化工进展 ›› 2023, Vol. 42 ›› Issue (S1): 94-103.DOI: 10.16085/j.issn.1000-6613.2023-0809
基于动力学分析的核桃壳最佳炭化工艺
- 西安交通大学热流科学与工程教育部重点实验室,陕西 西安 710049
-
收稿日期:2023-05-15修回日期:2023-06-21出版日期:2023-10-25发布日期:2023-11-30 -
通讯作者:王云刚 -
作者简介:刘阳(1995—),男,博士研究生,研究方向为生物质高效利用等。E-mail:707187439@qq.com。 -
基金资助:国家重点研发计划(2021YFC3001803);陕西省重点研发计划(2018ZDXM-SF-033);王宽诚基金会项目
Optimal carbonization process of walnut shell based on dynamic analysis
LIU Yang( ), WANG Yungang(
), WANG Yungang( ), XIU Haoran, ZOU Li, BAI Yanyuan
), XIU Haoran, ZOU Li, BAI Yanyuan
- Key Laboratory of Thermo-Fluid Science and Engineering (Ministry of Education), Xi'an Jiaotong University, Xi'an 710049, Shaanxi, China
-
Received:2023-05-15Revised:2023-06-21Online:2023-10-25Published:2023-11-30 -
Contact:WANG Yungang
摘要:
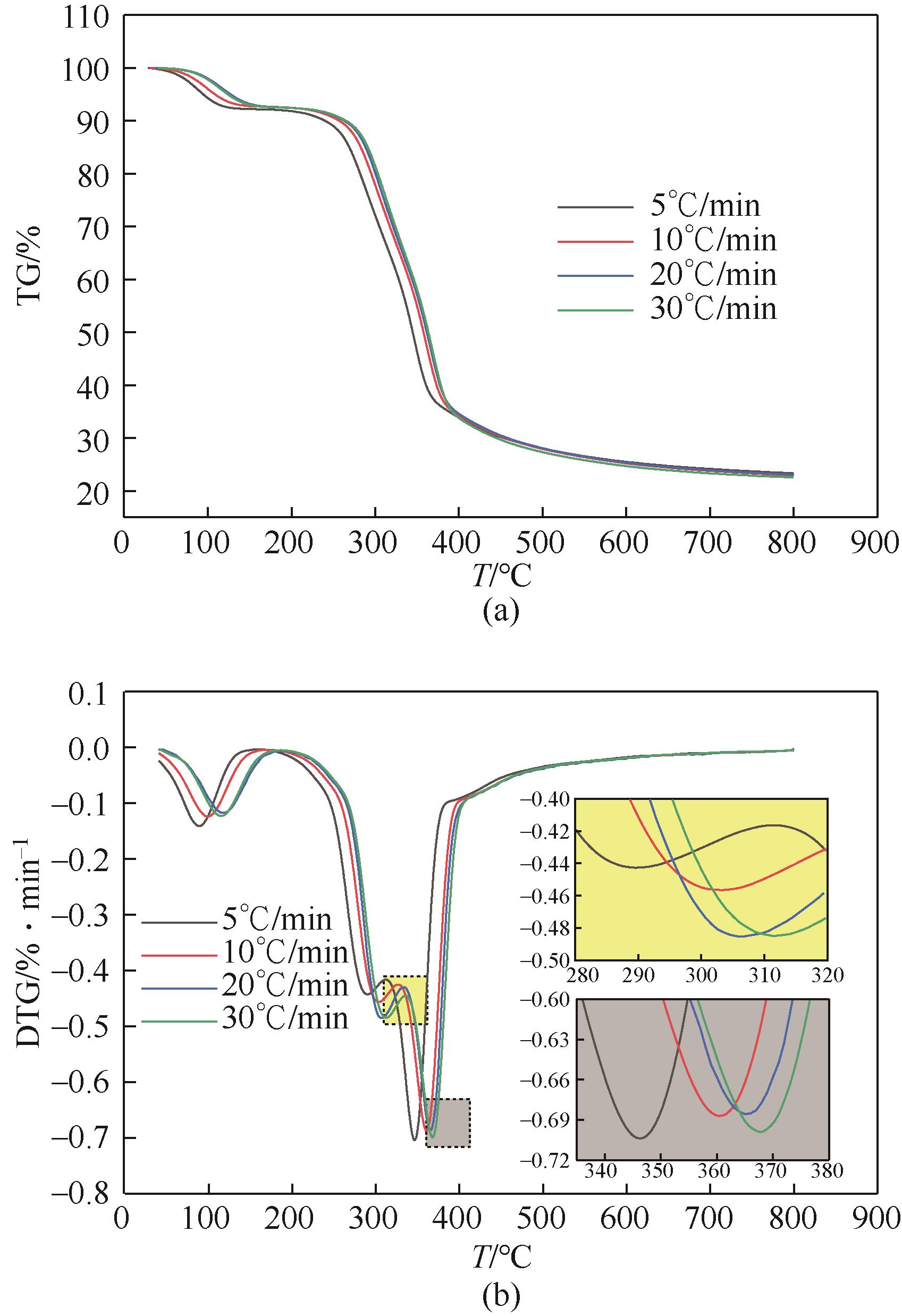
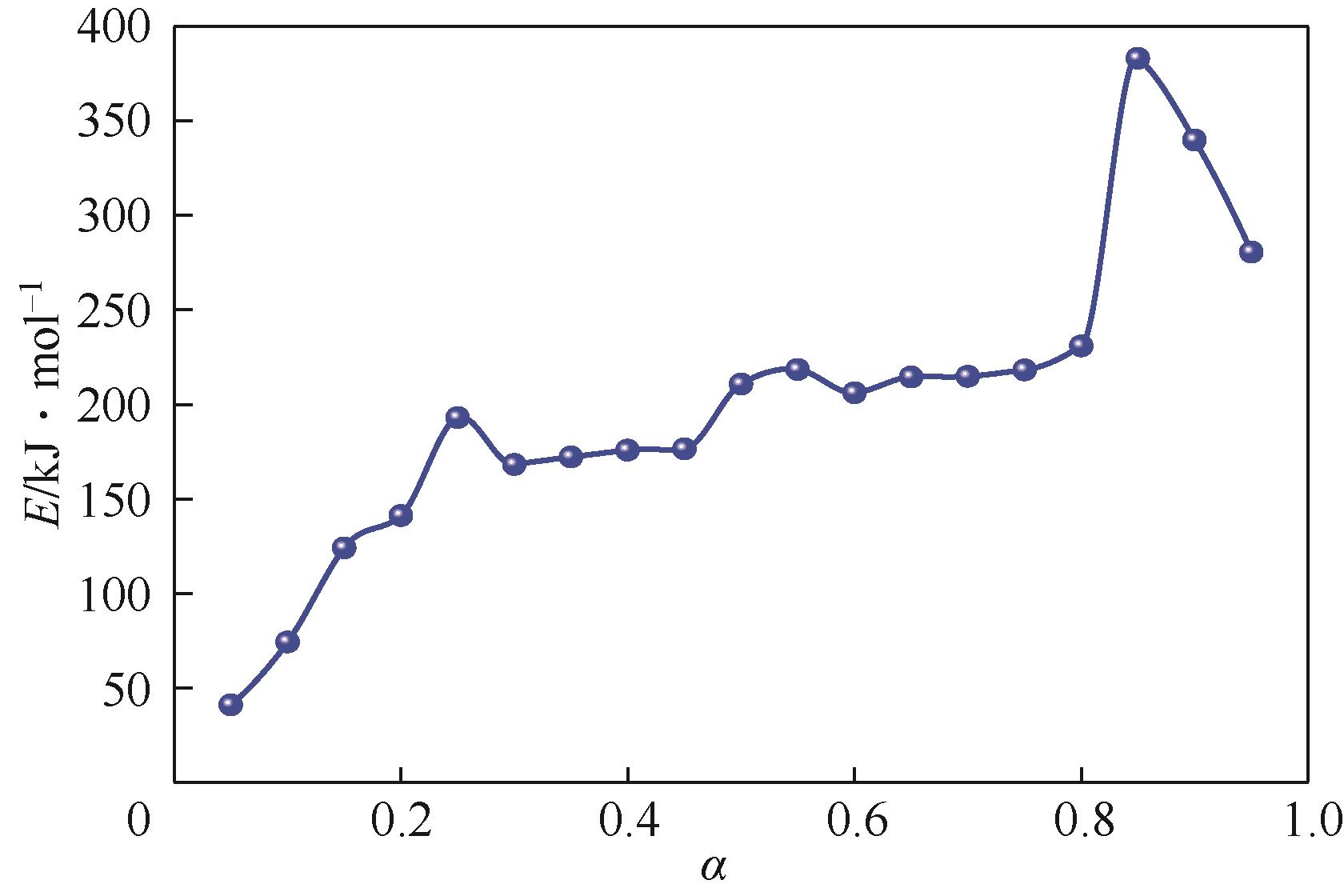
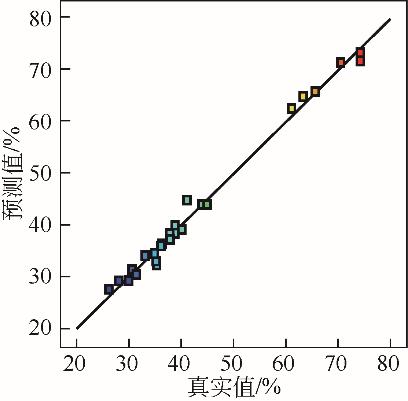
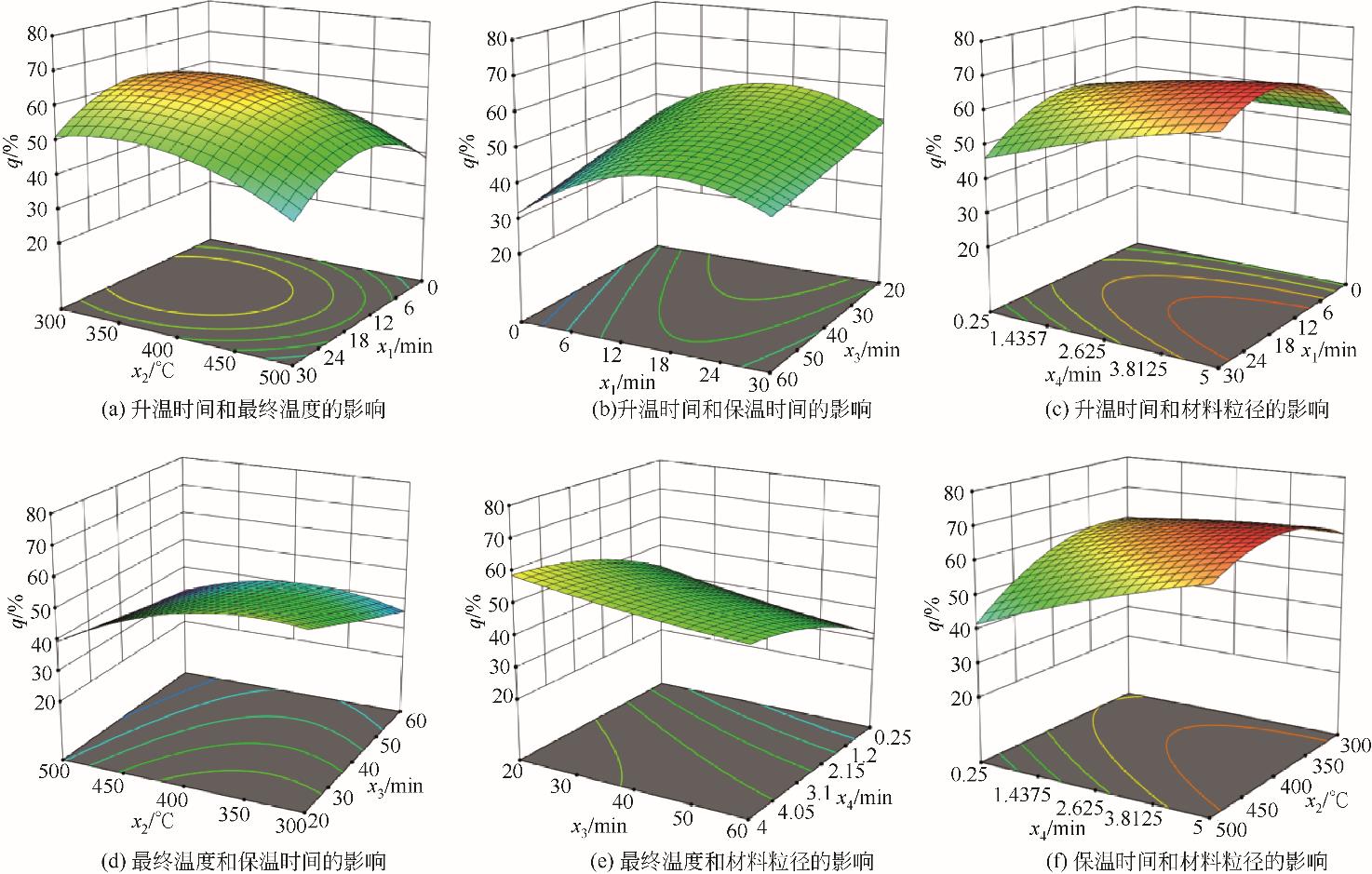
核桃壳产量多,固定碳含量极高且灰分少。选取核桃壳作为研究对象,对其炭化过程的热动力学参数进行分析,探究其炭化进程及原理,最终通过实验和响应面模拟分析得到核桃壳炭化的最佳工艺。研究发现,综合炭化特性指数随着升温速率的增大呈先增大后减小的趋势,且在10℃/min左右时指数达到峰值,此升温速率下炭化反应更剧烈。核桃壳的炭化过程是一个多阶段的复杂反应过程,其分阶段进行半纤维素、纤维素和木质素的分解,该过程活化能总体逐渐升高。最后通过模拟和实验分析得出最佳工况为:升温时间为14.8min,最终温度为324.7℃,保温时间为60min,材料粒径为5mm左右,最佳炭保留率为69.4%。
中图分类号:
引用本文
刘阳, 王云刚, 修浩然, 邹立, 白彦渊. 基于动力学分析的核桃壳最佳炭化工艺[J]. 化工进展, 2023, 42(S1): 94-103.
LIU Yang, WANG Yungang, XIU Haoran, ZOU Li, BAI Yanyuan. Optimal carbonization process of walnut shell based on dynamic analysis[J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 94-103.
| 原料 | 工业分析(质量分数)/% | 元素分析(质量分数)/% | Qnet·ar/MJ·kg -1 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Aad | Vad | Mad | FCad | C | H | O | N | S | ||
| 核桃壳 | 0.28 | 70.73 | 7.77 | 21.22 | 48.4 | 6.18 | 44.29 | 0.33 | 0.01 | 17.44 |
表1 核桃壳工业/元素分析
| 原料 | 工业分析(质量分数)/% | 元素分析(质量分数)/% | Qnet·ar/MJ·kg -1 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Aad | Vad | Mad | FCad | C | H | O | N | S | ||
| 核桃壳 | 0.28 | 70.73 | 7.77 | 21.22 | 48.4 | 6.18 | 44.29 | 0.33 | 0.01 | 17.44 |
| 参数 | 数值 | |||
|---|---|---|---|---|
| 5℃·min -1 | 10℃·min -1 | 20℃·min -1 | 30℃·min -1 | |
| Td/℃ | 228.5 | 247.8 | 259.2 | 267.3 |
| Te/℃ | 389.9 | 395.1 | 405.3 | 416.6 |
| TP/℃ | 346.3 | 361.7 | 365.2 | 367.7 |
| ΔWmax/% | 51.1 | 52.7 | 52.3 | 52.9 |
| η∞/% | 76.6 | 77.0 | 76.9 | 77.4 |
| (dW/dt)max/%·min -1 | 0.71 | 0.69 | 0.69 | 0.70 |
| (dW/dt)mean/%·min -1 | 0.1 | 0.1 | 0.1 | 0.1 |
| P/10 -5%3·min -2·℃ -1 | 9.8 | 10.1 | 9.5 | 9.3 |
表2 不同升温速率下核桃壳热解特性参数
| 参数 | 数值 | |||
|---|---|---|---|---|
| 5℃·min -1 | 10℃·min -1 | 20℃·min -1 | 30℃·min -1 | |
| Td/℃ | 228.5 | 247.8 | 259.2 | 267.3 |
| Te/℃ | 389.9 | 395.1 | 405.3 | 416.6 |
| TP/℃ | 346.3 | 361.7 | 365.2 | 367.7 |
| ΔWmax/% | 51.1 | 52.7 | 52.3 | 52.9 |
| η∞/% | 76.6 | 77.0 | 76.9 | 77.4 |
| (dW/dt)max/%·min -1 | 0.71 | 0.69 | 0.69 | 0.70 |
| (dW/dt)mean/%·min -1 | 0.1 | 0.1 | 0.1 | 0.1 |
| P/10 -5%3·min -2·℃ -1 | 9.8 | 10.1 | 9.5 | 9.3 |
| 转化率 | 拟合曲线 | 活化能E/kJ·mol -1 | 线性相关系数R2 |
|---|---|---|---|
| 0.05 | y=10.47x+21.07 | 40.92942 | 0.96724 |
| 0.1 | y=11.32x+18.37 | 74.23701 | 0.96137 |
| 0.15 | y=14.93x+18.52 | 123.99989 | 0.96492 |
| 0.2 | y=16.99x+21.47 | 141.1927 | 0.97774 |
| 0.25 | y=23.23x+31.85 | 192.93921 | 0.99983 |
| 0.3 | y=19.52x+24.87 | 168.19976 | 0.97851 |
| 0.35 | y=20.73x+26.42 | 172.21079 | 0.96724 |
| 0.4 | y=22.85x+29.43 | 175.81415 | 0.96137 |
| 0.45 | y=25.21x+32.78 | 176.44426 | 0.9587 |
| 0.5 | y=25.37x+32.41 | 210.77128 | 0.95368 |
| 0.55 | y=26.29x+33.33 | 218.43556 | 0.95423 |
| 0.6 | y=24.82x+30.51 | 206.15721 | 0.95912 |
| 0.65 | y=25.81x+30.69 | 214.45144 | 0.96901 |
| 0.7 | y=25.86x+31.39 | 214.83034 | 0.9575 |
| 0.75 | y=26.27x+31.62 | 218.23444 | 0.95142 |
| 0.8 | y=10.47x+21.07 | 230.85888 | 0.91083 |
| 0.85 | y=27.79x+33.49 | 383.15627 | 0.94912 |
| 0.9 | y=40.92x+47.70 | 339.92115 | 0.95142 |
| 0.95 | y=53.78x+57.11 | 280.62832 | 0.99983 |
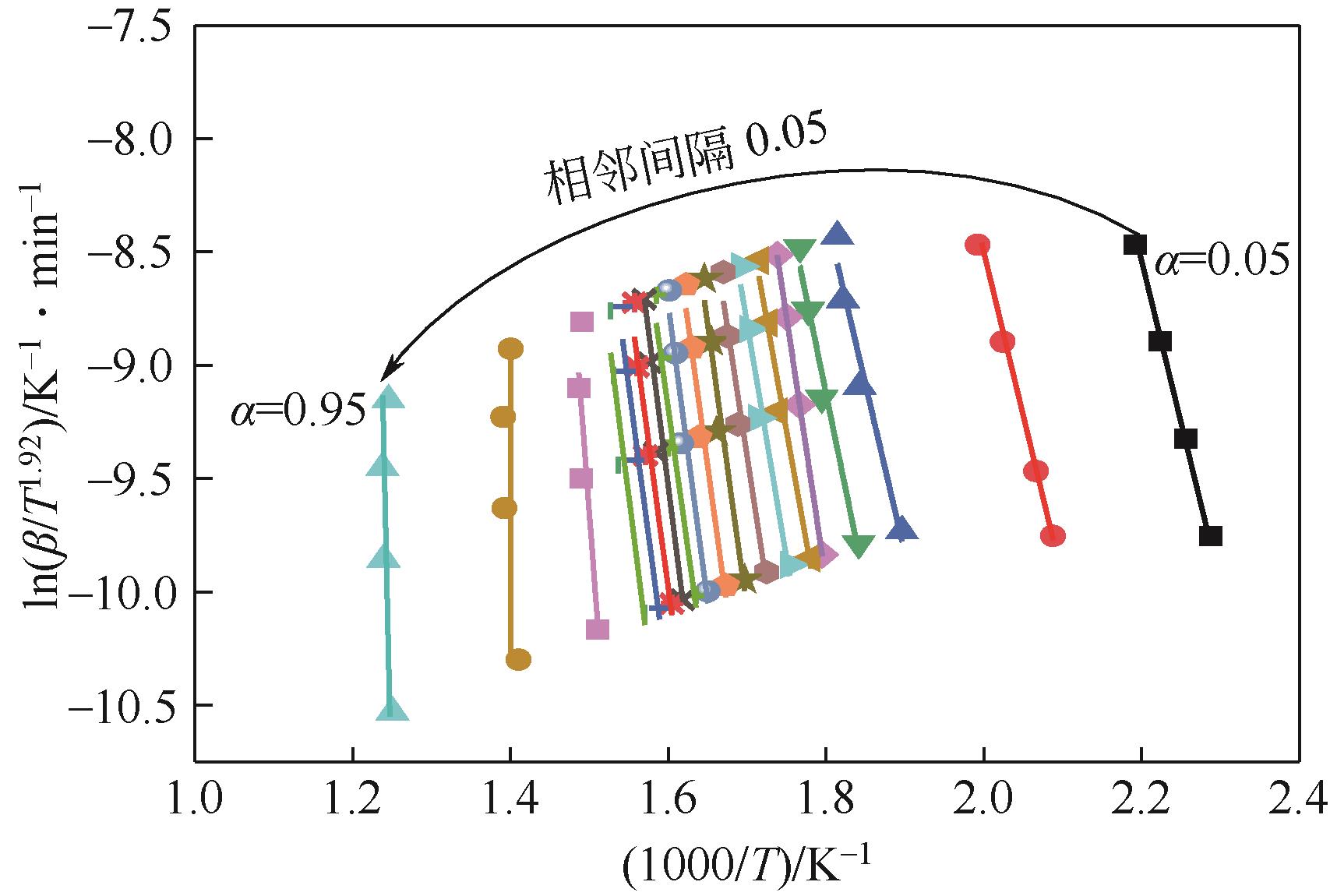
表3 不同升温速率下等转化率法得到的活化能
| 转化率 | 拟合曲线 | 活化能E/kJ·mol -1 | 线性相关系数R2 |
|---|---|---|---|
| 0.05 | y=10.47x+21.07 | 40.92942 | 0.96724 |
| 0.1 | y=11.32x+18.37 | 74.23701 | 0.96137 |
| 0.15 | y=14.93x+18.52 | 123.99989 | 0.96492 |
| 0.2 | y=16.99x+21.47 | 141.1927 | 0.97774 |
| 0.25 | y=23.23x+31.85 | 192.93921 | 0.99983 |
| 0.3 | y=19.52x+24.87 | 168.19976 | 0.97851 |
| 0.35 | y=20.73x+26.42 | 172.21079 | 0.96724 |
| 0.4 | y=22.85x+29.43 | 175.81415 | 0.96137 |
| 0.45 | y=25.21x+32.78 | 176.44426 | 0.9587 |
| 0.5 | y=25.37x+32.41 | 210.77128 | 0.95368 |
| 0.55 | y=26.29x+33.33 | 218.43556 | 0.95423 |
| 0.6 | y=24.82x+30.51 | 206.15721 | 0.95912 |
| 0.65 | y=25.81x+30.69 | 214.45144 | 0.96901 |
| 0.7 | y=25.86x+31.39 | 214.83034 | 0.9575 |
| 0.75 | y=26.27x+31.62 | 218.23444 | 0.95142 |
| 0.8 | y=10.47x+21.07 | 230.85888 | 0.91083 |
| 0.85 | y=27.79x+33.49 | 383.15627 | 0.94912 |
| 0.9 | y=40.92x+47.70 | 339.92115 | 0.95142 |
| 0.95 | y=53.78x+57.11 | 280.62832 | 0.99983 |
| 转化率范围 | 温度范围 | 反应 | 有效活化能Ea |
|---|---|---|---|
| α<0.11 | T0~T1 | 水分蒸发,低温易分解组分进行分解 | 从40.9kJ/mol上升至 86.2kJ/mol |
| 0.11≤α<0.44 | T1~T3 | 半纤维素分解出挥发分 | 从86.2kJ/mol上升至181.7kJ/mol再下降至173.6kJ/mol后续基本没有变化 |
| 0.44≤α<0.85 | T3~T5 | 纤维素分解出挥发分、木质素分解成炭 | 从173.6kJ/mol迅速上升至352.2kJ/mol |
| α≥0.85 | T5~Te | 木质素分解成炭 | 从352.2kJ/mol下降至280.4kJ/mol |
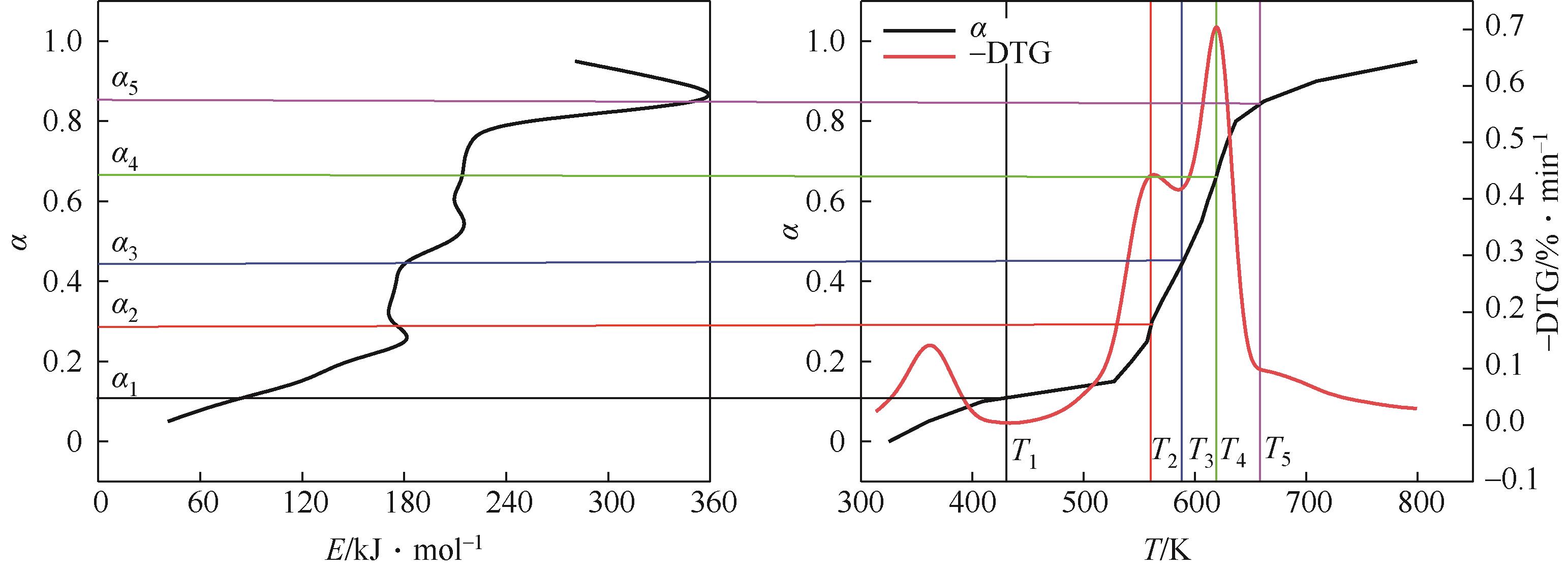
表4 核桃壳炭化热解的活化能与组分反应过程
| 转化率范围 | 温度范围 | 反应 | 有效活化能Ea |
|---|---|---|---|
| α<0.11 | T0~T1 | 水分蒸发,低温易分解组分进行分解 | 从40.9kJ/mol上升至 86.2kJ/mol |
| 0.11≤α<0.44 | T1~T3 | 半纤维素分解出挥发分 | 从86.2kJ/mol上升至181.7kJ/mol再下降至173.6kJ/mol后续基本没有变化 |
| 0.44≤α<0.85 | T3~T5 | 纤维素分解出挥发分、木质素分解成炭 | 从173.6kJ/mol迅速上升至352.2kJ/mol |
| α≥0.85 | T5~Te | 木质素分解成炭 | 从352.2kJ/mol下降至280.4kJ/mol |
| 因素 | 水平 | ||
|---|---|---|---|
| 低水平 (-1) | 中间水平 (0) | 高水平 (+1) | |
| X1 | 0 | 15 | 30 |
| X2 | 300 | 400 | 500 |
| X3 | 20 | 40 | 60 |
| X4 | 0.25 | 2.625 | 5 |
表5 响应面分析因素及水平
| 因素 | 水平 | ||
|---|---|---|---|
| 低水平 (-1) | 中间水平 (0) | 高水平 (+1) | |
| X1 | 0 | 15 | 30 |
| X2 | 300 | 400 | 500 |
| X3 | 20 | 40 | 60 |
| X4 | 0.25 | 2.625 | 5 |
| 方差来源 | 平方和 | 自由度 | 均方差 | F值 | P值 | 显著性 |
|---|---|---|---|---|---|---|
| R2=0.9913, Adj R2=0.9792, 信噪比=27.2898 | ||||||
| 模型 | 5387.31 | 14 | 384.81 | 81.80 | < 0.0001 | 显著 |
| X1 | 39.79 | 1 | 39.79 | 8.46 | 0.0156 | |
| X2 | 4338.08 | 1 | 4338.08 | 922.14 | < 0.0001 | |
| X3 | 119.20 | 1 | 119.20 | 25.34 | 0.0005 | |
| X4 | 110.84 | 1 | 110.84 | 23.56 | 0.0007 | |
| X1X2 | 4.62 | 1 | 4.62 | 0.9826 | 0.3449 | |
| X1X3 | 0.54 | 1 | 0.54 | 0.1148 | 0.7417 | |
| X1X4 | 2.04 | 1 | 2.04 | 0.4347 | 0.5246 | |
| X2X3 | 0.73 | 1 | 0.73 | 0.1554 | 0.7017 | |
| X2X4 | 23.38 | 1 | 23.38 | 4.97 | 0.0499 | |
| X3X4 | 0.02 | 1 | 0.02 | 0.0042 | 0.9498 | |
| X12 | 6.09 | 1 | 6.09 | 1.29 | 0.2818 | |
| X22 | 406.73 | 1 | 406.73 | 86.46 | < 0.0001 | |
| X32 | 5.57 | 1 | 5.57 | 1.18 | 0.3021 | |
| X42 | 3.09 | 1 | 3.09 | 0.6565 | 0.4367 | |
| 误差 | 47.04 | 10 | 4.70 | — | — | |
| 失拟项 | 28.93 | 3 | 6.58 | 7.43 | 0.0771 | 不显著 |
| 纯误差 | 1.32 | 4 | 0.97 | — | — | |
| 校正总和 | 5434.35 | 24 | — | — | — | |
表6 回归方程方差分析
| 方差来源 | 平方和 | 自由度 | 均方差 | F值 | P值 | 显著性 |
|---|---|---|---|---|---|---|
| R2=0.9913, Adj R2=0.9792, 信噪比=27.2898 | ||||||
| 模型 | 5387.31 | 14 | 384.81 | 81.80 | < 0.0001 | 显著 |
| X1 | 39.79 | 1 | 39.79 | 8.46 | 0.0156 | |
| X2 | 4338.08 | 1 | 4338.08 | 922.14 | < 0.0001 | |
| X3 | 119.20 | 1 | 119.20 | 25.34 | 0.0005 | |
| X4 | 110.84 | 1 | 110.84 | 23.56 | 0.0007 | |
| X1X2 | 4.62 | 1 | 4.62 | 0.9826 | 0.3449 | |
| X1X3 | 0.54 | 1 | 0.54 | 0.1148 | 0.7417 | |
| X1X4 | 2.04 | 1 | 2.04 | 0.4347 | 0.5246 | |
| X2X3 | 0.73 | 1 | 0.73 | 0.1554 | 0.7017 | |
| X2X4 | 23.38 | 1 | 23.38 | 4.97 | 0.0499 | |
| X3X4 | 0.02 | 1 | 0.02 | 0.0042 | 0.9498 | |
| X12 | 6.09 | 1 | 6.09 | 1.29 | 0.2818 | |
| X22 | 406.73 | 1 | 406.73 | 86.46 | < 0.0001 | |
| X32 | 5.57 | 1 | 5.57 | 1.18 | 0.3021 | |
| X42 | 3.09 | 1 | 3.09 | 0.6565 | 0.4367 | |
| 误差 | 47.04 | 10 | 4.70 | — | — | |
| 失拟项 | 28.93 | 3 | 6.58 | 7.43 | 0.0771 | 不显著 |
| 纯误差 | 1.32 | 4 | 0.97 | — | — | |
| 校正总和 | 5434.35 | 24 | — | — | — | |
| X1 | X2 | X3 | X4 | q | |
|---|---|---|---|---|---|
| 实验值 | 预测值 | ||||
| 14.8min | 324.7℃ | 60min | 5mm | 68.8% | 72.3% |
| 14.8min | 324.7℃ | 60min | 5mm | 70.9% | 72.3% |
| 14.8min | 324.7℃ | 60min | 5mm | 68.6% | 72.3% |
表7 模型验证
| X1 | X2 | X3 | X4 | q | |
|---|---|---|---|---|---|
| 实验值 | 预测值 | ||||
| 14.8min | 324.7℃ | 60min | 5mm | 68.8% | 72.3% |
| 14.8min | 324.7℃ | 60min | 5mm | 70.9% | 72.3% |
| 14.8min | 324.7℃ | 60min | 5mm | 68.6% | 72.3% |
| 1 | Umi Fazara MD ALI, AZMI Nur Hidayah, Khairuddin MD ISA, et al. Optimization study on preparation of amine functionalized sea mango (cerbera odollam) activated carbon for carbon dioxide (CO2) adsorption[J]. Combustion Science and Technology, 2018, 190: 1259-1282. |
| 2 | 王申宛, 郑晓燕, 校导, 等. 生物炭的制备、改性及其在环境修复中应用的研究进展[J]. 化工进展, 2020, 39(S2): 352-361. |
| WANG Shenwan, ZHENG Xiaoyan, XIAO Dao, et al. Research progress of production, modification and application in environment remediation of biochar[J]. Chemical Industry and Engineering Progress, 2020, 39(S2): 352-361. | |
| 3 | Endah AGUSTINA S, FIRMANSYAH. Design and performance test of drum kiln for durian peel carbonization[J]. IOP Conference Series: Earth and Environmental Science, 2020, 542(1): 012040. |
| 4 | 常秋连, 李文博, 赵鹏. 煤焦油渣炭化过程中孔结构及表面分形特征[J]. 化工进展, 2020, 39(10): 4305-4313. |
| CHANG Qiulian, LI Wenbo, ZHAO Peng. Structure and surface fractal characteristics of coal tar residue during carbonization[J]. Chemical Industry and Engineering Progress, 2020, 39(10): 4305-4313. | |
| 5 | 朱金陵, 何晓峰, 王志伟, 等. 玉米秸秆颗粒热解制炭的试验研究[J]. 太阳能学报, 2010, 31(7): 789-793. |
| ZHU Jinling, HE Xiaofeng, WANG Zhiwei, et al. Experimental study on pyrolysising and producting charcoal with corn straw pellet[J]. Acta Energiae Solaris Sinica, 2010, 31(7): 789-793. | |
| 6 | 朱赫男, 王志朴, 邢文龙, 等. 污泥与生物质共热解制备生物质炭工艺优化及吸附性能[J]. 化工进展, 2018, 37(S1): 199-204. |
| ZHU Henan, WANG Zhipu, XING Wenlong, et al. Process optimization and adsorption performance of biochars prepared by co-pyrolysis of sludge and biomasses[J]. Chemical Industry and Engineering Progress, 2018, 37(S1): 199-204. | |
| 7 | 胡福昌, 陈顺伟, 康志雄, 等. 竹材列管移动床连续干馏炭化的工业试验[J]. 林产化学与工业, 2005, 25(2): 47-51. |
| HU Fuchang, CHEN Shunwei, KANG Zhixiong, et al. An industrial test on continuous carbonization of bamboo in multitubular moving bed[J]. Chemistry & Industry of Forest Products, 2005, 25(2): 47-51. | |
| 8 | 杨莉, 付婧, 文子伟, 等. 6种低温生物质炭的制备及结构表征[J]. 吉林农业大学学报, 2021, 43(5): 565-573. |
| YANG Li, FU Jing, WEN Ziwei, et al. Preparation and structure characterization of six kinds of low temperature biochar[J]. Journal of Jilin Agricultural University, 2021, 43(5): 565-573. | |
| 9 | 徐大勇, 张苗, 杨伟伟, 等. 氧化铝改性污泥生物炭粒制备及其对Pb(Ⅱ)的吸附特性[J]. 化工进展, 2020, 39(3): 1153-1166. |
| XU Dayong, ZHANG Miao, YANG Weiwei, et al. Preparation of alumina modified sludge biocharcoal particles and their adsorption characteristics for Pb(Ⅱ)[J]. Chemical Industry and Engineering Progress, 2020, 39(3): 1153-1166. | |
| 10 | GHYSELS Stef, RONSSE Frederik, DICKINSON Dane, et al. Production and characterization of slow pyrolysis biochar from lignin-rich digested stillage from lignocellulosic ethanol production[J]. Biomass and Bioenergy, 2019, 122: 349-360. |
| 11 | 罗煜, 赵立欣, 孟海波, 等. 不同温度下热裂解芒草生物质炭的理化特征分析[J]. 农业工程学报, 2013, 29(13): 208-217. |
| LUO Yu, ZHAO Lixin, MENG Haibo, et al. Physio-chemical characterization of biochars pyrolyzed from miscanthus under two different temperatures[J]. Transactions of the Chinese Society of Agricultural Engineering, 2013, 29(13): 208-217. | |
| 12 | 高美. 生物质炭化成型燃料的制备及其燃烧性能的研究[D]. 哈尔滨: 黑龙江科技学院, 2010. |
| GAO Mei. The research of the fabrication and combustion characteristic of the biomass carbonized forming fuel [D].: Harbin: Heilongjiang University of Science and Technology, 2010. | |
| 13 | MEDIC D, DARR M, SHAH A, et al. Effects of torrefaction process parameters on biomass feedstock upgrading[J]. Fuel, 2012, 91(1): 147-154. |
| 14 | PIMCHUAI Anuphon, DUTTA Animesh, BASU Prabir. Torrefaction of agriculture residue to enhance combustible properties[J]. Energy & Fuels, 2010, 24(9): 4638-4645. |
| 15 | Po-Chih KUO, WU Wei, CHEN Wei-Hsin. Gasification performances of raw and torrefied biomass in a downdraft fixed bed gasifier using thermodynamic analysis[J]. Fuel, 2014, 117: 1231-1241. |
| 16 | 姚红宇, 唐光木, 葛春辉, 等. 炭化温度和时间与棉杆炭特性及元素组成的相关关系[J]. 农业工程学报, 2013, 29(7): 199-206. |
| YAO Hongyu, TANG Guangmu, GE Chunhui, et al. Characteristics and elementary composition of cotton stalk-char in different carbonization temperature and time[J]. Transactions of the Chinese Society of Agricultural Engineering, 2013, 29(7): 199-206. | |
| 17 | MA Yuhui, WANG Jing, ZHANG Yushan. TG-FTIR study on pyrolysis of Enteromorpha prolifera[J]. Biomass Conversion and Biorefinery, 2018, 8(1): 151-157. |
| 18 | FAN Fangyu, ZHENG Yunwu, HUANG Yuanbo, et al. Combustion kinetics of biochar prepared by pyrolysis of macadamia shells[J]. BioResources, 2017, 12(2): 68-71. |
| 19 | 梁嘉晋. 纤维素和半纤维素热解机理及其产物调控途径的研究[D]. 广州: 华南理工大学, 2016. |
| LIANG Jiajin. Mechanism researches of cellulose and hemicellulose pyrolysis and their products regulation[D]. Guangzhou: South China University of Technology, 2016. | |
| 20 | 钱卫. 低阶烟煤中低温热解及热解产物研究[D]. 北京: 中国矿业大学(北京), 2012. |
| QIAN Wei. Experimental study on medium-low temperature pyrolysis of low rank bituminous coal and characterization of pyrolysis-derived products[D]. Beijing: China University of Mining & Technology, Beijing, 2012. | |
| 21 | VYAZOVKIN Sergey, BURNHAM Alan K, CRIADO José M, et al. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data[J]. Thermochimica Acta, 2011, 520(1/2): 1-19. |
| 22 | 吴正锐. 黄松甸木耳菌糠的热解特性分析及动力学研究[D]. 吉林: 东北电力大学, 2020. |
| WU Zhengrui. Study on pyrolysis charcteristic and pyrolysis kinetics analysis of spent jew’s-ear substrate in huangsongdian[D]. Jilin: Northeast Dianli University, 2020. | |
| 23 | 任宁, 王昉, 张建军, 等. 热分析动力学研究方法的新进展[J]. 物理化学学报, 2020, 36(6): 12-18. |
| REN Ning, WANG Fang, ZHANG Jianjun, et al. Progress in thermal analysis kinetics[J]. Acta Physico-Chimica Sinica, 2020, 36(6): 12-18. | |
| 24 | TSAMBA Alberto J, YANG Weihong, BLASIAK Wlodzimierz. Pyrolysis characteristics and global kinetics of coconut and cashew nut shells[J]. Fuel Processing Technology, 2006, 87(6): 523-530. |
| 25 | YANG Haiping, YAN Rong, CHEN Hanping, et al. Characteristics of hemicellulose, cellulose and lignin pyrolysis[J]. Fuel, 2007, 86(12/13): 1781-1788. |
| [1] | 赵尧, 周志辉, 吴红丹, 胡传智, 张国春, 吴睿鹏. Silicalite-1分子筛膜渗透蒸发条件的响应面分析与优化[J]. 化工进展, 2023, 42(5): 2586-2594. |
| [2] | 王雪, 徐期勇, 张超. 木质纤维素类生物质水热炭化机理及水热炭应用进展[J]. 化工进展, 2023, 42(5): 2536-2545. |
| [3] | 冷南江, 马国光, 张涛, 雷洋, 彭豪, 熊祚帅, 陈玉婷. 高含有机硫天然气的净化研究与探索[J]. 化工进展, 2022, 41(10): 5342-5353. |
| [4] | 张乾, 向欣宁, 梁丽彤, 刘建伟, 王志青, 房倚天, 黄伟. 热重分析煤焦恒温气化过程中气体切换的影响[J]. 化工进展, 2022, 41(1): 166-173. |
| [5] | 邹星宇, 赵文霞, 刘勇, 许瑞梅. 炭化叶脉网络生长MOFs材料制备透明超级电容器[J]. 化工进展, 2022, 41(1): 350-358. |
| [6] | 徐杰, 黄群星, 孟详东, 郜华萍. 钙基添加剂对污水污泥在水热炭化过程中磷形态及生物有效性的影响[J]. 化工进展, 2021, 40(6): 3507-3514. |
| [7] | 黄冠华, 刘序彦, 房晨曦, 顾庆峰, 雷浩. 生物模板法制备磁性中空微球的方法和应用[J]. 化工进展, 2021, 40(5): 2613-2623. |
| [8] | 王犇, 王超, 尹进华. 微反应器内邻氨基苯甲酸甲酯的连续重氮化工艺[J]. 化工进展, 2021, 40(10): 5678-5691. |
| [9] | 郑晓园, 蒋正伟, 陈伟, 叶雨彤, 应芝, 纪莎莎, 王波. 污水污泥水热炭化过程中磷的迁移转化特性[J]. 化工进展, 2020, 39(5): 2017-2025. |
| [10] | 常秋连, 李文博, 赵鹏. 煤焦油渣炭化过程中孔结构及表面分形特征[J]. 化工进展, 2020, 39(10): 4305-4313. |
| [11] | 袁艳文, 赵立欣, 孟海波, 丛宏斌, 霍丽丽, 汤森. 生物质炭化热解气催化重整制取费-托合成气研究进展[J]. 化工进展, 2019, 38(s1): 152-158. |
| [12] | 王亮才,马欢欢,周建斌. 炭化工艺对脱水沼渣炭理化性质的影响[J]. 化工进展, 2019, 38(03): 1545-1551. |
| [13] | 卢亮, 陈军昊, 王树荣. 模拟生物油分子蒸馏的响应面法工况优化[J]. 化工进展, 2018, 37(07): 2605-2612. |
| [14] | 朱永红, 淡勇, 王莉莎, 李冬, 李稳宏. 煤基石脑油半再生催化重整制芳烃的工艺[J]. 化工进展, 2018, 37(03): 947-955. |
| [15] | 朱亚明, 何迎莹, 赵雪飞, 王莹, 郭海东, 高丽娟. AlCl3改性净化沥青的液相炭化[J]. 化工进展, 2017, 36(S1): 353-360. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||