化工进展 ›› 2025, Vol. 44 ›› Issue (8): 4352-4364.DOI: 10.16085/j.issn.1000-6613.2025-0068
• 微介观过程与材料的模拟与仿真 • 上一篇
基于分子动力学模拟的胺溶剂碳捕集过程自扩散系数
黄可儿( ), 刘佳豪, 李昊明, 周天航(
), 刘佳豪, 李昊明, 周天航( ), 高金森, 蓝兴英(
), 高金森, 蓝兴英( )
)
- 中国石油大学(北京)重质油全国重点实验室,北京 102249
-
收稿日期:2025-01-10修回日期:2025-03-28出版日期:2025-08-25发布日期:2025-09-08 -
通讯作者:周天航,蓝兴英 -
作者简介:黄可儿(2000—),女,博士研究生,研究方向为人工智能及化学产品设计。E-mail:huangkeer0728@163.com。 -
基金资助:国家自然科学基金(U22B20149)
Self-diffusion coefficients in the process of carbon capture by amine solvents based on molecular dynamics simulation
HUANG Ke’er( ), LIU Jiahao, LI Haoming, ZHOU Tianhang(
), LIU Jiahao, LI Haoming, ZHOU Tianhang( ), GAO Jinsen, LAN Xingying(
), GAO Jinsen, LAN Xingying( )
)
- State Key Laboratory of Heavy Oil Processing, China University of Petroleum (Beijing), Beijing 102249, China
-
Received:2025-01-10Revised:2025-03-28Online:2025-08-25Published:2025-09-08 -
Contact:ZHOU Tianhang, LAN Xingying
摘要:
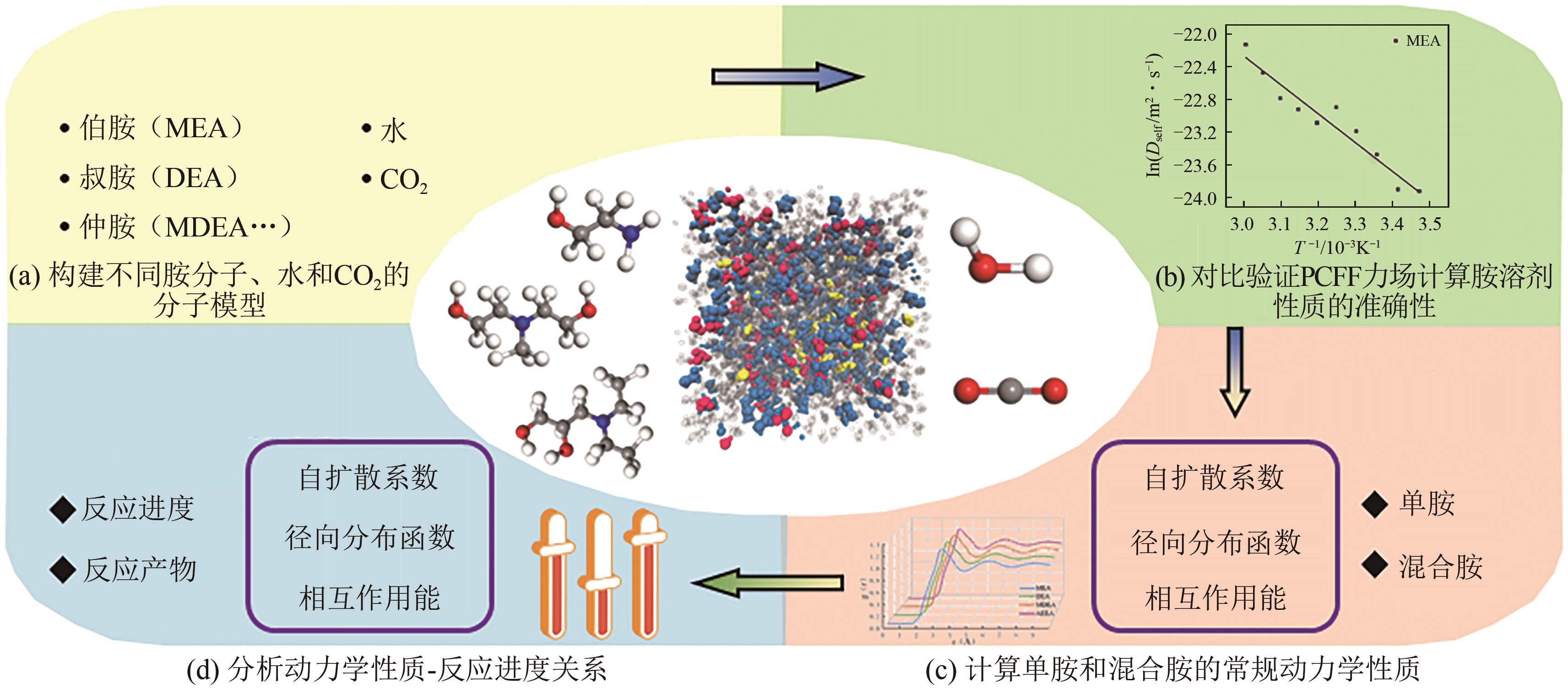
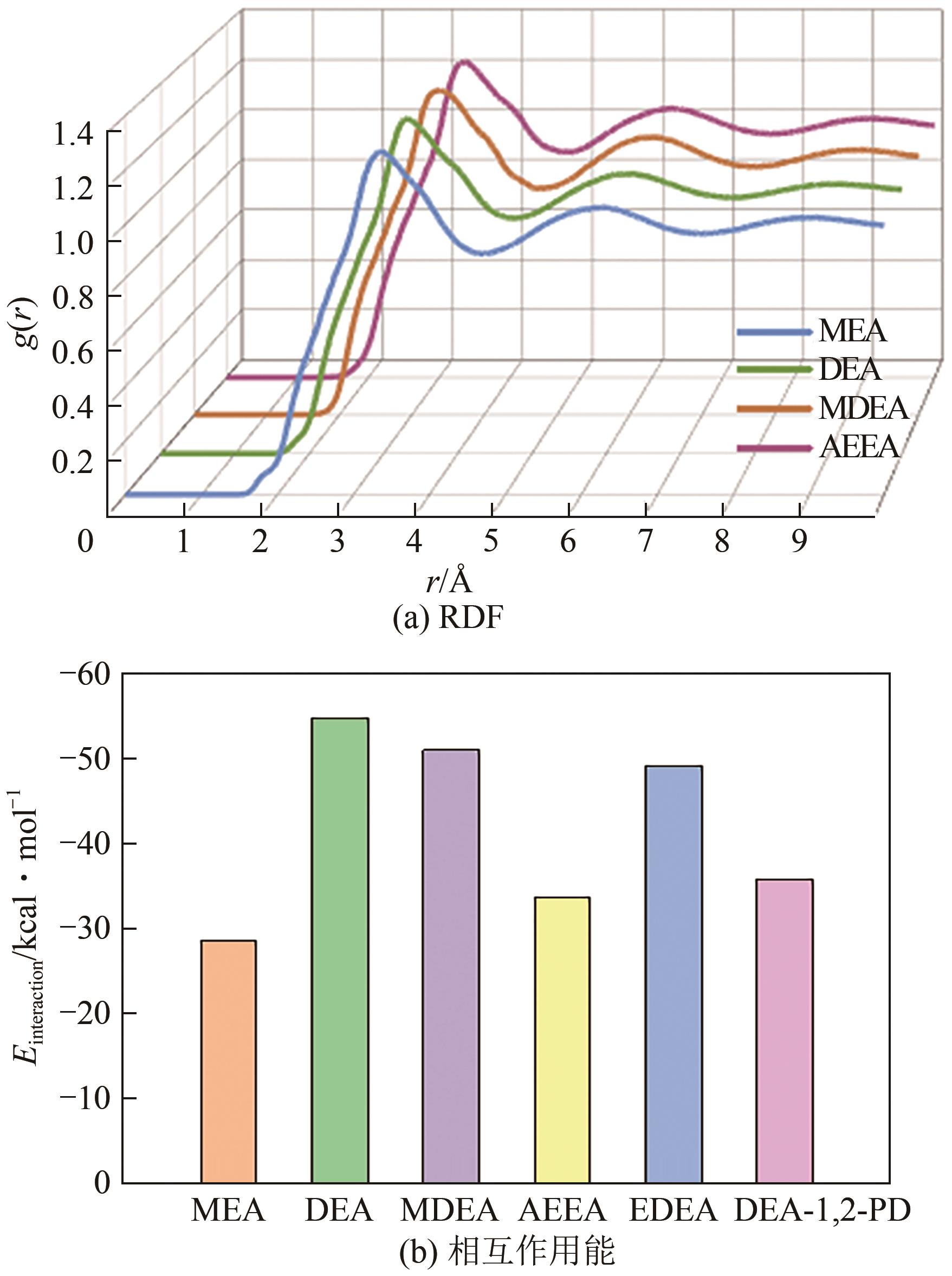
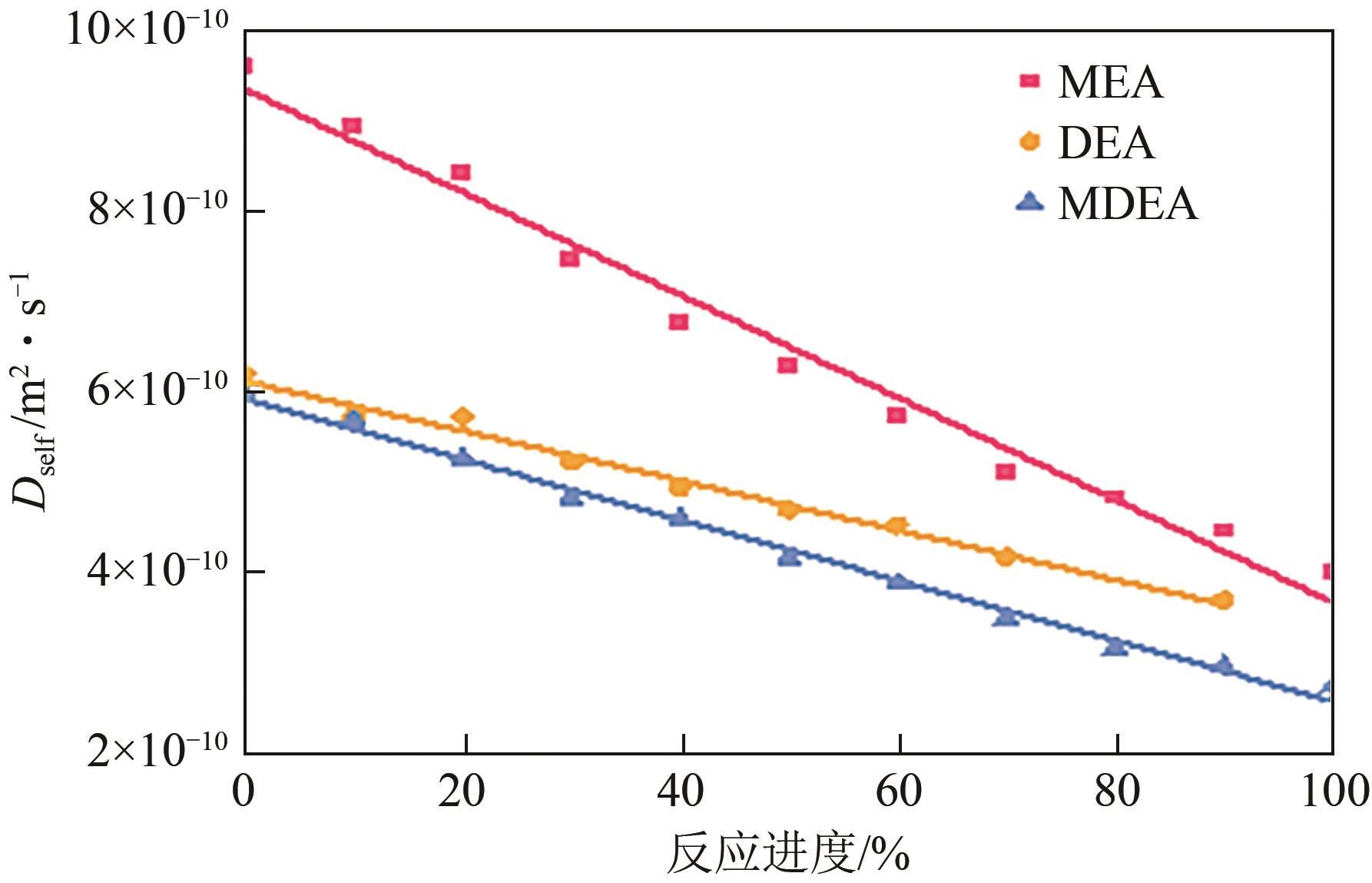
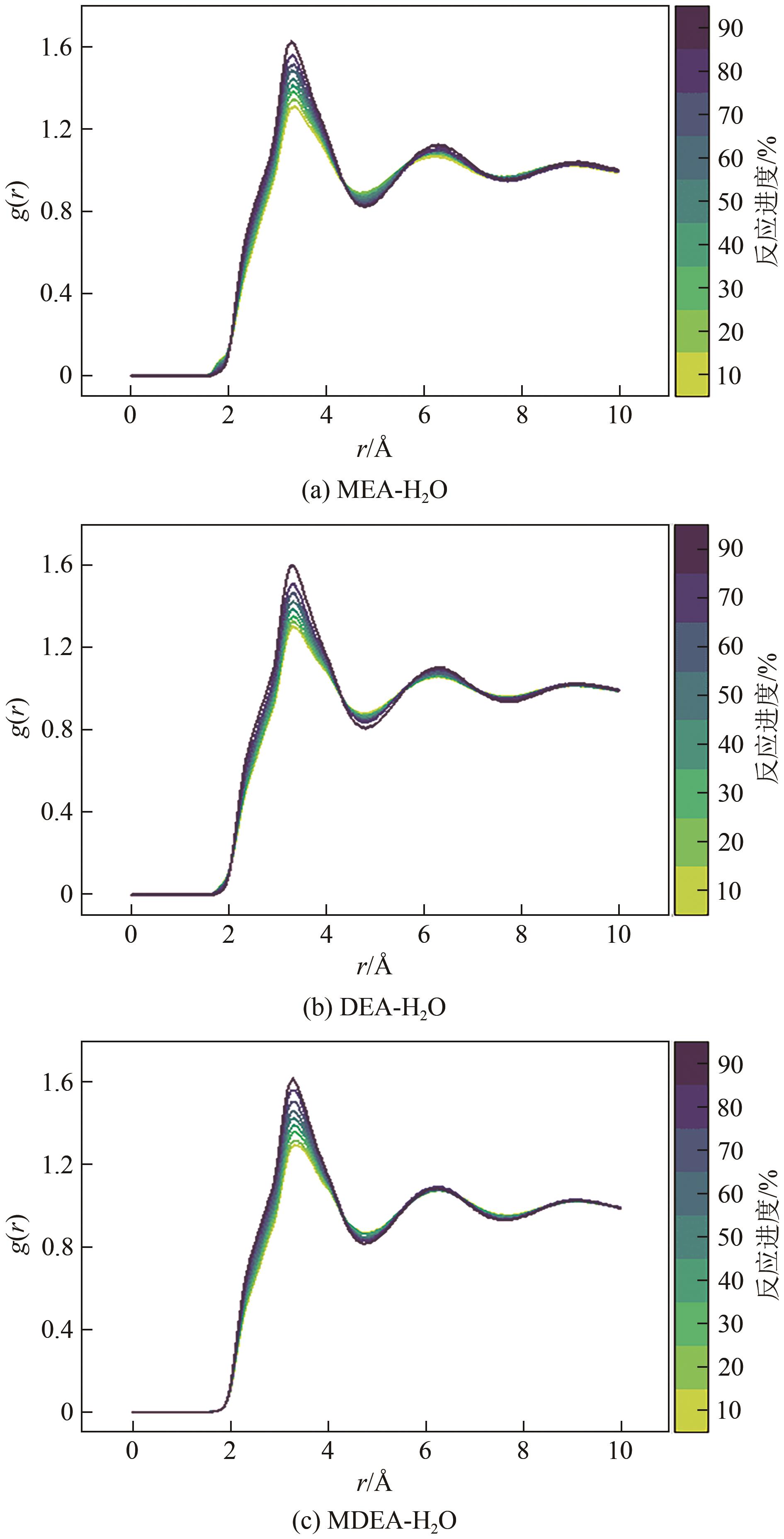
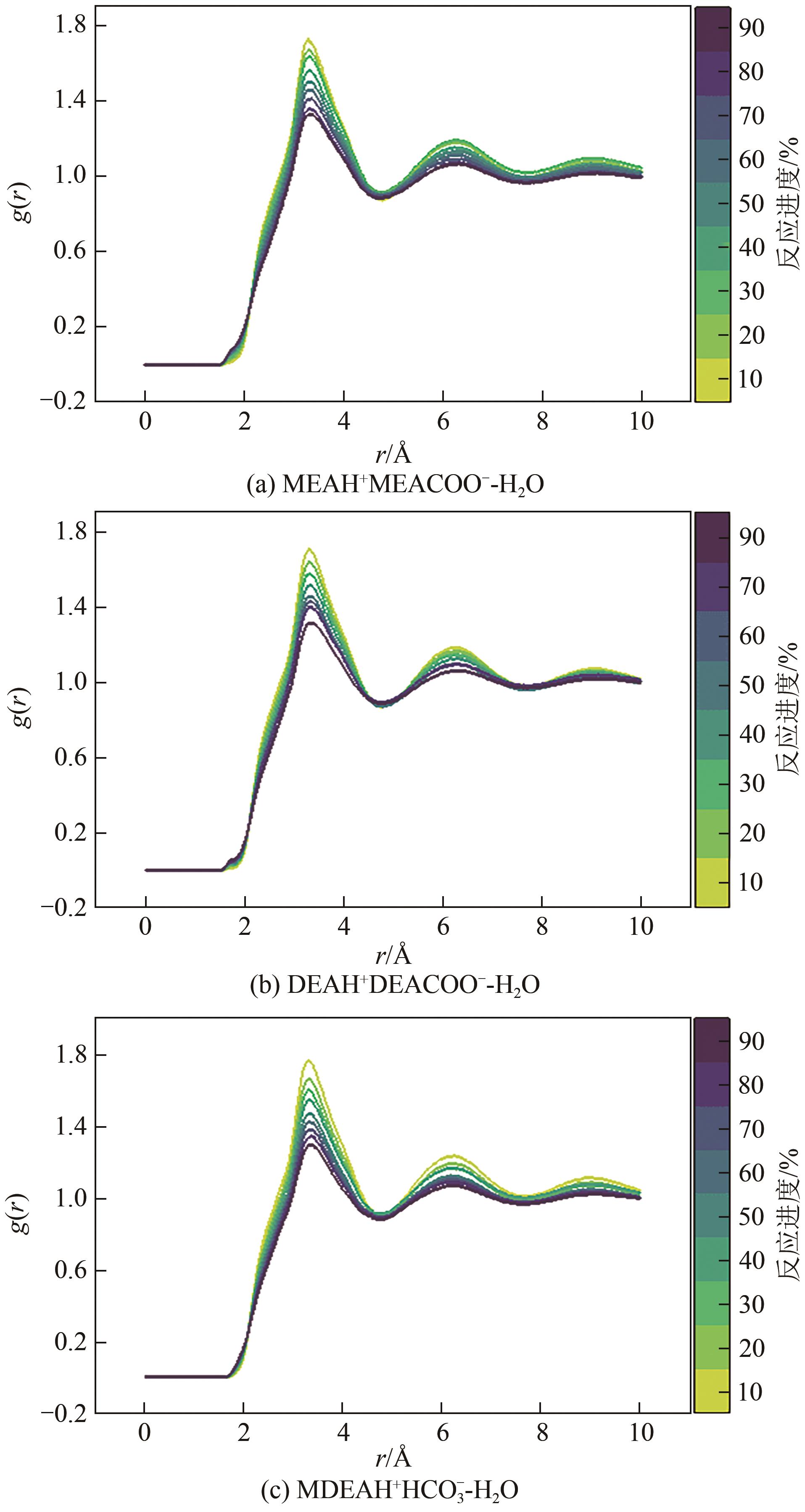
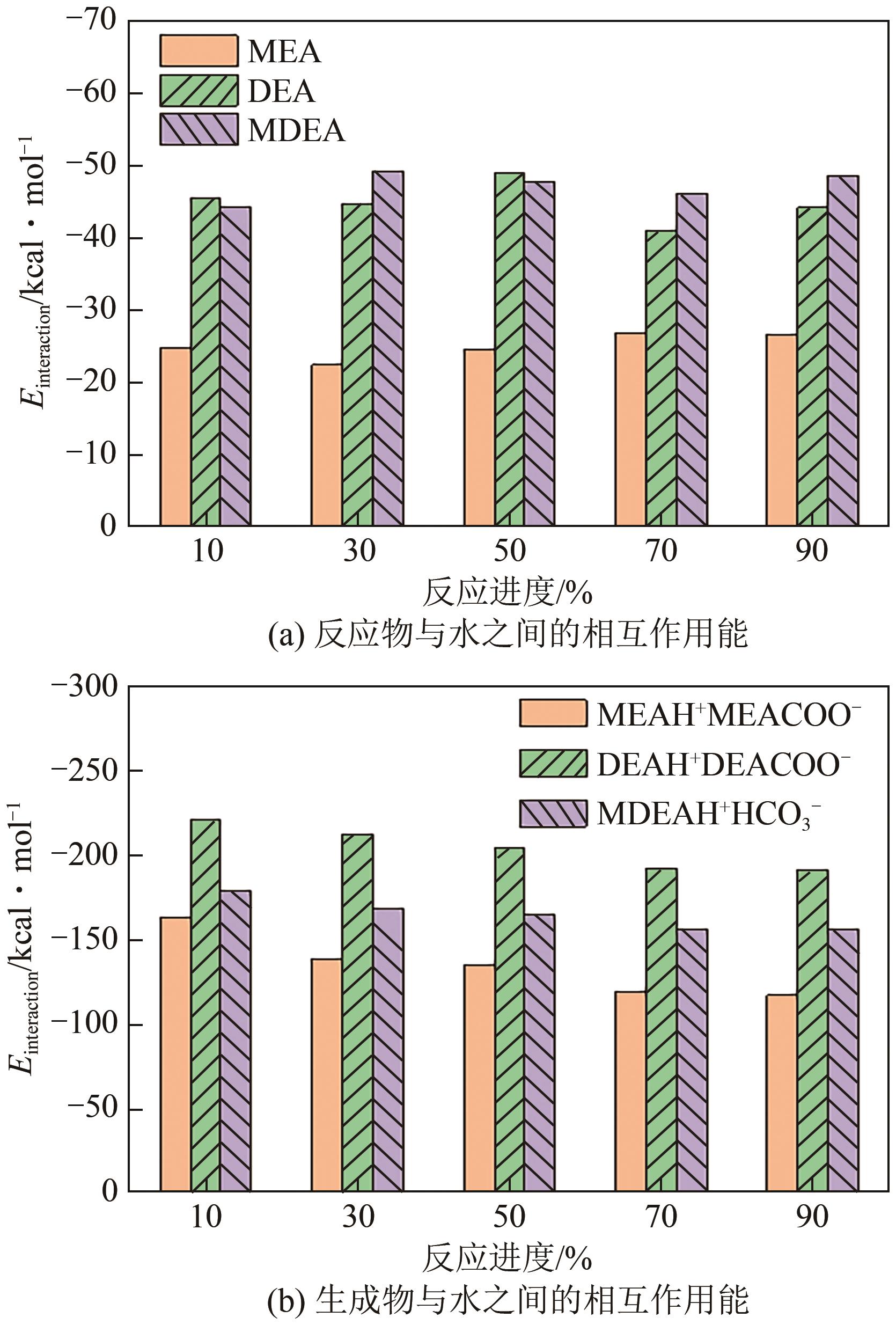
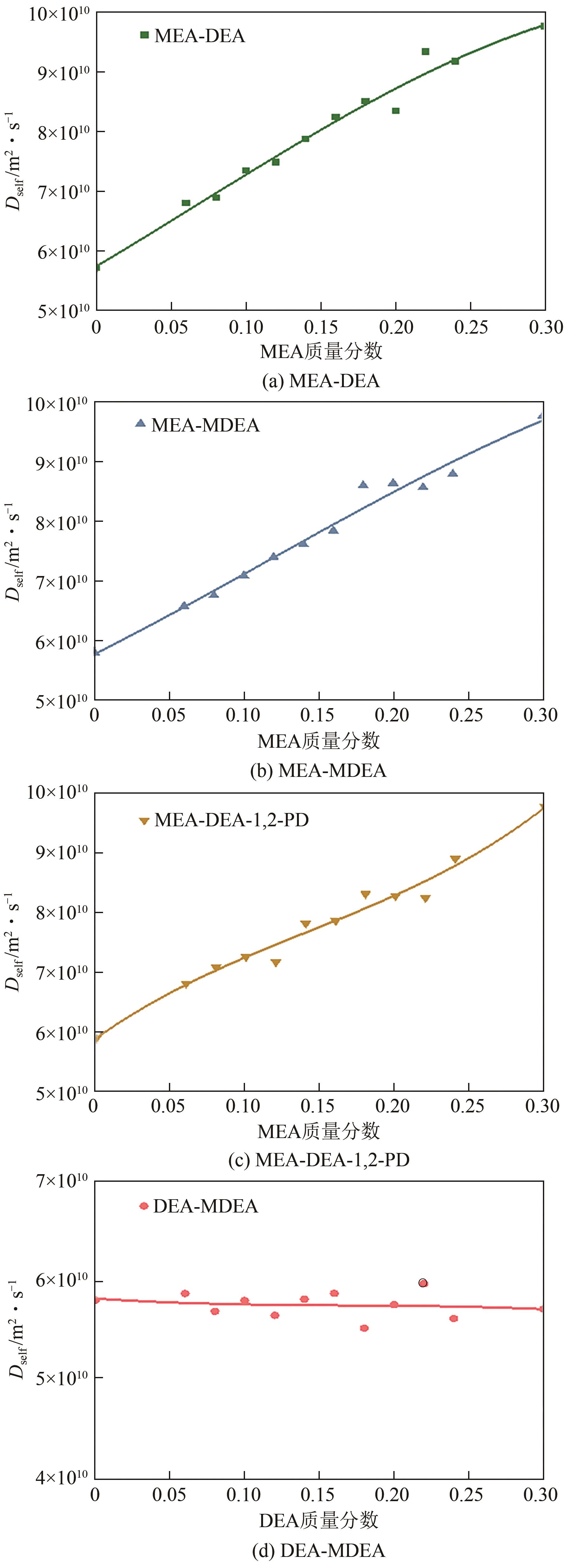
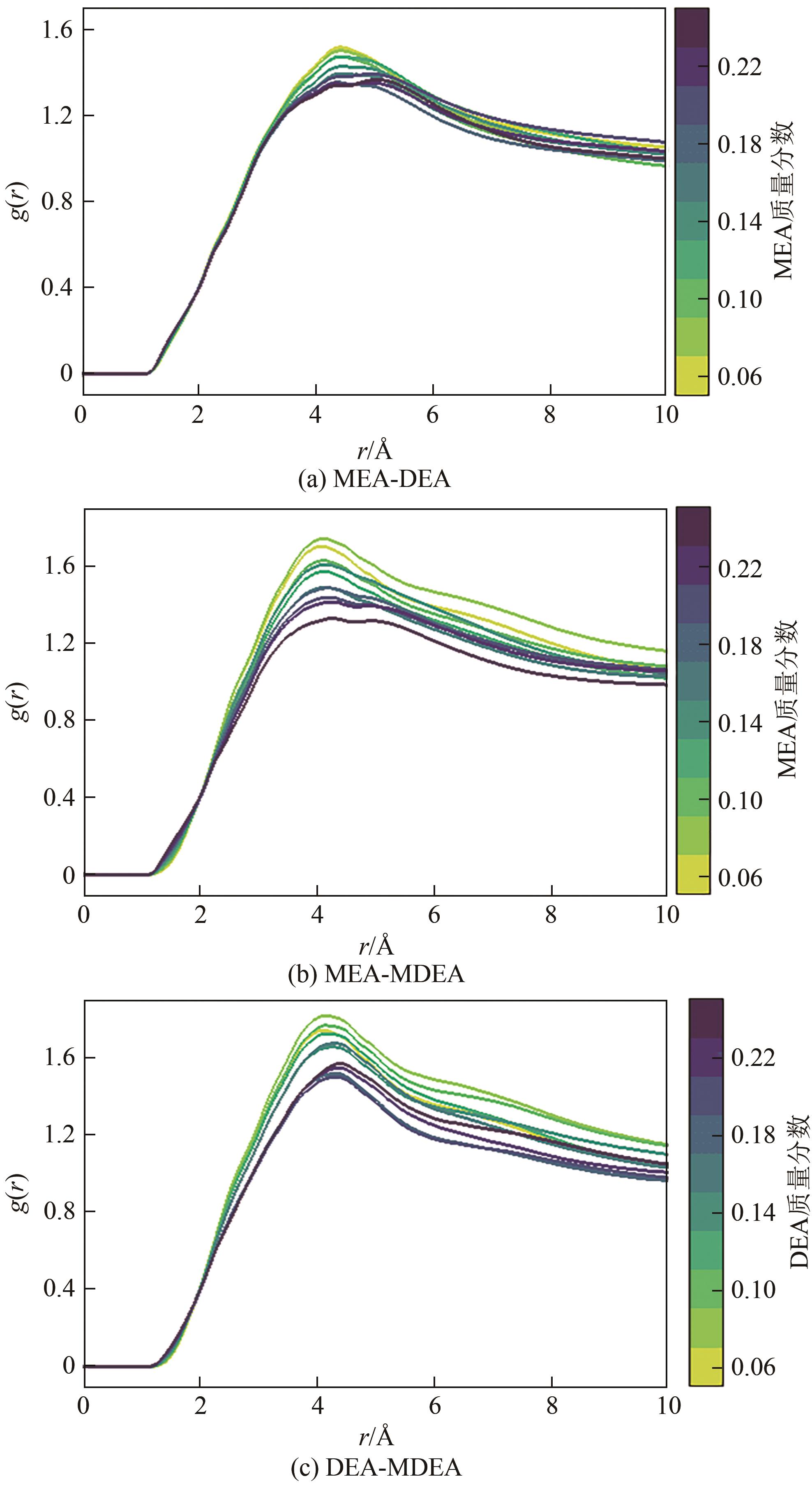
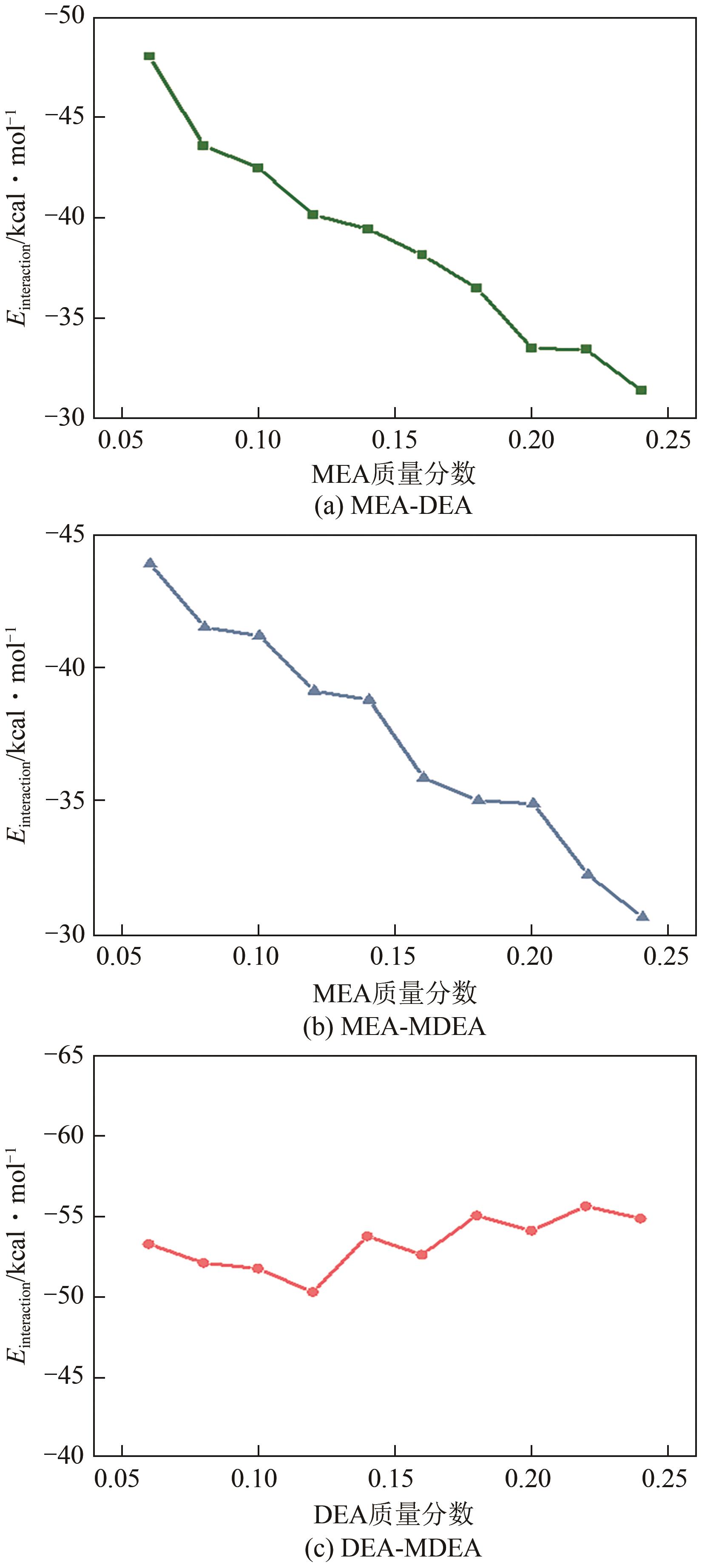
化学吸收法是当前广泛应用的CO2捕集方法之一,而采用胺溶剂进行化学吸收的技术较为成熟。胺溶剂碳捕集过程是典型具备“三传一反”特性的化工过程。在这一过程中,扩散系数是评估其传质过程的重要参数,同时影响着胺溶剂吸收CO2过程中的动力学性能评价和反应性能评价。由于传统的实验方法测量胺溶剂扩散系数较为烦琐,本文采用分子动力学模拟的方法进行研究。首先,研究发现通用小分子力场模型(PCFF)在胺溶剂水溶液体系下的模拟精度较低,为确保模拟的准确性,需要针对分子力场模型进行修正。结果表明,修正后的力场模型能准确描述甲基二乙醇胺(MDEA)溶剂中的分子间相互作用和自扩散系数,预测误差小于3%。随后,基于修正模型,本文计算了多种单胺溶剂的自扩散系数,并推导了自扩散系数与温度之间的定量关系。为探明胺溶剂动力学性质受反应进度变化产生的离子的影响,研究构建了不同反应进度下的模拟体系。基于结果,研究发现自扩散系数在反应过程中逐渐减小,且与反应进度呈线性关系。此外,对不同比例混合胺溶剂(如单乙醇胺与甲基二乙醇胺)的模拟显示,自扩散系数与MEA的质量分数近似呈线性关系。通过对分子间相互作用能和径向分布函数的进一步分析,本文阐明了胺溶剂自扩散系数的变化规律及其微观机制。综上,本文针对通用小分子力场模型进行了修正,并为优化胺溶剂的CO2捕集性能提供了重要的理论支持和模拟工具。
中图分类号:
引用本文
黄可儿, 刘佳豪, 李昊明, 周天航, 高金森, 蓝兴英. 基于分子动力学模拟的胺溶剂碳捕集过程自扩散系数[J]. 化工进展, 2025, 44(8): 4352-4364.
HUANG Ke’er, LIU Jiahao, LI Haoming, ZHOU Tianhang, GAO Jinsen, LAN Xingying. Self-diffusion coefficients in the process of carbon capture by amine solvents based on molecular dynamics simulation[J]. Chemical Industry and Engineering Progress, 2025, 44(8): 4352-4364.
| 项目 | 288K | 298K | 308K |
|---|---|---|---|
| 未修正力场结果/10-10m2·s-1 | 11.55 | 12.84 | 14.75 |
| 修正力场结果/10-10m2·s-1 | 0.41 | 0.64 | 1.15 |
| 阿伦尼乌斯方程拟合结果/10-10m2·s-1 | 0.41 | 0.61 | 0.89 |
| 实验结果/10-10m2·s-1 | 0.4 | 0.55 | 0.93 |
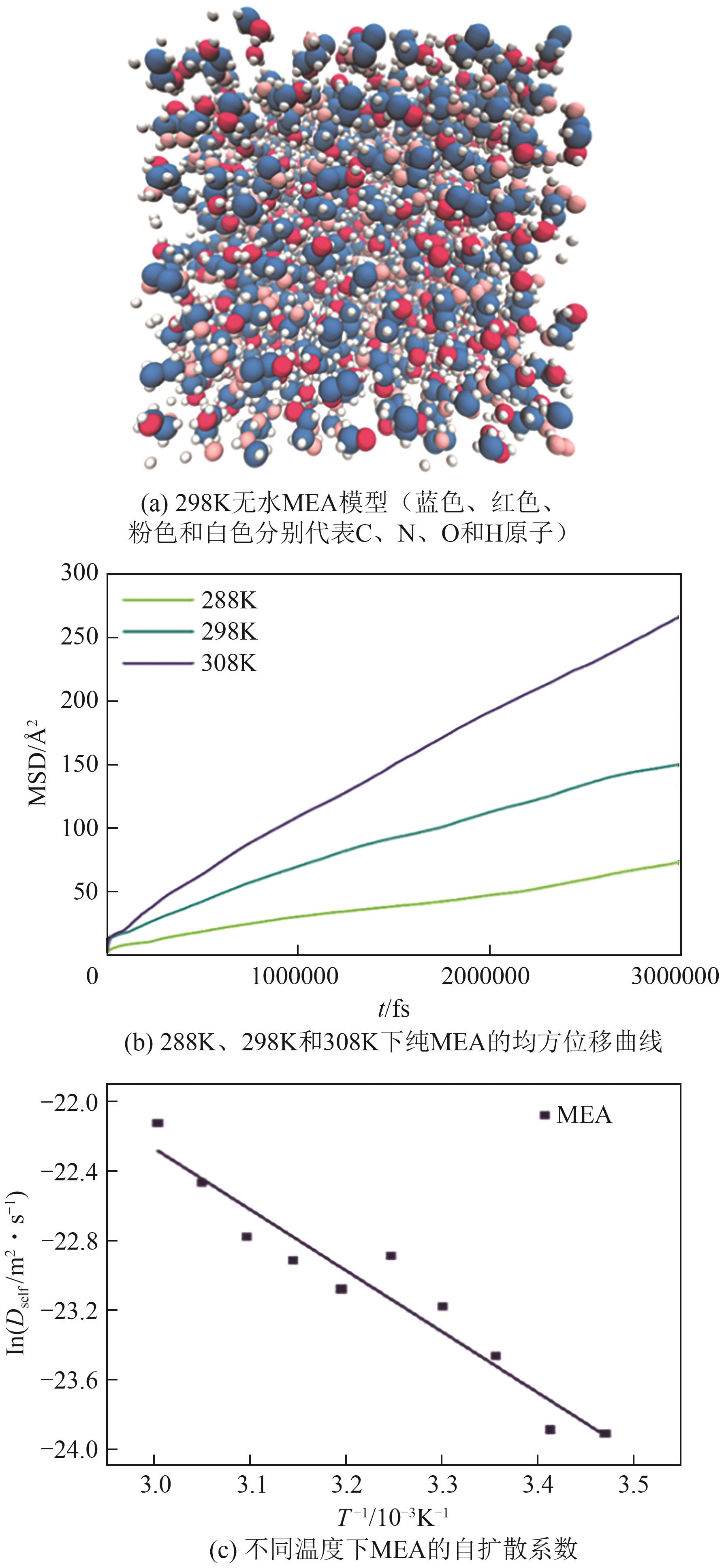
表1 无水MEA在288K、298K和308K下模拟计算的自扩散系数和实验值
| 项目 | 288K | 298K | 308K |
|---|---|---|---|
| 未修正力场结果/10-10m2·s-1 | 11.55 | 12.84 | 14.75 |
| 修正力场结果/10-10m2·s-1 | 0.41 | 0.64 | 1.15 |
| 阿伦尼乌斯方程拟合结果/10-10m2·s-1 | 0.41 | 0.61 | 0.89 |
| 实验结果/10-10m2·s-1 | 0.4 | 0.55 | 0.93 |
| 分子 | 原子 | ϵ/kcal·mol-1 | σ/Å |
|---|---|---|---|
| 反应物 | N | 0.325(修正值) | 4.070 |
| H(—CH2—、—NH2) | 0.013 | 1.098 | |
| C | 0.054 | 4.010 | |
| O | 1.200(修正值) | 3.535 | |
| H(—OH) | 0.020 | 2.955 | |
| H2O | H | 0.013 | 1.098 |
| O | 0.9864(修正值) | 3.608 | |
| CO2 | C | 0.064 | 4.010 |
| O | 0.060 | 3.535 | |
| 生成物 | N | 0.325(修正值) | 3.262 |
| H | 0.013 | 1.098 | |
| C | 0.120 | 3.908 | |
| O(—NHCOO—中第一个O) | 0.167 | 3.596 | |
| O(—NHCOO—中第二个O) | 1.200(修正值) | 3.535 |
表2 修正后的PCFF下反应物、H2O、CO2和生成物的力场参数
| 分子 | 原子 | ϵ/kcal·mol-1 | σ/Å |
|---|---|---|---|
| 反应物 | N | 0.325(修正值) | 4.070 |
| H(—CH2—、—NH2) | 0.013 | 1.098 | |
| C | 0.054 | 4.010 | |
| O | 1.200(修正值) | 3.535 | |
| H(—OH) | 0.020 | 2.955 | |
| H2O | H | 0.013 | 1.098 |
| O | 0.9864(修正值) | 3.608 | |
| CO2 | C | 0.064 | 4.010 |
| O | 0.060 | 3.535 | |
| 生成物 | N | 0.325(修正值) | 3.262 |
| H | 0.013 | 1.098 | |
| C | 0.120 | 3.908 | |
| O(—NHCOO—中第一个O) | 0.167 | 3.596 | |
| O(—NHCOO—中第二个O) | 1.200(修正值) | 3.535 |
| 结果 | 303K | 313K | 323K |
|---|---|---|---|
| 模拟值/10-10m2·s-1 | 4.76 | 5.81 | 7.12 |
| 实验值/10-10m2·s-1 | 4.49 | 5.76 | 7.27 |
表3 30% MDEA溶剂在303K、313K和323K下模拟计算的自扩散系数和实验值[27]
| 结果 | 303K | 313K | 323K |
|---|---|---|---|
| 模拟值/10-10m2·s-1 | 4.76 | 5.81 | 7.12 |
| 实验值/10-10m2·s-1 | 4.49 | 5.76 | 7.27 |
| 英文缩写 | 中文名称 | 分子式 | 结构式 | 分子量 |
|---|---|---|---|---|
| MEA | 单乙醇胺 | C2H7NO | NH2CH2CH2OH | 61.083 |
| DEA | 二乙醇胺 | C4H11NO2 | NH(CH2CH2OH)2 | 105.136 |
| AEEA | 羟乙基乙二胺 | C4H12N2O | NH2CH2CH2NHCH2CH2OH | 104.15 |
| MDEA | 甲基二乙醇胺 | C5H13NO2 | CH3N(CH2CH2OH)2 | 119.16 |
| EDEA | N-乙基二乙醇胺 | C6H15NO2 | CH3CH2N(CH2CH2OH)2 | 133.1888 |
| DEA-1,2-PD | 3-二乙氨基-1,2-丙二醇 | C7H17NO2 | (CH3CH2)2NCH2(CHOH)CH2OH | 147.215 |
表4 单胺分子信息
| 英文缩写 | 中文名称 | 分子式 | 结构式 | 分子量 |
|---|---|---|---|---|
| MEA | 单乙醇胺 | C2H7NO | NH2CH2CH2OH | 61.083 |
| DEA | 二乙醇胺 | C4H11NO2 | NH(CH2CH2OH)2 | 105.136 |
| AEEA | 羟乙基乙二胺 | C4H12N2O | NH2CH2CH2NHCH2CH2OH | 104.15 |
| MDEA | 甲基二乙醇胺 | C5H13NO2 | CH3N(CH2CH2OH)2 | 119.16 |
| EDEA | N-乙基二乙醇胺 | C6H15NO2 | CH3CH2N(CH2CH2OH)2 | 133.1888 |
| DEA-1,2-PD | 3-二乙氨基-1,2-丙二醇 | C7H17NO2 | (CH3CH2)2NCH2(CHOH)CH2OH | 147.215 |
| 不同单胺溶剂 | 自扩散系数公式 |
|---|---|
| MEA | |
| DEA | |
| MDEA | |
| AEEA | |
| EDEA | |
| DEA-1,2-PD |
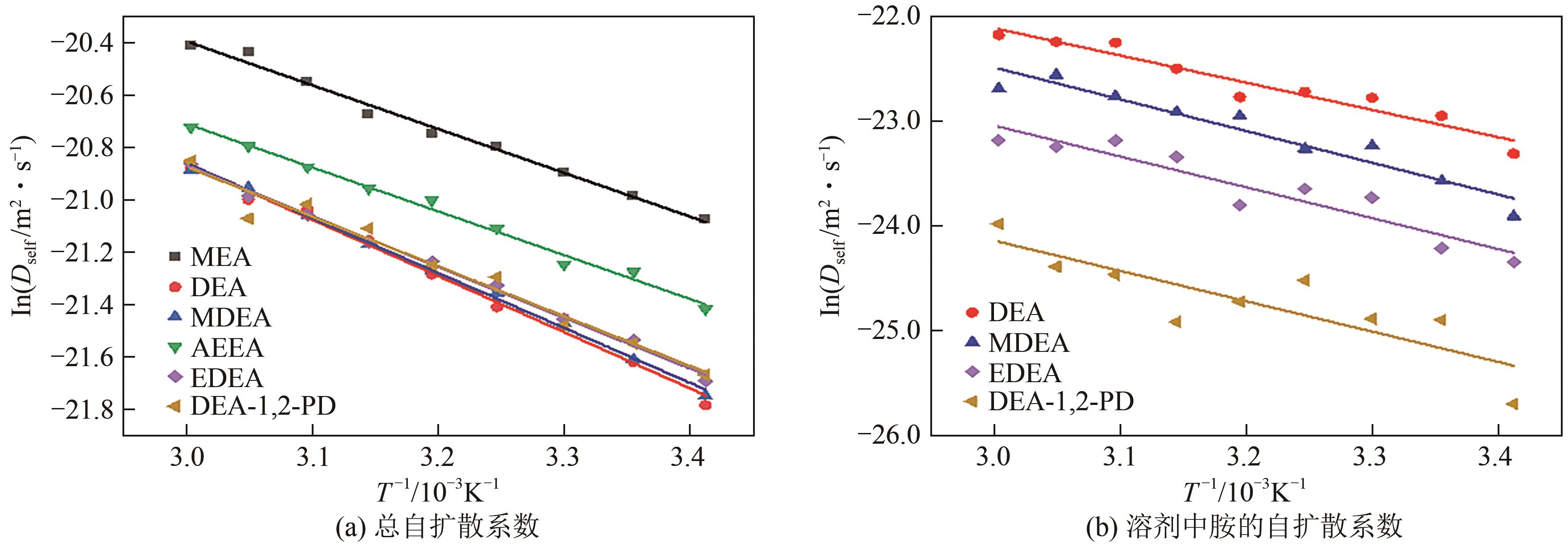
表5 30%不同单胺溶剂自扩散系数与温度之间的定量关系
| 不同单胺溶剂 | 自扩散系数公式 |
|---|---|
| MEA | |
| DEA | |
| MDEA | |
| AEEA | |
| EDEA | |
| DEA-1,2-PD |
| 反应进度/% | 分子个数 | |||||
|---|---|---|---|---|---|---|
| MEA | DEA | MDEA | MEAH+MEACOO- | DEAH+DEACOO- | MDEAH+HCO | |
| 0 | 253 | 147 | 130 | 0 | 0 | 0 |
| 10 | 227 | 133 | 117 | 13 | 7 | 13 |
| 20 | 203 | 117 | 104 | 25 | 15 | 26 |
| 30 | 177 | 103 | 91 | 38 | 22 | 39 |
| 40 | 151 | 89 | 78 | 51 | 29 | 52 |
| 50 | 127 | 73 | 65 | 63 | 37 | 65 |
| 60 | 101 | 59 | 52 | 76 | 44 | 78 |
| 70 | 77 | 45 | 39 | 88 | 51 | 91 |
| 80 | 51 | 29 | 26 | 101 | 59 | 104 |
| 90 | 25 | 15 | 13 | 114 | 66 | 117 |
| 100 | 0 | 0 | 0 | 126 | 74 | 130 |
表6 30%单胺溶剂不同反应进度的分子个数
| 反应进度/% | 分子个数 | |||||
|---|---|---|---|---|---|---|
| MEA | DEA | MDEA | MEAH+MEACOO- | DEAH+DEACOO- | MDEAH+HCO | |
| 0 | 253 | 147 | 130 | 0 | 0 | 0 |
| 10 | 227 | 133 | 117 | 13 | 7 | 13 |
| 20 | 203 | 117 | 104 | 25 | 15 | 26 |
| 30 | 177 | 103 | 91 | 38 | 22 | 39 |
| 40 | 151 | 89 | 78 | 51 | 29 | 52 |
| 50 | 127 | 73 | 65 | 63 | 37 | 65 |
| 60 | 101 | 59 | 52 | 76 | 44 | 78 |
| 70 | 77 | 45 | 39 | 88 | 51 | 91 |
| 80 | 51 | 29 | 26 | 101 | 59 | 104 |
| 90 | 25 | 15 | 13 | 114 | 66 | 117 |
| 100 | 0 | 0 | 0 | 126 | 74 | 130 |
| 质量分数 | 分子个数 | |||
|---|---|---|---|---|
| MEA | DEA | MDEA | DEA-1,2-PD | |
| 0.06 | 50 | 29 | 26 | 21 |
| 0.08 | 67 | 39 | 35 | 28 |
| 0.10 | 84 | 49 | 43 | 35 |
| 0.12 | 101 | 59 | 52 | 42 |
| 0.14 | 118 | 69 | 60 | 49 |
| 0.16 | 135 | 78 | 69 | 56 |
| 0.18 | 152 | 88 | 78 | 63 |
| 0.20 | 169 | 98 | 86 | 70 |
| 0.22 | 185 | 108 | 95 | 77 |
| 0.24 | 202 | 117 | 104 | 84 |
表7 混合胺溶剂中胺分子个数
| 质量分数 | 分子个数 | |||
|---|---|---|---|---|
| MEA | DEA | MDEA | DEA-1,2-PD | |
| 0.06 | 50 | 29 | 26 | 21 |
| 0.08 | 67 | 39 | 35 | 28 |
| 0.10 | 84 | 49 | 43 | 35 |
| 0.12 | 101 | 59 | 52 | 42 |
| 0.14 | 118 | 69 | 60 | 49 |
| 0.16 | 135 | 78 | 69 | 56 |
| 0.18 | 152 | 88 | 78 | 63 |
| 0.20 | 169 | 98 | 86 | 70 |
| 0.22 | 185 | 108 | 95 | 77 |
| 0.24 | 202 | 117 | 104 | 84 |
| [1] | D’ALESSANDRO Deanna M, SMIT Berend, LONG Jeffrey R. Carbon dioxide capture: Prospects for new materials[J]. Angewandte Chemie International Edition, 2010, 49(35): 6058-6082. |
| [2] | GAO Wanlin, LIANG Shuyu, WANG Rujie, et al. Industrial carbon dioxide capture and utilization: State of the art and future challenges[J]. Chemical Society Reviews, 2020, 49(23): 8584-8686. |
| [3] | ZHAO Xiaomeng, LI Xingyu, LU Houfang, et al. Predicting phase-splitting behaviors of an amine-organic solvent-water system for CO2 absorption: A new model developed by density functional theory and statistical and experimental methods[J]. Chemical Engineering Journal, 2021, 422: 130389. |
| [4] | Mai BUI, ADJIMAN Claire S, BARDOW André, et al. Carbon capture and storage (CCS): The way forward[J]. Energy & Environmental Science, 2018, 11(5): 1062-1176. |
| [5] | HEPBURN Cameron, ADLEN Ella, BEDDINGTON John, et al. The technological and economic prospects for CO2 utilization and removal[J]. Nature, 2019, 575(7781): 87-97. |
| [6] | MAKUL Natt. Towards computational CO2 capture and storage models[J]. The Global Environmental Engineers, 2021, 8: 55-69. |
| [7] | ROCHELLE Gary T. Amine scrubbing for CO2 capture[J]. Science, 2009, 325(5948): 1652-1654. |
| [8] | ZHANG Qi, BAHAMON Daniel, ALKHATIB Ismail I I, et al. Molecular insights into the CO2 absorption mechanism by superbase protic ionic liquids by a combined density functional theory and molecular dynamics approach[J]. Journal of Molecular Liquids, 2024, 394: 123683. |
| [9] | DUTCHER Bryce, FAN Maohong, RUSSELL Armistead G. Amine-based CO2 capture technology development from the beginning of 2013—A review[J]. ACS Applied Materials & Interfaces, 2015, 7(4): 2137-2148. |
| [10] | OSMAN Ahmed I, HEFNY Mahmoud, ABDEL MAKSOUD M I A, et al. Recent advances in carbon capture storage and utilisation technologies: A review[J]. Environmental Chemistry Letters, 2021, 19(2): 797-849. |
| [11] | SMIT Berend. Carbon capture and storage: Introductory lecture[J]. Faraday Discussions, 2016, 192: 9-25. |
| [12] | Alicia GARCÍA-ABUÍN, Diego GÓMEZ-DÍAZ, NAVAZA José M, et al. Carbon dioxide capture with tertiary amines. Absorption rate and reaction mechanism[J]. Journal of the Taiwan Institute of Chemical Engineers, 2017, 80: 356-362. |
| [13] | WANG M, LAWAL A, STEPHENSON P, et al. Post-combustion CO2 capture with chemical absorption: A state-of-the-art review[J]. Chemical Engineering Research and Design, 2011, 89(9): 1609-1624. |
| [14] | YU Y S, LU H F, WANG G X, et al. Characterizing the transport properties of multiamine solutions for CO2 capture by molecular dynamics simulation[J]. Journal of Chemical & Engineering Data, 2013, 58(6): 1429-1439. |
| [15] | CASTRO-ANAYA Luis E, OROZCO Gustavo A. Self-diffusion coefficients of amines, a molecular dynamics study[J]. Fluid Phase Equilibria, 2022, 553: 113301. |
| [16] | FENG Huajie, LIU Xin, GAO Wei, et al. Evolution of self-diffusion and local structure in some amines over a wide temperature range at high pressures: A molecular dynamics simulation study[J]. Physical Chemistry Chemical Physics, 2010, 12(45): 15007-15017. |
| [17] | SHARIF Maimoona, WU Xiaomei, YU Yunsong, et al. Estimation of diffusivity and intermolecular interaction strength of secondary and tertiary amine for CO2 absorption process by molecular dynamic simulation[J]. Molecular Simulation, 2022, 48(6): 484-494. |
| [18] | MELNIKOV Sergey M, STEIN Matthias. The effect of CO2 loading on alkanolamine absorbents in aqueous solutions[J]. Physical Chemistry Chemical Physics, 2019, 21(33): 18386-18392. |
| [19] | SNIJDER Erwin D, RIELE Marcel J M TE, VERSTEEG Geert F, et al. Diffusion coefficients of several aqueous alkanolamine solutions[J]. Journal of Chemical & Engineering Data, 1993, 38(3): 475-480. |
| [20] | BABA Hiromi, URANO Ryo, NAGAI Tetsuro, et al. Prediction of self-diffusion coefficients of chemically diverse pure liquids by all-atom molecular dynamics simulations[J]. Journal of Computational Chemistry, 2022, 43(28): 1892-1900. |
| [21] | HARUN N, MASIREN E E. Molecular dynamic simulation of amine-CO2 absorption process[J]. Indian Journal of Science and Technology, 2017, 10(2): 110382. |
| [22] | LIN Po-Hsun, Chih-Chiang KO, LI Menghui. Ternary diffusion coefficients of diethanolamine and N-methyldiethanolamine in aqueous solutions containing diethanolamine and N-methyldiethanolamine[J]. Fluid Phase Equilibria, 2009, 276(1): 69-74. |
| [23] | KIM Sunkyung, SHI Hu, LEE Jin Yong. CO2 absorption mechanism in amine solvents and enhancement of CO2 capture capability in blended amine solvent[J]. International Journal of Greenhouse Gas Control, 2016, 45: 181-188. |
| [24] | YIANNOURAKOU M, UNGERER P, LEBLANC B, et al. Molecular simulation of adsorption in microporous materials[J]. Oil & Gas Science and Technology-Revue d’IFP Energies Nouvelles, 2013, 68(6): 977-994. |
| [25] | MOOSAVI Fatemeh, ABDOLLAHI Farkhondeh, RAZMKHAH Mohammad. Carbon dioxide in monoethanolamine: Interaction and its effect on structural and dynamic properties by molecular dynamics simulation[J]. International Journal of Greenhouse Gas Control, 2015, 37: 158-169. |
| [26] | RODNIKOVA M N, SAMIGULLIN F M, SOLONINA I A, et al. Molecular mobility and the structure of polar liquids[J]. Journal of Structural Chemistry, 2014, 55(2): 256-262. |
| [27] | Chih-Chiang KO, CHANG Wen-Haur, LI Menghui. Ternary diffusion coefficients of monoethanolamine and N-methyldiethanolamine in aqueous solutions[J]. Journal of the Chinese Institute of Chemical Engineers, 2008, 39(6): 645-651. |
| [28] | ORLOV Alexey Alam, VALTZ Alain, COQUELET Christophe, et al. Computational screening methodology identifies effective solvents for CO2 capture[J]. Communications Chemistry, 2022, 5(1): 37. |
| [29] | ZHANG Shihan, SHEN Yao, SHAO Peijing, et al. Kinetics, thermodynamics, and mechanism of a novel biphasic solvent for CO2 capture from flue gas[J]. Environmental Science & Technology, 2018, 52(6): 3660-3668. |
| [30] | CHOWDHURY Firoz A, YAMADA Hidetaka, HIGASHII Takayuki, et al. CO2 capture by tertiary amine absorbents: A performance comparison study[J]. Industrial & Engineering Chemistry Research, 2013, 52(24): 8323-8331. |
| [1] | 刘燕燕, 李飞泉, 刘栋, 王俊涛, 罗雪. 纳观尺度下再生沥青-集料界面性质的分子模拟[J]. 化工进展, 2025, 44(8): 4302-4310. |
| [2] | 李艳平, 杨涛, 王洪勋, 张城, 温国胜, 韩治成, 蓝公家, 严大洲. 三氯氢硅在氢气氛中的热分解及还原体系的反应分子动力学模拟[J]. 化工进展, 2025, 44(8): 4322-4330. |
| [3] | 刘力涵, 王琪君, 王轩, 彭阳峰, 徐小飞. 丁苯橡胶应力软化的全原子分子动力学模拟[J]. 化工进展, 2025, 44(8): 4331-4340. |
| [4] | 齐妍, 常昊, 张磊. 基于分子动力学模拟的结构性产品配方设计方法[J]. 化工进展, 2025, 44(8): 4341-4351. |
| [5] | 秘一芳, 王保国, 王文强, 孙国金, 曹志海. 氮自掺杂蓝藻生物质基活性炭的制备及其CO2吸附性能[J]. 化工进展, 2025, 44(7): 4223-4232. |
| [6] | 段五华, 孙涛祥, 郑强. 工业规模核用离心萃取器的水力学和传质性能[J]. 化工进展, 2025, 44(7): 3709-3717. |
| [7] | 孙金磊, 廖丹葵, 陈小鹏, 童张法. 超重力-微界面法制备类球形纳米碳酸钙[J]. 化工进展, 2025, 44(7): 3757-3769. |
| [8] | 郑慧哲, 王浩泽, 蒋杰, 赵玲, 奚桢浩. 反应与传质耦合的PCTG共聚酯圆盘反应器建模与模拟[J]. 化工进展, 2025, 44(6): 3372-3381. |
| [9] | 马梓轩, 施瑞晨, 刘明杰, 杨莹杰, 宋子瑜, 梅晓鹏, 高晓峰, 洪龙城, 姚思宇, 张治国, 任其龙. 环烷烃催化制氢反应器的设计与性能优化: 前沿进展与挑战[J]. 化工进展, 2025, 44(5): 2919-2937. |
| [10] | 陈奥辉, 宋艳芳, 陈为, 魏伟. 多孔自支撑电极电催化还原二氧化碳[J]. 化工进展, 2025, 44(5): 2806-2810. |
| [11] | 戴月明, 周梅芳, 沈建华, 姜海波, 李春忠. TiO2纳米颗粒烧结机制分子动力学模拟[J]. 化工进展, 2025, 44(4): 2202-2214. |
| [12] | 窦玉, 王文选, 范春雷, 马吉亮, 梁财, 陈晓平. 脱硫石膏矿化CO2制备球霰石碳酸钙[J]. 化工进展, 2025, 44(4): 2328-2337. |
| [13] | 丁红兵, 柴旭天, 王世伟, 宋鑫宇, 孙宏军. 单液滴与多液滴撞击流动液膜的实验探究[J]. 化工进展, 2025, 44(4): 1888-1897. |
| [14] | 王佳琪, 刘佳兴, 魏皓琦, 周昕霖, 程传晓, 葛坤. 鼠李糖脂强化CO2水合物生成[J]. 化工进展, 2025, 44(4): 1998-2007. |
| [15] | 佘永璐, 徐强, 罗欣怡, 聂腾飞, 郭烈锦. 反应温度对光电极表面气泡动力学及传质特性的影响[J]. 化工进展, 2025, 44(3): 1243-1252. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||