化工进展 ›› 2024, Vol. 43 ›› Issue (11): 6155-6172.DOI: 10.16085/j.issn.1000-6613.2023-1778
• 工业催化 • 上一篇
碳基催化剂用于两电子氧还原合成过氧化氢的研究进展
- 清华大学化学工程系,北京 100084
-
收稿日期:2023-10-11修回日期:2024-01-26出版日期:2024-11-15发布日期:2024-12-07 -
通讯作者:王保国 -
作者简介:许琴(1998—),女,博士研究生,研究方向为电催化与储能。E-mail:xuq20@mails.tsinghua.edu.cn。 -
基金资助:国家自然科学基金(222278239)
Recent progress on carbon-based electrocatalysts for hydrogen peroxide production via two-electron oxygen reduction reaction
- Department of Chemical Engineering, Tsinghua University, Beijing 100084, China
-
Received:2023-10-11Revised:2024-01-26Online:2024-11-15Published:2024-12-07 -
Contact:WANG Baoguo
摘要:
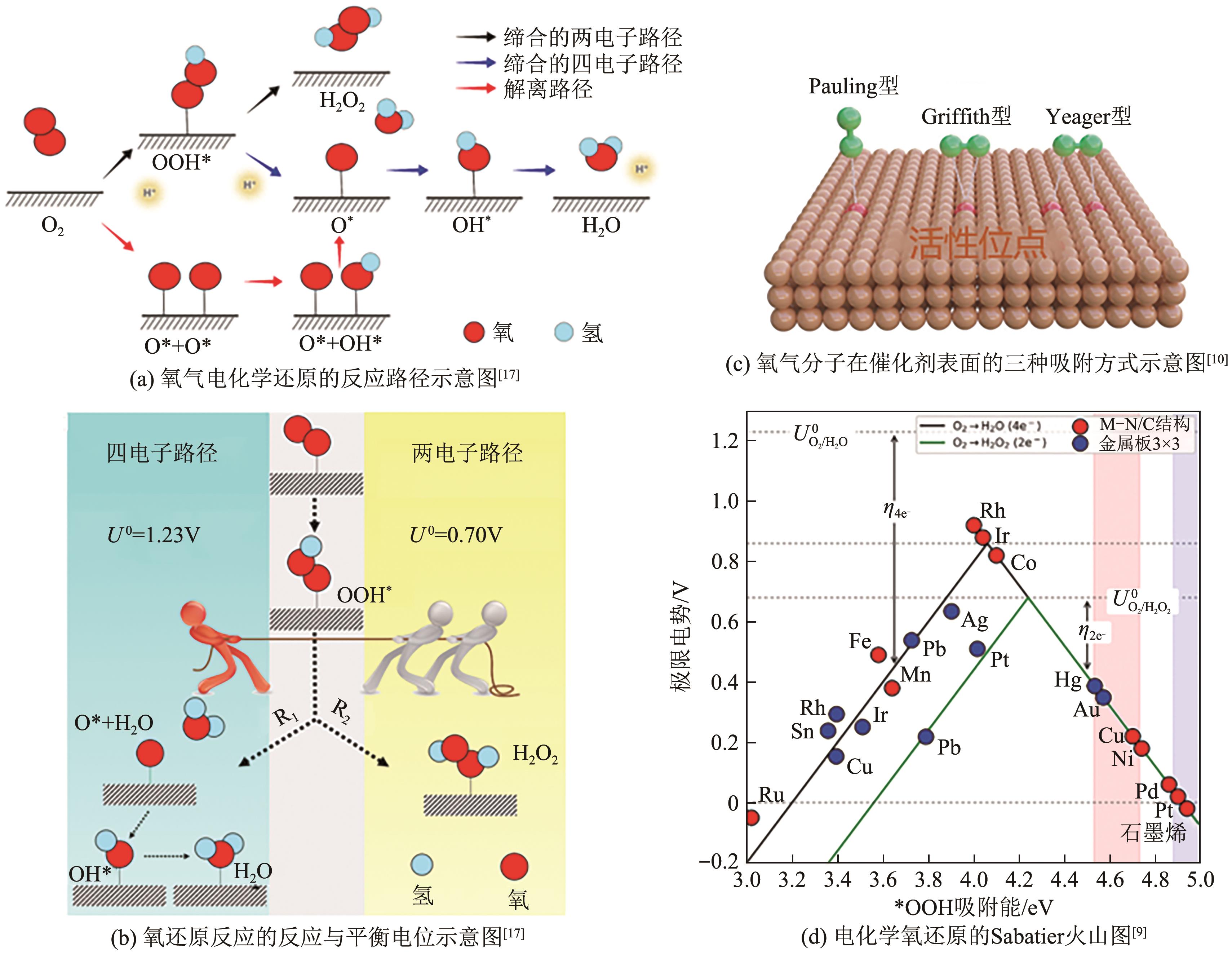
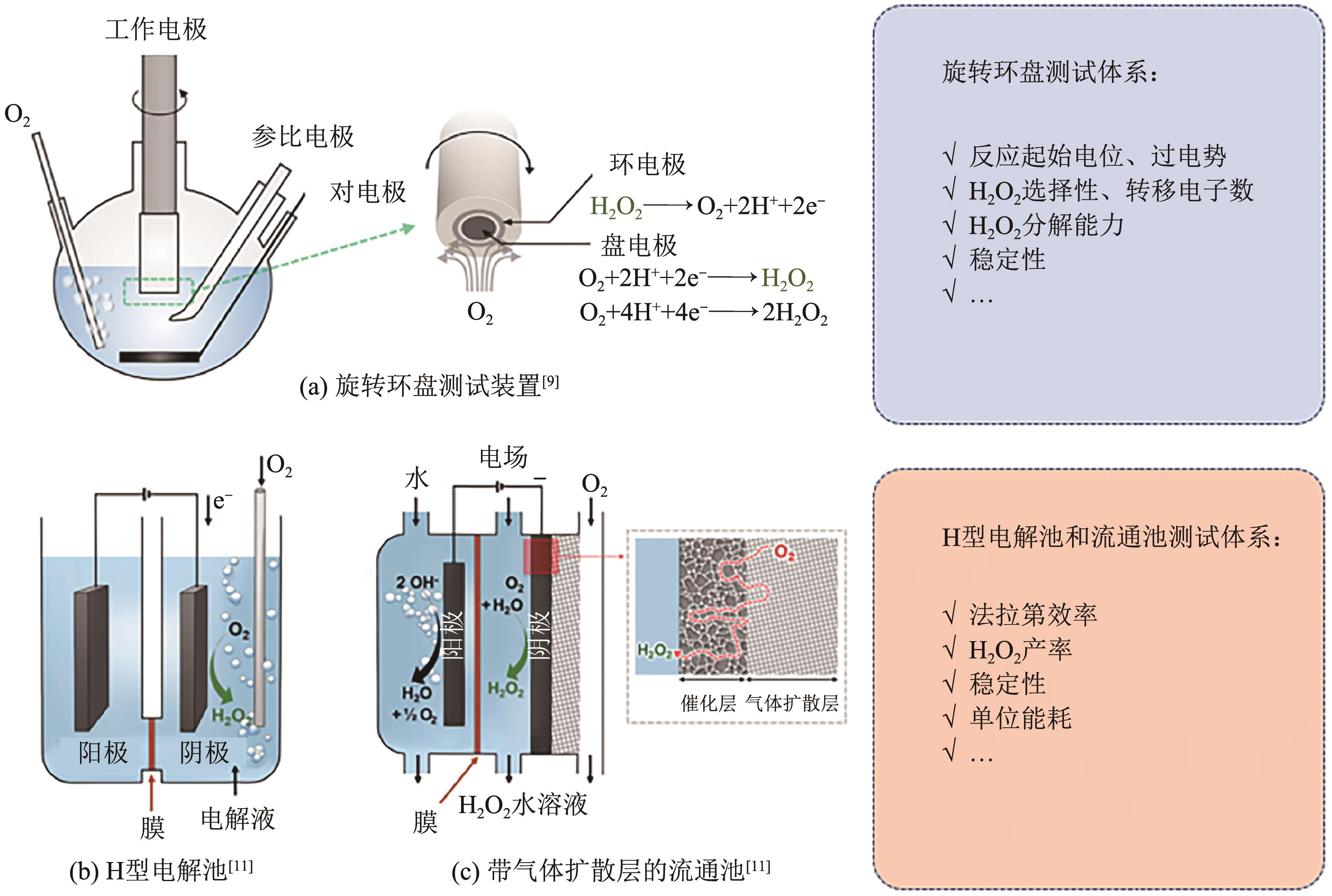
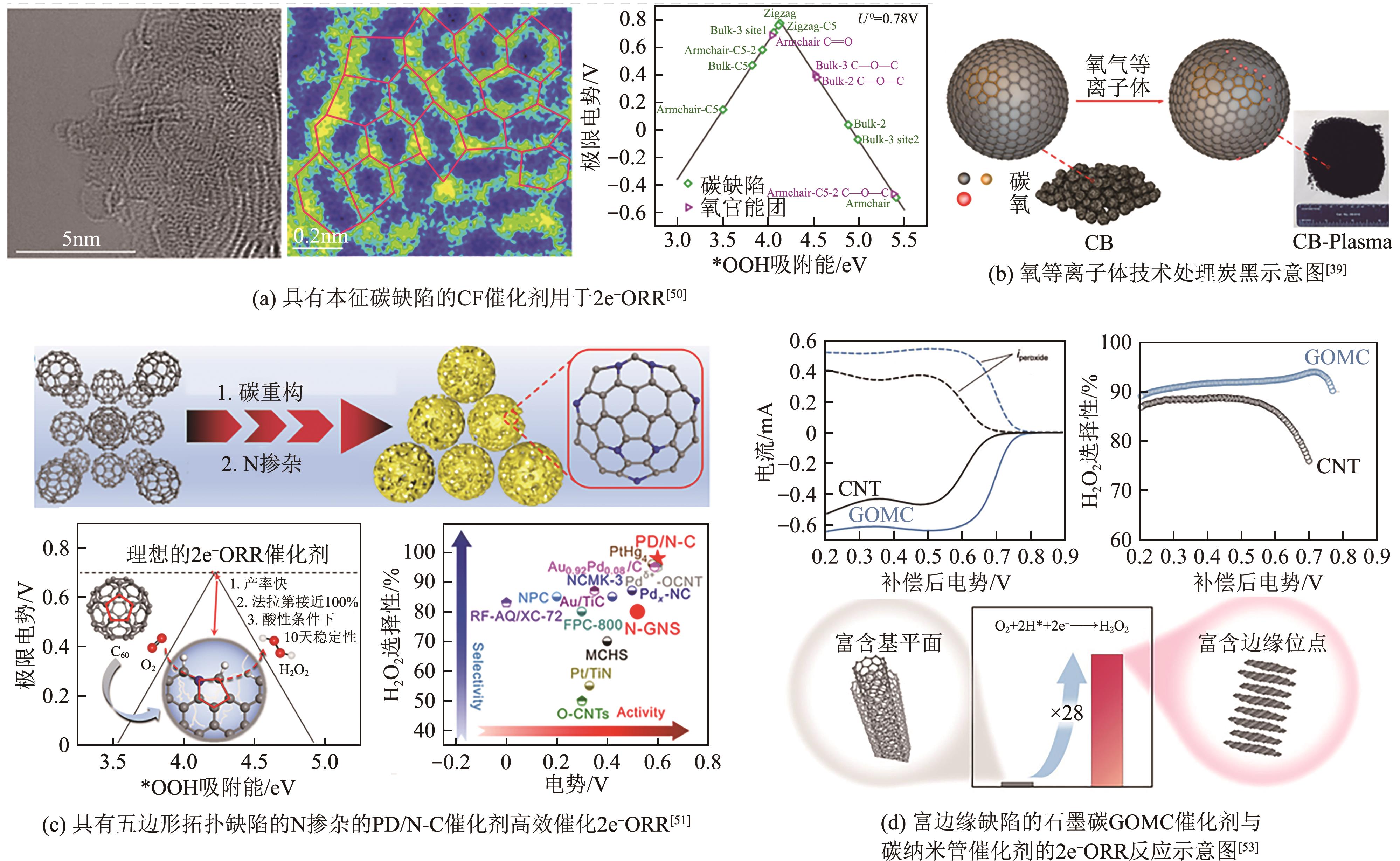
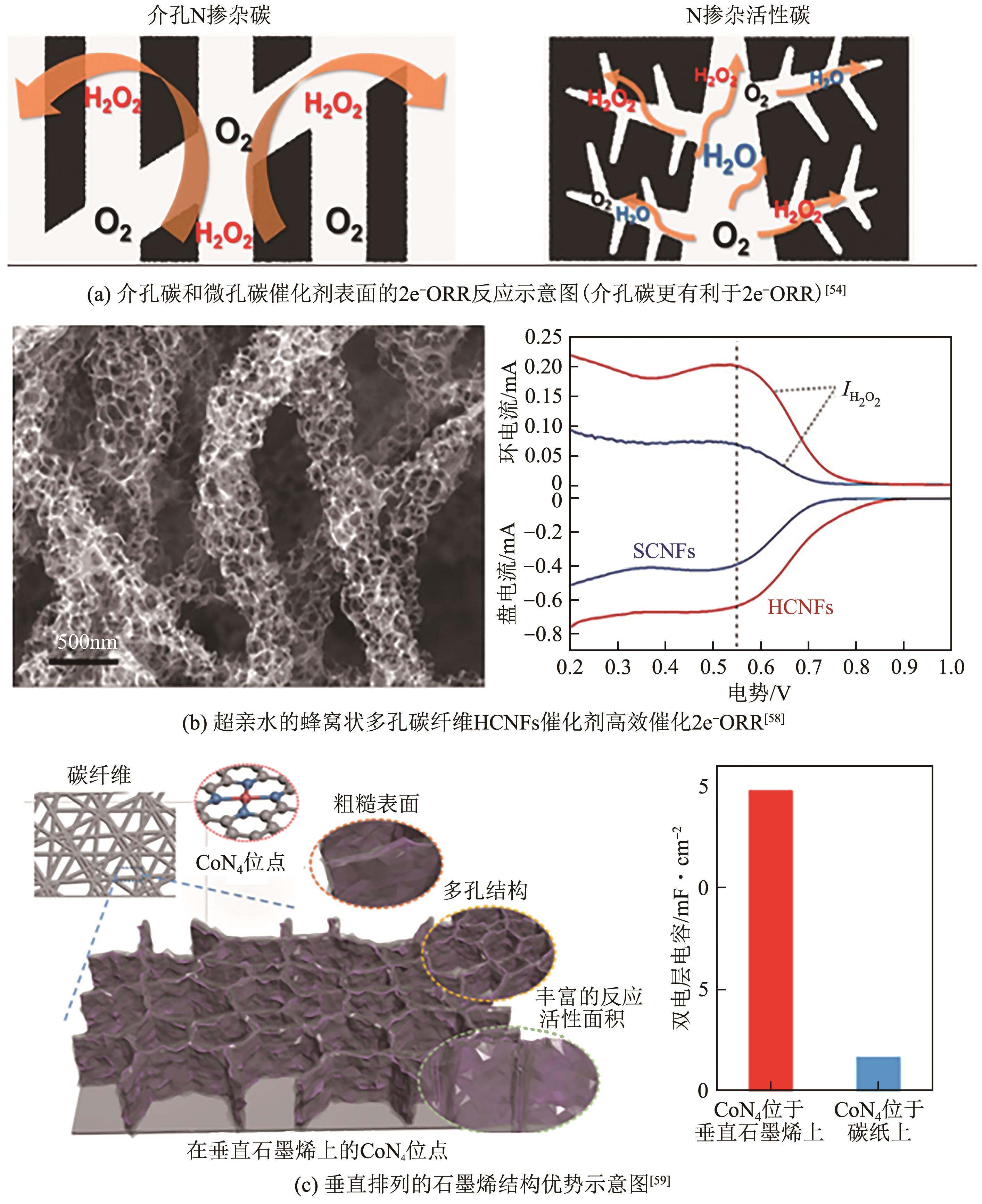
电化学两电子氧还原反应(2e-ORR)制备过氧化氢(H2O2)是一种可替代工业蒽醌法的绿色环保且安全高效的技术路线,具有广阔发展前景。然而,受限于缓慢的氧还原反应(ORR)动力学以及竞争的四电子反应过程,开发具有高氧还原反应活性以及两电子选择性的高性能催化剂是目前研究的核心关键。近年来,碳基材料因其储量丰富、成本低廉、结构易于调控等优势,被广泛应用于2e-ORR合成H2O2领域,并取得了显著的研究进展。基于此,本文首先简要介绍了氧还原过程的基本原理以及催化剂的一般评价方法,指出了构建高效催化剂的基本设计指导原则;其次重点综述了近年来碳基催化剂用于2e-ORR制备H2O2的最新研究进展,针对非金属碳基材料和过渡金属碳基材料两大类,细致讨论并总结了异相原子掺杂、缺陷工程、孔结构工程、单原子局部微环境调控等催化剂活性位点优化策略对提升催化性能的作用效果;最后,针对H2O2生产介质、催化剂结构-性能关系以及在工业级电流密度下稳定生产H2O2三方面,展望了两电子氧还原合成H2O2领域未来发展的挑战及方向。
中图分类号:
引用本文
许琴, 王保国. 碳基催化剂用于两电子氧还原合成过氧化氢的研究进展[J]. 化工进展, 2024, 43(11): 6155-6172.
XU Qin, WANG Baoguo. Recent progress on carbon-based electrocatalysts for hydrogen peroxide production via two-electron oxygen reduction reaction[J]. Chemical Industry and Engineering Progress, 2024, 43(11): 6155-6172.
| 1 | BU Yunfei, WANG Yaobin, HAN Gaofeng, et al. Carbon-based electrocatalysts for efficient hydrogen peroxide production[J]. Advanced Materials, 2021, 33(49): 2103266. |
| 2 | PERRY Samuel C, PANGOTRA Dhananjai, VIEIRA Luciana, et al. Electrochemical synthesis of hydrogen peroxide from water and oxygen[J]. Nature Reviews Chemistry, 2019, 3: 442-458. |
| 3 | ZHANG Jiayi, ZHANG Haochen, CHENG Mu-Jeng, et al. Tailoring the electrochemical production of H2O2: Strategies for the rational design of high-performance electrocatalysts[J]. Small, 2020, 16(15): e1902845. |
| 4 | TANG Junying, ZHAO Tianshuo, SOLANKI Devan, et al. Selective hydrogen peroxide conversion tailored by surface, interface, and device engineering[J]. Joule, 2021, 5: 1432-1461. |
| 5 | YAMAZAKI Shin-ichi, SIROMA Zyun, SENOH Hiroshi, et al. A fuel cell with selective electrocatalysts using hydrogen peroxide as both an electron acceptor and a fuel[J]. Journal of Power Sources, 2008, 178(1): 20-25. |
| 6 | MOUSAVI SHAEGH Seyed ALI, NGUYEN Nam-Trung, MOUSAVI EHTESHAMI Seyyed Mohsen, et al. A membraneless hydrogen peroxidefuel cell using Prussian Blue as cathode material[J]. Energy & Environmental Science, 2012, 5(8): 8225-8228. |
| 7 | LI Nan, HUANG Chuchu, WANG Xin, et al. Electrosynthesis of hydrogen peroxide via two-electron oxygen reduction reaction: A critical review focus on hydrophilicity/hydrophobicity of carbonaceous electrode[J]. Chemical Engineering Journal, 2022, 450: 138246. |
| 8 | ZHOU Wei, MENG Xiaoxiao, GAO Jihui, et al. Hydrogen peroxide generation from O2 electroreduction for environmental remediation: A state-of-the-art review[J]. Chemosphere, 2019, 225: 588-607. |
| 9 | YANG Sungeun, Arnau VERDAGUER-CASADEVALL, ARNARSON Logi, et al. Toward the decentralized electrochemical production of H2O2: A focus on the catalysis[J]. ACS Catalysis, 2018, 8(5): 4064-4081. |
| 10 | WANG Kun, HUANG Jianhao, CHEN Haixin, et al. Recent advances in electrochemical 2e oxygen reduction reaction for on-site hydrogen peroxide production and beyond[J]. Chemical Communications, 2020, 56(81): 12109-12121. |
| 11 | JUNG Euiyeon, SHIN Heejong, HOOCH ANTINK Wytse, et al. Recent advances in electrochemical oxygen reduction to H2O2: Catalyst and cell design[J]. ACS Energy Letters, 2020, 5(6): 1881-1892. |
| 12 | WANG Nan, MA Shaobo, ZUO Pengjian, et al. Recent progress of electrochemical production of hydrogen peroxide by two-electron oxygen reduction reaction[J]. Advanced Science, 2021, 8(15): e2100076. |
| 13 | ZHANG Xiao, XIA Yang, XIA Chuan, et al. Insights into practical-scale electrochemical H2O2 synthesis[J]. Trends in Chemistry, 2020, 2(10): 942-953. |
| 14 | MELCHIONNA Michele, FORNASIERO Paolo, PRATO Maurizio. The rise of hydrogen peroxide as the main product by metal-free catalysis in oxygen reductions[J]. Advanced Materials, 2019, 31(13): e1802920. |
| 15 | HUANG Xiao, SONG Min, ZHANG Jingjing, et al. Recent advances of electrocatalyst and cell design for hydrogen peroxide production[J]. Nano-Micro Letters, 2023, 15(1): 86. |
| 16 | BERL E. A new cathodic process for the production of H2O2 [J]. Transactions of the Electrochemical Society, 1939, 76(1): 359. |
| 17 | GUO Xiangyu, LIN Shiru, GU Jinxing, et al. Simultaneously achieving high activity and selectivity toward two-electron O2 electroreduction: The power of single-atom catalysts[J]. ACS Catalysis, 2019, 9(12): 11042-11054. |
| 18 | ZHANG Jincheng, YANG Hongbin, LIU Bin. Coordination engineering of single-atom catalysts for the oxygen reduction reaction: A review[J]. Advanced Energy Materials, 2021, 11(3): 2002473. |
| 19 | ZHAO Xunhua, LIU Yuanyue. Origin of selective production of hydrogen peroxide by electrochemical oxygen reduction[J]. Journal of the American Chemical Society, 2021, 143(25): 9423-9428. |
| 20 | PAULING LINUS. Nature of the iron-oxygen bond in oxyhæmoglobin[J]. Nature, 1964, 203: 182-183. |
| 21 | GRIFFITH J S, ROUGHTON F J W. On the magnetic properties of some haemoglobin complexes[J]. Proceedings of the Royal Society of London Series A Mathematical and Physical Sciences, 1956, 235(1200): 23-36. |
| 22 | YEAGER E. Electrocatalysts for O2 reduction[J]. Electrochimica Acta, 1984, 29(11): 1527-1537. |
| 23 | MEDFORD Andrew J, VOJVODIC Aleksandra, HUMMELSHØJ Jens S, et al. From the Sabatier principle to a predictive theory of transition-metal heterogeneous catalysis[J]. Journal of Catalysis, 2015, 328: 36-42. |
| 24 | XIA Chuan, KIM Jung Yoon, WANG Haotian. Recommended practice to report selectivity in electrochemical synthesis of H2O2 [J]. Nature Catalysis, 2020, 3: 605-607. |
| 25 | KIM Hyo Won, ROSS Michael B, KORNIENKO Nikolay, et al. Efficient hydrogen peroxide generation using reduced graphene oxide-based oxygen reduction electrocatalysts[J]. Nature Catalysis, 2018, 1: 282-290. |
| 26 | YAO Yongchao, WANG Huiqing, DONG Kai, et al. Shifting the O2 reduction pathway from H2O to H2O2 via in situ reconstruction of Ti2O3 nanoparticles[J]. Journal of Materials Chemistry A, 2023, 11(41): 22154-22160. |
| 27 | KULKARNI Ambarish, SIAHROSTAMI Samira, PATEL Anjli, et al. Understanding catalytic activity trends in the oxygen reduction reaction[J]. Chemical Reviews, 2018, 118(5): 2302-2312. |
| 28 | WANG Dawei, SU Dangsheng. Heterogeneous nanocarbon materials for oxygen reduction reaction[J]. Energy & Environmental Science, 2014, 7(2): 576-591. |
| 29 | HE Wenhui, WANG Ying, JIANG Chunhuan, et al. Structural effects of a carbon matrix in non-precious metal O2 - reduction electrocatalysts[J]. Chemical Society Reviews, 2016, 45(9): 2396-2409. |
| 30 | SUN Yanyan, SINEV Ilya, JU Wen, et al. Efficient electrochemical hydrogen peroxide production from molecular oxygen on nitrogen-doped mesoporous carbon catalysts[J]. ACS Catalysis, 2018, 8(4): 2844-2856. |
| 31 | SUN Yanyan, LI Shuang, JOVANOV Zarko Petar, et al. Structure, activity, and faradaic efficiency of nitrogen-doped porous carbon catalysts for direct electrochemical hydrogen peroxide production[J]. ChemSusChem, 2018, 11(19): 3388-3395. |
| 32 | ZHANG Junyu, ZHANG Gong, JIN Shengyao, et al. Graphitic N in nitrogen-Doped carbon promotes hydrogen peroxide synthesis from electrocatalytic oxygen reduction[J]. Carbon, 2020, 163: 154-161. |
| 33 | LI Laiquan, TANG Cheng, ZHENG Yao, et al. Tailoring selectivity of electrochemical hydrogen peroxide generation by tunable pyrrolic-nitrogen-carbon[J]. Advanced Energy Materials, 2020, 10(21): 2000789. |
| 34 | PENG Wei, LIU Jiaxin, LIU Xiaoqing, et al. Facilitating two-electron oxygen reduction with pyrrolic nitrogen sites for electrochemical hydrogen peroxide production[J]. Nature Communications, 2023, 14: 4430. |
| 35 | LU Zhiyi, CHEN Guangxu, SIAHROSTAMI Samira, et al. High-efficiency oxygen reduction to hydrogen peroxide catalysed by oxidized carbon materials[J]. Nature Catalysis, 2018, 1: 156-162. |
| 36 | XIA Chuan, XIA Yang, ZHU Peng, et al. Direct electrosynthesis of pure aqueous H2O2 solutions up to 20% by weight using a solid electrolyte[J]. Science, 2019, 366(6462): 226-231. |
| 37 | ZHANG Chang, LIU Wei, SONG Min, et al. Pyranoid-O-dominated graphene-like nanocarbon for two-electron oxygen reduction reaction[J]. Applied Catalysis B: Environmental, 2022, 307: 121173. |
| 38 | HAN Lei, SUN Yanyan, LI Shuang, et al. In-plane carbon lattice-defect regulating electrochemical oxygen reduction to hydrogen peroxide production over nitrogen-doped graphene[J]. ACS Catalysis, 2019, 9(2): 1283-1288. |
| 39 | WANG Zhe, LI Qinkun, ZHANG Chenhao, et al. Hydrogen peroxide generation with 100% faradaic efficiency on metal-free carbon black[J]. ACS Catalysis, 2021, 11(4): 2454-2459. |
| 40 | JUNG Euiyeon, SHIN Heejong, LEE Byoung-Hoon, et al. Atomic-level tuning of Co-N-C catalyst for high-performance electrochemical H2O2 production[J]. Nature Materials, 2020, 19(4): 436-442. |
| 41 | HAN Gaofeng, LI Feng, ZOU Wei, et al. Building and identifying highly active oxygenated groups in carbon materials for oxygen reduction to H2O2 [J]. Nature Communications, 2020, 11(1): 2209. |
| 42 | CHEN Shanyong, LUO Tao, CHEN Kejun, et al. Chemical identification of catalytically active sites on oxygen-doped carbon nanosheet to decipher the high activity for electro-synthesis hydrogen peroxide[J]. Angewandte Chemie International Edition, 2021, 60(30): 16607-16614. |
| 43 | XIA Yang, ZHAO Xunhua, XIA Chuan, et al. Highly active and selective oxygen reduction to H2O2 on boron-doped carbon for high production rates[J]. Nature Communications, 2021, 12(1): 4225. |
| 44 | CHEN Shucheng, CHEN Zhihua, SIAHROSTAMI Samira, et al. Designing boron nitride islands in carbon materials for efficient electrochemical synthesis of hydrogen peroxide[J]. Journal of the American Chemical Society, 2018, 140(25): 7851-7859. |
| 45 | JIA Nan, YANG Ting, SHI Shufeng, et al. N, F-codoped carbon nanocages: An efficient electrocatalyst for hydrogen peroxide electroproduction in alkaline and acidic solutions[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(7): 2883-2891. |
| 46 | XIANG Fei, ZHAO Xuhong, YANG Jian, et al. Enhanced selectivity in the electroproduction of H2O2 via F/S dual-doping in metal-free nanofibers[J]. Advanced Materials, 2023, 35(7): e2208533. |
| 47 | ZHU Jiawei, MU Shichun. Defect engineering in carbon-based electrocatalysts: Insight into intrinsic carbon defects[J]. Advanced Functional Materials, 2020, 30(25): 2001097. |
| 48 | JIANG Yufei, YANG Lijun, SUN Tao, et al. Significant contribution of intrinsic carbon defects to oxygen reduction activity[J]. ACS Catalysis, 2015, 5(11): 6707-6712. |
| 49 | CHEN Shucheng, CHEN Zhihua, SIAHROSTAMI Samira, et al. Defective carbon-based materials for the electrochemical synthesis of hydrogen peroxide[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(1): 311-317. |
| 50 | WANG Wang, ZHENG Yanxing, HU Youcheng, et al. Intrinsic carbon defects for the electrosynthesis of H2O2 [J]. The Journal of Physical Chemistry Letters, 2022, 13(38): 8914-8920. |
| 51 | ZHANG Chang, SHEN Wangqiang, GUO Kun, et al. A pentagonal defect-rich metal-free carbon electrocatalyst for boosting acidic O2 reduction to H2O2 production[J]. Journal of the American Chemical Society, 2023, 145(21): 11589-11598. |
| 52 | SUNG Lim June, HYUNG Kim Jae, JINWOO Woo, et al. Designing highly active nanoporous carbon H2O2 production electrocatalysts through active site identification[J]. Chem, 2021, 7(11): 3114-3130. |
| 53 | Young Jin SA, KIM Jae Hyung, Sang Hoon JOO. Active edge-site-rich carbon nanocatalysts with enhanced electron transfer for efficient electrochemical hydrogen peroxide production[J]. Angewandte Chemie International Edition, 2019, 58(4): 1100-1105. |
| 54 | PARK Jisung, NABAE Yuta, HAYAKAWA Teruaki, et al. Highly selective two-electron oxygen reduction catalyzed by mesoporous nitrogen-doped carbon[J]. ACS Catalysis, 2014, 4(10): 3749-3754. |
| 55 | LIU Yanming, QUAN Xie, FAN Xinfei, et al. High-yield electrosynthesis of hydrogen peroxide from oxygen reduction by hierarchically porous carbon[J]. Angewandte Chemie International Edition, 2015, 54(23): 6837-6841. |
| 56 | LEE Kyungbin, Jeonghoon LIM, LEE Michael J, et al. Structure-controlled graphene electrocatalysts for high-performance H2O2 production[J]. Energy & Environmental Science, 2022, 15(7): 2858-2866. |
| 57 | YAN Chenglu, MA Qiuting, WANG Fengyi, et al. Honeycomb-like phosphorus doped nickel/carbon: A highly efficient electrocatalyst for oxygen reduction to H2O2 [J]. Chemical Engineering Journal, 2022, 433: 133651. |
| 58 | DONG Kai, LIANG Jie, WANG Yuanyuan, et al. Honeycomb carbon nanofibers: A superhydrophilic O2-entrapping electrocatalyst enables ultrahigh mass activity for the two-electron oxygen reduction reaction[J]. Angewandte Chemie International Edition, 2021, 60(19): 10583-10587. |
| 59 | LIN Zeheng, ZHANG Qingran, PAN Jian, et al. Atomic Co decorated free-standing graphene electrode assembly for efficient hydrogen peroxide production in acid[J]. Energy & Environmental Science, 2022, 15(3): 1172-1182. |
| 60 | WANG Aiqin, LI Jun, ZHANG Tao. Heterogeneous single-atom catalysis[J]. Nature Reviews Chemistry, 2018, 2: 65-81. |
| 61 | ZHANG Longcheng, LIANG Jie, YUE Luchao, et al. N-doped carbon nanotubes supported CoSe2 nanoparticles: A highly efficient and stable catalyst for H2O2 electrosynthesis in acidic media[J]. Nano Research, 2022, 15(1): 304-309. |
| 62 | ZHANG Shijie, ZHENG Jingnan, LIN Junming, et al. Doped-nitrogen enhanced the performance of Nb2CT x on the electrocatalytic synthesis of H2O2 [J]. Nano Research, 2023, 16(5): 6120-6127. |
| 63 | CHENG Yaojia, WANG Hao, SONG Haoqiang, et al. Design strategies towards transition metal single atom catalysts for the oxygen reduction reaction—A review[J]. Nano Research Energy, 2023, 2: e9120082. |
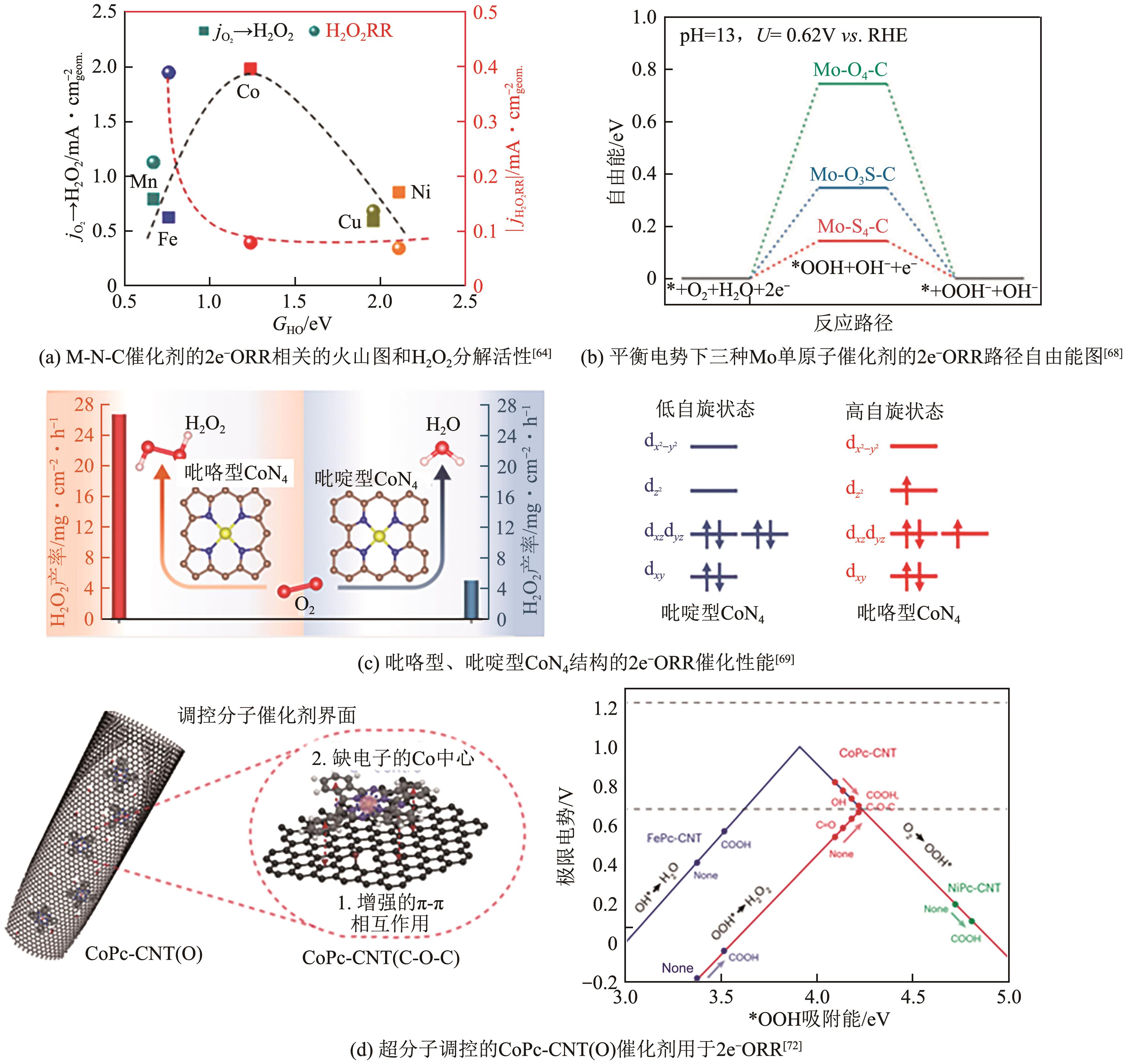
| 64 | SUN Yanyan, SILVIOLI Luca, SAHRAIE Nastaran Ranjbar, et al. Activity-selectivity trends in the electrochemical production of hydrogen peroxide over single-site metal-nitrogen-carbon catalysts[J]. Journal of the American Chemical Society, 2019, 141(31): 12372-12381. |
| 65 | ZHAO Xuan, YIN Qi, MAO Xinnan, et al. Theory-guided design of hydrogen-bonded cobaltoporphyrin frameworks for highly selective electrochemical H2O2 production in acid[J]. Nature Communications, 2022, 13(1): 2721. |
| 66 | SMITH Peter T, KIM Younghoon, BENKE Bahiru Punja, et al. Supramolecular tuning enables selective oxygen reduction catalyzed by cobalt porphyrins for direct electrosynthesis of hydrogen peroxide[J]. Angewandte Chemie International Edition, 2020, 59(12): 4902-4907. |
| 67 | LIU Wei, ZHANG Chang, ZHANG Jingjing, et al. Tuning the atomic configuration of Co-N-C electrocatalyst enables highly-selective H2O2 production in acidic media[J]. Applied Catalysis B: Environmental, 2022, 310: 121312. |
| 68 | LEE Byoung-Hoon, SHIN Heejong, RASOULI Armin Sedighian, et al. Supramolecular tuning of supported metal phthalocyanine catalysts for hydrogen peroxide electrosynthesis[J]. Nature Catalysis, 2023, 6: 234-243. |
| 69 | JIANG Kun, BACK Seoin, AKEY Austin J, et al. Highly selective oxygen reduction to hydrogen peroxide on transition metal single atom coordination[J]. Nature Communications, 2019, 10(1): 3997. |
| 70 | TANG Cheng, JIAO Yan, SHI Bingyang, et al. Coordination tunes selectivity: Two-electron oxygen reduction on high-loading molybdenum single-atom catalysts[J]. Angewandte Chemie (International Ed in English), 2020, 59(23): 9171-9176. |
| 71 | WANG Yulin, SHI Run, SHANG Lu, et al. High-efficiency oxygen reduction to hydrogen peroxide catalyzed by nickel single-atom catalysts with tetradentate N2 O2 coordination in a three-phase flow cell[J]. Angewandte Chemie (International Ed in English), 2020, 59(31): 13057-13062. |
| 72 | CHEN Shanyong, LUO Tao, LI Xiaoqing, et al. Identification of the highly active co-N4 coordination motif for selective oxygen reduction to hydrogen peroxide[J]. Journal of the American Chemical Society, 2022, 144(32): 14505-14516. |
| 73 | XIAO Chuqian, CHENG Ling, ZHU Yihua, et al. Super-coordinated nickel N4Ni1O2 site single-atom catalyst for selective H2O2 electrosynthesis at high current densities[J]. Angewandte Chemie International Edition, 2022, 61(38): 2206544. |
| 74 | CAO Peike, QUAN Xie, NIE Xiaowa, et al. Metal single-site catalyst design for electrocatalytic production of hydrogen peroxide at industrial-relevant currents[J]. Nature Communications, 2023, 14(1): 172. |
| 75 | FAN Wenjun, DUAN Zhiyao, LIU Wei, et al. Rational design of heterogenized molecular phthalocyanine hybrid single-atom electrocatalyst towards two-electron oxygen reduction[J]. Nature Communications, 2023, 14(1): 1426. |
| 76 | ZHANG Qingran, CHEN Yinguang, PAN Jian, et al. Electrosynthesis of hydrogen peroxide through selective oxygen reduction: A carbon innovation from active site engineering to device design[J]. Small, 2023, 19(40): e2302338. |
| 77 | WANG Jian, KIM Juwon, CHOI Subin, et al. A review of carbon-supported nonprecious metals as energy-related electrocatalysts[J]. Small Methods, 2020, 4(10): 2000621. |
| 78 | LIU Meihuan, SU Hui, CHENG Weiren, et al. Synergetic dual-ion centers boosting metal organic framework alloy catalysts toward efficient two electron oxygen reduction[J]. Small, 2022, 18(27): e2202248. |
| 79 | GAO Chen, MU Shengdong, YAN Rui, et al. Recent advances in ZIF-derived atomic metal-N-C electrocatalysts for oxygen reduction reaction: Synthetic strategies, active centers, and stabilities[J]. Small, 2022, 18(14): e2105409. |
| 80 | WANG Mengjun, ZHANG Nan, FENG Yonggang, et al. Partially pyrolyzed binary metal-organic framework nanosheets for efficient electrochemical hydrogen peroxide synthesis[J]. Angewandte Chemie International Edition, 2020, 59(34): 14373-14377. |
| 81 | WANG Yan, ZHOU Yitong, FENG Yi, et al. Synergistic electronic and pore structure modulation in open carbon nanocages enabling efficient electrocatalytic production of H2O2 in acidic medium[J]. Advanced Functional Materials, 2022, 32(17): 2110734. |
| 82 | LI Ruilong, YANG Shaokang, ZHANG Yida, et al. Short-range order in amorphous nickel oxide nanosheets enables selective and efficient electrochemical hydrogen peroxide production[J]. Cell Reports Physical Science, 2022, 3: 100788. |
| 83 | WU Zekun, WANG Tianzuo, ZOU Jijun, et al. Amorphous nickel oxides supported on carbon nanosheets as high-performance catalysts for electrochemical synthesis of hydrogen peroxide[J]. ACS Catalysis, 2022, 12(10): 5911-5920. |
| [1] | 张巍, 宋权斌, 周运河, 董梦瑶, 李婕, 伍乔, 付业昊, 梁垚城, 尹艳山, 成珊, 宋健. 全钒液流电池离子导电膜的选择性[J]. 化工进展, 2024, 43(9): 4859-4870. |
| [2] | 吴泽亮, 管琦卉, 陈世霞, 王珺. 炔烃选择性加氢制烯烃反应的研究进展[J]. 化工进展, 2024, 43(8): 4366-4381. |
| [3] | 卞维柏, 张睿轩, 潘建明. 无机金属锂离子筛材料制备方法研究进展[J]. 化工进展, 2024, 43(8): 4173-4186. |
| [4] | 石家汀, 王辉, 蒲凯凯, 赵婷, 聂丽君, 郑娜, 高宇航, 薛坤坤, 石建惠. 具有碳空位超薄g-C3N4纳米片可见光下产过氧化氢[J]. 化工进展, 2024, 43(7): 4148-4154. |
| [5] | 马文君, 张旭, 刘孟顺, 梁志远. 新兴湿法退役锂电池正极材料回收技术研究进展[J]. 化工进展, 2024, 43(4): 2077-2090. |
| [6] | 李开瑞, 高照华, 刘甜甜, 李静, 魏海生. 还原温度调变Rh/FePO4催化剂喹啉选择加氢性能[J]. 化工进展, 2024, 43(3): 1342-1349. |
| [7] | 闫守成, 张慧华, 徐倩倩, 王煜坤. 石墨烯复合载体催化剂在柴油车尾气NO脱除中的应用[J]. 化工进展, 2024, 43(3): 1456-1465. |
| [8] | 容凡丁, 丁泽相, 曹义风, 陈俐吭, 杨柳, 申福星, 杨启炜, 鲍宗必. 离子液体强化不饱和键差异化合物分离的研究进展[J]. 化工进展, 2024, 43(1): 198-214. |
| [9] | 钟丁磊, 黄铎, 应翔, 邱守添, 汪勇. 熔纺-选择性溶胀制备嵌段共聚物多通道中空纤维膜[J]. 化工进展, 2024, 43(1): 269-278. |
| [10] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [11] | 李化全, 王明华, 邱贵宝. 硫酸酸解钙钛矿相精矿的行为[J]. 化工进展, 2023, 42(S1): 536-541. |
| [12] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [13] | 杨建平. 降低HPPO装置反应系统原料消耗的PSE[J]. 化工进展, 2023, 42(S1): 21-32. |
| [14] | 王晋刚, 张剑波, 唐雪娇, 刘金鹏, 鞠美庭. 机动车尾气脱硝催化剂Cu-SSZ-13的改性研究进展[J]. 化工进展, 2023, 42(9): 4636-4648. |
| [15] | 朱传强, 茹晋波, 孙亭亭, 谢兴旺, 李长明, 高士秋. 固体高分子脱硝剂选择性非催化还原NO x 特性[J]. 化工进展, 2023, 42(9): 4939-4946. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||