化工进展 ›› 2025, Vol. 44 ›› Issue (6): 3224-3237.DOI: 10.16085/j.issn.1000-6613.2024-2128
• 专栏:化工过程强化 • 上一篇
聚乙烯常规及微波催化热解产物分布特征的研究进展
张莹1,2( ), 郑雪梅2,3, 马爱元2,3(
), 郑雪梅2,3, 马爱元2,3( ), 田时泓4(
), 田时泓4( )
)
- 1.大连大学环境与化学工程学院,辽宁 大连 116622
2.六盘水师范学院化学与材料工程学院,贵州 六盘水 553004
3.贵州省煤碳洁净利用重点实验室,贵州 六盘水 553004
4.中国科学院重庆绿色智能技术研究院 大气环境研究中心,重庆 400714
-
收稿日期:2024-12-31修回日期:2025-03-25出版日期:2025-06-25发布日期:2025-07-08 -
通讯作者:马爱元,田时泓 -
作者简介:张莹(2000—),女,硕士研究生,研究方向为塑料催化热解。E-mail:zy2000202109@163.com。 -
基金资助:贵州省煤炭洁净利用重点实验室(黔科合平台人才[2020]2001);六盘水市科技局科技发展项目(52020-2023-0-2-6);六盘水市科技局科技发展项目(52020-2024-0-2-8);重庆市北碚区科技人才与自主创新专项(2024-19)
Research progress based on conventional and microwave pyrolysis behavior of polyethylene
ZHANG Ying1,2( ), ZHENG Xuemei2,3, MA Aiyuan2,3(
), ZHENG Xuemei2,3, MA Aiyuan2,3( ), TIAN Shihong4(
), TIAN Shihong4( )
)
- 1.College of Environmental and Chemical Engineering, Dalian University, Dalian 116622, Liaoning, China
2.School of Chemistry and Materials Engineering, Liupanshui Normol University, Liupanshui 553004, Guizhou, China
3.Guizhou Provincial Key Laboratory of Coal Clean Utilization, Liupanshui 553004, Guizhou, China
4.Research Center for Atmospheric Environment, Chongqing Institute of Green and Intelligent Technology, Chinese Academy of Sciences, Chongqing 400714, China
-
Received:2024-12-31Revised:2025-03-25Online:2025-06-25Published:2025-07-08 -
Contact:MA Aiyuan, TIAN Shihong
摘要:
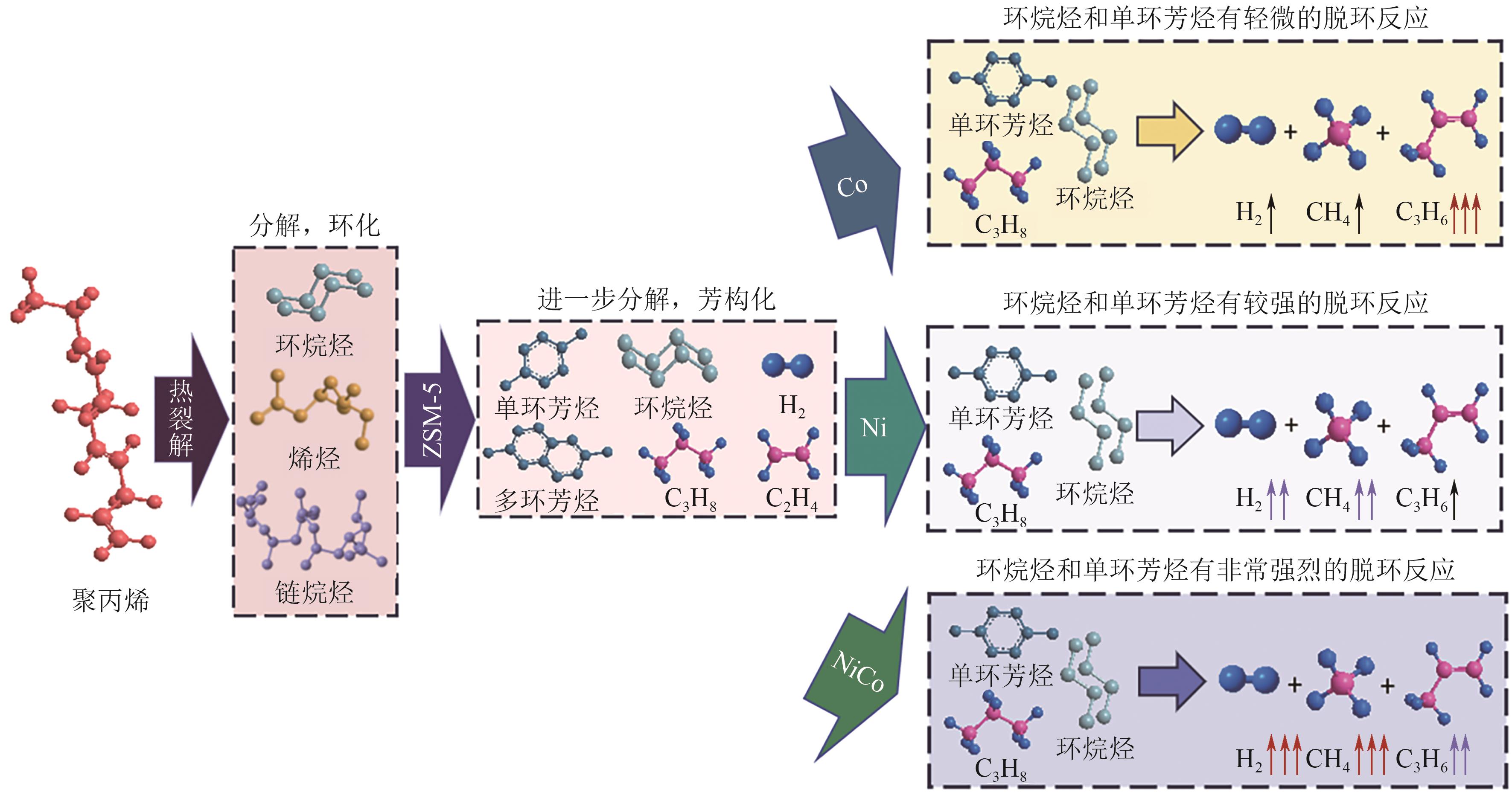
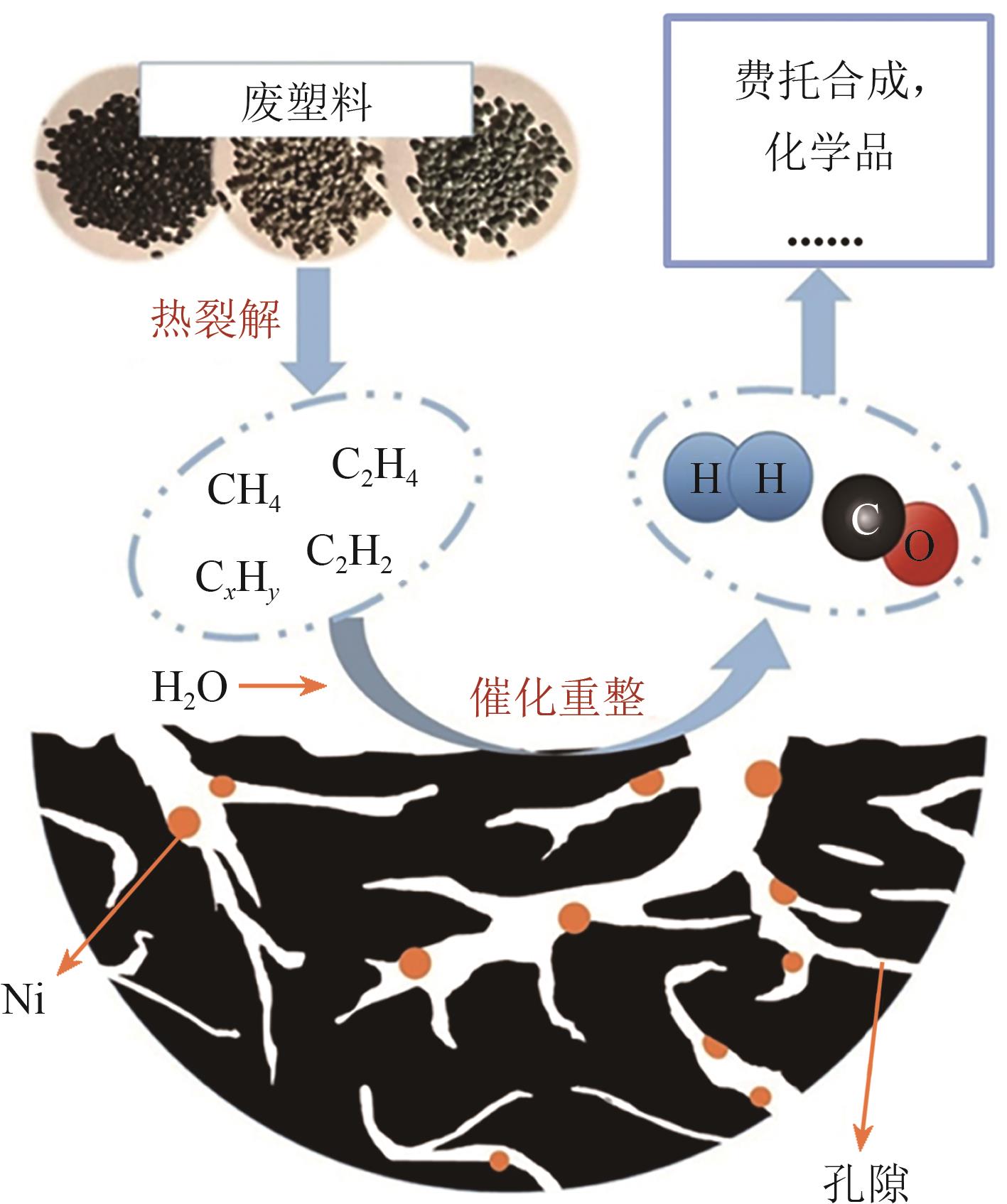
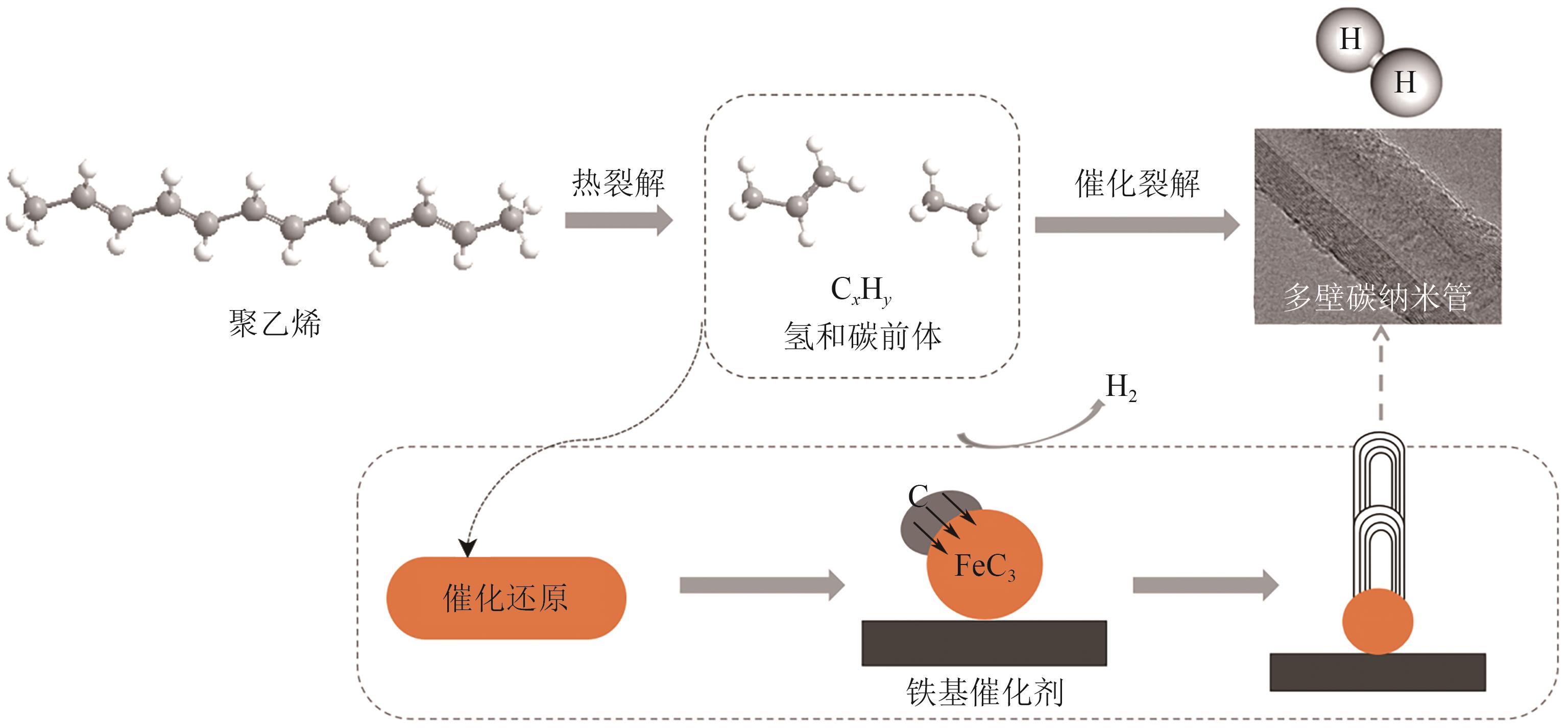
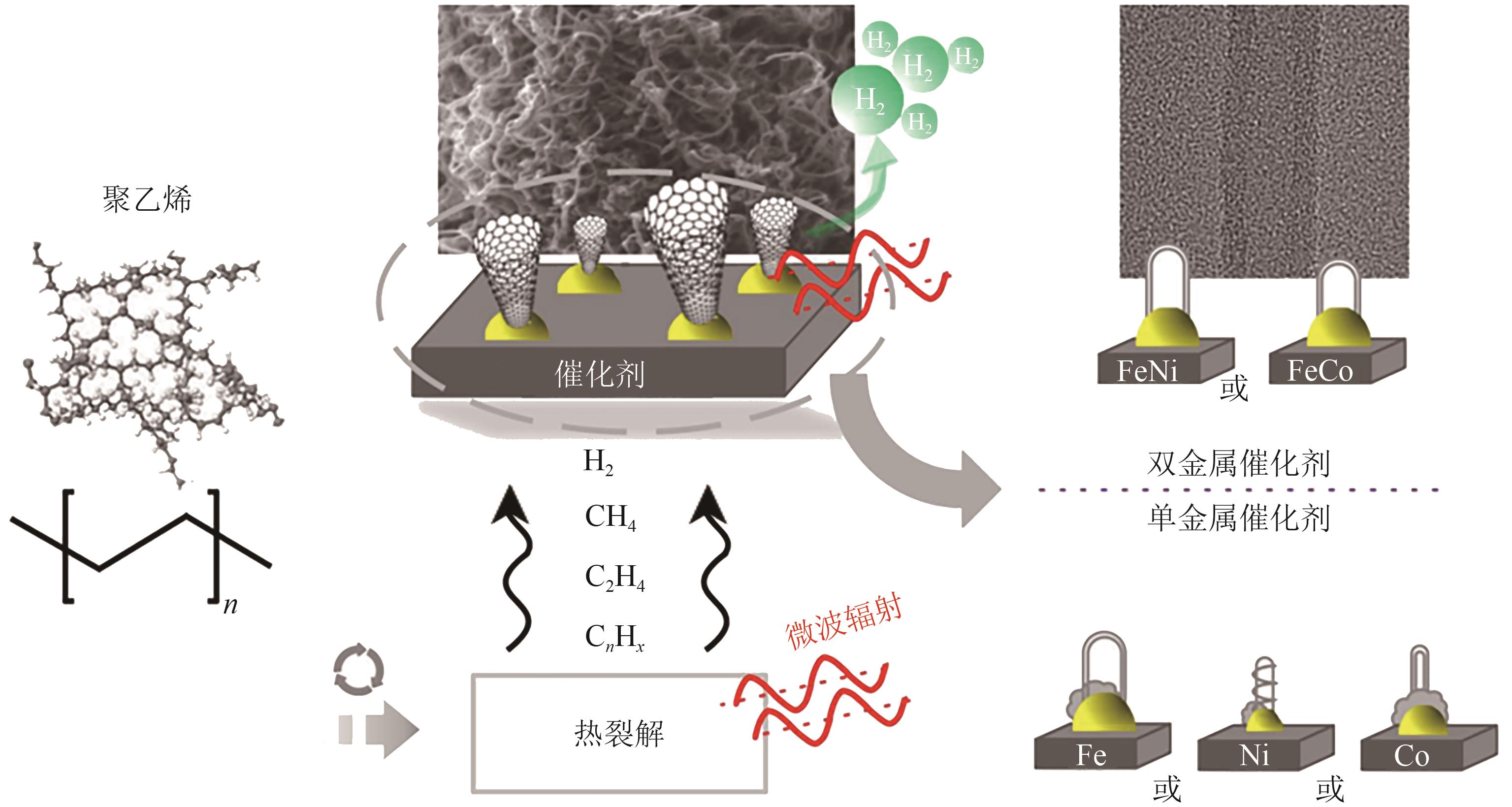
聚烯烃类塑料制品因碳氢链结构化学稳定性好、难自然降解,其处理方式备受关注。催化热解技术被认为是废塑料回收利用的绿色方法。区别于常规热解技术的低效能和低产率,微波催化热解因加热速率快、受热均匀、能量转换率高,能显著提升微波转化效率,提高高价值化学品的产率与品质。本文从常规和微波催化热解的工艺应用出发,系统阐述过渡金属(Fe、Co、Ni等)负载型催化剂对常规催化热解聚乙烯产生的气液固三相产物的影响,以及铁基复合金属催化剂和分子筛类催化剂对微波催化热解聚乙烯产生的氢气、碳纳米管和芳烃油的选择性差异,梳理了常规和微波催化热解废塑料的产物分布规律,对比了铁基复合催化剂对常规和微波催化热解产物的选择性,探讨了常规及微波催化热解废塑料的反应机理和发展趋势。针对微波催化热解废塑料催化剂的性能问题,提出开发具有良好吸波性能和催化能力的催化剂来提高微波利用率和催化活性,进一步改善微波催化废塑料高附加值产品的质量,最后对微波热解废塑料产物的可控性和纯度进行了展望。
中图分类号:
引用本文
张莹, 郑雪梅, 马爱元, 田时泓. 聚乙烯常规及微波催化热解产物分布特征的研究进展[J]. 化工进展, 2025, 44(6): 3224-3237.
ZHANG Ying, ZHENG Xuemei, MA Aiyuan, TIAN Shihong. Research progress based on conventional and microwave pyrolysis behavior of polyethylene[J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3224-3237.
| 催化剂 | 反应温度 | 塑料种类 | 氢气产率 | 液体产率 | 固体产率 | 文献 |
|---|---|---|---|---|---|---|
| Fe/Al2O3 | 800℃ | LDPE | 50.60% | — | — | [ |
| Ni/Al2O3 | 800℃ | LDPE | 36.60% | — | — | [ |
| Co/Al2O3 | 800℃ | LDPE | 31.30% | — | — | [ |
| Cu/Al2O3 | 800℃ | LDPE | 26.00% | — | — | [ |
| Fe/SiO2(L) | 800℃ | PP | 25.60mmol/g | — | — | [ |
| Ni/SiO2(L) | 800℃ | PP | 22.60mmol/g | — | — | [ |
| Ni-Fe/Al2O3 | 800℃ | mix-plastics | 31.80mmol/g | — | — | [ |
| Fe-Ni/MCM-41 | 800℃ | mix-plastics | 38.10mmol/g | — | — | [ |
| Ni-Co/ZSM-5 | 900℃ | PP | 28.70mmol/g | — | — | [ |
| Ni/ZSM-5 | 800℃ | HDPE | 64.50% | — | — | [ |
| Ga-Mo/HZSM-5 | 600℃ | HDPE | — | 82% aromatics | — | [ |
| Ni-WO3/Al2O3-Hβ | — | PE | — | 86.20% liquid | — | [ |
| Ni/HZSM-5 | 400℃ | HDPE | — | 28.90% aromatics | — | [ |
| Ni/Al2O3 | 800℃ | HDPE | 51.51mmol/g | 64.20% liquid | 1.98% carbon | [ |
| 1% Ni/Y | 600℃ | HDPE | — | 85% aromatics | — | [ |
| 1% Ga/Y | 600℃ | HDPE | — | 93% aromatics | — | [ |
| 1% Fe/Y | 600℃ | HDPE | — | 94% aromatics | — | [ |
| 1% Co/Y | 600℃ | HDPE | — | 80% aromatics | — | [ |
| Ga/HZSM-5 | — | LLDPE | — | 66.54% MAHs | [ | |
| CaO-Ga/HZSM-5 | — | LLDPE | — | 72.85% MAHs | — | [ |
| P-Ga/HZSM-5 | 550℃ | LDPE | — | 90.70% MAHs | — | [ |
| Ga-Na/HZSM-5 | 650℃ | PP | — | 67.20% aromatics | — | [ |
| Ni/AC | 700℃ | mix-plastics | 4.24% | — | 34.50% carbon | [ |
| Fe/AC | 700℃ | mix-plastics | 1.97% | — | 12.40% carbon | [ |
| Fe-Ni/Al2O3(Sg) | 800℃ | PP | 25.14mmol/g | 20.00% liquid | 36.00% carbon | [ |
| Fe/MgO | 900℃ | PP | — | — | 33.30% carbon | [ |
| Fe/Al2O3 | 800℃ | HDPE | 56.00% | 20.00% liquid | 36.90% carbon | [ |
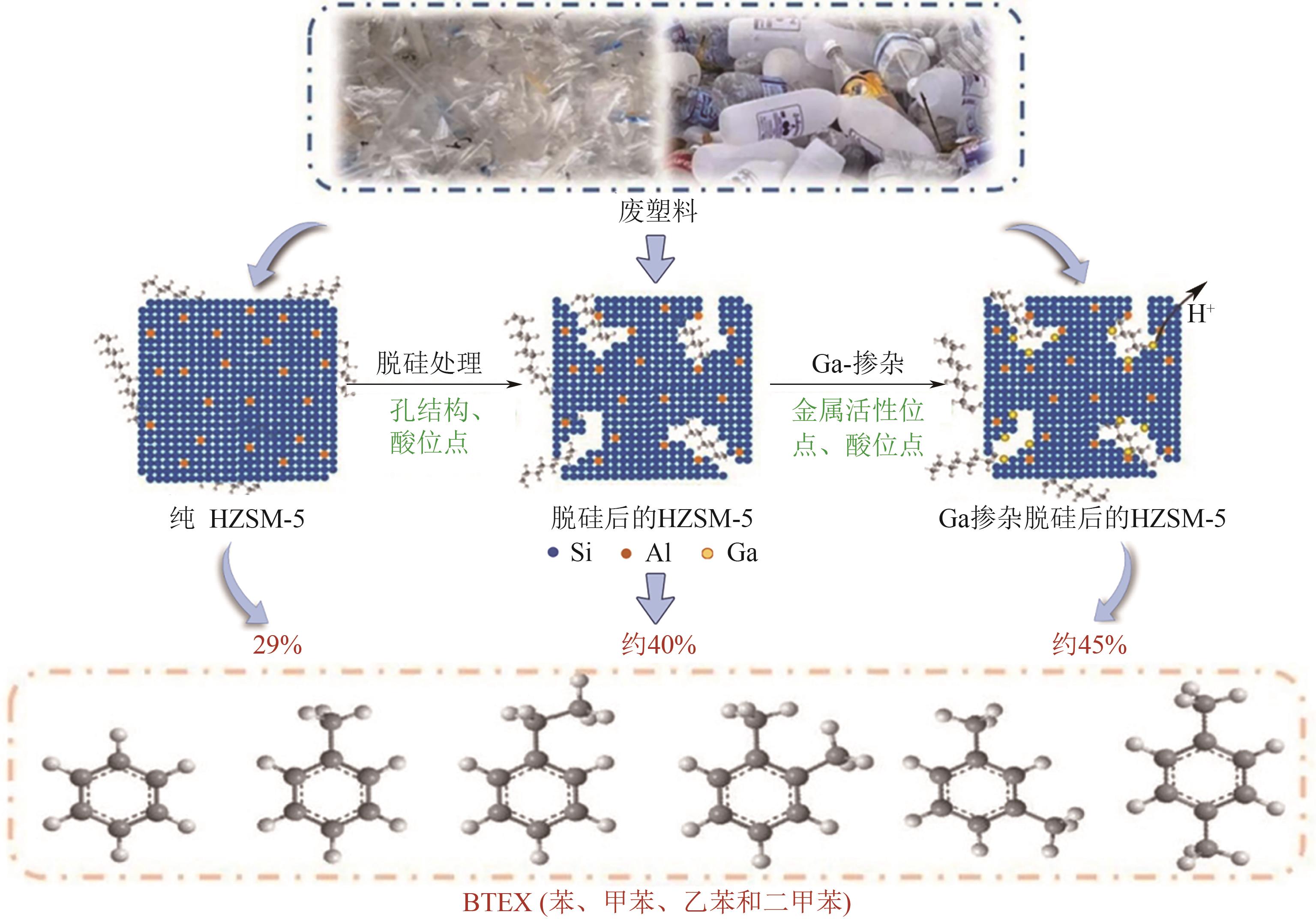
| Ni/Al2O3 | 800℃ | PE | 17.31mmol/g | 28.95% liquid | 25.64% carbon | [ |
| Ni/ZrO2 | 800℃ | PE | 22.01mmol/g | 40.22% liquid | 16.03% carbon | [ |
| Ni/TiO2 | 800℃ | PE | 20.74mmol/g | 30.72% liquid | 26.53% carbon | [ |
| Ni/Nb2O3 | 800℃ | PE | 12.04mmol/g | 34.84% liquid | 8.51% carbon | [ |
| Ni/Al2O3 | 800℃ | PE | 36.77mmol/g | 23.81% liquid | 36.54% carbon | [ |
| Fe-Ni-Mg | 800℃ | LDPE | 35.27mmol/g | 26.71% liquid | 32.30% carbon | [ |
| Ni-Co/Al2O3 | 950℃ | PE | — | — | 25.50% carbon | [ |
| Fe-Ni | 800℃ | mix-plastics | 73.93% | 8.80% liquid | 50.90% carbon | [ |
| Fe-Ni | 700℃ | PE | — | — | 20.00% carbon | [ |
| Fe/Al2O3 | 800℃ | PP | 58.70% | 18.10% liquid | 30.20% carbon | [ |
表1 常规催化热解聚乙烯的产物产率
| 催化剂 | 反应温度 | 塑料种类 | 氢气产率 | 液体产率 | 固体产率 | 文献 |
|---|---|---|---|---|---|---|
| Fe/Al2O3 | 800℃ | LDPE | 50.60% | — | — | [ |
| Ni/Al2O3 | 800℃ | LDPE | 36.60% | — | — | [ |
| Co/Al2O3 | 800℃ | LDPE | 31.30% | — | — | [ |
| Cu/Al2O3 | 800℃ | LDPE | 26.00% | — | — | [ |
| Fe/SiO2(L) | 800℃ | PP | 25.60mmol/g | — | — | [ |
| Ni/SiO2(L) | 800℃ | PP | 22.60mmol/g | — | — | [ |
| Ni-Fe/Al2O3 | 800℃ | mix-plastics | 31.80mmol/g | — | — | [ |
| Fe-Ni/MCM-41 | 800℃ | mix-plastics | 38.10mmol/g | — | — | [ |
| Ni-Co/ZSM-5 | 900℃ | PP | 28.70mmol/g | — | — | [ |
| Ni/ZSM-5 | 800℃ | HDPE | 64.50% | — | — | [ |
| Ga-Mo/HZSM-5 | 600℃ | HDPE | — | 82% aromatics | — | [ |
| Ni-WO3/Al2O3-Hβ | — | PE | — | 86.20% liquid | — | [ |
| Ni/HZSM-5 | 400℃ | HDPE | — | 28.90% aromatics | — | [ |
| Ni/Al2O3 | 800℃ | HDPE | 51.51mmol/g | 64.20% liquid | 1.98% carbon | [ |
| 1% Ni/Y | 600℃ | HDPE | — | 85% aromatics | — | [ |
| 1% Ga/Y | 600℃ | HDPE | — | 93% aromatics | — | [ |
| 1% Fe/Y | 600℃ | HDPE | — | 94% aromatics | — | [ |
| 1% Co/Y | 600℃ | HDPE | — | 80% aromatics | — | [ |
| Ga/HZSM-5 | — | LLDPE | — | 66.54% MAHs | [ | |
| CaO-Ga/HZSM-5 | — | LLDPE | — | 72.85% MAHs | — | [ |
| P-Ga/HZSM-5 | 550℃ | LDPE | — | 90.70% MAHs | — | [ |
| Ga-Na/HZSM-5 | 650℃ | PP | — | 67.20% aromatics | — | [ |
| Ni/AC | 700℃ | mix-plastics | 4.24% | — | 34.50% carbon | [ |
| Fe/AC | 700℃ | mix-plastics | 1.97% | — | 12.40% carbon | [ |
| Fe-Ni/Al2O3(Sg) | 800℃ | PP | 25.14mmol/g | 20.00% liquid | 36.00% carbon | [ |
| Fe/MgO | 900℃ | PP | — | — | 33.30% carbon | [ |
| Fe/Al2O3 | 800℃ | HDPE | 56.00% | 20.00% liquid | 36.90% carbon | [ |
| Ni/Al2O3 | 800℃ | PE | 17.31mmol/g | 28.95% liquid | 25.64% carbon | [ |
| Ni/ZrO2 | 800℃ | PE | 22.01mmol/g | 40.22% liquid | 16.03% carbon | [ |
| Ni/TiO2 | 800℃ | PE | 20.74mmol/g | 30.72% liquid | 26.53% carbon | [ |
| Ni/Nb2O3 | 800℃ | PE | 12.04mmol/g | 34.84% liquid | 8.51% carbon | [ |
| Ni/Al2O3 | 800℃ | PE | 36.77mmol/g | 23.81% liquid | 36.54% carbon | [ |
| Fe-Ni-Mg | 800℃ | LDPE | 35.27mmol/g | 26.71% liquid | 32.30% carbon | [ |
| Ni-Co/Al2O3 | 950℃ | PE | — | — | 25.50% carbon | [ |
| Fe-Ni | 800℃ | mix-plastics | 73.93% | 8.80% liquid | 50.90% carbon | [ |
| Fe-Ni | 700℃ | PE | — | — | 20.00% carbon | [ |
| Fe/Al2O3 | 800℃ | PP | 58.70% | 18.10% liquid | 30.20% carbon | [ |
| 催化剂 | 反应温度 | 塑料种类 | 氢气产率 | 液体产率 | 固体产率 | 文献 |
|---|---|---|---|---|---|---|
| Fe/Ni-CeO2@CNTs | 800℃ | HDPE | 91.50% | — | 20.00% carbon | [ |
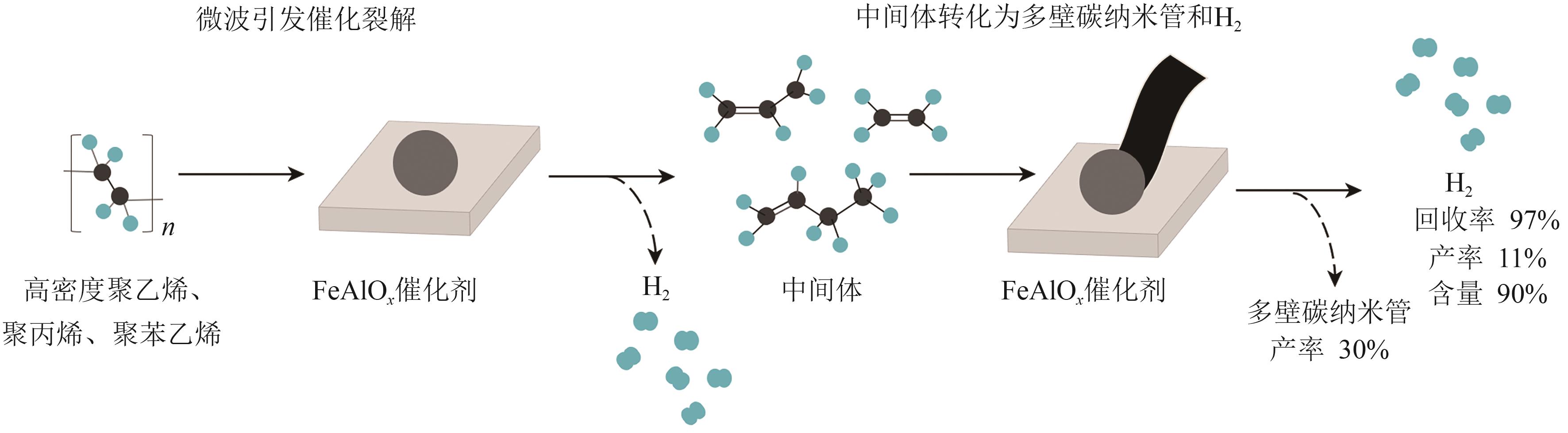
| FeAlO x | — | PE | 48.10mmol/g | — | — | [ |
| FeAlO x | 550℃ | HDPE | 55.60mmol/g | — | — | [ |
| 7%FeAlO x | 500℃ | HDPE | 86.30% | — | — | [ |
| 22%FeAlO x | 500℃ | HDPE | 93.70% | — | — | [ |
| 30%Fe/FeAlO x | 330℃ | HDPE | 47.03mmol/g | — | — | [ |
| Fe-Co-Al | 800℃ | LDPE | 61.39mmol/g | — | — | [ |
| Ni-Fe-Al | 700℃ | LDPE | 60.50mmol/g | — | — | [ |
| ZSM-5 | 450℃ | LDPE | — | 32.58% liquid | — | [ |
| ZSM-5 | 560℃ | LDPE | — | 47.40% liquid | — | [ |
| MCM-41/HY | 450℃ | LDPE | — | 63.75% liquid | — | [ |
| HZSM-5/MgO | 500℃ | LDPE | — | 90% aromatics | — | [ |
| NiO/HY | 450℃ | LDPE | — | 56.53% liquid | — | [ |
| Ni-Fe/Al2O3 | 800℃ | PP | 65.10% | 27.70% liquid | 33.50% carbon | [ |
| Ni-La | 800℃ | LDPE | — | — | 82.20% carbon | [ |
| Ni-Fe-Al | — | PP、LDPE、HDPE | 83.10% | — | — | [ |
| Fe-Ni | — | PE | 415.00mmol/g | — | — | [ |
| Ni1Fe3O x | 700℃ | LDPE | 60.20mmol/g | 1.20% liquid | 36.50% carbon | [ |
| Ni1Co3O x | 700℃ | LDPE | 63.20mmol/g | 0.80% liquid | 41.40% carbon | [ |
| Ni3Co2O x | 700℃ | LDPE | 63.50mmol/g | 0.80% liquid | 41.50% carbon | [ |
| Fe-Ni/SiC | 800℃ | LDPE | 73.89% | — | — | [ |
| Al2O3-NiFe2O4 | 400℃ | HDPE | 90.60% | 7.50% liquid | 78.00% carbon | [ |
| Al2O3-ZnFe2O4 | 400℃ | HDPE | 80.00% | 7.50% liquid | 71.50% carbon | [ |
| Al2O3-MgFe2O4 | 400℃ | HDPE | 75.50% | 6.25% liquid | 74.00% carbon | [ |
表2 微波催化热解聚乙烯类塑料的产物产率
| 催化剂 | 反应温度 | 塑料种类 | 氢气产率 | 液体产率 | 固体产率 | 文献 |
|---|---|---|---|---|---|---|
| Fe/Ni-CeO2@CNTs | 800℃ | HDPE | 91.50% | — | 20.00% carbon | [ |
| FeAlO x | — | PE | 48.10mmol/g | — | — | [ |
| FeAlO x | 550℃ | HDPE | 55.60mmol/g | — | — | [ |
| 7%FeAlO x | 500℃ | HDPE | 86.30% | — | — | [ |
| 22%FeAlO x | 500℃ | HDPE | 93.70% | — | — | [ |
| 30%Fe/FeAlO x | 330℃ | HDPE | 47.03mmol/g | — | — | [ |
| Fe-Co-Al | 800℃ | LDPE | 61.39mmol/g | — | — | [ |
| Ni-Fe-Al | 700℃ | LDPE | 60.50mmol/g | — | — | [ |
| ZSM-5 | 450℃ | LDPE | — | 32.58% liquid | — | [ |
| ZSM-5 | 560℃ | LDPE | — | 47.40% liquid | — | [ |
| MCM-41/HY | 450℃ | LDPE | — | 63.75% liquid | — | [ |
| HZSM-5/MgO | 500℃ | LDPE | — | 90% aromatics | — | [ |
| NiO/HY | 450℃ | LDPE | — | 56.53% liquid | — | [ |
| Ni-Fe/Al2O3 | 800℃ | PP | 65.10% | 27.70% liquid | 33.50% carbon | [ |
| Ni-La | 800℃ | LDPE | — | — | 82.20% carbon | [ |
| Ni-Fe-Al | — | PP、LDPE、HDPE | 83.10% | — | — | [ |
| Fe-Ni | — | PE | 415.00mmol/g | — | — | [ |
| Ni1Fe3O x | 700℃ | LDPE | 60.20mmol/g | 1.20% liquid | 36.50% carbon | [ |
| Ni1Co3O x | 700℃ | LDPE | 63.20mmol/g | 0.80% liquid | 41.40% carbon | [ |
| Ni3Co2O x | 700℃ | LDPE | 63.50mmol/g | 0.80% liquid | 41.50% carbon | [ |
| Fe-Ni/SiC | 800℃ | LDPE | 73.89% | — | — | [ |
| Al2O3-NiFe2O4 | 400℃ | HDPE | 90.60% | 7.50% liquid | 78.00% carbon | [ |
| Al2O3-ZnFe2O4 | 400℃ | HDPE | 80.00% | 7.50% liquid | 71.50% carbon | [ |
| Al2O3-MgFe2O4 | 400℃ | HDPE | 75.50% | 6.25% liquid | 74.00% carbon | [ |
| [1] | WECKHUYSEN Bert M. Creating value from plastic waste[J]. Science, 2020, 370(6515): 400-401. |
| [2] | SAMUEL POTTINGER A, GEYER Roland, BIYANI Nivedita, et al. Pathways to reduce global plastic waste mismanagement and greenhouse gas emissions by 2050[J]. Science, 2024, 386(6726): 1168-1173. |
| [3] | RODRIGUES M O, ABRANTES N, GONÇALVES F J M, et al. Impacts of plastic products used in daily life on the environment and human health: What is known?[J]. Environmental Toxicology and Pharmacology, 2019, 72: 103239. |
| [4] | KARTIK S, BALSORA Hemant Kumar, SHARMA Manisha, et al. Valorization of plastic wastes for production of fuels and value-added chemicals through pyrolysis—A review[J]. Thermal Science and Engineering Progress, 2022, 32: 101316. |
| [5] | WILLIAMS Paul T. Hydrogen and carbon nanotubes from pyrolysis-catalysis of waste plastics: A review[J]. Waste and Biomass Valorization, 2021, 12(1): 1-28. |
| [6] | LIU Zewei, XIE Ming, ZHOU Tao, et al. A review on liquid fuel produced from microwave-assisted pyrolysis of plastic waste[J]. Process Safety and Environmental Protection, 2024, 187: 833-844. |
| [7] | MUKHERJEE Ankita, Biswajit RUJ, SADHUKHAN Anup Kumar, et al. Applications of H2-Enriched syngas and slag products from plastic wastes via novel plasma dual-stage-arc pyrolysis[J]. Journal of Environmental Management, 2024, 370: 123025. |
| [8] | ZHANG Yeshui, WU Chunfei, NAHIL Mohamad A, et al. Pyrolysis-catalytic reforming/gasification of waste tires for production of carbon nanotubes and hydrogen[J]. Energy & Fuels, 2015, 29(5): 3328-3334. |
| [9] | ACOMB Jonathan C, WU Chunfei, WILLIAMS Paul T. The use of different metal catalysts for the simultaneous production of carbon nanotubes and hydrogen from pyrolysis of plastic feedstocks[J]. Applied Catalysis B: Environmental, 2016, 180: 497-510. |
| [10] | LIU Xiaotong, ZHANG Yeshui, NAHIL Mohamad A, et al. Development of Ni- and Fe-based catalysts with different metal particle sizes for the production of carbon nanotubes and hydrogen from thermo-chemical conversion of waste plastics[J]. Journal of Analytical and Applied Pyrolysis, 2017, 125: 32-39. |
| [11] | YAO Dingding, ZHANG Yeshui, WILLIAMS Paul T, et al. Co-production of hydrogen and carbon nanotubes from real-world waste plastics: Influence of catalyst composition and operational parameters[J]. Applied Catalysis B: Environmental, 2018, 221: 584-597. |
| [12] | YAO Dingding, YANG Haiping, HU Qiang, et al. Carbon nanotubes from post-consumer waste plastics: Investigations into catalyst metal and support material characteristics[J]. Applied Catalysis B: Environmental, 2021, 280: 119413. |
| [13] | FU Wenming, CHENG Yoke Wang, XU Dequan, et al. Reaction synergy of bimetallic catalysts on ZSM-5 support in tailoring plastic pyrolysis for hydrogen and value-added product production[J]. Applied Energy, 2024, 372: 123853. |
| [14] | ZHAI Haoshan, WANG Xuetao, LIU Mengjie, et al. Effect of reaction conditions on pyrolysis performance of waste plastics loaded with Ni/ZSM-5 catalyst[J]. Journal of the Energy Institute, 2024, 115: 101681. |
| [15] | WANG Tao, LIU Qian, ZHONG Wenqi, et al. Production of light aromatics by co-pyrolysis of lignite and plastics: Effect of metal loaded HZSM-5[J]. Journal of Analytical and Applied Pyrolysis, 2023, 170: 105927. |
| [16] | SUN Jie, WU Changdong, ZHOU Yuchen, et al. Noble metal-free tandem catalysis enables efficient upcycling plastic waste into liquid fuel components[J]. Chemical Engineering Journal, 2024, 500: 156988. |
| [17] | PAN Zeyou, XUE Xiangfei, ZHANG Changsen, et al. Production of aromatic hydrocarbons by hydro-liquefaction of high-density polyethylene (HDPE) over Ni/HZSM-5[J]. Journal of Analytical and Applied Pyrolysis, 2018, 136: 208-214. |
| [18] | YAO Dingding, YANG Haiping, CHEN Hanping, et al. Co-precipitation, impregnation and sol-gel preparation of Ni catalysts for pyrolysis-catalytic steam reforming of waste plastics[J]. Applied Catalysis B: Environmental, 2018, 239: 565-577. |
| [19] | AKUBO Kaltume, NAHIL Mohamad Anas, WILLIAMS Paul T. Aromatic fuel oils produced from the pyrolysis-catalysis of polyethylene plastic with metal-impregnated zeolite catalysts[J]. Journal of the Energy Institute, 2019, 92(1): 195-202. |
| [20] | FU Linchen, XIONG Qingang, WANG Qinhui, et al. Catalytic pyrolysis of waste polyethylene using combined CaO and Ga/ZSM-5 catalysts for high value-added aromatics production[J]. ACS Sustainable Chemistry & Engineering, 2022, 10(29): 9612-9623. |
| [21] | ZHANG Jiehan, MA Mingyu, CHEN Zhaohui, et al. Production of monocyclic aromatics and light olefins through ex-situ catalytic pyrolysis of low-density polyethylene over Ga/P/ZSM-5 catalyst[J]. Journal of the Energy Institute, 2023, 108: 101235. |
| [22] | LIU Ji, FU Hao, ZHOU Guanzheng, et al. Preparation of aromatic hydrocarbons from fast pyrolysis of waste medical mask catalyzed by modified HZSM-5[J]. Journal of Analytical and Applied Pyrolysis, 2023, 169: 105797. |
| [23] | Gerardo MARTÍNEZ-NARRO, PHAN Ha H, HASSAN Samaila, et al. Catalytic pyrolysis of plastic waste using metal-incorporated activated carbons for monomer recovery and carbon nanotube synthesis[J]. Journal of Environmental Chemical Engineering, 2024, 12(2): 112226. |
| [24] | YAO Dingding, Wang Chi-Hwa. Pyrolysis and in-line catalytic decomposition of polypropylene to carbon nanomaterials and hydrogen over Fe- and Ni-based catalysts[J]. Applied Energy, 2020, 265: 114819. |
| [25] | WU Qianru, Xuan LYU, XU Ningning, et al. Upcycling plastic polymers into single-walled carbon nanotubes from a magnesia supported iron catalyst[J]. Carbon, 2023, 215: 118492. |
| [26] | CAI Ning, LI Xiaoqiang, XIA Sunwen, et al. Pyrolysis-catalysis of different waste plastics over Fe/Al2O3 catalyst: High-value hydrogen, liquid fuels, carbon nanotubes and possible reaction mechanisms[J]. Energy Conversion and Management, 2021, 229: 113794. |
| [27] | LI Qinglin, SHAN Rui, WANG Shuxiao, et al. Production of carbon nanotubes via catalytic pyrolysis of waste plastics over Ni/Al2O3 catalyst: The influence of plastic types[J]. Journal of Analytical and Applied Pyrolysis, 2024, 177: 106318. |
| [28] | LI Qinglin, SHAN Rui, WANG Shuxiao, et al. Carbon nanotubes production from catalytic pyrolysis of polyethylene over nickel-based catalysts: The influence of support materials[J]. Fuel, 2023, 343: 127966. |
| [29] | YAO Dingding, LI He, MOHAN Babu Cadiam, et al. Conversion of waste plastic packings to carbon nanomaterials: Investigation into catalyst material, waste type, and product applications[J]. ACS Sustainable Chemistry & Engineering, 2022, 10(3): 1125-1136. |
| [30] | LIU Xingmin, XIE Wenjie, WIDENMEYER Marc, et al. Upcycling waste plastics into multi-walled carbon nanotube composites via NiCo2O4 catalytic pyrolysis[J]. Catalysts, 2021, 11(11): 1353. |
| [31] | YAO Dingding, WU Chunfei, YANG Haiping, et al. Co-production of hydrogen and carbon nanotubes from catalytic pyrolysis of waste plastics on Ni-Fe bimetallic catalyst[J]. Energy Conversion and Management, 2017, 148: 692-700. |
| [32] | LI Kezhuo, ZHANG Haijun, ZHENG Yangfan, et al. Catalytic preparation of carbon nanotubes from waste polyethylene using FeNi bimetallic nanocatalyst[J]. Nanomaterials, 2020, 10(8): 1517. |
| [33] | CAI Ning, XIA Sunwen, LI Xiaoqiang, et al. High-value products from ex-situ catalytic pyrolysis of polypropylene waste using iron-based catalysts: The influence of support materials[J]. Waste Management, 2021, 136: 47-56. |
| [34] | VATANKHAH Fatemeh, CARRILLO GARCÍA Adrián, CHAOUKI Jamal. Hydrogen and carbon nanotube production from microwave-assisted catalytic decomposition of plastic waste[J]. Chemical Engineering Journal, 2025, 503: 158189. |
| [35] | RAMZAN Fazeela, SHOUKAT Bilal, Muhammad Y NAZ, et al. Single step microwaves assisted catalytic conversion of plastic waste into valuable fuel and carbon nanotubes[J]. Thermochimica Acta, 2022, 715: 179294. |
| [36] | MA Yujun, WANG Wenliang, FU Yishuai, et al. A multifunctional SiC@HZSM-5@CoFe2O4 catalyst for synergistic co-pyrolysis of biomass and waste plastics by microwave irradiation[J]. Fuel, 2024, 359: 130407. |
| [37] | LI Sijie, XUE Yuan, LIN Yixi, et al. Synergistic activity of the Fe2O3/Al2O3 catalyst for hydrogen production through pyrolysis-catalytic decomposition of plastics[J]. ACS Sustainable Chemistry & Engineering, 2023, 11(27): 10108-10118. |
| [38] | ZHANG Peng, LIANG Cai, WU Mudi, et al. High-efficient microwave plasma discharging initiated conversion of waste plastics into hydrogen and carbon nanotubes[J]. Energy Conversion and Management, 2022, 268: 116017. |
| [39] | WANG Jun, PAN Yuhan, SONG Jiaxing, et al. A high-quality hydrogen production strategy from waste plastics through microwave-assisted reactions with heterogeneous bimetallic iron/nickel/cerium catalysts[J]. Journal of Analytical and Applied Pyrolysis, 2022, 166: 105612. |
| [40] | WANG Hui, ZHANG Bowen, LUO Pan, et al. Simultaneous achievement of high-yield hydrogen and high-performance microwave absorption materials from microwave catalytic deconstruction of plastic waste[J]. Processes, 2022, 10(4): 782. |
| [41] | Xiangyu JIE, LI Weisong, SLOCOMBE Daniel, et al. Microwave-initiated catalytic deconstruction of plastic waste into hydrogen and high-value carbons[J]. Nature Catalysis, 2020, 3(11): 902-912. |
| [42] | SHEN X, ZHAO Z, LI H, et al. Microwave-assisted pyrolysis of plastics with iron-based catalysts for hydrogen and carbon nanotubes production[J]. Materials Today Chemistry, 2022, 26: 101166. |
| [43] | YAO Liansheng, YI Baokui, ZHAO Xiqiang, et al. Microwave-assisted decomposition of waste plastic over Fe/FeAl2O4 to produce hydrogen and carbon nanotubes[J]. Journal of Analytical and Applied Pyrolysis, 2022, 165: 105577. |
| [44] | LI Wentao, QIAN Kezhen, YANG Zixu, et al. Promotion effect of cobalt doping on microwave-initiated plastic deconstruction for hydrogen production over iron catalysts[J]. Applied Catalysis B: Environmental, 2023, 327: 122451. |
| [45] | ZHANG Zhe, CHEN Huan, HU Wenheng, et al. Temperature-Dependent NiFeAl catalysts for efficient microwave-assisted catalytic pyrolysis of polyethylene into value-added hydrogen and carbon nanotubes[J]. Fuel, 2024, 366: 131390. |
| [46] | ZHANG Xuesong, LEI Hanwu, YADAVALLI Gayatri, et al. Gasoline-range hydrocarbons produced from microwave-induced pyrolysis of low-density polyethylene over ZSM-5[J]. Fuel, 2015, 144: 33-42. |
| [47] | ZHOU Nan, DAI Leilei, LV Yuancai, et al. Catalytic pyrolysis of plastic wastes in a continuous microwave assisted pyrolysis system for fuel production[J]. Chemical Engineering Journal, 2021, 418: 129412. |
| [48] | PENG Yujie, WANG Xiaofei, FAN Liangliang, et al. Conversion of low-density polyethylene into monocyclic aromatic hydrocarbons through continuous microwave pyrolysis with ex-situ dual-catalyst beds[J]. Journal of Cleaner Production, 2023, 418: 138039. |
| [49] | FAN Liangliang, CHEN Paul, ZHANG Yaning, et al. Fast microwave-assisted catalytic co-pyrolysis of lignin and low-density polyethylene with HZSM-5 and MgO for improved bio-oil yield and quality[J]. Bioresource Technology, 2017, 225: 199-205. |
| [50] | DING Kuan, LIU Shasha, HUANG Yong, et al. Catalytic microwave-assisted pyrolysis of plastic waste over NiO and HY for gasoline-range hydrocarbons production[J]. Energy Conversion and Management, 2019, 196: 1316-1325. |
| [51] | GRAVES Katherine A, HIGGINS Luke J R, NAHIL Mohamad A, et al. Structural comparison of multi-walled carbon nanotubes produced from polypropylene and polystyrene waste plastics[J]. Journal of Analytical and Applied Pyrolysis, 2022, 161: 105396. |
| [52] | JIA Jingbo, VEKSHA Andrei, Teik-Thye LIM, et al. In situ grown metallic nickel from X-Ni (X=La, Mg, Sr) oxides for converting plastics into carbon nanotubes: Influence of metal-support interaction[J]. Journal of Cleaner Production, 2020, 258: 120633. |
| [53] | HUANG Jijiang, VEKSHA Andrei, JIN JUN Thaddeus FOO, et al. Upgrading waste plastic derived pyrolysis gas via chemical looping cracking-gasification using Ni-Fe-Al redox catalysts[J]. Chemical Engineering Journal, 2022, 438: 135580. |
| [54] | ZHANG Peng, WU Mudi, LIANG Cai, et al. In-situ exsolution of Fe-Ni alloy catalysts for H2 and carbon nanotube production from microwave plasma-initiated decomposition of plastic wastes[J]. Journal of Hazardous Materials, 2023, 445: 130609. |
| [55] | ZHAO Jun, GAO Jianye, WANG Duanda, et al. Microwave-intensified catalytic upcycling of plastic waste into hydrogen and carbon nanotubes over self-dispersing bimetallic catalysts[J]. Chemical Engineering Journal, 2024, 483: 149270. |
| [56] | LUO Juan, GONG Guojin, MA Rui, et al. Study on high-value products of waste plastics from microwave catalytic pyrolysis: Construction and performance evaluation of advanced microwave absorption-catalytic bifunctional catalysts[J]. Fuel, 2023, 346: 128296. |
| [57] | SHOUKAT Bilal, HUSSAIN Hammad, YASIN NAZ Muhammad, et al. Microwaves assisted deconstruction of HDPE waste into structured carbon and hydrogen fuel using Al2O3-(Ni, Zn, Mg)Fe2O4 composite catalysts[J]. Thermal Science and Engineering Progress, 2024, 47: 102368. |
| [58] | YAO Lu, ZHU Jianhua, LI Shuyuan, et al. Analysis of liquid products and mechanism of thermal/catalytic pyrolysis of HDPE[J]. Journal of Thermal Analysis and Calorimetry, 2022, 147(24): 14257-14266. |
| [59] | TIAN Xiaojie, ZENG Zihong, LIU Zhihao, et al. Conversion of low-density polyethylene into monocyclic aromatic hydrocarbons by catalytic pyrolysis: Comparison of HZSM-5, Hβ, HY and MCM-41[J]. Journal of Cleaner Production, 2022, 358: 131989. |
| [60] | JIANG Hao, ZHANG Junjie, SHAO Jingai, et al. Desulfurization and upgrade of pyrolytic oil and gas during waste tires pyrolysis: The role of metal oxides[J]. Waste Management, 2024, 182: 44-54. |
| [61] | BELRHAZI Ilyass, SAIR Said, OUSALEH Hanane AIT, et al. Catalytic transformation of plastic waste: Harnessing zeolite for enhanced energy product yield in pyrolysis[J]. Energy Conversion and Management, 2024, 318: 118897. |
| [62] | CHEN Zezhou, ZHANG Xurui, CHE Lei, et al. Effect of volatile reactions on oil production and composition in thermal and catalytic pyrolysis of polyethylene[J]. Fuel, 2020, 271: 117308. |
| [63] | FU Linchen, LIN Huaping, ZHU Likai, et al. Enhancing catalytic performance for waste plastic upgrading: Simultaneous regulation of pore structure and acid sites in Ga-doped desilicated HZSM-5 catalysts[J]. Journal of Analytical and Applied Pyrolysis, 2023, 175: 106186. |
| [64] | DWIVEDI Uma, NAIK S N, PANT K K. High quality liquid fuel production from waste plastics via two-step cracking route in a bottom-up approach using bi-functional Fe/HZSM-5 catalyst[J]. Waste Management, 2021, 132: 151-161. |
| [65] | WANG Yazhuo, CHENG Leilei, GU Jing, et al. Catalytic pyrolysis of polyethylene for the selective production of monocyclic aromatics over the zinc-loaded ZSM-5 catalyst[J]. ACS Omega, 2022, 7(3): 2752-2765. |
| [66] | WAN MAHARI Wan Adibah, AWANG Syafikah, ZAHARIMAN Nur Alifah Zakirah, et al. Microwave co-pyrolysis for simultaneous disposal of environmentally hazardous hospital plastic waste, lignocellulosic, and triglyceride biowaste[J]. Journal of Hazardous Materials, 2022, 423: 127096. |
| [67] | SURIAPPARAO Dadi V, VINU R. Resource recovery from synthetic polymers via microwave pyrolysis using different susceptors[J]. Journal of Analytical and Applied Pyrolysis, 2015, 113: 701-712. |
| [68] | LOPEZ Gartzen, SANTAMARIA Laura. Microwaving plastic into hydrogen and carbons[J]. Nature Catalysis, 2020, 3(11): 861-862. |
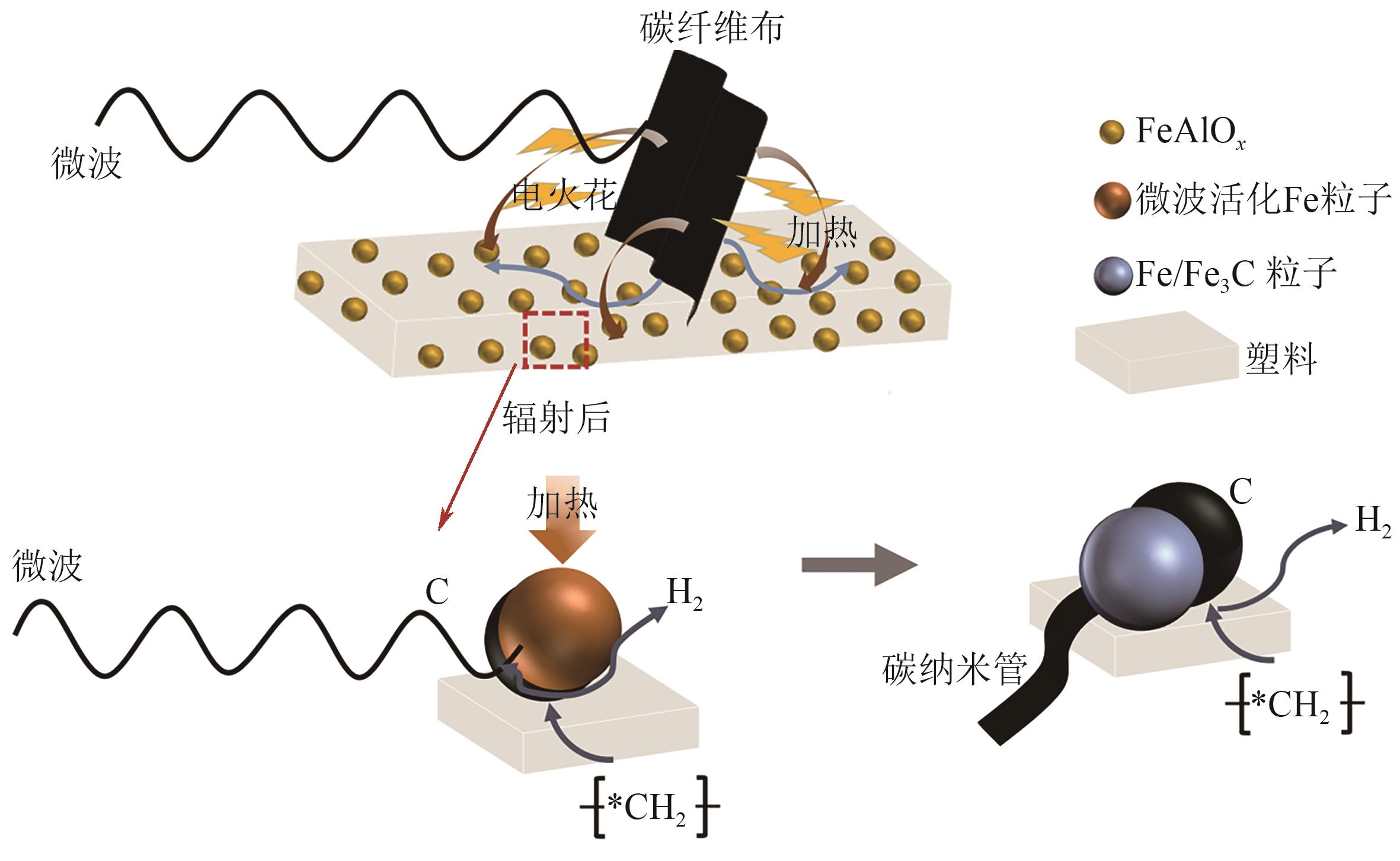
| [69] | ZHANG Bowen, WANG Hui, YANG Yiyun, et al. Microwave-carbon fiber cloth co-ignited catalytic degradation of waste plastic into high-yield hydrogen and carbon nanotubes[J]. Journal of Environmental Chemical Engineering, 2023, 11(3): 109710. |
| [70] | CHAO Yuwen, LIU Bingguo, RONG Qian, et al. Mechanism of microwave-assisted iron-based catalyst pyrolysis of discarded COVID-19 masks[J]. Waste Management, 2023, 155: 77-86. |
| [71] | CAI Ning, LIU Qingyu, LI Xiaoqiang, et al. Identify the impact of pyrolysis temperature on preparation of carbon nanotubes by catalytic reforming polypropylene[J]. Waste Management, 2024, 190: 161-168. |
| [1] | 姚如伟, 宋乐音, 牛琴琴, 李聪明. Na-S双助剂修饰铁基催化剂催化CO2加氢制C2+醇[J]. 化工进展, 2025, 44(6): 3154-3162. |
| [2] | 陈少伟, 陈奕, 牛江奇, 刘天奇, 黄建国, 陈焕浩, 范晓雷. 介质阻挡放电等离子体催化反应器研究进展及应用展望[J]. 化工进展, 2025, 44(6): 3175-3189. |
| [3] | 王家慧, 李培雅, 杨福胜, 王斌, 方涛. 有机液态储氢载体甲基环己烷脱氢研究进展[J]. 化工进展, 2025, 44(6): 3208-3223. |
| [4] | 石秀顶, 王永全, 曾静, 苏畅, 洪俊明. 纳米管状Co-N-C活化过碳酸盐降解四环素[J]. 化工进展, 2025, 44(6): 3041-3052. |
| [5] | 谢武强, 张岭, 贺杠, 蒋里锋, 郑晰瑞, 张和鹏. CoTBrPP-PTAB-Cu电催化还原CO2制甲烷[J]. 化工进展, 2025, 44(6): 3093-3100. |
| [6] | 曹湘洪, 周峰, 姜睿, 刘诗哲, 方向晨, 亢万忠, 乔金樑, 聂红. 加快我国生物基材料产业发展的对策[J]. 化工进展, 2025, 44(5): 2385-2393. |
| [7] | 丁阿静, 周巧巧, 顾学红. 膜反应器中杨木催化气化制清洁合成气[J]. 化工进展, 2025, 44(5): 2716-2723. |
| [8] | 何志勇. 分步脱羟/脱碳催化剂实现高效裂解甲醇制氢[J]. 化工进展, 2025, 44(5): 2724-2732. |
| [9] | 范晓娅, 赵镇, 彭强. 电催化二氧化碳和硝酸根共还原合成尿素研究进展[J]. 化工进展, 2025, 44(5): 2856-2869. |
| [10] | 苏俊杰, 刘苏, 周海波, 刘畅, 张琳, 王仰东, 谢在库. 用于CO2加氢直接制低碳烯烃的InZr/SAPO-34双功能催化剂[J]. 化工进展, 2025, 44(5): 2870-2878. |
| [11] | 汪柯, 胡登, 王星博, 孙楠楠, 魏伟. Fe x Co y Ca3Al双功能材料用于CO2捕集-转化一体化制合成气[J]. 化工进展, 2025, 44(5): 2888-2897. |
| [12] | 鲍婕, 余攀结, 马永德, 张宏伟, 蔡镇平, 曹彦宁, 黄宽, 江莉龙. Cu-ZrO2催化材料的制备及其棕榈酸甲酯加氢制脂肪醇性能[J]. 化工进展, 2025, 44(5): 2997-3008. |
| [13] | 朱慧红, 刘璐, 刘鹏, 李贺, 杨涛, 王继锋, 侯栓弟, 彭冲, 赵毅毅, 潘云翔. 劣质渣油加氢催化剂构筑及其催化性能提升机制[J]. 化工进展, 2025, 44(5): 3009-3016. |
| [14] | 张绎如, 韩东梅, 马伟芳. 铁基复合卤氧化铋磁性材料强化可见光催化处理难降解有机废水研究进展[J]. 化工进展, 2025, 44(4): 2258-2273. |
| [15] | 宋坤莉, 肖雷, 马丹丹, 肖朋, 杨莎莎, 石建稳. 超低温氨气选择性脱硝催化剂的研究进展[J]. 化工进展, 2025, 44(4): 2028-2035. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||