化工进展 ›› 2024, Vol. 43 ›› Issue (4): 2077-2090.DOI: 10.16085/j.issn.1000-6613.2023-0547
• 资源与环境化工 • 上一篇
新兴湿法退役锂电池正极材料回收技术研究进展
- 西安交通大学热流科学与工程教育部重点实验室,陕西 西安 710049
-
收稿日期:2023-04-07修回日期:2023-05-04出版日期:2024-04-15发布日期:2024-05-13 -
通讯作者:梁志远 -
作者简介:马文君(1998—),女,博士研究生,研究方向为锂电池正极材料回收和赤泥碱融合成分子筛。E-mail:277699450@qq.com。 -
基金资助:青年科技新星项目(S2022-ZC-XXXM-0112);陕西省重点研发计划(2023-YBGY-291)
Research progress of novel hydrometallurgy in recycling cathode materials from spent lithium-ion batteries
MA Wenjun( ), ZHANG Xu, LIU Mengshun, LIANG Zhiyuan(
), ZHANG Xu, LIU Mengshun, LIANG Zhiyuan( )
)
- Key Laboratory of Thermal Fluid Science and Engineering of MOE, Xi’an Jiaotong University, Xi’an 710049, Shaanxi, China
-
Received:2023-04-07Revised:2023-05-04Online:2024-04-15Published:2024-05-13 -
Contact:LIANG Zhiyuan
摘要:
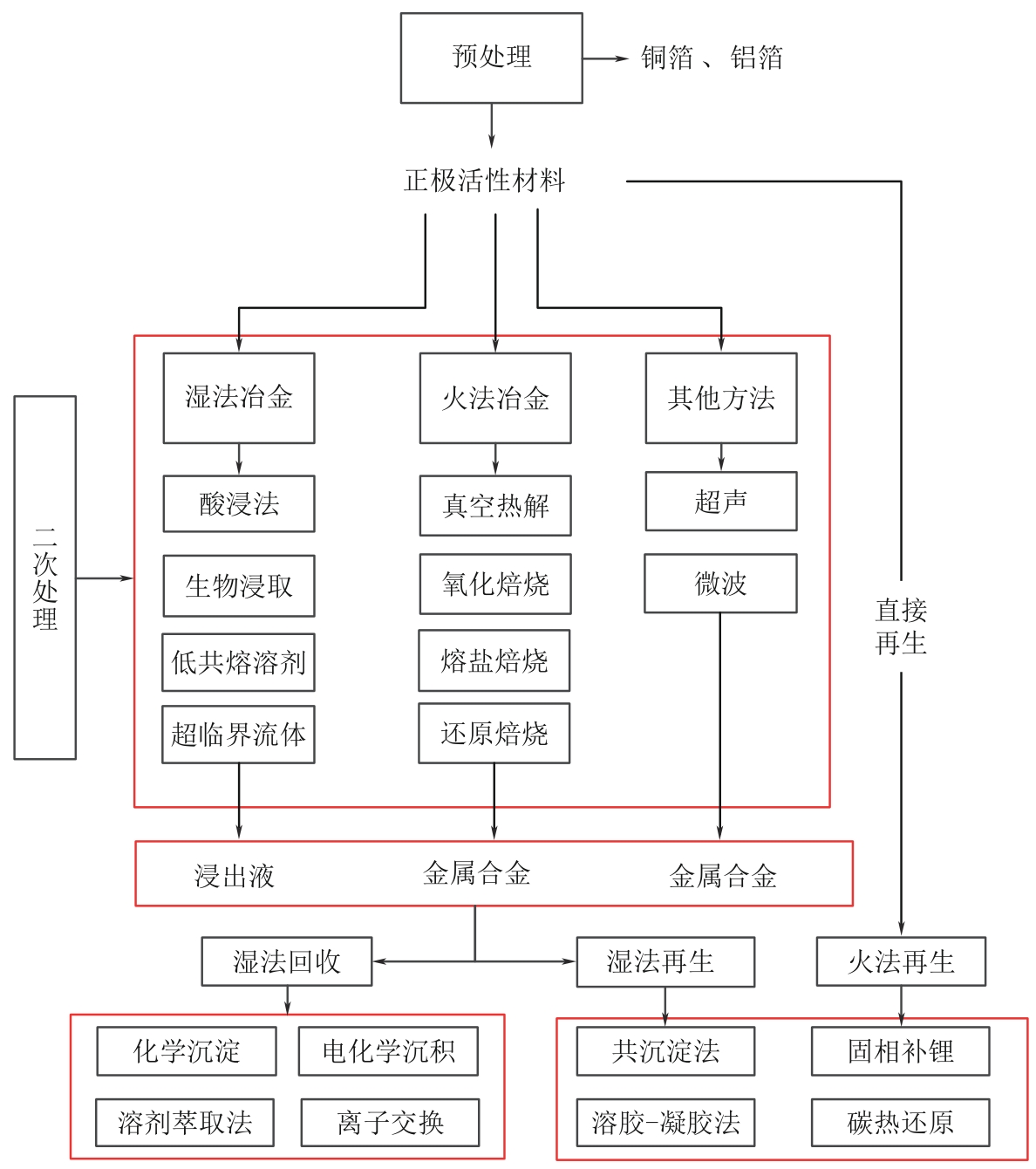
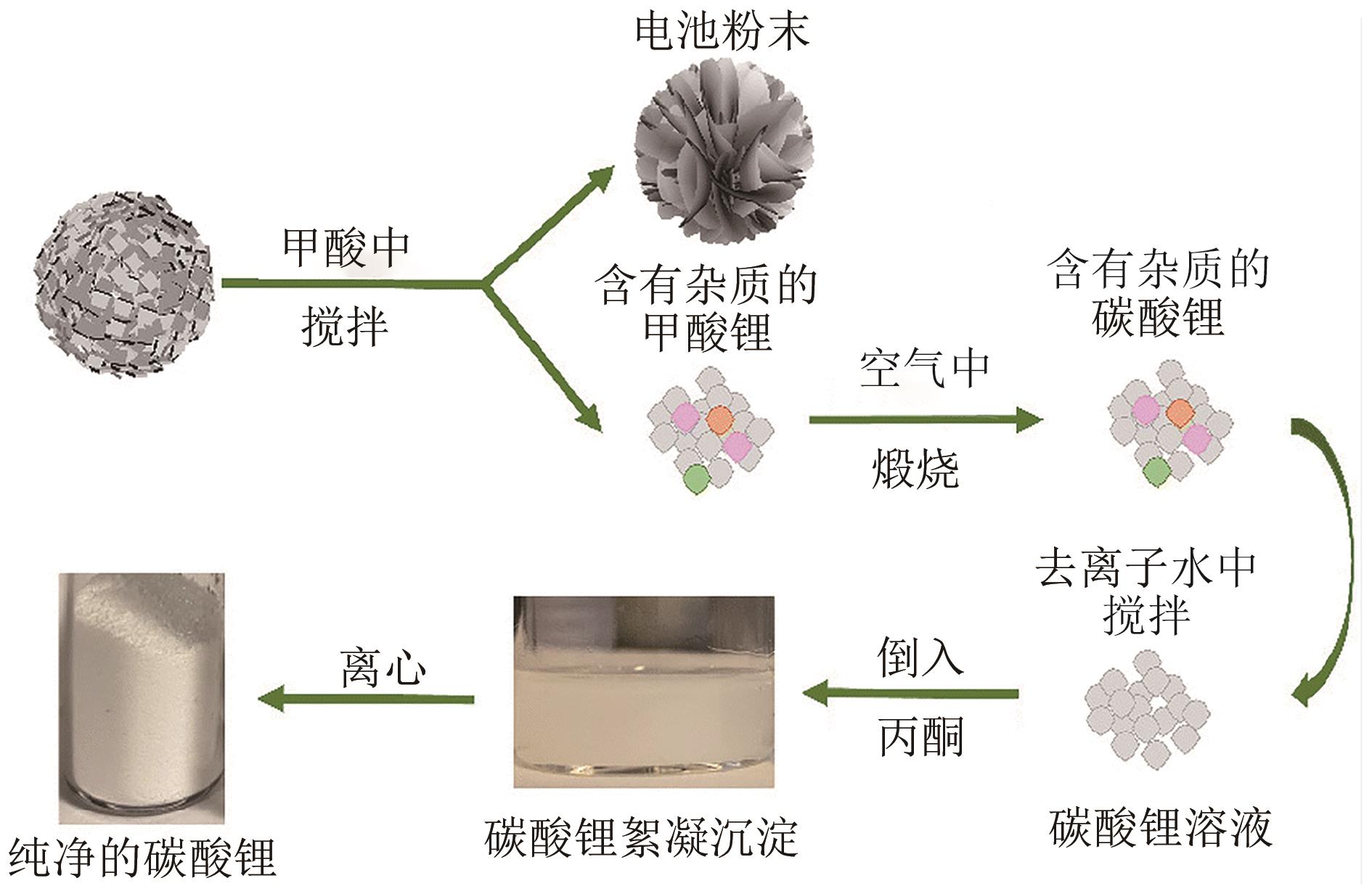
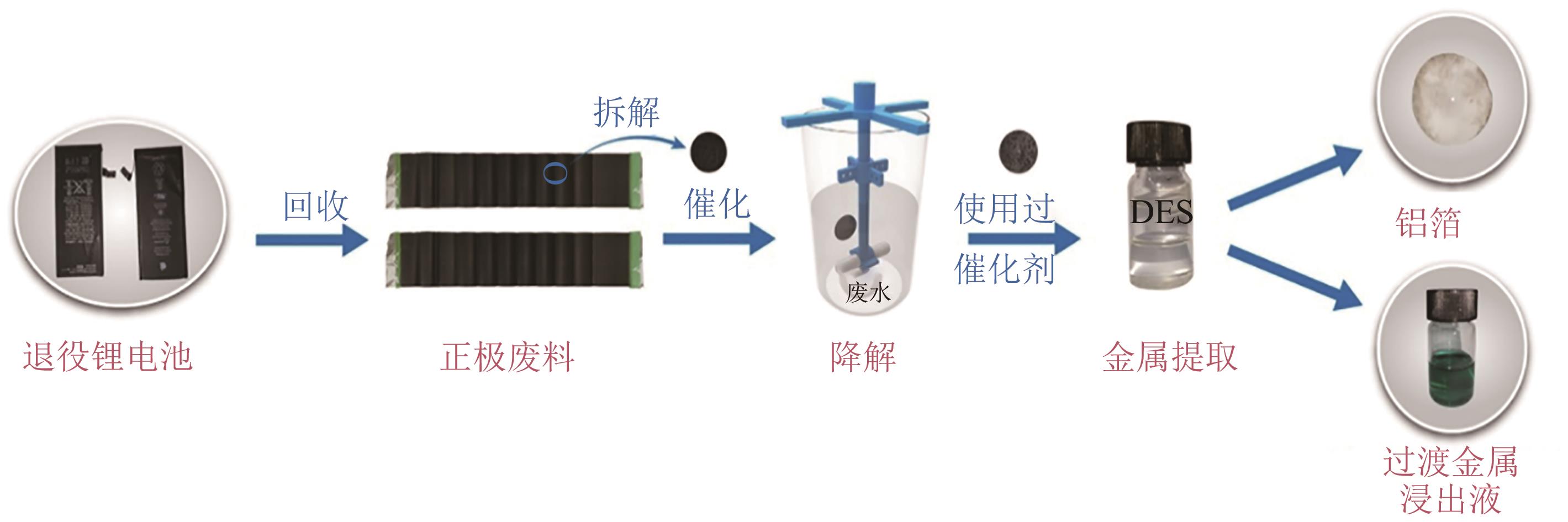
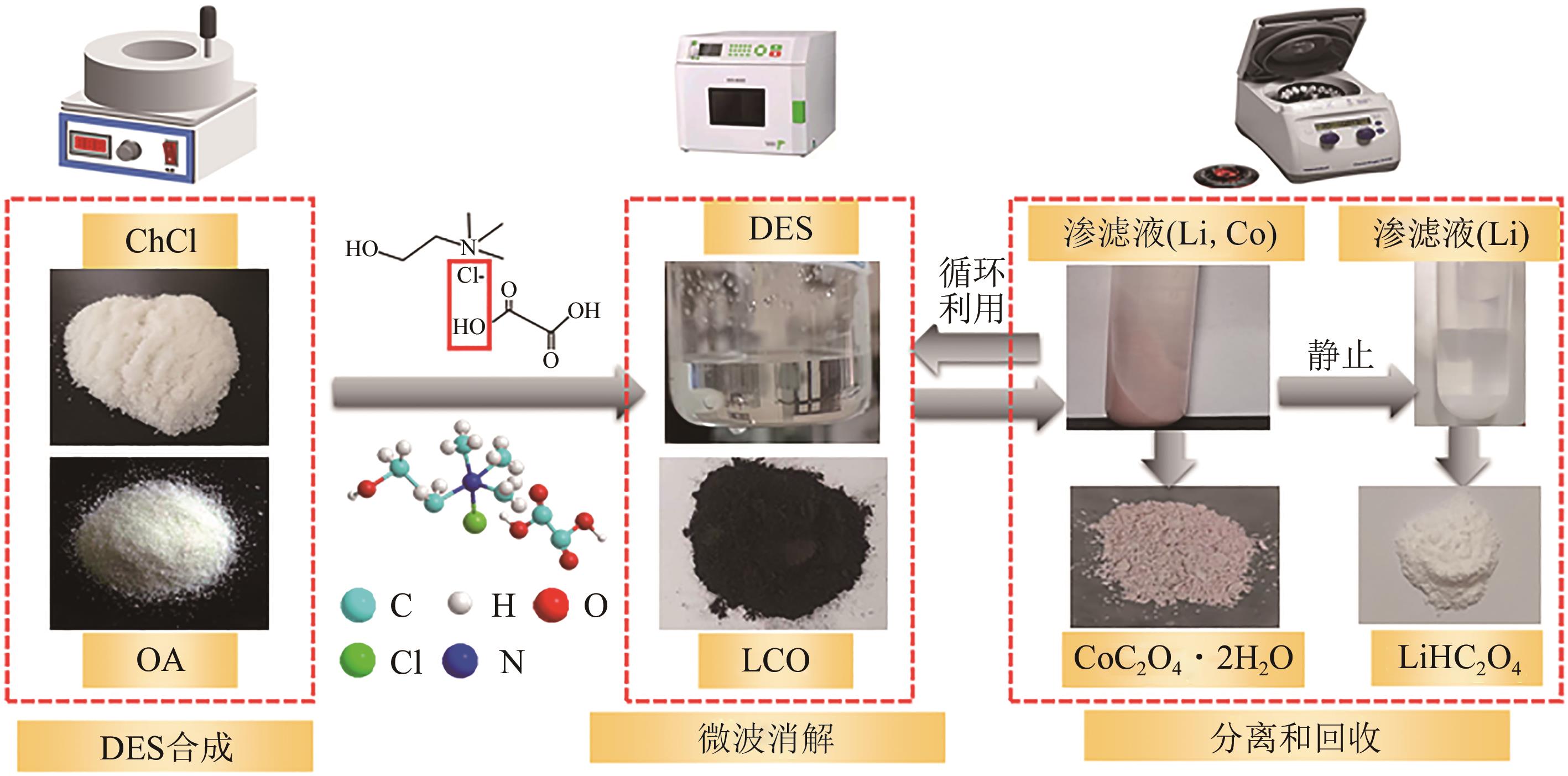
退役锂电池中的钴、镍、锂等稀有金属的绿色高效回收利用逐渐成为国内外研究的重点。传统酸浸法具有能源成本低、金属回收纯度高和效率高的优点,但使用腐蚀性强酸和昂贵萃取物,反应时间长且产生废酸、污泥和高盐溶液等二次废物。本文总结了传统酸浸法中绿色浸取剂和还原剂以及低共熔溶剂(DES)和超临界流体(SCF)两种新兴的湿法冶金技术对高效绿色回收锂电池正极材料的应用。阐明了微波超声辅助手段和选择性浸取回收工艺分别对改善浸取工况和简化分离回收程序的重要作用。并重点介绍了超临界水(SCW)和超临界二氧化碳(SC-CO2)两种超临界流体降解有机污染物、回收稀有金属并改善合成正极材料的应用,为高效、绿色、低成本回收退役锂电池中稀有金属提供了重要参考价值。
中图分类号:
引用本文
马文君, 张旭, 刘孟顺, 梁志远. 新兴湿法退役锂电池正极材料回收技术研究进展[J]. 化工进展, 2024, 43(4): 2077-2090.
MA Wenjun, ZHANG Xu, LIU Mengshun, LIANG Zhiyuan. Research progress of novel hydrometallurgy in recycling cathode materials from spent lithium-ion batteries[J]. Chemical Industry and Engineering Progress, 2024, 43(4): 2077-2090.
| 浸取剂 | 还原剂 | 最佳工况 | 浸取效率 | 参考文献 |
|---|---|---|---|---|
| 3mol/L硫酸 | 5%(体积分数)的乙醇 | 90℃,160min,20g/L | 99%的Co和Li | [ |
| 1.5mol/L柠檬酸 | 200mg橙皮粉 | 100℃,4h,25g/mL | 80%~99%的Li、Ni、Mn、Co | [ |
| 1.5mol/L苹果酸 | 600mg/g葡萄籽 | 180min,80℃,20g/L | 92%的Co,99%的Li | [ |
| 0.5mol/L硫酸 | LCO/LFP摩尔比为1∶1 | 20min,30g/L,50℃ | 99.9%的Li、Fe、P和92.4%的Co | [ |
表1 绿色还原剂在传统酸浸法回收锂电池正极材料中的应用
| 浸取剂 | 还原剂 | 最佳工况 | 浸取效率 | 参考文献 |
|---|---|---|---|---|
| 3mol/L硫酸 | 5%(体积分数)的乙醇 | 90℃,160min,20g/L | 99%的Co和Li | [ |
| 1.5mol/L柠檬酸 | 200mg橙皮粉 | 100℃,4h,25g/mL | 80%~99%的Li、Ni、Mn、Co | [ |
| 1.5mol/L苹果酸 | 600mg/g葡萄籽 | 180min,80℃,20g/L | 92%的Co,99%的Li | [ |
| 0.5mol/L硫酸 | LCO/LFP摩尔比为1∶1 | 20min,30g/L,50℃ | 99.9%的Li、Fe、P和92.4%的Co | [ |
| 浸取剂 | 氧化剂 | 最佳工况 | 浸取率 | 参考文献 |
|---|---|---|---|---|
| 0.3mol/L硫酸 | 过氧化氢 | H2SO4/Li摩尔比为0.57,60℃, 120min | 96.85%的Li,0.027%的Fe,1.95%的P | [ |
| 1.1倍摩尔量硫酸氢钠 | 2%(体积分数)过氧化氢 | 65℃,100g/L,15min | 99.84%的Li,0.048%的Fe | [ |
| 0.8mol/L醋酸 | 6%(体积分数)过氧化氢 | 120g/L,50℃,30min | 95.05%的Li | [ |
| 10倍质量比的柠檬酸钠 | 过氧化氢 | 5h,500r/min | 98.9%的Li | [ |
| 0.6mol/L硫酸 | 1.3MPa的O2 | H2SO4/Li摩尔比为0.52,90min | 97%的Li和1%的Fe | [ |
表2 选择性浸取回收工艺在LFP回收中的应用
| 浸取剂 | 氧化剂 | 最佳工况 | 浸取率 | 参考文献 |
|---|---|---|---|---|
| 0.3mol/L硫酸 | 过氧化氢 | H2SO4/Li摩尔比为0.57,60℃, 120min | 96.85%的Li,0.027%的Fe,1.95%的P | [ |
| 1.1倍摩尔量硫酸氢钠 | 2%(体积分数)过氧化氢 | 65℃,100g/L,15min | 99.84%的Li,0.048%的Fe | [ |
| 0.8mol/L醋酸 | 6%(体积分数)过氧化氢 | 120g/L,50℃,30min | 95.05%的Li | [ |
| 10倍质量比的柠檬酸钠 | 过氧化氢 | 5h,500r/min | 98.9%的Li | [ |
| 0.6mol/L硫酸 | 1.3MPa的O2 | H2SO4/Li摩尔比为0.52,90min | 97%的Li和1%的Fe | [ |
| 湿法冶金 技术 | 设备成本 | 试剂成本 | 选择性 | 回收效率 | 污染性 |
|---|---|---|---|---|---|
| 传统酸浸 | 较低 | 较低 | 较低 | 时间短 | 腐蚀性强,排放有毒气体 |
| 低共熔溶剂 | 较低 | 较高 | 较低 | 时间中等 | 低污染 |
| 超临界流体 | 较高 | 极低 | 较高 | 时间极短 | 可有效降解有机污染物 |
表3 湿法退役锂电池正极材料回收技术对比
| 湿法冶金 技术 | 设备成本 | 试剂成本 | 选择性 | 回收效率 | 污染性 |
|---|---|---|---|---|---|
| 传统酸浸 | 较低 | 较低 | 较低 | 时间短 | 腐蚀性强,排放有毒气体 |
| 低共熔溶剂 | 较低 | 较高 | 较低 | 时间中等 | 低污染 |
| 超临界流体 | 较高 | 极低 | 较高 | 时间极短 | 可有效降解有机污染物 |
| 1 | 何宏恺, 王粤威, 陈朝方, 等. 废旧动力锂电池回收利用技术的进展[J]. 广州化学, 2014, 39(4): 81-86. |
| HE Hongkai, WANG Yuewei, CHEN Chaofang, et al. Advances in waste power lithium battery recycling technology[J]. Guangzhou Chemistry, 2014, 39(4): 81-86. | |
| 2 | HUANG X. Research on short process recycling of ternary cathode materials for waste lithium ion power battery[D]. Beijing: Beijing General Research Institute for Nonferrous Metals, 2019. |
| 3 | ZHENG Xiaohong, ZHU Zewen, LIN Xiao, et al. A mini-review on metal recycling from spent lithium ion batteries[J]. Engineering, 2018, 4(3): 361-370. |
| 4 | LI L, LIANG Y, CHEN L, et al. Research progress on recovery of cobalt and nickel from waste batteries[J]. China Nonferrous Metallurgy, 2008, 3(4): 57-60. |
| 5 | YUE Lingping, LOU Ping, XU Guohua, et al. Regeneration of degraded LiNi0.5Co0.2Mn0.3O2 from spent lithium ion batteries[J]. Ionics, 2020, 26(6): 2757-2761. |
| 6 | 昝振峰. 废旧LiCoO2锂离子电池回收及再利用研究[D]. 哈尔滨: 哈尔滨工业大学, 2012. |
| ZAN Zhenfeng. Study on recycling of spent LiCoO2Lithium ion batteries[D]. Harbin: Harbin Institute of Technology, 2012. | |
| 7 | 王洪彩. 含钴废旧锂离子电池回收技术及中试工艺研究[D]. 哈尔滨: 哈尔滨工业大学, 2013. |
| WANG Hongcai. Study on recycling of spent lithium ion batteries containing cobalt and pilot scale experiment[D]. Harbin: Harbin Institute of Technology, 2013. | |
| 8 | GEORGI-MASCHLER T, FRIEDRICH B, WEYHE R, et al. Development of a recycling process for Li-ion batteries[J]. Journal of Power Sources, 2012, 207: 173-182. |
| 9 | 韩琳, 袁佳歆. 锂离子电池放电仿真电源的研制[J]. 电源技术, 2013, 37(5): 747-748, 752. |
| HAN Lin, YUAN Jiaxin. Research and development of lithium ion battery discharge simulation power supply[J]. Chinese Journal of Power Sources, 2013, 37(5): 747-748, 752. | |
| 10 | 宾智勇, 刘景槐, 吴海国, 等. 废旧锂离子电池的综合利用试验研究[J]. 湖南有色金属, 2008, 24(5): 27-31, 80. |
| Zhiyong BIN, LIU Jinghuai, WU Haiguo, et al. Study on comprehensive utilization of spent lithium ion secondary battery[J]. Hunan Nonferrous Metals, 2008, 24(5): 27-31, 80. | |
| 11 | 潘英俊. 以磷酸铁锂为正极材料的废旧锂离子电池回收及再利用[D]. 哈尔滨: 哈尔滨工业大学, 2012. |
| PAN Yingjun. Recycling and reuse of spent lithium-ion battery that is used LiFePO4 as cathode material[D]. Harbin: Harbin Institute of Technology, 2012. | |
| 12 | 张英杰, 宁培超, 杨轩, 等. 废旧三元锂离子电池回收技术研究新进展[J]. 化工进展, 2020, 39(7): 2828-2840. |
| ZHANG Yingjie, NING Peichao, YANG Xuan, et al. Research progress on the recycling technology of spent ternary lithium ion battery[J]. Chemical Industry and Engineering Progress, 2020, 39(7): 2828-2840. | |
| 13 | 李建波, 徐政, 纪仲光, 等. 废旧锂离子动力电池回收的研究现状[J]. 稀有金属, 2019, 43(2): 201-212. |
| LI Jianbo, XU Zheng, JI Zhongguang, et al. Overview on current technologies of recycling spent lithium-ion batteries[J]. Chinese Journal of Rare Metals, 2019, 43(2): 201-212. | |
| 14 | YE Luhan, LI Xin. A dynamic stability design strategy for lithium metal solid state batteries[J]. Nature, 2021, 593(7858): 218-222. |
| 15 | SUN Shuo, ZHAO Chenzi, YUAN Hong, et al. Eliminating interfacial O-involving degradation in Li-rich Mn-based cathodes for all-solid-state lithium batteries[J]. Science Advances, 2022, 8(47): eadd5189. |
| 16 | 李劲, 邵威, 毛洪仁. 废弃锂离子电池回收处理的污染物分析[J]. 化工进展, 2016, 35(5): 1529-1538. |
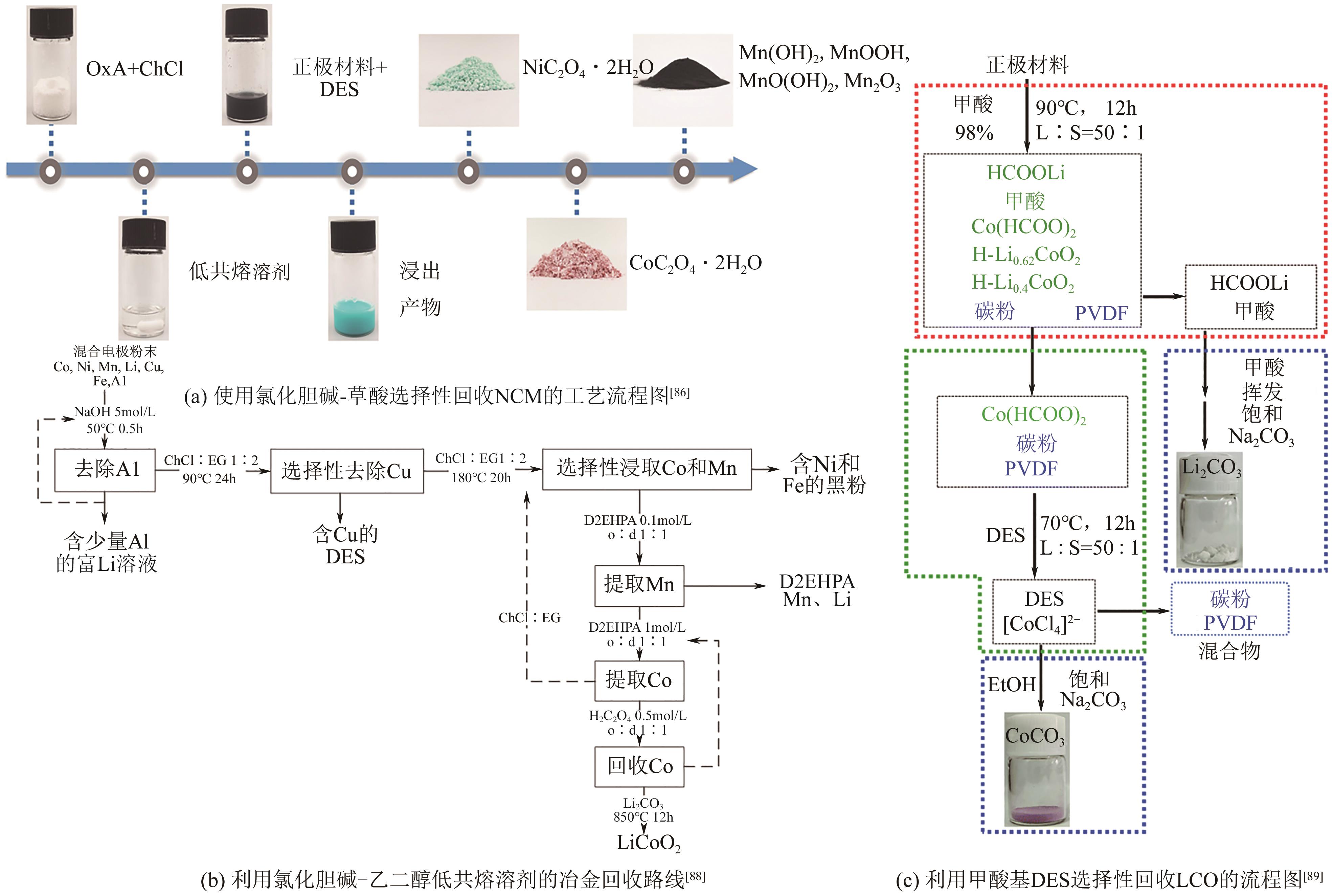
| LI Jin, SHAO Wei, MAO Hongren. Analysis of pollutants in the recycling of waste lithium batteries[J]. Chemical Industry and Engineering Progress, 2016, 35(5): 1529-1538. | |
| 17 | GRATZ E, WANG Y. Recycling all solid state batteries (ASSBs), comprises receiving recycling stream of ASSBs, introducing passivating substance, agitating batteries in recycling stream, and recovering charge material and electrolyte from agitated batteries: US2022416325-A1[P]. 2021-06-17. |
| 18 | OSADA N, MATSUSHITA S. Recovery method for recovering capacitance of all-solid-state battery involves heat processing all-solid-state battery at predetermined temperature range for couple of hours: JP2016126912-A[P]. 2016-07-11. |
| 19 | WANG Junxiong, JIA Kai, MA Jun, et al. Sustainable upcycling of spent LiCoO2 to an ultra-stable battery cathode at high voltage[J]. Nature Sustainability, 2023: 6: 797-805. |
| 20 | KAISER Doreen, Sandra PAVÓN, BERTAU Martin. Recovery of Al, Co, Cu, Fe, Mn, and Ni from spent LIBs after Li selective separation by the COOL-process. part 1: Leaching of solid residue from COOL-process[J]. Chemie Ingenieur Technik, 2021, 93(11): 1833-1839. |
| 21 | AALTONEN Miamari, PENG Chao, WILSON Benjamin, et al. Leaching of metals from spent lithium-ion batteries[J]. Recycling, 2017, 2(4): 20. |
| 22 | SHUVA Mohammad, Kurny ASW. Hydrometallurgical recovery of value metals from spent lithium ion batteries[J]. American Journal of Materials Engineering and Technology, 2013, 1(1): 8-12. |
| 23 | CHAN Ka Ho, ANAWATI John, MALIK Monu, et al. Closed-loop recycling of lithium, cobalt, nickel, and manganese from waste lithium-ion batteries of electric vehicles[J]. ACS Sustainable Chemistry & Engineering, 2021, 9(12): 4398-4410. |
| 24 | ZHAO Jingjing, ZHANG Beilei, XIE Hongwei, et al. Hydrometallurgical recovery of spent cobalt-based lithium-ion battery cathodes using ethanol as the reducing agent[J]. Environmental Research, 2020, 181: 108803. |
| 25 | PENG Chao, HAMUYUNI Joseph, WILSON Benjamin P, et al. Selective reductive leaching of cobalt and lithium from industrially crushed waste Li-ion batteries in sulfuric acid system[J]. Waste Management, 2018, 76: 582-590. |
| 26 | PAGNANELLI Francesca, MOSCARDINI Emanuela, ALTIMARI Pietro, et al. Leaching of electrodic powders from lithium ion batteries: Optimization of operating conditions and effect of physical pretreatment for waste fraction retrieval[J]. Waste Management, 2017, 60: 706-715. |
| 27 | PAGNANELLI Francesca, MOSCARDINI Emanuela, GRANATA Giuseppe, et al. Acid reducing leaching of cathodic powder from spent lithium ion batteries: Glucose oxidative pathways and particle area evolution[J]. Journal of Industrial and Engineering Chemistry, 2014, 20(5): 3201-3207. |
| 28 | SHIH Yu Jen, CHIEN Shih Kai, JHANG Syu Ruei, et al. Chemical leaching, precipitation and solvent extraction for sequential separation of valuable metals in cathode material of spent lithium ion batteries[J]. Journal of the Taiwan Institute of Chemical Engineers, 2019, 100: 151-159. |
| 29 | 宋佳丽, 孙峙, 高文芳, 等. 锂离子电池正极废料中有价元素的选择性回收及其动力学模型[J]. 过程工程学报, 2017, 17(4): 845-852. |
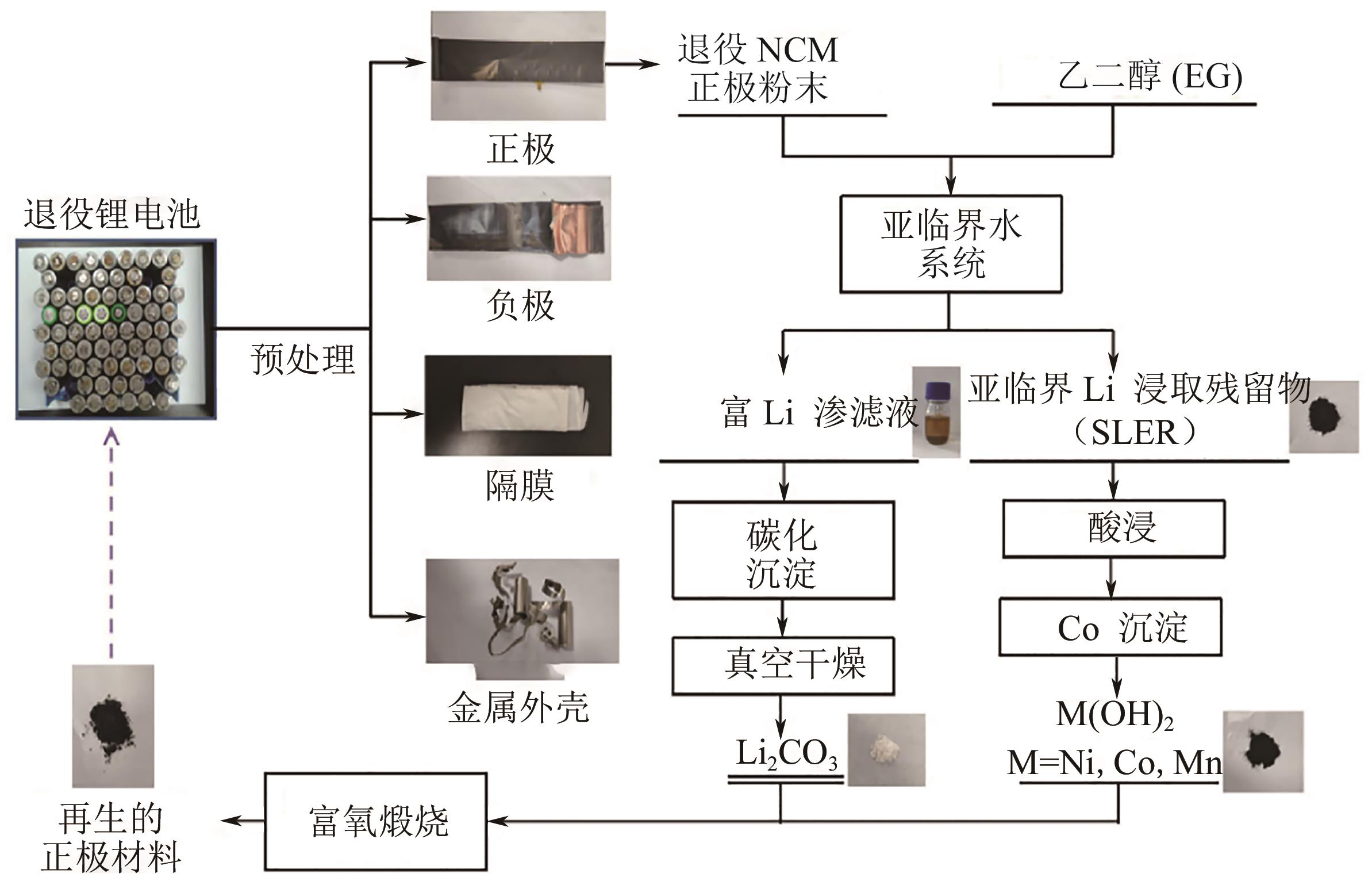
| SONG Jiali, SUN Sun, GAO Wenfang, et al. Selective recovery and kinetics of valuable elements from waste lithium-ion battery cathodes[J]. The Chinese Journal of Process Engineering, 2017, 17(4): 845-852. | |
| 30 | PINNA Eliana G, M-C RUIZ, OJEDA Manuel W, et al. Cathodes of spent Li-ion batteries: Dissolution with phosphoric acid and recovery of lithium and cobalt from leach liquors[J]. Hydrometallurgy, 2017, 167: 66-71. |
| 31 | GU Fu, GUO Jianfeng, YAO Xing, et al. An investigation of the current status of recycling spent lithium-ion batteries from consumer electronics in China[J]. Journal of Cleaner Production, 2017, 161: 765-780. |
| 32 | 曹玲, 刘雅丽, 康铎之, 等. 废旧锂电池中有价金属回收及三元正极材料的再制备[J]. 化工进展, 2019, 38(5): 2499-2505. |
| CAO Ling, LIU Yali, KANG Duozhi, et al. Recovery of valuable metals from spent lithium ion battery and the resynthesis of Li(Ni1/3Co1/3Mn1/3)O2 materials[J]. Chemical Industry and Engineering Progress, 2019, 38(5): 2499-2505. | |
| 33 | ZHANG Xihua, CAO Hongbin, XIE Yongbing, et al. A closed-loop process for recycling LiNi1/3Co1/3Mn1/3O2 from the cathode scraps of lithium-ion batteries: Process optimization and kinetics analysis[J]. Separation and Purification Technology, 2015, 150: 186-195. |
| 34 | LI Li, GE Jing, CHEN Renjie, et al. Environmental friendly leaching reagent for cobalt and lithium recovery from spent lithium-ion batteries[J]. Waste Management, 2010, 30(12): 2615-2621. |
| 35 | DOS SANTOS Caroline Santana, ALVES João Carlos, SILVA Stephany Pires DA, et al. A closed-loop process to recover Li and Co compounds and to resynthesize LiCoO2 from spent mobile phone batteries[J]. Journal of Hazardous Materials, 2019, 362: 458-466. |
| 36 | FU Yuanpeng, HE Yaqun, CHEN Hangchao, et al. Effective leaching and extraction of valuable metals from electrode material of spent lithium-ion batteries using mixed organic acids leachant[J]. Journal of Industrial and Engineering Chemistry, 2019, 79: 154-162. |
| 37 | WU Zhuoran, Tanto SOH, CHAN Jun jie, et al. Repurposing of fruit peel waste as a green reductant for recycling of spent lithium-ion batteries[J]. Environmental Science & Technology, 2020, 54(15): 9681-9692. |
| 38 | YAO Lu, FENG Yong, XI Guoxi. A new method for the synthesis of LiNi1/3Co1/3Mn1/3O2 from waste lithium ion batteries[J]. RSC Advances, 2015, 5(55): 44107-44114. |
| 39 | 张飞, 陆颖舟. 一步法回收和再生废旧钴酸锂电池中的钴酸锂[J]. 化工进展, 2019, 38(8): 3874-3880. |
| ZHANG Fei, LU Yingzhou. One-step recovery and regeneration of LiCoO2 from the spent lithium cobalt oxide battery[J]. Chemical Industry and Engineering Progress, 2019, 38(8): 3874-3880. | |
| 40 | WANG Bin, LIN Xinye, TANG Yuanyuan, et al. Recycling LiCoO2 with methanesulfonic acid for regeneration of lithium-ion battery electrode materials[J]. Journal of Power Sources, 2019, 436: 226828. |
| 41 | LI Li, QU Wenjie, ZHANG Xiaoxiao, et al. Succinic acid-based leaching system: A sustainable process for recovery of valuable metals from spent Li-ion batteries[J]. Journal of Power Sources, 2015, 282: 544-551. |
| 42 | FAN Ersha, SHI Pingchuan, ZHANG Xiaoxiao, et al. Glucose oxidase-based biocatalytic acid-leaching process for recovering valuable metals from spent lithium-ion batteries[J]. Waste Management, 2020, 114: 166-173. |
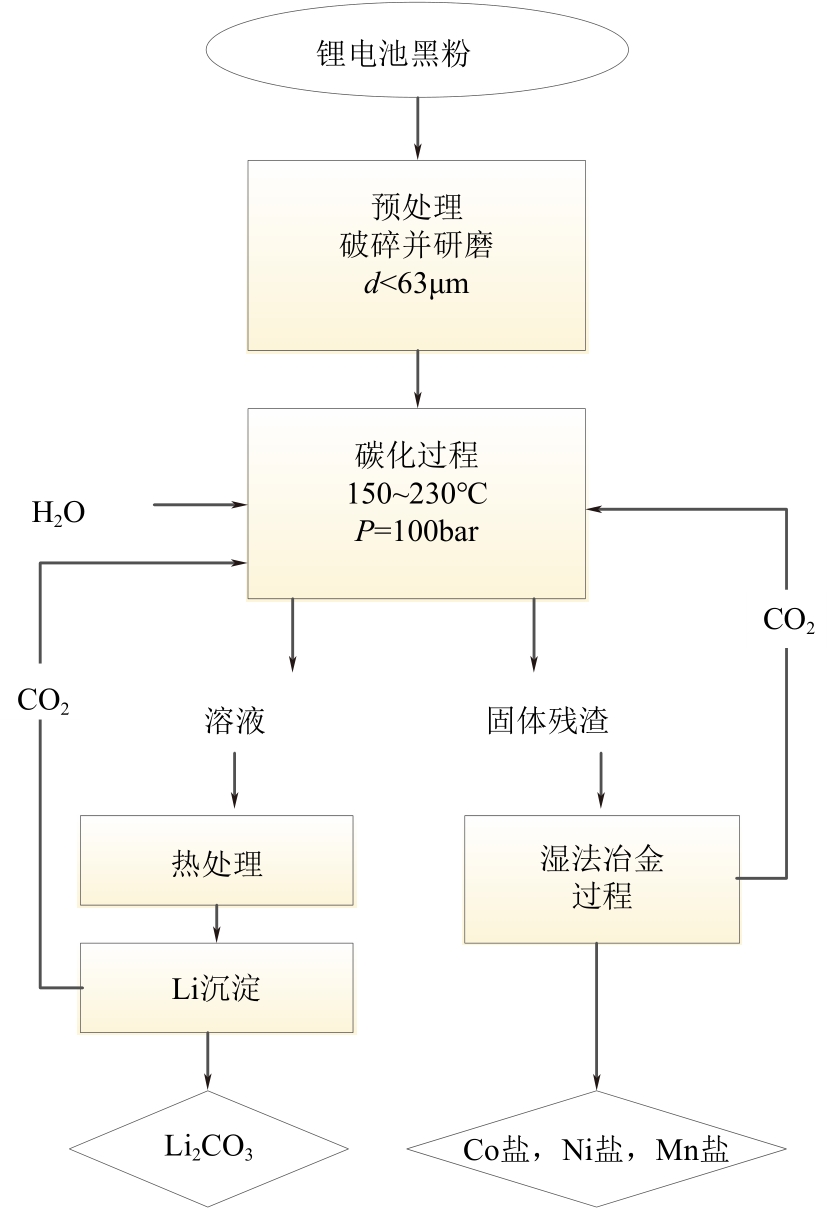
| 43 | 李飞. 废锂电池资源化技术及污染控制研究[D]. 成都: 西南交通大学, 2017. |
| LI Fei. Study on resource technology and pollution control of waste lithium battery[D]. Chengdu: Southwest Jiaotong University, 2017. | |
| 44 | CHEN Xiangping, GUO Chunxiu, MA Hongrui, et al. Organic reductants based leaching: A sustainable process for the recovery of valuable metals from spent lithium ion batteries[J]. Waste Management, 2018, 75: 459-468. |
| 45 | NAYAKA Girish Praveen, Karkala Vasantakumar PAI, MANJANNA Jayappa, et al. Use of mild organic acid reagents to recover the Co and Li from spent Li-ion batteries[J]. Waste Management, 2016, 51: 234-238. |
| 46 | ZHANG Xiaoxiao, LI Li, FAN Ersha, et al. Toward sustainable and systematic recycling of spent rechargeable batteries[J]. Chemical Society Reviews, 2018, 47(19): 7239-7302. |
| 47 | ZHANG Yingjie, MENG Qi, DONG Peng, et al. Use of grape seed as reductant for leaching of cobalt from spent lithium-ion batteries[J]. Journal of Industrial and Engineering Chemistry, 2018, 66: 86-93. |
| 48 | JIANG Youzhou, CHEN Xiangping, YAN Shuxuan, et al. Pursuing green and efficient process towards recycling of different metals from spent lithium-ion batteries through Ferro-chemistry[J]. Chemical Engineering Journal, 2021, 426: 131637. |
| 49 | MARAFI M, STANISLAUS A. Waste catalyst utilization: Extraction of valuable metals from spent hydroprocessing catalysts by ultrasonic-assisted leaching with acids[J]. Industrial & Engineering Chemistry Research, 2011, 50(16): 9495-9501. |
| 50 | MANDAL Ashis K, Ranjan SEN. An overview on microwave processing of material: A special emphasis on glass melting[J]. Materials and Manufacturing Processes, 2017, 32(1): 1-20. |
| 51 | ESMAEILI M, S-O RASTEGAR, BEIGZADEH R, et al. Ultrasound-assisted leaching of spent lithium ion batteries by natural organic acids and H2O2 [J]. Chemosphere, 2020, 254: 126670. |
| 52 | YANG Yongxia, MENG Xiangqi, CAO Hongbin, et al. Selective recovery of lithium from spent lithium iron phosphate batteries: A sustainable process[J]. Green Chemistry, 2018, 20(13): 3121-3133. |
| 53 | LI Huan, XING Shengzhou, LIU Yu, et al. Recovery of lithium, iron, and phosphorus from spent LiFePO4 batteries using stoichiometric sulfuric acid leaching system[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(9): 8017-8024. |
| 54 | GONG Rui, LI Chenchen, MENG Qi, et al. A sustainable closed-loop method of selective oxidation leaching and regeneration for lithium iron phosphate cathode materials from spent batteries[J]. Journal of Environmental Management, 2022, 319: 115740. |
| 55 | ZHANG Qiyue, FAN Ersha, LIN Jiao, et al. Acid-free mechanochemical process to enhance the selective recycling of spent LiFePO4 batteries[J]. Journal of Hazardous Materials, 2023, 443(Pt A): 130160. |
| 56 | WU Deyou, WANG Dongxing, LIU Zhiqiang, et al. Selective recovery of lithium from spent lithium iron phosphate batteries using oxidation pressure sulfuric acid leaching system[J]. Transactions of Nonferrous Metals Society of China, 2022, 32(6): 2071-2079. |
| 57 | HOU Jiahui, MA Xiaotu, FU Jinzhao, et al. A green closed-loop process for selective recycling of lithium from spent lithium-ion batteries[J]. Green Chemistry, 2022, 24(18): 7049-7060. |
| 58 | HARPER Gavin, SOMMERVILLE Roberto, KENDRICK Emma, et al. Recycling lithium-ion batteries from electric vehicles[J]. Nature, 2019, 575(7781): 75-86. |
| 59 | LIU Chunwei, LIN Jiao, CAO Hongbin, et al. Recycling of spent lithium-ion batteries in view of lithium recovery: A critical review[J]. Journal of Cleaner Production, 2019, 228: 801-813. |
| 60 | 张盈盈, 陆小华, 冯新, 等. 胆碱类低共熔溶剂的物性及应用[J]. 化学进展, 2013, 25(6): 881-892. |
| ZHANG Yingying, LU Xiaohua, FENG Xin, et al. Properties and applications of choline-based deep eutectic solvents[J]. Progress in Chemistry, 2013, 25(6): 881-892. | |
| 61 | TRAN Mai K, RODRIGUES Marco-Tulio F, KATO Keiko, et al. Deep eutectic solvents for cathode recycling of Li-ion batteries[J]. Nature Energy, 2019, 4: 339-345. |
| 62 | GOLMOHAMMADZADEH Rabeeh, RASHCHI Fereshteh, VAHIDI Ehsan. Recovery of lithium and cobalt from spent lithium-ion batteries using organic acids: Process optimization and kinetic aspects[J]. Waste Management, 2017, 64: 244-254. |
| 63 | MENG Qi, ZHANG Yingjie, DONG Peng. Use of glucose as reductant to recover Co from spent lithium ions batteries[J]. Waste Management, 2017, 64: 214-218. |
| 64 | SUN Liang, QIU Keqiang. Organic oxalate as leachant and precipitant for the recovery of valuable metals from spent lithium-ion batteries[J]. Waste Management, 2012, 32(8): 1575-1582. |
| 65 | WANG Shubin, ZHANG Zuotai, LU Zhouguang, et al. A novel method for screening deep eutectic solvent to recycle the cathode of Li-ion batteries[J]. Green Chemistry, 2020, 22(14): 4473-4482. |
| 66 | ROLDÁN-RUIZ María Jesús, FERRER María Luisa, GUTIÉRREZ María Concepción, et al. Highly efficient p-toluenesulfonic acid-based deep-eutectic solvents for cathode recycling of Li-ion batteries[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(14): 5437-5445. |
| 67 | RODRIGUEZ Nerea, MACHIELS Lieven, BINNEMANS Koen. p-toluenesulfonic acid-based deep-eutectic solvents for solubilizing metal oxides[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(4): 3940-3948. |
| 68 | LU Bing, DU Rong, WANG Gang, et al. High-efficiency leaching of valuable metals from waste Li-ion batteries using deep eutectic solvents[J]. Environmental Research, 2022, 212(Pt B): 113286. |
| 69 | VAN OSCH Dannie J G P, PARMENTIER Dries, DIETZ Carin H J T, et al. Removal of alkali and transition metal ions from water with hydrophobic deep eutectic solvents[J]. Chemical Communications, 2016, 52(80): 11987-11990. |
| 70 | MORINA Riccardo, CALLEGARI Daniele, MERLI Daniele, et al. Cathode active material recycling from spent lithium batteries: A green (circular) approach based on deep eutectic solvents[J]. ChemSusChem, 2022, 15(2): e202102080. |
| 71 | 巩珊珊, 吴彤, 王官格, 等. 基于高效回收废旧锂离子电池正极材料的低共熔溶剂的筛选[J]. 高等学校化学学报, 2021, 42(10): 3151-3159. |
| GONG Shanshan, WU Tong, WANG Guange, et al. Screening of deep eutectic solvent based on efficient recovery of spent lithium-ion battery cathode materials[J]. Chemical Journal of Chinese Universities, 2021, 42(10): 3151-3159. | |
| 72 | JOULIÉ M, BILLY E, LAUCOURNET R, et al. Current collectors as reducing agent to dissolve active materials of positive electrodes from Li-ion battery wastes[J]. Hydrometallurgy, 2017, 169: 426-432. |
| 73 | PENG Chao, LIU Fupeng, Arif T AJI, et al. Extraction of Li and Co from industrially produced Li-ion battery waste - Using the reductive power of waste itself[J]. Waste Management, 2019, 95: 604-611. |
| 74 | PEETERS Nand, BINNEMANS Koen, Sofía RIAÑO. Solvometallurgical recovery of cobalt from lithium-ion battery cathode materials using deep-eutectic solvents[J]. Green Chemistry, 2020, 22(13): 4210-4221. |
| 75 | LI Xuning, HUANG Xiang, XI Shibo, et al. Single cobalt atoms anchored on porous N-doped graphene with dual reaction sites for efficient fenton-like catalysis[J]. Journal of the American Chemical Society, 2018, 140(39): 12469-12475. |
| 76 | LONG Yangke, HUANG Yixuan, WU Huiyi, et al. Peroxymonosulfate activation for pollutants degradation by Fe-N-codoped carbonaceous catalyst: Structure-dependent performance and mechanism insight[J]. Chemical Engineering Journal, 2019, 369: 542-552. |
| 77 | GUO Hao, MIN Zijun, HAO Ying, et al. Sustainable recycling of LiCoO2 cathode scrap on the basis of successive peroxymonosulfate activation and recovery of valuable metals[J]. The Science of the Total Environment, 2021, 759: 143478. |
| 78 | RAMANUJAPURAM Anirudh, GORDON Daniel, MAGASINSKI Alexandre, et al. Degradation and stabilization of lithium cobalt oxide in aqueous electrolytes[J]. Energy & Environmental Science, 2016, 9(5): 1841-1848. |
| 79 | XU Zhiwen, SHAO Huaishuang, ZHAO Qinxin, et al. Use of microwave-assisted deep eutectic solvents to recycle lithium manganese oxide from Li-ion batteries[J]. JOM, 2021, 73(7): 2104-2110. |
| 80 | LIU Mengshun, MA Wenjun, ZHANG Xu, et al. Recycling lithium and cobalt from LIBs using microwave-assisted deep eutectic solvent leaching technology at low-temperature[J]. Materials Chemistry and Physics, 2022, 289: 126466. |
| 81 | MA Wenjun, LIU Mengshun, ZHANG Xu, et al. An efficient and precipitant-free approach to selectively recover lithium cobalt oxide made for cathode materials using a microwave-assisted deep eutectic solvent[J]. Energy & Fuels, 2023, 37(1): 724-734. |
| 82 | FAN Ersha, LI Li, WANG Zhenpo, et al. Sustainable recycling technology for Li-ion batteries and beyond: Challenges and future prospects[J]. Chemical Reviews, 2020, 120(14): 7020-7063. |
| 83 | WU Zhi, HUANG Rongrong, YU Hang, et al. Deep eutectic solvent synthesis of LiMnPO4/C nanorods as a cathode material for lithium ion batteries[J]. Materials, 2017, 10(2): 134. |
| 84 | WU Zhi, LONG Yun fei, Xiaoyan LYU, et al. Microwave heating synthesis of spindle-like LiMnPO4/C in a deep eutectic solvent[J]. Ceramics International, 2017, 43(8): 6089-6095. |
| 85 | LU Qingqiang, CHEN Linlin, LI Xiaowei, et al. Sustainable and convenient recovery of valuable metals from spent Li-ion batteries by a one-pot extraction process[J]. ACS Sustainable Chemistry & Engineering, 2021, 9(41): 13851-13861. |
| 86 | CHANG Xin, FAN Min, GU Chaofan, et al. Selective extraction of transition metals from spent LiNi x Co y Mn 1- x-y O2 cathode via regulation of coordination environment[J]. Angewandte Chemie International Edition, 2022, 61(24): 2202558. |
| 87 | THOMPSON Dana L, PATELI Ioanna M, LEI Chunhong, et al. Separation of nickel from cobalt and manganese in lithium ion batteries using deep eutectic solvents[J]. Green Chemistry, 2022, 24(12): 4877-4886. |
| 88 | SCHIAVI Pier Giorgio, ALTIMARI Pietro, BRANCHI Mario, et al. Selective recovery of cobalt from mixed lithium ion battery wastes using deep eutectic solvent[J]. Chemical Engineering Journal, 2021, 417: 129249. |
| 89 | CHEN Linlin, CHAO Yanhong, LI Xiaowei, et al. Engineering a tandem leaching system for the highly selective recycling of valuable metals from spent Li-ion batteries[J]. Green Chemistry, 2021, 23(5): 2177-2184. |
| 90 | LI Kuo, ZHANG Lingen, XU Zhenming. Decomposition behavior and mechanism of epoxy resin from waste integrated circuits under supercritical water condition[J]. Journal of Hazardous Materials, 2019, 374: 356-364. |
| 91 | CHEN Jingwei, MENG Tian, LENG Erwei, et al. Review on metal dissolution characteristics and harmful metals recovery from electronic wastes by supercritical water[J]. Journal of Hazardous Materials, 2022, 424(Pt D): 127693. |
| 92 | 刘肖贝, 张西华, 熊梅, 等. 退役锂电池放电废水特征有机污染物解析[J]. 化工进展, 2022, 41(10): 5619-5629. |
| LIU Xiaobei, ZHANG Xihua, XIONG Mei, et al. Analysis on the characteristic organic pollutants from discharge wastewater of spent lithium batteries[J]. Chemical Industry and Engineering Progress, 2022, 41(10): 5619-5629. | |
| 93 | O'BRIEN Christopher P, THIES Mark C, BRUCE David A. Supercritical water oxidation of the PCB congener 2-chlorobiphenyl in methanol solutions: A kinetic analysis[J]. Environmental Science & Technology, 2005, 39(17): 6839-6844. |
| 94 | VERIANSYAH Bambang, KIM Jae-Duck, LEE Youn-Woo. Simultaneous recovery of chromium and destruction of organics from LCD manufacturing process wastewater by supercritical water oxidation[J]. Journal of Cleaner Production, 2006, 15(10): 972-978. |
| 95 | MATSUMOTO Yuta, OSHIMA Yoshito. Au and Cu recovery from printed boards by decomposition of epoxy resin in supercritical water[J]. The Journal of Supercritical Fluids, 2014, 95: 462-467. |
| 96 | LI Kuo, XU Zhenming. Application of supercritical water to decompose brominated epoxy resin and environmental friendly recovery of metals from waste memory module[J]. Environmental Science & Technology, 2015, 49(3): 1761-1767. |
| 97 | XING Mingfei, ZHANG Fushen. Degradation of brominated epoxy resin and metal recovery from waste printed circuit boards through batch sub/supercritical water treatments[J]. Chemical Engineering Journal, 2013, 219: 131-136. |
| 98 | NSHIZIRUNGU Theoneste, AGARWAL Ashutosh, Young Tae JO, et al. Chlorinated polyvinyl chloride (CPVC) assisted leaching of lithium and cobalt from spent lithium-ion battery in subcritical water[J]. Journal of Hazardous Materials, 2020, 393: 122367. |
| 99 | NSHIZIRUNGU Theoneste, RANA Masud, Young-Tae JO, et al. Rapid leaching and recovery of valuable metals from spent Lithium Ion batteries (LIBs) via environmentally benign subcritical nickel-containing water over chlorinated polyvinyl chloride[J]. Journal of Hazardous Materials, 2020, 396: 122667. |
| 100 | MA Yayun, LIU Xiaojian, ZHOU Xiangyang, et al. Selective extraction of lithium from spent LiNi x Co y Mn z O2 cathode via in situ conversion of ethylene glycol in subcritical water system[J]. Chemical Engineering Journal, 2023, 451: 138535. |
| 101 | LIN Fengying, LIU Dagang, MAITI DAS Sonakshi, et al. Recent progress in heavy metal extraction by supercritical CO2 fluids[J]. Industrial & Engineering Chemistry Research, 2014, 53(5): 1866-1877. |
| 102 | CALGARO C O, SCHLEMMER D F, SILVA M D C R DA, et al. Fast copper extraction from printed circuit boards using supercritical carbon dioxide[J]. Waste Management, 2015, 45: 289-297. |
| 103 | LIU Yuanlong, MU Deying, DAI Yunkun, et al. Analysis on extraction behaviour of lithium-ion battery electrolyte solvents in supercritical CO2 by gas chromatography[J]. International Journal of Electrochemical Science, 2016, 11(9): 7594-7604. |
| 104 | KIM Seoa, BANG Jaeyeon, YOO Junsang, et al. A comprehensive review on the pretreatment process in lithium-ion battery recycling[J]. Journal of Cleaner Production, 2021, 294: 126329. |
| 105 | LIU Yuanlong, MU Deying, LI Ruhong, et al. Purification and characterization of reclaimed electrolytes from spent lithium-ion batteries[J]. The Journal of Physical Chemistry C, 2017, 121(8): 4181-4187. |
| 106 | Xaver MÖNNIGHOFF, FRIESEN Alex, KONERSMANN Benedikt, et al. Supercritical carbon dioxide extraction of electrolyte from spent lithium ion batteries and its characterization by gas chromatography with chemical ionization[J]. Journal of Power Sources, 2017, 352: 56-63. |
| 107 | XIE Ming, ZHANG Xiaoxue, DENG Sixu, et al. The effects of supercritical carbon dioxide treatment on the morphology and electrochemical performance of LiFePO4 cathode materials[J]. RSC Advances, 2013, 3(31): 12786-12793. |
| 108 | XIE Ming, ZHANG Xiaoxue, LAAKSO Jarmo, et al. New method of postmodifying the particle size and morphology of LiFePO4 via supercritical carbon dioxide[J]. Crystal Growth & Design, 2012, 12(5): 2166-2168. |
| 109 | KUNANUSONT Nattanai, SHIMOYAMA Yusuke. Porous carbon cathode assisted with ionogel binder fabricated from supercritical fluid technique toward Li-O2/CO2 battery application[J]. ACS Applied Energy Materials, 2020, 3(5): 4421-4431. |
| 110 | ZHANG Jingwen, ZHUO Linhai, ZHANG Leilei, et al. Synthesis and electrochemical properties of LiFePO4/C composite cathode material prepared by a new route using supercritical carbon dioxide as a solvent[J]. Journal of Materials Chemistry, 2011, 21(19): 6975-6980. |
| 111 | LIU Hongting, CHAN Ka HO, MALIK Monu, et al. Synthesis of LiNi0.6Co0.2Mn0.2O2 using supercritical carbon dioxide as a cathode material for lithium-ion batteries[J]. ChemElectroChem, 2023, 10(1): e202201021. |
| 112 | XIE Ming, ZHANG Xiaoxue, WANG Yazhou, et al. A template-free method to prepare porous LiFePO4 via supercritical carbon dioxide[J]. Electrochimica Acta, 2013, 94: 16-20. |
| 113 | FU Yuanpeng, SCHUSTER Jonas, PETRANIKOVA Martina, et al. Innovative recycling of organic binders from electric vehicle lithium-ion batteries by supercritical carbon dioxide extraction[J]. Resources, Conservation and Recycling, 2021, 172: 105666. |
| 114 | MARTIN G, SCHNEIDER A, VOIGT W, et al. Lithium extraction from the mineral zinnwaldite: Part Ⅱ: Lithium carbonate recovery by direct carbonation of sintered zinnwaldite concentrate[J]. Minerals Engineering, 2017, 110: 75-81. |
| 115 | RENTSCH Lars, MARTIN Gunther, BERTAU Martin, et al. Lithium extracting from zinnwaldite: Economical comparison of an adapted spodumene and a direct-carbonation process[J]. Chemical Engineering & Technology, 2018, 41(5): 975-982. |
| 116 | Sandra PAVÓN, KAISER Doreen, MENDE Robert, et al. The COOL-process—a selective approach for recycling lithium batteries[J]. Metals, 2021, 11(2): 259. |
| 117 | BERTUOL Daniel A, MACHADO Caroline M, SILVA Mariana L, et al. Recovery of cobalt from spent lithium-ion batteries using supercritical carbon dioxide extraction[J]. Waste Management, 2016, 51: 245-251. |
| 118 | ZHANG Jiakai, AZIMI Gisele. Recycling of lithium, cobalt, nickel, and manganese from end-of-life lithium-ion battery of an electric vehicle using supercritical carbon dioxide[J]. Resources, Conservation and Recycling, 2022, 187: 106628. |
| 119 | SUNARSO Jaka, ISMADJI Suryadi. Decontamination of hazardous substances from solid matrices and liquids using supercritical fluids extraction: A review[J]. Journal of Hazardous Materials, 2009, 161(1): 1-20. |
| 120 | RUIU Andrea, BOUILHAC Cécile, GIMELLO Olinda, et al. Synthesis and phase behavior of a platform of CO2-soluble functional gradient copolymers bearing metal-complexing units[J]. Polymers, 2022, 14(13): 2698. |
| 121 | 黄辉, 韩健峰, 王奕顺, 等. 富锂锰表面超临界CO2辅助包覆磷酸锰锂及其电化学性能[J]. 材料导报, 2018, 32(23): 4072-4078. |
| HUANG Hui, HAN Jianfeng, WANG Yishun, et al. Supercritical CO2 assisting cladding of LiMnPO4 on the surface of Li[Li0.2-Mn0.54Co0.13Ni0.13]O2 and its electrochemical properties[J]. Materials Review, 2018, 32(23): 4072-4078. | |
| 122 | 朱允. 超临界二氧化碳沉积制备锂硫电池复合正极材料[D]. 金华: 浙江师范大学, 2019. |
| ZHU Yun. Supercritical carbon dioxide deposition of composite cathode materials for lithium-sulfur battery[D]. Jinhua: Zhejiang Normal University, 2019. |
| [1] | 何金, 赖裕文, 李艳春, 周士林, 周勇, 高从堦. DES改变胺单体扩散速率制备高性能复合反渗透膜[J]. 化工进展, 2024, 43(4): 1972-1980. |
| [2] | 刘世达, 王海燕, 侯栓弟, 刘忠生, 廖昌建, 王宽岭. 我国石化储罐VOCs安全高效深度减排、回收和热氧化技术进展[J]. 化工进展, 2024, 43(4): 2063-2076. |
| [3] | 李开瑞, 高照华, 刘甜甜, 李静, 魏海生. 还原温度调变Rh/FePO4催化剂喹啉选择加氢性能[J]. 化工进展, 2024, 43(3): 1342-1349. |
| [4] | 闫守成, 张慧华, 徐倩倩, 王煜坤. 石墨烯复合载体催化剂在柴油车尾气NO脱除中的应用[J]. 化工进展, 2024, 43(3): 1456-1465. |
| [5] | 楚振普, 陈禹蒙, 李俊国, 孙庆轩, 刘科. 废旧锂离子电池负极石墨循环再生的研究进展[J]. 化工进展, 2024, 43(3): 1524-1534. |
| [6] | 容凡丁, 丁泽相, 曹义风, 陈俐吭, 杨柳, 申福星, 杨启炜, 鲍宗必. 离子液体强化不饱和键差异化合物分离的研究进展[J]. 化工进展, 2024, 43(1): 198-214. |
| [7] | 钟丁磊, 黄铎, 应翔, 邱守添, 汪勇. 熔纺-选择性溶胀制备嵌段共聚物多通道中空纤维膜[J]. 化工进展, 2024, 43(1): 269-278. |
| [8] | 卜祥宁, 任玺冰, 童正, 倪梦茜, 倪超, 谢广元. 功率超声对废旧锂离子电池资源化回收利用过程的影响研究进展[J]. 化工进展, 2024, 43(1): 514-528. |
| [9] | 李梦圆, 郭凡, 李群生. 聚乙烯醇生产中回收工段第三、第四精馏塔的模拟与优化[J]. 化工进展, 2023, 42(S1): 113-123. |
| [10] | 马伊, 曹世伟, 王家骏, 林立群, 邢延, 曹腾良, 卢峰, 赵振伦, 张志军. 低共熔溶剂回收废旧锂离子电池正极材料的研究进展[J]. 化工进展, 2023, 42(S1): 219-232. |
| [11] | 张杰, 王放放, 夏忠林, 赵光金, 马双忱. “双碳”目标下SF6排放现状、减排手段分析及未来展望[J]. 化工进展, 2023, 42(S1): 447-460. |
| [12] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [13] | 李化全, 王明华, 邱贵宝. 硫酸酸解钙钛矿相精矿的行为[J]. 化工进展, 2023, 42(S1): 536-541. |
| [14] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [15] | 王晋刚, 张剑波, 唐雪娇, 刘金鹏, 鞠美庭. 机动车尾气脱硝催化剂Cu-SSZ-13的改性研究进展[J]. 化工进展, 2023, 42(9): 4636-4648. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||