化工进展 ›› 2023, Vol. 42 ›› Issue (S1): 233-245.DOI: 10.16085/j.issn.1000-6613.2023-1040
变电吸附二氧化碳捕集技术研究进展
- 天津大学中低温热能高效利用教育部重点实验室,天津 300350
-
收稿日期:2023-06-25修回日期:2023-10-08出版日期:2023-10-25发布日期:2023-11-30 -
通讯作者:邓帅 -
作者简介:王胜岩(2000—),男,硕士研究生,研究方向为二氧化碳吸附。E-mail:wsywsy@tju.edu.cn。 -
基金资助:国家自然科学基金青年科学基金(52306265);天津市自然科学基金重点项目(22JCZDJC00540)
Research progress on carbon dioxide capture technology based on electric swing adsorption
WANG Shengyan( ), DENG Shuai(
), DENG Shuai( ), ZHAO Ruikai
), ZHAO Ruikai
- Key Laboratory of Efficient Utilization of Low and Medium Grade Energy (Tianjin University), Ministry of Education of China, Tianjin 300350, China
-
Received:2023-06-25Revised:2023-10-08Online:2023-10-25Published:2023-11-30 -
Contact:DENG Shuai
摘要:
基于变电吸附的碳捕集技术,通过“通电-断电”实现摆荡模式,利用电能焦耳效应产生热能,驱动吸附剂实现连续地吸附与再生。相对于变温吸附,其输入高品位电能,因此可驱动碳源、碳汇之间的大浓度差富集,近年来备受关注。然而,目前限制变电吸附碳捕集技术应用的主要问题是较高的能耗与较低的产率。据此,本文总结了近年来国内外变电吸附碳捕集技术的研究进展并提出了技术展望。首先讨论了变电吸附碳捕集的基本原理,其次综述了近十年变电吸附碳捕集技术中吸附剂、循环结构的研究进展及发展趋势,应用热力学第二定律效率对变电吸附碳捕集系统展开评价。最后,对变电吸附碳捕集技术发展趋势进行展望,变电吸附技术具备规模化竞争力的关键为:在改善吸附剂导电和捕集性能的基础上,改进吸附剂制备工艺,关注吸附剂的加热形式以及吸附腔体内的电阻分配,尝试与其他碳捕集技术耦合进行分级捕集,与可再生能源进行集成。
中图分类号:
引用本文
王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245.
WANG Shengyan, DENG Shuai, ZHAO Ruikai. Research progress on carbon dioxide capture technology based on electric swing adsorption[J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 233-245.
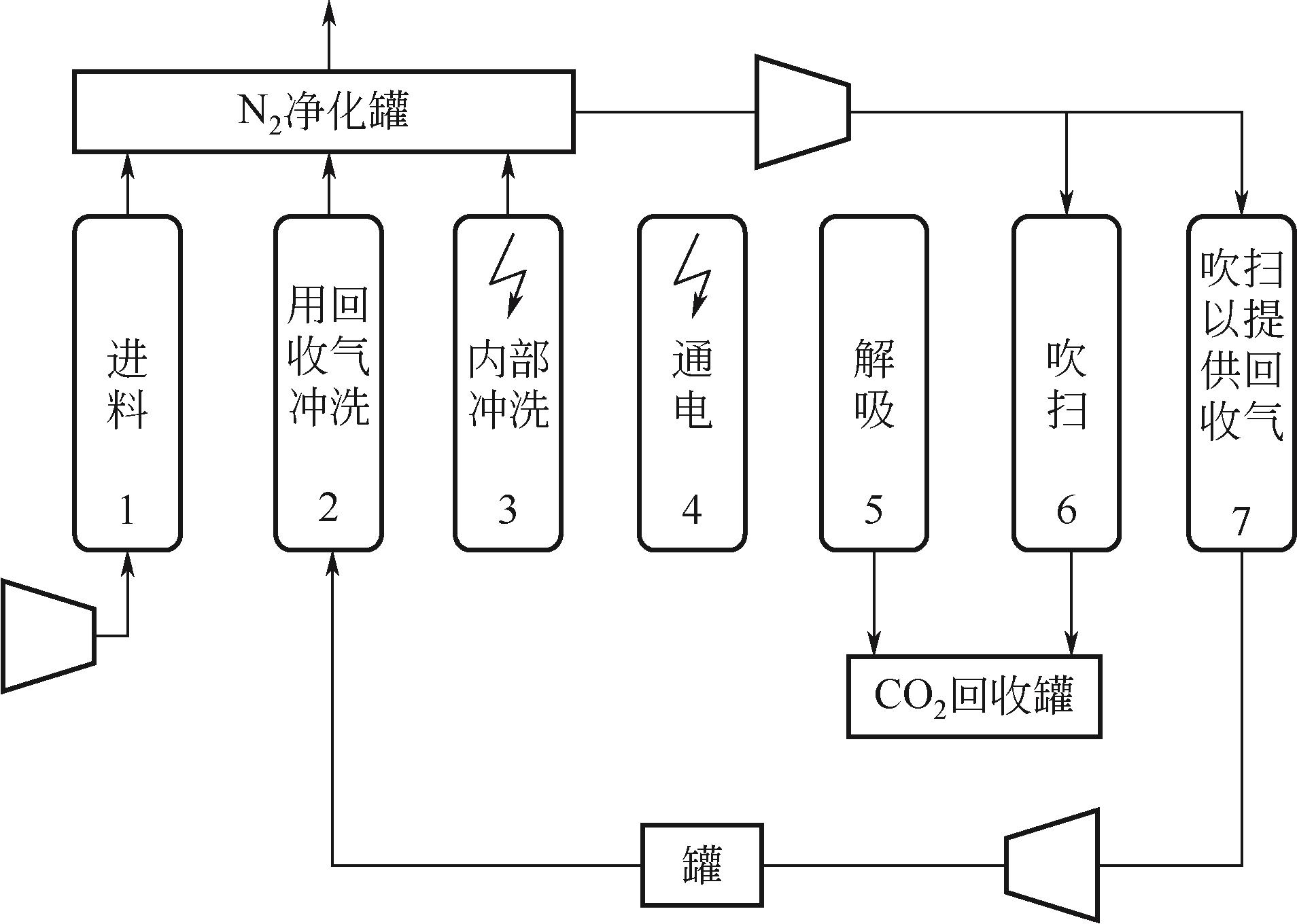
| 年份 | 材料 | 类型 | 比表 面积 /m2·g-1 | 总孔 容积 /cm3·g-1 | 平均孔径 /nm | 温度 /℃ | 压力 /bar | 入口气体成分 | CO2吸附量 /mol·kg-1 | 电阻率 /Ω·m |
|---|---|---|---|---|---|---|---|---|---|---|
| 2009 | 活性炭纤维 | 蜂窝整体 | 907 | 0.36 | 16.1 | 25 | 1 | 99.99%CO2 | 0.91 | — |
| 70%沸石/30%石墨 | 蜂窝整体 | — | — | — | 42 | 1.5 | 3.5%CO2、96.5%N2 | 1.2 | — | |
| 2010 | 浸渍胺的聚合树脂 | 挤压颗粒填充 | — | — | — | 40 | 1 | 99.99%CO2 | 3.7 | — |
| 2010 | 66%活性炭纤维/34% 酚醛树脂 | 蜂窝整体 | 998 | 0.6 | 1.6 | 20 | 1 | 99.99%CO2 | 2.9 | — |
| 2011 | 沥青基球形活性炭 | 挤压颗粒填充 | 1205 | 0.59 | — | 30 | 0.15 | 99.99%CO2 | 1.12 | — |
| 2013 | 氧化改性的酚醛树脂基炭 | 挤压颗粒填充 | 962 | 0.41 | — | 25 | 1 | 99.99%CO2 | 2.7 | — |
| 2013 | 18%沸石/82%活性炭 | 挤压颗粒填充 | — | — | — | 22 | 1 | 7.6%CO2、92.4%N2 | 0.82 | 0.00144-0.000001Ts |
| 2015 | 炭 | 蜂窝整体 | — | — | — | 30 | 1 | 0.3%CO2 | 3.45×10-3 | 5.02×10-2 |
| 2016 | 石墨烯薄膜 | 片状薄膜 | — | — | — | 25 | 1 | 99.99%CO2 | 11.59 | |
| 2016 | 石墨烯气凝胶 | 片状薄膜 | 165 | — | — | 25 | 1 | 99.99%CO2 | 21.36 | — |
| 2016 | 毫米级介孔碳球 | 挤压颗粒填充 | 36 | 0.13 | 30.1 | 75 | 1 | 15%CO2、85%N2 | 3.71 | — |
| 2017 | 22%酚醛树脂/78%H-ZSM-5-800 | 蜂窝整体 | 397 | 0.2 | — | 25 | 1.1 | 99.99%CO2 | 2.2 | 5.8 |
| 2017 | 多孔炭(UGIL-900) | 蜂窝整体 | 4200 | 2.41 | 2.3 | 25 | 1 | 99.99%CO2 | 2.2 | — |
| 2017 | 活性炭 | 蜂窝整体 | — | — | — | 20 | 1 | 99.99%CO2 | 3.5 | 0.00063-0.0000004954Ts |
| 2018 | 70%沸石NaUSY/30%活性炭 | 蜂窝整体 | 460 | 0.26 | — | 20 | 0.15 | 99.99%CO2 | 2.05 | 1.18×10-2 |
| 2018 | 活性炭 | 蜂窝整体 | — | — | — | — | — | — | — | 0.0011-0.000000856Ts |
| 2018 | 18%沸石13X/82%活性炭 | 蜂窝整体 | — | — | — | — | — | — | — | 0.0014-0.00000111Ts |
| 2019 | 浸渍PEI的碳化硅纤维 | 中空管束 | — | — | 500 | 30 | 1 | 15%CO2、85%N2 | 0.886 | 0.00125 |
| 2019 | 沸石填充的碳纳米管 | 中空管束 | — | 1.6 | 30 | 1 | 15%CO2、85%N2 | 11.14 | 0.0003 | |
| 2019 | 82%沸石H-ZSM-5/18%活性炭 | 蜂窝整体 | — | — | — | 20 | 0.15 | 99.99%CO2 | 1.2 | 3.4423-0.0067Ts |
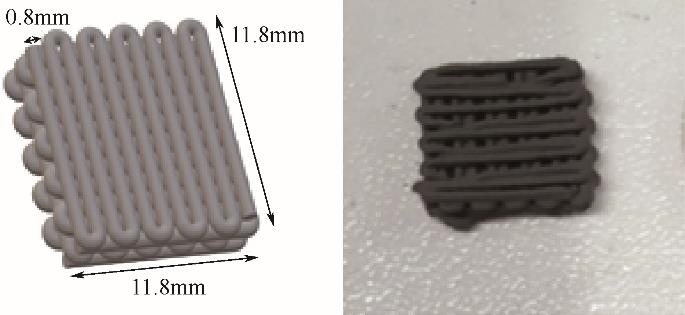
| 2019 | 70%沸石13X/30%活性炭 | 3D打印整体 | 2028 | 0.648 | — | 0 | 0.15 | 99.99%CO2 | 3.49 | 0.28 |
| 2020 | 50%沸石13X/50%活性炭 | 挤压颗粒填充 | — | — | 237 | 30 | 1 | 99.99%CO2 | 3.2 | 0.57-0.0885(Ts-293)0.3 |
| 2021 | 活性炭管 | 中空管束 | — | — | — | 35 | 1 | 99.99%CO2 | 1.22 | 0.0108 |
| 2021 | 50%沸石13X/50%活性炭 | 3D打印整体 | 819 | 0.43 | 1700 | 27 | 0.15 | 99.99%CO2 | 1.54 | 59.0619 |
| 2021 | 50%沸石13X/50%石墨 | 3D打印整体 | 316 | 0.15 | 1500 | 27 | 0.15 | 99.99%CO2 | 1.38 | 7.5398 |
| 2022 | 47.5%沸石47.5%活性炭5%黏合剂 | 3D打印整体 | 1985 | 0.88 | — | 30 | 1 | 15%CO2、85%N2 | 1.5 | — |
表1 吸附材料性能
| 年份 | 材料 | 类型 | 比表 面积 /m2·g-1 | 总孔 容积 /cm3·g-1 | 平均孔径 /nm | 温度 /℃ | 压力 /bar | 入口气体成分 | CO2吸附量 /mol·kg-1 | 电阻率 /Ω·m |
|---|---|---|---|---|---|---|---|---|---|---|
| 2009 | 活性炭纤维 | 蜂窝整体 | 907 | 0.36 | 16.1 | 25 | 1 | 99.99%CO2 | 0.91 | — |
| 70%沸石/30%石墨 | 蜂窝整体 | — | — | — | 42 | 1.5 | 3.5%CO2、96.5%N2 | 1.2 | — | |
| 2010 | 浸渍胺的聚合树脂 | 挤压颗粒填充 | — | — | — | 40 | 1 | 99.99%CO2 | 3.7 | — |
| 2010 | 66%活性炭纤维/34% 酚醛树脂 | 蜂窝整体 | 998 | 0.6 | 1.6 | 20 | 1 | 99.99%CO2 | 2.9 | — |
| 2011 | 沥青基球形活性炭 | 挤压颗粒填充 | 1205 | 0.59 | — | 30 | 0.15 | 99.99%CO2 | 1.12 | — |
| 2013 | 氧化改性的酚醛树脂基炭 | 挤压颗粒填充 | 962 | 0.41 | — | 25 | 1 | 99.99%CO2 | 2.7 | — |
| 2013 | 18%沸石/82%活性炭 | 挤压颗粒填充 | — | — | — | 22 | 1 | 7.6%CO2、92.4%N2 | 0.82 | 0.00144-0.000001Ts |
| 2015 | 炭 | 蜂窝整体 | — | — | — | 30 | 1 | 0.3%CO2 | 3.45×10-3 | 5.02×10-2 |
| 2016 | 石墨烯薄膜 | 片状薄膜 | — | — | — | 25 | 1 | 99.99%CO2 | 11.59 | |
| 2016 | 石墨烯气凝胶 | 片状薄膜 | 165 | — | — | 25 | 1 | 99.99%CO2 | 21.36 | — |
| 2016 | 毫米级介孔碳球 | 挤压颗粒填充 | 36 | 0.13 | 30.1 | 75 | 1 | 15%CO2、85%N2 | 3.71 | — |
| 2017 | 22%酚醛树脂/78%H-ZSM-5-800 | 蜂窝整体 | 397 | 0.2 | — | 25 | 1.1 | 99.99%CO2 | 2.2 | 5.8 |
| 2017 | 多孔炭(UGIL-900) | 蜂窝整体 | 4200 | 2.41 | 2.3 | 25 | 1 | 99.99%CO2 | 2.2 | — |
| 2017 | 活性炭 | 蜂窝整体 | — | — | — | 20 | 1 | 99.99%CO2 | 3.5 | 0.00063-0.0000004954Ts |
| 2018 | 70%沸石NaUSY/30%活性炭 | 蜂窝整体 | 460 | 0.26 | — | 20 | 0.15 | 99.99%CO2 | 2.05 | 1.18×10-2 |
| 2018 | 活性炭 | 蜂窝整体 | — | — | — | — | — | — | — | 0.0011-0.000000856Ts |
| 2018 | 18%沸石13X/82%活性炭 | 蜂窝整体 | — | — | — | — | — | — | — | 0.0014-0.00000111Ts |
| 2019 | 浸渍PEI的碳化硅纤维 | 中空管束 | — | — | 500 | 30 | 1 | 15%CO2、85%N2 | 0.886 | 0.00125 |
| 2019 | 沸石填充的碳纳米管 | 中空管束 | — | 1.6 | 30 | 1 | 15%CO2、85%N2 | 11.14 | 0.0003 | |
| 2019 | 82%沸石H-ZSM-5/18%活性炭 | 蜂窝整体 | — | — | — | 20 | 0.15 | 99.99%CO2 | 1.2 | 3.4423-0.0067Ts |
| 2019 | 70%沸石13X/30%活性炭 | 3D打印整体 | 2028 | 0.648 | — | 0 | 0.15 | 99.99%CO2 | 3.49 | 0.28 |
| 2020 | 50%沸石13X/50%活性炭 | 挤压颗粒填充 | — | — | 237 | 30 | 1 | 99.99%CO2 | 3.2 | 0.57-0.0885(Ts-293)0.3 |
| 2021 | 活性炭管 | 中空管束 | — | — | — | 35 | 1 | 99.99%CO2 | 1.22 | 0.0108 |
| 2021 | 50%沸石13X/50%活性炭 | 3D打印整体 | 819 | 0.43 | 1700 | 27 | 0.15 | 99.99%CO2 | 1.54 | 59.0619 |
| 2021 | 50%沸石13X/50%石墨 | 3D打印整体 | 316 | 0.15 | 1500 | 27 | 0.15 | 99.99%CO2 | 1.38 | 7.5398 |
| 2022 | 47.5%沸石47.5%活性炭5%黏合剂 | 3D打印整体 | 1985 | 0.88 | — | 30 | 1 | 15%CO2、85%N2 | 1.5 | — |
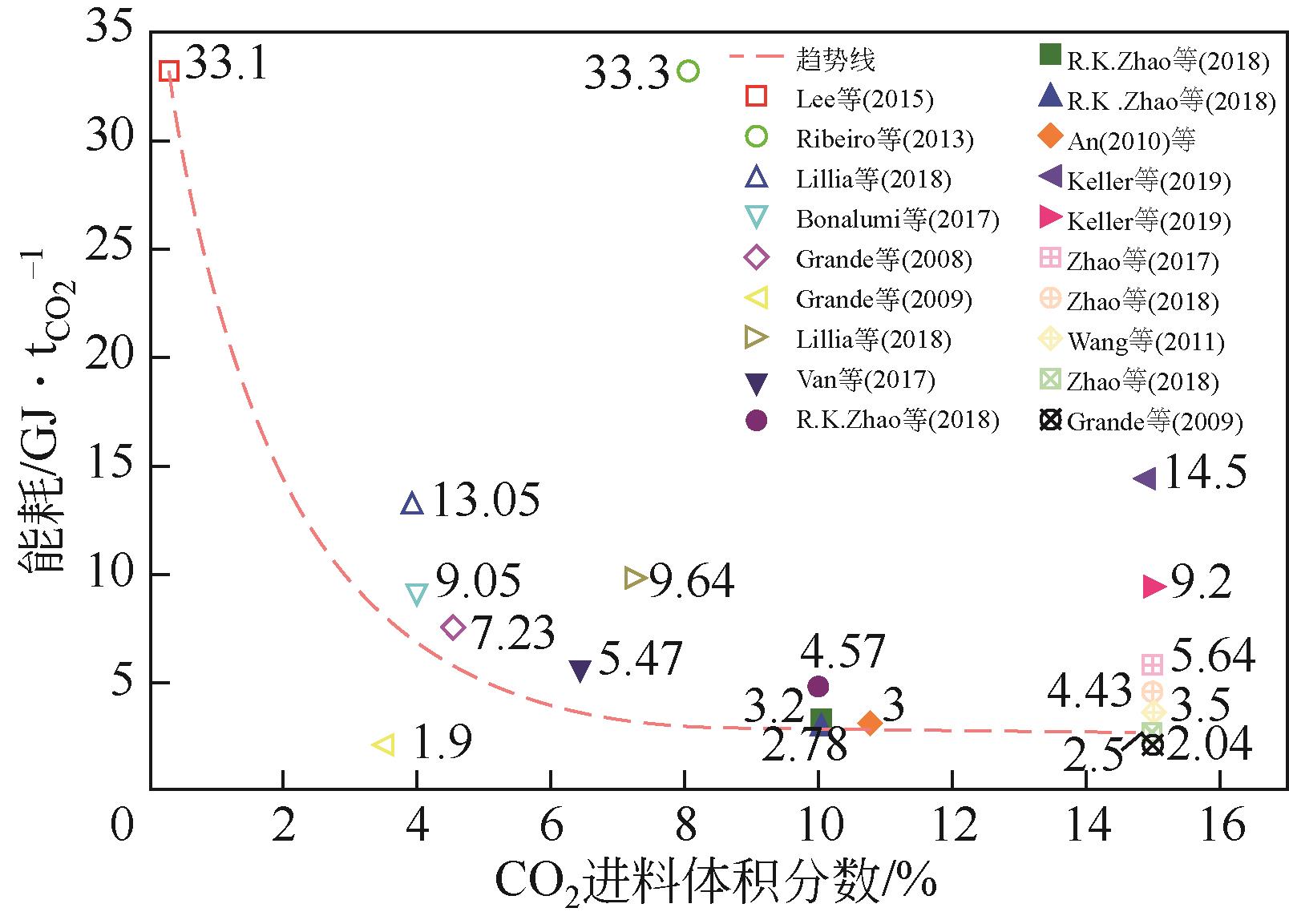
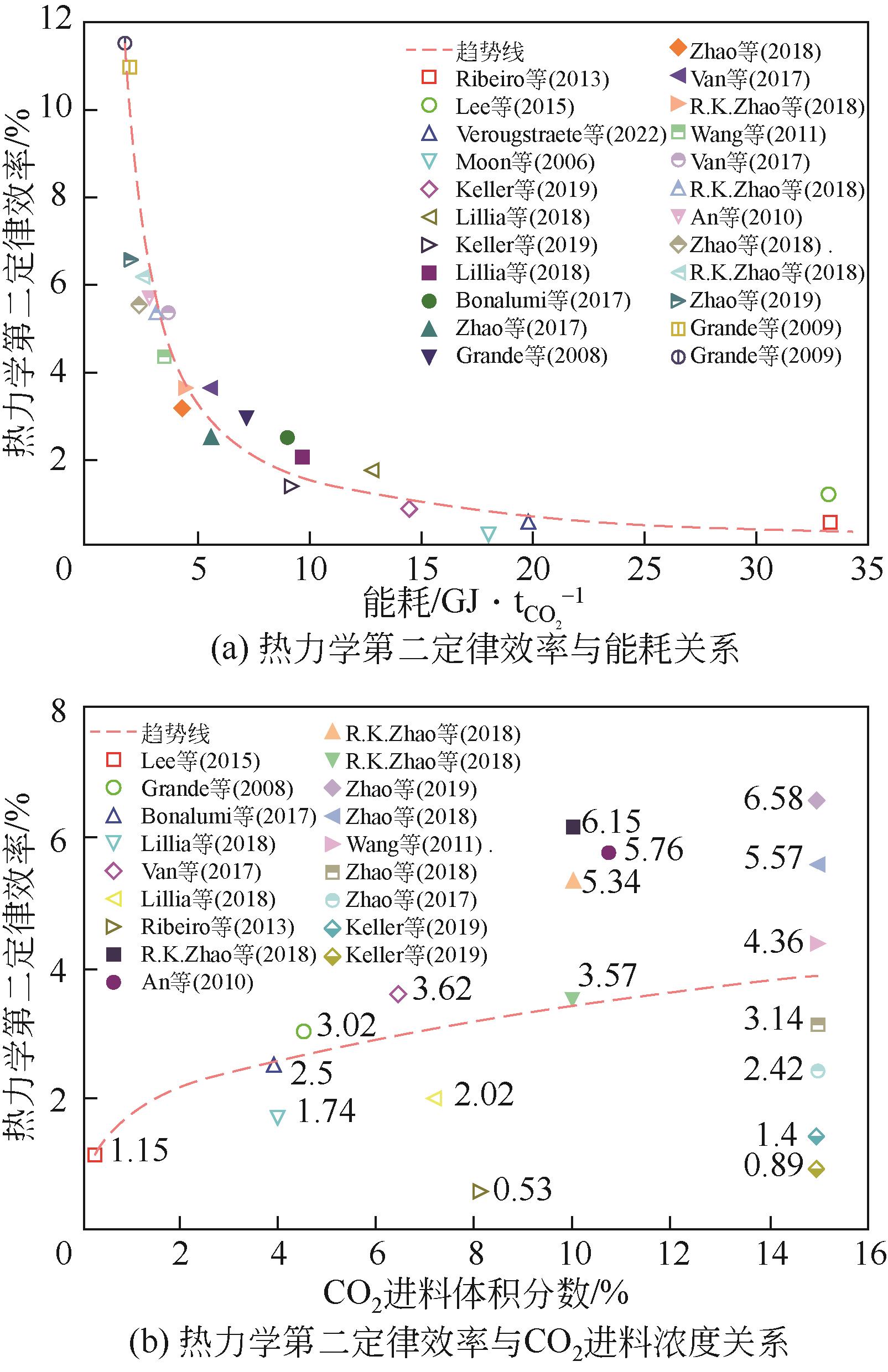
| 年份 | 材料 | 循环结构 | CO2进料 体积分数/% | 回收率/% | 纯度/% | 生产率 /kg·s-1·kg-1 | 能耗 / | η /% | η2nd① /% |
|---|---|---|---|---|---|---|---|---|---|
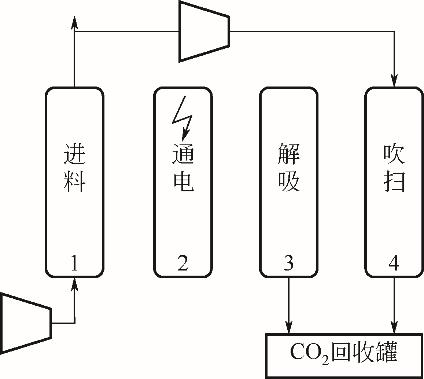
| 2006 | 活性炭纤维 | 五步VESA | 50 | 73.00 | — | — | 18.04 | — | 0.32 |
| 2008 | 活性炭 | 四步ESA | 4.51 | 89.52 | 16.36 | 1.54×10-3 | 7.23 | — | 3.02 |
| 2009 | 70%沸石/30%石墨 | 六步ESA | 3.5 | 79.50 | 78.93 | — | 2.04 | — | 11.03 |
| 2009 | 80%沸石13X/20%活性炭 | 七步ESA | 3.5 | 70.00 | 89.70 | — | 1.90 | — | 11.52 |
| 2010 | 66%活性炭纤维/34%酚醛树脂 | 四步ESA | 10.75 | 95.00 | — | — | 3.00 | — | 5.76 |
| 2011 | 沥青基球形活性炭 | 五步VESA | 15 | 95.00 | — | — | 3.50 | — | 4.36 |
| 2013 | 18%沸石/82%活性炭 | 六步ESA | 8.1 | 81.40 | 46.60 | — | 33.30 | 32.70 | 0.53 |
| 2015 | 炭 | 四步ESA | 0.3 | 95.00 | — | — | 33.10 | 64.00 | 1.15 |
| 2017 | 分子筛13X | 八步ESA | 3.96 | 89.90 | 95.10 | — | 9.05 | 2.50 | |
| 2017 | 活性炭 | 四步ESA | 15 | 76.00 | 52.00 | — | 5.64 | 47.00 | 2.42 |
| 2017 | 70%沸石13X/30%活性炭 | 四步ESA | 3.5 | 90.00 | — | — | 5.47 | — | 3.62 |
| 2017 | 70%TRI-PE-MCM-41/30%活性炭 | 四步ESA | 6.4 | 90.00 | — | — | 3.67 | — | 5.40 |
| 2018 | 80%沸石13X/20%活性炭 | 八步ESA | 3.96 | 91.20 | 94.70 | 9.60×10-3 | 13.05 | — | 1.74 |
| 2018 | 80%沸石13X/20%活性炭 | 八步ESA | 7.26 | 93.60 | 95.60 | 1.38×10-2 | 9.64 | — | 2.02 |
| 2018 | 70%NaUSY沸石/30%活性炭 | 四步ESA | 15 | 80.00 | 44.00 | 3.47×10-4 | 2.50 | 75.00 | 5.57 |
| 2018 | 活性炭 | 四步ESA | 15 | 80.00 | 53.00 | 5.86×10-4 | 4.43 | 18.40 | 3.14 |
| 2018 | 活性炭 | 四步ESA | 10 | — | — | — | 4.57 | 34.67 | 3.57 |
| 2018 | 18%沸石13X/82%活性炭 | 四步ESA | 10 | — | — | — | 3.20 | 39.51 | 5.34 |
| 2018 | 70%沸石NaUSY/30%活性炭 | 四步ESA | 10 | — | — | — | 2.78 | 56.22 | 6.15 |
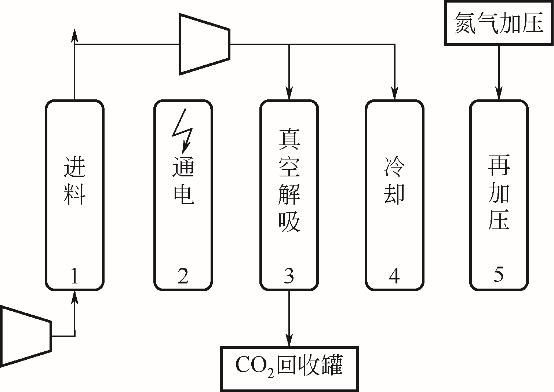
| 2019 | 浸渍PEI的碳化硅纤维 | 三步ESA | 15 | 62.00 | 81.00 | — | 14.50 | — | 0.89 |
| 2019 | 沸石填充的碳纳米管 | 三步ESA | 15 | 62.00 | 81.00 | — | 9.20 | — | 1.40 |
| 2019 | 82%沸石H-ZSM-5/18%活性炭 | 五步VESA | 15 | 72.00 | 33.00 | 4.80×10-4 | 2.04 | — | 6.58 |
| 2021 | 50%沸石13X/50%活性炭 | — | 15 | — | — | — | — | 19.00 | — |
| 2021 | 50%沸石13X/50%石墨 | — | 15 | — | — | — | — | 34.00 | — |
| 2022 | 47.5%沸石47.5%活性炭5%黏合剂 | — | 15 | — | — | 1.98×10-4 | — | — | — |
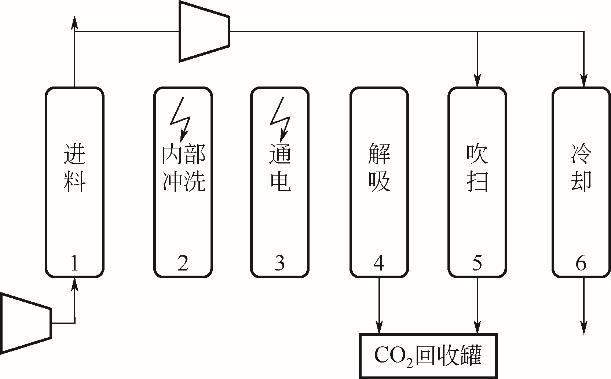
| 2022 | 50%硅酸钾/50%活性炭 | 四步ESA | 30 | 92.10 | — | 19.90 | — | 0.53 |
表2 ESA碳捕集系统热力学性能分析总结
| 年份 | 材料 | 循环结构 | CO2进料 体积分数/% | 回收率/% | 纯度/% | 生产率 /kg·s-1·kg-1 | 能耗 / | η /% | η2nd① /% |
|---|---|---|---|---|---|---|---|---|---|
| 2006 | 活性炭纤维 | 五步VESA | 50 | 73.00 | — | — | 18.04 | — | 0.32 |
| 2008 | 活性炭 | 四步ESA | 4.51 | 89.52 | 16.36 | 1.54×10-3 | 7.23 | — | 3.02 |
| 2009 | 70%沸石/30%石墨 | 六步ESA | 3.5 | 79.50 | 78.93 | — | 2.04 | — | 11.03 |
| 2009 | 80%沸石13X/20%活性炭 | 七步ESA | 3.5 | 70.00 | 89.70 | — | 1.90 | — | 11.52 |
| 2010 | 66%活性炭纤维/34%酚醛树脂 | 四步ESA | 10.75 | 95.00 | — | — | 3.00 | — | 5.76 |
| 2011 | 沥青基球形活性炭 | 五步VESA | 15 | 95.00 | — | — | 3.50 | — | 4.36 |
| 2013 | 18%沸石/82%活性炭 | 六步ESA | 8.1 | 81.40 | 46.60 | — | 33.30 | 32.70 | 0.53 |
| 2015 | 炭 | 四步ESA | 0.3 | 95.00 | — | — | 33.10 | 64.00 | 1.15 |
| 2017 | 分子筛13X | 八步ESA | 3.96 | 89.90 | 95.10 | — | 9.05 | 2.50 | |
| 2017 | 活性炭 | 四步ESA | 15 | 76.00 | 52.00 | — | 5.64 | 47.00 | 2.42 |
| 2017 | 70%沸石13X/30%活性炭 | 四步ESA | 3.5 | 90.00 | — | — | 5.47 | — | 3.62 |
| 2017 | 70%TRI-PE-MCM-41/30%活性炭 | 四步ESA | 6.4 | 90.00 | — | — | 3.67 | — | 5.40 |
| 2018 | 80%沸石13X/20%活性炭 | 八步ESA | 3.96 | 91.20 | 94.70 | 9.60×10-3 | 13.05 | — | 1.74 |
| 2018 | 80%沸石13X/20%活性炭 | 八步ESA | 7.26 | 93.60 | 95.60 | 1.38×10-2 | 9.64 | — | 2.02 |
| 2018 | 70%NaUSY沸石/30%活性炭 | 四步ESA | 15 | 80.00 | 44.00 | 3.47×10-4 | 2.50 | 75.00 | 5.57 |
| 2018 | 活性炭 | 四步ESA | 15 | 80.00 | 53.00 | 5.86×10-4 | 4.43 | 18.40 | 3.14 |
| 2018 | 活性炭 | 四步ESA | 10 | — | — | — | 4.57 | 34.67 | 3.57 |
| 2018 | 18%沸石13X/82%活性炭 | 四步ESA | 10 | — | — | — | 3.20 | 39.51 | 5.34 |
| 2018 | 70%沸石NaUSY/30%活性炭 | 四步ESA | 10 | — | — | — | 2.78 | 56.22 | 6.15 |
| 2019 | 浸渍PEI的碳化硅纤维 | 三步ESA | 15 | 62.00 | 81.00 | — | 14.50 | — | 0.89 |
| 2019 | 沸石填充的碳纳米管 | 三步ESA | 15 | 62.00 | 81.00 | — | 9.20 | — | 1.40 |
| 2019 | 82%沸石H-ZSM-5/18%活性炭 | 五步VESA | 15 | 72.00 | 33.00 | 4.80×10-4 | 2.04 | — | 6.58 |
| 2021 | 50%沸石13X/50%活性炭 | — | 15 | — | — | — | — | 19.00 | — |
| 2021 | 50%沸石13X/50%石墨 | — | 15 | — | — | — | — | 34.00 | — |
| 2022 | 47.5%沸石47.5%活性炭5%黏合剂 | — | 15 | — | — | 1.98×10-4 | — | — | — |
| 2022 | 50%硅酸钾/50%活性炭 | 四步ESA | 30 | 92.10 | — | 19.90 | — | 0.53 |
| 1 | 赵小令, 肖晋宇, 侯金鸣, 等. 中国二氧化碳捕集利用和封存技术经济性与规模预测[J]. 石油勘探与开发, 2023, 50(3): 657-668. |
| ZHAO Xiaoling, XIAO Jinyu, HOU Jinming, et al. Economic and scale prediction of CO2 capture, utilization and storage technologies in China[J]. Petroleum Exploration and Development, 2023, 50(3): 657-668. | |
| 2 | 武永光. CCUS技术进展和应用情况[J]. 当代化工研究, 2022(11): 118-120. |
| WU Yongguang. Advances and application of CCUS technology[J]. Modern Chemical Research, 2022(11): 118-120. | |
| 3 | REGUFE Maria João, FERREIRA Alexandre F P, LOUREIRO José Miguel, et al. Development of hybrid materials with activated carbon and zeolite 13X for CO2 capture from flue gases by electric swing adsorption[J]. Industrial & Engineering Chemistry Research, 2020, 59(26): 12197-12211. |
| 4 | 陆诗建, 刘苗苗, 刘玲, 等. 烟气胺法CO2捕集技术进展与未来发展趋势[J]. 化工进展, 2023, 42(1): 435-444. |
| LU Shijian, LIU Miaomiao, LIU Ling, et al. Progress and future development trend of amine method of CO2 capture technology from flue gas[J]. Chemical Industry and Engineering Progress, 2023, 42(1): 435-444. | |
| 5 | JIANG L, WANG R Q, GONZALEZ-DIAZ A, et al. Comparative analysis on temperature swing adsorption cycle for carbon capture by using internal heat/mass recovery[J]. Applied Thermal Engineering, 2020, 169: 114973. |
| 6 | MENDES Diogo N D L, GASPAR Ana, FERREIRA Isabel, et al. 3D-Printed hybrid zeolitic/carbonaceous electrically conductive adsorbent structures[J]. Chemical Engineering Research and Design, 2021, 174: 442-453. |
| 7 | 陈旭, 杜涛, 李刚, 等. 吸附工艺在碳捕集中的应用现状[J]. 中国电机工程学报, 2019, 39(S1): 155-163. |
| CHEN Xu, DU Tao, LI Gang, et al. Application of adsorption technology on carbon capture[J]. Proceedings of the CSEE, 2019, 39(S1): 155-163. | |
| 8 | FABUSS B M, DUBOIS W H. Carbon adsorption-electrodesorption process[C]//In: 63rd Annual Meeting of the Air Pollution Control Association. St. Louis, MO:1970. |
| 9 | GRANDE Carlos A, RIBEIRO Rui P P L, RODRIGUES Alírio E. Challenges of electric swing adsorption for CO2 capture[J]. ChemSusChem, 2010, 3(8): 892-898. |
| 10 | RIBEIRO R P P L, GRANDE C A, RODRIGUES A E. Electric swing adsorption for gas separation and purification: A review[J]. Separation Science and Technology, 2014, 49(13): 1985-2002. |
| 11 | ZHAO Qinghu, WU Fan, XIE Ke, et al. Synthesis of a novel hybrid adsorbent which combines activated carbon and zeolite NaUSY for CO2 capture by electric swing adsorption (ESA)[J]. Chemical Engineering Journal, 2018, 336: 659-668. |
| 12 | YANG Meng, MA Chao, XU Mimi, et al. Recent advances in CO2 adsorption from air: A review[J]. Current Pollution Reports, 2019, 5(4): 272-293. |
| 13 | MOON Seung-Hyun, SHIM Jae-Woon. A novel process for CO2/CH4 gas separation on activated carbon fibers—Electric swing adsorption[J]. Journal of Colloid and Interface Science, 2006, 298(2): 523-528. |
| 14 | GRANDE Carlos A, RIBEIRO Rui P P L, RODRIGUES Alírio E. CO2 Capture from NGCC power stations using electric swing adsorption (ESA)[J]. Energy & Fuels, 2009, 23(5): 2797-2803. |
| 15 | AN Hui, FENG Bo. Desorption of CO2 from activated carbon fibre–phenolic resin composite by electrothermal effect[J]. International Journal of Greenhouse Gas Control, 2010, 4(1): 57-63. |
| 16 | WANG Zhinan, ZHAN Liang, GE Ming, et al. Pith based spherical activated carbon for CO2 removal from flue gases[J]. Chemical Engineering Science, 2011, 66(22): 5504-5511. |
| 17 | PLAZA M G, THURECHT K J, PEVIDA C, et al. Influence of oxidation upon the CO2 capture performance of a phenolic-resin-derived carbon[J]. Fuel Processing Technology, 2013, 110: 53-60. |
| 18 | RIBEIRO R P P L, GRANDE C A, RODRIGUES A E. Activated carbon honeycomb monolith—Zeolite 13X hybrid system to capture CO2 from flue gases employing Electric Swing Adsorption[J]. Chemical Engineering Science, 2013, 104: 304-318. |
| 19 | LEE Tae Soo, CHO Jung Hyuk, CHI Suk Hwan. Carbon dioxide removal using carbon monolith as electric swing adsorption to improve indoor air quality[J]. Building and Environment, 2015, 92: 209-221. |
| 20 | SEVANTHI Ritesh, IRIN Fahmida, PARVIZ Dorsa, et al. Electrical Current stimulated desorption of carbon dioxide adsorbed on graphene based structures[J]. RSC Advances, 2016, 6(49): 43401-43407. |
| 21 | WANG Mei, YAO Liwen, WANG Jitong, et al. Adsorption and regeneration study of polyethylenimine-impregnated millimeter-sized mesoporous carbon spheres for post-combustion CO2 capture[J]. Applied Energy, 2016, 168: 282-290. |
| 22 | MASALA Alessio, VITILLO Jenny G, MONDINO Giorgia, et al. Conductive ZSM-5-based adsorbent for CO2 capture: Active phase vs monolith[J]. Industrial & Engineering Chemistry Research, 2017, 56(30): 8485-8498. |
| 23 | JALILOV Almaz S, LI Yilun, TIAN Jian, et al. Ultra-high surface area activated porous asphalt for CO2 capture through competitive adsorption at high pressures[J]. Advanced Energy Materials, 2017, 7(1): 1600693. |
| 24 | ZHAO Qinghu, WU Fan, HE Yingdian, et al. Impact of operating parameters on CO2 capture using carbon monolith by electrical swing adsorption technology (ESA)[J]. Chemical Engineering Journal, 2017, 327: 441-453. |
| 25 | ZHAO Ruikai, LIU Longcheng, ZHAO Li, et al. Thermodynamic analysis on carbon dioxide capture by electric swing adsorption (ESA) technology[J]. Journal of CO2 Utilization, 2018, 26: 388-396. |
| 26 | KELLER L, LOHAUS T, ABDULY L, et al. Electrical swing adsorption on functionalized hollow fibers[J]. Chemical Engineering Journal, 2019, 371: 107-117. |
| 27 | ZHAO Qinghu, WU Fan, Yuhan MEN, et al. CO2 capture using a novel hybrid monolith (H-ZSM5/activated carbon) as adsorbent by combined vacuum and electric swing adsorption (VESA)[J]. Chemical Engineering Journal, 2019, 358: 707-717. |
| 28 | REGUFE Maria João, FERREIRA Alexandre F P, LOUREIRO José Miguel, et al. Electrical conductive 3D-printed monolith adsorbent for CO2 capture[J]. Microporous and Mesoporous Materials, 2019, 278: 403-413. |
| 29 | ARAOZ María Emilse, MARCIAL Adrián Facundo, TREJO GONZALEZ José Adolfo, et al. Renewable and electroactive biomass-derived tubes for CO2 capture in agroindustrial processes[J]. ACS Sustainable Chemistry & Engineering, 2021, 9(23): 7759-7768. |
| 30 | PEREIRA Ana, FERREIRA Alexandre F P, RODRIGUES Alírio, et al. Evaluation of the potential of a 3D-printed hybrid zeolite 13X/activated carbon material for CO2/N2 separation using electric swing adsorption[J]. Chemical Engineering Journal, 2022, 450: 138197. |
| 31 | HIRST Edward A, ALISON Taylor, ROBERT Mokaya. A simple flash carbonization route for conversion of biomass to porous carbons with high CO2 storage capacity[J]. Journal of Materials Chemistry A, 2018, 6(26): 12393-12403. |
| 32 | HUANG Ning, Day Gregory, YANG Xinyu, et al. Engineering porous organic polymers for carbon dioxide capture[J]. Science China Chemistry, 2017, 60(8): 1007-1014. |
| 33 | QI Shichao, LIU Yu, PENG A, et al. Fabrication of porous carbons from mesitylene for highly efficient CO2 capture: A rational choice improving the carbon loop[J]. Chemical Engineering Journal, 2019, 361: 945-952. |
| 34 | SOLYANIKOVA A S, Yu Chayka M, BORYAK A V, et al. Composite electrodes of electrochemical capacitors based on carbon materials with different structure[J]. Russian Journal of Electrochemistry, 2014, 50(5): 419-428. |
| 35 | WU Junye, ZHU Xuancan, YANG Fan, et al. Shaping techniques of adsorbents and their applications in gas separation: A review[J]. Journal of Materials Chemistry A, 2022, 10(43): 22853-22895. |
| 36 | SAYSSET S, GREVILLOT G, LAMINE A S. In adsorption of volatile organic compounds on carbonaceous adsorbent and desorption by direct joule effect[C]//2nd European Congress of Chemical Engineering. Montpellier, France :1999. |
| 37 | MENKA Petkovska, DANIJELA Antov-Bozalo, Markovic ANA, et al. Multiphysics modeling of electric-swing adsorption system with in-vessel condensation[J]. Adsorption, 2007, 13(3): 357-372. |
| 38 | GRANDE C, RODRIGUES A. Electric Swing Adsorption for CO2 removal from flue gases[J]. International Journal of Greenhouse Gas Control, 2008, 2(2): 194-202. |
| 39 | PETERS Martin, GEITNER Christian, VERHOEVEN Birte Friederike. Experimental investigation of electrothermal desorption of steel mill gases from activated carbon[J]. Chemie Ingenieur Technik, 2022, 94(10): 1458-1465. |
| 40 | RAHIMI Mahshid, SINGH Jayant K, Florian MÜLLER-PLATHE. CO2 adsorption on charged carbon nanotube arrays: A possible functional material for electric swing adsorption[J]. The Journal of Physical Chemistry C, 2015, 119(27): 15232-15239. |
| 41 | KRETZSCHMAR Ansgar, SELMERT Victor, WEINRICH Henning, et al. Study of CO2 sorption kinetics on electrospun polyacrylonitrile-based carbon nanofibers[J]. Chemical Engineering & Technology, 2021, 44(7): 1168-1177. |
| 42 | Adilla Rashidi NOR, SUZANA Yusup, AZRY Borhan, et al. Experimental and modelling studies of carbon dioxide adsorption by porous biomass derived activated carbon[J]. Clean Technologies and Environmental Policy, 2014, 16(7): 1353-1361. |
| 43 | ERBEN Johannes, HEUBNER Alenica, THIELE Simon, et al. Activation of electrospun carbon fibers: The effect of fiber diameter on CO2 and steam reaction kinetics[J]. Journal of Polymer Research, 2021, 28(4): 1-14. |
| 44 | BONALUMI Davide, LILLIA Stefano, MANZOLINI Giampaolo, et al. Innovative process cycle with zeolite (MS13X) for post combustion adsorption[J]. Energy Procedia, 2017, 114: 2211-2218. |
| 45 | RIBEIRO R P P L, GRANDE C A, RODRIGUES A E. Electrothermal performance of an activated carbon honeycomb monolith[J]. Chemical Engineering Research and Design, 2012, 90(11): 2013-2022. |
| 46 | TRICKETT Christopher A, HELAL Aasif, AL-MAYTHALONY Bassem A, et al. The chemistry of metal-organic frameworks for CO2 capture, regeneration and conversion[J]. Nature Reviews Materials, 2017, 2: 17045. |
| 47 | LI Hailian, EDDAOUDI Mohamed, O’KEEFFE M, et al. Design and synthesis of an exceptionally stable and highly porous metal-organic framework[J]. Nature, 1999, 402(6759): 276-279. |
| 48 | MILLWARD Andrew R, YAGHI Omar M. Metal-organic frameworks with exceptionally high capacity for storage of carbon dioxide at room temperature[J]. Journal of the American Chemical Society, 2005, 127(51): 17998-17999. |
| 49 | QUAN Wenying, HOLMES Hannah E, ZHANG Fengyi, et al. Scalable formation of diamine-appended metal-organic framework hollow fiber sorbents for postcombustion CO2 capture[J]. JACS Au, 2022, 2: 1350-1358. |
| 50 | TAO Yingle, HUANG Guoshun, LI Qiangqiang, et al. Localized electrical induction heating for highly efficient synthesis and regeneration of metal-organic frameworks[J]. ACS Applied Materials & Interfaces, 2020, 12(3): 4097-4104. |
| 51 | ALAIN Goeppert, MIKLOS Czaun, SURYA Prakash G K, et al. Air as the renewable carbon source of the future: An overview of CO2 capture from the atmosphere[J]. Energy & Environmental Science, 2012, 5(7): 7833-7853. |
| 52 | ZHANG Zhonghua, MA Xiaoliang, WANG Dongxiang, et al. Development of silica-gel-supported polyethylenimine sorbents for CO2 capture from flue gas[J]. AIChE Journal, 2012, 58(8): 2495-2502. |
| 53 | CHAEHOON Kim, Sung Cho HAE, CHANG Shuai, et al. An ethylenediamine-grafted Y zeolite: A highly regenerable carbon dioxide adsorbent via temperature swing adsorption without urea formation[J]. Energy & Environmental Science, 2016, 9(5): 1803-1811. |
| 54 | MCDONALD Thomas M, MASON Jarad A, KONG Xueqian, et al. Cooperative insertion of CO2 in diamine-appended metal-organic frameworks[J]. Nature, 2015, 519(7543): 303-308. |
| 55 | HICKS Jason C, DRESE Jeffrey H, FAUTH Daniel J, et al. Designing adsorbents for CO2 capture from flue gas-hyperbranched aminosilicas capable of capturing CO2 reversibly[J]. Journal of the American Chemical Society, 2008, 130(10): 2902-2903. |
| 56 | YOUSSEF Belmabkhout, ABDELHAMID Sayari. Effect of pore expansion and amine functionalization of mesoporous silica on CO2 adsorption over a wide range of conditions[J]. Adsorption, 2009, 15(3): 318-328. |
| 57 | GOEPPERT Alain, CZAUN Miklos, Robert B MAY, et al. Carbon dioxide capture from the air using a polyamine based regenerable solid adsorbent[J]. Journal of the American Chemical Society, 2011, 133(50): 20164-20167. |
| 58 | YU Cheng-Hsiu, HUANG Chih-Hung, TAN Chung-Sung. A review of CO2 capture by absorption and adsorption[J]. Aerosol and Air Quality Research, 2012, 12(5): 745-769. |
| 59 | Rodrigo SERNA-GUERRERO, SAYARI Abdelhamid. Modeling adsorption of CO2 on amine-functionalized mesoporous silica. 2: Kinetics and breakthrough curves[J]. Chemical Engineering Journal, 2010, 161(1/2): 182-190. |
| 60 | LILLIA S, BONALUMI D, GRANDE C, et al. A comprehensive modeling of the hybrid temperature electric swing adsorption process for CO2 capture[J]. International Journal of Greenhouse Gas Control, 2018, 74: 155-173. |
| 61 | ZHAO Ruikai, DENG Shuai, LIU Yinan, et al. Carbon pump: Fundamental theory and applications[J]. Energy, 2017, 119: 1131-1143. |
| 62 | GRANDE Carlos A, RIBEIRO Rui P L, OLIVEIRA Eduardo L G, et al. Electric swing adsorption as emerging CO2 capture technique[J]. Energy Procedia, 2009, 1(1): 1219-1225. |
| 63 | VAN DER SPEK Mijndert, ANDREA Ramirez, Faaij ANDRÉ. Challenges and uncertainties of ex ante techno-economic analysis of low TRL CO2 capture technology: Lessons from a case study of an NGCC with exhaust gas recycle and electric swing adsorption[J]. Applied Energy, 2017, 208: 920-934. |
| 64 | VEROUGSTRAETE Brieuc, SCHOUKENS Matthias, SUTENS Ben, et al. Electrical swing adsorption on 3D-printed activated carbon monoliths for CO2 capture from biogas[J]. Separation and Purification Technology, 2022, 299: 121660. |
| [1] | 戴欢涛, 曹苓玉, 游新秀, 徐浩亮, 汪涛, 项玮, 张学杨. 木质素浸渍柚子皮生物炭吸附CO2特性[J]. 化工进展, 2023, 42(S1): 356-363. |
| [2] | 董佳宇, 王斯民. 超声强化对二甲苯结晶特性及调控机理实验[J]. 化工进展, 2023, 42(9): 4504-4513. |
| [3] | 白亚迪, 邓帅, 赵睿恺, 赵力, 杨英霞. 变温吸附碳捕集机组标准化测试方案探讨及性能实验[J]. 化工进展, 2023, 42(7): 3834-3846. |
| [4] | 张雪伟, 黄亚继, 许月阳, 程好强, 朱志成, 李金壘, 丁雪宇, 王圣, 张荣初. 碱性吸附剂对燃煤烟气中SO3的吸附特性[J]. 化工进展, 2023, 42(7): 3855-3864. |
| [5] | 陆洋, 周劲松, 周启昕, 王瑭, 刘壮, 李博昊, 周灵涛. CeO2/TiO2吸附剂煤气脱汞产物的浸出规律[J]. 化工进展, 2023, 42(7): 3875-3883. |
| [6] | 顾诗亚, 董亚超, 刘琳琳, 张磊, 庄钰, 都健. 考虑中间节点的碳捕集管路系统设计与优化[J]. 化工进展, 2023, 42(6): 2799-2808. |
| [7] | 杨许召, 李庆, 袁康康, 张盈盈, 韩敬莉, 吴诗德. 含Gemini离子液体低共熔溶剂热力学性质[J]. 化工进展, 2023, 42(6): 3123-3129. |
| [8] | 王久衡, 荣鼐, 刘开伟, 韩龙, 水滔滔, 吴岩, 穆正勇, 廖徐情, 孟文佳. 水蒸气强化纤维素模板改性钙基吸附剂固碳性能及强度[J]. 化工进展, 2023, 42(6): 3217-3225. |
| [9] | 马润梅, 杨海超, 李正大, 李双喜, 赵祥, 章国庆. 表面强化镀层对高速轴承腔密封端面变形及摩擦磨损影响分析[J]. 化工进展, 2023, 42(4): 1688-1697. |
| [10] | 符乐, 杨阳, 徐文青, 耿錾卜, 朱廷钰, 郝润龙. 新型相变有机胺吸收捕集CO2技术研究进展[J]. 化工进展, 2023, 42(4): 2068-2080. |
| [11] | 贺山明, 潘界昌, 徐国钻, 李文君, 梁勇. 粗钨酸钠溶液亚铁盐沉淀法除铬、钒的热力学分析及实验验证[J]. 化工进展, 2023, 42(4): 2171-2179. |
| [12] | 赵重阳, 赵磊, 石详文, 黄俊, 李治尧, 沈凯, 张亚平. O2/H2O/SO2 对改性富铁凹凸棒石高温吸附PbCl2 的影响[J]. 化工进展, 2023, 42(4): 2190-2200. |
| [13] | 王晓月, 张伟敏, 姚正阳, 郭晓宏, 李聪明. 逆水煤气变换反应研究进展[J]. 化工进展, 2023, 42(3): 1583-1594. |
| [14] | 尚玉, 肖满, 崔秋芳, 涂特, 晏水平. CO2捕集工艺中热再生气余热的PVDF/BN-OH平板复合膜回收特性[J]. 化工进展, 2023, 42(3): 1618-1628. |
| [15] | 孙潇, 朱光涛, 裴爱国. 氢液化装置产业化与研究进展[J]. 化工进展, 2023, 42(3): 1103-1117. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||