化工进展 ›› 2024, Vol. 43 ›› Issue (9): 4859-4870.DOI: 10.16085/j.issn.1000-6613.2023-1434
• 能源加工与技术 • 上一篇
全钒液流电池离子导电膜的选择性
张巍1,2( ), 宋权斌1,2(
), 宋权斌1,2( ), 周运河1,2, 董梦瑶1,2, 李婕1,2, 伍乔1,2, 付业昊1,2, 梁垚城1,2, 尹艳山1,2, 成珊1,2, 宋健3
), 周运河1,2, 董梦瑶1,2, 李婕1,2, 伍乔1,2, 付业昊1,2, 梁垚城1,2, 尹艳山1,2, 成珊1,2, 宋健3
- 1.长沙理工大学能源与动力工程学院,湖南 长沙 410114
2.可再生能源电力技术湖南省重点实验室,湖南 长沙 410114
3.湖南大唐节能有限公司,湖南 长沙 410021
-
收稿日期:2023-08-16修回日期:2023-11-20出版日期:2024-09-15发布日期:2024-09-30 -
通讯作者:张巍,宋权斌 -
作者简介:张巍(1974—),男,博士,副教授,硕士生导师,研究方向为能源高效清洁利用。E-mail:weizhang@csust.edu.cn
宋权斌(1972—),男,博士,副教授,硕士生导师,研究方向为高效清洁燃烧。E-mail:sqb@csust.edu.cn。 -
基金资助:国家自然科学基金(52006016);湖南省自然科学基金(2023JJ30047);湖南省教育厅科学研究项目重点项目(21A0216);湖南省教育厅优秀青年项目(20B041);长沙理工大学青年教师成长计划(2019QJCZ044)
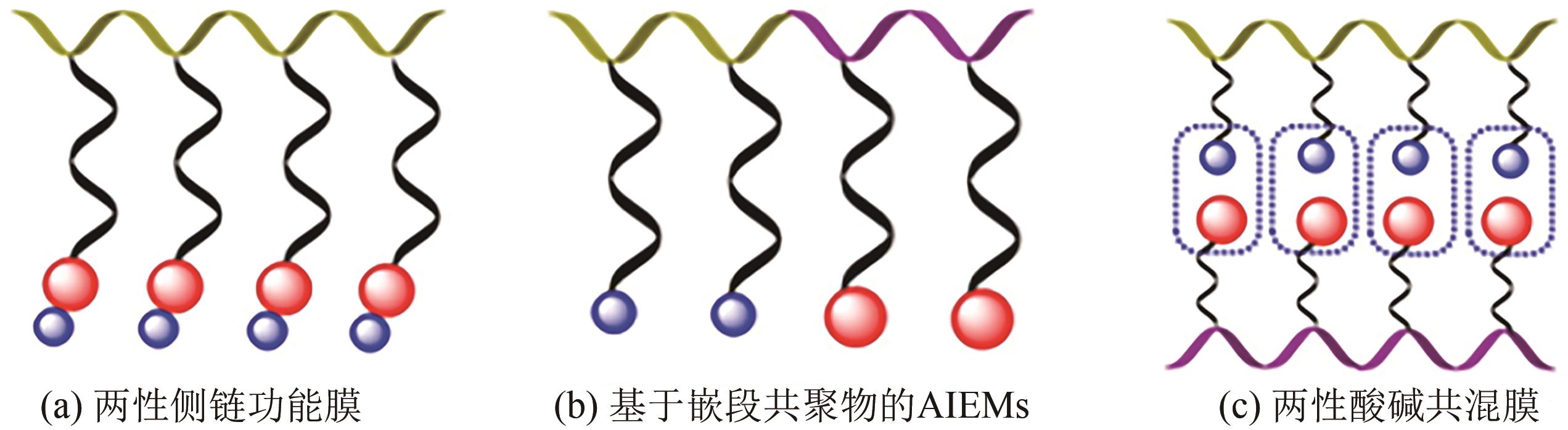
Selectivity of ion conductive membranes in all-vanadium flow battery
ZHANG Wei1,2( ), SONG Quanbin1,2(
), SONG Quanbin1,2( ), ZHOU Yunhe1,2, DONG Mengyao1,2, LI Jie1,2, WU Qiao1,2, FU Yehao1,2, LIANG Yaocheng1,2, YIN Yanshan1,2, CHENG Shan1,2, SONG Jian3
), ZHOU Yunhe1,2, DONG Mengyao1,2, LI Jie1,2, WU Qiao1,2, FU Yehao1,2, LIANG Yaocheng1,2, YIN Yanshan1,2, CHENG Shan1,2, SONG Jian3
- 1.College of Energy and Power Engineering, Changsha University of Science and Technology, Changsha 410114, Hunan, China
2.Key Laboratory of Renewable Energy and Electric Power Technology of Hunan Province, Changsha 410114, Hunan, China
3.Hunan Datang Energy Saving Company Limited, Changsha 410021, Hunan, China.
-
Received:2023-08-16Revised:2023-11-20Online:2024-09-15Published:2024-09-30 -
Contact:ZHANG Wei, SONG Quanbin
摘要:
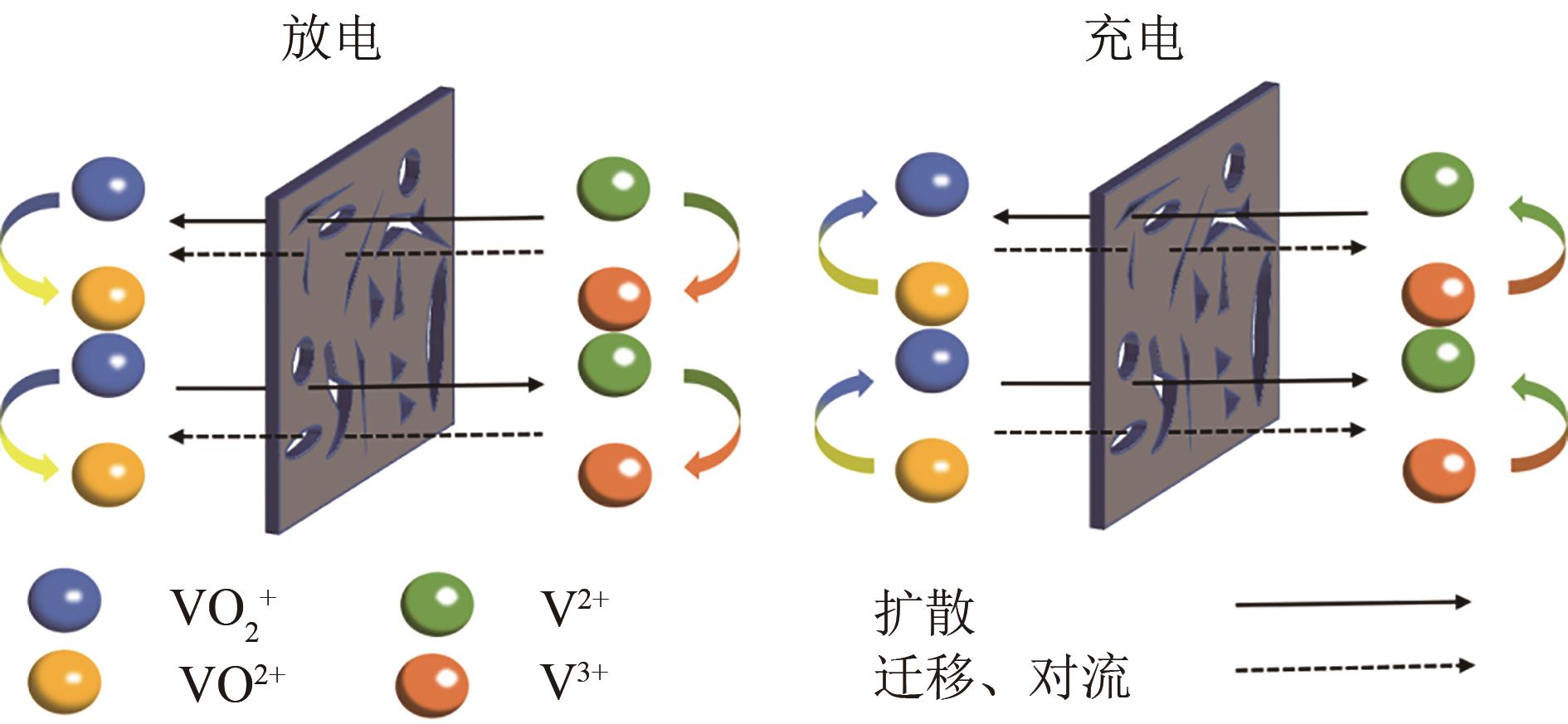
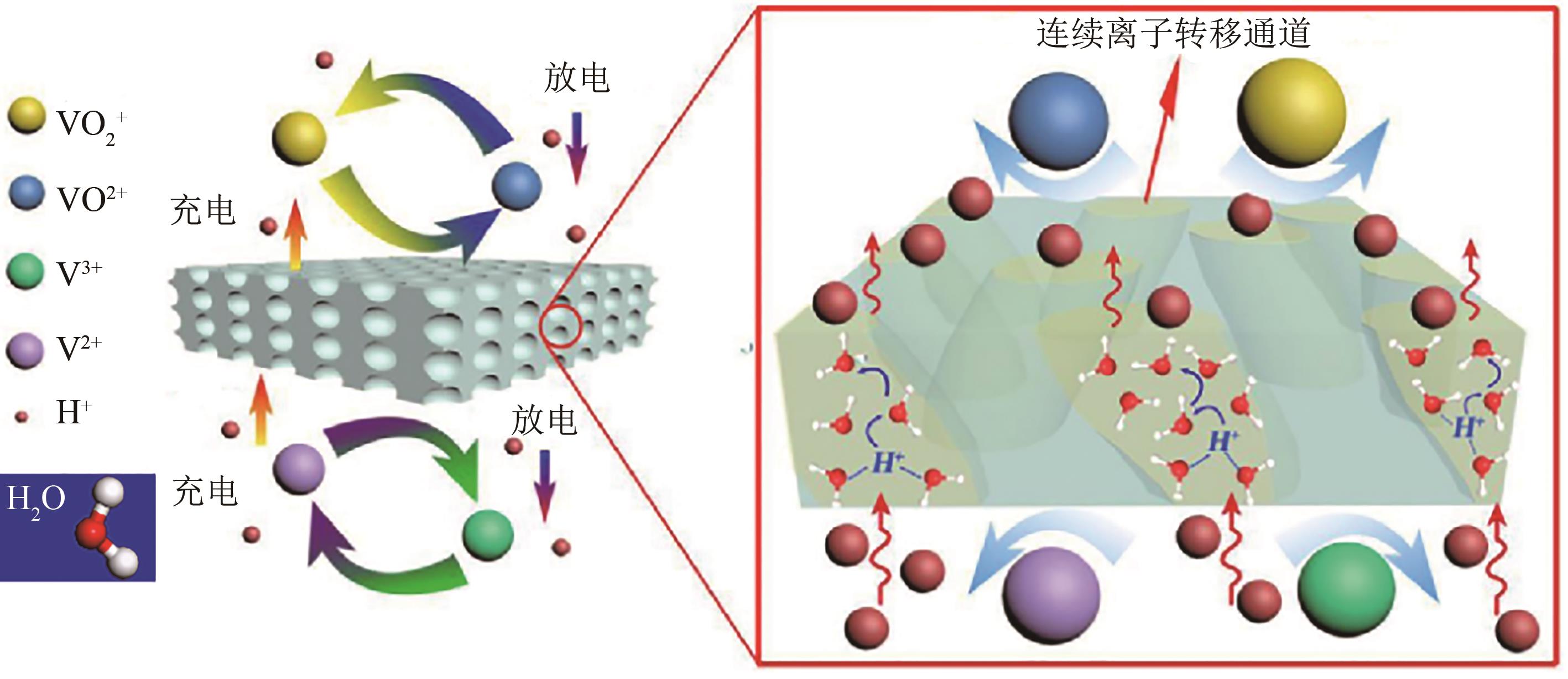
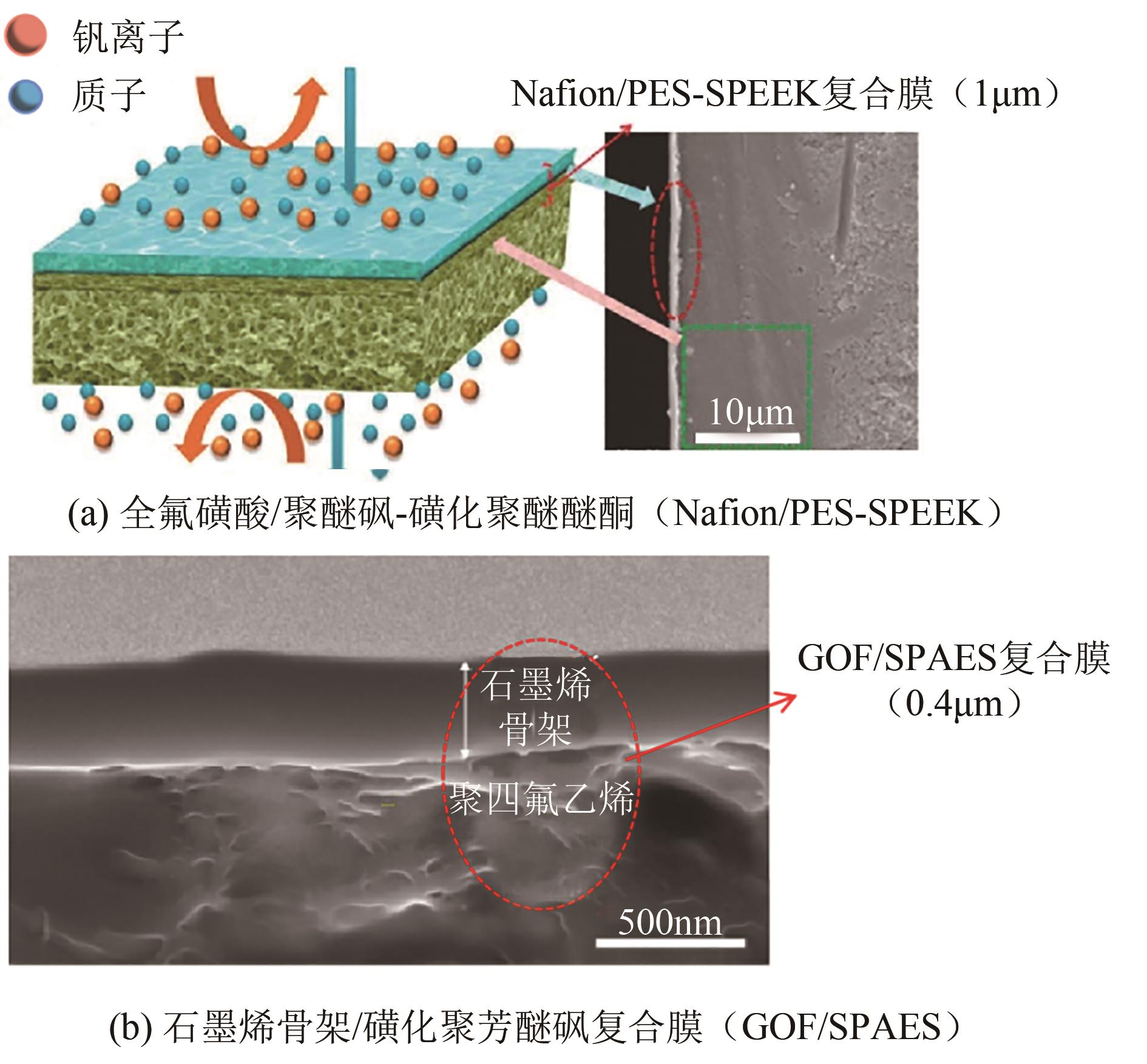
全钒液流电池(VFB)具有功率大、容量大、效率高、安全性能高的特点,近年来在储能应用方面受到广泛关注。离子导电膜作为VFB的关键组件,存在严重的钒离子交叉污染问题,易造成电池容量损失,降低电池使用寿命,因此深入了解VFB离子导电膜的选择性和质子传导对电池性能具有重要意义。本文综述了VFB中阳离子交换膜、阴离子交换膜、两性离子交换膜以及多孔膜等的研究进展,分析了离子导电膜上钒离子的渗透和质子的传输,重点总结了离子导电膜的改性、超薄复合膜设计、膜内微观结构优化及离子基团功能化对提高离子导电膜选择性和电导率的影响,较为全面地阐述了当前VFB离子导电膜选择性和电导率之间的平衡问题,为开发高性能、低成本、长寿命的离子导电膜,促进其商业化发展提供了参考依据,并展望了基于质子传导机制的氢键网络结构、多孔导电膜、低成本的超薄复合膜以及利用分子动力学模拟离子跨膜来提高VFB离子导电膜选择性的研究方向。
中图分类号:
引用本文
张巍, 宋权斌, 周运河, 董梦瑶, 李婕, 伍乔, 付业昊, 梁垚城, 尹艳山, 成珊, 宋健. 全钒液流电池离子导电膜的选择性[J]. 化工进展, 2024, 43(9): 4859-4870.
ZHANG Wei, SONG Quanbin, ZHOU Yunhe, DONG Mengyao, LI Jie, WU Qiao, FU Yehao, LIANG Yaocheng, YIN Yanshan, CHENG Shan, SONG Jian. Selectivity of ion conductive membranes in all-vanadium flow battery[J]. Chemical Industry and Engineering Progress, 2024, 43(9): 4859-4870.
| 膜类型 | 电池性能 | |||||
|---|---|---|---|---|---|---|
| 库仑效率/% | 电压效率/% | 能量效率/% | 电流密度/mA·cm-2 | 循环次数 | 容量保持率/% | |
| 阳离子交换膜 | ||||||
| Nafion 117[ | 93 | 85 | 79 | 80 | 200 | 50 |
| Nafion/SiO2[ | >N117 | >N117 | 79.9 | 20 | 100 | — |
| Nafion/PBI[ | 97.72 | 83 | 81.31 | 200 | 300 | 73.25 |
| 阴离子交换膜 | ||||||
| P-TPN1[ | 99.9 | 80.2 | 80.1 | 80 | 150 | 70 |
| PSf-MIm[ | 90.7 | — | 82.4 | 120 | 4800 | 65.9 |
| PTP-CHPTMA[ | 99 | 91.3 | 90 | 60 | 580 | 38.1 |
| PBI-BPTMA[ | 99 | 83 | 82.7 | 80 | 200 | 55.9 |
| 两性离子交换膜 | ||||||
| N/Ng-PSBMA-20%[ | 91 | 93 | 84.8 | 60 | 100 | 95 |
| 双功能侧链[ | 96 | 92 | 80.11 | 80 | 50 | 81.97 |
| PPO-TTA[ | 94.3 | 95 | 90.3 | 80 | 50 | 48 |
| 60SPAEK-6F-co-10%BI-cld[ | 99.1 | 89.8 | 89 | 30 | 220 | — |
| S/APP-5%[ | 96.4 | 86.1 | 83 | 60 | 50 | 47 |
| 多孔导电膜 | ||||||
| PES/SPEEK[ | 98 | 92 | 90 | 80 | >500 | — |
| SPC-40[ | 92 | 87 | 93.5 | 80 | 300 | 39.9 |
| 多孔PBI[ | 99.29 | 83 | 81.93 | 200 | — | — |
| CMPSF/TMA[ | >99 | >80 | >80 | 80 | >1500 | >N115 |
| PVDF-HFP/PVP[ | 98 | 89 | 88 | 80 | 160 | >N115 |
表 1 不同离子导电膜电池性能比较
| 膜类型 | 电池性能 | |||||
|---|---|---|---|---|---|---|
| 库仑效率/% | 电压效率/% | 能量效率/% | 电流密度/mA·cm-2 | 循环次数 | 容量保持率/% | |
| 阳离子交换膜 | ||||||
| Nafion 117[ | 93 | 85 | 79 | 80 | 200 | 50 |
| Nafion/SiO2[ | >N117 | >N117 | 79.9 | 20 | 100 | — |
| Nafion/PBI[ | 97.72 | 83 | 81.31 | 200 | 300 | 73.25 |
| 阴离子交换膜 | ||||||
| P-TPN1[ | 99.9 | 80.2 | 80.1 | 80 | 150 | 70 |
| PSf-MIm[ | 90.7 | — | 82.4 | 120 | 4800 | 65.9 |
| PTP-CHPTMA[ | 99 | 91.3 | 90 | 60 | 580 | 38.1 |
| PBI-BPTMA[ | 99 | 83 | 82.7 | 80 | 200 | 55.9 |
| 两性离子交换膜 | ||||||
| N/Ng-PSBMA-20%[ | 91 | 93 | 84.8 | 60 | 100 | 95 |
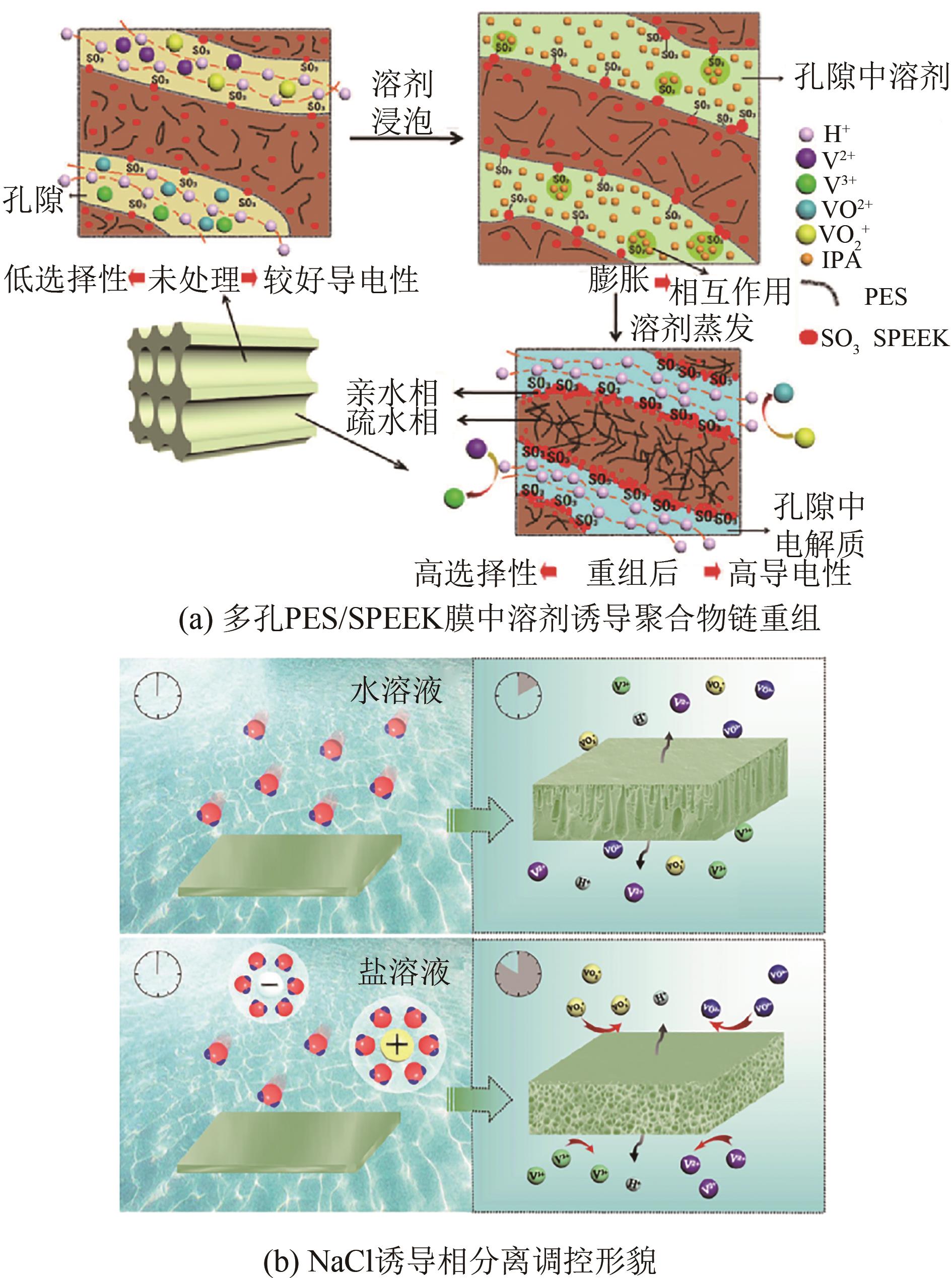
| 双功能侧链[ | 96 | 92 | 80.11 | 80 | 50 | 81.97 |
| PPO-TTA[ | 94.3 | 95 | 90.3 | 80 | 50 | 48 |
| 60SPAEK-6F-co-10%BI-cld[ | 99.1 | 89.8 | 89 | 30 | 220 | — |
| S/APP-5%[ | 96.4 | 86.1 | 83 | 60 | 50 | 47 |
| 多孔导电膜 | ||||||
| PES/SPEEK[ | 98 | 92 | 90 | 80 | >500 | — |
| SPC-40[ | 92 | 87 | 93.5 | 80 | 300 | 39.9 |
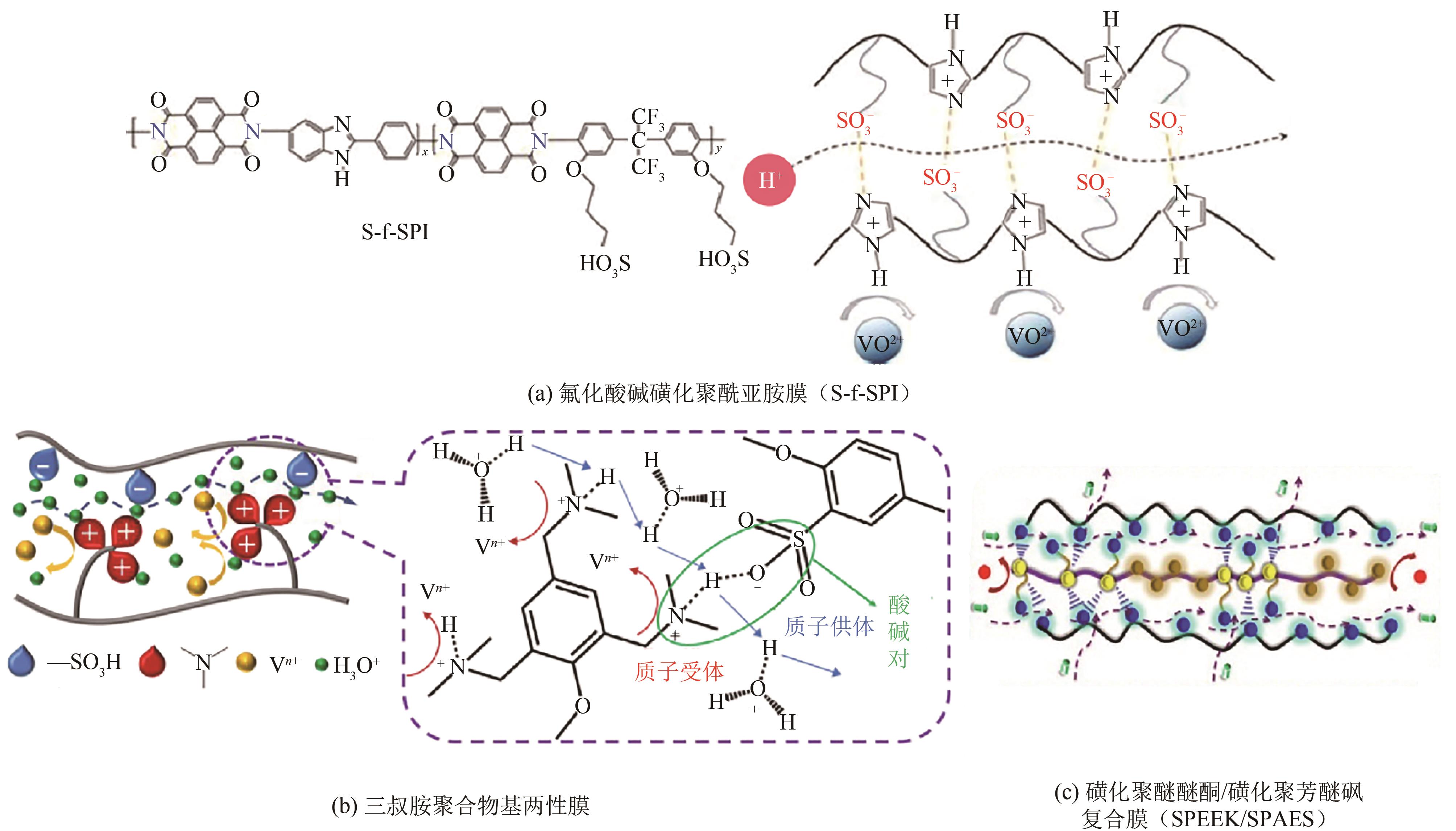
| 多孔PBI[ | 99.29 | 83 | 81.93 | 200 | — | — |
| CMPSF/TMA[ | >99 | >80 | >80 | 80 | >1500 | >N115 |
| PVDF-HFP/PVP[ | 98 | 89 | 88 | 80 | 160 | >N115 |
| 33 | DAI Jicui, ZHANG Hongqiang, SUI Zhaobin, et al. Study on Nafion/Nafion-g-poly(sulfobetaine methacrylate)-blended amphoteric membranes for vanadium redox flow battery[J]. Ionics, 2020, 26(2): 801-811. |
| 34 | LIU Lei, WANG Chao, HE Zhenfeng, et al. Bi-functional side chain architecture tuned amphoteric ion exchange membranes for high-performance vanadium redox flow batteries[J]. Journal of Membrane Science, 2021, 624: 119118. |
| 35 | ZHANG Huaqing, YAN Xiaoming, GAO Li, et al. Novel triple tertiary amine polymer-based hydrogen bond network inducing highly efficient proton-conducting channels of amphoteric membranes for high-performance vanadium redox flow battery[J]. ACS Applied Materials & Interfaces, 2019, 11(5): 5003-5014. |
| 36 | LIAO J B, LU M Z, CHU Y Q, et al. Ultra-low vanadium ion diffusion amphoteric ion-exchange membranes for all-vanadium redox flow batteries[J]. Journal of Power Sources, 2015, 282: 241-247. |
| 37 | WANG Gang, ZHANG Miaomiao, HE Zhenhua, et al. Novel amphoteric ion exchange membranes by blending sulfonated poly(ether ether ketone) with ammonium polyphosphate for vanadium redox flow battery applications[J]. Journal of Applied Polymer Science, 2021, 138(25): e50592. |
| 38 | LU Wenjing, YUAN Zhizhang, ZHAO Yuyue, et al. High-performance porous uncharged membranes for vanadium flow battery applications created by tuning cohesive and swelling forces[J]. Energy & Environmental Science, 2016, 9(7): 2319-2325. |
| 39 | JEON Choongseop, CHOI Chanyong, KIM Hee-Tak, et al. Achieving fast proton transport and high vanadium ion rejection with uniformly mesoporous composite membranes for high-efficiency vanadium redox flow batteries[J]. ACS Applied Energy Materials, 2020, 3(6): 5874-5881. |
| 40 | LU Wenjing, YUAN Zhizhang, ZHAO Yuyue, et al. Advanced porous PBI membranes with tunable performance induced by the polymer-solvent interaction for flow battery application[J]. Energy Storage Materials, 2018, 10: 40-47. |
| 41 | ZHAO Yuyue, ZHANG Huamin, XIAO Chuanhai, et al. Highly selective charged porous membranes with improved ion conductivity[J]. Nano Energy, 2018, 48: 353-360. |
| 42 | LU Wenjing, SHI Dingqin, ZHANG Huamin, et al. Highly selective core-shell structural membrane with cage-shaped pores for flow battery[J]. Energy Storage Materials, 2019, 17: 325-333. |
| 43 | KUSHNER Douglas I, CROTHERS Andrew R, KUSOGLU Ahmet, et al. Transport phenomena in flow battery ion-conducting membranes[J]. Current Opinion in Electrochemistry, 2020, 21: 132-139. |
| 44 | CROTHERS Andrew R, DARLING Robert M, KUSHNER Douglas I, et al. Theory of multicomponent phenomena in cation-exchange membranes: Part Ⅲ. transport in vanadium redox-flow-battery separators[J]. Journal of the Electrochemical Society, 2020, 167(1): 013549. |
| 45 | SUN Chenxi, CHEN Jian, ZHANG Huamin, et al. Investigations on transfer of water and vanadium ions across Nafion membrane in an operating vanadium redox flow battery[J]. Journal of Power Sources, 2010, 195(3): 890-897. |
| 46 | CHOI Chanyong, KIM Soohyun, KIM Riyul, et al. A review of vanadium electrolytes for vanadium redox flow batteries[J]. Renewable and Sustainable Energy Reviews, 2017, 69: 263-274. |
| 47 | LEMMERMANN Torben, BECKER Maik, STEHLE Maria, et al. In situ and in operando detection of redox reactions with integrated potential probes during vanadium transport in ion exchange membranes[J]. Journal of Power Sources, 2022, 533: 231343. |
| 48 | DARLING Robert M, WEBER Adam Z, TUCKER Michael C, et al. The influence of electric field on crossover in redox-flow batteries[J]. Journal of the Electrochemical Society, 2015, 163(1): A5014-A5022. |
| 49 | LUO Qingtao, LI Liyu, NIE Zimin, et al. In-situ investigation of vanadium ion transport in redox flow battery[J]. Journal of Power Sources, 2012, 218: 15-20. |
| 50 | 孙炼, 王洪磊, 余金山, 等. 金属有机框架质子导体及其质子交换膜的研究进展[J]. 化学学报, 2020, 78(9): 888-900. |
| SUN Lian, WANG Honglei, YU Jinshan, et al. Recent progress on proton-conductive metal-organic frameworks and their proton exchange membranes[J]. Acta Chimica Sinica, 2020, 78(9): 888-900. | |
| 51 | XIN Li, ZHANG Dezhu, QU Kai, et al. Zr-MOF-enabled controllable ion sieving and proton conductivity in flow battery membrane[J]. Advanced Functional Materials, 2021, 31(42): 2104629. |
| 52 | TAN Qinglong, LU Shanfu, SI Jiangju, et al. A bunch-like tertiary amine grafted polysulfone membrane for VRFBs with simultaneously high proton conductivity and low vanadium ion permeability[J]. Macromolecular Rapid Communications, 2017, 38(8): 1600710. |
| 53 | LONG Jun, XU Wenjie, XU Shoubin, et al. A novel double branched sulfonated polyimide membrane with ultra-high proton selectivity for vanadium redox flow battery[J]. Journal of Membrane Science, 2021, 628: 119259. |
| 54 | SUN Chuanyu, NEGRO Enrico, Keti VEZZÙ, et al. Hybrid inorganic-organic proton-conducting membranes based on SPEEK doped with WO3 nanoparticles for application in vanadium redox flow batteries[J]. Electrochimica Acta, 2019, 309: 311-325. |
| 55 | ZHANG Yue, ZHOU Xinjie, XUE Rui, et al. Proton exchange membranes with ultra-low vanadium ions permeability improved by sulfated zirconia for all vanadium redox flow battery[J]. International Journal of Hydrogen Energy, 2019, 44(12): 5997-6006. |
| 1 | PONCE DE LEÓN C, FRÍAS-FERRER A, GONZÁLEZ-GARCÍA J, et al. Redox flow cells for energy conversion[J]. Journal of Power Sources, 2006, 160(1): 716-732. |
| 2 | 李彦, 徐铜文. 全钒液流电池用离子交换膜的研究进展[J]. 化工学报, 2015, 66(9): 3296-3304. |
| LI Yan, XU Tongwen. Development of ion exchange membrane for all-vanadium redox flow battery[J]. CIESC Journal, 2015, 66(9): 3296-3304. | |
| 3 | 王保国. 电化学能源转化膜与膜过程研究进展[J]. 膜科学与技术, 2020, 40(1): 179-187. |
| WANG Baoguo. A review on membranes and membrane processes for electrochemical energy conversion applications[J]. Membrane Science and Technology, 2020, 40(1): 179-187. | |
| 4 | DAI Qing, LIU Zhiqiang, HUANG Ling, et al. Thin-film composite membrane breaking the trade-off between conductivity and selectivity for a flow battery[J]. Nature Communications, 2020, 11(1): 13. |
| 5 | 王斐然, 蒋峰景. 全钒液流电池离子导电膜[J]. 化学进展, 2021, 33(3): 462-470. |
| WANG Feiran, JIANG Fengjing. Ion-vonducting membrane for vanadium redox flow batteries[J]. Progress in Chemistry, 2021, 33(3): 462-470. | |
| 6 | KOH P W, YULIATI L, LEE S L. Effect of transition metal oxide doping (Cr, Co, V) in the photocatalytic activity of TiO2 for congo red degradation under visible light[J]. Jurnal Teknologi, 2014, 69(5): 45-50. |
| 7 | Dennis DÜERKOP, WIDDECKE Hartmut, SCHILDE Carsten, et al. Polymer membranes for all-vanadium redox flow batteries: A review[J]. Membranes, 2021, 11(3): 214. |
| 8 | Zhensheng MAI, ZHANG Huamin, LI Xianfeng, et al. Nafion/polyvinylidene fluoride blend membranes with improved ion selectivity for vanadium redox flow battery application[J]. Journal of Power Sources, 2011, 196(13): 5737-5741. |
| 9 | ZHANG Daishuang, WANG Qian, PENG Sangshan, et al. An interface-strengthened cross-linked graphene oxide/Nafion212 composite membrane for vanadium flow batteries[J]. Journal of Membrane Science, 2019, 587: 117189. |
| 56 | DAI Wenjing, SHEN Yi, LI Zhaohua, et al. SPEEK/Graphene oxide nanocomposite membranes with superior cyclability for highly efficient vanadium redox flow battery[J]. Journal of Materials Chemistry A, 2014, 2(31): 12423-12432. |
| 57 | KIM Jihoon, LEE Yongkyu, JEON Jae-Deok, et al. Ion-exchange composite membranes pore-filled with sulfonated poly(ether ether ketone) and Engelhard titanosilicate-10 for improved performance of vanadium redox flow batteries[J]. Journal of Power Sources, 2018, 383: 1-9. |
| 58 | Yeonho AHN, KIM Dukjoon. Ultra-low vanadium ion permeable electrolyte membrane for vanadium redox flow battery by pore filling of PTFE substrate[J]. Energy Storage Materials, 2020, 31: 105-114. |
| 59 | LI Jinchao, LIU Jun, XU Wenjie, et al. A sulfonated polyimide/nafion blend membrane with high proton selectivity and remarkable stability for vanadium redox flow battery[J]. Membranes, 2021, 11(12): 946. |
| 60 | FU Zhimin, LIU Jinying, LIU Qifeng. SPEEK/PVDF/PES composite as alternative proton exchange membrane for vanadium redox flow batteries[J]. Journal of Electronic Materials, 2016, 45(1): 666-671. |
| 61 | WU Jine, DAI Qing, ZHANG Huamin, et al. Recent development in composite membranes for flow batteries[J]. ChemSusChem, 2020, 13(15): 3805-3819. |
| 62 | LI Yun, LI Xianfeng, CAO Jingyu, et al. Composite porous membranes with an ultrathin selective layer for vanadium flow batteries[J]. Chemical Communications, 2014, 50(35): 4596-4599. |
| 63 | PADDISON Stephen J, PAUL Reginald. The nature of proton transport in fully hydrated Nafion® [J]. Physical Chemistry Chemical Physics, 2002, 4(7): 1158-1163. |
| 64 | TUNG Siu On, FISHER Sydney L, KOTOV Nicholas A, et al. Nanoporous aramid nanofibre separators for nonaqueous redox flow batteries[J]. Nature Communications, 2018, 9: 4193. |
| 65 | SHIN Dong Won, GUIVER Michael D, LEE Young Moo. Hydrocarbon-based polymer electrolyte membranes: Importance of morphology on ion transport and membrane stability[J]. Chemical Reviews, 2017, 117(6): 4759-4805. |
| 66 | KIM Soohyun, CHOI Junghoon, CHOI Chanyong, et al. Pore-size-tuned graphene oxide frameworks as ion-selective and protective layers on hydrocarbon membranes for vanadium redox-flow batteries[J]. Nano Letters, 2018, 18(6): 3962-3968. |
| 67 | YUAN Zhizhang, ZHU Xiangxue, LI Mingrun, et al. A highly ion-selective zeolite flake layer on porous membranes for flow battery applications[J]. Angewandte Chemie, 2016, 55(9): 3058-3062. |
| 10 | LIU Jiaman, YU Liwei, CAI Xingke, et al. Sandwiching h-BN monolayer films between sulfonated poly(ether ether ketone) and nafion for proton exchange membranes with improved ion selectivity[J]. ACS Nano, 2019, 13(2): 2094-2102. |
| 11 | ZHANG Bengui, WANG Qi, GUAN Shanshan, et al. High performance membranes based on new 2-adamantane containing poly(aryl ether ketone) for vanadium redox flow battery applications[J]. Journal of Power Sources, 2018, 399: 18-25. |
| 12 | CHEN Dongyang, HICKNER Michael A, AGAR Ertan, et al. Selective anion exchange membranes for high coulombic efficiency vanadium redox flow batteries[J]. Electrochemistry Communications, 2013, 26: 37-40. |
| 13 | CHEN Dongyang, HICKNER Michael A. Degradation of imidazolium-and quaternary ammonium-functionalized poly(fluorenyl ether ketone sulfone) anion exchange membranes[J]. ACS Applied Materials & Interfaces, 2012, 4(11): 5775-5781. |
| 14 | SHUKLA Geetanjali, SHAHI Vinod K. Amine functionalized graphene oxide containing C16 chain grafted with poly(ether sulfone) by DABCO coupling: Anion exchange membrane for vanadium redox flow battery[J]. Journal of Membrane Science, 2019, 575: 109-117. |
| 15 | LIU Lei, WANG Chao, HE Zhenfeng, et al. An overview of amphoteric ion exchange membranes for vanadium redox flow batteries[J]. Journal of Materials Science & Technology, 2021, 69: 212-227. |
| 16 | NIBEL Olga, ROJEK Tomasz, SCHMIDT Thomas J, et al. Amphoteric ion-exchange membranes with significantly improved vanadium barrier properties for all-vanadium redox flow batteries[J]. ChemSusChem, 2017, 10(13): 2767-2777. |
| 17 | QIU Jingyi, ZHAI Maolin, CHEN Jinhua, et al. Performance of vanadium redox flow battery with a novel amphoteric ion exchange membrane synthesized by two-step grafting method[J]. Journal of Membrane Science, 2009, 342(1/2): 215-220. |
| 18 | LIU Shuai, WANG Lihua, DING Yue, et al. Novel sulfonated poly(ether ether keton)/polyetherimide acid-base blend membranes for vanadium redox flow battery applications[J]. Electrochimica Acta, 2014, 130: 90-96. |
| 19 | LI Zhaohua, DAI Wenjing, YU Lihong, et al. Sulfonated poly(ether ether ketone)/mesoporous silica hybrid membrane for high performance vanadium redox flow battery[J]. Journal of Power Sources, 2014, 257: 221-229. |
| 20 | ZHOU X L, ZHAO T S, AN L, et al. Modeling of ion transport through a porous separator in vanadium redox flow batteries[J]. Journal of Power Sources, 2016, 327: 67-76. |
| 21 | ZHANG Hongzhang, ZHANG Huamin, LI Xianfeng, et al. Nanofiltration (NF) membranes: The next generation separators for all vanadium redox flow batteries (VRBs)?[J]. Energy & Environmental Science, 2011, 4(5): 1676-1679. |
| 68 | VIJAYAKUMAR M, LUO Qingtao, LLOYD Ralph, et al. Tuning the perfluorosulfonic acid membrane morphology for vanadium redox-flow batteries[J]. ACS Applied Materials & Interfaces, 2016, 8(50): 34327-34334. |
| 69 | MA Yanjiao, LI Lv, MA Lingling, et al. Cyclodextrin templated nanoporous anion exchange membrane for vanadium flow battery application[J]. Journal of Membrane Science, 2019, 586: 98-105. |
| 70 | LU Wenjing, YUAN Zhizhang, LI Mingrun, et al. Solvent-induced rearrangement of ion-transport channels: A way to create advanced porous membranes for vanadium flow batteries[J]. Advanced Functional Materials, 2017, 27(4): 1604587. |
| 71 | JIANG Fengjing, ZHANG Yue, WANG Feiran, et al. Finely controlled swelling: A shortcut to construct ion-selective channels in polymer membranes[J]. Polymer, 2021, 225: 123793. |
| 72 | QIAO Lin, ZHANG Huamin, LU Wenjing, et al. Advanced porous membranes with tunable morphology regulated by ionic strength of nonsolvent for flow battery[J]. ACS Applied Materials & Interfaces, 2019, 11(27): 24107-24113. |
| 73 | JIA Chao, LI Lei, LIU Ying, et al. Highly compressible and anisotropic lamellar ceramic sponges with superior thermal insulation and acoustic absorption performances[J]. Nature Communications, 2020, 11(1): 3732. |
| 74 | HERZ H G, KREUER K D, MAIER J, et al. New fully polymeric proton solvents with high proton mobility[J]. Electrochimica Acta, 2003, 48(14/15/16): 2165-2171. |
| 75 | WANG Jingtao, HE Yakun, ZHAO Liping, et al. Enhanced proton conductivities of nanofibrous composite membranes enabled by acid-base pairs under hydrated and anhydrous conditions[J]. Journal of Membrane Science, 2015, 482: 1-12. |
| 76 | SCHUSTER M, RAGER T, NODA A, et al. About the choice of the protogenic group in PEM separator materials for intermediate temperature, low humidity operation: A critical comparison of sulfonic acid, phosphonic acid and imidazole functionalized model compounds[J]. Fuel Cells, 2005, 5(3): 355-365. |
| 77 | HE Zhenhua, WANG Gang, WEI Shiguo, et al. A novel fluorinated acid-base sulfonated polyimide membrane with sulfoalkyl side-chain for vanadium redox flow battery[J]. Electrochimica Acta, 2021, 399: 139434. |
| 78 | QIAN Penghua, WANG Haixia, ZHANG Lei, et al. An enhanced stability and efficiency of SPEEK-based composite membrane influenced by amphoteric side-chain polymer for vanadium redox flow battery[J]. Journal of Membrane Science, 2022, 643: 120011. |
| 79 | PANG Bo, ZHANG Qi, YAN Xiaoming, et al. Superior acidic sulfate ester group based high conductive membrane for vanadium redox flow battery[J]. Journal of Power Sources, 2021, 506: 230203. |
| 22 | LI Yun, ZHANG Huamin, LI Xianfeng, et al. Porous poly(ether sulfone) membranes with tunable morphology: Fabrication and their application for vanadium flow battery[J]. Journal of Power Sources, 2013, 233: 202-208. |
| 23 | ZHANG Hongzhang, ZHANG Huamin, ZHANG Fengxiang, et al. Advanced charged membranes with highly symmetric spongy structures for vanadium flow battery application[J]. Energy & Environmental Science, 2013, 6(3): 776-781. |
| 24 | XU Wanxing, LI Xianfeng, CAO Jingyu, et al. Morphology and performance of poly(ether sulfone)/sulfonated poly(ether ether ketone) blend porous membranes for vanadium flow battery application[J]. RSC Advances, 2014, 4(76): 40400-40406. |
| 25 | DAI Jicui, DONG Yichao, GAO Peng, et al. A sandwiched bipolar membrane for all vanadium redox flow battery with high coulombic efficiency[J]. Polymer, 2018, 140: 233-239. |
| 26 | JIANG Bo, WU Lantao, YU Lihong, et al. A comparative study of Nafion series membranes for vanadium redox flow batteries[J]. Journal of Membrane Science, 2016, 510: 18-26. |
| 27 | XI Jingyu, WU Zenghua, QIU Xinping, et al. Nafion/SiO2 hybrid membrane for vanadium redox flow battery[J]. Journal of Power Sources, 2007, 166(2): 531-536. |
| 28 | ZHAO Yingying, ZHANG Denghua, ZHAO Lina, et al. Excellent ion selectivity of Nafion membrane modified by PBI via acid-base pair effect for vanadium flow battery[J]. Electrochimica Acta, 2021, 394: 139144. |
| 29 | CHEN Dongju, CHEN Xiaoli, DING Lifang, et al. Advanced acid-base blend ion exchange membranes with high performance for vanadium flow battery application[J]. Journal of Membrane Science, 2018, 553: 25-31. |
| 30 | XING Yi, GENG Kang, CHU Xiaomeng, et al. Chemically stable anion exchange membranes based on C2-protected imidazolium cations for vanadium flow battery[J]. Journal of Membrane Science, 2021, 618: 118696. |
| 31 | TANG Weiqin, MU Tong, CHE Xuefu, et al. Highly selective anion exchange membrane based on quaternized poly(triphenyl piperidine) for the vanadium redox flow battery[J]. ACS Sustainable Chemistry & Engineering, 2021, 9(42): 14297-14306. |
| 32 | TANG Weiqin, YANG Yunfei, LIU Xinli, et al. Long side-chain quaternary ammonium group functionalized polybenzimidazole based anion exchange membranes and their applications[J]. Electrochimica Acta, 2021, 391: 138919. |
| [1] | 王庆泰, 张赛, 王杰敏. 全钒液流电池多孔电极非均匀压缩的数值模拟[J]. 化工进展, 2024, 43(6): 2940-2949. |
| [2] | 林明杰, 李士洋, 马俊梅, 高从堦, 薛立新. 聚酰胺/醋酸纤维素复合正渗透膜的制备及相转化工艺参数的优化[J]. 化工进展, 2024, 43(3): 1418-1427. |
| [3] | 赵国珂, 张杨, 刘轶群. 膜法分离一/二价阳离子的研究进展[J]. 化工进展, 2024, 43(3): 1363-1373. |
| [4] | 冯江涵, 宋钫. 阴离子交换膜电解池的研究进展[J]. 化工进展, 2023, 42(7): 3501-3509. |
| [5] | 赵王瑞, 刘燕, 张伟, 邓会宁. Fe3+诱导聚多巴胺-聚乙烯亚胺电沉积制备单价选择性膜[J]. 化工进展, 2023, 42(3): 1508-1514. |
| [6] | 郭志鹏, 卜宪标, 李华山, 龚宇烈, 王令宝. 基于热-流-化耦合作用的单井增强地热系统性能分析[J]. 化工进展, 2023, 42(2): 711-721. |
| [7] | 蔡铭威, 王知, 卢小闯, 庄俊伟, 吴嘉豪, 张诗洋, 闵永刚. 聚酰亚胺薄膜在氢气分离中的研究进展[J]. 化工进展, 2023, 42(10): 5232-5248. |
| [8] | 张洪铭, 卢炯元, 王三反. 燃料电池用阴离子交换膜分子结构研究进展[J]. 化工进展, 2022, 41(S1): 318-330. |
| [9] | 黄明, 祖韵秋, 高亢, 韦韡, 张娜, 朱华平, 刘春太. 大丝束CF/EP汽车地板VARTM模拟与高温力学性能[J]. 化工进展, 2022, 41(5): 2546-2554. |
| [10] | 董林, 陈青柏, 王建友, 李鹏飞, 王进. 电渗析苦咸水淡化技术研究进展[J]. 化工进展, 2022, 41(4): 2102-2114. |
| [11] | 张群, 陈重军, 谢嘉玮, 邹馨怡. 高盐废水微生物脱盐池处理研究进展[J]. 化工进展, 2022, 41(2): 974-980. |
| [12] | 张传保, 王彦玲, 陈孟鑫, 梁诗南, 史文静. 耐高温胍胶压裂液及其对储层的伤害机理研究进展[J]. 化工进展, 2022, 41(11): 5912-5924. |
| [13] | 王锡民, 魏潇然, 冯瑛, 黄国勇, 王春霞. 碳基全固态离子选择性电极材料研究进展[J]. 化工进展, 2022, 41(10): 5456-5464. |
| [14] | 王敏键, 陈四国, 邵敏华, 魏子栋. 氢燃料电池电催化剂研究进展[J]. 化工进展, 2021, 40(9): 4948-4961. |
| [15] | 李旅, 巩守涛, 马艳娇, 张奎博, 张凤祥. 自由体积和微孔调控的阴离子交换膜制备及应用研究进展[J]. 化工进展, 2020, 39(6): 2105-2114. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||