |
| TBTA | DEA | 1∶6 | 303.15 | 6000 | 0.050 | [ |
| TBAB | MDEA | 1∶3 | 303.15 | 8100 | 0.020 | [ |
| TBAB | MDEA | 1∶4 | 303.15 | 7000 | 0.020 | [ |
| ChCl | PG | 1∶2 | 303.15 | 9000 | 0.025 | [ |
| ChCl | GLY | 1∶2 | 303.15 | 11000 | 0.030 | [ |
| ChCl | MA | 1∶1 | 303.15 | 10000 | 0.025 | [ |
| TMG | PhAc | 1∶2 | 313.15 | 400 | (38.1±1.2)mg/g | [ |
| TMG | GA | 1∶2 | 313.15 | 400 | (2.84±0.19)mg/g | [ |
| TMG | EA | 1∶2 | 313.15 | 400 | (0.52±0.04)mg/g | [ |
表3 NADESs的CO2捕集性能
| HBA | HBD | HBA∶HBD | T/K | P/kPa | CO2溶解度(摩尔分数) | 文献 |
|---|---|---|---|---|---|---|
| ChCl | EG | 1∶2 | 303.15 | 10000 | 0.046 | [ |
| ChCl | DEG | 1∶3 | 303.15 | 8200 | 0.028 | [ |
| ChCl | DEG | 1∶4 | 303.15 | 8300 | 0.028 | [ |
| TBAB | EG | 1∶2 | 303.15 | 7800 | 0.031 | [ |
| TBAB | EG | 1∶3 | 303.15 | 8800 | 0.039 | [ |
| TBAB | EG | 1∶4 | 303.15 | 8300 | 0.032 | [ |
| TBAB | DEG | 1∶2 | 303.15 | 7200 | 0.034 | [ |
| TBAB | DEG | 1∶3 | 303.15 | 6900 | 0.040 | [ |
| TBAB | DEG | 1∶4 | 303.15 | 9000 | 0.084 | [ |
| ChCl | MDEA | 1∶6 | 303.15 | 7100 | 0.160 | [ |
| ChCl | MDEA | 1∶7 | 303.15 | 5900 | 0.120 | [ |
| ChCl | DEA | 1∶6 | 303.15 | 8000 | 0.078 | [ |
| TBTA | DEA | 1∶6 | 303.15 | 6000 | 0.050 | [ |
| TBAB | MDEA | 1∶3 | 303.15 | 8100 | 0.020 | [ |
| TBAB | MDEA | 1∶4 | 303.15 | 7000 | 0.020 | [ |
| ChCl | PG | 1∶2 | 303.15 | 9000 | 0.025 | [ |
| ChCl | GLY | 1∶2 | 303.15 | 11000 | 0.030 | [ |
| ChCl | MA | 1∶1 | 303.15 | 10000 | 0.025 | [ |
| TMG | PhAc | 1∶2 | 313.15 | 400 | (38.1±1.2)mg/g | [ |
| TMG | GA | 1∶2 | 313.15 | 400 | (2.84±0.19)mg/g | [ |
| TMG | EA | 1∶2 | 313.15 | 400 | (0.52±0.04)mg/g | [ |
| HBA | HBD | 助剂 | HBA∶HBD∶助剂 | T/K | P/kPa | CO2溶解度(质量分数)/% | 文献 |
|---|---|---|---|---|---|---|---|
| ChCl | Gly | DBN | 1∶2∶3 | RT③ | 101 | 9.6 | [ |
| ChCl | Gly | DBN | 1∶2∶6 | RT | 101 | 10.3 | [ |
| ChCl | Gly | DBN | 1∶2∶7 | RT | 101 | 10.5 | [ |
| ChCl | Gly | DBN | 1∶2∶8 | RT | 101 | 10.3 | [ |
| ChCl | Gly | DBN | 1∶3∶10 | RT | 101 | 10.4 | [ |
| ChCl | Gly | DBU | 1∶2∶6 | RT | 101 | 3.55 | [ |
| ChCl | Gly | MTBD | 1∶2∶6 | RT | 101 | 10 | [ |
| ChCl | MEA | 1∶5 | 303.15 | 101 | 25.23 | [ | |
| BmimCl | Im | DBN | 1∶1∶1 | 298.15 | 101 | (1.02) ① | [ |
| BmimCl | Im | DBN | 1∶1∶2 | 298.15 | 101 | (0.97) ① | [ |
| BmimCl | Im | DBN | 1∶2∶1 | 298.15 | 101 | (1.07) ① | [ |
| DBN | DMLU | 2∶1 | 318.15 | 101 | 4.27;2.47 ② | [ | |
| DBN | DMU | 2∶1 | 318.15 | 101 | 17.34;16.8 ② | [ | |
| [HDBU][Im] | EG | 7∶3 | 313.15 | 101 | 0.141 | [ | |
| [HDBU][Ind] | EG | 7∶3 | 313.15 | 101 | 0.117 | [ | |
| [HDBU][Triz] | EG | 7∶3 | 313.15 | 101 | 0.108 | [ | |
| HmimCl | AP | 1∶1 | RT | 101 | 2.0 (0.04) ① | [ | |
| HmimCl | AP | 1∶2 | RT | 101 | 9.5 (0.21) ① | [ | |
| HmimCl | AP | 1∶3 | RT | 101 | 13.9 (0.30) ① | [ | |
| HmimCl | AP | 1∶4 | RT | 101 | 19.4 (0.37) ① | [ | |
| HmimCl | PEHA | 1∶4 | RT | 101 | 8.4 (0.4) ① | [ | |
| HmimCl | DETA | 1∶4 | RT | 101 | 22.8 (0.55) ① | [ | |
| HmimCl | EDA | 1∶1 | RT | 101 | 9.0 (0.19) ① | [ | |
| HmimCl | EDA | 1∶2 | RT | 101 | 25.0 (0.45) ① | [ | |
| HmimCl | EDA | 1∶3 | RT | 101 | 26.7 (0.45) ① | [ | |
| HmimCl | EDA | 1∶4 | RT | 101 | 30.8 (0.50) ① | [ | |
| HmimCl | TEPA | 1∶4 | RT | 101 | 9.9 (0.39) ① | [ | |
| MEAC | AP | 1∶1 | RT | 101 | 15.8 (0.28) ① | [ | |
| MEAC | AP | 1∶2 | RT | 101 | 21 (0.37) ① | [ | |
| MEAC | AP | 1∶3 | RT | 101 | 24.3 (0.42) ① | [ | |
| MEAC | AP | 1∶4 | RT | 101 | 26.3 (0.46) ① | [ | |
| MEAC | DETA | 1∶4 | RT | 101 | 25.5 (0.57) ① | [ | |
| MEAC | EDA | 1∶1 | RT | 101 | 23.5 (0.38) ① | [ | |
| MEAC | EDA | 1∶2 | RT | 101 | 30.9 (0.47) ① | [ | |
| MEAC | EDA | 1∶3 | RT | 101 | 36.5 (0.54) ① | [ | |
| MEAC | EDA | 1∶3 | 303.15 | 101 | 33.7 | [ | |
| MEAC | EDA | 1∶4 | RT | 101 | 39.0 (0.57) ① | [ | |
| MEAC | PEHA | 1∶4 | RT | 101 | 12.7 (0.59) ① | [ | |
| MEAC | TEPA | 1∶4 | RT | 101 | 16.6 (0.63) ① | [ | |
| [N2222][Im] | EG | 1∶2 | 298.15 | 101 | 12.9 (0.94) ① | [ | |
| [N2222][Triz] | EG | 1∶2 | 298.15 | 101 | 12.5 (0.92) ① | [ | |
| [P2222][Im] | EG | 1∶2 | 298.15 | 101 | 11.8 (0.91) ① | [ | |
| [P2222][Triz] | EG | 1∶2 | 298.15 | 101 | 11.8 (0.91) ① | [ | |
| MEACl | EDA | 1∶3 | 303.15 | 101 | 31.5 | [ | |
| TBAB | AMP | 1∶3 | RT | 101 | 10.5 (0.35) ① | [ | |
| TBAB | AMP | 1∶4 | RT | 101 | 12.2 (0.38) ① | [ | |
| TBAB | AP | 1∶2 | RT | 101 | 11.1 (0.43) ① | [ | |
| TBAB | AP | 1∶3 | RT | 101 | 15.6 (0.49) ① | [ | |
| TBAB | AP | 1∶4 | RT | 101 | 18.1 (0.51) ① | [ | |
| TEAC | DA | 1∶3 | 303.15 | 101 | 24.2 | [ | |
| [TETA]Cl | DG | 1∶2 | 313.15 | 101 | 0.159 | [ | |
| [TEPA]Cl | thymol | 1∶3 | 313.15 | 101 | 0.088 (1.355) ① | [ | |
| [TETA]Cl | EG | 1∶3 | 313.15 | 101 | 0.175 | [ | |
| [TETA]Cl | thymol | 1∶3 | 313.15 | 101 | 0.09 (1.298) ① | [ | |
| UEC | EDA | 1∶3 | 303.15 | 101 | 17.8 | [ | |
| TPAC | EA | 1∶6 | 298.15 | 20.0 | 0.276 | [ | |
| TPAC | AC | 1∶4 | 298.15 | 20.0 | 0.240 | [ | |
| TPAC | AC | 1∶7 | 298.15 | 20.0 | 0.435 | [ | |
| TBAB | OCT+OA | 1∶2 | 298.15 | 16.46 | 0.285 | [ | |
| TPAC | DecA | 1∶2 | 298.15 | 19.9 | 0.299 | [ | |
| TMP | thymol | 1∶3 | 313.15 | 10.13 | 1.355 | [ |
表4 功能化DESs的CO2捕集性能
| HBA | HBD | 助剂 | HBA∶HBD∶助剂 | T/K | P/kPa | CO2溶解度(质量分数)/% | 文献 |
|---|---|---|---|---|---|---|---|
| ChCl | Gly | DBN | 1∶2∶3 | RT③ | 101 | 9.6 | [ |
| ChCl | Gly | DBN | 1∶2∶6 | RT | 101 | 10.3 | [ |
| ChCl | Gly | DBN | 1∶2∶7 | RT | 101 | 10.5 | [ |
| ChCl | Gly | DBN | 1∶2∶8 | RT | 101 | 10.3 | [ |
| ChCl | Gly | DBN | 1∶3∶10 | RT | 101 | 10.4 | [ |
| ChCl | Gly | DBU | 1∶2∶6 | RT | 101 | 3.55 | [ |
| ChCl | Gly | MTBD | 1∶2∶6 | RT | 101 | 10 | [ |
| ChCl | MEA | 1∶5 | 303.15 | 101 | 25.23 | [ | |
| BmimCl | Im | DBN | 1∶1∶1 | 298.15 | 101 | (1.02) ① | [ |
| BmimCl | Im | DBN | 1∶1∶2 | 298.15 | 101 | (0.97) ① | [ |
| BmimCl | Im | DBN | 1∶2∶1 | 298.15 | 101 | (1.07) ① | [ |
| DBN | DMLU | 2∶1 | 318.15 | 101 | 4.27;2.47 ② | [ | |
| DBN | DMU | 2∶1 | 318.15 | 101 | 17.34;16.8 ② | [ | |
| [HDBU][Im] | EG | 7∶3 | 313.15 | 101 | 0.141 | [ | |
| [HDBU][Ind] | EG | 7∶3 | 313.15 | 101 | 0.117 | [ | |
| [HDBU][Triz] | EG | 7∶3 | 313.15 | 101 | 0.108 | [ | |
| HmimCl | AP | 1∶1 | RT | 101 | 2.0 (0.04) ① | [ | |
| HmimCl | AP | 1∶2 | RT | 101 | 9.5 (0.21) ① | [ | |
| HmimCl | AP | 1∶3 | RT | 101 | 13.9 (0.30) ① | [ | |
| HmimCl | AP | 1∶4 | RT | 101 | 19.4 (0.37) ① | [ | |
| HmimCl | PEHA | 1∶4 | RT | 101 | 8.4 (0.4) ① | [ | |
| HmimCl | DETA | 1∶4 | RT | 101 | 22.8 (0.55) ① | [ | |
| HmimCl | EDA | 1∶1 | RT | 101 | 9.0 (0.19) ① | [ | |
| HmimCl | EDA | 1∶2 | RT | 101 | 25.0 (0.45) ① | [ | |
| HmimCl | EDA | 1∶3 | RT | 101 | 26.7 (0.45) ① | [ | |
| HmimCl | EDA | 1∶4 | RT | 101 | 30.8 (0.50) ① | [ | |
| HmimCl | TEPA | 1∶4 | RT | 101 | 9.9 (0.39) ① | [ | |
| MEAC | AP | 1∶1 | RT | 101 | 15.8 (0.28) ① | [ | |
| MEAC | AP | 1∶2 | RT | 101 | 21 (0.37) ① | [ | |
| MEAC | AP | 1∶3 | RT | 101 | 24.3 (0.42) ① | [ | |
| MEAC | AP | 1∶4 | RT | 101 | 26.3 (0.46) ① | [ | |
| MEAC | DETA | 1∶4 | RT | 101 | 25.5 (0.57) ① | [ | |
| MEAC | EDA | 1∶1 | RT | 101 | 23.5 (0.38) ① | [ | |
| MEAC | EDA | 1∶2 | RT | 101 | 30.9 (0.47) ① | [ | |
| MEAC | EDA | 1∶3 | RT | 101 | 36.5 (0.54) ① | [ | |
| MEAC | EDA | 1∶3 | 303.15 | 101 | 33.7 | [ | |
| MEAC | EDA | 1∶4 | RT | 101 | 39.0 (0.57) ① | [ | |
| MEAC | PEHA | 1∶4 | RT | 101 | 12.7 (0.59) ① | [ | |
| MEAC | TEPA | 1∶4 | RT | 101 | 16.6 (0.63) ① | [ | |
| [N2222][Im] | EG | 1∶2 | 298.15 | 101 | 12.9 (0.94) ① | [ | |
| [N2222][Triz] | EG | 1∶2 | 298.15 | 101 | 12.5 (0.92) ① | [ | |
| [P2222][Im] | EG | 1∶2 | 298.15 | 101 | 11.8 (0.91) ① | [ | |
| [P2222][Triz] | EG | 1∶2 | 298.15 | 101 | 11.8 (0.91) ① | [ | |
| MEACl | EDA | 1∶3 | 303.15 | 101 | 31.5 | [ | |
| TBAB | AMP | 1∶3 | RT | 101 | 10.5 (0.35) ① | [ | |
| TBAB | AMP | 1∶4 | RT | 101 | 12.2 (0.38) ① | [ | |
| TBAB | AP | 1∶2 | RT | 101 | 11.1 (0.43) ① | [ | |
| TBAB | AP | 1∶3 | RT | 101 | 15.6 (0.49) ① | [ | |
| TBAB | AP | 1∶4 | RT | 101 | 18.1 (0.51) ① | [ | |
| TEAC | DA | 1∶3 | 303.15 | 101 | 24.2 | [ | |
| [TETA]Cl | DG | 1∶2 | 313.15 | 101 | 0.159 | [ | |
| [TEPA]Cl | thymol | 1∶3 | 313.15 | 101 | 0.088 (1.355) ① | [ | |
| [TETA]Cl | EG | 1∶3 | 313.15 | 101 | 0.175 | [ | |
| [TETA]Cl | thymol | 1∶3 | 313.15 | 101 | 0.09 (1.298) ① | [ | |
| UEC | EDA | 1∶3 | 303.15 | 101 | 17.8 | [ | |
| TPAC | EA | 1∶6 | 298.15 | 20.0 | 0.276 | [ | |
| TPAC | AC | 1∶4 | 298.15 | 20.0 | 0.240 | [ | |
| TPAC | AC | 1∶7 | 298.15 | 20.0 | 0.435 | [ | |
| TBAB | OCT+OA | 1∶2 | 298.15 | 16.46 | 0.285 | [ | |
| TPAC | DecA | 1∶2 | 298.15 | 19.9 | 0.299 | [ | |
| TMP | thymol | 1∶3 | 313.15 | 10.13 | 1.355 | [ |
| [1] | ROGELJ Joeri, HUPPMANN Daniel, KREY Volker, et al. A new scenario logic for the Paris Agreement long-term temperature goal[J]. Nature, 2019, 573(7774): 357-363. |
| [2] | LIU Helei, TANTIKHAJORNGOSOL Puttipong, CHAN Christine, et al. Technology development and applications of artificial intelligence for post-combustion carbon dioxide capture: Critical literature review and perspectives[J]. International Journal of Greenhouse Gas Control, 2021, 108: 103307. |
| [3] | YOUNAS M, SOHAIL M, LEONG L K, et al. Feasibility of CO2 adsorption by solid adsorbents: A review on low-temperature systems[J]. International Journal of Environmental Science and Technology, 2016, 13(7): 1839-1860. |
| [4] | CHEN Pao chi, LAI Yanlin. Optimization in the stripping process of CO2 gas using mixed amines[J]. Energies, 2019, 12(11): 2202. |
| [5] | GAO Wanlin, LIANG Shuyu, WANG Rujie, et al. Industrial carbon dioxide capture and utilization: State of the art and future challenges[J]. Chemical Society Reviews, 2020, 49(23): 8584-8686. |
| [6] | WU Shu-Yii, LIU Yongfang, CHU Chen-Yeon, et al. Optimal absorbent evaluation for the CO2 separating process by absorption loading, desorption efficiency, cost, and environmental tolerance[J]. International Journal of Green Energy, 2015, 12(10): 1025-1030. |
| [7] | OLAJIRE Abass A. CO2 capture and separation technologies for end-of-pipe applications—A review[J]. Energy, 2010, 35(6): 2610-2628. |
| [8] | ZHOU Shan, WANG Shujuan, CHEN Changhe. Thermal degradation of monoethanolamine in CO2 capture with acidic impurities in flue gas[J]. Industrial & Engineering Chemistry Research, 2012, 51(6): 2539-2547. |
| [9] | Se-Young OH, BINNS Michael, CHO Habin, et al. Energy minimization of MEA-based CO2 capture process[J]. Applied Energy, 2016, 169: 353-362. |
| [10] | LEUNG Dennis Y C, CARAMANNA Giorgio, Mercedes MAROTO-VALER M. An overview of current status of carbon dioxide capture and storage technologies[J]. Renewable and Sustainable Energy Reviews, 2014, 39: 426-443. |
| [11] | FYTIANOS Georgios, VEVELSTAD Solrun J, KNUUTILA Hanna K. Degradation and corrosion inhibitors for MEA-based CO2 capture plants[J]. International Journal of Greenhouse Gas Control, 2016, 50: 240-247. |
| [12] | KITTEL J, IDEM R, GELOWITZ D, et al. Corrosion in MEA units for CO2 capture: Pilot plant studies[J]. Energy Procedia, 2009, 1(1): 791-797. |
| [13] | Gregorio GARCÍA, ATILHAN Mert, APARICIO Santiago. Interfacial properties of deep eutectic solvents regarding to CO2 capture[J]. The Journal of Physical Chemistry C, 2015, 119(37): 21413-21425. |
| [14] | GHAEDI Hosein, AYOUB Muhammad, SUFIAN Suriati, et al. Measurement and correlation of physicochemical properties of phosphonium-based deep eutectic solvents at several temperatures (293.15K-343.15K) for CO2 capture[J]. The Journal of Chemical Thermodynamics, 2017, 113: 41-51. |
| [15] | PAIVA Alexandre, CRAVEIRO Rita, AROSO Ivo, et al. Natural deep eutectic solvents-solvents for the 21st century[J]. ACS Sustainable Chemistry & Engineering, 2014, 2(5): 1063-1071. |
| [16] | 白佳乐, 燕童凡, 谷嘉诚, 等. 低共熔溶剂的性质与应用研究进展[J]. 工业催化, 2024, 32(9): 26-32. |
| BAI Jiale, YAN Tongfan, GU Jiacheng, et al. Research progress on the properties and applications of deep eutectic solvents[J]. Industrial Catalysis, 2024, 32(9): 26-32. | |
| [17] | ZHANG Qinghua, DE OLIVEIRA VIGIER Karine, ROYER Sébastien, et al. Deep eutectic solvents: Syntheses, properties and applications[J]. Chemical Society Reviews, 2012, 41(21): 7108-7146. |
| [18] | FRANCISCO María, VAN DEN BRUINHORST Adriaan, ZUBEIR Lawien F, et al. A new low transition temperature mixture (LTTM) formed by choline chloride+lactic acid: Characterization as solvent for CO2 capture[J]. Fluid Phase Equilibria, 2013, 340: 77-84. |
| [19] | LERON Rhoda B, CAPARANGA Alvin, LI Menghui. Carbon dioxide solubility in a deep eutectic solvent based on choline chloride and urea at T =303.15-343.15K and moderate pressures[J]. Journal of the Taiwan Institute of Chemical Engineers, 2013, 44(6): 879-885. |
| [20] | ZHANG Yingying, JI Xiaoyan, LU Xiaohua. Choline-based deep eutectic solvents for CO2 separation: Review and thermodynamic analysis[J]. Renewable and Sustainable Energy Reviews, 2018, 97: 436-455. |
| [21] | KUMAR K, KESHRI S, BHARTI A, et al. Solubility of gases in choline chloride-based deep eutectic solvents from molecular dynamics simulation[J]. Industrial & Engineering Chemistry Research, 2022, 61(13): 4659-4671. |
| [22] | Emad ALI, HADJ-KALI Mohamed K, MULYONO Sarwono, et al. Analysis of operating conditions for CO2 capturing process using deep eutectic solvents[J]. International Journal of Greenhouse Gas Control, 2016, 47: 342-350. |
| [23] | Emad ALI, HADJ-KALI Mohamed K, MULYONO Sarwono, et al. Solubility of CO2 in deep eutectic solvents: Experiments and modelling using the Peng-Robinson equation of state[J]. Chemical Engineering Research and Design, 2014, 92(10): 1898-1906. |
| [24] | SARMAD Shokat, XIE Yujiao, MIKKOLA Jyri-Pekka, et al. Screening of deep eutectic solvents (DESs) as green CO2 sorbents: From solubility to viscosity[J]. New Journal of Chemistry, 2017, 41(1): 290-301. |
| [25] | ZUBEIR Lawien F, VAN OSCH Dannie J G P, ROCHA Marisa A A, et al. Carbon dioxide solubilities in decanoic acid-based hydrophobic deep eutectic solvents[J]. Journal of Chemical and Engineering Data, 2018, 63(4): 913-919. |
| [26] | CHOI Young Hae, VAN SPRONSEN Jaap, DAI Yuntao, et al. Are natural deep eutectic solvents the missing link in understanding cellular metabolism and physiology?[J]. Plant Physiology, 2011, 156(4): 1701-1705. |
| [27] | LIU Yang, BRENT FRIESEN J, MCALPINE James B, et al. Natural deep eutectic solvents: Properties, applications, and perspectives[J]. Journal of Natural Products, 2018, 81(3): 679-690. |
| [28] | FLORINDO Catarina, BRANCO Prof Luís C, MARRUCHO Prof Dr Isabel M. Quest for green-solvent design: From hydrophilic to hydrophobic (deep) eutectic solvents[J]. ChemSusChem, 2019, 12(8): 1549-1559. |
| [29] | TOMÉ Luciana I N, Vanessa BAIÃO, SILVA Wanderson DA, et al. Deep eutectic solvents for the production and application of new materials[J]. Applied Materials Today, 2018, 10: 30-50. |
| [30] | ZHANG Man, ZHANG Xingyilong, LIU Yingying, et al. Insights into the relationships between physicochemical properties, solvent performance, and applications of deep eutectic solvents[J]. Environmental Science and Pollution Research International, 2021, 28(27): 35537-35563. |
| [31] | PADUCH Roman, Martyna KANDEFER-SZERSZEŃ, TRYTEK Mariusz, et al. Terpenes: Substances useful in human healthcare[J]. Archivum Immunologiae et Therapiae Experimentalis, 2007, 55(5): 315-327. |
| [32] | HAIDER Mohd Belal, Divyam JHA, MARRIYAPPAN SIVAGNANAM Balathanigaimani, et al. Thermodynamic and kinetic studies of CO2 capture by glycol and amine-based deep eutectic solvents[J]. Journal of Chemical & Engineering Data, 2018, 63(8): 2671-2680. |
| [33] | ESPINO Magdalena, DE LOS ÁNGELES FERNÁNDEZ María, GOMEZ Federico J V, et al. Natural designer solvents for greening analytical chemistry[J]. TrAC Trends in Analytical Chemistry, 2016, 76: 126-136. |
| [34] | MULIA Kamarza, PUTRI Sylvania, KRISANTI Elsa, et al. Natural deep eutectic solvents (NADES) as green solvents for carbon dioxide capture[C]//International Conference on Chemistry, Chemical Process and Engieering (IC3PE), 2017. |
| [35] | CRAIG Stuart AS. Betaine in human nutrition[J]. The American Journal of Clinical Nutrition, 2004, 80(3): 539-549. |
| [36] | VRANOVA Valerie, REJSEK Klement, FORMANEK Pavel. Aliphatic, cyclic, and aromatic organic acids, vitamins, and carbohydrates in soil: A review[J]. The Scientific World Journal, 2013, 2013(1): 524239. |
| [37] | SIANI Gabriella, TIECCO Matteo, DI PROFIO Pietro, et al. Physical absorption of CO2 in betaine/carboxylic acid-based Natural Deep Eutectic Solvents[J]. Journal of Molecular Liquids, 2020, 315: 113708. |
| [38] | Leonhard L SZE, PANDEY Shubha, RAVULA Sudhir, et al. Ternary deep eutectic solvents tasked for carbon dioxide capture[J]. ACS Sustainable Chemistry & Engineering, 2014, 2(9): 2117-2123. |
| [39] | ZHANG Na, HUANG Zhaohe, ZHANG Haiming, et al. Highly efficient and reversible CO2 capture by task-specific deep eutectic solvents[J]. Industrial & Engineering Chemistry Research, 2019, 58(29): 13321-13329. |
| [40] | JIANG Bin, MA Jingwen, YANG Na, et al. Superbase/acylamido-based deep eutectic solvents for multiple-site efficient CO2 absorption[J]. Energy & Fuels, 2019, 33(8): 7569-7577. |
| [41] | SHUKLA Shashi Kant, MIKKOLA Jyri-Pekka. Intermolecular interactions upon carbon dioxide capture in deep-eutectic solvents[J]. Physical Chemistry Chemical Physics, 2018, 20(38): 24591-24601. |
| [42] | CUI Ge, Meng LYU, YANG Dezhong. Efficient CO2 absorption by azolide-based deep eutectic solvents[J]. Chemical Communications, 2019, 55(10): 1426-1429. |
| [43] | TRIVEDI Tushar J, LEE Ji Hoon, LEE Hyeon Jeong, et al. Deep eutectic solvents as attractive media for CO2 capture[J]. Green Chemistry, 2016, 18(9): 2834-2842. |
| [44] | ZHANG Kai, HOU Yucui, WANG Yiming, et al. Efficient and reversible absorption of CO2 by functional deep eutectic solvents[J]. Energy & Fuels, 2018, 32(7): 7727-7733. |
| [45] | GU Yanxue, HOU Yucui, REN Shuhang, et al. Hydrophobic functional deep eutectic solvents used for efficient and reversible capture of CO2 [J]. ACS Omega, 2020, 5(12): 6809-6816. |
| [46] | HAIDER Mohd Belal, Divyam JHA, KUMAR Rakesh, et al. Ternary hydrophobic deep eutectic solvents for carbon dioxide absorption[J]. International Journal of Greenhouse Gas Control, 2020, 92: 102839. |
| [47] | CAO Lingdi, HUANG Junhua, ZHANG Xiangping, et al. Imidazole tailored deep eutectic solvents for CO2 capture enhanced by hydrogen bonds[J]. Physical Chemistry Chemical Physics, 2015, 17(41): 27306-27316. |
| [48] | LI Zhuo, WANG Lili, LI Changping, et al. Absorption of carbon dioxide using ethanolamine-based deep eutectic solvents[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(12): 10403-10414. |
| [49] | GURKAN B, GOODRICH B F, MINDRUP E M, et al. Molecular design of high capacity, low viscosity, chemically tunable ionic liquids for CO2 capture[J]. The Journal of Physical Chemistry Letters, 2010, 1(24): 3494-3499. |
| [50] | LIU Fan, SHEN Yao, SHEN Li, et al. Novel amino-functionalized ionic liquid/organic solvent with low viscosity for CO2 capture[J]. Environmental Science & Technology, 2020, 54(6): 3520-3529. |
| [51] | LEE Yunyang, PENLEY Drace, KLEMM Aidan, et al. Deep eutectic solvent formed by imidazolium cyanopyrrolide and ethylene glycol for reactive CO2 separations[J]. ACS Sustainable Chemistry & Engineering, 2021, 9(3): 1090-1098. |
| [52] | Ahmad AL-BODOUR, ALOMARI Noor, Alberto GUTIÉRREZ, et al. High-pressure carbon dioxide solubility in terpene based deep eutectic solvents[J]. Journal of Environmental Chemical Engineering, 2022, 10(5): 108237. |
| [53] | SONG Xueyi, YUAN Junjie, YANG Chen, et al. Carbon dioxide separation performance evaluation of amine-based versus choline-based deep eutectic solvents[J]. Renewable and Sustainable Energy Reviews, 2023, 184: 113499. |
| [54] | XIN Kun, VAN SINT ANNALAND Martin. Diffusivities and solubilities of carbon dioxide in deep eutectic solvents[J]. Separation and Purification Technology, 2023, 307: 122779. |
| [55] | DENG Dongshun, JIANG Yaotai, LIU Xiaobang, et al. Investigation of solubilities of carbon dioxide in five levulinic acid-based deep eutectic solvents and their thermodynamic properties[J]. The Journal of Chemical Thermodynamics, 2016, 103: 212-217. |
| [56] | Iwona CICHOWSKA-KOPCZYŃSKA, NOWOSIELSKI Bartosz, Dorota WARMIŃSKA. Deep eutectic solvents: Properties and applications in CO2 separation[J]. Molecules, 2023, 28(14): 5293. |
| [57] | FAN Jing, ZHANG Xin, HE Nan, et al. Physical absorption and thermodynamic modeling of CO2 in new deep eutectic solvents[J]. Journal of Molecular Liquids, 2024, 402: 124752. |
| [58] | LI Xiaoyong, HOU Minqiang, HAN Buxing, et al. Solubility of CO2 in a choline chloride + urea eutectic mixture[J]. Journal of Chemical & Engineering Data, 2008, 53(2): 548-550. |
| [59] | LI Ziliang, ZHONG Fuyu, HUANG Jiyong, et al. Sugar-based natural deep eutectic solvents as potential absorbents for NH3 capture at elevated temperatures and reduced pressures[J]. Journal of Molecular Liquids, 2020, 317: 113992. |
| [60] | ADEYEMI Idowu, ABU-ZAHRA Mohammad R M, ALNASHEF Inas. Experimental study of the solubility of CO2 in novel amine based deep eutectic solvents[J]. Energy Procedia, 2017, 105: 1394-1400. |
| [61] | SHUKLA Shashi Kant, NIKJOO Dariush, MIKKOLA Jyri-Pekka. Is basicity the sole criterion for attaining high carbon dioxide capture in deep-eutectic solvents?[J]. Physical Chemistry Chemical Physics, 2020, 22(3): 966-970. |
| [62] | QIAN Wenbin, HAO Jin, ZHU Mingjian, et al. Development of green solvents for efficient post-combustion CO2 capture with good regeneration performance[J]. Journal of CO2 Utilization, 2022, 59: 101955. |
| [63] | CHEN Yanfei, AI Ning, LI Guihua, et al. Solubilities of carbon dioxide in eutectic mixtures of choline chloride and dihydric alcohols[J]. Journal of Chemical & Engineering Data, 2014, 59(4): 1247-1253. |
| [64] | LU Meizhen, HAN Guoqiang, JIANG Yaotai, et al. Solubilities of carbon dioxide in the eutectic mixture of levulinic acid (or furfuryl alcohol) and choline chloride[J]. The Journal of Chemical Thermodynamics, 2015, 88: 72-77. |
| [65] | CHEN Mingzhe, XU Jinming. CO2 capture mechanism by deep eutectic solvents formed by choline prolinate and ethylene glycol[J]. Molecules, 2023, 28(14): 5461. |
| [66] | ZHANG Kaiqing, WANG Rui. A critical review on new and efficient adsorbents for CO2 capture[J]. Chemical Engineering Journal, 2024, 485: 149495. |
| [67] | RUAN Jiawei, CHEN Lifang, QI Zhiwen. Deep eutectic solvents as a versatile platform toward CO2 capture and utilization[J]. Green Chemistry, 2023, 25(21): 8328-8348. |
| [68] | WANG Ze, WANG Zonghua, CHEN Jie, et al. The influence of hydrogen bond donors on the CO2 absorption mechanism by the bio-phenol-based deep eutectic solvents[J]. Molecules, 2021, 26(23): 7167. |
| [69] | PISHRO Khatereh ALI, MURSHID Ghulam, MJALLI Farouq Sabri, et al. Investigation of CO2 solubility in monoethanolamine hydrochloride based deep eutectic solvents and physical properties measurements[J]. Chinese Journal of Chemical Engineering, 2020, 28(11): 2848-2856. |
| [70] | RUAN Jiawei, YE Xiangzhu, WANG Ruizhuan, et al. Experimental and theoretical study on efficient CO2 absorption coordinated by molecules and ions of DBN and 1,2,4-triazole formed deep eutectic solvents[J]. Fuel, 2023, 334: 126709. |
| [71] | FOORGINEZHAD Sahar, JI Xiaoyan. Development of monoethanolamine chloride-ethylene diamine deep eutectic solvent for ffficient carbon dioxide capture[J]. Separation and Purification Technology, 2024, 347: 127593. |
| [72] | ZHEKENOV Temirlan, TOKSANBAYEV Nursultan, KAZAKBAYEVA Zhanna, et al. Formation of type Ⅲ Deep Eutectic Solvents and effect of water on their intermolecular interactions[J]. Fluid Phase Equilibria, 2017, 441: 43-48. |
| [73] | ZHANG Yue, HAN Rui, ZHOU Shujun, et al. Amine-based deep eutectic solvents for CO2 capture: Experiments and molecular thermodynamics[J]. Separation and Purification Technology, 2025, 359: 130559. |
| [74] | LI Guihua, DENG Dongshun, CHEN Yanfei, et al. Solubilities and thermodynamic properties of CO2 in choline-chloride based deep eutectic solvents[J]. The Journal of Chemical Thermodynamics, 2014, 75: 58-62. |
| [75] | SU Wen cheng, WONG David Shan Hill, LI Meng hui. Effect of water on solubility of carbon dioxide in (aminomethanamide + 2-hydroxy-N,N,N-trimethylethanaminium chloride)[J]. Journal of Chemical & Engineering Data, 2009, 54(6): 1951-1955. |
| [76] | XIE Yujiao, DONG Haifeng, ZHANG Suojiang, et al. Effect of water on the density, viscosity, and CO2 solubility in choline chloride/urea[J]. Journal of Chemical & Engineering Data, 2014, 59(11): 3344-3352. |
| [77] | FETISOV Evgenii O, HARWOOD David B, KUO I-Feng William, et al. First-principles molecular dynamics study of a deep eutectic solvent: Choline chloride/urea and its mixture with water[J]. The Journal of Physical Chemistry B, 2018, 122(3): 1245-1254. |
| [78] | SHAH Dhawal, MJALLI Farouq S. Effect of water on the thermo-physical properties of reline: An experimental and molecular simulation based approach[J]. Physical Chemistry Chemical Physics, 2014, 16(43): 23900-23907. |
| [79] | Alberto GUTIÉRREZ, ATILHAN Mert, APARICIO Santiago. Molecular dynamics study on water confinement in deep eutectic solvents[J]. Journal of Molecular Liquids, 2021, 339: 116758. |
| [80] | Kai TÖPFER, PASTI Andrea, Anuradha DAS, et al. Structure, organization, and heterogeneity of water-containing deep eutectic solvents[J]. Journal of the American Chemical Society, 2022, 144(31): 14170-14180. |
| [81] | WENG Lindong, TONER Mehmet. Janus-faced role of water in defining nanostructure of choline chloride/glycerol deep eutectic solvent[J]. Physical Chemistry Chemical Physics, 2018, 20(35): 22455-22462. |
| [82] | ABDRABOU Hossam K, ALNASHEF Inas, ZAHRA Mohammad ABU, et al. Experimental investigation of novel ternary amine-based deep eutectic solvents for CO2 capture[J]. PLoS One, 2023, 18(6): e0286960. |
| [83] | HUAN Qun, ZHANG Yan, WIBOWO Haryo, et al. Study on regeneration characteristics of choline chloride-monoethanolamine deep eutectic solvent after capturing CO2 from biogas[J]. Separation and Purification Technology, 2022, 302: 122064. |
| [84] | LIU Xiangwei, AO Qian, SHI Shengyou, et al. CO2 capture by alcohol ammonia based deep eutectic solvents with different water content[J]. Materials Research Express, 2022, 9(1): 015504. |
| [85] | GUTIÉRREZ María C, FERRER María L, REYES MATEO C, et al. Freeze-drying of aqueous solutions of deep eutectic solvents: A suitable approach to deep eutectic suspensions of self-assembled structures[J]. Langmuir, 2009, 25(10): 5509-5515. |
| [86] | ZHANG Yuqi, ZHU Chunying, FU Taotao, et al. CO2 absorption performance of ChCl-MEA deep eutectic solvent in microchannel[J]. Journal of Environmental Chemical Engineering, 2022, 10(6): 108792. |
| [87] | AL-DAWSARI Jiyad N, Abdelbasset BESSADOK-JEMAI, WAZEER Irfan, et al. Fitting of experimental viscosity to temperature data for deep eutectic solvents[J]. Journal of Molecular Liquids, 2020, 310: 113127. |
| [88] | ALIZADEH Vahideh, ESSER Lars, KIRCHNER Barbara. How is CO2 absorbed into a deep eutectic solvent?[J]. The Journal of Chemical Physics, 2021, 154(9): 094503. |
| [89] | YAN Mi, HUAN Qun, ZHANG Yan, et al. Effect of operating parameters on CO2 capture from biogas with choline chloride-monoethanolamine deep eutectic solvent and its aqueous solution[J]. Biomass Conversion and Biorefinery, 2024, 14(1): 283-297. |
| [1] | 张文静, 黄致新, 李士腾, 邓帅, 李双俊. 生物质碳气凝胶CO2吸附剂研究进展[J]. 化工进展, 2025, 44(9): 5018-5032. |
| [2] | 齐妍, 常昊, 张磊. 基于分子动力学模拟的结构性产品配方设计方法[J]. 化工进展, 2025, 44(8): 4341-4351. |
| [3] | 汪柯, 胡登, 王星博, 孙楠楠, 魏伟. Fe x Co y Ca3Al双功能材料用于CO2捕集-转化一体化制合成气[J]. 化工进展, 2025, 44(5): 2888-2897. |
| [4] | 付紫君, 宋学行, 沈群, 王晓波, 顾佳名, 汪丹峰, 魏伟, 孙楠楠. 基于LCA的CO2捕集-甲烷化一体化技术碳足迹分析[J]. 化工进展, 2025, 44(5): 2879-2887. |
| [5] | 陆诗建, 张娟娟, 杨菲, 刘玲, 陈思铭, 康国俊, 房芹芹. 化学吸收法胺液逃逸控制技术研究进展[J]. 化工进展, 2024, 43(8): 4562-4570. |
| [6] | 马文君, 张旭, 刘孟顺, 梁志远. 新兴湿法退役锂电池正极材料回收技术研究进展[J]. 化工进展, 2024, 43(4): 2077-2090. |
| [7] | 何金, 赖裕文, 李艳春, 周士林, 周勇, 高从堦. DES改变胺单体扩散速率制备高性能复合反渗透膜[J]. 化工进展, 2024, 43(4): 1972-1980. |
| [8] | 秦雪, 赵传文, 黄浦, 曾鹏鑫, 孙健, 郭亚飞. 石墨盘法Na2CO3基CO2成型吸附剂制备及性能优化[J]. 化工进展, 2024, 43(12): 7095-7104. |
| [9] | 苏辉辉, 王恩禄, 徐逸飞. 液体吸收剂捕集燃烧后CO2的研究进展[J]. 化工进展, 2024, 43(10): 5734-5747. |
| [10] | 陈崇明, 陈秋, 宫云茜, 车凯, 郁金星, 孙楠楠. 分子筛基CO2吸附剂研究进展[J]. 化工进展, 2023, 42(S1): 411-419. |
| [11] | 马伊, 曹世伟, 王家骏, 林立群, 邢延, 曹腾良, 卢峰, 赵振伦, 张志军. 低共熔溶剂回收废旧锂离子电池正极材料的研究进展[J]. 化工进展, 2023, 42(S1): 219-232. |
| [12] | 杨莹, 侯豪杰, 黄瑞, 崔煜, 王兵, 刘健, 鲍卫仁, 常丽萍, 王建成, 韩丽娜. 利用煤焦油中酚类物质Stöber法制备碳纳米球用于CO2吸附[J]. 化工进展, 2023, 42(9): 5011-5018. |
| [13] | 杨志强, 曾纪珺, 马义丁, 尉涛, 赵波, 刘英哲, 张伟, 吕剑, 李兴文, 张博雅, 唐念, 李丽, 孙东伟. 六氟化硫替代气体的研究现状及未来发展趋势[J]. 化工进展, 2023, 42(8): 4093-4107. |
| [14] | 王久衡, 荣鼐, 刘开伟, 韩龙, 水滔滔, 吴岩, 穆正勇, 廖徐情, 孟文佳. 水蒸气强化纤维素模板改性钙基吸附剂固碳性能及强度[J]. 化工进展, 2023, 42(6): 3217-3225. |
| [15] | 陆诗建, 张媛媛, 吴文华, 杨菲, 刘玲, 康国俊, 李清方, 陈宏福, 王宁, 王风, 张娟娟. 百万吨级CO2捕集项目亚硝胺污染物扩散健康风险评估[J]. 化工进展, 2023, 42(6): 3209-3216. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||